Abstract
The adult heart is a vital and highly specialized organ of the human body, with limited capability of self-repair and regeneration in case of injury or disease. Engineering biomimetic cardiac tissue to regenerate the heart has been an ambition in the field of tissue engineering, tracing back to the 1990s. Increased understanding of human stem cell biology and advances in process engineering have provided an unlimited source of cells, particularly cardiomyocytes, for the development of functional cardiac muscle, even though pluripotent stem cell-derived cardiomyocytes poorly resemble those of the adult heart. This review outlines key biology-inspired strategies reported to improve cardiomyocyte maturation features and current biofabrication approaches developed to engineer clinically relevant cardiac tissues. It also highlights the potential use of this technology in drug discovery science and disease modeling as well as the current efforts to translate it into effective therapies that improve heart function and promote regeneration.
Subject terms: Tissue engineering, Induced pluripotent stem cells, Stem-cell research, Stem-cell biotechnology, Regenerative medicine
Introduction
Cardiovascular diseases (CVDs) have been recognized as a major concern for global health among noncommunicable diseases1, and despite half-century of advances in cardiovascular science and preventive medicine, CVDs continue to yield high mortality and morbidity rates worldwide2. Revascularization interventions or pharmacological treatment allow in many cases to salvage the heart and prevent transplantation, even though such strategies are not recognized as a definitive cure3.
The adult human heart has a limited regenerative capacity4 and so regenerative medicine therapies have shed new hopes to repair or replace damaged hearts. The marginal benefits reported by cell-based5–8 and cell-free9–11 approaches have grossly been associated with inappropriate delivery and retention, as well as ineffective therapeutic efficacy12. In the past decades, tremendous advances have been witnessed in the field of tissue engineering (TE), especially concerning stem cell engineering, the development of functional biomaterials and biomimetic scaffolds, and the implementation of biofabrication tools for the generation of complex biological structures with high resolution13. This progress has set a solid background to rethink current therapeutic approaches and design new bioengineering therapies14. Nonetheless, creating fully matured and functional cardiac tissue in vitro is still challenging. Despite this, it is becoming possible to robustly engineer miniaturized tissue versions able to compete, if not replace, commonly used platforms to assess drug safety and model human physiopathology to guide efficient drug discovery15.
This review highlights recent progress in cardiac stem cell bioengineering, with a particular focus on the identification of essential requirements for the design of clinically relevant human cardiac tissues: (i) the cell sources, (ii) bioinspired strategies to generate cardiomyocytes (CMs) with improved maturity, and (iii) biofabrication methodologies. The potential use of this technology in drug discovery science and disease modeling as well as the current efforts to translate it into effective heart regeneration therapies is also discussed.
Cell sources to engineer cardiac tissue
Cardiac muscle is the functional unit of the heart, as it is involved in force generation and propagation of electrical signals through the CM-rich tissue to allow rhythmic pump contraction. Human cardiac muscle is composed of various cell types, the most abundant being CMs, pericytes, endothelial cells, and fibroblasts. The proportion of each cell population in the heart is still a matter of discussion, but there are evidences that CMs occupy the majority of the heart volume (roughly 75%) and account for 30–40% of all cells16. State-of-the-art analyses of single-cell/nuclei RNA-sequencing transcriptomic data of six heart regions across several unrelated individuals have clarified that CMs are the most prevalent cardiac cell population in the heart, while also highlighting a surprising cellular heterogeneity among CM, pericyte, endothelial, and fibroblast cell populations17. It appears that the cellular composition of cardiac muscle and its transcriptional signature varies across the heart in order to ensure that each anatomical region is highly specialized in a given function. Such complexity undeniably elevates the challenge of engineering artificial cardiac muscle.
Typically, engineering a contractile cardiac muscle requires foremost a source of functional CM. The first engineered cardiac tissue models relied on nonhuman cell cultures of different species, including cell lines of rat embryonic myoblasts (H9c218) and mouse CMs (HL-119) as well as primary CMs isolated from chicken embryos20 and neonatal rats21. Despite limitations regarding human tissue availability, human adult CMs can be isolated from myocardial biopsies22; however, soon after isolation, CMs undergo a profound structural and functional remodeling, leading to cell dedifferentiation and loss of viability23. Recently, applying near-physiologic electromechanical stimuli during 24 h proved capable of inactivating CM remodeling after isolation24. Long-term culture (up to 4 months) of adult human myocardial muscle strips subjected to biomimetic stimuli similarly prevented pronounced CM remodeling, even though changes at the structural, mechanical, and gene expression levels were detected25. Immortalized adult human ventricular CM cell lines have also been established by the fusion of primary cells with SV40-transformed human fibroblasts to create cardiac models26, but their poor recapitulation of human physiology discourages its use.
Due to their high self-renewal capacity and ability to generate any cell type of the human body, pluripotent stem cells (PSCs) are becoming an attractive alternative for cardiac TE. These PSCs can either be derived from the inner cell mass of the blastocyst, named embryonic stem cells (ESCs)27, or by forced expression of pluripotency genes through the delivery of the “reprogramming factors” Oct3/4, Sox2, Klf4, and c-Myc to somatic cells, termed induced PSCs (iPSCs)28,29. In particular, human iPSC (hiPSC) derivatives better capture patient-specific physiology30,31, besides surpassing the ethical concerns associated with human ESC (hESC). Acquired knowledge on stem cell biology together with the advances in PSC technology has allowed the establishment of robust differentiation protocols capable of obtaining every subtype of CM (ventricular, atrial, and pacemaker) with a high degree of purity32. Briefly, temporal modulation of the Wnt/β-catenin signaling pathway is usually performed by first promoting activation for mesoderm formation and subsequent inhibition for cardiac specification33,34. The use of small molecules (e.g., CHIR99021, IWP2, IWR1) to control the Wnt/β-catenin signaling pathway is currently preferred to growth factors/recombinant proteins due to their better diffusion properties and moderate cost32,35,36.
A lot of effort has been put to evaluate the maturity state of human PSC-derived CMs (hPSC-CMs) and it is unanimous that, after differentiation, these cells are more similar to fetal rather than adult CMs (Fig. 1). Adult ventricular CMs display a remarkably organized structure with sophisticated intracellular organelles optimized for contraction. Among these components are densely packed aligned sarcomeres37,38 within a hypertrophic39 (usually) binucleated40 cell; the sarcolemma and a vast network of transverse tubules (T-tubules)41,42 along the Z-lines to allow for fast depolarization; a functional longitudinal sarcoplasmic or endoplasmic reticulum (SR/ER) and terminal cisternae positioned near T-tubules adapted for quick calcium kinetics43; a high density of mitochondria near the contractile apparatus to improve ATP consumption44; and intercalated discs with well-developed cell junctions to promote electrical coupling45,46. hPSC-CMs have not shown this level of cellular complexity47–52, as they are less fit for contraction53,54 and are incapable of matching the metabolic demands of adult CMs55–57 needed for significant force generation58–61, which is apparent in their negative force–frequency relationship (FFR)62,63 and minimal post-rest potentiation (PRP)62. Due to a dissimilar ion-channel density, hPSC-CMs exhibit a distinct action potential (AP) profile from adult CMs64,65, resulting in asynchronous and spontaneous beating rate66, less negative resting membrane potential (RMP)67,68, slower upstroke velocity69, and irregular AP duration67,69,70. In fact, the immature ultrastructure of hPSC-CMs not only affects calcium handling71 due to an under-developed excitation–contraction coupling (ECC) machinery50,51,62,72 but it also slows AP propagation73,74 owing to the lack of organized electromechanical junctions compared to adult cardiac tissue75,76. Furthermore, these differences are seen at a transcriptomic level, with hPSC-CMs showing preferential expression of fetal sarcomeric gene isoforms (myosin heavy chain: MYH6 > MYH7; troponin I: TNNI1 > TNNI3; titin: TTN-N2BA > TTN-N2B) as well as lower expression of genes related with electrophysiological function (e.g., KCNJ2, KCND3, KCNJ8, KCNH7, SCN5A, HCN4, GJA1, JPH2) and ECC (e.g., CACNA1C, RYR2, ATP2A2, CASQ2, SLC8A1, PLN, ITPR3, CAV3, BIN1)77.
Fig. 1. Differences between human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs) and ventricular cardiomyocytes from adult cardiac tissue.
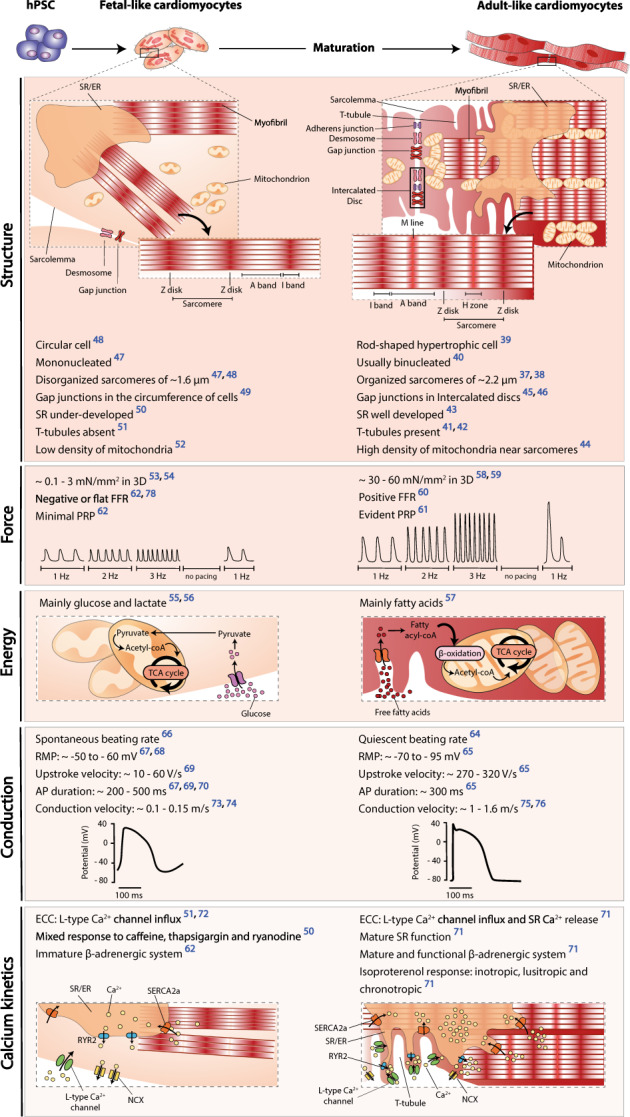
hPSC-CMs obtained from the differentiation of pluripotent stem cells present fetal-like features in respect to structural and ultrastructural organization, contractile force, metabolism, and electrophysiological function. AP action potential, ECC excitation–contraction coupling, FFR force–frequency relationship, hPSCs human pluripotent stem cells, NCX sodium–calcium exchanger, PRP post-rest potentiation, RMP resting membrane potential, RYR2 ryanodine receptor type 2, SERCA2a sarcoplasmic/endoplasmic reticulum calcium-ATPase 2a, SR/ER sarcoplasmic/endoplasmic reticulum.
The maturation defects of hPSC-CMs have gained increased attention over the past decade since their immaturity greatly limits their application in several areas of precision medicine. A better understanding of cardiac development has allowed the identification of relevant players that partake in the transformation of the fetal heart into a competent and efficient organ. Complete maturation of hPSC-CMs has yet to be reported; however, nature-inspired biological strategies and/or engineering solutions proposed over the past few years have undoubtedly made significant contributions to improve the maturation of these cells.
Bioinspired strategies to improve hPSC-CM maturity
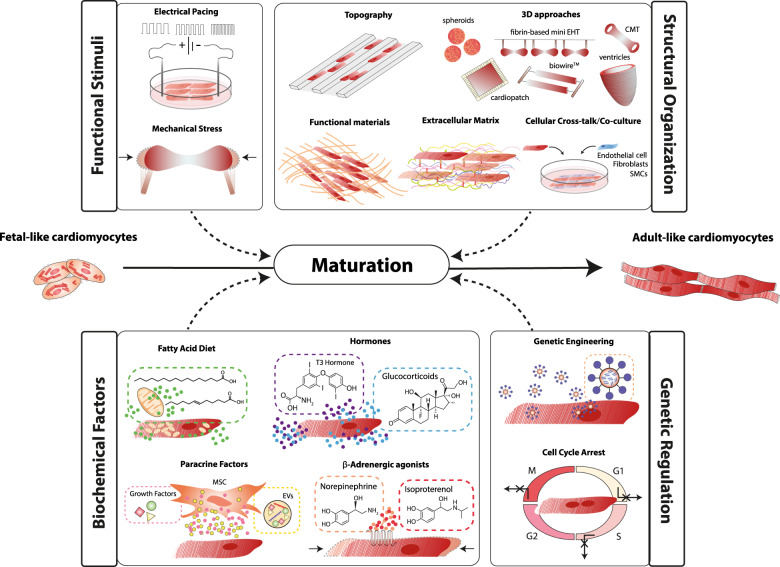
During cardiac development, several environmental factors including mechanical forces, electrical stimuli, biochemical factors’ gradients, extracellular matrix (ECM) remodeling, and heterotypic interactions drive CM maturation78. Maturation is the last phase of cardiac development and corresponds to a series of orchestrated phenotypic events that prepare the fetal heart for efficient and competent life-long pumping function. Some of these events are initiated in utero, such as physiologic hypertrophic growth, sarcomeric isoform switch, cell cycle exit, and a glycolytic-to-oxidative metabolic switch79. However, the highly specialized cellular machinery of adult CMs only fully develops years after birth46. Even though prolonging time in culture of hPSC-CMs (up to 1 year) improves structural and functional maturation47,80,81, these cells still show signs of a fetal phenotype. Early-stage hPSC-CMs transplanted in animal hearts were able to acquire adult-like features82,83, providing evidence of their plasticity and ability to be matured if the right environment is ensured. By attempting to mimic nature, various bioengineering approaches have been proposed to promote hPSC-CM maturation (Fig. 2).
