Abstract
We herein report four cases of diffuse bronchiolitis proven by a transbronchial lung cryobiopsy (TBLC). Based on various aspects, including the pathological findings, we definitively diagnosed two patients with diffuse panbronchiolitis (DPB) and the other two with primary ciliary dyskinesia (PCD). One of the DPB patients had more severe peribronchiolar fibrosis than the other, and the disease course was refractory to macrolide therapy. One of the PCD patients was additionally diagnosed with combined constrictive bronchiolitis. This report highlights the importance of a TBLC in the differentiation of bronchiolitis, suggesting its utility for helping pulmonologists formulate a treatment strategy.
Keywords: transbronchial lung cryobiopsy, diffuse panbronchiolitis, primary ciliary dyskinesia, constrictive bronchiolitis
Introduction
Bronchiolitis refers to a nonspecific cellular and mesenchymal reaction of the bronchioles. Developing a straightforward classification is difficult because the differential diagnosis of bronchiolitis is varied, and its etiology is mixed, including diffuse panbronchiolitis (DPB), primary ciliary dyskinesia (PCD), nontuberculous mycobacteria infection, immunodeficiency, connective tissue disease, diffuse aspiration bronchiolitis, follicular bronchiolitis, bronchiolitis obliterans, eosinophilic bronchiolitis, and others (1). Furthermore, there are many diseases that, in addition to causing bronchiolitis, also cause diseases proximal (e.g., bronchiectasis) or distal (e.g., organizing pneumonia) to the bronchioles. As a result, some avoid defining the precise site of involvement and instead refer to the peripheral airways (<2 mm in diameter) as “small airways” (2). Therefore, the definitive diagnosis of a specific bronchiolitis entity may require a histopathologic evaluation.
The recently introduced transbronchial lung cryobiopsy (TBLC) has advantages of a higher diagnostic yield than a conventional transbronchial lung biopsy, good pathological inter-observer agreement, and a better safety profile than a surgical lung biopsy. Therefore, the usage of TBLCs has spread in the diagnostic algorithm of several thoracic diseases, especially interstitial lung diseases (3). We recently hypothesized that TBLC would provide a large specimen that allows for the pathological evaluation of bronchial-to-alveolar lesions, allowing us to analyze both the lung parenchyma and broncho-bronchiolar lesions together (4). However, there are few studies and little evidence describing the utility of a TBLC in cases of bronchiolitis.
Therefore, in the present report, we assessed the utility of a TBLC in patients with diffuse bronchiolitis and provided additional evidence concerning the pathological picture of this disease.
Case Reports
Patient 1
The patient was a 70-year-old man in whom an abnormal shadow on chest X-ray had been noted during a routine medical examination, and he was later referred to our hospital. The patient revealed that he had had mild symptoms of a wet cough for a long time. He had a history of smoking and chronic sinusitis and no obvious history of infertility.
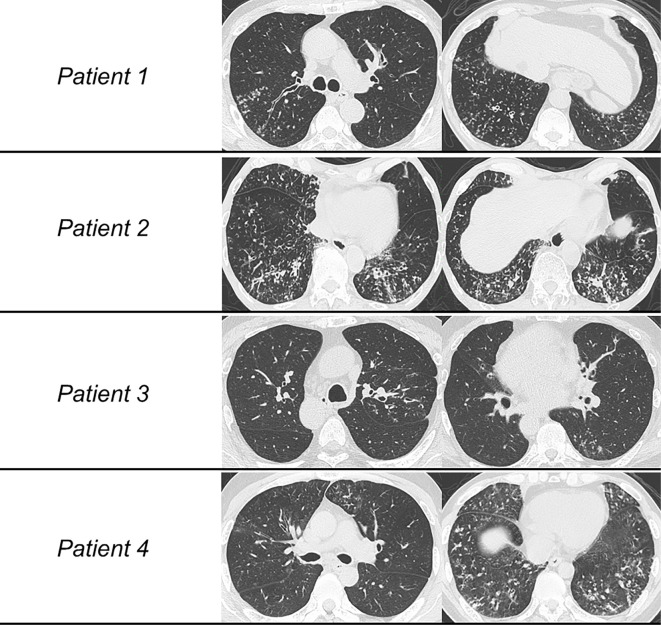
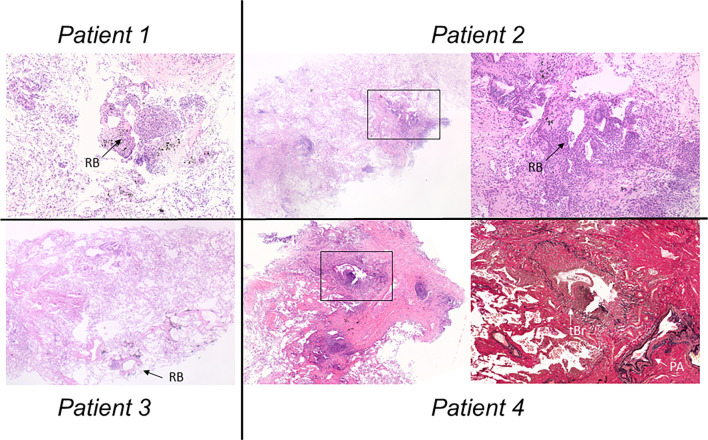
