Abstract
We herein report a 56-year-old woman who developed allergic bronchopulmonary aspergillosis (ABPA) possibly due to fungal exposure after disastrous heavy rainfall in Western Japan in 2018. She was diagnosed with ABPA complicated with asthma, increased peripheral blood eosinophil count, elevation of specific immunoglobulin E for Aspergillus fumigatus, positive Aspergillus fumigatus precipitation antibody reaction test results, and notable chest computed tomography findings. After treatment with benralizumab, her symptoms, peripheral blood eosinophil count, radiological findings, and respiratory function dramatically improved. The administration of benralizumab appears to be an effective treatment strategy for ABPA.
Keywords: allergic bronchopulmonary aspergillosis, benralizumab, bronchial asthma, Aspergillus fumigatus, heavy rainfall
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is an immunological pulmonary disease characterized by systemic and airway eosinophilia, elevated serum immunoglobulin (Ig) E levels, lung infiltration, bronchiectasis, and mucoid impaction of the central bronchi due to a complex hypersensitivity reaction to Aspergillus fumigatus (1). The complete immune mechanism behind ABPA is not well understood. To our knowledge, Aspergillus sp. colonizing the airways can induce type I and III hypersensitivity reactions with production of specific IgE/G antibodies to A. fumigatus (2). The immune response of ABPA patients to A. fumigatus is thought to be caused by interleukin (IL)-4 and IL-5 released from T helper 2 cells, which are involved in type 2 inflammation (3). IL-4 stimulates B cells to produce IgE, and IL-5 induces eosinophil activation, resulting in mucoid impaction and bronchial impairment.
Benralizumab is a monoclonal antibody that works against the alpha-chain of the IL-5 receptor. It binds to the IL-5 receptor-alpha-chain of eosinophils, blocks the binding of IL-5 to eosinophils, and inhibits the action of IL-5. In addition, binding to eosinophils activates natural killer (NK) cells, which directly remove eosinophils via apoptosis by inducing antibody-dependent cellular cytotoxicity (ADCC) (4). Thus, benralizumab exerts novel effects to treat severe bronchial asthma by inducing a rapid decrease in blood and sputum eosinophil counts. Given the pathogenesis of bronchial asthma, benralizumab seems likely to be a suitable therapeutic agent for ABPA as well.
In July 2018, heavy rain caused flooding and water damage in Western Japan, including Okayama Prefecture. This disaster resulted in 224 deaths, the complete destruction of 6,758 buildings, and flooding of 8,567 dwellings above floor level (5). Water damage can potentially increase the risk of aspiration of water, dust, and soil containing microbes such as Legionella, Escherichia coli, Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Burkholderia cepacian, Scedosporium, and Aspergillus sp. during recovery work (6).
We herein report a case of ABPA complicated with asthma that was considered to have developed due to continuous fungal exposure caused by disastrous heavy rainfall in Western Japan in 2018 and was successfully treated with benralizumab.
Case Report
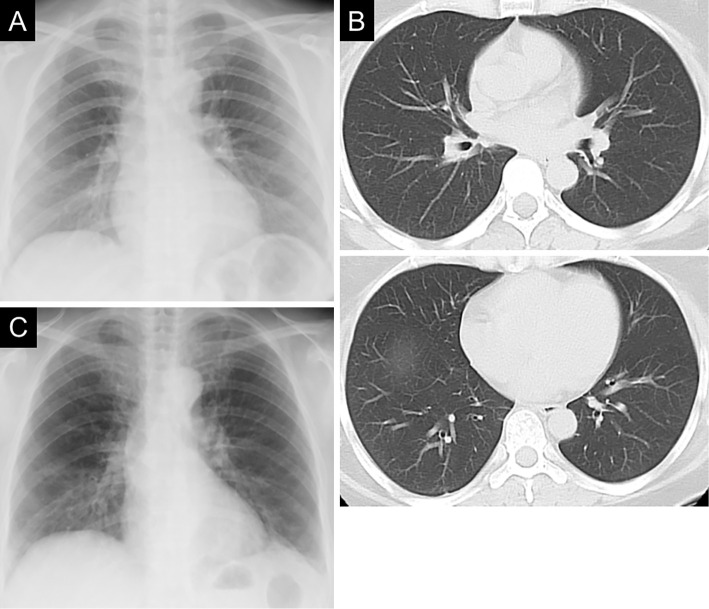
A 56-year-old woman was admitted to our hospital with complaints of a wet cough and dyspnea on exertion in December 2019. She was a caregiver who was working at a nearby nursing home and had a medical history of dyslipidemia and bronchial asthma with eosinophilia, which had been diagnosed in 2006 without any positive results for antigen-specific IgE and then treated with inhaled corticosteroid/long-acting β-agonist. After that treatment, her asthmatic symptoms were relatively stable, and no asthmatic attacks occurred. Chest radiography and computed tomography (CT) showed no abnormal findings (Fig. 1A, B).