Fig. 2. Bioengineering strategies to improve the maturation of cardiomyocytes derived from human pluripotent stem cells (hPSC-CMs).
hPSC-CMs can develop adult-like features with training based on functional stimuli, cues to guide their structural organization, relevant biochemical factors, and by manipulating the genetic program. CMT cardiac microtissue, EHT engineered heart tissue, EV extracellular vesicle, MSC mesenchymal stem cell, SMC smooth muscle cell, T3 triiodothyronine.
Functional stimuli
In cardiac tissue, CMs are responsive to external electrical stimuli and mechanical forces to perform their function, and these are initiated early during cardiac embryogenesis.
Electrical excitability is a key feature of CMs, dictating electromechanical coupling. The effect of exogenous electrical stimuli on CM maturation was first tested using neonatal rat CMs seeded in collagen sponges and exposed to pulse waves at 1 Hz for up to 8 days, resulting in cell alignment and elongation along the electrical field as well as the formation of intercalated discs with gap junctions expressing connexin-4384,85. Both neonatal rat CMs86,87 and hPSC-CMs88 have also shown a more mature phenotype after being stimulated with pulse frequencies >1 Hz. Interestingly, applying a supraphysiological pacing regimen up to 1 week (increasing pulse frequency from 1 to 6 Hz) to hPSC-CMs seeded in a collagen matrix markedly contributed to cell alignment, establishment of an organized contractile ultrastructure, improved SR/ER function, and electrophysiological profile89.
In turn, mechanical stress induces CM rhythmic contraction, which has a direct impact on physiologic hypertrophic growth, cell elongation, tissue alignment, and force generation. By casting neonatal rat CMs in a ring-shaped mixture of collagen and Matrigel® exposed to mechanical stretch, it was demonstrated for the first time the importance of applying stress to induce both ultrastructural and functional maturation of CM90. Since cylindrical casting molds provided little tissue deflection, mechanical stimulation via cyclic stretch is now achieved using fibrin-based hydrogels in flexible posts91 or in thin elastomeric frames92, allowing for auxotonic muscle contraction. These technologies were first established using rodent cells, but were successfully translated to hPSC-CMs62,93. hPSC-CMs show alignment along the hydrogel and sarcomeric organization62, a metabolic shift towards fatty acid consumption94, improved calcium drug sensitivity95, and force development62, even though the force generated (up to ~1 mN/mm2) is still relatively inferior to those of adult cardiac muscle strips (~30–60 mN/mm2)58,59. More recently, modulation of cyclic96 and passive stretching97 of hPSC-CMs revealed an impact on tissue functional performance, indicating that there might be ways to further improve tissue force generation. Of note, more complex systems simulating the hemodynamic loads of the cardiac cycle have also been used to improve hPSC-CM maturation98.
In addition, CMs can be electrically and mechanically stimulated simultaneously99–101, thereby enhancing maturation. In a recent work, hPSC-CMs subjected to combined cyclic stretch in elastomeric posts and a supraphysiological pacing regimen of 2 weeks (increasing pulse frequency from 2 to 6 Hz) resulted in these cells displaying adult-like features never reported before, such as a vast network of T-tubules, inotropic response to isoproterenol, a positive FFR, and noticeable PRP101. This represents the most advanced strategy reported for hPSC-CM maturation so far, although these more mature hPSC-CMs still presented inadequate contractile force generation ability (up to ~4 mN/mm2) when compared to adult counterparts.
Structural organization
Cardiac tissue function depends on a highly anisotropic architecture with orderly aligned myofibrils along the direction of muscle contraction, which is impossible to replicate when culturing CMs on standard and simple plastic two-dimensional (2D) surfaces. Promoting this level of cellular organization can be achieved using grooved patterned substrates where hPSC-CMs already showed elongation along the line patterns, electromechanical coupling with neighboring CMs, and electrophysiological improvements68,102. Of note, substrate stiffness, groove width, and cell density seem to have an impact on the structural maturation of hPSC-CMs103. In fact, the stiffness of a substrate can by itself modulate the mechanical load experienced by hPSC-CMs, affecting the cytoskeleton organization104 and contractility102. In addition, various natural (collagen20,89, fibrin91, and Matrigel®90,105) or synthetic (polyesters106,107, polyethylene glycol108, polylactones109, and elastomers110) biomaterials can be used to guide cardiac tissue structural organization, even though their properties (mechanical, topographical, and biocompatibility) influence the degree of maturation obtained. Unfortunately, none of these biomaterials can fully recapitulate the architecture and functional composition of the cardiac ECM. Still, artificial ECM111 or decellularized myocardial ECM112–114 has been reported to promote hPSC-CM maturation without biomimetic regimens.
Throughout cardiac development, CMs are in direct contact with other cell types. Even though non-myocytes occupy a relatively small volume in cardiac tissue (~25%), they are necessary for CM survival, contractile performance, cell–cell communication, vascular supply, and ECM deposition115. Coculture of hPSC-CMs with endothelial cells, either on Matrigel®-coated surfaces116,117 or Matrigel® hydrogels118, resulted in higher expression of proteins related with CM maturity116, contributed for ultrastructural organization116,117, improved contractility118, and calcium handling116,118.
Assembling hPSC-CMs into three-dimensional (3D) tissues greatly improves cell–cell communication with more physiological relevance, promoting structural, metabolic, and functional maturation. Engineered tissues can assume various architectures, such as cell sheets119, spheroids120,121 and organoids122, muscle strips in the form of rings90,123, ribbons63,124 or cables89,92, patches125,126, and even ventricle analogs127. A recent study using a patch platform reported, without exogenous stimulation, advanced hPSC-CM mechanical maturity with a contractile force closer to adult ventricular tissue (~17–22 mN/mm2)126, even though it had slightly negative FFR and an organized ECC apparatus without pronounced T-tubulation. Nonetheless, 3D approaches are extremely versatile and can be combined with other strategies to enhance maturation and tissue performance (e.g., intensive electrical training101, dynamic culture92, coculture with other cell types128–130). For instance, culturing fibroblasts and hPSC-CMs in muscle strips with a ratio of 1:3 improved cell–cell communication and tissue remodeling128, in addition to also contributing to hPSC-CM maturation. Intriguingly, the electrical pacing of hPSC-CM muscle strips in flexible posts requires a supporting network of fibroblasts, otherwise the tissue loses its integrity when electrically stimulated101,131. Due to the increasing relevance of 3D maturation-based strategies to the field, we will further detail 3D tissue fabrication methodologies in this review (see “Fabrication of biomimetic cardiac tissues”).
Biochemical factors
Several soluble and biological factors have been proposed to induce hPSC-CM maturation, such as the type of carbon substrate and hormones. In vivo, CM maturation is characterized by a metabolic shift from glycolysis to preferential fatty acid oxidation, a consequence of cellular hypertrophy and higher energetic demands56. hPSC-CMs display metabolic substrate plasticity that can be modulated to promote fatty acid β-oxidation132,133. Our group has demonstrated that hPSC-CM structural, functional, and metabolic maturation can be improved in fatty acid-containing medium with galactose to prevent lipotoxicity134, when compared with glucose-rich medium. Among hormones, triiodothyronine (T3) is essential during cardiac development135, and has revealed to play a key role in inducing hPSC-CM maturation136. Furthermore, by combining T3 with dexamethasone, a glucocorticoid hormone, noticeable improvements of SR/ER contribution in ECC and T-tubulation were detected, which otherwise was not seen with each hormone alone137.
Biological factors such as those secreted by neighboring cells within the cardiac niche can also positively impact CM maturation state. Recently, hPSC-CM and mesenchymal stem cell (MSC) coculture promoted cellular cross-talk via the release of cytokines and extracellular vesicles capable of modulating hPSC-CM sarcomeric organization, electrophysiology, and metabolism138. Another study used a spherical microtissue containing hPSC-CMs as well as cardiac-specific endothelial cells and fibroblasts, and revealed that this tri-cellular interaction upregulated the cAMP/β-adrenergic pathway130. An increase in intracellular cAMP levels consequently affected the assembly of connexin-43 gap junctions, besides improving hPSC-CM structural, contractile, metabolic, and electrophysiologic features. Taken together, by identifying key biological factors capable of inducing CM maturation, these studies underline the importance of heterotypic cell interaction in the cardiac microenvironment.
A less conventional approach to induce CM maturation has been the use of β-adrenergic agonists, namely norepinephrine139, isoproterenol140, and phenylephrine141,142. These compounds are capable of causing structural changes and cellular hypertrophy by inducing intensive workload, even though these effects are likely dose-dependent to avoid pathological remodeling and cytotoxicity.
Genetic regulation
Adult CMs have defined gene regulation mechanisms, ultimately dictating cardiac tissue phenotype and maturation. Overexpressing KCNJ2 during differentiation resulted in hPSC-CMs with hyperpolarized RMP and eliminated the AP proarrhythmic trait during repolarization, even though calcium handling and structural features were not improved due to early loss of automaticity143. In turn, calcium kinetics were significantly improved in hPSC-CMs by forced expression of CSQ2144. Instead of manipulating the transcriptome, altering the epigenetic state of hPSC-CMs using histone H3 deacetylase inhibitors also showed to promote adult-like expression of calcium handling and conduction genes145,146, even though reports regarding electrophysiologic improvements are inconsistent. Priming cells with polyinosinic-polycytidylic acid at the cardiac progenitor stage of differentiation strikingly accelerated structural, electrical, and metabolic maturation of hPSC-CMs, and this acceleration of the developmental clock was associated with increased H3K9ac activation that promoted earlier transcription of cardiac myofilament genes and the Notch ligand JAG1147. In another study, the inhibition of aberrantly upregulated factors involved in aerobic glycolysis promoted an hPSC-CM metabolic shift towards oxidative phosphorylation, and consequently features of functional maturation133. Moreover, the comprehensive analyses of micro-RNA (miR) levels throughout hPSC-CMs’ prolonged culture have identified those that become differentially expressed148,149, suggesting alternative routes to modulate gene expression to induce maturation. Let-7 family miRs150, miR-1151,152, miR-499152, and mir-200c153, and a combination of miRs (miR-125b, miR-199a, miR-221, and miR-222)154 have proved useful to improve hPSC-CM maturation features.
Soon after birth, CMs become nonproliferative cells due to extensive hypertrophic growth and remarkable sarcomere re-organization hindering cytokinesis. Ultimately, these changes result in an increase in binucleation and polyploidy. A recent work revealed that knockout of the ECM protein agrin in neonatal mice resulted in the loss of CM proliferation alongside sarcomere assembly and maturation155. As expected, administration of agrin to hPSC-CMs avoided loss of proliferation, but negatively affected gene expression and conduction velocity155. By promoting cell cycle cessation, mitomycin C treatment similarly increased sarcomerogenesis in neonatal rat-derived PSC-CMs156. Further work will be needed to elucidate how cell cycle arrest can promote hPSC-CM maturation other than structural improvements.
Fabrication of biomimetic cardiac tissues
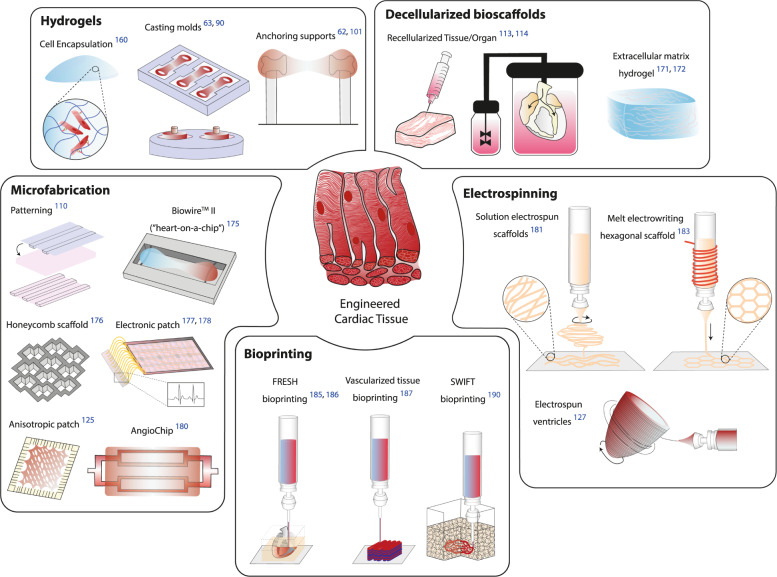
Inducing the maturation of hPSC-CMs is still a challenge in the field of cardiac TE; however, considerable progress has been achieved by seeking innovative approaches. Moving from simple beating cells to physiologically relevant tissues will require further efforts to engineer the complexity of the in vivo conditions. In the following section, we highlight the most promising techniques adopted to date to grow biomimetic cardiac tissue in the laboratory (Fig. 3). A more detailed overview of these current biofabrication methodologies, their advantages and disadvantages for translation to TE and regenerative medicine fields can be found in ref. 157.
Fig. 3. Overview of state-of-the-art biomimetic cardiac tissues and corresponding fabrication approaches.
Engineered cardiac tissues range from hydrogels capable of encouraging muscle fiber organization, decellularized scaffolds for tissue/organ bioengineering, microfabricated systems for promoting tissue anisotropy and vascularization, electrospun fibers for close mimicry of tissue architecture, and even printable tissue/miniaturized organs. FRESH Freeform Reversible Embedding of Suspended Hydrogels, SWIFT Sacrificial Writing Into Functional Tissue.
Hydrogel method
Hydrogels are the most widely used polymers for cardiac TE due to their biocompatible physical and chemical properties, including high water content, efficient gas and mass transfer (i.e., adequate exchange of oxygen and metabolites), ability to be molded into several geometries, easy to be modified/functionalized in order to include cell-binding sites (e.g., RGD integrin-binding domains and ECM proteins), and scalability158,159. Cardiac tissues can be fabricated by joint gelation of cells and a polymer160 or by having a casting mold to confine the cells in a given polymeric organization63,90. Typically, natural hydrogels89,91,101 are preferred for engineering cardiac tissues by promoting cell assembly and subsequent maturation, but functionalized synthetic polymers106 or hybrid biomaterials161,162 are also suitable options. Cells often self-organized within hydrogels in ways that are different from those found in native tissues, and so it can be advantageous to tailor the hydrogel’s microarchitecture to encourage anisotropic rather than isotropic tissue growth, which can be done by relying on simple methods such as freeze-drying, solvent casting, gas foaming, and unidirectional freezing163. Hydrogels are generally soft, with inferior mechanical compliance in comparison to the native heart tissue. Anchoring supports are useful not only to retain hydrogel conformation but also to provide a more adequate mechanical load to CM62,101 and facilitate force measurement87,131,164. Impressively, the resulting tissues can be several millimeters long (~7–9 mm62,101).
