Abstract
Anti-lipopolysaccharide (LPS) antibody administration has the potential benefits of neutralizing and consequently controlling rumen-derived LPS during subacute ruminal acidosis. Four Holstein bulls were used in this crossover study with a 2-week wash-out period. Anti-LPS antibody (0 or 4 g) was administered once daily for 14 days. Significantly lower ruminal LPS and higher 1-h mean ruminal pH were identified in the 4 g group. However, blood metabolites, acute-phase proteins, cytokines, and hepatic transcriptomes were not different between the two groups. Therefore, anti-LPS antibody administration mitigated ruminal LPS release and pH depression without accompanying responses in acute-phase inflammation or hepatic transcriptomic expression.
Keywords: Anti-lipopolysaccharide antibody, cattle, liver transcriptome, rumen fermentation, subacute ruminal acidosis
INTRODUCTION
The occurrence of ruminal acidosis or subacute ruminal acidosis (SARA) in cattle fed a high-grain diet causes higher acidity, resulting in higher lipopolysaccharide (LPS) activity in the rumen [1]. The increased ruminal LPS translocates to the bloodstream and can provoke inflammatory and acute-phase protein (APP) responses in cattle [2]. Furthermore, transcriptomic analysis of liver tissue revealed that the metabolic consequences of uncontrolled inflammation induced by LPS challenge can be particularly harmful during the early stages of lactation, when there is a marked degree of body fat mobilization [3]. Therefore, LPS neutralization and related roles of liver cells are important in cattle fed a high-grain diet.
Previously, in vitro (peptide-bound bead method) [4] and in vivo (mouse model) [5] studies using LPS-binding peptides were performed to neutralize LPS. However, there has been little research regarding anti-LPS antibody administration in cattle despite the potential benefits of neutralizing and consequently controlling rumen-derived LPS. Therefore, we investigated the effects of ruminal anti-LPS antibody administration on rumen fermentation and LPS activity, as well as hepatic transcriptomic adaptation during SARA challenge.
MATERIALS AND METHODS
Anti-LPS antibody preparation
Anti-LPS antibody was produced under patented and proprietary procedures (EW Nutrition Japan, Japan) as described previously [6]. Briefly, 1 mL antigen (1 × 109 CFU/g inactivated whole Escherichia coli O139) was injected intramuscularly into egg-laying hens (Hy-Line W36). Then, the egg yolk was separated to prepare the product, yielding 1 g of the product bound to 0.25 g purified LPS from E. coli O111. We determined the amount of anti-LPS antibody based on previously reported ruminal LPS concentrations (up to 5 μg/mL) in growing Holstein steers (body weight 330–380 kg) with a rumen volume of approximately 100 L [7].
Animals and experimental design
The experimental protocol was approved by the Iwate University Laboratory Animal Care and Use Committee (A201453-1; Japan). Four rumen-fistulated Holstein bulls (5–6 months of age; 162 ± 9 kg) were used in a crossover study with a 2-week washout period. Cattle were fed a roughage diet (orchard and timothy mixed hay: 5.6–7.0 kg/day) during the first 11 days (days −11 to −1; pre-challenge), followed by a high-grain diet (50% concentrate and 50% soybean flakes: 3.0–3.6 and 3.0–3.8 kg/day, respectively) for 2 days (days 0 and 1; SARA challenge), and then a roughage diet for 1 day (day 2; post-challenge). The high-grain diet contained 80.5% total digestible nutrients, 15.7% crude protein, 12.8% acid detergent fiber, and 25.7% neutral detergent fiber.
The cattle were administered 0 (control group) or 4 g anti-LPS antibody per head once daily via the rumen fistula for 14 consecutive days. The diets were supplied daily at 800 and 1,630 h in 2 equal portions. The feed composition and amounts were based on the requirements of the Japanese Feeding Standard for Dairy Cattle.
Sampling and measurements
Ruminal pH was measured using a radio transmission system (YCOW-S; DKK-TOA, Japan) as described previously [8]. Rumen fluid and blood samples were collected at 800 and 1,400 h on days −1, 0, and 1 and at 800 h on day 2. Then, fluid and blood samples were prepared for further analysis as described previously [9].