Laboratory examinations revealed significantly high serum levels of cold agglutinin titer (Table). Blood human leucocyte antigen (HLA) testing indicated the presence of HLA-A11. Respiratory function testing showed airway obstruction, with a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of 61%. High-resolution computed tomography (HRCT) showed the diffuse distribution of centrilobular nodular shadows and bronchial thickening predominantly in the lower lung (Fig. 1). A TBLC of four random bronchi (right B2a, B6b, B9a, and B10b) showed mononuclear cell infiltration with foamy macrophages around the respiratory bronchioles (Fig. 2). Electron microscopy of the bronchial mucosa showed no structural abnormalities. Given the clinical and histological findings referenced in the diagnostic criteria (5), we diagnosed the patient with DPB. His respiratory symptoms and radiological shadows improved with the continuous administration of low-dose erythromycin at 600 mg/day.
Table.
Clinical Characteristics.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Diagnosis | DPB | DPB | PCD | PCD |
| Age, years/sex | 70/man | 71/man | 52/man | 40/man |
| Smoking status | Ex-smoker (SI 50) | Ex-smoker (SI 100) | Ex-smoker (SI 50) | Never |
| Chronic sinusitis | + | + | + | + |
| Childhood episodes of respiratory infection | - | - | - | - |
| Other information | - | - | Infertility, visceral inversion | Infertility |
| Laboratory findings | ||||
| Cold agglutinin reaction (titer) | +(256) | - (32) | - (32) | - (32) |
| Autoantibodies | - | - | - | - |
| HTLV-1 and HIV antibodies | - | - | - | - |
| HLA | A11/A24/B52/B60 | A2/A24/B51/B61 | A26/A33/B44/B61 | A24/B54/B59 |
| Sputum or broncho-lavage culture | Haemophilus influenzae | Non-significant | Pseudomonas sp. Staphylococcus aureus | Haemophilus influenzae Streptococcus pneumoniae |
| Pulmonary function | ||||
| VC, % predicted | 88 % | 122 % | 103 % | 102 % |
| FEV1/FVC ratio (%) | 61 % | 64 % | 82 % | 68 % |
| DLco, % predicted | 152 % | 104 % | 84 % | 67 % |
| Ciliary structure under electron microscope | Normal | Normal | Complete defects of inner dynein arm | Partial defects of outer and inner dynein arm |
| Pathological findings by a cryobiopsy | Respiratory bronchioles with various inflammation cells and foamy macrophages | Bronchiolitis with high infiltration and peribronchiolar fibrosis | Mild respiratory bronchiolitis | Bronchiolitis obliterans involving terminal and respiratory bronchioles |
| Effects of macrolide therapy for six months | Good | Slightly worsened | Slightly improved | No change |
DPB: diffuse panbronchiolitis, PCD: primary ciliary dyskinesia, SI: smoking index, HTLV-1: human T-cell leukemia virus type 1, HIV: human immunodeficiency virus, HLA: human leukocyte antigen, VC: vital capacity, FVC: forced vital capacity, FEV1: forced expiratory volume in 1 second, DLco, diffusing capacity of carbon monoxide
Figure 1.
High-resolution computed tomography (HRCT) images of the four patients. (Patient 1) Centrilobular nodular shadows and bronchial thickening are diffusely distributed predominantly in the lower lungs. (Patient 2) HRCT showed not only diffuse centrilobular nodules but also mosaic perfusion indicative of air trapping and mild consolidation. (Patient 3) Centrilobular nodules and bronchiectasis were seen predominantly in the dorsal left upper lung and left S6. (Patient 4) Diffuse distribution of centrilobular nodules, bronchial thickening, and air trapping were seen predominantly in the lower lungs.