Decellularized bioscaffolds
The main aim of decellularization methodologies is to generate scaffolds with native ECM ultrastructure and composition while removing all cells and genetic material present in the native tissue. Several decellularization protocols have been described for nearly all tissues and organs in the body, and include physical, chemical, and/or enzymatic approaches. Discussing the effects of these agents is beyond the scope of this review (reviewed in refs. 165,166); however, it is important to highlight that the choice of optimal protocol for tissue decellularization depends on many factors, including tissue source, cellularity, composition, density, thickness, and donor age, and these overall impact the efficiency of the decellularization process and the resultant ECM composition.
Cardiac tissue or whole heart decellularization is typically accomplished by using ionic and nonionic detergents. Decellularization of a whole rat heart through perfusion167 and of thin slices of human myocardial tissue168 using sodium dodecyl sulfate (SDS) resulted in lower DNA content and maintenance of native ECM structure, when compared with approaches using other chemicals. Typically, decellularizing human cardiac tissue114 requires a higher SDS exposure time than animal-derived cardiac tissue169,170, and the reasons for this can be manyfold, such as higher collagen and lipidic content, a stiffer ECM, or donor age. Because of this, it is becoming clear that interspecies differences may hinder the use of decellularization protocols optimized in animals for human tissue. The most immediate application of decellularized myocardial sheets (~200–400 µm thickness113,114), muscle strips (15 mm length, 2.5 mm diameter114), or whole heart114 is scaffold recellularization. Even though myocardial sheets and strips were successfully recellularized with CMs, recellularization of a human heart is an unmet goal and the best attempt so far has been an ~500 million CM intramyocardial injection to an ~5 cm3 volume of an acellular heart scaffold114, which is way below the billions of CMs necessary to repopulate this organ. Nevertheless, isolated ECM can be broken down, solubilized with pepsin, and then assembled into a nanofibrous hydrogel171, inevitably destroying the structural and mechanical architecture of the ECM scaffold. Interestingly, decellularized human cardiac ECM requires additional steps of lipid removal before hydrogel formation172. Soluble ECM in 3D settings promoted CM maturation112, but has a wider application for scaffold functionalization and has shown versatility to be implemented in advanced manufacturing techniques169,173,174. Despite its promising applications, decellularized human cardiac ECM is scarce due to donor organ shortage, and therefore research often relies on off-the-shelf animal-derived matrices.
Microfabrication
Advances in microfabrication have allowed detailed engineering of material features in order to resemble tissue in vivo conditions. In particular, photolithography enables the precise transfer of geometrical shapes from a mask onto a substrate to obtain defined topographies for anisotropic tissue organization, while with soft lithography, substrates can be designed to include microchannels and complex microfluidic networks that are useful to recreate tissue vasculature. However, manufacturing such platforms can be time consuming, requires specialized equipment and facilities, and production may be costly.
The simpler systems use microfabrication to pattern material substrates, so as to ease CM spreading and sarcomere axial alignment. For instance, one of the initial attempts used polydimethylsiloxane (PDMS) thin films that contained an array of alternating 20-µm-wide lines, and these grooves substantially improved CM alignment and showed compliance during the cardiac cycle110. Currently, more sophisticated patterned culture platforms have been established, which synergize well with biochemical cues. In fact, circular chambers (ranging from 200 to 600 µm diameter), obtained through plasma etching of polyethylene glycol substrates, allowed geometrical confinement of hiPSC colonies and mediated morphogenesis of cardiac microchambers during differentiation122. Patterned culture chambers can also be useful to support hydrogel-based cardiac tissue formation (~3–4 mm length) and its long-term maturation (e.g., BiowireTM II175). Nonetheless, microfabrication methodologies can be harnessed to create complex tissues similar to the native myocardium. Pioneering work used laser microablation of poly(glycerol sebacate) membranes to produce an accordion-like honeycomb scaffold, which was designed by overlapping two 200 µm × 200 µm squares at a 45° angle176. The unconventional design was purposely conceived to closely resemble the heart anisotropy, and proved successful in guiding CM alignment and improving their mechano-microenvironment. In another study, a porous photomask (2.5 cm × 2.5 cm) was crafted based on cardiac fiber orientation of a specific epicardial plane retrieved from a 3D reconstruction of the human ventricle125. This mask was used to mold a PDMS substrate that allowed a patch-like cardiac tissue formation in a developmentally mimetic fashion. More contemporary improvements have advanced even further cardiac patch design by using conductive biomaterials with built-in nanoelectronics177,178 (~20 mm × 5 mm), which are practical for online electrophysiological recording and electromechanical pacing.
Simple fabrication technologies, such as hydrogels, are unable to integrate organized vasculature within engineered tissue; however, microfabricated cardiac tissues can be assembled with perfusable and highly branched endothelialized channels179,180 by relying on simple soft lithography approaches. In particular, the AngioChip device (5 mm × 3.1 mm × 150–300 µm), a biodegradable elastomer assembled with layer-by-layer 3D stamping, reported unprecedented functional vascularization of cardiac tissues, allowing perfusion through an open lumen, capillary outgrowth, enhanced permeability, structural integrity, and matrix remodeling180.
Despite the lack of standardized culture modes across these systems, remarkable tissue-level complexity can be achieved, as well as adequate tissue function for high-throughput analysis175.
Electrospinning
Electrospinning is a versatile method to fabricate ECM-like scaffolds as braided, woven, or knitted fibrous networks, from both naturally derived and synthetic materials. In solution electrospinning, the polymer is gravitationally forced to pass through an electrostatic field to generate a fibrous meshwork. Even though electrospun fibers frequently display inappropriate porosity compromising cell spreading and infiltration, certain parameters, like voltage and polymer flow rate, can be readily adjusted to fabricate the desired fibrous architecture. We have used solution electrospinning to produce poly(ε-caprolactone) (PCL) electrospun scaffolds (~2 cm × 2.5 cm × 229 nm), and the resulting fibers displayed an appropriate architecture that allowed CM alignment181. Furthermore, coating the scaffold with piezoelectric microfibers provided suitable electrical stimuli to the cells.
Over the past few years, there have been proposed other electrospinning-based techniques, which aimed at improving certain drawbacks of the traditional method, such as dependency on the polymer’s solution conductivity, slow deposition rates, and imprecise fabrication of 3D structures. Producing nano- to sub-micrometer scale fibers with a fast rate can be achieved using a high-speed rotating reservoir that propels centrifugal polymer deposition in a cylindrical collector182. The resulting polyester scaffold (25 mm diameter) was shown to promote CM alignment. A more recent approach used an ellipsoidal mandrel to collect electrospun PCL gelatin-coated nanofibers127. The ventricle-inspired structure (truncated ellipsoid; 4.5 mm diameter, 9 mm height) promoted CM laminar alignment, even though the ejection fractions and contractile work were ~50–250 and ~108 smaller than the physiologic values for a human ventricle, respectively. Another promising methodology is melt electrospinning (also known as melt electrowriting), which allows a more controlled scaffold fabrication by precise collector or nozzle positioning, similar to 3D printing. Despite being unsuitable for cell encapsulation purposes, a relatively recent study demonstrated that a foldable PCL melt electrospun hexagonal-shaped scaffold (8 mm diameter, 300 µm thick) contributed to CM alignment and maturation183. Future improvements to these techniques or integrating existing expertise in multistep biofabrication approaches could allow their widespread use for cardiac TE.
Bioprinting
Bioprinting is an emerging biofabrication method based on precise layer-by-layer deposition of biomaterials, biochemicals, and living cells, collectively named bioinks184. High-fidelity tissue and organ surrogates can be generated with the aid of medical imaging modalities, namely computed tomography and magnetic resonance imaging.
Synthetic materials were amongst the first to be printed, but now research focuses on using naturally derived materials, such as ECM hydrogels derived from decellularized tissue. In particular, cardiac-specific ECM-derived bioinks, when pressure extruded through a printing nozzle, have successfully generated grid-type structures in the form of rectangles169 (~5 mm × 5 mm × 1 mm) or circles173 (10 mm diameter, 0.6 mm thick), such that it resembles a patch. Extrusion bioprinting is one of the most popular methods to directly print cell-laden hydrogel-based cardiac tissue in an organized and reproducible fashion, besides allowing printing with high cell densities184, which is a necessary requirement to print more complex structures. Aside from the above-mentioned simple tissue constructs, extrusion bioprinting can be used to create more complex tissue models, but freeform printing of such structures requires a supporting sacrificial material. A novel method termed “FRESH” (i.e., Freeform Reversible Embedding of Suspended Hydrogels) achieved extrusion-based freeform printing by embedding the bioink in a thermally reversible supportive hydrogel during printing185,186, and this allowed fabrication of high-resolution 3D anisotropic structures, including a beating ventricle (truncated ellipsoid; 5.7 mm diameter, 8 mm height) and an acellular neonatal-sized heart analog (37 mm diameter, 55 mm height)186. Another study used a similar approach, but with an enzymatic or chemical reversible supportive hydrogel, and was able to print a heart-shaped model (14 mm diameter, 20 mm height) using an omental ECM biomaterial in combination with CMs and endothelial cells174. Further improvements are needed to create a functional tissue since the cells were reported to be arranged as nonaligned aggregates after 1 day in culture.
Another advantage of bioprinting is the possibility of creating tissues with inherent perfusable vasculature187–189. Direct extrusion-based bioprinting was used to manufacture one of the first vascularized cardiac tissues, in which the resulting endothelialized grid-type structure (~5.5 mm × 3.5 mm × 0.75 mm) was seeded with CMs and perfused with a microfluidic device187. Bioprinting a vascular network greatly improves the thickness these tissues can achieve without becoming necrotic (>1 cm188), but they often lack physiologically relevant cell numbers (~1 × 108 cell/mL). A new innovative method termed “SWIFT” (i.e., Sacrificial Writing Into Functional Tissue) relies on assembling up to 400,000 spheroids/organoids as a tissue matrix and then extrudes a sacrificial ink throughout the compact granular tissue, which can later be evacuated and the resulting lumen perfused190, despite lacking robustly endothelialized channels. This clever technique allowed the assembly of a cardiac tissue with 240,000 million cells (6 mm top width, 4.2 mm bottom width, 12 mm height, 4.2 mm depth) that fuse and beat synchronously over 7 days in culture. Stereolithography, a higher resolution bioprinting methodology that uses a light projector to solidify layer-by-layer the bioink, has recently been used to create entangled vascular networks to perfuse an alveolar sac model189. Due to its exceptional level of functionality, translating this approach to cardiac TE could also be worthwhile.
It is important to take into account that additive printing with a high level of detail requires a slow printing velocity, affecting the scalability of current methods. To overcome this limitation, a recent study demonstrated that tissue can be printed within seconds with high fidelity by irradiating multiple light patterns representing projections of the object to be printed onto a photopolymer191. Despite being a promising technology, its potential use for cardiac TE still needs to be evaluated.
Unlocking cardiac tissue engineering for precision medicine
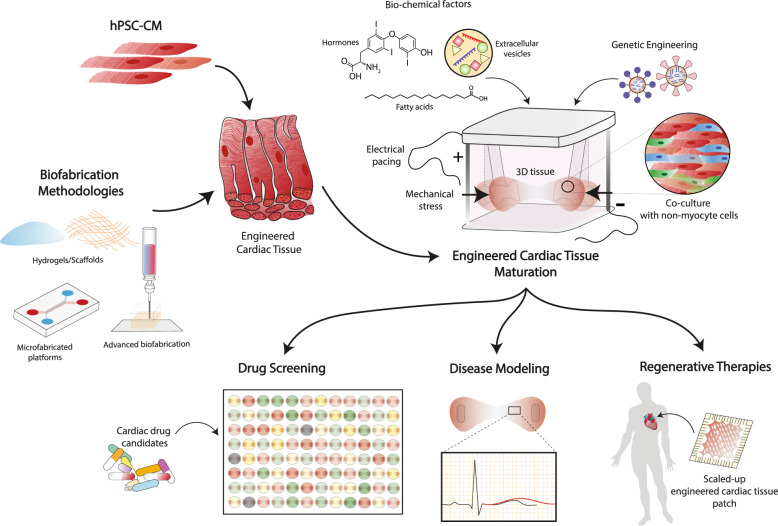
The advances in hPSC biology together with the development of novel materials and biofabrication methods have enabled the production of patient-specific cardiac tissues, which in turn opens up the possibility of fulfilling the promise of personalized medicine (Fig. 4). This section includes a brief overview of the current state-of-the-art applications of cardiac TE for drug discovery, disease modeling, and regenerative therapies.
Fig. 4. Streamlining engineered cardiac tissues for precision medicine applications.
Tissue engineering expertise enables the generation of physiologically relevant cardiac tissues grown from human pluripotent stem cell-derived cardiomyocytes (hPSC-CMs). Designing a proper maturation strategy will allow the maturation of hPSC-derived cardiac tissues to an adult-like phenotype, which can later on be used for patient-specific drug screening, disease modeling, and regenerative therapies.
