Summary
Striatal dopamine and smartphone behavior have both been linked with behavioral variability. Here, we leverage day-to-day logs of natural, unconstrained smartphone behavior and establish a correlation between a measure of smartphone social activity previously linked with behavioral variability and a measure of striatal dopamine synthesis capacity using [18F]-DOPA PET in (N = 22) healthy adult humans. Specifically, we find that a higher proportion of social app interactions correlates with lower dopamine synthesis capacity in the bilateral putamen. Permutation tests and penalized regressions provide evidence that this link between dopamine synthesis capacity and social versus non-social smartphone interactions is specific. These observations provide a key empirical grounding for current speculations about dopamine's role in digital social behavior.
Subject areas: Behavioral neuroscience, Biological sciences, Neuroscience
Graphical abstract
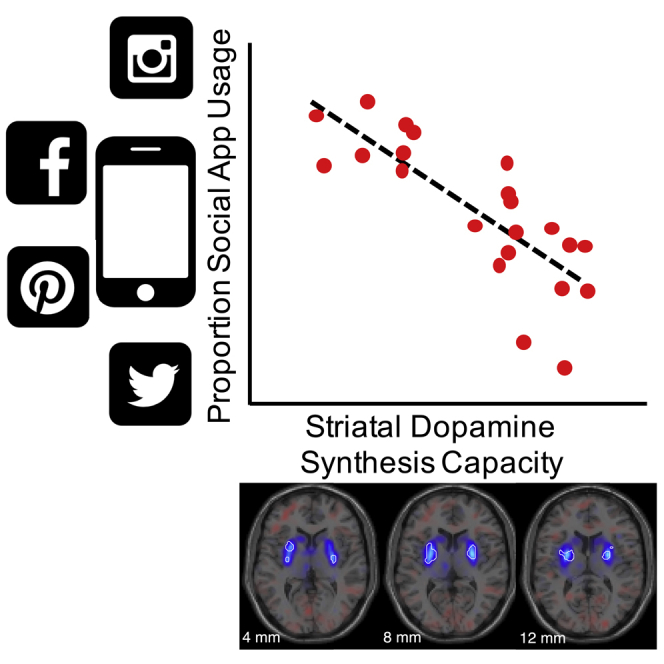
Highlights
-
•
Putamen dopamine synthesis capacity correlates with smartphone social app use.
-
•
The correlation parallels a prior link between social app use and motor variability.
-
•
It is selective to social app use, controlling for multiple smartphone use factors.
Behavioral neuroscience; Biological sciences; Neuroscience
Introduction
Striatal dopamine plays key roles in cognition and behavior. Beyond gross motor and cognitive disturbances associated with cell loss in Parkinson disease, striatal dopamine tone has been linked with increasing vigor (Niv et al., 2007) and regulating behavioral variability (Costa, 2011; Galea et al., 2013; Mikhael et al., 2021) as a function of reward context. How dopamine function relates to naturalistic behavior outside the confines of laboratory settings and precisely controlled reward schemes, however, is rarely studied.
Smartphones offer a powerful tool for examining rich, spontaneous, real-world behavior, yet studies have only begun to leverage their potential (Reeves et al., 2020). In one recent, intriguing example, naturalistic smartphone behaviors correlated with individual differences in sensorimotor variability in a lab-based task (Balerna and Ghosh, 2018). Specifically, passively recorded logs of smartphone interactions revealed that participants who touched their screens more frequently in their daily life were also less variable in highly constrained button tapping in the lab. Interestingly, however, participants touching social apps more frequently showed “greater” sensorimotor variability. Why low-level motor function should track social versus non-social smartphone interactions remains unclear. Yet, given prior evidence implicating dopamine transmission in (social context related) behavioral variability (e.g., Galea et al., 2013; Leblois et al., 2010; MacDonald et al., 2012) and speculations about dopamine's role in social media use (Burhan and Moradzadeh, 2020), we here ask whether smartphone social interactions index individual differences in striatal dopamine function.