Figure 2.
Histological images. (Patient 1) The lesion was characterized by an accumulation of inflammatory cell infiltration with foamy macrophages around the respiratory bronchioles in the right B9a [Hematoxylin and Eosin (H&E) staining, ×4]. (RB: respiratory bronchiole) (Patient 2) Respiratory bronchioles with the infiltration of lymphocytes and plasma cells. In addition, a fibrotic lesion indicative of peribronchiolar fibrosis was seen from the respiratory bronchioles to the alveoli at the right bronchus B9b (left image: H&E staining, ×2.5) (right image: high-power magnification view of the square, ×10). (Patient 3) Mild mononuclear cell infiltration was seen around the respiratory bronchioles without fibrotic changes at left bronchus 6b (H&E staining, ×2.5). (Patient 4) A terminal bronchiole revealed mononuclear cell infiltration at right bronchus B9a (left image: H&E staining, ×4). A high-power magnification view of the square shows fibrous obliterans of the bronchiolar lumen with remaining elastic lamina consistent with constrictive bronchiolitis (right image: elastica van Gieson stain, ×10) (tBr: terminal bronchiole, PA: pulmonary artery).
Patient 2
A 71-year-old man who was an ex-smoker presented with cough and sputum persisting over 2 years. He had suffered from chronic rhinitis and sinusitis from his 30s and had no history of infertility. Respiratory function testing revealed airway obstruction, as indicated by a FEV1/FVC ratio of 64% (Table). HRCT showed not only diffuse centrilobular nodules but also mosaic perfusion indicative of air trapping and mild consolidation (Fig. 1). A TBLC of three random bronchi (right B9b, B10b, and B10c) showed respiratory bronchioles with infiltrations of lymphocytes and plasma cells. In addition, peribronchiolar fibrosis was observed from the respiratory bronchioles to the alveoli (Fig. 2). Electron microscopy of the bronchial mucosa showed no structural abnormalities. Given the clinical and histological findings referenced in the diagnostic criteria (5), we diagnosed the patient with DPB. The patient's respiratory symptoms and radiological shadows showed no change with low-dose erythromycin (600 mg/day) for 3 months, so he was switched to continuous azithromycin at 250 mg/day. However, by six months later, his symptoms still had not improved, and the radiological shadows had mildly worsened.
Patient 3
A 52-year-old man who was an ex-smoker was referred to our hospital because of repeated episodes of pneumonia and chronic sputum production for a long time. He had chronic sinusitis and an obvious history of male infertility. His sputum culture was positive for Pseudomonas species and Staphylococcus aureus. Respiratory function test results were normal (Table). HRCT showed visceral inversion. In addition, centrilobular nodules and bronchiectasis were seen predominantly in the dorsal left upper lung and left S6 (Fig. 1). A TBLC of three random bronchi (left B2a, B2b, and B6b) revealed mild infiltration around the respiratory bronchioles without fibrotic change (Fig. 2). Electron microscopy of the bronchial mucosa showed complete defects of inner dynein arms. Based on the American Thoracic Society (ATS) clinical practice guideline (6), we diagnosed the patient with PCD. The patient's respiratory symptoms and radiological shadows slightly improved with low-dose erythromycin at 600 mg/day.
Patient 4
A 40-year-old man who had never smoked presented with chronic sputum for a long time. He had chronic sinusitis and a history of infertility. His sputum culture was positive for Haemophilus influenzae and Streptococcus pneumoniae. Respiratory function testing revealed obstructive disorder and diffusion impairment; his FEV1/FVC ratio was 68%, and %predicted DLco was 67% (Table). HRCT revealed the diffuse distribution of centrilobular nodules, bronchial thickening, and air trapping predominantly in the lower lung (Fig. 1). A TBLC of three random bronchi (right B9a, B9b, and B10a) showed the accumulation of inflammatory cell infiltration around the terminal bronchioles. In addition, some of the these specimens showed submucosal dense fibrosis with luminal narrowing and remaining elastic lamina. These pathological findings indicated constrictive bronchiolitis (Fig. 2). Electron microscopy of the bronchial mucosa showed partial defects of the outer and inner dynein arms. In accordance with the ATS clinical practice guideline (6), we diagnosed the patient with PCD with constrictive bronchiolitis. The patient's respiratory symptoms and radiological shadows have shown no change despite low-dose erythromycin at 600 mg/day.