Cardiac tissue engineering for drug discovery and disease modeling
It is well known that cardiovascular safety liabilities remain a major cause of drug attrition, resulting in early termination of pipeline candidates or withdrawal of marketed drugs192. Commonly used platforms to assess safety include small animal models, primarily mice, and ion-channel overexpressing cell lines193,194, which fail to truly predict human drug response effects (proarrhythmic, cardiotonic, and cardiotoxic) due to differences in cardiovascular physiopathology. Thus, cardiac TE-based solutions, such as spheroids120,195, microtissues63,124, and “heart-on-a-chip”/microfluidic devices175,196,197, have been emerging as advanced culture systems, capable of improving the physiologic relevance over traditional 2D in vitro culture platforms. In these systems, hPSC-CMs have shown higher predictive insight regarding drug mechanistic (e.g., inotropic response101,175,198 and ECC blockage101,175) and toxic (e.g., loss of contractile function198 and apoptosis195) effects. Nonetheless, additional efforts are required to improve the reliability of these systems. For instance, a recent study demonstrated that linsitinib, a promising drug candidate to treat Ewing sarcoma, significantly reduced tumor viability and cardiac function when both of these engineered tissues were cultured separately under perfusion in an organ-on-a-chip system. However, when they were cultured in an integrated way in the same platform, linsitinib showed poor tumor response and less cardiotoxicity, which was in agreement with the unfortunate clinical trial outcome of this drug199. Testing the targeted or off-targeted effect of new cardiovascular drugs will require advancing the physiological relevance in which cardiac engineered tissues are cultured. In addition, the complexity of engineered cardiac tissues is still incompatible with a rapid screen of large libraries of compounds, despite their smaller size in relation to a whole tissue/organ. Although some platforms have shown the ability to be scaled-down124,200,201, high-throughput culture modes and reproducible assessment of tissue functionality are still lacking202.
Expediting drug development will further require reliable models for a better understanding of disease mechanisms that guide target drug validation/discovery. In this context, hPSCs, as stated above, have emerged as attractive tools and a preferred source for obtaining cells for in vitro models, since these cells can be derived from patients using minimally invasive procedures, and preserve the genetic and phenotypic characteristics of the donors, including pathological traits responsible for disease onset and progression203,204. Several studies have been using 2D hiPSC-CM systems to investigate a plethora of genetic mutations associated with genetic heart diseases, including the main inherited cardiomyopathies (i.e., familial hypertrophic cardiomyopathy205, dilated cardiomyopathy203,206, arrhythmogenic cardiomyopathy207, and left ventricular noncompaction cardiomyopathy208) and channelopathies (e.g., type-2 long-QT syndrome209 and Timothy syndrome210). Others are modeling diseases without a known disease-causing genetic variant, such as idiosyncratic drug-induced cardiotoxicity204,211,212. In parallel with the use of patient-derived hPSCs, recent advances in targeted gene editing using the CRISPR-Cas9 systems have been performed on hPSCs213–215. Employing this technology, any wild-type cell can be altered to harbor a specific mutation, and a disease-causing mutation can be corrected in patient-derived cells, generating an isogenic control cell line. To the best of our knowledge, there are 13 completed or active clinical studies (www.clinicaltrials.gov) with the goal of generating patient-specific CMs to study the molecular mechanisms involved in several cardiac diseases, such as genetic and hypertrophic cardiomyopathies, inherited arrhythmias and valvulopathies, and cardiac fibrosis, among others (Table 1).
Table 1.
Human clinical studies for the generation of disease and/or patient-specific models of cardiac diseases (Trial Identifier: www.clinicaltrials.gov).
| Study title | Condition(s) | Study type | Trial identifier |
|---|---|---|---|
| Individualized early risk assessment for heart diseases (IndivuHeart) | Hypertrophic cardiomyopathy; dilated cardiomyopathy | Observational | NCT02417311 |
| Investigating hereditary cardiac disease by reprogramming skin cells to heart muscle (CLUE) | Electrophysiology of iPSC-CM | Observational | NCT01865981 |
| Modeling and pharmacological targeting of genetic cardiomyopathy in children via iPSC-CM (DMDstem) | Familial cardiomyopathy | Interventional | NCT03696628 |
| Molecular mechanism identification in inherited arrhythmias and valvulopathies from iPSC (Diag-iPS) | Inherited arrhythmias and valvulopathies | Interventional | NCT01734356 |
| Evaluating cardiovascular phenotypes using iPSC | Coronary artery disease | Observational | NCT01517425 |
| Generation of Marfan syndrome and Fontan cardiovascular models using patient-specific iPSC | Marfan’s syndrome | Observational | NCT02815072 |
| Translational approaches to septic cardiomyopathy (TASC01) | Cardiomyopathies, sepsis, septic shock | Observational | NCT03252613 |
| CIQTP prolongation: role and mechanism in sudden cardiac death (IQARE-SCD) | Sudden cardiac death | Observational | NCT03387072 |
| Derivation of iPSC to heritable cardiac arrhythmias | Inherited cardiac arrhythmias; long QT syndrome; Brugada syndrome; catecholaminergic polymorphic ventricular tachycardia; early repolarization syndrome; arrhythmogenic cardiomyopathy; hypertrophic cardiomyopathy; dilated cardiomyopathy; muscular dystrophies (Duchenne, Becker, myotonic dystrophy) | Observational | NCT02413450 |
| Early MRI detection of myocardial deterioration as a preventive, disease staging, and prognostic biomarker in insulin resistance | Cardiomyopathies; insulin resistance; non-ischemic cardiomyopathy; cardiac fibrosis; diabetes | Observational | NCT03509441 |
| Characterization of patients with uncommon presentations and/or uncommon diseases associated with the cardiovascular system | Cardiomyopathy; Li-Fraumeni syndrome; Parkinson’s disease; atherosclerosis; cardiovascular capacity | Observational | NCT01143454 |
| UTHealth Turner syndrome research registry | Turner syndrome | Observational | NCT03185702 |
iPSC induced pluripotent stem cells, iPSC-CM induced pluripotent stem cells derived cardiomyocytes, MRI magnetic resonance imaging.
Notwithstanding, the use of engineered cardiac tissues as disease models is a relatively new concept. hPSC-CM tissue platforms harboring genetic mutations characteristic of hypertrophic cardiomyopathy216, dilated cardiomyopathy217, and mitochondrial cardiomyopathy of Barth syndrome218 have reported abnormalities in cardiac function congruent with a pathological phenotype. More recently, chamber-specific arrhythmia was demonstrated in a ring-shaped cardiac tissue containing both ventricular and atrial hPSC-CMs219. Manipulating engineered tissue microenvironment to induce pathological changes, such as CM remodeling of myocardium failure220, excessive ECM deposition of cardiac fibrosis221, and thrombus formation222, could similarly provide invaluable insights into mechanistic studies or predict patient-specific disease progression. Even though promising, disease-carrying hPSC-CMs still present an immature phenotype, thus possibly confounding identified disease hallmarks and identified mechanisms. For instance, genetically engineered hPSC-CM microtissues carrying mutations that truncate the sarcomere protein titin, a known common cause of dilated cardiomyopathy, exhibited reduced contractility and decreased sarcomere length, among other abnormalities217, none of which were detected in adult samples223. As a matter of fact, cardiac tissue structural maturity imposed by intensive electrical training was necessary to model pathological ventricular hypertrophy101,175. Even with their current limitations, the identified pathomechanisms in diseased engineered cardiac tissues can, in some cases, be translated to patient care in a personalized way. Recent research found out diltiazem, an l-type calcium channel blocker, was effective to correct disease-specific cardiac abnormalities in patient-derived engineered muscle strips with a rare form of hypertrophic cardiomyopathy. Diltiazem was further translated into patient care and ameliorated the prolonged QTc interval after 1 month of treatment224. These encouraging results give hope that emerging TE platforms could be extremely useful to reverse the growing burden associated with cardiovascular disorders.
Cardiac tissue engineering for heart regenerative therapy
Regenerative cardiac cell therapy broadly aims to achieve two complementary goals: (i) the direct cell replacement of the injured myocardium with contractile CMs and (ii) paracrine modulation of endogenous repair processes, such as angiogenesis, inflammation, apoptosis, and fibrosis, with both contractile and noncontractile cells14,225,226. To the best of our knowledge, there are over 40 completed clinical trials exploring the therapeutic potential of cell-based therapies for cardiac regeneration and repair (www.clinicaltrials.gov). Pioneer studies tested non-cardiomyogenic cell populations that can easily be prepared for clinical applications, namely bone marrow-derived mononuclear cells (BM-MNCs) or MSCs, which in spite of their limited regenerative potential, may stimulate endogenous regenerative responses225,227. Second-generation cell candidates require a more refined isolation process, and ex vivo amplification procedures, but have higher regenerative potential, such as cardiac-derived progenitor cells and hPSC-CMs225,227. However, the clinical outcome of most of these studies remains insufficient, with evidence suggesting the major common obstacles to be the low engraftment and survival rate of transplanted cells in the host tissue, and the scarcity of endogenous cells with repair capacity225,228–230.
Cardiac TE has proven to be an effective method to improve the delivery, engraftment, and differentiation of stem cells in injured hearts, and therefore has gained much attention in recent years. Cardiac tissue surrogates developed to date are typically engineered cardiac muscle strips231–233, epicardial patches126,234–236, or cardiogenic scaffolds237, even though other strategies can be employed238–243, such as acellular hydrogels to improve infarct mechanical integrity241–243. A summary of relevant cardiac TE preclinical studies in animal models is highlighted in Table 2. When transplanted into infarcted or defected animal hearts, hPSC-CM-based tissues led to longer-term cell retentions231, progressively matured in vivo231, (imperfectly) electrically coupled with the host tissue233, reversed infarction-associated changes (e.g., ameliorated cardiac remodeling by decreasing the amount of fibrosis241–243, enhanced vascularization126,233,242), and overall improved left ventricular function231,240,243. Recent advances have allowed rapid fabrication of clinically relevant sized human cardiac tissues able to maintain functionality preimplantation126,234, thus being suitable for large animal preclinical studies and future clinical applications.
Table 2.
Selected in vivo preclinical studies on cardiac tissue engineering.
| Tissue engineering strategy | Specific tissue engineering approach | Cell source | Animal model | Disease model | Major achievements | Ref. |
|---|---|---|---|---|---|---|
| Engineered heart tissue | CM and collagen type I macroscale ring EHT | hESC-CM | Rat |
MI IR 60 min |
Cardiac function recovery: no significant changes in LVEDV and LVESV at 4 weeks; progressive improvement in LVEF at 4 weeks Assessment methods: echocardiography and MRI |
231 |
| Engineered heart tissue | Fibrin EHT | hiPSC-CM, -EC | Guinea pig | Cryo-injury |
Cardiac function recovery: absence of pro-arrhythmogenic effects at day 28 Assessment methods: echocardiography |
232 |
| Engineered heart tissue | Fibrin EHT | hiPSC-CM, -EC | Guinea pig | Cryo-injury |
Cardiac function recovery: improved LV function at day 28; EHT vascularization and electrical coupling with host heart tissue Assessment methods: ecochardiography |
233 |
| Cardiac patch | 3D-printed patch composed of hyaluronic acid/gelatin-based matrix. | Human CPC | Mouse | MI |
Cardiac function recovery: reduction in LVEDV and LVESV at 4 weeks; reduction of infarct fibrosis Assessment methods: MRI and histology |
237 |
| Cardiac patch | Fibrin patch with nylon frame | hiPSC-CM | Rat | N/A |
Cardiac function recovery: patches failed to electrically couple with the recipient’s hearts; patch vascularization by host vessels; no immune rejection Assessment methods: electromechanical optical dual mapping, dorsal window chamber assay, and histology |
126 |
| Cardiac patch | Cardiac muscle patch | hiPSC-CM, -EC, and -SMCs | Pig |
MI IR 60 min |
Cardiac function recovery: LVEF and LVEDV improvements at 4 weeks; no spontaneous arrhythmias were detected 2 weeks post-acute MI phase Assessment methods: MRI and electrocardiography |
235 |
| Cardiac patch | 3D fibrin patch loaded with insulin growth factor-encapsulated microspheres | hiPSC-CM, -EC, and -SMC | Pig | MI |
Cardiac function recovery: improved LVEF at 4 weeks; reduction in LV wall stress; reduction in infarct size Assessment methods: ecochardiography and histology |
234 |
| Cardiac patch | Conductive patch composed of chitosan, phytic acid, and aniline | Acellular | Rat | MI |
Cardiac function recovery: improvements in LVEF and LVFS at 2 weeks; absence of pro-arrhythmogenic effects Assessment methods: echocardiography |
236 |
| Cardiac patch | Viscoelastic starch patch designed by finite-element simulation | Acellular | Rat | MI |
Cardiac function recovery: decreased LVIDD and LVIDS at 4 weeks; improved LVEF and LVFS at 4 weeks; reduction in infarct size; reduction of myocardial hypertrophy Assessment methods: echocardiography, histology, and immunofluorescence microscopy |
243 |
| Microspheres | Gelatin MSs | CPC and CPC + MS | Mouse | MI |
Cardiac function recovery: improved LVEF at day 28; reduction in LVESV and LVEDV Assessment methods: MRI |
238 |
| Nanofibers | Poly(d,l-lactic-co-glycolic acid) polymer nanofibers | hiPSC-CM | Rat | MI |
Cardiac function recovery: improvements in LVEF, LVFS, and LVESD at 4 weeks; no immune rejection Assessment methods: echocardiography and histology |
239 |
| Cell sheets | Cell sheet | hiPSC-CM | Pig | MI |
Cardiac function recovery: improvements in LVEF, LVEDV, and LVESV at 4 and 8 weeks Assessment methods: echocardiography and cardiac MSCT |
240 |
| Biomaterials | Injectable alginate hydrogel | Acellular | Rat | MI |
Cardiac function recovery: LVFS improvement at 8 weeks after injection, either in recent or old infarcts Assessment methods: echocardiography |
241 |
| Biomaterials | Solubilized porcine myocardial ECM injectable hydrogel | Acellular | Pig | MI |
Cardiac function recovery: LVEF, LVEDV, and LVESV improvements at 12 weeks; reduced infarct size at 3 weeks Assessment methods: echocardiography and NOGA |
242 |
CM cardiomyocytes, CPC cardiac progenitor cell, EC endothelial cells, ECM extracellular matrix, hESC-CM human embryonic stem cells derived cardiomyocytes, hiPSC human induced pluripotent stem cells, hiPSC-CM human induced pluripotent stem cell-derived cardiomyocytes, IR ischemia–reperfusion, LV left ventricular, LVEDV left ventricular end-diastolic volume, LVEF left ventricular ejection fraction, LVESD left ventricular end-systolic dimension, LVESV left ventricular end-systolic volume, LVFS left ventricular fractional shortening, LVIDD left ventricular internal diastolic diameter, LVIDS left ventricular internal systolic diameter, MI myocardial infarction, MRI magnetic resonance imaging, MS microspheres, MSCT multislice computer tomography, SMC smooth muscle cells.