Figure 1.
Chest X-ray film (A) and computed tomography scans (B) of the patient taken at the time of the diagnosis of bronchial asthma in 2006, and chest radiography findings on admission to our hospital in December 2019 (C). Ground-glass attenuation in both lung fields (especially in the lower fields) was observed on admission, whereas no abnormal findings were observed at the onset of bronchial asthma.
In July 2018, a disastrous heavy rainfall struck Western Japan, where she lives. Her home and workplace were flooded above the ground level with soil and water. Thereafter, her asthmatic symptoms gradually worsened. In June 2019, she underwent chest CT in a nearby hospital; the scans indicated the presence of sinusitis and chronic bronchitis. Although the symptoms were initially improved with antibiotics, controlling her asthmatic symptoms was difficult. In November 2019, chest CT showed that the thickening of the bronchial wall had worsened, and she was referred to our hospital.
On admission to our hospital, she was conscious, and her vital signs were as follows: temperature, 36.2°C; pulse rate, 80 beats/minute; blood pressure, 133/85 mmHg; respiratory rate, 16 breaths/minute; and oxygen saturation, 95% while breathing room air. No jugular vein dilation, heart murmur, or skin rash was observed. However, she presented with end-expiratory wheezes in both lungs with mild bilateral leg edema. She was slightly obese, with a body mass index of 28.5.
Laboratory findings showed that her peripheral blood eosinophil count was high (1,317 /μL), and her total serum IgE levels were elevated (986.0 IU/mL) (Table). In addition, she also tested positive for Aspergillus antibodies and had high Aspergillus-specific IgE (Class 4). Candia- or Alternaria- specific IgE were also slightly elevated. Her serum C-reactive protein and sialylated carbohydrate antigen KL-6 levels were slightly elevated, whereas her anti-neutrophil cytoplasmic antibody, β-D-glucan, and aspergillus galactomannan results were normal. A blood gas analysis showed that she had mild hypoxemia. Bacterial culture with a sputum sample detected only normal flora. However, a cytological examination revealed approximately 80% eosinophils in the visible field of the sputum sample.
Table.
Laboratory Findings on Admission to Our Hospital.
| Hematology | TP | 7.3 | g/dL | PR3-ANCA | <0.1 | U/mL | |||||||
| WBC | 7,400 | /μL | ALB | 3.9 | g/dL | MPO-ANCA | <1.0 | U/mL | |||||
| Seg | 46.7 | % | CRE | 0.88 | mg/dL | Mycoplasma Ab | <×40 | ||||||
| Mon | 5.0 | % | BUN | 16 | mg/dL | Candida Ag | (-) | ||||||
| Lym | 29.5 | % | Na | 141 | mEq/L | Cryptococcus Ag | (-) | ||||||
| Eos | 17.8 | % | K | 3.8 | mEq/L | β-D-glucan | 7.0 | pg/mL | |||||
