Abstract
It has been suggested that the human species may be undergoing an evolutionary transition in individuality (ETI). But there is disagreement about how to apply the ETI framework to our species, and whether culture is implicated as either cause or consequence. Long-term gene–culture coevolution (GCC) is also poorly understood. Some have argued that culture steers human evolution, while others proposed that genes hold culture on a leash. We review the literature and evidence on long-term GCC in humans and find a set of common themes. First, culture appears to hold greater adaptive potential than genetic inheritance and is probably driving human evolution. The evolutionary impact of culture occurs mainly through culturally organized groups, which have come to dominate human affairs in recent millennia. Second, the role of culture appears to be growing, increasingly bypassing genetic evolution and weakening genetic adaptive potential. Taken together, these findings suggest that human long-term GCC is characterized by an evolutionary transition in inheritance (from genes to culture) which entails a transition in individuality (from genetic individual to cultural group). Thus, research on GCC should focus on the possibility of an ongoing transition in the human inheritance system.
Keywords: culture, gene–culture coevolution, evolutionary transition, inheritance, human evolution
1. Introduction
The human species may be undergoing an evolutionary transition in individuality (ETI) [1–6]. The evolutionary transitions framework explains how new levels of biological organization (such as multicellularity, or eusociality) emerge from subsidiary units (such as cells or individuals) through the formation of cooperative groups [6–10]. First proposed by Maynard Smith & Szathmáry [3], evolutionary transitions are thought to unfold via a shift in the dominant level of selection from competitive individuals to well-integrated functional groups [8,11]. These transitions exhibit a common set of patterns, including new divisions of labour, the loss of full individual autonomy and reproductive control, and the rise of new routes of information transmission [6,7,10]. Humans exhibit many patterns thought to be characteristic of an ETI, including the scale of our cooperation with non-kin, the prominence of human language and our complex, full-time division of labour. Consequently, it has been repeatedly hypothesized that human evolution is in some way characterized by an evolutionary transition [1–6]. However, there is little consensus on how to apply an evolutionary transition framework to humans. Here, we review research and evidence on the human ETI to clarify the roles of culture and genes in a human evolutionary transition.
One line of research applies the ETI framework to humans by focusing solely on biological and genetic evidence. We might call such a transition a ‘genetic ETI’. Research in this theme commonly concludes that humans have begun, but not completed, such an evolutionary transition [2,5,6], having evolved some characteristics of superorganisms but not others. For example, while humans are highly interdependent, sharing resources in large cooperative groups, we also remain highly autonomous and capable of individual reproduction. Stearns [5] reasons that factors such as migration (which reduces genetic differentiation between groups) and trade (which increases interdependence between groups) are likely to undermine the force of genetic group selection necessary to complete a transition. Szathmáry [6] concludes that human ‘group structure is too transitory to allow for a major transition in evolution in a purely biological sense’. Kesebir [12] also argues that, given the fluidity of human groups and our capacity to hold multiple group identities, the superorganism concept is an inappropriate description of human evolutionary status. Viewed from this perspective, aspects of human society, social organization and social cognition may impede a human genetic ETI.
A different strain of research suggests that human society may also undergo evolutionary transitions through cultural processes. We might therefore consider a ‘cultural ETI’, in which changes in cultural and social organization are facilitated by cultural evolution, as distinct from a ‘genetic ETI’ in which changes in biological organization are facilitated solely by genetic evolution. In a cultural ETI, the scale of cultural and social organization shifts from single humans, or smaller social units to larger groups composed of many such units via cultural evolution operating without necessarily changing genes. Thus, before a cultural ETI, the predominant levels of control, adaptation and inheritance of cultural traits would be at the subsidiary level (e.g. single humans, families, regional polities), while after a cultural ETI, these would be at the level of the cultural group (e.g. groups, clans, nations). This strain of research focuses on cultural patterns as evidence [1,2,4,6,12]. For more than a century, anthropologists and sociologists have debated whether society represents a novel level of organization (i.e. a ‘superorganic’ entity). Spencer [13,14] saw society as a superorganic, emergent property of interacting individuals, as did Kroeber [15], who drew on Darwinian principles to explain social change. Recently, cultural evolutionists have argued that human society constitutes a ‘crude superorganism’ [16], with effective but imperfect mechanisms to achieve unity of action and resolve conflict within a group [12]. Within evolutionary anthropology, a long-term process of cultural selection acting on social groups is considered a primary hypothesis for the emergence of societal features with group-level functionality [17]. Cultural group selection has been proposed as an explanation for large, hierarchical societies [18,19]. Gowdy & Krall [2,20] suggest that the emergence of hierarchical agricultural societies represents a major transition to an ‘ultrasocial’, rather than fully eusocial, state. Powers et al. [4] argue that society has experienced multiple evolutionary transitions in the emergence of large, complex, cooperative societies facilitated by the human ability to create institutions, which make cooperation individually beneficial and facilitate division of labour. From this perspective, cultural ETIs may occur quite readily, but the implications for genetic and biological organization are unclear and under-developed.
In summary, there is a general consensus that humans exhibit signs of being involved in an evolutionary transition in individuality. But there is significant disagreement about the status and details of a human ETI. Research in biology suggests that culture and social organization may be impeding a genetic ETI, while research in anthropology suggests that multiple cultural ETIs may have occurred, yet they remain somehow disjunct from a genetic ETI. These two approaches to the question of human ETI differ in their evaluation of its status, because they differ in their relevant definitions and nature of evidence. We believe these differences have obscured a deeper understanding of human evolution and highlight our lack of knowledge about how genes and culture are likely to interact in the long term. We therefore organize this review around three emergent themes in human sociobiological research. First, cultural inheritance exhibits greater adaptive capacity and generates more group-level adaptations than genetic inheritance. Second, cultural evolution determines the outcomes of gene–culture coevolution (GCC) more frequently than genetic evolution. Finally, patterns in long-term GCC point to an evolutionary transition in both inheritance and individuality (ETII) from genetic individuals to cultural groups. We argue that this synthesis resolves much of the apparent disagreement and confusion around a possible human ETI.
