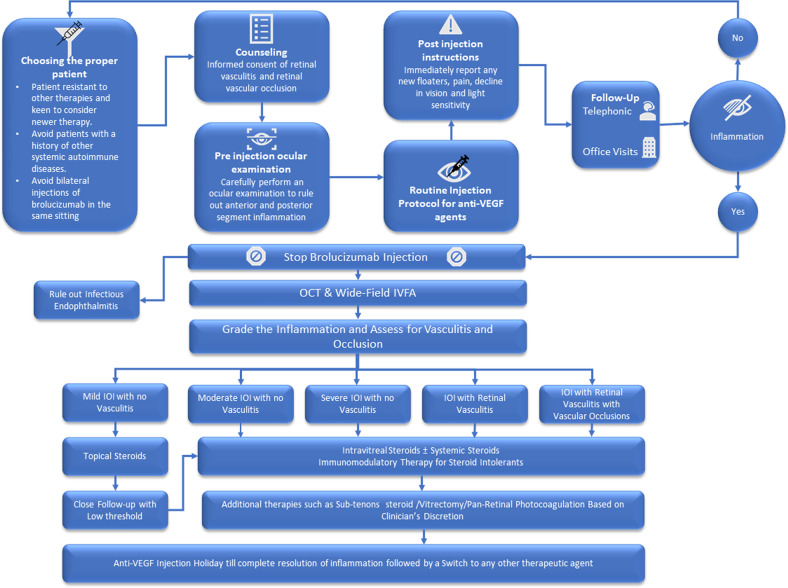
Brolucizumab is the newest anti-vascular endothelial growth factor (anti-VEGF) approved for the management of neovascular age-related macular degeneration (nAMD) in more than 40 countries, including approval from the Food and Drug Administration (FDA), USA [1, 2]. After brolucizumab received FDA approval, it was noticed in clinical practice that there were new adverse events (retinal vasculitis and retinal vascular occlusion) associated with intraocular inflammation (IOI), which was not initially reported in the HAWK and HARRIER trials [3]. The drug has demonstrated excellent macular drying efficacy with superior resolution of intraretinal fluid and subretinal fluid compared to aflibercept in the trials and anecdotally in the real-world setting [4, 5]. Furthermore, it provided the opportunity to extend the treatment interval for up to 12 weeks [4]. As brolucizumab is now available to be used, it is important to understand the probable workflow that can help clinicians to incorporate the drug in their practice with minimizing the risk. The index manuscript will provide a stepwise approach on how to use brolucizumab in the current nAMD treatment landscape guided by available scientific evidence. The proposed approach is prepared based on a literature search on PubMed using keywords brolucizumab and vasculitis. Out of the nine articles, six were related to patients who developed retinal vasculitis. Furthermore, expert opinion from a recent, international panel discussion (with leading retina and uveitis specialists from Australia, Austria, India, Israel, Italy, and United States) at a recent virtual meeting (Brolucizumab Global Symposium, October 17, 2020) was considered [6]. These six articles and an expert panel discussion formed the basis to design this eight-step “Avoiding brolucizumab-related adverse events by scrutinizing available evidence” (A BRAVE SAVE) recommendation (Fig. 1). Limitation of the protocol “A BRAVE SAVE” is that these suggestions are based on a consensus as there is very limited evidence of outcomes from real-world usage of this drug.
Fig. 1.
Avoiding Brolucizumab Related Adverse Event by Scrutinizing Available Evidence (A BRAVE-SAVE) Recommendation. OCT Optical coherence tomography, IVFA Intravenous fluorescein angiography.
Step 1
Choosing the proper patient: As of now, there is no clear patient profile that has emerged to be more or less susceptible to IOI and related retinal adverse events. We suggest that at this point that the drug be avoided patients with only one good seeing eye. The suitable patients would be the ones resistant to other therapies who are keen to consider a newer drug knowing its safety profile.