Discussion
We herein report four cases of diffuse bronchiolitis (two with DPB and two with PCD) histologically confirmed by a TBLC. The knowledge obtained from biopsy specimens in patients with bronchiolitis may provide useful information to pulmonologists. The present cases therefore have important implications.
First, a TBLC can be useful in patients with bronchiolitis when histological changes need to be confirmed for the differential diagnosis. Although our patients were suspected of having DPB or PCD because they had chronic sinusitis, a specific HLA [e.g., HLA-A11 or -B54 for DPB (7)], and/or ciliary structures, the differential diagnosis of bronchiolitis comprises varied and mixed etiologies (e.g. combined DPB with nontuberculous mycobacteria or connective tissue disease-related bronchiolitis with diffuse aspiration bronchiolitis) in clinical practice. Histologically, connective tissue disease-related bronchiolitis, including follicular bronchiolitis and bronchiolitis obliterans, can show lymphoid hyperplasia along the bronchiole or peribronchiolar lymphocytic infiltration (8). Diffuse aspiration bronchiolitis shows bronchiolocentric organizing pneumonia with giant cells that contain material consistent with aspirated food (9). Destruction of bronchial cartilage and the smooth muscle layer, obstruction of the airway by granulomas, and ulceration of the bronchial mucosa are frequently observed in nontuberculous mycobacteria infection (10). However, it is important to characterize the lymphocyte and macrophage accumulation around respiratory bronchioles in order to elucidate the pathogenesis of DPB (11).
While our understanding of the prevalence of type-specific bronchiolitis other than diffuse bronchiolitis among populations with PCD is insufficient at present, the pathology in PCD-related bronchiolitis has been suggestive of DPB (12). Interestingly, Thalanayar and Holguin reported a case of follicular bronchiolitis in a patient with PCD in which they proposed a revised approach to managing airway disease, including the type-specific bronchiolitis noted in this subset of PCD patients (13). The pathological findings in our four cases were compatible with DPB or PCD, and one patient showed the presence of constrictive bronchiolitis with PCD. Given these findings, a more accurate diagnosis of bronchiolitis is needed for pathological evaluation, although a surgical lung biopsy (SLB) is not routinely performed for patients with bronchiolitis.
A recent report showed that the diagnostic performance of a conventional transbronchial lung biopsy (TBLB) is low, and a TBLC is a promising technique that substantially expands the pulmonary armamentarium in the diagnosis of bronchiolitis (14). Indeed, a case series of patients with constrictive bronchiolitis diagnosed with a cryobiopsy was reported (15), suggesting that the usefulness of a TBLC for diffuse bronchiolitis (e.g. DPB and PCD) may be comparable to that of an SLB. Regarding the risks associated with a TBLC, studies in diffuse lung disease thus far have reported rates of 0-7% for pneumothorax requiring chest tube thoracostomy and rare incidents of serious hemorrhaging (15,16). A TBLC is a robust and low-invasive method for the characterization of small airway diseases and is associated with a low rate of complications and good diagnostic confidence (14). In addition, a TBLC provides a large specimen that allows for the pathological evaluation of bronchial-to-alveolar lesions (4,15). Therefore, a TBLC may be more useful than an SLB or conventional TBLB for allowing pulmonologists to accurately diagnose patients with bronchiolitis.
Second, the pathological findings may predict the therapeutic response and determine the medication to be administered. Among the two patients with DPB in the present study, Patient 2 had more severe peribronchiolar fibrosis than Patient 1, and Patient 2's disease course was refractory to macrolide therapy. Some patients with DPB are refractory to macrolides (17), and to some extent, we could predict the refractory course of macrolides in Patient 2. Although there is little evidence supporting any particular therapeutic strategy for PCD-related bronchiolitis because the pathophysiology, symptoms, and prognosis differ among patients with PCD (18), macrolide therapy may be beneficial in some cases, as in Patient 3 (19). Notably, a TBLC pathologically proved the presence of constrictive bronchiolitis in Patient 4. On reviewing the bronchiolitis patterns previously reported in PCD patients, to our knowledge, combined constrictive bronchiolitis has not been reported thus far. Although we cannot definitively prove this, we speculate that anti-inflammatory therapy (e.g., corticosteroid, mycophenolate mofetil, and cyclophosphamide) may be effective in cases of PCD because an autoimmune response may be associated with constrictive bronchiolitis (20). To improve the prognosis of bronchiolitis, further research may be important for determining the correlation between the pathological findings and therapeutic decision-making strategies.