2. The role of culture in human evolution
Human culture constitutes a second system of adaptive inheritance in humans. Composed of socially transmitted information including language, beliefs, norms, institutions and technology, culture has a great impact on how people survive and adapt in a given environment [21]. Cultural evolution is also mechanistically distinct from genetic evolution in multiple ways [22–24]. For example, while genetic inheritance is primarily vertical and non-strategic for the recipient, cultural inheritance often occurs through strategic social learning, includes many cultural models and can occur in vertical, horizontal or oblique directions relative to genetic lineages [23,24]. Furthermore, while genetic variation is largely random, cultural variation can be ‘guided’ by intentional innovation [22], and the accumulation of cultural variation may be more rapid (see [25] versus [26]). Thus, culture provides a non-genetic system of adaptive inheritance [27] that is fundamentally distinct from genetic inheritance at a structural level (figure 1). These structural differences have two major implications for our inquiry: adaptive capacity and group structure.
Figure 1.
Cultural inheritance is not parallel to genetic inheritance. Genetic material is physically replicated, directly transmitted and passively inherited by offspring. Cultural traits, on the other hand, are transmitted via an active process of reconstructive phenotypic inference and selective imitation by the learner. (Online version in colour.)
(a) . Adaptive capacity
Cultural inheritance may hold greater adaptive potential than genetic inheritance due to its mechanistic differences. Indeed, the primary explanation for the emergence of the human cultural inheritance system itself is that it provides a more flexible and rapid system of behavioural evolution than genetics alone allow. Evidence [28] and theory [29] support the assertion that cultural evolution is more rapid than genetic evolution [27,28,30,31], even when measured on comparable scales [30,31]. One simple reason for this difference is that the ‘generation time’, G, of cultural transmission can be orders of magnitude shorter than that of genetic transmission [30]. In humans, the average time between the birth of parents and the birth of their offspring, genetic G, ranges from roughly 2 to 3 decades, while cultural G, the average time between learning a piece of information and transmitting it, ranges from seconds to decades. Thus, it is reasonable to presume that cultural inheritance may provide greater adaptive capacity than genetic inheritance.
Indeed, there is strong evidence that culture is a major adaptive force in the evolution of many animal species, among which humans show both the strongest evidence and the greatest impacts of GCC [32]. Human culture is by far the most complex and extensive form of culture, and its impact on human genetics is correspondingly profound [33,34]. Humans are thought to have acquired significant genetic changes as a result of long-term GCC, including dramatic digestive changes, the emergence of docility and reduced aggression [35], modified vocal tracts [36], the cognitive apparatus for social learning [22,37] and norm internalization [38]. Apparent genetic accommodation of cultural evolution in humans supports the proposal that cultural evolution may be more adaptive than genetic evolution. It is still further supported by the correspondence between the growth in the scale and complexity of our social systems, and emergence of our species as the dominant ecological force on Earth [39]. Far beyond simply altering human evolution, this evidence suggests that human cultural inheritance is of global evolutionary significance.
(b) . Group structure
Human culture is also more group structured than are human genes [40]. First, culturally organized groups are typically more powerful than individuals. This simple fact has evolutionary significance because it means that culturally organized groups may proliferate, even at the expense of average individual fitness. Importantly, group selection on cultural variation is facilitated by mechanisms that have no genetic parallel, including conformity [41–44] and social marking [45–47], as well as emergent processes within groups such as equilibrium selection on institutions [48]. Second, competition between culturally organized groups facilitates the evolution of cooperation within groups [49,50] leading to the expansion of human cooperation and prosocial tendencies, often with a genetic basis [51]. This pattern is supported by cross-cultural evidence through human history [17] across geographical regions [50], and by quantitative evidence from violent group conflict [52,53]. Third, culturally organized groups appear to solve adaptive problems more readily than individuals through the compounding value of social learning and cultural transmission in groups [54,55]. Societies may operate to make each of their members more innovative than they would otherwise be individually [56]. Indeed, larger groups with shared culture may achieve group-level cultural adaptations more rapidly than smaller groups. In Oceania, for example, population size predicts technological complexity in the absence of environmental variability [57]. Similarly, languages with more speakers tend to be more efficient from an information theoretic perspective [56], likely because the rate of language evolution increases with population size [58]. In summary, group-level cultural adaptation appears to be a major force in human evolution generally [17,50,52,59], even while group selection is rare in genetic systems.
Thus, cultural evolution is generally believed to exhibit three pertinent characteristics relative to genetic evolution: it tends to be more rapid, it holds greater general adaptive capacity and it generates group-level adaptation. These effects also appear to shape the long-term patterns of GCC.
3. Patterns of gene–culture coevolution
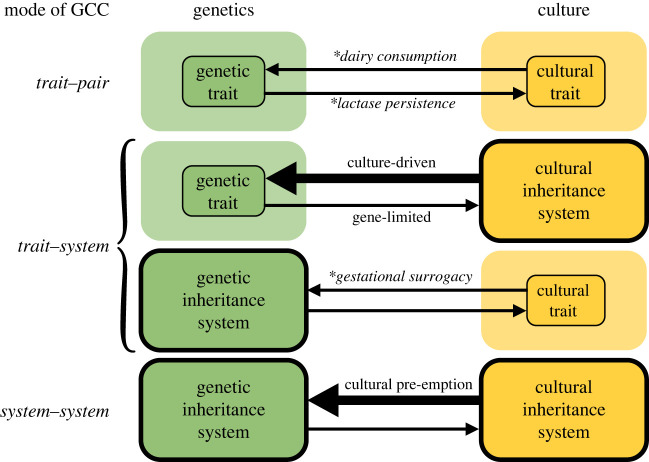
As indicated earlier, differing genetic and cultural interpretations of a human ETI remain unresolved, in part because our understanding of the links between genes and culture remains limited. The various patterns of long-term GCC have never been systematically compared, and the relevant evidence remains largely unevaluated. How often are genes and culture mutually reinforcing or in conflict? When they conflict, what are the most common outcomes? We review the existing GCC literature and evidence to address these questions. Theory and evidence can be categorized according to the nature of the reciprocal effects between inheritance systems (figure 2), from specific interactions between a pair of genetic and cultural traits (trait–pair GCC) to long-term interactions between a trait on the one hand and an entire inheritance system on the other (trait–system GCC), to the longest-term interactions between the systems of inheritance themselves (system–system GCC).