A series of cases by Witkun et al. showed that 20% of patients who had a history of inflammation had underlying autoimmune diseases such as multiple sclerosis, Reynaulds phenomenon, hypothyroidism, Graves’ disease, and psoriasis [7]. In another case series reported by Baumel et al. 58% cases had presence of arthritis, multiple sclerosis, and hypothyroidism, all of which have auto-immune pathophysiology [8]. Furthermore, two case reports of brolucizumab-related vasculitis reported a history of arthritis and hypothyroidism [9, 10]. Also, the number of females was significantly higher in the cases reported with vasculitis in the published literature.
With limited evidence, it would be preferable to avoid patients with a history of systemic autoimmune diseases. In addition, clinicians should avoid bilateral injections of brolucizumab in the same sitting to avoid risk to both eyes in case of any untoward ocular adverse event.
Step 2
Counseling: Once the clinician intends to use brolucizumab, there should be a clear mention of retinal vasculitis and retinal occlusion with the potential to result in permanent vision loss in the informed consent. Furthermore, the patient should be verbally well-informed about the chances of developing retinal vasculitis in 1/200 patients [8].
Step 3
Pre-injection ocular examination: Clinicians should carefully perform an ocular examination before the injection and rule out any pre-existing inflammation in both anterior and posterior segments. If there are any signs of acute or chronic ocular inflammation, anti-VEGF agents other than brolucizumab should be used in preference.
Step 4
Injection procedure: Injection can be performed as per the prevailing standard injection protocol similar to other anti-VEGFs.
Step 5
Postinjection instructions: Patients should be instructed to report immediately if they experience any signs and symptoms of possible inflammation such as new floaters, pain, a decline in vision, and light sensitivity. Since the injection frequency is likely to be in the 8–12 week range based on the pivotal trials and the onset of the inflammation is delayed and unpredictable, office and clinics staff should be aware of the potential symptoms and recommend urgent evaluation if such symptoms are reported by the patient regardless of when the injection was performed relative to the onset of the symptoms.
Step 6
Injection protocol: Rather than 3-monthly loading injections in all patients, individualized, less frequent injection protocols adopted early on in the course of treatment should be considered. The ongoing Treat-to-control Regimen in Patients With Neovascular Age-related Macular Degeneration (TALON) trial may help us to understand better the efficacy and safety of the drug with less frequent injections [11].
Step 7
Management if the patient develops IOI/retinal vasculitis/retinal vascular occlusion: How to manage the patients was very well highlighted by Baumal et al. With any signs of IOI, the subsequent injection of brolucizumab must be avoided. The following is a summary of the recommendations [12].
(7a) Rule out infectious endophthalmitis, which will have an acute onset within a week compared to the delayed and subacute presentation of sterile IOI.
(7b) Optical coherence tomography and intravenous fluorescein angiography (preferable wide-field or peripheral sweeps) are the key diagnostic modalities. Any patient with IOI should be suspected of having vasculitis and images should be carefully analyzed to determine the involvement of vessels even in cases of mild inflammation. Indocyanine Green Angiography to assess choroidal ischemia can be utilized at the clinician’s discretion.
(7c) If no vascular involvement is seen and IOI is mild then close follow-up with intensive topical steroids treatment should be considered. There should be close follow-up with a low threshold more intensive steroid therapy if the condition worsens.
(7d) In cases of moderate to severe IOI or vasculitis or any form of vascular occlusion, intravitreal steroid injection along with systemic steroids should be considered. Immunomodulators, vitrectomy, and pan-retinal photocoagulation are the other options to be utilized per the clinician’s discretion.
In addition, when indicated, the patients should be evaluated for underlying diseases such as giant cell arteritis or collagen vascular diseases that may have led to the ocular vaso-occlusive disease.
Step 8
Future course of treating nAMD: Good responders can be continued to be treated with brolucizumab using the same workflow. Clinicians should wait for the complete resolution of inflammation and then treat them with other anti-VEGFs according to the clinician’s choice (no preference to any particular drug). If there is no worsening of nAMD, an injection holiday can be considered for some time with close follow-up.
Globally, the experience is not sufficient to formulate a definitive guideline on brolucizumab use with the current safety signals. However, some of the publications in the recent past have highlighted a few points to consider as a guide at this time. Hopefully, we will have a better understanding of the suitable patient profile, risk, and management in the future once clinical experience grows and the manufacturer provides further guidance on the potential cause of inflammation.