| Bas | 1.0 | % | Cl | 106 | mEq/L | Aspergillus Ag | 0.5 | ||||||
| RBC | 471 | ×104/μL | Serology | Aspergillus Ab | (+) | ||||||||
| Hgb | 14.0 | g/dL | CRP | 1.36 | mg/dL | Aspergillus specific IgE | 34.1 | UA/mL | |||||
| Hct | 41.8 | % | HbA1c | 6.4 | % | Class 4 | |||||||
| PLT | 27.5 | ×104/μL | IgG | 1,744 | mg/dL | Alternaria specific IgE | 3.19 | UA/mL | |||||
| ESR | 41 | mm/1h | IgA | 126 | mg/dL | Candida specific IgE | 2.06 | UA/mL | |||||
| Biochemistry | IgM | 69 | mg/dL | Aspergillus precipitating antibody testing | (+) | ||||||||
| T-Bil | 0.4 | mg/dL | IgE | 986.0 | IU/mL | Blood gas analysis | |||||||
| AST | 15 | U/L | KL-6 | 701 | U/mL | pH | 7.435 | ||||||
| ALT | 14 | U/L | SP-D | 102.5 | ng/mL | pCO2 | 40.9 | Torr | |||||
| LDH | 230 | U/L | RF | 18 | U/mL | pO2 | 65.8 | Torr | |||||
| ALP | 255 | U/L | ANA | <×40 | HCO3- | 27.4 | mEq/L | ||||||
| γ-GTP | 18 | U/L | SaO2 | 94.2 | % | ||||||||
WBC: white blood cell, RBC: red blood cell, Hgb: hemoglobin, Hct: hematocrit, PLT: platelet, ESR: erythrocyte sedimentation rate, T-Bil: total bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: γ-glutamyl transpeptidase, TP: total protein, ALB: albumin, CRE: creatinine, BUN: blood urea nitrogen, CRP: C reactive protein, HbA1c: hemoglobin A1c, IgG: immunoglobulin G, IgA: immunoglobulin A, IgM: immunoglobulin M, IgE: immunoglobulin E, KL-6: krebs von den lungen-6, SP-D: surfactant protein-D, RF: rheumatoid factor, ANA: antinuclear antibody, PR3-ANCA: proteinase 3-anti-neutrophil cytoplasmic antibody, MPO-ANCA: myeloperoxidase-anti-neutrophil cytoplasmic antibody
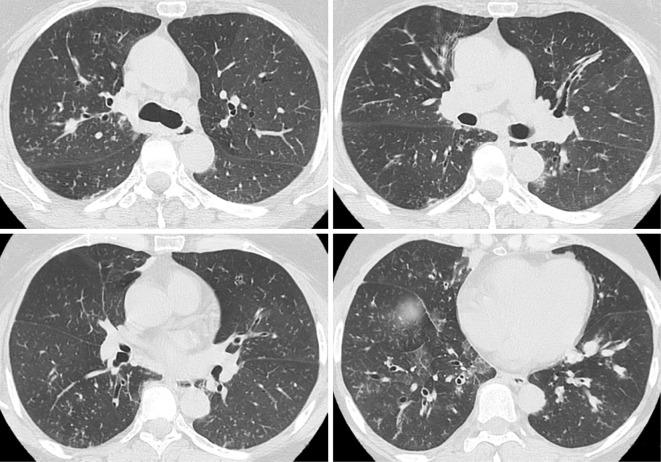
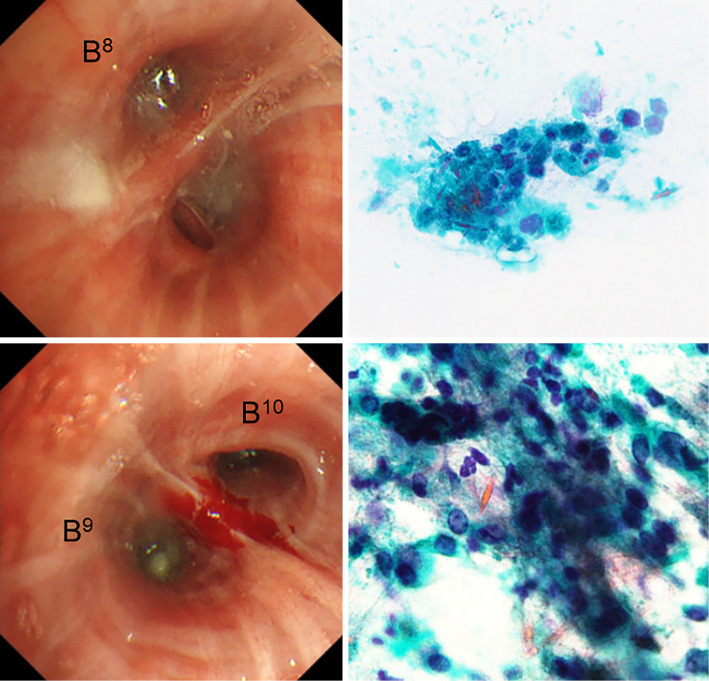
Chest radiography showed ground-glass attenuation in both lung fields but without a dilated heart (Fig. 1C). Chest CT showed bilateral thickening of the bronchi, ground-glass attenuation, centrilobular nodules, and mosaic attenuation (Fig. 2). Bronchiectasis with high-attenuation mucoid impaction was also observed (Fig. 3). On admission, pulmonary function tests showed a decline in the vital capacity (1.88 L) and forced vital capacity (FVC; 1.84 L), in addition to a reduced forced expiratory volume (FEV1.0) in 1 second (FEV1.0/FVC; 70.24%), percent predicted FEV1.0 (%FEV1.0; 56.6%), and peak expiratory flow (PEF; 5.29 L/s). The fractional nitric oxide concentration in her exhaled breath was 21 ppb. A bronchoscopic examination performed on the fourth day of hospitalization revealed bilateral mucous plugs in the lower lobe bronchi (Fig. 4), in which Charcot-Leyden crystals were microscopically observed. Bacterial culture did not detect anything significant in sucking sputum or brushing samples, with no obvious fungus noted on a pathological examination.