Based on our experience, a TBLC may be a viable diagnostic modality in patients with diffuse bronchiolitis, but our results will need to be confirmed on a larger scale. A TBLC may help pulmonologists more accurately diagnose patients with bronchiolitis and determine an appropriate medication strategy.
All patients provided their written informed consent and allowed their medical data to be cited.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Meyer KC, Raghu G, Verleden GM, et al. Clinical Practice Guideline: The Diagnosis, Management, and Prevention of Bronchiolitis. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J 44: 1479-1503, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Meissner HC. Bronchiolitis. In: Principles and Practice of Pediatric Infectious Diseases. 5th ed. Long S, Prober C, Fischer M, Eds. PA: Elsevier, Philadelphia, 2018: 1588-1662. [Google Scholar]
- 3.Hetzel J, Maldonado F, Ravaglia C, et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the cryobiopsy working group on safety and utility and a call for standardization of the procedure. Respiration 95: 188-200, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Yamakawa H, Takemura T, Tsumiyama E, et al. IgG4-related bronchial gland inflammation proven by transbronchial cryobiopsy. Am J Respir Crit Care Med 201: 1554-1556, 2020. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald JE, King TE Jr, Lynch DA, Tuder RM, Schwarz MI. Diffuse panbronchiolitis in the United States. Am J Respir Crit Care Med 154: 497-503, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro AJ, Davis SD, Polineni D, et al. Diagnosis of primary ciliary dyskinesia. An official American thoracic society clinical practice guideline. Am J Respir Crit Care Med 197: e24-e39, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keicho N, Hijikata M. Genetic predisposition to diffuse panbronchiolitis. Respirology 16: 581-588, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Howling SJ, Hansell DM, Wells AU, Nicholson AG, Flint JD, Müller NL. Follicular bronchiolitis: thin-section CT and histologic findings. Radiology 212: 637-642, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Papiris SA, Malagari K, Manali ED, et al. Bronchiolitis: adopting a unifying definition and a comprehensive etiological classification. Expert Rev Respir Med 7: 289-306, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Fujita J, Ohtsuki Y, Shigeto E, et al. Pathological findings of bronchiectases caused by Mycobacterium avium intracellulare complex. Respir Med 97: 933-938, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Kudoh S, Keicho N. Diffuse panbronchiolitis. Clin Chest Med 33: 297-305, 2012. [DOI] [PubMed] [Google Scholar]
- 12.Homma S, Kawabata M, Kishi K, et al. Bronchiolitis in Kartagener's syndrome. Eur Respir J 14: 1332-1339, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Thalanayar PM, Holguin F. Follicular bronchiolitis in primary ciliary dyskinesia. Australas Med J 7: 294-297, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirol Aflah Syazatul S, Piciucchi S, Tomassetti S, Ravaglia C, Dubini A, Poletti V. Cryobiopsy in the diagnosis of bronchiolitis: a retrospective analysis of twenty-three consecutive patients. Sci Rep 10: 10906, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lentz RJ, Fessel JP, Johnson JE, Maldonado F, Miller RF, Rickman OB. Transbronchial cryobiopsy can diagnose constrictive bronchiolitis in veterans of recent conflicts in the Middle East. Am J Respir Crit Care Med 193: 806-808, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lentz RJ, Argento AC, Colby TV, Rickman OB, Maldonado F. Transbronchial cryobiopsy for diffuse parenchymal lung disease: a state-of-the-art review of procedural techniques, current evidence, and future challenges. J Thorac Dis 9: 2186-2203, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med 157: 1829-1832, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Lucas JS, Gahleitner F, Amorim A, et al. Pulmonary exacerbations in patients with primary ciliary dyskinesia: an expert consensus definition for use in clinical trials. ERJ Open Res 5: pii:00147-2018, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kido T, Yatera K, Yamasaki K, et al. Two cases of primary ciliary dyskinesia with different responses to macrolide treatment. Intern Med 51: 1093-1098, 2012. [DOI] [PubMed] [Google Scholar]
- 20.Cortot AB, Cottin V, Miossec P, Fauchon E, Thivolet-Béjui F, Cordier JF. Improvement of refractory rheumatoid arthritis-associated constrictive bronchiolitis with etanercept. Respir Med 99: 511-514, 2005. [DOI] [PubMed] [Google Scholar]