Compliance with ethical standards
Conflict of interest
AS: Consultant: for Novartis, Allergan, Bayer and Intas. BDK: Clinical research: Alcon, Alimera, Allegro, Allergan, Apellis, Clearside, Genentech, GSK, Ionis, jCyte, Novartis, Regeneron, ThromboGenics; Consultant: Alimera, Allegro, Allergan, Cell Care, Dose, Eyedaptic, Galimedix, Genentech, Glaukos, Interface Biologics, jCyte, Novartis, Ophthotech, Regeneron, Revana, Theravance Biopharma. FB: Consultant: Allergan, Bayer, Boehringer- Ingelheim, Fidia Sooft, Hofmann La Roche, Novartis, NTC Pharma, Sifi, Thrombogenics, Zeiss. CDR: Consultant: Allergan, Chengdu Kanghong, Genentech/Roche, Novartis, Kodiak, Notal, Merck, Takeda, Adverum, Graybug, Eyepoint; research support: Allergan, Chengdu Kanghong, Genentech/Roche, Novartis, Kodiak, Iveric, Adverum, Regeneron, RegenXBio. DB: Consultant: Genentech, Allergan, Roche, Regeneron, Bayer, Novartis. QDN: Consultant/Scientific Advisory Boards: EyePoint, Genentech, Gilead, Regeneron, Santen. The other authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Beovu [US prescribing information]. East Hanover, NJ: Novartis; 2019. Accessed Oct 2020.
- 2.https://www.globenewswire.com/news-release/2020/09/14/2092794/0/en/European-Medicines-Agency-EMA-approves-safety-label-update-for-Novartis-Beovu.html. Accesses Oct 2020.
- 3.Sharma A, Kumar N, Parachuri N, Singh S, Bandello F, Kuppermann BD, et al. Brolucizumab-related retinal vasculitis: emerging disconnect between clinical trials and real world. Eye (Lond). 2020. 10.1038/s41433-020-01227-w. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 4.Dugel PU, Koh A, Ogura Y, Jaffe GJ, Schmidt-Erfurth U, Brown DM, et al. HAWK and HARRIER Study Investigators. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of Brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A, Kumar N, Parachuri N, Sadda SR, Corradetti G, Heier J, et al. Brolucizumab-early real-world experience: BREW study. Eye. 2020. 10.1038/s41433-020-1111-x. [DOI] [PMC free article] [PubMed]
- 6.One Technosoft. Brolucizumab Global Symposium—6.30 pm—17 Oct. 2020. https://www.youtube.com/watch?v=a-UtDlgYTKk.
- 7.Witkin AJ, Hahn P, Murray TG, Arevalo JF, Blinder KJ, Choudhry N, et al. Occlusive retinal vasculitis following intravitreal brolucizumab. J Vitreoretin Dis. 2020;4:269–79. doi: 10.1177/2474126420930863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumal CR, Spaide RF, Vajzovic L, Freund KB, Walter SD, John V, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127:1345–59. doi: 10.1016/j.ophtha.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Haug SJ, Hien DL, Uludag G, Ngoc TTT, Lajevardi S, Halim MS, et al. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18:100680. doi: 10.1016/j.ajoc.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Chea S, Matsumiya W, Halim MS, Yaşar Ç, Kuang G, et al. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep. 2020;18:100687. doi: 10.1016/j.ajoc.2020.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Study to assess the efficacy and safety of brolucizumab 6mg compared to Aflibercept 2 mg in a treat to control regimen (TALON). ClinicalTrials.gov. Accessed Nov 11 2020. https://clinicaltrials.gov/ct2/show/NCT04005352.
- 12.Baumal CR, Bodaghi B, Singer M, Tanzer DJ, Seres A, Joshi MR.et al. Expert opinion on management of intraocular inflammation, retinal vasculitis, and/or vascular occlusion after brolucizumab treatment. Ophthalmol Retina. 2020. 10.1016/j.oret.2020.09.020. [DOI] [PubMed]