Figure 2.
Computed tomography scans of the patient’s chest acquired on admission to our hospital in December 2019 showed bilateral thickening of the bronchi, ground-glass attenuation, centrilobular nodules, and mosaic attenuation.
Figure 3.
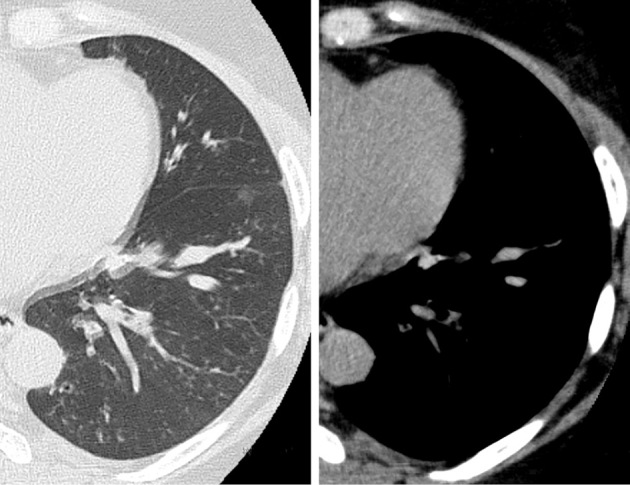
High-attenuation mucus plaque in the left lower lobe was detected in addition to bronchiectasis.
Figure 4.
A bronchoscopic examination showed a mucous plug in the left lower lobe bronchus (B8, B9, and B10). Although Aspergillus fumigatus was not cultured in sucking sputum or brushing samples, Charcot-Leyden crystal was detected on a cytological examination.
The patient had a history of bronchial asthma with eosinophilia. She also had elevated specific IgE antibody levels for A. fumigatus and serum IgE antibody levels. Her chest CT findings were consistent with those of ABPA, and the precipitation antibody reaction test against A. fumigatus returned a positive result (the results of the Aspergillus precipitation antibody reaction test performed at the Medical Mycology Research Center, Chiba University were positive). Although she had already started a regimen of inhaled corticosteroids, her peripheral blood eosinophil count was elevated in the absence of systemic corticosteroid administration. Therefore, we diagnosed her with ABPA according to the criteria established by the International Society for Human Animal Mycology (ISHAM), even though her total IgE level was <1,000 IU/mL (7). Following an environmental survey, A. fumigatus was cultured in a swab sample obtained from a bedroom (back of the closet) and dressing room in her home, suggesting that continuous inhalation of Aspergillus was responsible for the development of ABPA.
We first proposed treatment with systemic corticosteroids as a standard therapy for ABPA. However, the patient wanted to avoid the use of systemic corticosteroids due to concerns about adverse events, such as central obesity and moon face, which were cosmetically problematic for her. In addition, her serum hemoglobin A1c level was also mildly elevated (Table). Therefore, we decided to treat her with benralizumab using the following regimen: 30 mg subcutaneously every 4 weeks for the first 3 doses, followed by administration every 8 weeks.