Among relevant risks that have to be taken into account in these therapies are the development of arrhythmias244,245, uncontrolled proliferation, and unwanted differentiation of cells that may lead to tumor formation in the case of hPSC-based therapies245–248, or to calcification of the host myocardium by MSCs or BM-MNCs247,249. Another potential issue is the development of an adverse immune response against the cells and materials employed in the constructs250. Designing tissues with a more controlled cell composition, defined architectural structure and developing strategies favoring muscular maturation before implantation could further improve their regenerative capability.
Despite showing promising outcomes in preclinical settings, the potential use of cardiac TE-based therapies at a clinical level is still in its infancy. Preliminary data from first-in-man clinical trials are providing evidence that cardiac TE-based therapies are generally safe251,252, but do not provide relevant functional benefits. We are aware of five completed and two active phase 1/2 studies mainly aiming at evaluating the safety and feasibility of cardiac TE therapeutic interventions, but also collecting preliminary efficacy data (Table 3).
Table 3.
Human clinical trial for cardiac tissue engineering (Trial Identifier: www.clinicaltrials.gov).
| Study title | Condition | Intervention material | Phase and status | Trial identifier | Refs. |
|---|---|---|---|---|---|
| IK-5001 for the Prevention of Remodeling of the Ventricle and Congestive Heart Failure After Acute Myocardial Infarction (PRESERVATION-1) | Acute MI; congestive HF; ST-elevation MI | IK-5001 (sodium alginate and calcium gluconate hydrogel) |
Phase 1 Completed |
NCT01226563 | 256–258 |
| A randomized, controlled study to evaluate Algisyl-LVR™ as a method of left ventricular Augmentation for Heart Failure (AUGMENT-HF) | HF; dilated cardiomyopathy | Algisyl (calcium alginate hydrogel) |
Phase 2 Completed |
NCT01311791 | 259–261 |
| Epicardial infarct repair using CorMatrix®-ECM: clinical feasibility study | Acute coronary syndrome; HF | CorMatrix-ECM |
Phase 1 Completed |
NCT02887768 | 252,254 |
| A study of VentriGel in post-MI patients | MI; HF; left ventricular remodeling | Ventrigel (ECM hydrogel) |
Phase 1 Completed |
NCT02305602 | 255 |
| Transplantation of hESC-derived progenitors in severe heart failure | Ischemic heart disease | hESC-derived CD15+Isl-1+ progenitors embedded into a fibrin patch |
Phase 1 Completed |
NCT02057900 | 251,262 |
| Randomized study of coronary revascularization surgery with an injection of WJ-MSCs and placement of an epicardial patch | Cardiovascular diseases; HF; coronary artery disease | ECM patch with WJ-MSC |
Phase 1 and 2 Active, not yet recruiting |
NCT04011059 | – |
| Safety and efficacy of iPSC-derived engineered human myocardium as Biological Ventricular Assist Tissue in Terminal Heart Failure (BioVAT-HF) | HF | Engineered heart muscle |
Phase 1 and 2 Active, recruiting |
NCT04396899 | – |
ECM extracellular matrix, hESC human embryonic stem cells, HF heart failure, MI myocardial infarction, WJ-MSC Wharton’s jelly-derived mesenchymal stem cells.
The first studies being conducted are focused on the hypothesis that biomaterials could act as an internal wall support to increase infarct wall thickness, thereby improving cardiac function. Results from PRESERVATION I and AUGMENT-HF trials were, however, disappointing, with minor improvements in symptoms and clinical status for patients with advanced heart failure (HF), but no significant changes in left ventricle (LV) remodeling. Aiming to provide not only mechanical support for the prevention of infarct expansion but also to restore ECM homeostasis and architecture, other strategies relied on the epicardial or myocardial application of ECM patches or hydrogels. In particular, CorMatrix®-ECM patches, derived from porcine small intestinal submucosa (SIS-ECM), were applied on the epicardium of patients undergoing coronary artery bypass grafting, after an acute ischemic event. Porcine SIS-ECM has been used for over 20 years in a range of cardiovascular applications, but of all the commercially available SIS-ECM products, CorMatrix® is the only medical device with clearance, even though there are few reports regarding its use for myocardial regeneration after acute myocardial infarction in humans253. No results for this study have been published so far; however, based on preliminary data obtained in rodent and porcine models, the patches are expected to not only prevent infarct expansion and LV dilation but also restore ECM homeostasis and architecture252,254. Another recently completed study evaluated the effect of VentriGel, a myocardium-specific ECM hydrogel, on patients in a 60- to 3-year window since their first acute ST-elevation myocardial infarction. Using a minimally invasive procedure, the gel was injected using a NOGA-MyoStar catheter, allowing for an intramyocardial delivery of the therapeutic gel on mapped infarcted areas. Overall, VentriGel showed encouraging safety results, with no deaths or patients who discontinued from the study, nor adverse events reported as a direct consequence of the treatment255. Although efficacy was not a primary outcome, there were suggestions of improvements, including in the 6-min walk test distance and decreases in New York Heart Association functional classification, across the entire cohort of patients255. Improvements in LV remodeling data, viable mass, and ventricular natriuretic peptide levels were mainly observed in patients who had a myocardial infarction >12 months before treatment255.
A distinctive approach has been the delivery of clinical-grade hESC-derived cardiovascular progenitor cells in fibrin patches for the treatment of severe ischemic left ventricular dysfunction251. Compared with biomaterial-only methods, cell patches showed equal safety profiles, with no complications that could be specifically ascribed to the cellular graft, namely, no detection of teratomas that could be developed from residual pluripotent cells, no occurrence of arrhythmias, and no major alloimmunization events. Still, following a similar trend as previous strategies, no astonishing results were obtained in terms of efficacy, although all patients were symptomatically improved. A newly posted study aims at determining the safety of Wharton’s jelly-derived MSCs from human umbilical cord seeded on ECM patches, and also test its efficacy to promote neomyogenesis in patients with previous myocardial infarction. Another active and recently recruiting promising clinical trial will evaluate the efficacy of an engineered heart muscle (EHM) to sustainably remuscularize and improve myocardial performance in patients with advanced HF with reduced ejection fraction (≤35%). The EHM, a construct generated from hiPSC-CMs and stromal cells in a bovine collagen type I hydrogel, will be the first of its kind to be tested in humans.
Despite the marginally positive outcome of all these clinical trials, this does not necessarily imply a lack of therapeutic value and may result instead from an inadequate choice of biomaterial and/or cell type or inaccuracies in dosing and/or delivery. A better understanding underlying the mechanism of action of these advanced medicinal products may contribute to further improvements down the road.
Conclusions
To date, inducing maturation of hPSC-CMs is a challenge in the field, limiting their use in clinical settings. Considerable progress has been achieved by continuously seeking innovative ways to improve hPSC-CM maturation state, and, currently, it is already possible to obtain cardiac tissue with adult-like gene signatures, remarkable ultrastructural organization, functional contractile machinery, and improved electrophysiology. Even though in vitro maturation may not follow the in vivo paradigm, identifying the spatio-temporal mechanisms dictating tissue development and designing a combinatorial strategy capable of addressing such complexity might be required to engineer fully matured biomimetic cardiac tissues. Given the interdisciplinarity of TE, from stem cell biology to stem cell bioprocessing, material science, and advanced biofabrication, taking advantage of this knowledge would be beneficial to grow physiologically relevant cardiac tissues in vitro. Recreating cardiac tissue complexity is still challenging, limiting the size of the engineered tissue possible to be obtained, and thus redirecting their application towards other areas rather than the clinical transplantation arena. Benchmarked “on-a-chip” engineered cardiac tissues, although promising, still need to show standardization and high-throughput compatibility to contribute to more reliable results than commonly used platforms, as well as reduction of costs to expedite drug development. Successful attempts at scaling up engineered cardiac tissues have made it possible to investigate their regenerative efficacy in large animal studies, and the results of pioneering TE-based clinical trials are eagerly awaited. While there are still few clinical trials, these have been an important step in the ability to engineer artificial constructs that mimic some of the important compositional, architectural, and functional properties of human cardiac tissues. Even if it is not unrealistic to foresee a translation of engineered cardiac tissues for in vitro clinical studies, their potential to remuscularize failing hearts and integrate the host tissue without adverse effects, despite a better retention, is still uncertain, and the causes associated with mild improvements unknown. Nonetheless, advances in the field of cardiac TE have undoubtedly imposed a new paradigm in cardiovascular science, paving the way one day for restoring or even replacing damaged hearts.
Acknowledgements
We acknowledge funding received from EU-funded project BRAV3 (H2020, ID:874827); Fundação para a Ciência e Tecnologia (FCT)-funded projects NETDIAMOND (SAICTPAC/0047/2015), MetaCardio (PTDC/BTMSAL/32566/2017) and Neocoronary (PTDC/MEC-CAR/29590/2017); iNOVA4Health, refs. UIDB/04462/2020 and UIDP/04462/2020, a program financially supported by FCT/Ministério da Educação e Ciência, through national funds. A.F.L. was supported by FCT Grant PD/BD/139078/2018.
Author contributions
M.F.T. and A.F.L. wrote the manuscript. P.M.A. and M.S. reviewed and edited the manuscript and were responsible for funding acquisition.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bloom, D. E. et al. The Global Economic Burden of Non-communicable Diseases. Geneva: World Economic Forum (2011).
- 2.Roth GA, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jessup M, Brozena S. Heart failure. N. Engl. J. Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menasché P, et al. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 6.Assmus B, et al. Clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ. Heart Fail. 2010;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]
- 7.Strauer BE, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary stem cell transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur. J. Heart Fail. 2010;12:721–729. doi: 10.1093/eurjhf/hfq095. [DOI] [PubMed] [Google Scholar]
- 8.Menasché P, et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 2015;36:2011–2017. doi: 10.1093/eurheartj/ehv189. [DOI] [PubMed] [Google Scholar]
- 9.Kastrup J, et al. Direct intramyocardial plasmid vascular endothelial growth factor-A 165 gene therapy in patients with stable severe angina pectoris: a randomized double-blind placebo-controlled study: the Euroinject One trial. J. Am. Coll. Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 10.Zohlnhofer, D. et al. Stem cell mobilization by granulocyte colony-stimulating factor in patients. JAMA295, 1003–1010 (2006). [DOI] [PubMed]
- 11.Gao R, et al. A phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J. Am. Coll. Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- 12.Chien KR, et al. Regenerating the field of cardiovascular cell therapy. Nat. Biotechnol. 2019;37:232–237. doi: 10.1038/s41587-019-0042-1. [DOI] [PubMed] [Google Scholar]
- 13.Khademhosseini A, Langer R. A decade of progress in tissue engineering. Nat. Protoc. 2016;11:1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 14.Madonna, R. et al. ESC Working Group on Cellular Biology of the Heart Position paper for Cardiovascular Research: tissue engineering and cell-based therapies for cardiac repair in ischemic heart disease and heart failure. Cardiovasc. Res.115, 488–500 (2019). [DOI] [PMC free article] [PubMed]
- 15.Zhao, Y. et al. Towards chamber specific heart-on-a-chip for drug testing applications. Adv. Drug Deliv. Rev. 165, 60–76 (2020). [DOI] [PMC free article] [PubMed]
- 16.Zhou P, Pu WT. Recounting cardiac cellular composition. Circ. Res. 2016;118:368–370. doi: 10.1161/CIRCRESAHA.116.308139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Litviňuková, M. et al. Cells of the adult human heart. Nature588, 466–472 (2020). [DOI] [PMC free article] [PubMed]
- 18.Hescheler J, et al. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ. Res. 1991;69:1476–1486. doi: 10.1161/01.RES.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 19.Claycomb WC, et al. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl Acad. Sci. USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eschenhagen T, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 21.Carrier RL, et al. Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization. Biotechnol. Bioeng. 1999;64:580–589. doi: 10.1002/(SICI)1097-0290(19990905)64:5<580::AID-BIT8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 22.Watson SA, et al. Preparation of viable adult ventricular myocardial slices from large and small mammals. Nat. Protoc. 2017;12:2623–2639. doi: 10.1038/nprot.2017.139. [DOI] [PubMed] [Google Scholar]
- 23.Banyasz T, et al. Transformation of adult rat cardiac myocytes in primary culture. Exp. Physiol. 2008;93:370–382. doi: 10.1113/expphysiol.2007.040659. [DOI] [PubMed] [Google Scholar]
- 24.Watson, S. A. et al. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat. Commun. 10, 1–15 (2019). [DOI] [PMC free article] [PubMed]
- 25.Fischer C, et al. Long-term functional and structural preservation of precision-cut human myocardium under continuous electromechanical stimulation in vitro. Nat. Commun. 2019;10:1–12. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson MM, et al. Novel cell lines derived from adult human ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 2005;39:133–147. doi: 10.1016/j.yjmcc.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell126, 663–676 (2006). [DOI] [PubMed]
- 29.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Chen IY, Matsa E, Wu JC. Induced pluripotent stem cells: at the heart of cardiovascular precision medicine. Nat. Rev. Cardiol. 2016;13:333–349. doi: 10.1038/nrcardio.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue, H., Nagata, N., Kurokawa, H. & Yamanaka, S. iPS cells: a game changer for future medicine. EMBO J.33, 409–417 (2014). [DOI] [PMC free article] [PubMed]
