Abstract
A 44-year-old patient progressed from nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) cirrhosis. She was diagnosed with NAFL via a liver biopsy. At 56 years old, she was diagnosed with NASH stage 3 via a second liver biopsy. One year later, she was diagnosed with NASH cirrhosis via a third liver biopsy. This is the first study to report the gradual deterioration of liver histology shown via three liver biopsies and fibrosis markers in a patient who progressed from NAFL to NASH cirrhosis. Following menopause, it is necessary to be aware of the rapid development of liver fibrosis.
Keywords: biopsy, histology, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, cirrhosis
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a common chronic liver disease found worldwide (1). It includes nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). Approximately 20% of individuals with NAFLD have NASH, and some cases may progress to cirrhosis and hepatocellular carcinoma (HCC) (2,3). However, the changes over time in the histology of NAFLD and liver fibrosis markers are not well documented.
Fibrosis progresses at a speed of 0.07 stages/year in NAFL and 0.14 stages/year in NASH, so NAFL progresses by 1 stage every 14 years and NASH by 1 stage every 7 years (4). Various factors affect the speed of progression; therefore, in this study, we examined the changes in liver histology and clinical findings in a case that progressed from NAFL to NASH over 14 years.
These findings suggest that the progression of liver fibrosis in postmenopausal women may accelerate and that caution should be practiced when treating NASH.
Case Report
A 44-year-old woman diagnosed with liver dysfunction and fatty liver at a medical checkup visited our hospital. She had no family history of NASH and no history of medication; she did not drink or smoke. The following measurements were obtained on admission: height, 152 cm; weight, 62 kg; body mass index (BMI), 27.6 kg/m2; blood pressure, 120/78 mmHg; pulse, 63 bpm; and temperature, 36.3°C. She was alert, and there was no evidence of anemia or jaundice in the bulbar conjunctiva. She had a soft, flat abdomen; her liver and spleen were not palpable. Furthermore, she had no abdominal distension, tenderness, or edema.
Blood tests revealed elevated serum liver enzyme levels: aspartate aminotransferase (AST), 22 IU/L; alanine aminotransferase (ALT), 38 U/L; and γ-glutamyltransferase (γ-GTP), 75 U/L. Her serum triglyceride level had reached 203 mg/dL. She displayed no insulin resistance. Her fasting blood glucose level was 110 mg/dL, her glycosylated hemoglobin (HbA1c) level was 5.6%, and her homeostasis model assessment-insulin resistance level (HOMA-IR) level was 0.9. She did not have elevated levels of leptin or high-sensitivity C-reactive protein, and her liver fibrosis marker data were as follows: type IV collagen 7S, 3.5 ng/mL; hyaluronic acid, 37 ng/mL; procollagen III peptide (P-III-P), 0.6 U/mL; and fibrosis-4 (FIB4 index), 0.74 (Table). All levels were within normal limits. Her antinuclear antibody serum concentration was normal, and other markers associated with causes of liver dysfunction, such as hepatitis B surface antigen negative, anti-hepatitis C virus antibody negative, and serum concentrations of immunoglobulin (Ig) G, IgM, and anti-mitochondrial antibody, were all within normal ranges as well.
Table.
Characteristics of the Patient with NASH during the Course of Three Biopsies.