Figure 2.
Patterns of GCC may be categorized based on the scale of cause and effect, which varies from specific traits to inheritance systems. Arrow thickness indicates the weight of evidence for total strength or frequency of each causal pathway. Example traits marked with asterisks. (Online version in colour.)
(a) . Trait–pair gene–culture coevolution
Most narrowly, GCC refers to a process of reciprocal adaptation between genetic and cultural traits within a single species. This occurs, for example, when a genetic trait carries a fitness advantage (e.g. adult expression of the lactase enzyme) which is enhanced by a cultural trait (e.g. dairy farming and consumption), enabling the two to spread in tandem [60,61]. Trait–pair GCC is a special case of genetic assimilation, the process by which plastic responses or behavioural innovations are solidified later by genetic evolution [62,63]. Trait–pair GCC relies on a functioning system of cultural inheritance. As a result, trait–pair GCC may be more prevalent in recent human evolution than in early humans with limited culture. Trait–pair GCC may also connect multiple trait pairs. For example, the evolution of cultural traits to improve subsistence through hunting, cooking and agriculture have dramatically altered the human diet (trait–pair 1). These changes to human diet appear to have modified the human bite configuration (trait–pair 2) which, in turn, influenced the variety of sound types humans could produce [36]. This final step, from genetic morphological traits to increased sound variety (and thus evolutionary potential), in human language is also an example of trait–system GCC.
(b) . Trait–system gene–culture coevolution
Trait–system GCC refers to the evolutionary interaction between a specific set of traits and an entire inheritance system. Much of the GCC research has focused on how specific genetic factors might coevolve with the human cultural inheritance system as a whole. However, there is disagreement between theorists about the nature of the long-term constraints on GCC. On the one hand, in their theory of GCC, Lumsden & Wilson [64] conclude that in the long term, culture could not subvert the genetic traits responsible for the cultural learning apparatus itself, and that ‘genetic natural selection operates in such a way as to keep culture on a leash’. Durham [65] argued a similar point. Thus, gene-limited GCC is the process in which specific genetic factors constrain the evolution of the entire system of cultural inheritance. On the other hand, Boyd & Richerson's [22] models of GCC do not assume a backstop genetic constraint on culture, but allow beneficial cultural traits to facilitate the evolution of increasingly flexible cultural learning systems without end. Boyd and Richerson argue that cultural evolution creates novel social and physical environments, which change selective pressures for genetic variation [66], a process they term culture-driven GCC. We gather the empirical evidence for both patterns of trait–system GCC below.
(i) . Evidence for gene-limited gene–culture coevolution
In gene-limited GCC, specific genetic factors constrain cultural inheritance generally. For example, cultural variation may be limited by genetically determined cognitive and psychological abilities.
Genes and languages coevolve [67], and genes may limit language evolution. For example, early human populations may not have been able to pronounce fricatives such as ‘f’ and ‘v’ [36] because of genetic limitations on bite configuration. Similarly, human languages tend to develop colour terms in a particular order (typically: black and white, then red, then yellow or green) [68,69]. This homology is believed to derive from universal aspects of the human perception system and may therefore be an example of gene-limited GCC. This limitation is weak, however, as genes appear not to influence the extent, complexity or sophistication of colour terms, but merely their order of emergence.
Perhaps, the best evidence for gene-limited GCC in the human lineage comes from a period in early human evolution during which stone tools remained unchanged for nearly 2 Myr [70,71]. The earliest current evidence for stone tool use dates to 3.3 Ma [72], marking the beginning of the lower palaeolithic, during which the accumulation of novel stone tool variation was extremely slow. This leads archaeologists to suspect that human cultural evolution as a whole was limited by the genetic factors determining human capacity for the culture [70]. This is strong evidence of genetic limitation for a significant period of human evolution. However, this limitation eventually gave way to accelerating increases in stone tool complexity, signalling an increase in the genetic capacity for cultural transmission, probably as a result of the benefits of cultural traits for enhanced stone tool production and use. Overall, we do not find much evidence of significant gene-limited GCC, and the evidence we do find suggests that gene-limitation is decreasing over the long term. Most of the evidence for trait–system GCC comes instead from culture-driven GCC.
(ii) . Evidence for culture-driven gene–culture coevolution
In culture-driven GCC, the adaptive advantages of cultural inheritance drive the evolution of specific genetic traits. For example, theoretical models [73,74] suggest that the tripling of human brain size (a specific set of genetic traits) resulted from the fitness benefits of the increased ability to store and process adaptive cultural information (cultural inheritance, generally). Cross-species evidence supports this assertion [75]. Culture-driven GCC also probably honed psychological and cognitive capacities to elaborate, transmit and accumulate cultural traits [21,76,77]. Further examples of culture-driven GCC may extend throughout human evolution and across the human lifespan [78]. For example, human longevity may have evolved as a result of the expanding ability to accumulate and transmit beneficial cultural adaptations, knowledge and capital [79]. It is therefore probably not a coincidence that increases in the human lifespan in the Upper Palaeolithic (approx. 30 ka) correspond with the emergence of behaviourally modern humans [80]. And like longevity, menopause [81] may have emerged because it favoured the transmission of adaptive cultural knowledge from older women to the young over the cost of ceased genetic reproduction [82]. Going further still, cultural evolution may sometimes cause outcomes that are maladaptive at the individual genetic level [83–85], such as reduced fertility [86,87], so long as they increase adaptive outcomes via cultural learning. Thus, there is significant evidence for culture-driven GCC.