- 32.Burridge PW, et al. Chemically defned generation of human cardiomyocytes. Nat. Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueno S, et al. Biphasic role for Wnt/β-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl Acad. Sci. USA. 2007;104:9685–9690. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lian, X. et al. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl Acad. Sci. USA109, E1848–E1857 (2012). [DOI] [PMC free article] [PubMed]
- 35.Wang H, Hao J, Hong CC. Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/β-catenin signaling. ACS Chem. Biol. 2011;6:192–197. doi: 10.1021/cb100323z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lian X, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat. Protoc. 2013;8:162–175. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Squire JM. Architecture and function in the muscle sarcomere. Curr. Opin. Struct. Biol. 1997;7:247–257. doi: 10.1016/S0959-440X(97)80033-4. [DOI] [PubMed] [Google Scholar]
- 38.Gerdes AM, et al. Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy. Circulation. 1992;86:426–430. doi: 10.1161/01.CIR.86.2.426. [DOI] [PubMed] [Google Scholar]
- 39.Mollova M, et al. Cardiomyocyte proliferation contributes to heart growth in young humans. Proc. Natl Acad. Sci. USA. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olivetti G, et al. Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart. J. Mol. Cell. Cardiol. 1996;28:1463–1477. doi: 10.1006/jmcc.1996.0137. [DOI] [PubMed] [Google Scholar]
- 41.Wei S, et al. T-tubule remodeling during transition from hypertrophy to heart failure. Circ. Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaprielian RR, Stevenson S, Rothery SM, Cullen MJ, Severs NJ. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation. 2000;101:2586–2594. doi: 10.1161/01.CIR.101.22.2586. [DOI] [PubMed] [Google Scholar]
- 43.Pinali C, Bennett H, Davenport JB, Trafford AW, Kitmitto A. Three-dimensional reconstruction of cardiac sarcoplasmic reticulum reveals a continuous network linking transverse-tubules: this organization is perturbed in heart failure. Circ. Res. 2013;113:1219–1230. doi: 10.1161/CIRCRESAHA.113.301348. [DOI] [PubMed] [Google Scholar]
- 44.Porter GA, et al. Bioenergetics, mitochondria, and cardiac myocyte differentiation. Prog. Pediatr. Cardiol. 2011;31:75–81. doi: 10.1016/j.ppedcard.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saffitz JE, Kanter HL, Green KG, Tolley TK, Beyer EC. Tissue-specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ. Res. 1994;74:1065–1070. doi: 10.1161/01.RES.74.6.1065. [DOI] [PubMed] [Google Scholar]
- 46.Peters NS, et al. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994;90:713–725. doi: 10.1161/01.CIR.90.2.713. [DOI] [PubMed] [Google Scholar]
- 47.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Snir M, et al. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2003;285:2355–2363. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 49.Vreeker, A. et al. Assembly of the cardiac intercalated disk during pre- and postnatal development of the human heart. PLoS ONE9, e94722 (2014). [DOI] [PMC free article] [PubMed]
- 50.Liu J, Fu JD, Siu CW, Li RA. Functional sarcoplasmic reticulum for calcium handling of human embryonic stem cell-derived cardiomyocytes: insights for driven maturation. Stem Cells. 2007;25:3038–3044. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 51.Lieu DK, et al. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 2009;18:1493–1500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dai, D. F., Danoviz, M. E., Wiczer, B., Laflamme, M. A. & Tian, R. Mitochondrial maturation in human pluripotent stem cell derived cardiomyocytes. Stem Cells Int. 2017, 5153625 (2017). [DOI] [PMC free article] [PubMed]
- 53.Schaaf, S. et al. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS ONE6, e26397 (2011). [DOI] [PMC free article] [PubMed]
- 54.Sasaki, D. et al. Contractile force measurement of human induced pluripotent stem cell-derived cardiac cell sheet-tissue. PLoS ONE13, e0198026 (2018). [DOI] [PMC free article] [PubMed]
- 55.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J. Cardiovasc. Pharmacol. 2010;56:130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 56.Chung S, et al. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 2007;4:60–67. doi: 10.1038/ncpcardio0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kolwicz SC, Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Der Velden J, et al. Isometric tension development and its calcium sensitivity in skinned myocyte-sized preparations from different regions of the human heart. Cardiovasc. Res. 1999;42:706–719. doi: 10.1016/S0008-6363(98)00337-X. [DOI] [PubMed] [Google Scholar]
- 59.Hasenfuss G, et al. Energetics of isometric force development in control and volume-overload human myocardium. Comparison with animal species. Circ. Res. 1991;68:836–846. doi: 10.1161/01.RES.68.3.836. [DOI] [PubMed] [Google Scholar]
- 60.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation. 1992;85:1743–1750. doi: 10.1161/01.CIR.85.5.1743. [DOI] [PubMed] [Google Scholar]
- 61.Pieske B, et al. Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy: functional evidence for alterations in intracellular Ca2+ handling. J. Clin. Invest. 1996;98:764–776. doi: 10.1172/JCI118849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mannhardt I, et al. Human engineered heart tissue: analysis of contractile force. Stem Cell Rep. 2016;7:29–42. doi: 10.1016/j.stemcr.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huebsch N, et al. Miniaturized iPS-cell-derived cardiac muscles for physiologically relevant drug response analyses. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antzelevitch C, Burashnikov A. Overview of basic mechanisms of cardiac arrhythmia. Card. Electrophysiol. Clin. 2011;3:23–45. doi: 10.1016/j.ccep.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dangman KH, et al. Electrophysiologic characteristics of human ventricular and Purkinje fibers. Circulation. 1982;65:362–368. doi: 10.1161/01.CIR.65.2.362. [DOI] [PubMed] [Google Scholar]
- 66.Satin J, et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J. Physiol. 2004;559:479–496. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mummery C, et al. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–2740. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 68.Ribeiro MC, et al. Functional maturation of human pluripotent stem cell derived cardiomyocytes invitro - correlation between contraction force andelectrophysiology. Biomaterials. 2015;51:138–150. doi: 10.1016/j.biomaterials.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 69.Scheel O, et al. Action potential characterization of human induced pluripotent stem cell-derived cardiomyocytes using automated patch-clamp technology. Assay Drug Dev. Technol. 2014;12:457–469. doi: 10.1089/adt.2014.601. [DOI] [PubMed] [Google Scholar]
- 70.Prajapati, C., Pölönen, R. P. & Aalto-Setälä, K. Simultaneous recordings of action potentials and calcium transients from human induced pluripotent stem cell derived cardiomyocytes. Biol. Open7, bio035030 (2018). [DOI] [PMC free article] [PubMed]
- 71.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 72.Zhang, G. Q., Wei, H., Lu, J., Wong, P. & Shim, W. Identification and characterization of calcium sparks in cardiomyocytes derived from human induced pluripotent stem cells. PLoS ONE8, e55266 (2013). [DOI] [PMC free article] [PubMed]
- 73.Riedel M, et al. Functional and pharmacological analysis of cardiomyocytes differentiated from human peripheral blood mononuclear-derived pluripotent stem cells. Stem Cell Rep. 2014;3:131–141. doi: 10.1016/j.stemcr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thompson SA, et al. Engraftment of human embryonic stem cell derived cardiomyocytes improves conduction in an arrhythmogenic in vitro model. J. Mol. Cell. Cardiol. 2012;53:15–23. doi: 10.1016/j.yjmcc.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Trautwein W, Kassebaum DG, Nelson RM, Hecht HH. Electrophysiological study of human heart muscle. Circ. Res. 1962;10:306–312. doi: 10.1161/01.RES.10.3.306. [DOI] [PubMed] [Google Scholar]
- 76.Kupersmith J, Krongrad E, Waldo AL. Conduction intervals and conduction velocity in the human cardiac conduction system. Studies during open-heart surgery. Circulation. 1973;47:776–785. doi: 10.1161/01.CIR.47.4.776. [DOI] [PubMed] [Google Scholar]
- 77.Synnergren J, Améen C, Jansson A, Sartipy P. Global transcriptional profiling reveals similarities and differences between human stem cell-derived cardiomyocyte clusters and heart tissue. Physiol. Genomics. 2012;44:245–258. doi: 10.1152/physiolgenomics.00118.2011. [DOI] [PubMed] [Google Scholar]
- 78.Scuderi, G. J. & Butcher, J. Naturally engineered maturation of cardiomyocytes. Front. Cell Dev. Biol.5, 50 (2017). [DOI] [PMC free article] [PubMed]
- 79.Kannan, S. & Kwon, C. Regulation of cardiomyocyte maturation during critical perinatal window. J. Physiol.598, 2941–2956 (2020). [DOI] [PMC free article] [PubMed]
- 80.Kamakura T, et al. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ. J. 2013;77:1307–1314. doi: 10.1253/circj.CJ-12-0987. [DOI] [PubMed] [Google Scholar]
- 81.Ebert A, et al. Proteasome-dependent regulation of distinct metabolic states during long-term culture of human ipsc-derived cardiomyocytes. Circ. Res. 2019;125:90–103. doi: 10.1161/CIRCRESAHA.118.313973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shiba, Y. et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature538, 388–391 (2016). [DOI] [PubMed]
- 83.Kadota S, Pabon L, Reinecke H, Murry CE. In vivo maturation of human induced pluripotent stem cell-derived cardiomyocytes in neonatal and adult rat hearts. Stem Cell Rep. 2017;8:278–289. doi: 10.1016/j.stemcr.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Radisic M, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl Acad. Sci. USA. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tandon N, et al. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sathaye A, Bursac N, Sheehy S, Tung L. Electrical pacing counteracts intrinsic shortening of action potential duration of neonatal rat ventricular cells in culture. J. Mol. Cell. Cardiol. 2006;41:633–641. doi: 10.1016/j.yjmcc.2006.06.076. [DOI] [PubMed] [Google Scholar]
- 87.Godier-Furnémont AFG, et al. Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation. Biomaterials. 2015;60:82–91. doi: 10.1016/j.biomaterials.2015.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroll K, et al. Electro-mechanical conditioning of human iPSC-derived cardiomyocytes for translational research. Prog. Biophys. Mol. Biol. 2017;130:212–222. doi: 10.1016/j.pbiomolbio.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Nunes SS, et al. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods. 2013;10:781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zimmermann WH, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 91.Hansen A, et al. Development of a drug screening platform based on engineered heart tissue. Circ. Res. 2010;107:35–44. doi: 10.1161/CIRCRESAHA.109.211458. [DOI] [PubMed] [Google Scholar]
- 92.Jackman CP, Carlson AL, Bursac N. Dynamic culture yields engineered myocardium with near-adult functional output. Biomaterials. 2016;111:66–79. doi: 10.1016/j.biomaterials.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Breckwoldt K, et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 2017;12:1177–1197. doi: 10.1038/nprot.2017.033. [DOI] [PubMed] [Google Scholar]
- 94.Ulmer BM, et al. Contractile work contributes to maturation of energy metabolism in hiPSC-derived cardiomyocytes. Stem Cell Rep. 2018;10:834–847. doi: 10.1016/j.stemcr.2018.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Uzun, A. U. et al. Ca2+-currents in human induced pluripotent stem cell-derived cardiomyocytes effects of two different culture conditions. Front. Pharmacol. 7, 300 (2016). [DOI] [PMC free article] [PubMed]
- 96.Leonard A, et al. Afterload promotes maturation of human induced pluripotent stem cell derived cardiomyocytes in engineered heart tissues. J. Mol. Cell. Cardiol. 2018;118:147–158. doi: 10.1016/j.yjmcc.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abilez OJ, et al. Passive stretch induces structural and functional maturation of engineered heart muscle as predicted by computational modeling. Stem Cells. 2018;36:265–277. doi: 10.1002/stem.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rogers AJ, Fast VG, Sethu P. Biomimetic cardiac tissue model enables the adaption of human induced pluripotent stem cell cardiomyocytes to physiological hemodynamic loads. Anal. Chem. 2016;88:9862–9868. doi: 10.1021/acs.analchem.6b03105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hirt MN, et al. Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation. J. Mol. Cell. Cardiol. 2014;74:151–161. doi: 10.1016/j.yjmcc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 100.Ruan JL, et al. Mechanical stress conditioning and electrical stimulation promote contractility and force maturation of induced pluripotent stem cell-derived human cardiac tissue. Circulation. 2016;134:1557–1567. doi: 10.1161/CIRCULATIONAHA.114.014998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ronaldson-Bouchard K, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239–243. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ribeiro AJS, et al. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl Acad. Sci. USA. 2015;112:12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rodriguez ML, et al. Substrate stiffness, cell anisotropy, and cell-cell contact contribute to enhanced structural and calcium handling properties of human embryonic stem cell-derived cardiomyocytes. ACS Biomater. Sci. Eng. 2019;5:3876–3888. doi: 10.1021/acsbiomaterials.8b01256. [DOI] [PubMed] [Google Scholar]
- 104.Wang PY, Yu J, Lin JH, Tsai WB. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011;7:3285–3293. doi: 10.1016/j.actbio.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 105.Feaster, T. K. et al. Matrigel mattress: a method for the generation of single contracting human-induced pluripotent stem cell-derived cardiomyocytes. Circ. Res.117, 995–1000 (2015). [DOI] [PMC free article] [PubMed]
- 106.Papadaki M, et al. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am. J. Physiol. Heart Circ. Physiol. 2001;280:168–178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y, et al. Engineering a freestanding biomimetic cardiac patch using biodegradable poly(lactic-co-glycolic acid) (PLGA) and human embryonic stem cell-derived ventricular cardiomyocytes (hESC-VCMs) Macromol. Biosci. 2015;15:426–436. doi: 10.1002/mabi.201400448. [DOI] [PubMed] [Google Scholar]
- 108.Jongpaiboonkit L, et al. An adaptable hydrogel array format for 3-dimensional cell culture and analysis. Biomaterials. 2008;29:3346–3356. doi: 10.1016/j.biomaterials.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chun YW, et al. Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiomyocytes. Biomaterials. 2015;67:52–64. doi: 10.1016/j.biomaterials.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feinberg AW, et al. Muscular thin films for building actuators and powering devices. Science. 2007;317:1366–1370. doi: 10.1126/science.1146885. [DOI] [PubMed] [Google Scholar]
