Abstract
Therapeutic programmed cell death protein 1 (PD-1) blockade enhances T cell mediated anti-tumor immunity, but many patients do not respond, and a significant proportion develops inflammatory toxicities. To develop better therapeutics and to understand the signaling pathways downstream of PD-1 we performed phosphoproteomic interrogation of PD-1 to identify key mediators of PD-1 signaling. Hereby, supporting data of the research article “VRK2 inhibition synergizes with PD-1 blockade to improve T cell responses” are presented. In the primary publication, we proposed that VRK2 is a unique therapeutic target and that combination of VRK2 inhibitors with PD-1 blockade may improve cancer immunotherapy. Here, we provide data on the effect of other kinases on PD-1 signaling utilizing shRNA knockdown of the different kinases in Jurkat T cells. In addition, we used VRK2 inhibition by a pharmacologic approach in the MC38 tumor mouse model, to show the combined outcome of anti PD-1 treatment with VRK2 inhibition. These data provide additional targets downstream PD-1 and point toward methods of testing the effect of the inhibition of these targets on tumor progression in vivo.
Keywords: PD-1, T CELL, TCR, VRK2
Specifications Table
| Subject | Immunology |
| Specific subject area | Cancer immunology, checkpoint inhibitor, immunotherapy, cell signaling |
| Type of data | Graph Figure |
| How data were acquired | RNA expression was determined by RT-PCR in QuantStudio 3 RT-PCR system. Concentrations of IL-2 were determined by specific ELISA kits in TEKAN microplate readers. Cell viability was measured with PrestoBlue (Invitrogen) in TEKAN microplate readers. T cells subsets were evaluated by flow cytometry in a MACSQuantR Analyzer 10. Statistical analysis was performed using GrapPad Prism 7 software. |
| Data format | Analysed |
| Parameters for data collection | Jurkat T cell lines were treated with anti-CD3 + anti-CD28 or anti-CD3 + anti-CD28 + recombinant PD-L2 coated beads. MC38 tumor cells were treated with Puromycin or AZD-7762. In vivo: mice harbouring MC38 tumors were orally administered with vehicle control, AZD-7762, Prexasertib and PF-477,736 (mice in each condition). In a second experiment, mice were orally administered with vehicle control or AZD-7762 and injected IP with vehicle control or 200 μg anti-PD-1 at day 0 and day 7 (4 mice in each group), two independent experiments. |
| Description of data collection | Total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen). Media was collected following centrifugation at 500 g for 5 min to remove floating cells from the media, followed by IL-2 ELISA (Biolegend). Tumor growth was monitored by external measurement using callipers. The volume of tumor masses was calculated with the following equation: 0.5 × Length × Width2. Spleens were harvested 17 days post-treatment initiation, followed by tissue dissociated on a mesh. Splenic cells were stained with anti-mouse antibodies for flow cytometry analysis. |
| Data source location | Columbia Center for Translational Immunology, Columbia University Medical Center, New York, NY 10,032, USA |
| Data accessibility | Data is provided in the article and the related research article. |
| Related research article | Michael Peled, Kieran Adam, Adam Mor. VRK2 inhibition synergizes with PD-1 blockade to improve T cell responses. Immunology Letters 2021 May;233:42–47. |
Value of the Data
-
•
The data point to new protein targets downstream of PD-1 that can serve as drug targets for cancer immunotherapy.
-
•
These data can help researchers to evaluate novel therapeutic targets downstream of PD-1, that their blockade may help cancer patients.
-
•
These data can spark a search of specific pharmacological VRK2 inhibitors.
1. Data Description
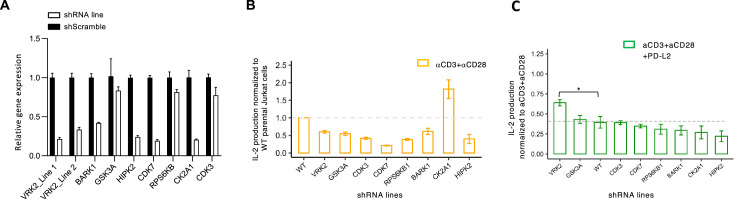
We have recently described potential kinases that may mediate PD-1 signaling in T lymphocytes based on phosphoproteome analysis of PD-1-activated T cells [1]. To assess if these kinases indeed facilitate signaling downstream of PD-1, knock-down of these kinases was induced by lentiviral transduction of kinase-specific shRNAs in Jurkat T cell lines (Fig. 1A). While all the cell lines secreted IL-2 following T cell receptor (TCR) activation with anti-CD3 + anti-CD28 antibodies (Fig. 1B), a combined TCR and PD-1 stimulation demonstrated that Vaccinia Related kinase 2 (VRK2) knocked down cells were the least inclined to PD-1 mediated inhibition of IL-2 secretion (Fig. 1C).
