Abstract
A 77-year-old man was treated with a DPP-4 inhibitor for type 2 diabetes. Hypoglycemia occurred frequently, and an examination revealed a tumor with a maximum diameter of 140 mm in both lobes of the liver. Western immunoblotting detected a high-molecular-weight form of insulin-like growth factor-II, and non-islet cell tumor hypoglycemia was diagnosed. Although prednisolone 40 mg was started, hypoglycemia continued to occur frequently. Surgical tumor removal was not indicated, so lenvatinib was initiated. Hypoglycemia improved quickly, and the tumor shrank until it had partially disappeared. His condition continued to improve, and he was discharged.
Keywords: non-islet cell tumor hypoglycemia, hypoglycemia, hepatocellular carcinoma, IGF-II, lnevatinib
Introduction
Intrinsic hypoglycemia has several triggers that do not include the use of oral hypoglycemic agents or insulin. Insulinoma, insulin autoimmune syndrome, and non-islet cell tumor hypoglycemia (NICTH) are all known causes of intrinsic hypoglycemia.
NICTH was first reported to cause hypoglycemia in 1988 as a result of the high-molecular-weight insulin-like growth factor-II (big IGF-II) produced by leiomyosarcoma (1). Since then, the occurrence of NICTH as a result of big IGF-II-producing tumors has been reported (2,3). Treatment with tumor resection or corticosteroid administration is considered the main therapy for NICTH (4). However, in the current case of NICTH, the response to steroids was ineffective. We herein report the efficacy of chemotherapy using lenvatinib in the early stages of administration. The current case is unusual, given that the use of lenvatinib has not been previously reported to be effective for NICTH due to liver cancer.
Case Report
A 77-year-old man who was sweating and trembling was transported to our emergency room. He had been diagnosed with type 2 diabetes at 42 years old and been temporarily given insulin therapy. However, his glycemic control was good, and treatment was continued with only a single DPP-4 inhibitor. About one month before being hospitalized, the patient began sweating and was frequently trembling. His blood glucose measurement showed hypoglycemia, so he was treated with oral glucose. His hypoglycemia gradually worsened, and he was transported to the emergency room because of hypoglycemic attacks.
The patient was conscious, but a laboratory examination demonstrated a low blood glucose level of 52 mg/dL. His symptoms resolved with the intravenous administration of glucose. He was hospitalized to determine the cause of and to treat his repeated hypoglycemia.
The patient had been eating three meals a day before admission. The DPP-4 inhibitor from admission was discontinued, but his hypoglycemia did not improve, so he was determined to be negative for drug-induced hypoglycemia. The laboratory data on admission are shown in the Table 1. In the endocrinological examination, only serum insulin and C-peptide levels were examined during hypoglycemia; both were suppressed. Other endocrinological examination were performed after the intravenous administration of glucose. According to the results, no insulinoma, insulin autoimmune syndrome and decreased levels of insulin antagonist hormones were observed. Enhanced computed tomography (CT) revealed multiple tumors in the liver with a maximum diameter of 140 mm, as well as hematomas in the center of tumor where the contrast leaked out. Ascites was present on the liver surface and in the rectovesical pouch. Left unilateral pleural effusion was also present (Fig. 1).
Table 1.
Laboratory data.