- 111.Herron, T. J. et al. Extracellular matrix-mediated maturation of human pluripotent stem cell-derived cardiac monolayer structure and electrophysiological function. Circ. Arrhythmia Electrophysiol.9, e003638 (2016). [DOI] [PMC free article] [PubMed]
- 112.Fong AH, et al. Three-dimensional adult cardiac extracellular matrix promotes maturation of human induced pluripotent stem cell-derived cardiomyocytes. Tissue Eng. Part A. 2016;22:1016–1025. doi: 10.1089/ten.tea.2016.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garreta E, et al. Myocardial commitment from human pluripotent stem cells: rapid production of human heart grafts. Biomaterials. 2016;98:64–78. doi: 10.1016/j.biomaterials.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Guyette JP, et al. Bioengineering human myocardium on native extracellular matrix. Circ. Res. 2016;118:56–72. doi: 10.1161/CIRCRESAHA.115.306874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian Y, Morrisey EE. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ. Res. 2012;110:1023–1034. doi: 10.1161/CIRCRESAHA.111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abecasis B, et al. Unveiling the molecular crosstalk in a human induced pluripotent stem cell-derived cardiac model. Biotechnol. Bioeng. 2019;116:1245–1252. doi: 10.1002/bit.26929. [DOI] [PubMed] [Google Scholar]
- 117.Dunn, K. K. et al. Coculture of endothelial cells with human pluripotent stem cell-derived cardiac progenitors reveals a differentiation stage-specific enhancement of cardiomyocyte maturation. Biotechnol. J.14, 1800725 (2019). [DOI] [PMC free article] [PubMed]
- 118.Burridge PW, et al. Multi-cellular interactions sustain long-term contractility of human pluripotent stem cell-derived cardiomyocytes. Am. J. Transl. Res. 2014;6:724–735. [PMC free article] [PubMed] [Google Scholar]
- 119.Shimizu, T. et al. Fabrication of pulsatile cardiac tissue grafts using a novel 3-dimensional cell sheet manipulation technique and temperature-responsive cell culture surfaces. Circ. Res.90, e40–e48 (2002). [DOI] [PubMed]
- 120.Correia C, et al. 3D aggregate culture improves metabolic maturation of human pluripotent stem cell derived cardiomyocytes. Biotechnol. Bioeng. 2018;115:630–644. doi: 10.1002/bit.26504. [DOI] [PubMed] [Google Scholar]
- 121.Eng G, et al. Autonomous beating rate adaptation in human stem cell-derived cardiomyocytes. Nat. Commun. 2016;7:1–10. doi: 10.1038/ncomms10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ma, Z. et al. Self-organizing human cardiac microchambers mediated by geometric confinement. Nat. Commun. 6, 1–10 (2015). [DOI] [PMC free article] [PubMed]
- 123.Goldfracht I, et al. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater. 2019;92:145–159. doi: 10.1016/j.actbio.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 124.Boudou T, et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng. Part A. 2012;18:910–919. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bian W, Badie N, Himel HD, Bursac N. Robust T-tubulation and maturation of cardiomyocytes using tissue-engineered epicardial mimetics. Biomaterials. 2014;35:3819–3828. doi: 10.1016/j.biomaterials.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shadrin, I. Y. et al. Cardiopatch platform enables maturation and scale-up of human pluripotent stem cell-derived engineered heart tissues. Nat. Commun. 8, 1–15 (2017). [DOI] [PMC free article] [PubMed]
- 127.Macqueen LA, et al. A tissue-engineered scale model of the heart ventricle. Nat. Biomed. Eng. 2018;2:930–941. doi: 10.1038/s41551-018-0271-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Thavandiran, N. et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc. Natl Acad. Sci. USA110, E4698–E4707 (2013). [DOI] [PMC free article] [PubMed]
- 129.Abecasis B, et al. Toward a microencapsulated 3D hiPSC-derived in vitro cardiac microtissue for recapitulation of human heart microenvironment features. Front. Bioeng. Biotechnol. 2020;8:1163. doi: 10.3389/fbioe.2020.580744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Giacomelli, E. et al. Human-iPSC-derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell26, 862–879 (2020). [DOI] [PMC free article] [PubMed]
- 131.Ronaldson-Bouchard, K. et al. Engineering of human cardiac muscle electromechanically matured to an adult-like phenotype. Nat. Protoc.14, 2781–2817 (2019). [DOI] [PMC free article] [PubMed]
- 132.Yang X, et al. Fatty acids enhance the maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cell Rep. 2019;13:657–668. doi: 10.1016/j.stemcr.2019.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu D, et al. Metabolic maturation of human pluripotent stem cellderived cardiomyocytes by inhibition of HIF1α and LDHA. Circ. Res. 2018;123:1066–1079. doi: 10.1161/CIRCRESAHA.118.313249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Correia C, et al. Distinct carbon sources affect structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Sci. Rep. 2017;7:1–17. doi: 10.1038/s41598-017-08713-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li M, et al. Thyroid hormone action in postnatal heart development. Stem Cell Res. 2014;13:582–591. doi: 10.1016/j.scr.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 136.Yang X, et al. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J. Mol. Cell. Cardiol. 2014;72:296–304. doi: 10.1016/j.yjmcc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Parikh SS, et al. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ. Res. 2017;121:1323–1330. doi: 10.1161/CIRCRESAHA.117.311920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yoshida S, et al. Maturation of human induced pluripotent stem cell-derived cardiomyocytes by soluble factors from human mesenchymal stem cells. Mol. Ther. 2018;26:2681–2695. doi: 10.1016/j.ymthe.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deng, X. F., Rokosh, D. G. & Simpson, P. C. Autonomous and growth factor-induced hypertrophy in cultured neonatal mouse cardiac myocytes: comparison with rat. Circ. Res.87, 781–788 (2000). [DOI] [PubMed]
- 140.Sucharov CC, et al. A β1-adrenergic receptor CaM kinase II-dependent pathway mediates cardiac myocyte fetal gene induction. Am. J. Physiol. Heart Circ. Physiol. 2006;291:1299–1308. doi: 10.1152/ajpheart.00017.2006. [DOI] [PubMed] [Google Scholar]
- 141.Patrizio M, et al. cAMP-mediated β-adrenergic signaling negatively regulates Gq-coupled receptor-mediated fetal gene response in cardiomyocytes. J. Mol. Cell. Cardiol. 2008;45:761–769. doi: 10.1016/j.yjmcc.2008.09.120. [DOI] [PubMed] [Google Scholar]
- 142.Földes G, et al. Modulation of human embryonic stem cell-derived cardiomyocyte growth: a testbed for studying human cardiac hypertrophy? J. Mol. Cell. Cardiol. 2011;50:367–376. doi: 10.1016/j.yjmcc.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lieu DK, et al. Mechanism-based facilitated maturation of human pluripotent stem cell-derived cardiomyocytes. Circ. Arrhythmia Electrophysiol. 2013;6:191–201. doi: 10.1161/CIRCEP.111.973420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu J, et al. Facilitated maturation of Ca2+ handling properties of human embryonic stem cell-derived cardiomyocytes by calsequestrin expression. Am. J. Physiol. Cell Physiol. 2009;297:152–159. doi: 10.1152/ajpcell.00060.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chow, M. Z. et al. Epigenetic regulation of the electrophysiological phenotype of human embryonic stem cell-derived ventricular cardiomyocytes: insights for driven maturation and hypertrophic growth. Stem Cells Dev.22, 2678–2690 (2013). [DOI] [PMC free article] [PubMed]
- 146.Otsuji, T. G., Kurose, Y., Suemori, H., Tada, M. & Nakatsuji, N. Dynamic link between histone H3 acetylation and an increase in the functional characteristics of human ESC/iPSC-derived cardiomyocytes. PLoS ONE7, e45010 (2012). [DOI] [PMC free article] [PubMed]
- 147.Biermann M, et al. Epigenetic priming of human pluripotent stem cell‐derived cardiac progenitor cells accelerates cardiomyocyte maturation. Stem Cells. 2019;37:910–923. doi: 10.1002/stem.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Babiarz JE, et al. Determination of the human cardiomyocyte mRNA and miRNA differentiation network by fine-scale profiling. Stem Cells Dev. 2012;21:1956–1965. doi: 10.1089/scd.2011.0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kumar N, et al. Assessment of temporal functional changes and miRNA profiling of human iPSC-derived cardiomyocytes. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-49653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuppusamy KT, et al. Let-7 family of microRNA is required for maturation and adult-like metabolism in stem cell-derived cardiomyocytes. Proc. Natl Acad. Sci. USA. 2015;112:E2785–E2794. doi: 10.1073/pnas.1424042112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lu TY, et al. Overexpression of microRNA-1 promotes cardiomyocyte commitment from human cardiovascular progenitors via suppressing WNT and FGF signaling pathways. J. Mol. Cell. Cardiol. 2013;63:146–154. doi: 10.1016/j.yjmcc.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fu, J. D. et al. Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyocytes. PLoS ONE6, e27417 (2011). [DOI] [PMC free article] [PubMed]
- 153.Poon ENY, et al. Integrated transcriptomic and regulatory network analyses identify microRNA-200c as a novel repressor of human pluripotent stem cell-derived cardiomyocyte differentiation and maturation. Cardiovasc. Res. 2018;114:894–906. doi: 10.1093/cvr/cvy019. [DOI] [PubMed] [Google Scholar]
- 154.Lee DS, et al. Defined microRNAs induce aspects of maturation in mouse and human embryonic-stem-cell-derived cardiomyocytes. Cell Rep. 2015;12:1960–1967. doi: 10.1016/j.celrep.2015.08.042. [DOI] [PubMed] [Google Scholar]
- 155.Bassat E, et al. The extracellular matrix protein agrin promotes heart regeneration in mice. Nature. 2017;547:179–184. doi: 10.1038/nature22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhou Y, et al. Comparative gene expression analyses reveal distinct molecular signatures between differentially reprogrammed cardiomyocytes. Cell Rep. 2017;20:3014–3024. doi: 10.1016/j.celrep.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moroni L, et al. Biofabrication strategies for 3D in vitro models and regenerative medicine. Nat. Rev. Mater. 2018;3:21–37. doi: 10.1038/s41578-018-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Malda J, et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 2013;25:5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 160.Lee S, et al. Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials. 2017;131:111–120. doi: 10.1016/j.biomaterials.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kerscher P, et al. Direct hydrogel encapsulation of pluripotent stem cells enables ontomimetic differentiation and growth of engineered human heart tissues. Biomaterials. 2016;83:383–395. doi: 10.1016/j.biomaterials.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Grover, G. N., Rao, N. & Christman, K. L. Myocardial matrix-polyethylene glycol hybrid hydrogels for tissue engineering. Nanotechnology25, 014011 (2014). [DOI] [PMC free article] [PubMed]
- 163.Bajaj P, Schweller RM, Khademhosseini A, West JL, Bashir R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Annu. Rev. Biomed. Eng. 2014;16:247–276. doi: 10.1146/annurev-bioeng-071813-105155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rodriguez, M. L. et al. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J. Biomech. Eng.136, 051005 (2014). [DOI] [PMC free article] [PubMed]
- 165.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 166.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–3243. doi: 10.1016/j.biomaterials.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ott HC, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 168.Oberwallner B, et al. Preparation of cardiac extracellular matrix scaffolds by decellularization of human myocardium. J. Biomed. Mater. Res. A. 2014;102:3263–3272. doi: 10.1002/jbm.a.35000. [DOI] [PubMed] [Google Scholar]
- 169.Pati F, et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014;5:1–11. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perea-Gil I, et al. A cell-enriched engineered myocardial graft limits infarct size and improves cardiac function: pre-clinical study in the porcine myocardial infarction model. JACC Basic Transl. Sci. 2016;1:360–372. doi: 10.1016/j.jacbts.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Seif-Naraghi SB, Salvatore MA, Schup-Magoffin PJ, Hu DP, Christman KL. Design and characterization of an injectable pericardial matrix gel: a potentially autologous scaffold for cardiac tissue engineering. Tissue Eng. Part A. 2010;16:2017–2027. doi: 10.1089/ten.tea.2009.0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johnson TD, et al. Human versus porcine tissue sourcing for an injectable myocardial matrix hydrogel. Biomater. Sci. 2014;2:735–744. doi: 10.1039/C3BM60283D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bejleri, D. et al. A bioprinted cardiac patch composed of cardiac-specific extracellular matrix and progenitor cells for heart repair. Adv. Healthc. Mater.7, 1800672 (2018). [DOI] [PMC free article] [PubMed]
- 174.Noor, N. et al. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv. Sci. 6, 1900344 (2019). [DOI] [PMC free article] [PubMed]
- 175.Zhao, Y. et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell176, 913–927 (2019). [DOI] [PMC free article] [PubMed]
- 176.Engelmayr GC, et al. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tian B, et al. Macroporous nanowire nanoelectronic scaffolds for synthetic tissues. Nat. Mater. 2012;11:986–994. doi: 10.1038/nmat3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Feiner R, et al. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat. Mater. 2016;15:679–685. doi: 10.1038/nmat4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chiu, L. L. Y., Montgomery, M., Liang, Y., Liu, H. & Radisic, M. Perfusable branching microvessel bed for vascularization of engineered tissues. Proc. Natl Acad. Sci. USA109, E3414–E3423 (2012). [DOI] [PMC free article] [PubMed]
- 180.Zhang B, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nat. Mater. 2016;15:669–678. doi: 10.1038/nmat4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gouveia PJ, et al. Flexible nanofilms coated with aligned piezoelectric microfibers preserve the contractility of cardiomyocytes. Biomaterials. 2017;139:213–228. doi: 10.1016/j.biomaterials.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 182.Badrossamay MR, McIlwee HA, Goss JA, Parker KK. Nanofiber assembly by rotary jet-spinning. Nano Lett. 2010;10:2257–2261. doi: 10.1021/nl101355x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Castilho, M. et al. Melt electrowriting allows tailored microstructural and mechanical design of scaffolds to advance functional human myocardial tissue formation. Adv. Funct. Mater.28, 1803151 (2018).