In this study, we recorded touchscreen interactions to assess a relationship between smartphone social behavior—proxied by the app in use—and striatal dopamine function (see STAR Methods). Specifically, healthy young adult participants (N = 22 from a larger study, see STAR Methods; ages 18 to 33; 9 women) completed a ([18F]-DOPA) positron emission tomography (PET) scan to quantify their striatal dopamine synthesis capacity. We then examined touchscreen logs over weeks (median: 32 days, 10th–90th percentile: 26–50 days) of normal, daily use, outside of the PET scanner. Our analysis focused on measures linked with motor variability from the prior smartphone monitoring study (Balerna and Ghosh, 2018), including overall usage (interactions per day) and proportion of social interactions (proportion of all interactions that occurred on social apps—e.g. chat and messenger apps). Given prior work linking striatal dopamine function and motoric vigor (Niv et al., 2007), we also analyzed peak daily smartphone interaction speed (fastest inter-touch intervals performed every day; see Figure S1 for descriptive statistics).
Results
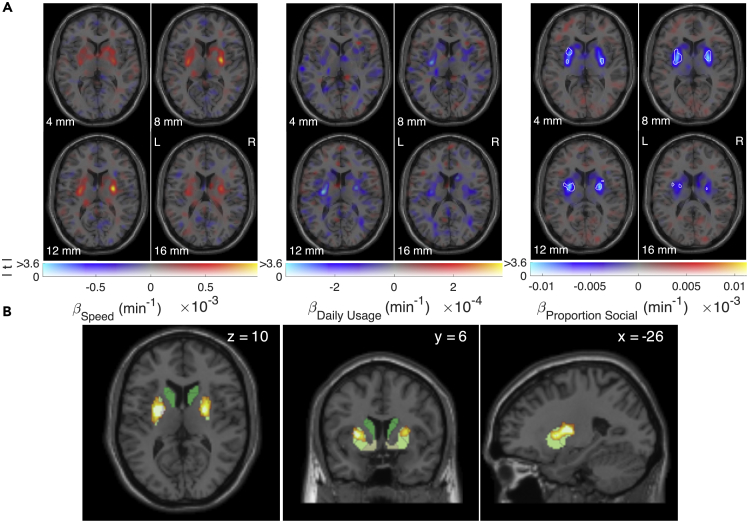
After aligning participants' PET scans, we performed a voxel-wise multiple regression to ask where each of these three measures reflected individual differences in dopamine synthesis capacity as quantified via Gjedde-Patlak analyses (Patlak et al., 1983). The fitted model (Figure 1A) revealed positive regression weights of striatal dopamine synthesis capacity on our proxy of vigor (interaction speed—fastest daily inter-touch intervals), negative weights on interactions per day, and negative weights on the proportion of social interactions.
Figure 1.
Whole-brain regression of dopamine synthesis capacity on speed, daily usage, and proportion of social interactions
(A) Whole-brain individual difference regression weights of dopamine synthesis capacity predicted by smartphone interaction speed, interactions per day, and proportion of social interactions. White boundaries encompass voxels at (uncorrected) p < 0.001, indicating that bilateral putamen synthesis capacity reflects proportion of social interactions; display format from (Zandbelt, 2017).
(B) Proportion social interaction clusters (PFWE < 0.05, small volume correction) resulting from permutation-based, threshold-free cluster enhancement (Smith and Nichols, 2009). Clusters overlap an independently defined segmentation of the striatum including the dorsal and medial caudate nucleus (dark green), anterior and posterior putamen (yellow-green), and ventral striatum (pale yellow). Note that similar clusters were obtained following thresholded (p < 0.001) cluster formation (see Figure S2).
Relationships with speed and interactions per day did not survive cluster corrections. However, the negative relationship between the proportion of social interactions and dopamine synthesis capacity was reliable in bilateral posterior putamen. Both threshold-free (Figure 1B) and thresholded cluster forming methods (Figure S3) confirmed a reliable correlation in the putamen in both hemispheres. We restricted our search to a dopamine-rich region encompassing the midbrain and striatum (Figure S2; (Sescousse et al., 2018)) and thus retained clusters falling below threshold of PFWE < 0.05, small volume corrected (Supplemental information).