| [Peripheral blood] | [Biochemical examination] | Blood glucose | 56 | mg/dL | |||||||||
| WBC | 10.3×103 | /μL | TP | 6.4 | g/dL | HbA1c (HPLC) | 5.0 | % | |||||
| RBC | 3.64×106 | /μL | Alb | 2.6 | g/dL | Glycoalbumin | 21.1 | % | |||||
| Hb | 11.2 | g/dL | T-bil | 1.23 | mg/dL | ||||||||
| Ht | 33.6 | % | D-bil | 0.4 | mg/dL | [Endocrinological examination] | |||||||
| MCV | 92.1 | fL | AST | 79 | U/L | GH | 0.23 | ng/mL | |||||
| MCH | 30.6 | pg | ALT | 66 | U/L | IGF-I | 12 | ng/mL | |||||
| MCHC | 33.3 | % | LDH | 428 | U/L | TSH | 1.48 | μU/mL | |||||
| Plt | 47.5×104 | /μL | ALP | 1,120 | U/L | FT3 | 2.61 | ng/dL | |||||
| γ-GTP | 235 | U/L | FT4 | 0.99 | μg/dL | ||||||||
| [Coagulation study] | AMY | 71 | U/L | ACTH | 9.7 | pg/mL | |||||||
| PT | 45.1 | % | CK | 68 | U/L | Serum Cortisol | 22.7 | μg/dL | |||||
| PT-INR | 1.64 | BUN | 10 | mg/dL | Urine Cortisol | 112 | μg/day | ||||||
| APTT | 37.7 | s | Cr | 0.71 | mg/dL | PRA | 3.3 | ng/mL/h | |||||
| D-dimer | 4.9 | μg/mL | eGFR | 81 | mL/min/1.73 m2 | PAC | 38.3 | pg/mL | |||||
| UA | 2.9 | mg/dL | IAA | <0.4 | U/mL | ||||||||
| CRP | 0.07 | mg/dL | Anti-GAD-Ab | <0.5 | U/mL | ||||||||
| TC | 220 | mg/dL | Urine CPR | 15.2 | μg/day | ||||||||
| TG | 49 | mg/dL | Serum CPR* | 0.07 | ng/mL | ||||||||
| LDL-C | 153 | mg/dL | Serum insulin* | <0.3 | μU/mL | ||||||||
| HDL-C | 45 | mg/dL | |||||||||||
| Na | 139 | mEq/L | [Infectious disease and tumor marker] | ||||||||||
| K | 2.9 | mEq/L | HBs-Ag | (-) | |||||||||
| Cl | 95 | mEq/L | HCV-Ab | (-) | |||||||||
| Ca | 7.8 | mg/dL | AFP | 13.8 | ng/mL | ||||||||
| P | 3.9 | mg/dL | CEA | 4.4 | ng/mL | ||||||||
| CA19-9 | 5.3 | U/mL | |||||||||||
| ANA | negative | NSE | 33.8 | ng/mL | |||||||||
| AMA-M2 | negative | Pro-GRP | 52.8 | pg/mL | |||||||||
| ASMA | negative | ||||||||||||
*Serum insulin and C-peptide were examined during hypoglycemia.
Figure 1.

Serum IGF-II electrophoresis by Western immunoblotting. Big IGF-II corresponding to 11-18 kDa was observed. IGF-II: insulin-like growth factor-II, *hM: human
In addition to the presence of a large tumor in the liver, IGF-I was suppressed to just 12 ng/mL (standard range of IGF-I in a 77-year-old man: 48-177 ng/mL), so we considered a diagnosis of hypoglycemia because the tumor was producing the macromolecule IGF-II. Western immunoblotting results using patient sera were compared with using 7.5 kDa normal IGF-II. Big IGF-II corresponding to 18 kDa was detected (Fig. 2), and we confirmed a diagnosis of NICTH.
Figure 2.
Time course of treatment and trends in blood glucose levels during hospitalization. The red zone indicates blood glucose below 70 mg/dL. Hypoglycemia disappeared early after the administration of lenvatinib.
During hospitalization, when the blood glucose level was less than 70 mg/dL, glucose was administered regardless of hypoglycemic symptoms. The DPP-4 inhibitor he had been taking at admission was stopped, and the patient continued to eat a full diet of ≥1,600 kcal/day on free feeding. However, hypoglycemia was frequently observed after admission, and severe hypoglycemia with a blood glucose level of <30 mg/dL was also observed. Thus, continuous infusion of 10% glucose solution 1,000 mL/day was given in addition to free feeding, but this did not improve the frequency of hypoglycemia. Since the presence of large serum IGF-II was confirmed, prednisolone (PSL) 40 mg/day was administered starting at day 12. Despite these measures, the baseline blood glucose level continued to increase, and hypoglycemia was frequently observed thereafter (Fig. 3).
Figure 3.
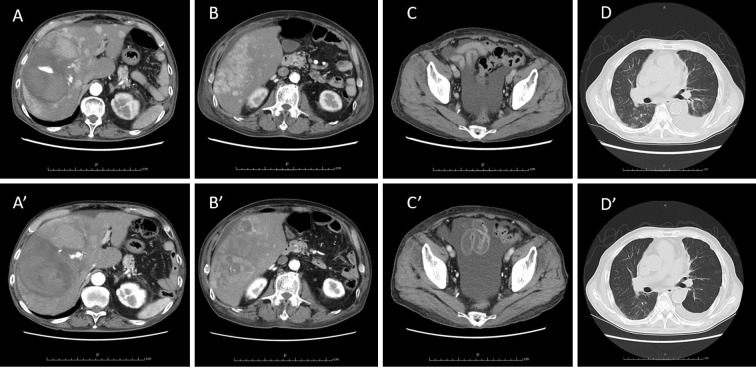
CT images before and after treatment. Upper panels A to D show findings treatment, while lower panels A’ to D’ show findings on day 27, when hypoglycemia disappeared. Before the administration of lenvatinib, multiple tumors with a maximum diameter of 140 mm were present in the liver, along with hematomas in the center of the tumor where the contrast leaked out. Ascites was present on the liver surface and in the rectovesical pouch. Left unilateral pleural effusion was also present. After treatment, the multiple tumors in the liver partially disappeared and shrank over the course of treatment. Pleural effusion almost disappeared, but the volume of ascites remained unchanged.