Thus far, we have only explored trait–system GCC in which genetic traits influence cultural inheritance as a whole; however, the reverse is also possible: specific cultural traits may influence the entire system of genetic inheritance, in a more extreme form of culture-driven GCC. The most salient examples of this pattern are medical and reproductive. Scientific medical practice is generally considered a cultural adaptation with clear advantages. However, scientific medicine can also act to obviate natural selection by promoting the health and reproduction of individuals with otherwise harmful genetic conditions. In doing so, scientific medicine may tend to weaken the genetic determination of phenotype and fitness. One example is the evolution of the Caesarean section procedure, a cultural adaptation to treat dangerous and deadly birth complications. The success and spread of the Caesarean procedure has marginally relaxed genetic selection in humans, slightly increasing the likelihood that a daughter born by Caesarean will herself require one [88]. Another example is that of gestational surrogacy, in which couples who cannot bear children themselves elect to have another woman gestate and birth their genetic offspring through the implantation of an egg fertilized in vitro [89]. Gestational surrogacy is a cultural adaptation to make genetic reproduction possible where it would otherwise be impossible. Importantly, both of these cultural solutions require deep and complex group-level cultural adaptations in the form of medical science and practice. Consequently, this type of trait–system GCC between specific cultural traits and genetic inheritance is a relatively new phenomenon. As Mitteroecker [90] argues, GCC operates differently now that humans are evolving almost exclusively within modern societies.
Taking stock, the evidence from the types of GCC we have considered suggests that the greater adaptive capacity of culture typically determines the outcome of GCC, whether the trait is cultural and the system genetic (e.g. with Caesarean section) or whether the trait is genetic and the system cultural (e.g. with brain size). We believe this is a central clue in understanding the hypothetical human evolutionary transition, for which we must address the long-term coevolution of the inheritance systems themselves.
(c) . System–system gene–culture coevolution
In system–system GCC, the basic differences between mechanisms of inheritance themselves determine coevolutionary outcomes. Rindos [91] developed a model showing how plastically adaptive culture could relax and remove selection on genes, eroding the amount of adaptive information stored genetically. Laland [29] has also shown theoretically that if cultural evolution is sufficiently rapid, it may act to pre-empt and slow genetic evolution. That is, in solving adaptive challenges before genetic evolution takes place, cultural inheritance may reduce the opportunity for natural selection on genes and weaken the adaptive value of information stored in genetic inheritance in the long term. This process is the opposite of genetic assimilation, in which a plastic trait becomes genetically encoded. We call this mode of GCC cultural pre-emption.
The temporal and population scale of cultural pre-emption make data hard to collect, but direct evidence is nonetheless ubiquitous, and theory supports its plausibility. Rendell et al. [74] modelled a process of runaway cultural niche construction in which cultural changes to the environment forestalled both genetic evolution and mutualistic GCC. The overall effect of cultural pre-emption is to reduce the fraction of adaptive information stored in genes and inherited genetically and to increase that fraction in culture.
In summary, the literature and evidence strongly suggest that culture tends to drive GCC, no matter which type or scale is examined. This comes as a natural consequence of the relative speed, group structure and adaptive capacity of cultural evolution. We can therefore posit a crucial hypothesis about system–system GCC: culture is gradually replacing genetics as the primary human system of inheritance. This hypothesis helps clarify the human ETI.
4. Rethinking the human evolutionary transition
The role of culture as the predominant driver of GCC suggests that culture is coming to replace genetics as the primary system of human inheritance. Thus, culture may be the ultimate cause of a human ETI. We can therefore present a reformulated theory of the human evolutionary transition.
(a) . An evolutionary transition in inheritance and individuality
Evolutionary transitions typically involve changes in both individuality and inheritance. Originally, Maynard Smith and Szathmáry [3] suggested that new modes of information transmission (i.e. inheritance) tend to arise during the transitions we now label ETIs. Jablonka [92] was the first to detail how evolutionary transitions in individuality may often require a corresponding transition in inheritance system. Jablonka argued that most transitions entail the emergence of new inheritance mechanisms in which information about cooperation, differentiation and integration of subsidiary units may accumulate and guide development. For example, epigenetic inheritance systems were a necessary and critical part of the transition to multicellularity, as they allowed for regulation, cooperation and differentiation between cells. Jablonka [92] argued that similar inheritance transitions occurred with the evolution of each new level of biological organization, including the emergence of protocells (from fragmentation to replication of genetic material), prokaryotic cells (inheritance of epigenetic information in the cytosol), eukaryotic cells (inheritance of organelles, chromosomes), multicellularity (further mechanisms of epigenetic inheritance and differentiation, gametes) and finally cultural groups (imitation, teaching and the transmission of artefacts).
It follows, then, that humans are experiencing an evolutionary transition in individuality from single human to cultural group because culture is replacing genes as the primary human inheritance system, and cultural adaptations are heavily group structured. The mechanistic argument for an evolutionary transition in inheritance and individuality (ETII) is as follows:
-
—
Culture tends to be a faster and stronger adaptive medium than genetics, and, therefore, tends to determine the outcomes of GCC. In humans, genetic limitations on GCC appear to be declining, while cultural adaptation is pre-empting genetic evolution to resolve adaptive challenges with increasing strength and frequency. Consequently, culture is replacing genetics as a primary system of inheritance, which means that an increasing share of adaptive information is stored in culture compared with genes.
-
—
As a result, the characteristic adaptive scale of culture is becoming increasingly important in human evolution. Human culture is more group structured than human genes. Cultural group selection has driven the genetic evolution of cognitive and psychological traits for cooperation and social learning, which make group-level cultural adaptations possible. Group-level cultural adaptations have emerged for defence, shelter, food provision, health and education. Consequently, an increasing share of adaptive human traits is stored in cultural groups when compared with genetic individuals.
Mechanistically, there may be no specific group size necessary for an ETII. The size and complexity of cultural groups are highly labile traits themselves, have varied over human evolution and may be under intense selection. Thus, we expect these quantities to change. For example, corporations and nations both vary in size across five orders of magnitude, and the two categories of group fully interpenetrate. Currently, cultural groups may be fluid, nested or overlapping. But the important evolutionary features are those traits and forces that determine survival and reproduction. Thus, membership in cultural groups need not be mutually exclusive or permanent, so long as group survival and reproduction are facilitated. However, if cultural group competition increases, groups may gain a fitness advantage by increasing size or monopolizing human membership, which might cause group boundaries to align and solidify.