The concentrations of total volatile fatty acids (VFAs), NH3-N, lactic acid, and individual VFAs (acetic, propionic, and butyric acids) were determined [9]. Rumen LPS activity was measured by kinetic limulus amebocyte lysate assay (Pyrochrome with Glucashield; Seikagaku, Japan) [9]. For APP analyses, the plasma concentrations of LPS binding protein (LBP), haptoglobin (HP), and serum amyloid A (SAA) were measured using commercial kits [9]. Concentrations of plasma tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and interleukin-6 (IL-6) were measured by sandwich enzyme-linked immunosorbent assay [9].
Transcriptome analysis of liver tissue
Liver tissue was biopsied at 800 h on day 2 in the 0 and 4 g groups. Preparation of RNA samples for microarray analysis and data processing were performed as described previously [10]. The entire microarray data set has been deposited in the Gene Expression Omnibus database with the following accession numbers: platform, GPL22091; samples, GSM 3901089 to GSM 3901115; series, GSE133152.
Statistical analysis
The normality of the distributions of variables was assessed using the Shapiro-Wilk test. The significance of differences among groups was evaluated using the unpaired t-test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables (Prism ver. 8.10; GraphPad Software, USA). Mixed-model repeated-measures analysis of variance, using time as a fixed effect, followed by Dunnett's multiple comparison method was performed to determine the significance of within-group differences. The microarray data were analyzed using the unpaired t-test with Benjamini-Hochberg false discovery rate (FDR) multiple testing correction (FDR corrected p < 0.10) (GeneSpring 12.0; Agilent Technologies, USA). In all analyses, p < 0.05 was taken to indicate statistical significance.
RESULTS
Ruminal LPS, pH, VFAs, and blood metabolites
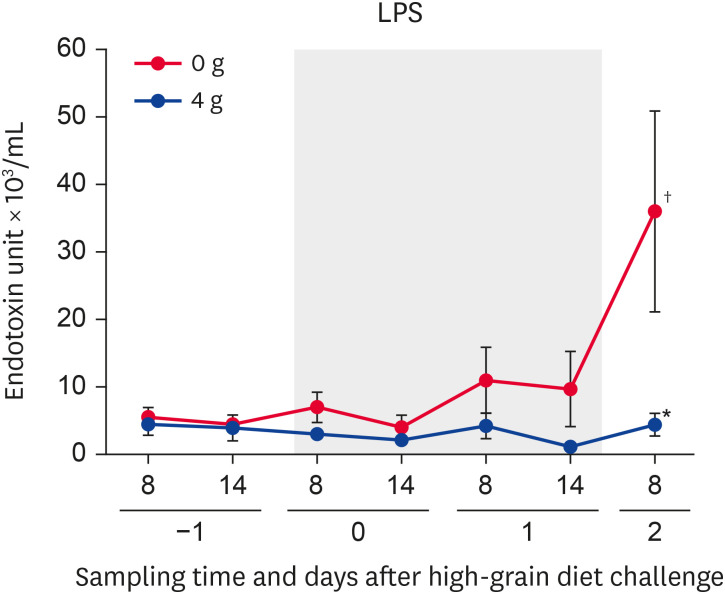
The ruminal LPS activity (0 g anti-LPS antibody) changed significantly (p < 0.05) during the SARA challenge. The ruminal LPS activity was significantly (p < 0.05) increased on day 2 (800 h) compared with day −1 (800 h). Significantly (p < 0.05) lower LPS activity was identified on day 2 (800 h) in the 4 g group compared with the 0 g group (Fig. 1).
Fig. 1.
Changes in ruminal LPS activity in Holstein bulls. Anti-LPS antibody was administered once daily at a dose of 0 or 4 g. Days −1, 0, 1, and 2 denote observations during the pre-challenge (day −1), subacute ruminal acidosis challenge (days 0 and 1; gray squares), and post-challenge (day 2) periods. Sampling times of day are shown as 8 (800 h) and 14 (1,400 h). Values represent means ± SE.
*Significant difference between the 0 and 4 g groups at that time point (p < 0.05); †Significant changes during the SARA challenge period (p < 0.05).
LPS, lipopolysaccharide.