Hepatocellular carcinoma was confirmed to be associated with NICTH, and tumor resection was considered. But the tumor was scattered in both hepatic lobes, and retention of moderate or more ascites was recognized, so chemotherapy was started. In this case, the Child-Pugh classification was B (9 points), and concomitant medication of lenvatinib 8 mg/day, which is indicated for hepatocellular carcinoma that cannot be removed, was started on day 16 of hospitalization. The frequency of hypoglycemia decreased as early as 3 days after the start of lenvatinib and had disappeared by day 24. Thereafter, 10% glucose was discontinued, and PSL was gradually reduced, but no additional hypoglycemia was observed. Even after the reduction of lenvatinib to 4 mg, no further hypoglycemia was observed.
Contrast-enhanced CT was re-examined. The parenchymal tumor was showed to be markedly reduced, and part of the tumor had disappeared (Fig. 4). The hematomas were slightly increased. Pleural effusion had also decreased, and no other findings, such as the development of distant metastasis, were noted. Chemotherapy with lenvatinib was continued. By the time he was discharged, the PSL dose had been reduced to 10 mg twice daily in the morning and evening, but no additional hypoglycemia was observed. If this state could be maintained without hypoglycemia, we planned continue to reduce the dose after discharge.
At discharge, the blood glucose level before breakfast was approximately 80-140 mg/dL, the day and evening blood glucose levels were approximately 130-180 mg/dL, and the early morning and night blood glucose levels were approximately 70-110 mg/dL. The patient still discontinued the use of the antidiabetic drugs and continued to maintain dietary therapy, including split meals, to prevent the reoccurrence of hypoglycemia.
Discussion
NICTH is a relatively rare cause of spontaneous hypoglycemia (4,5). The causative tumors are hepatocellular carcinoma, derived from mesenchymal tumors, including leiomyosarcoma, mesothelioma, and extravascular cell tumors, and gastric cancer. Approximately 70% are large tumors with a maximum diameter of 10 cm (6,7). NICTH-related IGF-II is known to be a large molecule. Although the molecular weight of ordinary IGF-II is about 7.5 kDa, high-molecular-weight IGF-II is typically 11-18 kDa (1).
IGF is classified into IGF-I and IGF-II with structures that are similar to those of proinsulin, and both have a hypoglycemic effect, although they are only 1/10 as potent as insulin in vivo (8). Approximately 70-80% of normal IGF-II forms a trimer with IGF binding protein (IGFBP) and acid labile subunit (ALS) in vivo and exists in a state with poor physiological activity. However, many high-molecular-weight IGF-II molecules, such as that described in this case, lack a high affinity with ALS. As a result, they pass through the capillary walls because they exist as dimers (IGF-IGFBP) with IGFBP and free IGF-II. IGF-II is said to bind to the insulin receptor in the target tissue and cause hypoglycemia (8,9). In addition, IGF-II acts to suppress GH, and reduced GH reduces ALS and IGFBP so that the portion of free high-molecular-weight IGF-II increases and hypoglycemia worsens (10). In healthy individuals, big IGF-II in the blood is considered to account for approximately 10% to 20% of the total IGF-II, but an increased ratio of high-molecular-weight IGF-II is believed to be associated with hypoglycemia (11). These results suggest that measuring only the total concentration of IGF-II in the blood may not be sufficient for diagnosing NICTH resulting from big IGF-II-producing tumors (7). In addition IGF-I can be suppressed, as in the present case, and the serum insulin concentration, GH secretion, and serum potassium levels are suppressed and very low in many cases (12-14). However, IGF-I is released by the coaction of platelets and hepatocytes, and IGF-I shows a decreasing trend in patients with cirrhosis. IGF-I may thus serves as a reference value in cases with a reduce liver function reserve.