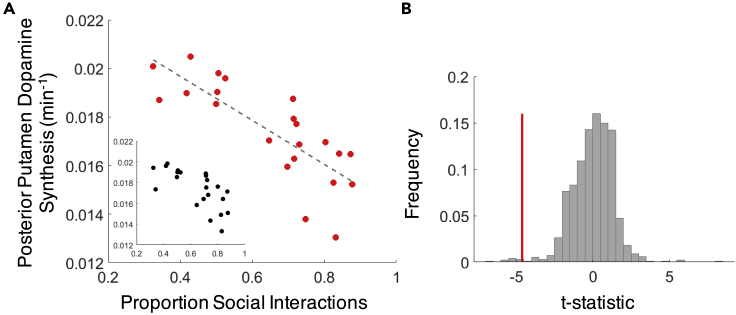
To further probe the relationship with the proportion of social interactions, we extracted mean dopamine synthesis capacity values from all voxels within the bilateral posterior putamen as defined by an independent, functional connectivity-based parcellation of the striatum (Piray et al., 2017). A robust multiple regression confirmed a negative relationship between individuals' mean dopamine synthesis capacity and the z-scored proportion of their smartphone interactions devoted to social apps (Figure 2A; = −1.5 × 10−3 min−1, t(18) = −4.8, p = 1.3 × 10−4) surviving correction for multiple comparisons across the five independently defined striatal sub-regions (PBonferroni = 6.5 × 10−4). Permutation testing confirmed that the slope of the relationship between dopamine synthesis capacity and social app categorization was extreme compared to 1000 random permutations of social versus non-social app category labels (Figure 2B). Neither speed ( = 7.9 × 10−4 min−1, t(18) = 2.3, p = 0.031) nor overall smartphone usage ( = −3.2 × 10−4 min−1, t(18) = −1.0, p = 0.32) survived multiple comparison corrections in their relationship to dopamine synthesis capacity in the bilateral posterior putamen. Collectively, this multiple regression model explains considerable between-subjects variance in posterior putamen dopamine synthesis capacity (R2 = 0.55).
Figure 2.
Individual differences in proportion of social app interactions covary with dopamine synthesis capacity in the bi-lateral posterior putamen
(A) Inverse relationship between individual differences in proportion social interactions and dopamine synthesis capacity (adjusted response plot of the multiple regression including speed and daily usage; inset shows unadjusted data). Dashed line is the estimated linear fit.
(B) The observed multiple regression slope (red) is extreme compared to the relationship observed in on 1000 random permutations of app category labels (gray).
A key feature of social activity is that it relies heavily on text messaging, raising the possibility that the observed relationship reflected intensive keypad typing rather than social interactions. However, several non-social apps (e.g., Notes) also rely on typing. Therefore, we created a category of “typing” apps to control for this effect. We added typing apps—along with age and gender of the participant—as explanatory variables in a lasso regression for parameter selection. Social activity survived the lasso regression (1000 bootstraps; Figure S3), suggesting that the amount of typing, per se, cannot explain the link between social activity and dopamine synthesis capacity. Thus, social interactions track between-subjects variance in dopamine synthesis capacity better than typing, age, and gender.
Discussion
We find a strong and focal relationship between dopamine synthesis capacity in the bilateral posterior putamen and smartphone social app use, which is also specific to social app use. This result supports the hypothesis that smartphone-based social behavior indexes striatal dopamine function. This result moreover highlights the potential for using naturalistic, passively tracked smartphone data to make inferences about individual differences in neuromodulatory function, although clearly replication studies are necessary, particularly given the small sample size (Button et al., 2013).
Our result also informs current speculations about digital social behavior and striatal dopamine function (Park and Kim, 2017; Turel et al., 2014). Consider, for example, problematic social media use. Excessive social media use has been associated with reduced ventral striatal gray matter volume (He et al., 2017; Montag et al., 2018), suggesting that problematic digital behavior (as in internet addiction) is driven by aberrant reward processing structures. We find a higher proportion of social app usage among those with lower dopamine synthesis capacity. Our results dovetail, albeit in a healthy sample, with the findings that individuals with attention deficit hyperactivity disorder have both lower dopamine synthesis capacity (Ernst et al., 1998; Ludolph et al., 2008) and are more prone to social media addiction (Andreassen et al., 2016). The fact that one of the attention deficit hyperactivity disorder studies showed reduced dopamine function specifically in the putamen (Ludolph et al., 2008) converges with the locus of our result—although we did not a priori predict social app use to relate to putamen dopamine function over other striatal sub-regions. Interestingly, “higher” rather than “lower” dopamine synthesis capacity has been associated with other behavioral addictions (e.g. gambling [Holst et al., 2018]; though see [Majuri et al., 2017]) and behavioral disinhibition (Lawrence and Brooks, 2014) indicating that dopamine synthesis capacity may interact with other factors in conferring risk for problematic social media use. One such factor may be D2 receptor density, which is positively correlated with trait extraversion in healthy adults (Baik et al., 2012).