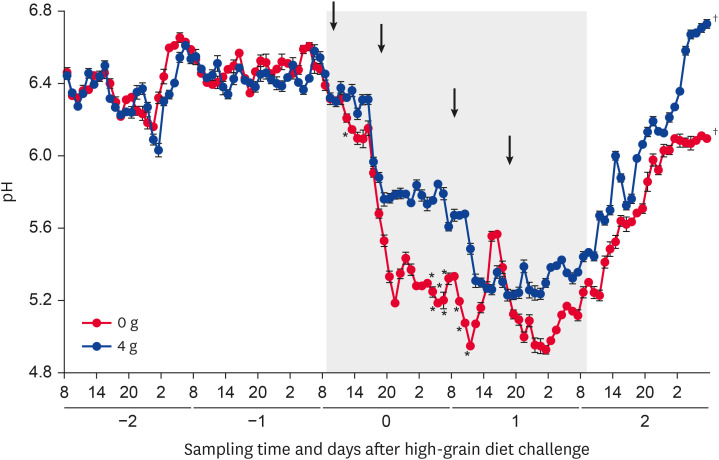
The 1-h mean ruminal pH (0 and 4 g anti-LPS antibody) changed significantly (p < 0.05) during the SARA challenge. The 1-h mean ruminal pH was significantly (p < 0.05) higher on days 0 (1,300 h) and 1 (300–700 h and 900–1,100 h) in the 4 g compared with 0 g group (Fig. 2).
Fig. 2.
Diurnal changes in the 1-h mean ruminal pH in Holstein bulls. Anti-lipopolysaccharide antibody was administered once daily at a dose of 0 or 4 g. Days −2, −1, 0, 1, and 2 denote observations during the pre-challenge (days −2 and −1), subacute ruminal acidosis challenge (days 0 and 1; gray squares), and post-challenge (day 2) periods. Arrows indicate feeding of a high-grain diet (800 and 1,630 h). Sampling times of day are shown as 8 (800 h), 14 (1,400 h), 20 (2,000 h), and 2 (200 h). Values represent means ± SE.
*Significant difference between the 0 and 4 g groups at that time point (p < 0.05); †Significant changes during the SARA challenge period (p < 0.05).
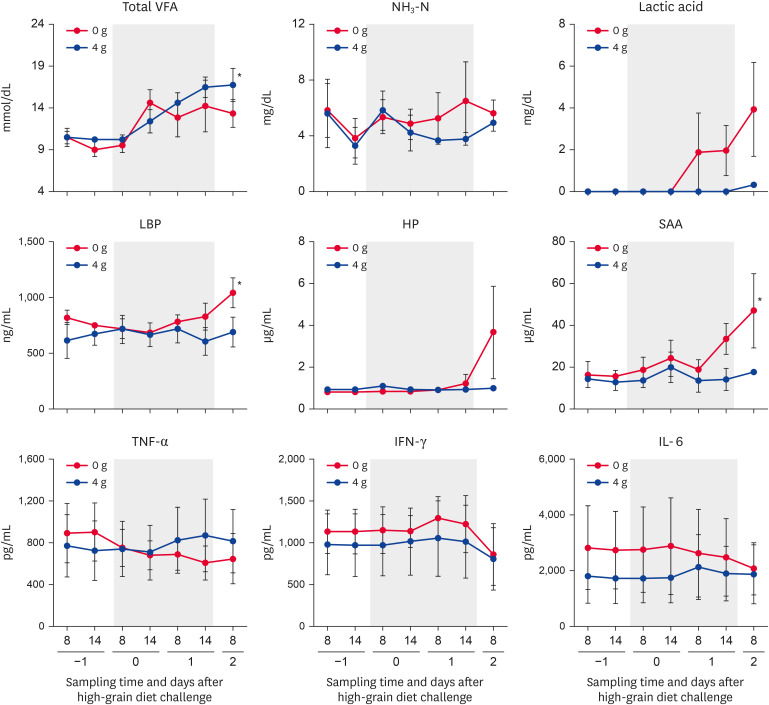
During the SARA challenge, the total VFA concentration (4 g group) was significantly (p < 0.05) increased on days 1 (1,400 h) and 2 (800 h) compared with day −1 (800 h) (Fig. 3). However, no significant change in the NH3-N or lactic acid concentration was detected during the SARA challenge period.
Fig. 3.
Changes in the levels of rumen fermentation parameters, peripheral blood APPs, and cytokines. The levels of rumen fermentation parameters (total VFAs, NH3-N, and lactic acid), peripheral blood APPs (LBP, HP, SAA), and cytokines (TNF-α, IFN-γ, IL-6) were determined in the groups administered 0 and 4 g of anti-lipopolysaccharide antibody. Days −1, 0, 1, and 2 denote observations during the pre-challenge (day −1), subacute ruminal acidosis challenge (days 0 and 1; gray squares), and post-challenge (day 2) periods. Sampling times of day are shown as 8 (800 h) and 14 (1,400 h). Values represent means ± SE.