This conceptual model (figure 3) resolves multiple inconsistencies in current literature on the human ETI. First, recall that the literature on the human ETI is somewhat divided: some find evidence for one or more cultural ETIs, suggesting that the dominant organizational level of cultural adaptation has expanded over human evolution; others find insufficient evidence for a genetic ETI because humans are not sufficiently isolated in groups to enable genetic group selection [5]. The ETII model resolves this apparent conflict as it is compatible with both sets of evidence; if culture is replacing genes as an inheritance system then the shift in the level of selection is not from genetic individual to genetic group, but from genetic individual to cultural group.
Figure 3.
An ETII entails an inheritance transition driven by the greater adaptive capacity of culture, which causes an individuality transition via a change in the dominant level of selection. (Online version in colour.)
Second, the ETII bears on the suggestion that the human transition is stalled because human populations are not sufficiently group structured [5], halting the process of genetic group selection. However, if the weight of adaptive information is shifting from genetic individuals to cultural groups, genetic group structure is not important: cultural group structure is. One consequence of this is that cultural groups can compete even as genetic groups homogenize [93]. For example, distinct genetic groups can dissolve through population mixing even while members continue to belong to distinct and competing cultural groups [94]. Thus, a culture-driven human ETII is possible despite increasing genetic mixture.
This touches on another often-confused issue in the human evolution literature, that cultural group selection relies on the extinction of human groups (i.e. ‘hard’ group selection). It does not. Group-level cultural evolution may continue without the loss of individual life (i.e. with ‘soft’ group selection), so long as cultural groups determine social outcomes. Moreover, if cultural groups influence reproductive outcomes, such as with the advancement of scientific medicine, then group-level culture increases its role in human evolution while weakening that of individuals' genes.
The ETII hypothesis enables a straightforward evaluation of the status of a human evolutionary transition. The majority of evidence and research suggest that humans are experiencing an ongoing ETII, driven by a shift to culture as a primary mechanism of inheritance, and characterized by an evolutionary transition from genetically organized individuals to culturally organized groups. The evidence suggests that the transition has not stopped but is instead accelerating as group-level cultural adaptations accumulate and individual genetic inheritance is rendered increasingly irrelevant.
Today, a growing fraction of human adaptation comes from those group-level cultural traits such as food production, defence, education and healthcare, all of which have become increasingly integrated and coordinated. Thus, while there is still major variation in human fitness due to genetic factors within societies, societal factors play an important role in determining individual health and fitness [95]. Although far from any hypothetical endpoint, the human ETII is ongoing and accelerating.
5. Conclusion
Our review has uncovered a pattern of hypothetical causal connections that resolves what appears to be contradictory evidence within the literature on a human evolutionary transition. The literature suggests that group-level cultural evolution is more adaptive and more rapid than genetic evolution in humans. This difference has caused an increasing fraction of human life to be mediated by culturally evolved group-level practices and technology, and a decreasing fraction by genetic traits. Available evidence suggests that this trend is ongoing and accelerating. We note that both cultural and environmental change are far from equilibrium, perhaps partly as a result of the human ETII. We speculate that, in the long term, culture will continue to grow in influence over human evolution, until genes become secondary structures that hold human biological design blueprints but are ultimately governed by culture. If genes hold culture on a leash, culture is dragging them straight off the trail.
Supplementary Material
Acknowledgements
We thank Brian Olsen, Mike Kinnison, Peter Richerson and Richard McElreath for inspiration, and Laurel Fogarty for her help in refining our thinking. The Department of Human Behavior, Ecology and Culture at the Max Planck Institute for Evolutionary Anthropology, the Evolution of Cooperation research group at the University of Bristol, and the EEHBC research group at UC Davis all provided useful feedback.
Data accessibility
This article has no additional data.
Authors' contributions
T.M.W. and Z.T.W. developed the ideas and wrote the paper. All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This research was supported in part by USDA award ME022008, and NSF awards SES-1352361 and EPS-2019470.
References
- 1.Andersson C, Törnberg P. 2008. Toward a macroevolutionary theory of human evolution: the social protocell. Biol. Theory 14, 86-102. ( 10.1007/s13752-018-0313-y). [DOI] [Google Scholar]
- 2.Gowdy J, Krall L. 2014. Agriculture as a major evolutionary transition to human ultrasociality. J. Bioecon. 16, 179-202. ( 10.1007/s10818-013-9156-6) [DOI] [Google Scholar]
- 3.Maynard Smith J, Szathmáry E. 1995. The major transitions in evolution. Oxford, UK: WH Freeman Spektrum. [Google Scholar]