In sum, our findings highlight the promise of naturalistic smartphone behavioral data both for elucidating rich, real-world social behavior and the neural mechanisms that support them.
Limitations of the study
Our discovery, while promising, warrants future replication studies to confirm that proportion social interactions is related to dopamine synthesis capacity specifically in the posterior putamen and not to other regions like the ventral striatum. We note that low sample size prevents strong inferences about one striatal sub-region over another, especially considering the moderate correlation in dopamine synthesis capacity between regions (for the posterior putamen and ventral striatum = 0.57). Thus, replication studies with larger sample sizes are critical. Multi-model imaging techniques may also prove especially informative: e.g. PET studies quantifying multiple facets of striatal dopamine signaling including synthesis capacity and D2 receptor density (Berry et al., 2017). Functional MRI can also provide critical information about the functional networks to which striatal sub-regions belong, with implications for anhedonia and impulsivity (Hamilton et al., 2018; Piray et al., 2017).
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| Smartphone derivative and analyzed data | This paper | https://doi.org/10.34973/vjwg-0214 |
| List of Social versus Non-social Apps | Arko Ghosh | Table S1 |
| Software and algorithms | ||
| FreeSurfer v7.1 | Laboratory for Computational Neuroimaging | https://surfer.nmr.mgh.harvard.edu/ |
| Statistical Parameteric Mapping v12 | The Wellcome Center for Human Neuroimaging | https://www.fil.ion.ucl.ac.uk/spm/ |
| MATLAB vR2020b | MathWorks | https://www.mathworks.com/products/matlab.html |
| TapCounter | QuantActions | https://quantactions.com/ |
| Slice Display | Bram Zandbelt | 10.6084/m9.figshare.4742866 |
| R v4.0.3 | The Comprehensive R Archive Network | https://cran.r-project.org/ |
| clickR v0.5.27 | David Hervas Marin | https://cran.r-project.org/ |
| MASS package v7.3-53.1 | Brian Ripley | http://www.stats.ox.ac.uk/pub/MASS4/ |
Resource availability
Lead contact
Further information and requests should be directed to the lead contact, Andrew Westbrook (andrew.westbrook@brown.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
Smartphone derivative measures links to PET measures and analysis code can all be found in the Donders Data Repository at https://doi.org/10.34973/vjwg-0214.
Experimental model and subject details
We invited participants from a pool of healthy, young adults in the Radboud University community (Nijmegen, Netherlands) who had completed a PET scan as part of a larger pharmaco-imaging study (N = 96 participants) on the influence of catecholamines on cognitive control. A full list of exclusion criteria and study measures collected for the larger study is registered at https://www.trialregister.nl/trial/5959. The study was approved by the regional research ethics committee (Commissie Mensgebonden Onderzoek, region Arnhem-Nijmegen; 2016/2646; ABR: NL57538.091.16). In total, N = 27 participants responded that they were willing and able to participate. After giving informed consent, a proprietary app (TapCounter, QuantActions; Lausanne, Switzerland) was installed on their smartphone (software limited to non-iPhones only) and activated so that their smartphone use data (personal interactions with the device) could be passively recorded and uploaded to a cloud-based server.
We initially planned to record approximately 3 weeks of data for all participants, but participants had independent control over when the app was removed from their phone, thus the recording interval varied. Data loss on some days for some participants also meant that final recording interval varied across participants with total usable days ranging from 8 to 467 days (median: 32 days, 10th–90th percentile: 26–50 days). Also, 5 participants were subsequently excluded due to excessive data loss from logging and connectivity issues and thus our final sample size was N = 22. Our final sample of N = 22 were ages 18 to 33; 9 women). The lag between PET scan and app installation ranged from 92 days prior to 658 days after the PET scan (mean lag was 328 days after the PET scan).