Fig. 1.
Kinase knockdown. (A) RT-qPCR analysis for kinase expression and shRNA knockdown efficiency of the indicated kinases in Jurkat T cells (n = 3). IL-2 production from shRNA knockdown Jurkat T cells lines following stimulation with anti-CD3 + anti-CD28 (B) or anti-CD3 + anti-CD28 + recombinant PD-L2-Fc (C), as indicated. Bar graphs show mean ± SEM of n = 3 independent experiments performed in triplicates.
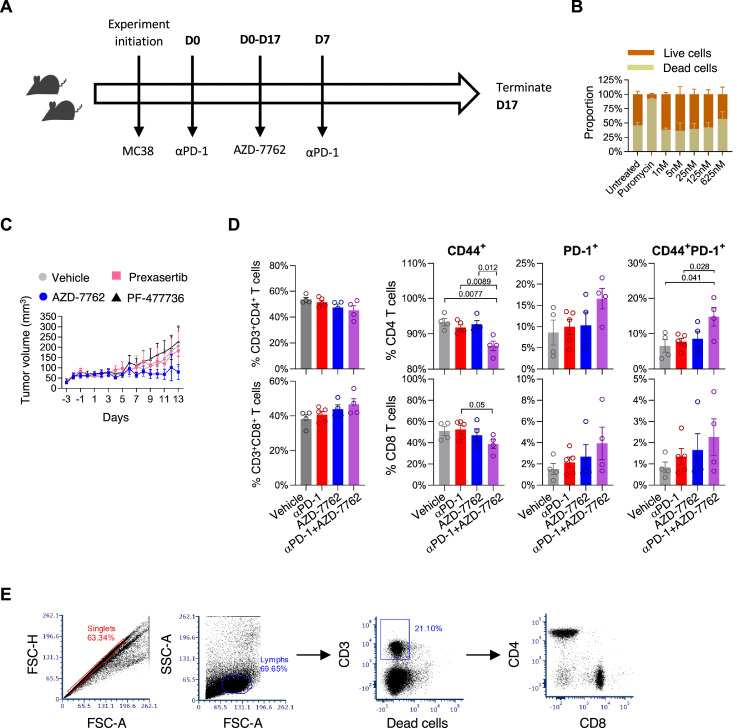
Following these results, we assessed if pharmacologic inhibition of VRK2 in the MC38 syngeneic mouse tumor model could enhance T cell responses by targeting the PD-1 pathway and potentially augmenting T cell activation with PD-1 blockade (Fig. 2A). To this end we used AZD-7762, an inhibitor of VRK2 and the checkpoint kinases (CHK1 and CHK2) [1]. This agent did not cause additional cytotoxicity in MC38 tumor cells in vitro (Fig. 2B). However, AZD-7762 significantly decreased tumor volume compared to vehicle or anti-PD-1 antibody treatment [2]. To exclude the possibility that AZD-7762 acts primarily via CHKs related mechanism in the tumor cells, tumor growth was determined in response to treatment with two CHK-specific inhibitors, Prexasertib (LY2606368) [3] and PF-477,736 [4]. Indeed, AZD-7762 treatment significantly decreased tumor volume compared to these drugs (Fig. 2C). Anti-PD-1 treatment is currently approved for many solid malignancies, however only a minority of the patients respond. Thus, we assessed the effect of a combined treatment of AZD-7762 with PD-1 blockade. The combined treatment of AZD-7762 with PD-1 blockade enhanced anti-tumor immune responses compared with either treatments alone, as demonstrated by the increased percentage of activated (CD44+PD-1+) T cells in the spleens (Fig. 2D).
Fig. 2.