VFA, volatile fatty acid; LBP, lipopolysaccharide binding protein; HP, haptoglobin; SAA, serum amyloid A; TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; IL-6, interleukin-6; APP, acute-phase protein.
*Significant changes during the SARA challenge period (p < 0.05).
The levels of peripheral blood APPs (LBP, HP, and SAA) were increased in the 0 g group, compared with the 4 g group, during the later part of the SARA challenge period (Fig. 3). The LBP and SAA concentrations in the 0 g group increased significantly (p < 0.05) during the SARA challenge. However, no significant changes in the levels of HP or cytokines (TNF-α, IFN-γ, and IL-6) were detected during the SARA challenge period.
Microarray analysis of liver tissue
There were no significantly differentially expressed genes (FDR corrected p < 0.10), including genes encoding APPs and cytokines, between the 0 and 4 g groups.
DISCUSSION
In the present study, ruminal LPS release and pH depression were alleviated by administration of anti-LPS antibody during SARA challenge. It is plausible that the anti-LPS antibody, showing high binding affinity to LPS, may affect living gram-negative bacteria, consistent with other studies using avian-derived polyclonal antibodies against Fusobacterium necrophorum and Streptococcus bovis in crossbred steers [11] and anti-LPS-enriched colostrum in a mouse model [5]. However, the present study verified the selective suppression of ruminal LPS activity, without significant changes in the rumen fermentation or blood metabolite profiles.
Once rumen-derived LPS enters the circulation, it activates Kupffer cells to release proinflammatory cytokines, such as TNF-α, IFN-γ, and IL-6, into the systemic circulation and triggers the secretion of APPs, such as LBP, HP, and SAA [12,13]. However, oral administration of anti-LPS-enriched colostrum alleviated immune-mediated colitis by lessening bowel inflammation in a mouse model, suggesting that the gut microbiome may serve as a target for regulatory T-cell-based immunotherapy [5]. In the present study, we found significant changes in the LBP and SAA concentrations only in the 0 g group. Furthermore, gradual increases in LBP, HP, and SAA concentrations during the later parts of the SARA challenge period were observed in the 0 g compared with 4 g group, suggesting the need for longer-term observations. Therefore, we postulated that the significantly lower ruminal LPS levels on day 2 in the administration groups may play a suppressive role in the acute-phase inflammatory responses of the peripheral blood in comparison with the 0 g group during SARA challenge.
In the present study, no differences in hepatic gene expression were detected between the 0 and 4 g groups although the ruminal LPS activity was significantly lower in the 4 g group. This was consistent with previous reports showing no significant effects of a single and mild episode of SARA on the ruminal epithelial barrier function immediately after the episode [14] and with the lack of significant differences in peripheral blood hepatic enzyme levels (aspartate aminotransferase and γ-glutamyltransferase) in the present study. That is, ruminal LPS release by SARA challenge may have a limited effect on hepatic responsiveness, likely due to relatively low LPS activity compared with a previous study (36.0 endotoxin units [EU] × 103/mL on day 2 in the present study vs. 47.17 and 79.04 EU × 103/mL in low- and high-grain-fed dairy cows, respectively [15]). Furthermore, the present study was performed over a short period to evaluate liver adaptation to both SARA challenge and anti-LPS antibody administration. Therefore, further studies are required to verify the effects of anti-LPS antibody administration on alterations in the hepatic transcriptome using longer-term challenge models.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Mizuguchi H, Sato S.
- Data curation: Kizaki K, Kimura A.
- Formal analysis: Kim YH, Kushibiki S, Ikuta K.
- Validation: Kizaki K, Kimura A, Kushibiki S, Kim YH.
- Visualization: Kim YH.
- Writing - original draft: Mizuguchi H. Kim YH, Sato S.
- Writing - review & editing: Mizuguchi H, Kim YH, Sato S.