- 4.Powers ST, van Schaik CP, Lehmann L. 2016. How institutions shaped the last major evolutionary transition to large-scale human societies. Phil. Trans. R. Soc. B 371, 20150098. ( 10.1098/rstb.2015.0098) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stearns SC. 2007. Are we stalled part way through a major evolutionary transition from individual to group? Evolution 61, 2275-2280. ( 10.1111/j.1558-5646.2007.00202.x) [DOI] [PubMed] [Google Scholar]
- 6.Szathmáry E. 2015. Toward major evolutionary transitions theory 2.0. Proc. Natl Acad. Sci. USA 112, 10 104-10 111. ( 10.1073/pnas.1421398112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calcott B, Sterelny K (eds). 2011. The major transitions in evolution revisited, 1st edn. Cambridge, MA: The MIT Press. See https://ebookcentral.proquest.com/lib/umaine/reader.action?docID=3339240&ppg=180. [Google Scholar]
- 8.Michod RE. 2000. Darwinian dynamics: evolutionary transitions in fitness and individuality. Princeton, NJ: Princeton University Press. [Google Scholar]
- 9.Queller DC, Strassmann JE. 2009. Beyond society: the evolution of organismality. Phil. Trans. R. Soc. B 364, 3143-3155. ( 10.1098/rstb.2009.0095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.West SA, Fisher RM, Gardner A, Kiers ET. 2015. Major evolutionary transitions in individuality. Proc. Natl Acad. Sci. USA 112, 10 112-10 119. ( 10.1073/pnas.1421402112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okasha S. 2005. Multilevel selection and the major transitions in evolution. Philos. Sci. 72, 1013-1025. ( 10.1086/508102) [DOI] [Google Scholar]
- 12.Kesebir S. 2012. The superorganism account of human sociality: how and when human groups are like beehives. Pers. Soc. Psychol. Rev. 16, 233-261. ( 10.1177/1088868311430834) [DOI] [PubMed] [Google Scholar]
- 13.Simon WM. 1960. Herbert Spencer and the ‘Social Organism’. J. Hist. Ideas. 21, 294-299. ( 10.2307/2708202) [DOI] [Google Scholar]
- 14.Spencer H. 1896. The principles of sociology. New York, NY: D Appleton and Company. [Google Scholar]
- 15.Kroeber AL. 1917. The superorganic. Am. Anthropol. 19, 163-213. ( 10.1525/aa.1917.19.2.02a00010) [DOI] [Google Scholar]
- 16.Richerson PJ, Boyd R. 1999. Complex societies: the evolutionary origins of a crude superorganism. Hum. Nat. 10, 253-289. ( 10.1007/s12110-999-1004-y) [DOI] [PubMed] [Google Scholar]
- 17.Richerson P, et al. 2016. Cultural group selection plays an essential role in explaining human cooperation: a sketch of the evidence. Behav. Brain Sci. 39, e30 (19 pages). [DOI] [PubMed] [Google Scholar]
- 18.Turchin P, Gavrilets S. 2009. Evolution of complex hierarchical societies. Soc. Hist. Evol. 8, 167-198. [Google Scholar]
- 19.Turchin P, Currie TE, Turner EAL, Gavrilets S. 2013. War, space, and the evolution of Old World complex societies. Proc. Natl Acad. Sci. USA 110, 16 384-16 389. ( 10.1073/pnas.1308825110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gowdy J, Krall L. 2016. The economic origins of ultrasociality. Behav. Brain Sci. 39, e92. ( 10.1017/S0140525X1500059X) [DOI] [PubMed] [Google Scholar]
- 21.Richerson PJ, Boyd R. 2005. Not by genes alone: how culture transformed human evolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 22.Boyd R, Richerson PJ. 1985. Culture and the evolutionary process. Chicago, IL: University of Chicago Press. [Google Scholar]
- 23.Cavalli-Sforza LL, Feldman MW. 1981. Cultural transmission and evolution: a quantitative approach. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 24.Mesoudi A. 2011. Cultural evolution: how Darwinian theory can explain human culture and synthesize the social sciences. Chicago, IL: University of Chicago Press. [Google Scholar]
- 25.Adamic LA, Lento TM, Adar E, Ng PC. 2016. Information evolution in social networks. In Proc. Ninth ACM Int. Conf. on Web Search and Data Mining—WSDM ’16, pp. 473-482. San Francisco, CA: Association for Computing Machinery. [Google Scholar]
- 26.Scally A. 2016. The mutation rate in human evolution and demographic inference. Curr. Opin Genet. Dev. 41, 36-43. ( 10.1016/j.gde.2016.07.008) [DOI] [PubMed] [Google Scholar]
- 27.Boyd R. 2017. A different kind of animal: how culture transformed our species. Princeton, NJ: Princeton University Press. [Google Scholar]
- 28.Mathew S, Perreault C. 2015. Behavioural variation in 172 small-scale societies indicates that social learning is the main mode of human adaptation. Proc. R. Soc. B 282, 20150061. ( 10.1098/rspb.2015.0061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laland KN. 1992. A theoretical investigation of the role of social transmission in evolution. Ethol. Sociobiol. 13, 87-113. ( 10.1016/0162-3095(92)90020-5) [DOI] [Google Scholar]
- 30.Lambert B, Kontonatsios G, Mauch M, Kokkoris T, Jockers M, Ananiadou S, Leroi AM. 2020. The pace of modern culture. Nat. Hum. Behav. 4, 352-360. ( 10.1038/s41562-019-0802-4) [DOI] [PubMed] [Google Scholar]
- 31.Perreault C. 2012. The pace of cultural evolution. PLoS ONE 7, e45150. ( 10.1371/journal.pone.0045150) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whitehead H, Laland KN, Rendell L, Thorogood R, Whiten A. 2019. The reach of gene–culture coevolution in animals. Nat. Commun. 10, 2405. ( 10.1038/s41467-019-10293-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laland KN, Odling-Smee J, Myles S. 2010. How culture shaped the human genome: bringing genetics and the human sciences together. Nat. Rev. Genet. 11, 137-148. ( 10.1038/nrg2734) [DOI] [PubMed] [Google Scholar]
- 34.Stearns SC, Byars SG, Govindaraju DR, Ewbank D. 2010. Measuring selection in contemporary human populations. Nat. Rev. Genet. 11, 611-622. ( 10.1038/nrg2831) [DOI] [PubMed] [Google Scholar]
- 35.Gibbons A. 2014. How we tamed ourselves—and became modern. Science 346, 405-406. ( 10.1126/science.346.6208.405) [DOI] [PubMed] [Google Scholar]
- 36.Blasi DE, Moran S, Moisik SR, Widmer P, Dediu D, Bickel B. 2019. Human sound systems are shaped by post-Neolithic changes in bite configuration. Science 363, eaav3218. ( 10.1126/science.aav3218) [DOI] [PubMed] [Google Scholar]