| Number of liver biopsies | First | Second | Third | |||
|---|---|---|---|---|---|---|
| Weight (kg) | 62 | 58.5 | 59.4 | |||
| BMI (kg/m2) | 27.6 | 25.5 | 25.9 | |||
| Platelet count (×104) | 21.2 | 11.6 | 10.1 | |||
| ALT (U/L) | 38 | 78 | 93 | |||
| AST (U/L) | 22 | 77 | 74 | |||
| γ-GTP (U/L) | 180 | 260 | 410 | |||
| Albumin (g/dL) | 4.5 | 4.1 | 4.2 | |||
| Total cholesterol (mg/dL) | 201 | 150 | 139 | |||
| Triglyceride (mg/dL) | 203 | 125 | 71 | |||
| LDL-C (mg/dL) | 143 | 77 | 73 | |||
| HLD-C (mg/dL) | 40 | 57 | 56 | |||
| Fast glucose (mg/dL) | 110 | 131 | 142 | |||
| Hemoglobin A1c (%) | 5.6 | 6.0 | 6.8 | |||
| Serum iron (µg/dL) | 188 | 70 | 85 | |||
| Ferritin (ng/mL) | 52 | 214 | 193 | |||
| HOMA-IR score | 0.9 | 7.2 | 10.8 | |||
| Leptin (ng/mL) | 8.3 | 23.6 | 21.3 | |||
| Adiponectin (µg/L) | 5.6 | 5.7 | 7.1 | |||
| hsCRP (mg/mL) | 0.042 | 0.052 | 0.025 | |||
| WFA+M2BP (C.O.I) | - | 0.37 | 0.5 | |||
| P-III-P (U/mL) | 0.6 | 1.27 | 1.35 | |||
| Type IV collagen7S (ng/mL) | 3.5 | 5.7 | 7.1 | |||
| Hyaluronic acid (ng/mL) | 37 | 151 | 206 | |||
| FIB4 index | 0.74 | 4.2 | 4.33 |
ALT: alanine aminotransferase, AST: aspartate aminotransferase, γ-GTP: γ-glutamyltransferase, FIB4 Index: Fibrosis-4 index, HLD-C: high-density lipoprotein cholesterol, HOMA-IR: homeostasis model assessment-insulin resistance, hs-CRP: high-sensitivity C-reactive protein, LDL-C: low-density lipoprotein cholesterol, NASH: nonalcoholic steatohepatitis, P-III-P: procollagen-III-peptide, WFA+M2BP: Wisteria floribunda agglutinin Mac-2 binding protein
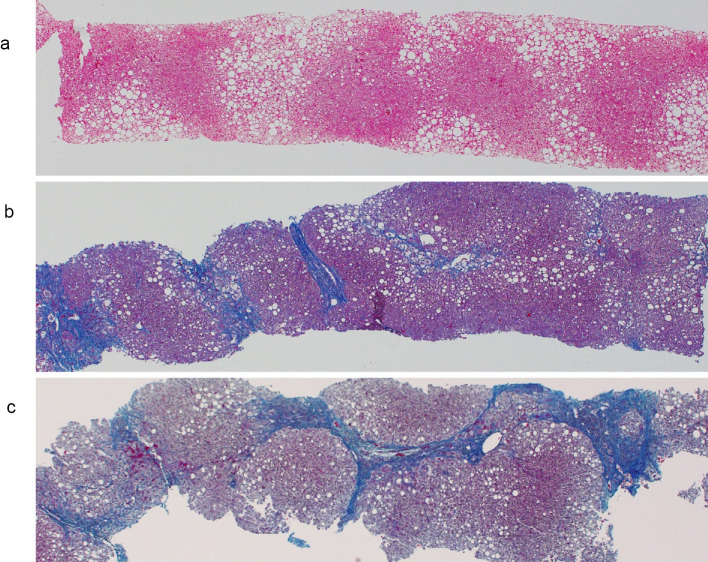
Abdominal ultrasonography revealed remarkable fatty liver with findings of an uneven hepatic surface, hepatorenal contrast, sound attenuation, and vascular blurring. She had dyslipidemia but did not have hypertension or diabetes. A histologic examination of a transcutaneous liver biopsy specimen followed by Hematoxylin and Eosin (H&E) staining and Azan staining showed mild lobular inflammation and the presence of macrovesicular hepatocellular steatosis. However, a liver biopsy indicated no hepatocyte ballooning degeneration or perisinusoidal fibrosis. Consequently, the patient was diagnosed with NAFL [Brunt classification (5), stage 0, grade 1; NAS (NAFLD activity score) 3 (6), (steatosis 2, lobular inflammation 1, ballooning 0); fatty liver inhibition of progression (FLIP) algorithm, NAFL (7)] (Fig. 1a, b). Subsequently, she had a checkup with her family doctor once a year.
Figure 1.
Azan staining of liver biopsy tissues at the initial presentation (a), after 12 years (b), and after 14 years (c) (×20). (a) There is no fibrosis surrounding the hepatocytes, portal area, or central venous area (stage 0). (b) Fibrosis persists around the portal area, central vein, and hepatocytes, with a clear increase in the degree of fibrosis (stage 3). (c) Liver fibrosis is increased around the hepatocytes, portal vein region, and central vein region and diagnosed as cirrhosis (stage 4).