Method details
Smartphone variables and their distributions
Smartphone data contained the following raw values: the time-stamp of the touchscreen interaction, the label of the app in use at the time of the interaction, and screen on/off times. From these, we computed our three measures of interest: 1) smartphone usage, 2) proportion of social interactions and 3) interaction speed. Smartphone usage was quantified as the square root-transformed total number of interactions to reduce non-normality, divided by the number of days of recording. Interaction speed was estimated as follows: the shortest 25th percentile of the inter-event intervals was accumulated in 24 h bins, the inverse of the median of these accumulated values was used.
Participants’ scores for our three key independent variables (speed, overall usage, and proportion social touches) are all distributed over a wide range, and within-subject variance is consistent across participants when the data are accumulated over a day (Figure S1).
Apps and their classification
App labels were used to determine the proportion of social interactions: number of interactions on social apps versus all interactions. Apps were rated as social if: 1) their main purpose was for communication among users, 2) the app facilitates interactions through messages, posts, and/or chat, 3) users can create personal profiles, 4) the app facilitates the development of a social network, 5) the app is intended to be used with a social app, or cannot be used without one. Otherwise, an app was classified as non-social (e.g. news or weather apps).
All apps used in the study were rated by hand as either social or non-social, and examined for inter-rater consistency. To resolve any inconsistent classifications across raters, the raters first attempted consensus by sharing their reasoning and when a consensus was not possible the app was labeled as uncategorized. As noted, a complete list of apps can be downloaded as Table S1.
Dopamine synthesis capacity measurement
Dopamine synthesis capacity was measured using the radiotracer [18F]-fluoro-DOPA (F-DOPA) and a Siemens mCT PET-CT scanner (4 x 4 mm voxels, 5 mm slice thickness). Participants received 150 mg carbidopa to reduce decarboxylase activity and 400 mg entacapone to reduce peripheral COMT activity, 50 minutes prior to the start of the scan. Participants were administered a low dose CT scan used for attenuation correction, followed by a bolus injection of 185 MBq (5 mCi) max F-DOPA into the antecubital vein. Over 89 minutes, we collected 4 1-minute frames, 3 2-minute frames, 3 3-minute frames, and 14 5-minute frames. Data were reconstructed with weighted attenuation correction, time-of-flight correction, correction for scatter, and smoothed with a 3 mm full-width-half-max kernel. Presynaptic dopamine synthesis capacity was quantified per voxel as F-DOPA influx rate (Ki; min-1) using Gjedde-Patlak linear graphical analysis (Patlak et al., 1983) for the frames of 24—89 minutes, with cerebellar gray matter as the reference region, which was obtained via FreeSurfer segmentation. Ki maps were spatially normalized to MNI space and smoothed using an 8 mm FWHM Gaussian kernel.
FreeSurfer segmentation was performed using high-resolution anatomical MRI scans collected on a separate date from the PET scan. Specifically, participants completed a structural T1-weighted magnetization prepared, rapid-acquisition gradient echo sequence MRI scan (TR 2300 ms, TE 3.03 ms, flip angle 8°, 192 sagittal slices, 1 mm thick, field of view 256 mm, voxel size 1x1x1 mm), scanned by a Siemens MAGNETOM Skyra 3 Tesla MR scanner.
Quantification and statistical analysis
Voxel-wise multiple regression and correction
We identified bi-lateral clusters of the putamen surviving FWE p < 0.05 small volume correction for multiple comparisons. For our analyses, the small volume was defined by a mask of dopamine-rich regions encompassing the midbrain, brainstem, and the basal ganglia (Figure S2). The mask was defined from the distribution of dopamine synthesis capacity signal across the full sample from the larger parent study of N = 94 participants receiving a [18F]-DOPA PET scan (N = 100 were recruited; 6 were removed for data loss and participant drop outs). Specifically, following (Sescousse et al., 2018), we defined a volume encompassing all voxels where dopamine synthesis capacity was 3 standard deviations above the mean signal across the whole brain. While dopamine synthesis capacity can be measured outside of this region, the signal is much stronger in the volume than elsewhere and hence we reasoned that any relationship with smartphone behavior would arise within this small volume.