- 37.Henrich J, McElreath R. 2003. The evolution of cultural evolution. Evol. Anthropol. Issues News Rev. 12, 123-135. ( 10.1002/evan.10110) [DOI] [Google Scholar]
- 38.Gintis H. 2003. The Hitchhiker's guide to altruism: gene-culture coevolution, and the internalization of norms. J. Theor. Biol. 220, 407-418. ( 10.1006/jtbi.2003.3104) [DOI] [PubMed] [Google Scholar]
- 39.Steffen W, Crutzen PJ, McNeill JR. 2007. The Anthropocene: are humans now overwhelming the great forces of nature. AMBIO J. Hum. Environ. 36, 614-621. ( 10.1579/0044-7447(2007)36[614:TAAHNO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 40.Bell AV. 2010. Why cultural and genetic group selection are unequal partners in the evolution of human behavior. Commun. Integr. Biol. 3, 159-161. ( 10.4161/cib.3.2.10528) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Claidière N, Whiten A. 2012. Integrating the study of conformity and culture in humans and nonhuman animals. Psychol. Bull. 138, 126. ( 10.1037/a0025868) [DOI] [PubMed] [Google Scholar]
- 42.Coultas JC. 2004. When in Rome … an evolutionary perspective on conformity. Group Process. Intergroup Relat. 7, 317-331. ( 10.1177/1368430204046141) [DOI] [Google Scholar]
- 43.Morgan TJH, Laland KN. 2012. The biological bases of conformity. Front. Neurosci. 6, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whiten A, Horner V, de Waal FBM. 2005. Conformity to cultural norms of tool use in chimpanzees. Nature 437, 737-740. ( 10.1038/nature04047) [DOI] [PubMed] [Google Scholar]
- 45.Boyd R, Richerson PJ. 1987. The evolution of ethnic markers. Cult. Anthropol. 2, 65-79. ( 10.1525/can.1987.2.1.02a00070) [DOI] [Google Scholar]
- 46.McElreath R, Boyd R, Richerson PJ. 2003. Shared norms and the evolution of ethnic markers. Curr. Anthropol. 44, 122-129. ( 10.1086/345689) [DOI] [Google Scholar]
- 47.Nettle D, Dunbar RIM. 1997. Social markers and the evolution of reciprocal exchange. Curr. Anthropol. 38, 93-99. ( 10.1086/204588) [DOI] [Google Scholar]
- 48.Bowles S, Choi J-K, Hopfensitz A. 2003. The co-evolution of individual behaviors and social institutions. J. Theor. Biol. 223, 135-147. ( 10.1016/S0022-5193(03)00060-2) [DOI] [PubMed] [Google Scholar]
- 49.Henrich J. 2004. Cultural group selection, coevolutionary processes and large-scale cooperation. J. Econ. Behav. Organ. 53, 3-35. ( 10.1016/S0167-2681(03)00094-5) [DOI] [Google Scholar]
- 50.Francois P, Fujiwara T, van Ypersele T. 2018. The origins of human prosociality: cultural group selection in the workplace and the laboratory. Sci. Adv. 4, eaat2201. ( 10.1126/sciadv.aat2201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chudek M, Henrich J. 2011. Culture–gene coevolution, norm-psychology and the emergence of human prosociality. Trends Cogn. Sci. 15, 218-226. ( 10.1016/j.tics.2011.03.003) [DOI] [PubMed] [Google Scholar]
- 52.Handley C, Mathew S. 2020. Human large-scale cooperation as a product of competition between cultural groups. Nat. Commun. 11, 1-9. ( 10.1038/s41467-020-14416-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zefferman MR, Mathew S. 2015. An evolutionary theory of large-scale human warfare: group-structured cultural selection. Evol. Anthropol. Issues News Rev. 24, 50-61. ( 10.1002/evan.21439) [DOI] [PubMed] [Google Scholar]
- 54.Brahm F, Poblete J. 2019. The evolution of productive organizations. See https://papers.sioe.org/paper/2311.html. [DOI] [PubMed] [Google Scholar]
- 55.Smaldino PE, Richerson PJ. 2013. Human cumulative cultural evolution as a form of distributed computation. In Handbook of human computation (ed. Michelucci P), pp. 979-992. New York, NY: Springer New York. [Google Scholar]
- 56.Muthukrishna M, Henrich J. 2016. Innovation in the collective brain. Phil. Trans. R. Soc. B 371, 20150192. ( 10.1098/rstb.2015.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kline MA, Boyd R. 2010. Population size predicts technological complexity in Oceania. Proc. R. Soc. B 277, 2559-2564. ( 10.1098/rspb.2010.0452) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bromham L, Hua X, Fitzpatrick TG, Greenhill SJ. 2015. Rate of language evolution is affected by population size. Proc. Natl Acad. Sci. USA 112, 2097-2102. ( 10.1073/pnas.1419704112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Richerson P, Henrich J. 2012. Tribal social instincts and the cultural evolution of institutions to solve collective action problems. Cliodynamics J. Theor. Math. Hist. 3, 38-80. ( 10.21237/c7clio3112453). [DOI] [Google Scholar]
- 60.Gerbault P, et al. 2011. Evolution of lactase persistence: an example of human niche construction. Phil. Trans. R. Soc. B 366, 863-877. ( 10.1098/rstb.2010.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gerbault P, Moret C, Currat M, Sanchez-Mazas A. 2009. Impact of selection and demography on the diffusion of lactase persistence. PLoS ONE 4, e6369. ( 10.1371/journal.pone.0006369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lande R. 2009. Adaptation to an extraordinary environment by evolution of phenotypic plasticity and genetic assimilation. J. Evol. Biol. 22, 1435-1446. ( 10.1111/j.1420-9101.2009.01754.x) [DOI] [PubMed] [Google Scholar]
- 63.Pigliucci M. 2006. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 209, 2362-2367. ( 10.1242/jeb.02070) [DOI] [PubMed] [Google Scholar]
- 64.Lumsden CJ, Wilson EO. 1981. Genes, mind, and culture: the coevolutionary process. Cambridge, MA: Harvard University Press. [Google Scholar]
- 65.Durham WH. 1991. Coevolution: genes, culture, and human diversity. Stanford, CA: Stanford University Press. [Google Scholar]