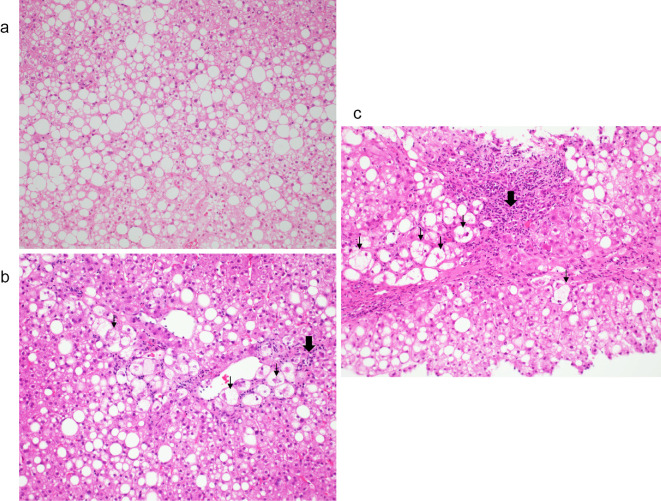
Twelve years and six months later, at 56 years old, the patient had an abnormal ALT level. She was menopausal at 50 years old and visited our hospital again. She had hypertension and diabetes in addition to dyslipidemia. At that time, she had been taking medication for diabetes dipeptidyl peptidase [(DPP4) inhibitor] and hypertension (Ca antagonist) for the past two years. Her body weight decreased to 58.5 kg (BMI, 25.5 kg/m2). However, the serum level of ALT, AST, HbA1c, and leptin and liver fibrosis markers increased as follows: type IV collagen 7S, 5.7 ng/mL; hyaluronic acid, 151 ng/mL; P-III-P, 1.27 U/mL; and FIB4 index, 4.2. Liver histology showed that the fibrosis, grade, and ballooning had progressed to stage 3, grade 2, and NAS 6 (steatosis 2, lobular inflammation 2, ballooning 2), respectively, and she had progressed from NAFL to NASH stage 3 (Fig. 2a, b; Table; Supplementary material).
Figure 2.
Hematoxylin and Eosin staining of liver biopsy tissues at the initial presentation (a), after 12 years (b), and after 14 years (c) (×40). (a) Approximately 80% of the tissues show fat deposits and macrovesicles to medium-sized fat droplets. No hepatocyte balloon-like cells are observed (grade 0). (b) Fatty deposits in the liver are decreased to approximately 40% of the tissue. Lipid droplets, ballooning, and Mallory bodies are also observed. Inflammatory cell infiltrates, mainly comprising lymphocytes, are distributed in the portal region (grade 2). (c) Fatty deposits in the liver comprise approximately 40% of the tissue. Lipid droplets, ballooning, and Mallory bodies are also observed. Inflammatory cell infiltrates are distributed in the portal area (grade 2). (↓) Inflammatory cell, (⬇) Ballooning cell
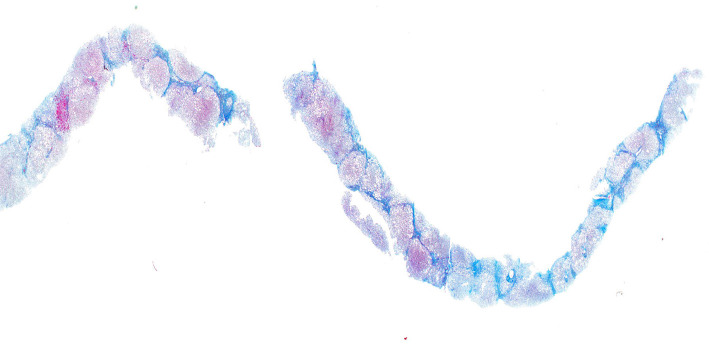
About 14 years after the first visit to our hospital, at 58 years old, she underwent a third liver biopsy at her request. The duration between the second and third liver biopsies was one year. Her body weight had not considerably changed between the second and third liver biopsies, but serum ALT levels remained high, and the HbA1c level had also increased. Liver histology revealed the progression of fatty changes, inflammation, and fibrosis; her ratings were stage 4, grade 2, and NAS 6 (steatosis 2, lobular inflammation 2, and ballooning 2, respectively; Fig. 1c, 2c, 3), respectively. Liver histology showed lipid droplets, ballooning, and Mallory bodies. Furthermore, liver fibrosis had increased and progressed by one stage in a year, leading to cirrhosis. With the progression from NAFL to NASH, type IV, the collagen 7S, P-III-P, FIB4 index, and serum ferritin levels had also increased. In particular, between the second and third liver biopsies, the type IV collagen 7S, P-III-P, and FIB4 index had increased significantly.