Cluster corrected whole-brain analysis
Complementing our threshold-free cluster enhancement methods in the main text, we also used classic threshold cluster forming methods. We used a seed threshold of p < 0.001, with 10 voxels extent, then applied small volume correction and retained clusters surviving FWE at PFWE < 0.05. The resulting clusters (Figure S3) were very similar to those obtained by threshold-free cluster enhancement.
Multiple regression on dopamine synthesis averages
After extracting region-averaged dopamine synthesis capacity in striatal subregions, we regressed posterior putamen dopamine synthesis capacity on the three smartphone explanatory variables: (a) smartphone usage, (b) speed, and (c) proportion of social touches via robust regression using the rlm function (MASS package v7.3-51.4) in R.
Lasso regressions
While we found a relationship between dopamine synthesis capacity in the posterior putamen and the proportion of touchscreen interactions on social apps, we were also curious whether such a relationship is specific to the posterior putamen and to social app use rather than other potentially explanatory variables. To examine these questions, we extracted mean dopamine synthesis capacity from all voxels in five striatal sub-regions, defined independently (via functional connectivity profiles: (Piray et al., 2017)): the anterior (aPut) and posterior putamen (pPut), the medial (mCaud) and dorsal caudate nucleus (dCaud), and the ventral striatum (vStr). We then fit lasso regression models, for each of these regions, with the following predictors: total smartphone usage, proportion of social interactions, and interaction speed. We also included the proportion of typing app interactions, participant age and gender. We fit lasso regression models in MATLAB (MathWorks, Natick, USA) for parameter selection. Only those parameters which resulted in coefficients different from 0 based on 0.5 and 99.5 percentile range of the bootstrapped (1000) lasso coefficients were considered as meaningful contributors to the regression.
Permutation testing (via app label shuffling) revealed that the proportion of social taps significantly predicts dopamine synthesis capacity in our model of the posterior putamen, but not in other regions (Figure S4). Moreover, the only reliable predictor in the posterior putamen model was social taps, further demonstrating the specificity of our results. Note that we also considered the possibility that gaming app use was related to dopamine synthesis capacity, but variance was highly restricted (13 participants showed no gaming at all). Nevertheless, and unsurprisingly given the limited variance, a parallel analysis incorporating gaming app use as a predictor in a lasso regression yielded no evidence of a relationship between the proportion of gaming app interactions and dopamine synthesis capacity.
Frequency versus proportion of social app use
Our core analysis was focused on the proportion of smartphone interactions devoted to social apps – a variable which was motivated by prior work relating the proportion of social app use to sensorimotor variability (Balerna and Ghosh, 2018) and the implication of dopamine in sensorimotor variability (Costa, 2011; Galea et al., 2013; Mikhael et al., 2021). We used proportion rather than the count of daily interactions using social apps because a proportion measure provides complementary information to overall count of daily interactions (which is also included in our multiple regression models). However, we also tested whether the count of social app interactions was a better predictor than proportion. First, we tested whether each predictor, on its own, relates to dopamine synthesis capacity in separate robust regression models. We found that the proportion of social app interactions is reliably related to dopamine synthesis capacity ( = -1.2×10-3 min-1, t(20) = -3.7, p = 1.5×10-3), but count is not ( = -2.4×10-5 min-1, t(20) = -0.06, p = 0.95). To pit them directly against each other, we fit a third robust regression model including regressors for speed along with both proportion and count of social app interactions (excluding the overall count of daily interactions which is highly correlated with the count of social app interactions). We found that dopamine synthesis capacity related to speed ( = 8.1×10-4 min-1, t(18) = 2.3, p = 2.8×10-2) and proportion social app interactions ( = -1.4×10-3 min-1, t(18) = -4.9, p = 1.1×10-4) but not count ( = -3.6×10-4 min-1, t(18) = -1.1, p = 0.29). Thus, in our data set, the proportion of social app usage covaries more reliably with dopamine synthesis capacity.
Acknowledgments
We thank the individuals who participated in this study. We also thank Dr. Henk van Steenbergen for introducing authors A.W. and A.G., thereby initiating this research. This study was supported by NWO VICI Grant, 453-14-015 (2015/01379/VI) to R.C.; Grants from Holcim Stiftung and Velux Stiftung (No. 1283) to A.G., and NIH Grant F32MH115600 to A.W.