- 66.Richerson PJ, Boyd R, Henrich J. 2010. Gene-culture coevolution in the age of genomics. Proc. Natl Acad. Sci. USA 107, 8985-8992. ( 10.1073/pnas.0914631107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Creanza N, Ruhlen M, Pemberton TJ, Rosenberg NA, Feldman MW, Ramachandran S. 2015. A comparison of worldwide phonemic and genetic variation in human populations. Proc. Natl Acad. Sci. USA 112, 1265-1272. ( 10.1073/pnas.1424033112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berlin B, Kay P. 1991. Basic color terms: their universality and evolution. Berkeley, CA: University of California Press. [Google Scholar]
- 69.Haynie HJ, Bowern C. 2016. Phylogenetic approach to the evolution of color term systems. Proc. Natl Acad. Sci. USA 113, 13 666-13 671. ( 10.1073/pnas.1613666113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ambrose SH. 2001. Paleolithic technology and human evolution. Science 291, 1748-1753. ( 10.1126/science.1059487) [DOI] [PubMed] [Google Scholar]
- 71.Semaw S. 2000. The world's oldest stone artefacts from Gona, Ethiopia: their implications for understanding stone technology and patterns of human evolution between 2.6–1.5 million years ago. J. Archaeol. Sci. 27, 1197-1214. [Google Scholar]
- 72.Harmand S, et al. 2015. 3.3-million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature 521, 310-315. ( 10.1038/nature14464) [DOI] [PubMed] [Google Scholar]
- 73.Muthukrishna M, Doebeli M, Chudek M, Henrich J. 2018. The cultural brain hypothesis: how culture drives brain expansion, sociality, and life history. PLoS Comput. Biol. 14, e1006504. ( 10.1371/journal.pcbi.1006504) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rendell L, Fogarty L, Laland KN. 2011. Runaway cultural niche construction. Phil. Trans. R. Soc. B 366, 823-835. ( 10.1098/rstb.2010.0256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Navarrete A, van Schaik CP, Isler K. 2011. Energetics and the evolution of human brain size. Nature 480, 91-93. ( 10.1038/nature10629) [DOI] [PubMed] [Google Scholar]
- 76.Henrich J, et al. 2016. Understanding cumulative cultural evolution. Proc. Natl Acad. Sci. USA 113, E6724-E6725. ( 10.1073/pnas.1610005113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Altman A, Mesoudi A. 2019. Understanding agriculture within the frameworks of cumulative cultural evolution, gene-culture co-evolution, and cultural niche construction. Hum. Ecol. 47, 483-497. ( 10.1007/s10745-019-00090-y) [DOI] [Google Scholar]
- 78.Richerson PJ, Boyd R. 2020. The human life history is adapted to exploit the adaptive advantages of culture. Phil. Trans. R. Soc. B 375, 20190498. ( 10.1098/rstb.2019.0498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kaplan HS, Robson AJ. 2002. The emergence of humans: the coevolution of intelligence and longevity with intergenerational transfers. Proc. Natl Acad. Sci. USA 99, 10 221-10 226. ( 10.1073/pnas.152502899) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caspari R, Lee S-H. 2004. Older age becomes common late in human evolution. Proc. Natl Acad. Sci. USA 101, 10 895-10 900. ( 10.1073/pnas.0402857101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hawkes K. 2003. Grandmothers and the evolution of human longevity. Am. J. Hum. Biol. 15, 380-400. ( 10.1002/ajhb.10156) [DOI] [PubMed] [Google Scholar]
- 82.Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell AF. 2004. Fitness benefits of prolonged post-reproductive lifespan in women. Nature 428, 178-181. ( 10.1038/nature02367) [DOI] [PubMed] [Google Scholar]
- 83.Lehmann L, Feldman MW. 2009. Coevolution of adaptive technology, maladaptive culture and population size in a producer–scrounger game. Proc. R. Soc. B 276, 3853-3862. ( 10.1098/rspb.2009.0724). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Logan MH, Qirko HN. 1996. An evolutionary perspective on maladaptive traits and cultural conformity. Am. J. Hum. Biol. 8, 615-629. () [DOI] [PubMed] [Google Scholar]
- 85.Henrich J. 2004. Demography and cultural evolution: why adaptive cultural processes produced maladaptive losses in Tasmania. Am. Antiq. 69, 197-221. ( 10.2307/4128416) [DOI] [Google Scholar]
- 86.Newson L, Postmes T, Lea SG, Webley P. 2005. Why are modern families small? Toward an evolutionary and cultural explanation for the demographic transition. Pers. Soc. Psychol. Rev. 9, 360-375. ( 10.1207/s15327957pspr0904_5) [DOI] [PubMed] [Google Scholar]
- 87.Newson L, Richerson PJ. 2009. Why do people become modern? A Darwinian explanation. Popul. Dev. Rev. 35, 117-158. ( 10.1111/j.1728-4457.2009.00263.x) [DOI] [Google Scholar]
- 88.Mitteroecker P, Windhager S, Pavlicev M. 2017. Cliff-edge model predicts intergenerational predisposition to dystocia and Caesarean delivery. Proc. Natl Acad. Sci. USA 114, 11 669-11 672. ( 10.1073/pnas.1712203114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brinsden PR. 2003. Gestational surrogacy. Hum. Reprod. Update. 9, 483-491. ( 10.1093/humupd/dmg033) [DOI] [PubMed] [Google Scholar]
- 90.Mitteroecker P. 2019. How human bodies are evolving in modern societies. Nat. Ecol. Evol. 3, 324-326. ( 10.1038/s41559-018-0773-2) [DOI] [PubMed] [Google Scholar]
- 91.Rindos D. 1986. The genetics of cultural anthropology: toward a genetic model for the origin of the capacity for culture. J. Anthropol. Archaeol. 5, 1-38. ( 10.1016/0278-4165(86)90009-7) [DOI] [Google Scholar]
- 92.Jablonka E. 1994. Inheritance systems and the evolution of new levels of individuality. J. Theor. Biol. 170, 301-309. ( 10.1006/jtbi.1994.1191) [DOI] [PubMed] [Google Scholar]
- 93.Barth F. 1970. Ethnic groups and boundaries: the social organization of culture difference. Boston, MA: Little, Brown. [Google Scholar]
- 94.van Dorp L, et al. 2018. Genetic legacy of state centralization in the Kuba Kingdom of the Democratic Republic of the Congo. Proc. Natl Acad. Sci. USA 116, 593-598. ( 10.1073/pnas.1811211115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.UN. 2019. Human development index ranking. See http://hdr.undp.org/en/content/2019-human-development-index-ranking.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.