Figure 3.
Azan staining of the liver biopsy tissue 14 years later. Pseudolobules were formed, and the patient was diagnosed with cirrhosis (×100).
The report protocol was in accordance with the Declaration of Helsinki. Informed consent was provided by the patient.
Discussion
A few detailed studies have investigated the histology associated with liver fibrosis progression. The present case is valuable because there was progression from NAFL to NASH cirrhosis over time, and liver biopsies repeatedly confirmed changes in the histology and fibrosis markers.
Singh et al. performed a systematic review and meta-analysis of 11 paired biopsy cohort studies that included 411 patients with >2,145 person-years of follow-up (8). Approximately 30% of patients had advanced fibrosis, and 70% remained stable or showed improvement in terms of the fibrosis stage. Overall, the annual fibrosis progression rate was 1 stage of progression every 14 years for patients with NAFL and 1 stage of progression every 7 years for those with NASH. Nasr et al. conducted a biochemical, clinical, and histological study of 129 patients with NAFLD who had been enrolled in a prospective cohort study from 1988 to 1993 and followed up for 19.8 years (9). They reported that 12 patients (9.3%) developed end-stage liver disease, and 44 (34%) developed advance fibrosis. Furthermore, of the 113 patients with low baseline fibrosis (stage 3), 21 (16%) developed advanced fibrosis.
Although several studies have reported the changes and rates of liver fibrosis, including the present report (2-4,8,9), to our knowledge, there has been no time-course evaluation actually showing the liver histology. Furthermore, no marked differences have been observed in the age, sex, or clinical, histological, or biochemical factors between patients with and without liver fibrosis.
As per a previous study, 80% patients with NAFL and NASH display slow liver fibrosis progression, whereas 20% display rapid liver fibrosis progression (9). It is speculated that the speed of fibrosis progression is related to genetic factors, including patatin-like phospholipase domain containing (PNPLA) 3 (10,11), the HOMA-IR (12), obesity severity, the onset and deterioration of diabetes (13,14), sex differences, and menopausal factors (15). NAFLD development involves a combination of various factors that vary from case to case and need to be considered on an individual basis.
In the present case, NAFL rapidly progressed to NASH in only 14 years. The first-second liver biopsy showed a progression of 0.24 stage/year, and the second-third liver biopsy showed a progression of 1 stage/year. Apparently, the fibrosis progressed rapidly in the second and third liver biopsies. PNPLA3 is CG and definitely a factor in menopausal disorders, and there is no doubt that this increases the speed of progression.
Estrogen is important for the regulation of glucose metabolism in menopausal women because it exerts a protective effect on pancreatic beta cells and plays an important role in regulating appetite and improving insulin resistance in insulin target organs. Estrogen may also play an important role in the progression of NAFL and NASH. Postmenopausal women are at an increased risk of NAFLD and might show metabolic features of insulin resistance. For example, increased total and visceral adiposity in peri- and postmenopausal women is associated with an increased risk of insulin resistance, dyslipidemia, hypertension, diabetes, and cardiovascular disease. Furthermore, postmenopausal women with NAFLD are at an increased risk of portal inflammation, ballooning, and fibrosis owing to their inability to suppress oxidative stress and fibrosis by lowering their estrogen level (16). Estrogen replacement therapy has some beneficial effects in patients with liver fibrosis (17). We predict that the speed of fibrosis will increase 5-10 years post-menopause, and this should be explored in future research.
Although the present patient did not gain weight, she developed diabetes, underwent menopause, and experienced rapid liver fibrosis progression over 14 years. We have observed many cases with liver fibrosis progression post-menopause, so we must be alert for liver fibrosis when treating NAFLD, even if the weight is controlled post-menopause,. In the present case, we observed the persistent elevation of the levels of ALT and liver fibrosis markers (FIB4 index and ferritin). The platelet count decreased from 21.2×104 to 10.1×104. These findings may be indicative of fibrosis progression.