The primary choice for the curative treatment of NICTH is complete resection of the tumor (13). However, as described above, many tumors are large, with a maximum diameter over 10 cm. In these cases, partial excision of the tumor or radio-chemotherapy is generally selected, although the effect is limited. In addition to direct treatment for tumors, divided meals, an increased calorie intake, continuous intravenous nutritional administration, and treatment with intractable glucagon (or corticosteroids if refractory) should be considered (15). Furthermore, in some cases, the combined use of human GH injection and corticosteroid administration is effective, but there is a concern that the total serum IGF-II may be increased, and its effectiveness is inconsistent (15,16). The administration of corticosteroids has been suggested to not only promote gluconeogenesis and increase insulin resistance in tissues but also directly decrease IGF-II production from tumors (17). Furthermore, since IGF-II is a tumor-promoting growth factor, suppression of IGF-II production using glucocorticoids may inhibit the growth of the tumor itself (15). A PSL dose of 30-60 mg/day is recommended, and the dose should be examined for each case based on the size of the tumor.
The current case is a type 2 diabetic patient who had been treated with a single DPP-4 inhibitor as a therapeutic agent. The oral compliance was good, no overdose was observed, and the frequency of hypoglycemia did not improve even after drug discontinuation. Therefore, drug-induced hypoglycemia was considered unlikely. Although various endocrinological examinations were performed to determine the cause of hypoglycemia, reduced insulin antagonist hormone levels, such as pituitary-adrenal insufficiency, were not detected. In addition, the serum insulin levels during hypoglycemia were below the measurement sensitivity, and insulinoma was negative. Furthermore, the patient was negative for anti-insulin antibody and insulin autoimmune syndrome. The patient ate three meals a day before admission and continued to stably consume 1,600 kcal/day, without alcohol or excessive exercising. Therefore, alcoholic hypoglycemia and insufficient calorie intake, reactive hypoglycemia due to excessive carbohydrate intake, and increased calorie consumption due to exercise were also negative. In addition to the presence of a large liver tumor, IGF-I was suppressed, although IGF-I may be reduced in patients with decreased liver functional reserve; further, the blood insulin levels were decreased, and hypokalemia was noted. Given these findings, NICTH due to a big IGF-II-producing tumor was suspected and this possibility was examined.
The present patient had hepatocellular carcinoma with a decreased liver functional reserve. We suspected that in addition to NICTH, the decrease in glycogen storage due to the decreased liver function (18) had made hypoglycemia occur more frequently. However, the therapeutic effects of steroids were poor, and after the disappearance of hypoglycemia, the Child-Pugh classification was not improved, remaining 9 points as it had been before lenvatinib had been started. In addition, the pleural effusion almost disappeared, but the ascites volume remained unchanged. These results indicate that there was no improvement in the liver function reserve. The initiation of anticancer chemotherapy and the effect of the consequent reduction in the hepatocellular carcinoma size were thus deemed to have contributed significantly to the improvement of hypoglycemia, rather than the hypoglycemia being improved by an improving liver function.
No previous study has shown that the administration of lenvatinib for hepatocellular carcinoma with NICTH resulted in the rapid improvement of hypoglycemia. We searched in PubMed for cases of NICTH due to hepatocellular carcinoma using the following keywords: “hepatocellular carcinoma,” “IGF” and “hypoglycemia.” This search found 11 cases that attempted treatment for repeated hypoglycemia including the present case (Table 2) (19-29). Almost all cases were in an advanced or terminal stage, and the therapeutic effect against hypoglycemia and hepatocellular carcinoma was only temporary. In a few cases, corticosteroids were effective for hypoglycemia. Some cases of terminal stage hepatocellular carcinoma have demonstrated adrenal insufficiency including those with adrenal metastases (30). In those cases in particular, corticosteroids may temporarily improve hypoglycemia, but hypoglycemia often recur due to the progression of hepatocellular carcinoma, so radical treatment may be crucial.
Table 2.