Viability and T cell phenotypic analysis following treatment with AZD-7762, PF-477,736 and Prexasertib. (A) Graphical representation of MC38 mouse tumor protocol used in this study. (B) MC38 viability following treatment with AZD-7762 (n = 2). (C) MC38 tumor growth curves of mice treated with the indicated drugs. Drugs were orally administered, daily, and the data represent mean ± SEM of tumor volume measured in five mice from each group. (D) Surface phenotype of splenic T cells at day 17 post-tumor implantation. Four mice from two independent experiments. (E) Gating strategy: single cells were selected based on forward and side scatter intensities. Lymphocytes were gated based on forward distribution following positive staining for CD3 expression and exclusion of live dead staining. Subsequently, CD4 cells and CD8 cells were selected bases on specific staining, excluding double negative cells.
2. Experimental Design, Materials and Methods
2.1. General reagents
RPMI 1640 medium, DMEM, Dulbecco's PBS, and FBS were purchased from Life Technologies. Opti-MEMI was purchased from Invitrogen. Ficoll-Paque was purchased from Stem Cell. Puromycin was obtained from Sigma-Aldrich.
2.2. Cell culture, transfection, and stimulation
In vitro T cell cultures were maintained in complete RPMI, containing 10% FBS, MEM nonessential amino acids, 1 mM sodium pyruvate, 100 IU/ml of penicillin, 100 µg/ml streptomycin and GlutaMAX-I. Human Jurkat T cells were obtained from the American Type Culture Collection and maintained in RPMI 1640 medium supplemented with 10% FBS and 100 U/ml penicillin and streptomycin. MC38 cells were provided by Kerafast and maintained in DMEM medium supplemented with 10% FBS and 100 U/ml penicillin and streptomycin. HEK 293T cells were obtained from the American Type Culture Collection and maintained in 5% CO2 at 37 °C in DMEM media supplemented with 10% FBS and 100 U/ml penicillin and streptomycin. Cells were stimulated with magnetic beads (ratio of 1:5 cells per bead), which were conjugated with the following protein combinations (the ratio in parentheses indicates the relative concentration of each protein): anti-CD3/anti-CD28/IgG1 (1:1:2), or anti-CD3/anti-CD28/PD-L2-Fc (1:1:2).
2.3. Antibodies
Anti-CD3 (UCHT1), and recombinant PD-L2-Fc were purchased from Acros. IgG1 (isotype control) was purchased from Jackson ImmunoResearch. Anti-CD28 (CD28.2) was purchased from eBioscience. Anti-mouse antibodies were purchased from BioLegend: CD3-AF488 (clone 17A2), CD8-PercpCy5.5 (clone 53–6.7), CD4-PE (clone GK1.5), CD44-BV421 (clone IM7), PD-1-PECy7 (clone RPM1–30).
2.4. Cytokine secretion
IL-2 concentrations in the supernatant were measured by enzyme linked immunosorbent assay (ELISA) from BioLegend.
2.5. Knocking down PD-1 related kinases
Kinases were stably knocked down in Jurkat T cells by short hairpin RNA using Mission shRNA plasmids (Sigma-Aldrich). Lentiviral particles were generated by transfecting HEK 293T cells with pMD2G, psPAX2, and the shRNA plasmid using SuperFect (Qiagen). T cells were transduced by centrifugation and selected with puromycin. The fowling shRNA sequences were used:
VRK2 (#1): CCGGCTGGAGGATTTGGATTGATATCTCGAGATATCAATCCAAATCCTCCAGTTTTTTG.
VRK2 (#2): CCGGGGGAAGAAGTTACAGATTTATCTCGAGATAAATCTGTAACTTCTTCCCTTTTT.
BARK1: CCGGGCATCATGCATGGCTACATGTCTCGAGACATGTAGCCATGCATGATGCTTTTTTG.
GSK3A: CCGGCCATAGCCCATCAAGCTCCTGCTCGAGCAGGAGCTTGATGGGCTATGGTTTTTTG.
HIPK2: CCGGCCCACAGCACACACGTCAAATCTCGAGATTTGACGTGTGTGCTGTGGGTTTTTTG.
CDK7: CCGGGCTGTAGAAGTGAGTTTGTAACTCGAGTTACAAACTCACTTCTACAGCTTTTT.
RPS6KB: CCGGAGCACAGCAAATCCTCAGACACTCGAGTGTCTGAGGATTTGCTGTGCTTTTTT.
CK2A1: CCGGATTACCTGCAGGTGGAATATTCTCGAGAATATTCCACCTGCAGGTAATTTTTTG.
CDK3: CCGGTCACCCAGCTGCCTGACTATACTCGAGTATAGTCAGGCAGCTGGGTGATTTTTG.