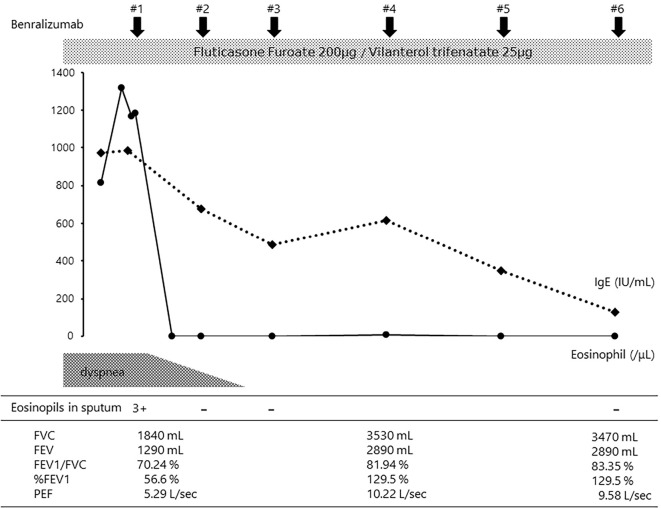
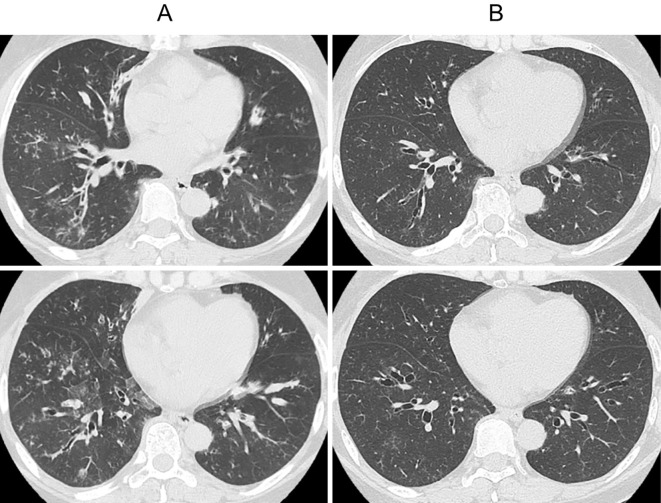
The benralizumab treatment was started on the eighth day of hospitalization. The patient's peripheral blood eosinophil count immediately decreased to almost 0 /μL within several days, and her subjective symptoms, including the wet cough and exertional dyspnea, disappeared. Her total serum IgE level also decreased and was within the normal range after the fourth dose of benralizumab. Eosinophils were not observed in the patient's sputum two weeks after the first dose of benralizumab (Fig. 5). Chest CT also showed significant improvements in the bronchial wall thickening and ground-glass attenuation associated with chronic bronchitis. The mucus plaque also disappeared after three cycles of benralizumab treatment (Fig. 6). The FVC, FEV1.0, %FEV1.0, and PEF values were significantly improved compared with those before the treatment.
Figure 5.
Clinical course after admission to our hospital. Benralizumab treatment led to a dramatic improvement in sputum and the peripheral blood eosinophil count. The total serum IgE level gradually decreased to within the normal range. As a result, the symptoms of exertional dyspnea immediately improved. Improvement in the respiratory function was also recognized.
Figure 6.
Computed tomography images of the chest before (A) and after three cycles of benralizumab (B). Bronchial wall thickening and ground-glass attenuation were significantly improved by benralizumab. The mucus plaque also disappeared.
The patient has been maintained on a regimen of benralizumab and is being followed up at our outpatient clinic regularly. She currently performs full daily work without exhibiting any respiratory symptoms.
Discussion
The present report presents the details of a case of ABPA complicated with asthma that developed probably due to continuous fungal exposure caused by disastrous heavy rainfall and was successfully treated with benralizumab. In July 2018, the patient survived the disastrous heavy rainfall that occurred in Western Japan, but her home and workplace were flooded. None of her respiratory symptoms occurred before the event; therefore, we suspected the possibility of ABPA due to increased fungal exposure associated with the disaster. The patient was considered to have bronchial asthma characterized by type 2 inflammation. We speculated that the increased frequency of exposure to Aspergillus sp. caused the secretion of IL-4 and IL-5 and the production of IgE and eosinophils, resulting in mucus production, deterioration of airway hyperresponsiveness, and smooth muscle hyperplasia (4,8). As a result, her asthmatic symptoms worsened.