- 184.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32:773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 185.Hinton, T. J. et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 1, e1500758 (2015). [DOI] [PMC free article] [PubMed]
- 186.Lee A, et al. 3D bioprinting of collagen to rebuild components of the human heart. Science. 2019;365:482–487. doi: 10.1126/science.aav9051. [DOI] [PubMed] [Google Scholar]
- 187.Zhang YS, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. 2016;110:45–59. doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl Acad. Sci. USA. 2016;113:3179–3184. doi: 10.1073/pnas.1521342113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Grigoryan B, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364:458–464. doi: 10.1126/science.aav9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Skylar-Scott, M. A. et al. Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels. Sci. Adv. 5, eaaw2459 (2019). [DOI] [PMC free article] [PubMed]
- 191.Bernal, P. N. et al. Volumetric bioprinting of complex living-tissue constructs within seconds. Adv. Mater. 31, 1904209 (2019). [DOI] [PubMed]
- 192.Fordyce CB, et al. Cardiovascular drug development: is it dead or just hibernating? J. Am. Coll. Cardiol. 2015;65:1567–1582. doi: 10.1016/j.jacc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 193.Braam SR, Passier R, Mummery CL. Cardiomyocytes from human pluripotent stem cells in regenerative medicine and drug discovery. Trends Pharmacol. Sci. 2009;30:536–545. doi: 10.1016/j.tips.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 194.Magdy T, Schuldt AJT, Wu JC, Bernstein D, Burridge PW. Human induced pluripotent stem cell (hiPSC)-derived cells to assess drug cardiotoxicity: opportunities and problems. Annu. Rev. Pharmacol. Toxicol. 2018;58:83–103. doi: 10.1146/annurev-pharmtox-010617-053110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Polonchuk L, et al. Cardiac spheroids as promising in vitro models to study the human heart microenvironment. Sci. Rep. 2017;7:1–12. doi: 10.1038/s41598-017-06385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Marsano A, et al. Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip. 2016;16:599–610. doi: 10.1039/C5LC01356A. [DOI] [PubMed] [Google Scholar]
- 198.Feric NT, et al. Engineered cardiac tissues generated in the biowire II: a platform for human-based drug discovery. Toxicol. Sci. 2019;172:89–97. doi: 10.1093/toxsci/kfz168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chramiec A, et al. Integrated human organ-on-a-chip model for predictive studies of anti-tumor drug efficacy and cardiac safety. Lab Chip. 2020;20:4357–4372. doi: 10.1039/D0LC00424C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lind JU, et al. Cardiac microphysiological devices with flexible thin-film sensors for higher-throughput drug screening. Lab Chip. 2017;17:3692–3703. doi: 10.1039/C7LC00740J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao, Y. et al. A multimaterial microphysiological platform enabled by rapid casting of elastic microwires. Adv. Healthc. Mater.8, 1801187 (2019). [DOI] [PubMed]
- 202.Zhang B, Radisic M. Organ-on-A-chip devices advance to market. Lab Chip. 2017;17:2395–2420. doi: 10.1039/C6LC01554A. [DOI] [PubMed] [Google Scholar]
- 203.Sun, N. et al. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci. Transl. Med. 4, 130ra47 (2012). [DOI] [PMC free article] [PubMed]
- 204.Burridge PW, et al. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat. Med. 2016;22:547–556. doi: 10.1038/nm.4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Lan F, et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell. 2013;12:101–113. doi: 10.1016/j.stem.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lee J, et al. Activation of PDGF pathway links LMNA mutation to dilated cardiomyopathy. Nature. 2019;572:335–340. doi: 10.1038/s41586-019-1406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Te Riele ASJM, et al. Multilevel analyses of SCN5A mutations in arrhythmogenic right ventricular dysplasia/cardiomyopathy suggest non-canonical mechanisms for disease pathogenesis. Cardiovasc. Res. 2017;113:102–111. doi: 10.1093/cvr/cvw234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kodo K, et al. iPSC-derived cardiomyocytes reveal abnormal TGF-β signalling in left ventricular non-compaction cardiomyopathy. Nat. Cell Biol. 2016;18:1031–1042. doi: 10.1038/ncb3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Itzhaki, I. et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature471, 225–230 (2011). [DOI] [PubMed]
- 210.Yazawa M, et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature. 2011;471:230–236. doi: 10.1038/nature09855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Matsa E, et al. Transcriptome profiling of patient-specific human iPSC-cardiomyocytes predicts individual drug safety and efficacy responses in vitro. Cell Stem Cell. 2016;19:311–325. doi: 10.1016/j.stem.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sharma, A. et al. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci. Transl. Med. 9, eaaf2584 (2017). [DOI] [PMC free article] [PubMed]
- 213.Smith JGW, et al. Isogenic pairs of hiPSC-CMs with hypertrophic cardiomyopathy/LVNC-associated ACTC1 E99K mutation unveil differential functional deficits. Stem Cell Rep. 2018;11:1226–1243. doi: 10.1016/j.stemcr.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.de la Roche, J. et al. Comparing human iPSC-cardiomyocytes versus HEK293T cells unveils disease-causing effects of Brugada mutation A735V of NaV1.5 sodium channels. Sci. Rep.9, 1–14 (2019). [DOI] [PMC free article] [PubMed]
- 215.Wei H, Zhang XH, Clift C, Yamaguchi N, Morad M. CRISPR/Cas9 gene editing of RyR2 in human stem cell-derived cardiomyocytes provides a novel approach in investigating dysfunctional Ca2+ signaling. Cell Calcium. 2018;73:104–111. doi: 10.1016/j.ceca.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mosqueira D, et al. CRISPR/Cas9 editing in human pluripotent stem cell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy. Eur. Heart J. 2018;39:3879–3892. doi: 10.1093/eurheartj/ehy249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hinson JT, et al. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science. 2015;349:982–986. doi: 10.1126/science.aaa5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang G, et al. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat. Med. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Goldfracht I, et al. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020;11:1–15. doi: 10.1038/s41467-019-13868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.McCain ML, Sheehy SP, Grosberg A, Goss JA, Parker KK. Recapitulating maladaptive, multiscale remodeling of failing myocardium on a chip. Proc. Natl Acad. Sci. USA. 2013;110:9770–9775. doi: 10.1073/pnas.1304913110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sadeghi, A. H. et al. Engineered 3D cardiac fibrotic tissue to study fibrotic remodeling. Adv. Healthc. Mater.6, 1601434 (2017). [DOI] [PMC free article] [PubMed]
- 222.Zhang YS, et al. Bioprinted thrombosis-on-a-chip. Lab Chip. 2016;16:4097–4105. doi: 10.1039/C6LC00380J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Vikhorev PG, et al. Abnormal contractility in human heart myofibrils from patients with dilated cardiomyopathy due to mutations in TTN and contractile protein genes. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-13675-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Prondzynski, M. et al. Disease modeling of a mutation in α‐actinin 2 guides clinical therapy in hypertrophic cardiomyopathy. EMBO Mol. Med.11, e11115 (2019). [DOI] [PMC free article] [PubMed]
- 225.Madonna R, et al. Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur. Heart J. 2016;37:1789–1798. doi: 10.1093/eurheartj/ehw113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sluijter, J. P. G. et al. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc. Res. 114, 19–34 (2018). [DOI] [PMC free article] [PubMed]
- 227.Eschenhagen T, et al. Cardiomyocyte regeneration: a consensus statement. Circulation. 2017;136:680–686. doi: 10.1161/CIRCULATIONAHA.117.029343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Terrovitis J, et al. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J. Am. Coll. Cardiol. 2009;54:1619–1626. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gaetani R, et al. Cardiospheres and tissue engineering for myocardial regeneration: potential for clinical application. J. Cell. Mol. Med. 2010;14:1071–1077. doi: 10.1111/j.1582-4934.2010.01078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hou, D. et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation112, I-150–I-156 (2005). [DOI] [PubMed]
- 231.Riegler J, et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 2015;117:720–730. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Pecha S, et al. Human iPS cell-derived engineered heart tissue does not affect ventricular arrhythmias in a guinea pig cryo-injury model. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-46409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Weinberger, F. et al. Cardiac repair in guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med.8, 363ra148 (2016). [DOI] [PubMed]
- 234.Gao L, et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018;137:1712–1730. doi: 10.1161/CIRCULATIONAHA.117.030785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mawad D, et al. A conducting polymer with enhanced electronic stability applied in cardiac models. Sci. Adv. 2016;2:e1601007. doi: 10.1126/sciadv.1601007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Ye L, et al. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750–761. doi: 10.1016/j.stem.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gaetani R, et al. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–348. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 238.Feyen DAM, et al. Gelatin microspheres as vehicle for cardiac progenitor cells delivery to the myocardium. Adv. Healthc. Mater. 2016;5:1071–1079. doi: 10.1002/adhm.201500861. [DOI] [PubMed] [Google Scholar]
- 239.Li J, et al. Human pluripotent stem cell-derived cardiac tissue-like constructs for repairing the infarcted myocardium. Stem Cell Rep. 2017;9:1546–1559. doi: 10.1016/j.stemcr.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kawamura, M. et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation126, S29–S37 (2012). [DOI] [PubMed]
- 241.Landa N, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 242.Seif-Naraghi, S. B. et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci. Transl. Med. 5, 173ra25 (2013). [DOI] [PMC free article] [PubMed]
- 243.Lin X, et al. A viscoelastic adhesive epicardial patch for treating myocardial infarction. Nat. Biomed. Eng. 2019;3:632–643. doi: 10.1038/s41551-019-0380-9. [DOI] [PubMed] [Google Scholar]
- 244.Almeida SO, Skelton RJ, Adigopula S, Ardehali R. Arrhythmia in stem cell transplantation. Card. Electrophysiol. Clin. 2015;7:357–370. doi: 10.1016/j.ccep.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Nguyen PK, Neofytou E, Rhee JW, Wu JC. Potential strategies to address the major clinical barriers facing stem cell regenerative therapy for cardiovascular disease: a review. JAMA Cardiol. 2016;1:953–962. doi: 10.1001/jamacardio.2016.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Madonna, R. Human-induced pluripotent stem cells: in quest of clinical applications. Mol. Biotechnol.52, 193–203 (2012). [DOI] [PubMed]
- 247.Breitbach M, et al. Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 2007;110:1362–1369. doi: 10.1182/blood-2006-12-063412. [DOI] [PubMed] [Google Scholar]
- 248.Blin G, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J. Clin. Invest. 2010;120:1125–1139. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yoon YS, Park JS, Tkebuchava T, Luedeman C, Losordo DW. Unexpected severe calcification after transplantation of bone marrow cells in acute myocardial infarction. Circulation. 2004;109:3154–3157. doi: 10.1161/01.CIR.0000134696.08436.65. [DOI] [PubMed] [Google Scholar]
- 250.Chung L, Maestas DR, Housseau F, Elisseeff JH. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017;114:184–192. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 251.Menasché P, et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 2018;71:429–438. doi: 10.1016/j.jacc.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 252.Mewhort HEM, et al. Epicardial infarct repair with bioinductive extracellular matrix promotes vasculogenesis and myocardial recovery. J. Heart Lung Transplant. 2016;35:661–670. doi: 10.1016/j.healun.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 253.Yanagawa, B., Rao, V., Yau, T. M. & Cusimano, R. J. Potential myocardial regeneration with cormatrix ECM: a case report. J. Thorac. Cardiovasc. Surg. 147, e41–e43 (2014). [DOI] [PubMed]
- 254.Mewhort HEM, Turnbull JD, Meijndert HC, Ngu JMC, Fedak PWM. Epicardial infarct repair with basic fibroblast growth factor-enhanced CorMatrix-ECM biomaterial attenuates postischemic cardiac remodeling. J. Thorac. Cardiovasc. Surg. 2014;147:1650–1659. doi: 10.1016/j.jtcvs.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 255.Traverse JH, et al. First-in-man study of a cardiac extracellular matrix hydrogel in early and late myocardial infarction patients. JACC Basic Transl. Sci. 2019;4:659–669. doi: 10.1016/j.jacbts.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Frey N, et al. Intracoronary delivery of injectable bioabsorbable scaffold (IK-5001) to treat left ventricular remodeling after ST-elevation myocardial infarction: a first-in-man study. Circ. Cardiovasc. Interv. 2014;7:806–812. doi: 10.1161/CIRCINTERVENTIONS.114.001478. [DOI] [PubMed] [Google Scholar]
- 257.Rao SV, et al. Bioabsorbable intracoronary matrix for prevention of ventricular remodeling after myocardial infarction. J. Am. Coll. Cardiol. 2016;68:715–723. doi: 10.1016/j.jacc.2016.05.053. [DOI] [PubMed] [Google Scholar]
- 258.Rao, S. V. et al. A randomized, double-blind, placebo-controlled trial to evaluate the safety and effectiveness of intracoronary application of a novel bioabsorbable cardiac matrix for the prevention of ventricular remodeling after large ST-segment elevation myocardial infarction: Rationale and design of the PRESERVATION I trial. Am. Heart J.170, 929–937 (2015). [DOI] [PubMed]
- 259.Anker SD, et al. A prospective comparison of alginate-hydrogel with standard medical therapy to determine impact on functional capacity and clinical outcomes in patients with advanced heart failure (AUGMENT-HF trial) Eur. Heart J. 2015;36:2297–2309. doi: 10.1093/eurheartj/ehv259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mann DL, et al. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur. J. Heart Fail. 2016;18:314–325. doi: 10.1002/ejhf.449. [DOI] [PubMed] [Google Scholar]
- 261.Lee RJ, et al. The feasibility and safety of Algisyl-LVRTM as a method of left ventricular augmentation in patients with dilated cardiomyopathy: initial first in man clinical results. Int. J. Cardiol. 2015;199:18–24. doi: 10.1016/j.ijcard.2015.06.111. [DOI] [PubMed] [Google Scholar]
- 262.Puymirat E, et al. Can mesenchymal stem cells induce tolerance to cotransplanted human embryonic stem cells? Mol. Ther. 2009;17:176–182. doi: 10.1038/mt.2008.208. [DOI] [PMC free article] [PubMed] [Google Scholar]