Author contributions
Conceptualization, R.C., A.G., and A.W.; data curation, A.G., R.v.d.B., J.I.M., L.H., and A.W.; formal analysis, A.G., R.v.d.B., and A.W.; funding acquisition, R.C., A.G., and A.W.; investigation, A.G. and A.W.; project administration, A.W. and J.I.M.; software, A.G., R.v.d.B., and A.W.; writing, R.C., A.G., and A.W.; supervision of the PET measurements, R.C.
Declarations of interests
A.G. is a co-founder of QuantActions Ltd, Lausanne, Switzerland. This company focuses on converting smartphone taps to mental health indicators. Software and data collection services from QuantActions were used to monitor smartphone activity.
Inclusion and diversity
We worked to ensure gender balance in the recruitment of human subjects. One or more of the authors of this paper self-identifies as a member of the LGBTQ+ community. The author list of this paper includes contributors from the location where the research was conducted who participated in the data collection, design, analysis, and/or interpretation of the work.
Published: May 21, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2021.102497.
Supplemental information
References
- Andreassen C.S., Billieux J., Griffiths M.D., Kuss D.J., Demetrovics Z., Mazzoni E., Pallesen S. The relationship between addictive use of social media and video games and symptoms of psychiatric disorders: a large-scale cross-sectional study. Psych. Addict. Behav. 2016;30:252–262. doi: 10.1037/adb0000160. [DOI] [PubMed] [Google Scholar]
- Baik S., Yoon H.S., Kim S.E., Kim S.H. Extraversion and striatal dopaminergic receptor availability in young adults. Neuroreport. 2012;23:251–254. doi: 10.1097/WNR.0b013e3283507533. [DOI] [PubMed] [Google Scholar]
- Balerna M., Ghosh A. The details of past actions on a smartphone touchscreen are reflected by intrinsic sensorimotor dynamics. Npj Digit. Med. 2018;1:1–5. doi: 10.1038/s41746-017-0011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry A.S., Shah V.D., Furman D.J., White R.L., III, Baker S.L., O’Neil J.P., Janabi M., D’Esposito M., Jagust W.J. Dopamine synthesis capacity is associated with D2/3 receptor binding but not dopamine release. Neuropsychopharmacology. 2017;43:1201–1211. doi: 10.1038/npp.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burhan R., Moradzadeh J. Neurotransmitter dopamine (DA) and its role in the development of social media addiction. J. Neurol. Neurophys. 2020;11:507–508. [Google Scholar]
- Button K.S., Ioannidis J.P.A., Mokrysz C., Nosek B.A., Flint J., Robinson E.S.J., Munafò M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Costa R.M. A selectionist account of de novo action learning. Curr. Opin. Neurobiol. 2011;21:579–586. doi: 10.1016/j.conb.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Ernst M., Zametkin A.J., Matochik J.A., Jons P.H., Cohen R.M. DOPA decarboxylase activity in attention deficit hyperactivity disorder adults. A [Fluorine-18]Fluorodopa Positron Emission Tomographic Study. J. Neurosci. 1998;18:5901–5907. doi: 10.1523/JNEUROSCI.18-15-05901.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea J.M., Ruge D., Buijink A., Bestmann S., Rothwell J.C. Punishment-induced behavioral and neurophysiological variability reveals dopamine-dependent selection of kinematic movement parameters. J. Neurosci. 2013;33:3981–3988. doi: 10.1523/JNEUROSCI.1294-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Sacchet M.D., Hjørnevik T., Chin F.T., Shen B., Kämpe R., Park J.H., Knutson B.D., Williams L.M., Borg N. Striatal dopamine deficits predict reductions in striatal functional connectivity in major depression: a concurrent 11C-raclopride positron emission tomography and functional magnetic resonance imaging investigation. Transl. Psychiatry. 2018;8:1–10. doi: 10.1038/s41398-018-0316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Turel O., Brevers D., Bechara A. Excess social media use in normal populations is associated with amygdala-striatal but not with prefrontal morphology. Psychiat Res. Neuroim. 2017;269:31–35. doi: 10.1016/j.pscychresns.2017.09.003. [DOI] [PubMed] [Google Scholar]