Disaster-related pneumonia includes cases that occur during the time of a disaster as well as during the subsequent recovery work. Tsunami lung occurs when people are swept by tsunami waves and is caused by the aspiration of saltwater and soil containing microbes. For example, there was a report of infectious diseases caused by Aspergillus inhalation due to the tsunami that occurred after the Great East Japan Earthquake on March 11, 2011 (9). Regarding the disastrous heavy rainfall that occurred in Western Japan in 2018, Oda et al. reported that Legionella pneumonia occurred during recovery work, possibly caused by exposure to water and soil after the flood disaster (5). In addition, Oshikata et al. reported a case of allergic bronchopulmonary mycosis that developed due to exposure to Eurotium herbariorum after the Great East Japan Earthquake, in which mycofloral surveillance was performed and high counts of Eurotium were detected (6). The present case seems to be similar and interesting in that the presence of the antigen was proven through an environmental survey. Indoor mold levels typically increase after natural disasters, flooding, and water damage. Therefore, it is important to recognize the risk of disaster-related pulmonary disease, and individuals who have bronchial asthma should avoid microbial exposure if they are victims of a natural disaster.
At present, there are several immunomodulatory biologic agents approved and available for the treatment of severe bronchial asthma. These agents include omalizumab (anti-IgE monoclonal antibody), dupilumab (anti-IL-4 receptor alpha monoclonal antibody), mepolizumab (anti-IL-5 monoclonal antibody), reslizumab (anti-IL-5 receptor monoclonal antibody), and benralizumab (10). Omalizumab has been used in the treatment of ABPA (11). However, it was reported that some patients did not respond to omalizumab properly (12). In contrast, five ABPA cases reported in a published series were successfully treated with mepolizumab. All of those patients were pretreated with systemic corticosteroids, which could not be tapered or stopped due to recurrent disease. Interestingly, two of the patients in those cases were administered omalizumab followed by mepolizumab. Altman et al. reported that combination therapy with omalizumab and mepolizumab improved ABPA, suggesting that together, they might have a synergistic effect. In that report, omalizumab led to a reduction in the use of corticosteroids, inhibited the recurrence of ABPA, and improved respiratory symptoms. However, improvement of oxygenation and CT findings was not achieved with omalizumab alone (13). In addition, Hirota et al. reported that, in a patient with ABPA, radiological improvement and the improvement of coexisting eosinophilic sinusitis and otitis media was unable to be obtained with omalizumab (14), suggesting that the potential efficacy of omalizumab for ABPA is probably inferior to that of mepolizumab.
Benralizumab is a humanized anti-IL-5 receptor alpha antibody that binds to the IL-5 receptor-alpha-chain of eosinophils, blocks the binding of IL-5 to eosinophils, and inhibits the action of IL-5 (8). In addition, it binds with high affinity to the RIIIa region of the Fcγ receptor found on NK cells, macrophages, and neutrophils, thus strongly inducing the ADCC of eosinophils and basophils (15,16). Acting directly on a specific cell receptor instead of indirectly through a mediator enables the more effective depletion of target cells. Thus, benralizumab induces fast and almost complete depletion of eosinophils at a rate exceeding 95%, which is higher than the rates of depletion induced by mepolizumab (84%) and reslizumab (82%) (16). Recent studies have shown that luminal eosinophils undergo cytolytic extracellular trap cell death (ETosis) and release filamentous chromatin fibers by direct interaction with A. fumigatus (2). This specific form of cell death may induce the release of toxic granule proteins and damage-associated molecular patterns, cause epithelial damage, and further reduce mucus clearance. Thus, ETosis is considered to contribute to the formation of mucoid impaction in ABPA. The inhibition of eosinophilic activation by targeting the IL-5 receptor is therefore considered a more reasonable strategy than administering omalizumab for ABPA.
To our knowledge, there are only a few case reports on the efficacy of benralizumab for ABPA (3,17,18). In the case reported by Soeda et al., the patient had ABPA exacerbation in addition to bronchial asthma and was treated with benralizumab (3). The therapy achieved not only symptomatic relief but rapid and dramatic improvement of radiological findings and a depletion of the peripheral eosinophil count as well. In addition, the patient in that case was worried about the adverse events of systemic corticosteroids and itraconazole and refused to be treated with them. Similarly, the patient in the present case wanted to avoid using systemic corticosteroids due to her history of diabetes and obesity. In fact, our patient experienced no intolerable adverse events and her chest CT findings, pulmonary function, and symptoms remarkably improved. We selected benralizumab rather than mepolizumab in the present case. This was because benralizumab seemed to have be more suitable for this patient in an outpatient setting, as it could be administered every eight weeks following the first three doses, whereas mepolizumab required administration every four weeks. We believe that benralizumab is useful, tolerable, and worthwhile for the treatment of ABPA.