References
- 1.Watanabe Y, Kim YH, Kushibiki S, Ikuta K, Ichijo T, Sato S. Effects of active dried Saccharomyces cerevisiae on ruminal fermentation and bacterial community during the short-term ruminal acidosis challenge model in Holstein calves. J Dairy Sci. 2019;102(7):6518–6531. doi: 10.3168/jds.2018-15871. [DOI] [PubMed] [Google Scholar]
- 2.Danscher AM, Thoefner MB, Heegaard PM, Ekstrøm CT, Jacobsen S. Acute phase protein response during acute ruminal acidosis in cattle. Livest Sci. 2011;135(1):62–69. [Google Scholar]
- 3.Minuti A, Zhou Z, Graugnard DE, Rodriguez-Zas SL, Palladino AR, Cardoso FC, et al. Acute mammary and liver transcriptome responses after an intramammary Escherichia coli lipopolysaccharide challenge in postpartal dairy cows. Physiol Rep. 2015;3(4):e12388. doi: 10.14814/phy2.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki MM, Matsumoto M, Omi H, Kobayashi T, Nakamura A, Kishi H, et al. Interaction of peptide-bound beads with lipopolysaccharide and lipoproteins. J Microbiol Methods. 2014;100:137–141. doi: 10.1016/j.mimet.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Ben Ya'acov A, Lichtenstein Y, Zolotarov L, Ilan Y. The gut microbiome as a target for regulatory T cell-based immunotherapy: induction of regulatory lymphocytes by oral administration of anti-LPS enriched colostrum alleviates immune mediated colitis. BMC Gastroenterol. 2015;15(1):154. doi: 10.1186/s12876-015-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zúñiga A, Yokoyama H, Albicker-Rippinger P, Eggenberger E, Bertschinger HU. Reduced intestinal colonisation with F18-positive enterotoxigenic Escherichia coli in weaned pigs fed chicken egg antibody against the fimbriae. FEMS Immunol Med Microbiol. 1997;18(3):153–161. doi: 10.1111/j.1574-695X.1997.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 7.Motoi Y, Oohashi T, Hirose H, Hiramatsu M, Miyazaki S, Nagasawa S, et al. Turbidimetric-kinetic assay of endotoxin in rumen fluid or serum of cattle fed rations containing various levels of rolled barley. J Vet Med Sci. 1993;55(1):19–25. doi: 10.1292/jvms.55.19. [DOI] [PubMed] [Google Scholar]
- 8.Sato S, Kimura A, Anan T, Yamagishi N, Okada K, Mizuguchi H, et al. A radio transmission pH measurement system for continuous evaluation of fluid pH in the rumen of cows. Vet Res Commun. 2012;36(1):85–89. doi: 10.1007/s11259-012-9518-x. [DOI] [PubMed] [Google Scholar]
- 9.Takemura K, Shingu H, Mizuguchi H, Kim YH, Sato S, Kushibiki S. Effects of forage feeding on rumen fermentation, plasma metabolites, and hormones in Holstein calves during pre- and postweaning periods1. J Anim Sci. 2019;97(5):2220–2229. doi: 10.1093/jas/skz088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim YH, Toji N, Kizaki K, Kushibiki S, Ichijo T, Sato S. Effects of dietary forage and calf starter on ruminal pH and transcriptomic adaptation of the rumen epithelium in Holstein calves during the weaning transition. Physiol Genomics. 2016;48(11):803–809. doi: 10.1152/physiolgenomics.00086.2016. [DOI] [PubMed] [Google Scholar]
- 11.DiLorenzo N, Diez-Gonzalez F, DiCostanzo A. Effects of feeding polyclonal antibody preparations on ruminal bacterial populations and ruminal pH of steers fed high-grain diets. J Anim Sci. 2006;84(8):2178–2185. doi: 10.2527/jas.2005-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckel EF, Ametaj BN. Invited review: role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J Dairy Sci. 2016;99(8):5967–5990. doi: 10.3168/jds.2015-10727. [DOI] [PubMed] [Google Scholar]
- 13.Zhao C, Liu G, Li X, Guan Y, Wang Y, Yuan X, et al. Inflammatory mechanism of Rumenitis in dairy cows with subacute ruminal acidosis. BMC Vet Res. 2018;14(1):135. doi: 10.1186/s12917-018-1463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penner GB, Oba M, Gäbel G, Aschenbach JR. A single mild episode of subacute ruminal acidosis does not affect ruminal barrier function in the short term. J Dairy Sci. 2010;93(10):4838–4845. doi: 10.3168/jds.2010-3406. [DOI] [PubMed] [Google Scholar]
- 15.Abaker JA, Xu TL, Jin D, Chang GJ, Zhang K, Shen XZ. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J Dairy Sci. 2017;100(1):666–678. doi: 10.3168/jds.2016-10871. [DOI] [PubMed] [Google Scholar]