Published Data on Patients with NICTH That Attempted to Treatment for Repeated Hypoglycemia.
| Reference | Age | Sex | Tumor size | Most effective therapy for hypoglycemia |
||||
|---|---|---|---|---|---|---|---|---|
| Present case | 77 | M | Diffuse tumor, Maximum 14 cm | Lenvatinib | ||||
| 19 | 38 | M | Diffuse tumor | Corticosteroids | ||||
| 20 | 33 | M | Maximum 20 cm | none | ||||
| 21 | 35 | F | Diffuse tumor | Transcatheter arterial infusion | ||||
| 22 | 73 | M | Diffuse tumor, Maximum 13 cm | Transcatheter arterial embolization | ||||
| 23 | 77 | M | Diffuse tumor | none | ||||
| 24 | 72 | F | Maximum 10 cm | Split meal | ||||
| 25 | 36 | F | - | Growth hormone and Corticosteroids | ||||
| 26 | 60 | F | Diffuse tumor | Transcatheter arterial embolization | ||||
| 27 | 76 | F | Diffuse tumor | IVH | ||||
| 28 | - | - | - | Transcatheter arterial infusion | ||||
| 29 | 62 | M | Maximum 14 cm | none |
M: male, F: female
-: not available, none: tried some treatments but no effective, IVH: intravenous hyperalimentation
Lenvatinib is an oral tyrosine kinase inhibitor with a selective inhibitory activity. It is involved in tumor angiogenesis and tumor malignant transformation by vascular endothelial growth factor (VEGF) 1-3, fibroblast growth factor receptor (FGFR) 1-4, platelet-derived growth factor (PDGF) α, rearranged during Transfection (RET). In particular, lenvatinib has high selectivity for kinases such as VEGF and FGF which are important for angiogenesis, and its strong inhibitory activity against FGFR4 is effective for preventing the malignant transformation of hepatocellular carcinoma and suppressing invasive metastasis (31). Therefore, lenvatinib is expected to exert an excellent antitumor effect against hepatocellular carcinoma, with a high expected response rate and rapid appearance of effects. (31,32). In the present case, the liver function reserve had already decreased, so performing hepatectomy or transcatheter arterial chemoembolization (TACE) have further reduce the liver function reserve, which may have led to the exacerbation of hypoglycemia. Lenvatinib therefor seems to be well-indicated for unresectable NICTH with a reduce liver function reserve as in this case.
Author's disclosure of potential Conflicts of Interest (COI).
Hiroyuki Ito: Honoraria, Eli Lilly Japan and Nippon Boehringer Ingelheim.
References
- 1.Daughaday WH, Emanuele MA, Brooks MH, Barbato AL, Kapadia M, Rotwein P. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med 319: 1434-1440, 1988. [DOI] [PubMed] [Google Scholar]
- 2.Versluis J, Valk G, van Rossum H, Tesselaar M. Non-islet cell tumour hypoglycaemia in a patient with a well-differentiated gastric neuroendocrine tumour. BMJ Case Rep Sep 16: e231069, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jannin A, Espiard S, Benomar K, et al. Non-islet-cell tumour hypoglycaemia (NICTH): about a series of 6 cases. Ann Endocrinol (Paris) 80: 21-25, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Takayama-Hasumi S, Eguchi Y, Sato A, Morita C, Hirata Y. Insulin autoimmune syndrome is the third leading cause of spontaneous hypoglycemic attacks in Japan. Diabetes Res Clin Pract 10: 211-214, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Marks V, Teale JD. Tumors producing hypoglycemia. Endocr Relat Cancer 5: 111-129, 1998. [Google Scholar]
- 6.Kalebi AY, Hale MJ, Wong ML, Hoffman T, Murray J. Surgically cured hypoglycemia secondary to pleural solitary fibrous tumour: case report and update review on the Doege-Potter syndrome. J Cardiothorac Surg 4: 45, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dynkevich Y, Rother K, Whitford I, et al. Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. Endocr Rev 34: 798-826, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Bond JJ, Meka S, Baxter RC. Binding characteristics of pro-insulin-like growth factor-II from cancer patients: binary and ternary complex formation with IGF binding proteins-1 to -6. J Endocrinol 165: 253-260, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Frystyk J, Skjaerbaek C, Zapf J, Orskov H. Increased levels of circulating free insulin-like growth factors in patient with non-islet cell tumour hypoglycaemia. Diabetologia 41: 589-594, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Phillips LS, Robertson DG. Insulin-like growth factors and non-islet cell tumor hypoglycemia. Metabolism 42: 1091-1101, 1993. [DOI] [PubMed] [Google Scholar]