NAFL progresses to NASH and NASH cirrhosis; however, it is important to assess the changes in liver histology and blood tests, presence or absence of lifestyle-related diseases, age, sex, and if possible, changes in body composition. We previously reported the improvement in liver histology from NASH to normal liver after weight loss with a low-calorie diet and aerobic exercise in patients with NASH (18). In the future, the standard method of evaluating fibrosis in NAFL and NASH is expected to change from a liver biopsy to noninvasive diagnostic methods, such as magnetic resonance elastography (19) and vibration-controlled transient elastography (VCTE) (20). However, liver histology reveals fibrosis, inflammation, fat, and the changes in detail as well as the pathological condition, wherein steatohepatitis is added to other liver diseases, such as metabolic-associated fatty liver disease (21). Therefore, a liver biopsy will likely continue to be an important test, depending on the case.
In conclusion, we encountered a valuable case wherein changes in the liver tissue were confirmed over time by performing three liver biopsies to assess the progression of NAFL to NASH over 14 years in a patient with cirrhosis. The present report shows that the liver tissue changes can be assessed using liver fibrosis markers, and menopause may be a cause of rapid liver fibrosis. Based on the extensive experience, including this case, postmenopausal liver fibrosis is likely to progress rapidly, and we believe it is important to provide guidance to postmenopausal women. In the future, we plan to study the progression of liver fibrosis in postmenopausal women.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1975 Declaration of Helsinki, as revised in 2008 (5).
The authors state that they have no Conflict of Interest (COI).
Supplementary Material
Histological evaluation of biopsied tissues.
References
- 1.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 69: 2672-2682, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44: 865-873, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Rafiq N, Bai C, Fang Y, et al. Long-term follow up of patients with nonalcoholic fatty liver. Clin Gastroenterol Hepatol 7: 234-238, 2009. [DOI] [PubMed] [Google Scholar]
- 4.Harrison SA. Nonalcoholic fatty liver disease and fibrosis progression: the good, the bad, and the unknown. Clin Gastroenterol Hepatol 13: 655-657, 2015. [DOI] [PubMed] [Google Scholar]
- 5.Kliner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41: 1313-1321, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Brunt EM, Kleiner DE, Wilson LA, et al. NASH Clinical Research Network (CRN). Nonalcoholic fatty liver disease (NAFLD) activity score and the histologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 53: 810-820, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beddosa P. FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 60: 565-575, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver versus nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired biopsy studies. Clin Gastroenterol Hepatol 13: 643-654, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasr P, Ignatova S, Kechagias S, et al. Natural history of nonalcoholic fatty liver disease: a prospective follow -up study with serial biopsies. Hepatology Commun 27: 199-210, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi T, Sumida Y, Umemura A, et al. Genetic polymorphisms of the human PNPLA3 gene are strongly associated with severity of non-alcoholic fatty liver disease in Japanese. PLoS One 7: e38322, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seko Y, Sumida Y, Tanaka S, et al. Development of hepatocellular carcinoma in Japanese patients with biopsy-proven non-alcoholic fatty liver disease: association between PNPLA3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatology Res 47: 1083-1092, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Fujii H, Imajyo K, Yoneda N, et al. HOMA-IR: Independent predictor of progressive cirrhosis in non-diabetic non-alcoholic fatty liver disease. J Gastroenterol Hepatol 34: 1390-1395, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Jarvis H, Craig D, Barker R, et al. Metabolic risk factor and incident advanced fibrosis in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of population- based observational studies. PLoS Med 17: e1003100, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sumida Y, Shima T, Mitsumoto Y, et al. Epidemiology: pathogenesis, and diagnostic strategy of diabetic liver disease in Japan. Int J Mol Sci 21: 4337, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klair JS, Yang JD, Abdelmalek MF, et al. A longer duration of estrogen deficiency increase fibrosis risk among postmenopausal women nonalcoholic fatty liver disease. Hepatology 64: 85-91, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Womens Health (Lond) 5: 191-203, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Yang JD, Abdelmalek MF, Pang H, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology 59: 1406-1414, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawanaka M, Nouso K, Yano S, et al. Case report of diet-related improvement of non-alcoholic steatohepatitis evaluated by four consecutive liver biopsies. Hepatology Res 47: 480-484, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology 150: 626-637, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Yoneda M, Yoneda M, Mawatari H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis 40: 371-378, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction - associated fatty liver disease: an international expert consensus statement. J Hepatol 73: 202-209, 2020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histological evaluation of biopsied tissues.