- Holst R.J.van, Sescousse G., Janssen L.K., Janssen M., Berry A.S., Jagust W.J., Cools R. Increased striatal dopamine synthesis capacity in gambling addiction. Biol. Psychiatry. 2018;83:1036–1043. doi: 10.1016/j.biopsych.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence A.D., Brooks D.J. Ventral striatal dopamine synthesis capacity is associated with individual differences in behavioral disinhibition. Front Behav. Neurosci. 2014;8:86. doi: 10.3389/fnbeh.2014.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblois A., Wendel B.J., Perkel D.J. Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors. J. Neurosci. 2010;30:5730–5743. doi: 10.1523/JNEUROSCI.5974-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludolph A.G., Kassubek J., Schmeck K., Glaser C., Wunderlich A., Buck A.K., Reske S.N., Fegert J.M., Mottaghy F.M. Dopaminergic dysfunction in attention deficit hyperactivity disorder (ADHD), differences between pharmacologically treated and never treated young adults: a 3,4-dihdroxy-6-[18F]fluorophenyl-l-alanine PET study. Neuroimage. 2008;41:718–727. doi: 10.1016/j.neuroimage.2008.02.025. [DOI] [PubMed] [Google Scholar]
- MacDonald S.W.S., Karlsson S., Rieckmann A., Nyberg L., Bäckman L. Aging-related increases in behavioral variability: relations to losses of dopamine D1 receptors. J. Neurosci. 2012;32:8186–8191. doi: 10.1523/JNEUROSCI.5474-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majuri J., Joutsa J., Johansson J., Voon V., Alakurtti K., Parkkola R., Lahti T., Alho H., Hirvonen J., Arponen E. Dopamine and opioid neurotransmission in behavioral addictions: a comparative PET study in pathological gambling and binge eating. Neuropsychopharmacology. 2017;42:1169–1177. doi: 10.1038/npp.2016.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhael J.G., Lai L., Gershman S.J. Rational inattention and tonic dopamine. PLoS Comput. Biol. 2021;17:e1008659. doi: 10.1371/journal.pcbi.1008659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C., Zhao Z., Sindermann C., Xu L., Fu M., Li J., Zheng X., Li K., Kendrick K.M., Dai J., Becker B. Internet Communication Disorder and the structure of the human brain: initial insights on WeChat addiction. Sci. Rep. 2018;8:2155. doi: 10.1038/s41598-018-19904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y., Daw N.D., Joel D., Dayan P. Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191:507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- Park H.S., Kim S.E. Internet addiction and PET. In: Montag C., Reuter M., editors. Internet Addiction. Spinger International Publishing; 2017. pp. 81–92. [Google Scholar]
- Patlak C.S., Blasberg R.G., Fenstermacher J.D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Piray P., den Ouden H.E.M., van der Schaaf M.E., Toni I., Cools R. Dopaminergic modulation of the functional ventrodorsal architecture of the human striatum. Cereb. Cortex. 2017;27:485–495. doi: 10.1093/cercor/bhv243. [DOI] [PubMed] [Google Scholar]
- Reeves B., Robinson T., Ram N. Time for the human screenome project. Nature. 2020;577:314–317. doi: 10.1038/d41586-020-00032-5. [DOI] [PubMed] [Google Scholar]
- Sescousse G., Ligneul R., Holst R.J.van, Janssen L.K., Boer F.de, Janssen M., Berry A.S., Jagust W.J., Cools R. Spontaneous eye blink rate and dopamine synthesis capacity: preliminary evidence for an absence of positive correlation. Eur. J. Neurosci. 2018;47:1081–1086. doi: 10.1111/ejn.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Turel O., He Q., Xue G., Xiao L., Bechara A. Examination of neural systems sub-serving facebook “addiction”. Psychol. Rep. 2014;115:675–695. doi: 10.2466/18.PR0.115c31z8. [DOI] [PubMed] [Google Scholar]
- Zandbelt B. Slice display [WWW Document] 2017. 10.6084/m9.figshare.4742866
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Smartphone derivative measures links to PET measures and analysis code can all be found in the Donders Data Repository at https://doi.org/10.34973/vjwg-0214.