2.6. RT-PCR analysis
Total RNA was extracted using the RNeasy Plus Mini Kit (Qiagen). RNA (500 ng) was used for cDNA synthesis using SuperScript II First Strand Synthesis (Invitrogen). Human kinases and HPRT Taqman Primer/Probes were used for all Taqman Gene Expression Assays with the Taqman Universal PCR Master Mix (Applied Biosystems). Quantitative gene expression analyses were performed with Applied Biosystems 7300 Real-Time PCR. Gene expression was analyzed by the ΔΔCt method.
2.7. Mice, MC38 tumor inoculation and T cell analysis
One million (1 × 106) MC38 cells were used for inoculation, implanted subcutaneously in the right hind flank of mice. Tumor growth was monitored using electronic callipers and calculated according to the formula: V = Length × Width2 × 0.52. For T cell phenotypic analysis by flow cytometry, spleens were harvested 17 days post-treatment initiation. Splenic cells were stained with anti-mouse antibodies for flow cytometry analysis. To deplete T cells, mice received intraperitoneal injection of 200 µg anti-CD4 (BioXcell BE0003) and 200 µg anti-CD8 (BioXcell BE0061) antibodies in PBS, a second dose of the antibodies was administered two days later. To assess cytotoxicity, MC38 cells were thawed, seeded at a density of 3 × 104 in a flat bottom 96-well plate and treated overnight at 37 °C and 5% CO2 with AZD-7762 (MCE HY-10,992) at the indicated concentrations. Cell viability was measured with PrestoBlue (Invitrogen). AZD-7762 and PF-4,777,736 were given I.P. at 25 mg/kg and 10 mg/kg respectively. Prexasertib was given S.C. at 10 mg/kg. All drugs were given for 12 days.
2.8. Statistical analysis
GraphPad Prism software was used for statistical analysis. Unpaired Student's t-test was used to compare differences between the means of two groups and a two-tailed p-value ≤ 0.05 was considered statistically significant, where *p < 0.05. To compare the effects of different treatments on tumor volume, we used repeated measures two-way ANOVA and Tukey's multiple comparisons test with individual variances computed for each comparison.
Ethics Statement
All animal experiments comply with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). IACUC approval # AAAW7464.
CRediT Author Statement
Michael Peled: Conceptualization, Investigation, Methodology, Writing – original draft; Kieran Adam: Investigation, Methodology; Adam Mor: Supervision, Investigation, Conceptualization, Methodology, Writing – reviewing & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships which have or could be perceived to have influenced the work reported in this article.
Acknowledgments
We acknowledge Anna Tocheva for technical assistance with the flow cytometry experiments, data analysis, discussion, and figures generation. This work was supported by grants from the NIH (AI125640, CA231277, AI150597), and the Cancer Research Institute.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.dib.2021.107168.
Appendix. Supplementary materials
References
- 1.Zabludoff S.D., Deng C., Grondine M.R., Sheehy A.M., Ashwell S., Caleb B.L. AZD7762, a novel checkpoint kinase inhibitor, drives checkpoint abrogation and potentiates DNA-targeted therapies. Mol. Cancer Ther. 2008;7(9):2955–2966. doi: 10.1158/1535-7163.MCT-08-0492. PubMed PMID: 18790776. [DOI] [PubMed] [Google Scholar]
- 2.Peled M., Tocheva A.S., Adam K., Mor A. VRK2 inhibition synergizes with PD-1 blockade to improve T cell responses. Immunol. Lett. 2021;233:42–47. doi: 10.1016/j.imlet.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King C., Diaz H.B., McNeely S., Barnard D., Dempsey J., Blosser W. LY2606368 Causes replication catastrophe and antitumor effects through CHK1-dependent mechanisms. Mol. Cancer Ther. 2015;14(9):2004–2013. doi: 10.1158/1535-7163.MCT-14-1037. PubMed PMID: 26141948. [DOI] [PubMed] [Google Scholar]
- 4.Blasina A., Hallin J., Chen E., Arango M.E., Kraynov E., Register J. Breaching the DNA damage checkpoint via PF-00477736, a novel small-molecule inhibitor of checkpoint kinase 1. Mol. Cancer Ther. 2008;7(8):2394–2404. doi: 10.1158/1535-7163.MCT-07-2391. PubMed PMID: 18723486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.