- 11.de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocrine Related Cancer 14: 979-993, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor-II producing non-islet-cell tumor hypoglycemia. Growth Horm IGF Res 16: 211-216, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Bodnar TW, Acevedo MJ, Pietropaolo M. Management of non-islet-cell tumor hypoglycemia: a clinical review. J Clin Endocrinol Metab 99: 713-722, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoff AO, Vassilopoulou-Sellin R. The role of glucagon administration in the diagnosis and treatment of patients with tumor hypoglycemia. Cancer 82: 1585-1592, 1998. [PubMed] [Google Scholar]
- 15.Teale ID, Wark G. The effectiveness of different treatment options for non-islet cell tumour hypoglycaermia. Clin Endocrinol (Oxf) 60: 457-460, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Bourcigaux N, Arnault-Ouary G, Christol R, Périn L, Charbonnel B, Le Bouc Y. Treatment of hypoglycemia using combined glucocorticoid and recombinant human growth hormone in a patient with a metastatic non-islet cell tumor hypoglycemia. Clin Ther 27: 246-257, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Baxter RC, Holman SR, Corbould A, Stranks S, Ho PJ, Braund W. Regulation of the insulin-like growth factors and their binding proteins by glucocorticoid and growth hormone in nonislet cell tumor hypoglycemia. J Clin Endocrinol Metab 80: 2700-2708, 1995. [DOI] [PubMed] [Google Scholar]
- 18.Sorlini M, Benini F, Cravarezza P, Romanelli G. Hypoglycemia, an atypical early sign of hepatocellular carcinoma. J Gastrointest Cancer 41: 209-211, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Rana P, Kim B. A unique case of IGF-2 induced hypoglycemia associated with hepatocellular carcinoma. Case Rep Endocrinol 2019: 4601484, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garla V, Sonani H, Palabindala V, Gomez-Sanchez C, Subauste J, Lien LF. Non-islet cell hypoglycemia: case series and review of the literature. Front Endocrinol (Lausanne) 10: 316, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakata R, Kadoya R, Sugimura N. Production of insulin-like growth factor-I in hepatocellular carcinoma with recurrent hypoglycemia: a case report. Nihon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterol) 114: 256-263, 2017(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 22.Naruse H, Shimoyama N, Satoh T, et al. Non-islet cell tumor hypoglycemia caused by a big insulin-like growth factor II - producing hepatocellular carcinoma: an autopsy case report. Nihon Shokakibyo Gakkai Zasshi (Jpn J Gastroenterol) 113: 2057-2066, 2016(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 23.Okushin K, Asaoka Y, Fukuda I, et al. IGF-II Producing hepatocellular carcinoma treated with sorafenib: metabolic complications and a foresight to molecular targeting therapy to the IGF signal. Case Rep Gastroenterol 6: 784-789, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara K, Yamamoto M, Sugimoto T. A case of Alzheimer disease with hepatocellular carcinoma whose dementia was manifested by non-islet cell tumor hypoglycemia. Intern Med 99: 1648-1649, 2010. [Google Scholar]
- 25.Van Wijngaarden P, Janssen JA, de Man RA. Hepatocellular carcinoma complicated by non-islet cell tumor hypoglycemia. Ned Tijdschr Geneeskd 146: 859-862, 2002. [PubMed] [Google Scholar]
- 26.Inoue R, Ito Y, Nishimiya M, Miyazaki S, Moriuchi A, Sakata T. A case of IGF-II producing liver hepatocellular carcinoma with hypoglycemia. Nihon Naika Gakkai Zasshi (J Jpn Soc Intern Med) 86: 1033-1035, 1997(in Japanese). [PubMed] [Google Scholar]
- 27.Ishida S, Noda M, Kuzuya N, et al. Big insulin-like growth factor II-producing hepatocellular carcinoma associated with hypoglycemia. Intern Med 34: 1201-1206, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Hunter SJ, Daughaday WH, Callender ME, et al. A case of hepatoma associated with hypoglycaemia and overproduction of IGF-II (E-21): beneficial effects of treatment with growth hormone and intrahepatic adriamycin. Clin Endocrinol (Oxf) 41: 397-401, 1994. [DOI] [PubMed] [Google Scholar]
- 29.Yonei Y, Tanaka M, Ozawa Y, et al. Primary hepatocellular carcinoma with severe hypoglycemia: involvement of insulin-like growth factors. Liver 12: 90-93, 1992. [DOI] [PubMed] [Google Scholar]
- 30.Toshinari T, Yukihiro N, Haruhisa Y, et al. Adrenal insufficiency due to metastatic hepatocellular carcinoma. Endocrine J 46: 591-596, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Kudo M, Finn R, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391: 1163-1173, 2018. [DOI] [PubMed] [Google Scholar]
- 32.Kuzuya T, Ishigami M, Ito T, et al. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol Res 50: 374-381, 2020. [DOI] [PubMed] [Google Scholar]