In conclusion, we successfully managed an interesting case in which the introduction of benralizumab was dramatically effective for treating ABPA complicated with asthma following disastrous heavy rainfall in Western Japan in 2018. Individuals with bronchial asthma may develop ABPA during the recovery phase of natural disasters. In addition, the administration of benralizumab seems to be a viable alternative therapeutic strategy to systemic corticosteroids for the treatment of ABPA. Further investigations are warranted to confirm the usefulness of benralizumab for treating ABPA.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We are grateful to Dr. Katsuhiko Kamei (Medical Mycology Research Center, Chiba University) for examining the Aspergillus precipitation antibody reaction test.
References
- 1.Oguma T, Taniguchi M, Shimoda T, et al. Allergic bronchopulmonary aspergillosis in Japan: a nationwide survey. Allergol Int 67: 79-84, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Ueki S, Hebisawa A, Kitani M, Asano K, Neves JS. Allergic bronchopulmonary aspergillosis-a luminal hypereosinophilic disease with extracellular trap cell death. Front Immunol 9: 2346, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soeda S, Kono Y, Tsuzuki R, et al. Allergic bronchopulmonary aspergillosis successfully treated with benralizumab. J Allergy Clin Immunol Pract 7: 1633-1635, 2019. [DOI] [PubMed] [Google Scholar]
- 4.Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med 377: 965-976, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Oda N, Hirahara T, Fujioka Y, Mitani R, Takata I. Legionella pneumonia following the heavy rain event of July 2018 in Japan. Intern Med 58: 2831-2834, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oshikata C, Watanabe M, Saito A, et al. Allergic bronchopulmonary mycosis due to exposure to Eurotium herbariorum after the Great East Japan Earthquake. Prehosp Disaster Med 32: 688-690, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Chakrabarti A, Shah A, et al. ABPA complicating asthma ISHAM working group. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 43: 850-873, 2013. [DOI] [PubMed] [Google Scholar]
- 8.Moss RB. The use of biological agents for the treatment of fungal asthma and allergic bronchopulmonary aspergillosis. Ann N Y Acad Sci 1272: 49-57, 2012. [DOI] [PubMed] [Google Scholar]
- 9.Kawakami Y, Tagami T, Kusakabe T, et al. Disseminated aspergillosis associated with Tsunami lung. Respir Care 57: 1674-1678, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Schoettler N, Strek ME. Recent advances in severe asthma. Chest 157: 516-528, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Der Ent CK, Hoekstra H, Rijkers GT. Successful treatment of allergic bronchopulmonary aspergillosis with recombinant anti-IgE antibody. Thorax 62: 276-277, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aydin Ö, Sözener Z.Ç, Soyyiğit Ş, et al. Omalizumab in the treatment of allergic bronchopulmonary aspergillosis: one center's experience with 14 cases. Allergy Asthma Proc 36: 493-500, 2015. [DOI] [PubMed] [Google Scholar]
- 13.Altman MC, Lenington J, Bronson S, Ayars AG. Combination omalizumab and mepolizumab therapy for refractory allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol Pract 5: 1137-1139, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Hirota S, Kobayashi Y, Ishiguro T, et al. Allergic bronchopulmonary aspergillosis successfully treated with mepolizumab: case report and review of the literature. Respir Med Case Rep 26: 59-62, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghazi A, Trikha A, Calhoun WJ. Benralizumab - a humanized mAb to IL-5Rα with enhanced antibody-dependent cell-mediated cytotoxicity - a novel approach for the treatment of asthma. Expert Opin Biol Ther 12: 113-118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González ID, Benítez FM, Quirce S. Benralizumab: a new approach for the treatment of severe eosinophilic asthma. J Investig Allergol Clin Immunol 29: 84-93, 2019. [DOI] [PubMed] [Google Scholar]
- 17.Bernal-Rubio L, de-la-Hoz Caballer B, Almonacid-Sánchez C, González-de-Olano D. Successful treatment of allergic bronchopulmonary aspergillosis with benralizumab after no response to omalizumab. J Investig Allergol Clin Immunol 30: 378-379, 2020. [DOI] [PubMed] [Google Scholar]
- 18.Tomomatsu K, Sugino Y, Okada N, Tanaka J, Oguma T, Asano K. Rapid clearance of mepolizumab-resistant bronchial mucus plugs inallergic bronchopulmonary aspergillosis with benralizumab treatment. Allergol Int 2020 in press. [DOI] [PubMed] [Google Scholar]