Abstract
Background and aims:
No consensus exists on how aggressively to treat relapsing–remitting multiple sclerosis (RRMS) nor on the timing of the treatment. The objective of this study was to evaluate disability trajectories in RRMS patients treated with an early intensive treatment (EIT) or with a moderate-efficacy treatment followed by escalation to higher-efficacy disease modifying therapy (ESC).
Methods:
RRMS patients with ⩾5-year follow-up and ⩾3 visits after disease modifying therapy (DMT) start were selected from the Italian MS Registry. EIT group included patients who received as first DMT fingolimod, natalizumab, mitoxantrone, alemtuzumab, ocrelizumab, cladribine. ESC group patients received the high efficacy DMT after ⩾1 year of glatiramer acetate, interferons, azathioprine, teriflunomide or dimethylfumarate treatment. Patients were 1:1 propensity score (PS) matched for characteristics at the first DMT. The disability trajectories were evaluated by applying a longitudinal model for repeated measures. The effect of early versus late start of high-efficacy DMT was assessed by the mean annual Expanded Disability Status Scale (EDSS) changes compared with baseline values (delta-EDSS) in EIT and ESC groups.
Results:
The study cohort included 2702 RRMS patients. The PS matching procedure produced 363 pairs, followed for a median (interquartile range) of 8.5 (6.5–11.7) years. Mean annual delta-EDSS values were all significantly (p < 0.02) higher in the ESC group compared with the EIT group. In particular, the mean delta-EDSS differences between the two groups tended to increase from 0.1 (0.01–0.19, p = 0.03) at 1 year to 0.30 (0.07–0.53, p = 0.009) at 5 years and to 0.67 (0.31–1.03, p = 0.0003) at 10 years.
Conclusion:
Our results indicate that EIT strategy is more effective than ESC strategy in controlling disability progression over time.
Keywords: big data, disability trajectories, disease registry, multiple sclerosis
Introduction
The most important goal of multiple sclerosis (MS) therapy is prevention of long-term disability accumulation. 1 The therapeutic scenario for relapsing–remitting MS (RRMS) has widely expanded during the past 20 years. Choosing the MS therapy has become increasingly complex, due to the difficulties in weighing the risk/benefit ratio of several different available disease modifying therapies (DMTs). To date, except for individuals expressing poor clinical and radiological features at baseline, the most applied treatment algorithm for early naïve MS is based on an escalation strategy. 2 According to this approach, patients start with safe moderate-efficacy DMTs and switch to high-efficacy immunotherapies with more complex safety profiles in the case of first treatment failure. The superiority of high-efficacy DMTs, such as natalizumab, alemtuzumab, ocrelizumab, cladribrine, mitoxantrone or fingolimod, in reducing measures of clinical and MRI disease activity in comparison with the traditional first-line MS therapies, such as interferon (IFN) beta, glatiramer acetate (GA) or teriflunomide, have been consistently proven by different randomized clinical trials (RCTs)3–7 and/or observational studies.8,9 Moreover, indirect comparisons from extension arms and subgroup analyses of randomized trials suggest that high-efficacy therapies are associated with improved control of relapse activity when initiated earlier after MS onset. 10
Whether patients initiating therapy with high-efficacy DMTs derive a greater long-term benefit on disability accumulation than those who start with moderate-efficacy agents remains a matter of debate.
Recent observational studies showed evidence that early initiation of highly effective therapy in RRMS may provide more benefit that an escalation approach in decreasing the risk of developing secondary progression and disability accrual, at least in a medium term of 5–6 years of follow-up.11–14
In this study, we compared the long-term effect of an early versus a late start (escalation approach) of high-efficacy DMTs on disability trajectories in a large population of naïve RRMS who started the first treatment within the first year from the disease onset and were longitudinally followed up to 10 years.
Methods
Study design
In this retrospective observational cohort study, patient data were prospectively collected from the Italian MS Register (IMSR). 15
Data extraction was executed in November 2019. The IMSR was approved by the ethical committee at the “Azienda Ospedaliero – Universitaria – Policlinico of Bari” (Study REGISTRO SM001 – approved on 8 July 2016) and by local ethics committees in all participating centers. Patients signed an informed consent that allows to use their clinical data for research purposes. According to the Registry rules, on 5 February 2018, the Scientific Committee of the Italian MS Registry granted the approval to conduct this project and extract and use the registry data.
Population
Patients with relapsing onset MS, 16 with a follow-up duration of at least 5 years, a first visit within 3 years from disease onset and at least three Expanded Disability Status Scale (EDSS) score evaluations after the first DMT start were extracted from the IMSR database.
Furthermore, it was required that the treatment exposure to the first DMT was at least 2 years for natalizumab, fingolimod, IFN β products, GA, teriflunomide and dimethylfumarate; at least 3 months for mitoxantrone and at least two courses of alemtuzumab, ocrelizumab and cladribine.
Patients meeting the eligibility criteria were divided into two groups: the early intensive treatment (EIT) group if their first treatment was one of the six high-efficacy DMTs (natalizumab, alemtuzumab, mitoxantrone, fingolimod, cladribine or ocrelizumab) and the escalation treatment (ESC) group if their first therapy was a moderate-efficacy DMT (IFN beta, GA, teriflunomide, dimethylfumarate or azathioprine) followed by escalation to a high-efficacy DMT, due to a lack of efficacy, after at least 1 year of treatment.17,18
The data collection tool of the IMSR allows to enter “DMT stop cause” using different possibilities. In this study we selected patients who have discontinued the first DMT because of a “lack of efficacy”. This term can be used to identify both clinical relapse and evidence of inflammatory activity from magnetic resonance imaging (MRI) (T1 gadolinium-enhanced lesions and/or new or enlarging T2 lesions).
The decision about the first DMT’s prescription and how and when to switch was the responsibility of the treating neurologist at each MS center. DMT prescriptions have been made following national and European prescription labels.
Outcome
The main objective was to compare the effect of early (EIT) versus late (ESC) start of high-efficacy DMTs on disability trajectories measured by the mean annual EDSS changes compared with baseline values (delta-EDSS) up to 10 years of follow-up.
Statistical analysis
Categorical data were expressed as frequency and proportion. Continuous data were expressed as mean and standard deviation (SD).
To minimize the imbalance between the groups, patients were 1:1 propensity score (PS) matched for baseline covariates at the time of the first DMT start. The multivariable logistic regression model to estimate a patient’s probability of being assigned to the EIT group included the following covariates: sex, age, clinical onset (monofocal/multifocal), time to first DMT, relapses before DMT start (Yes/No), EDSS before DMT start, number of visits after DMT start. In order to achieve a better balance between the two groups, age and number of visits were included in the model using the logarithmic form of them.
A 5-to-1 greedy matching algorithm without replacement was used for each pairwise comparison to identify a unique matched control with delayed treatment for each early-treated patient. 19 The quality of the match in each pair of matched cohorts was assessed with absolute standardized mean difference (SMD). SMD less than 10% was considered acceptable.
Then, in the matched cohort, the follow-up time from the first DMT start was segmented into 6-month periods. The 6-month period is the best compromise between information loss and EDSS density. When no disability data were recorded, previously recorded disability score was carried out, until the last available EDSS score. The disability trajectories were evaluated by applying a linear mixed model for repeated measures (LMMRM) with an autoregressive variance-covariance structure. LMMRM with an autoregressive correlation-type matrix makes an assumption of missing at random and accounts for both missingness at random and potential correlation within participants, because it allows evaluation of all individuals, including participants with incomplete data. 20
The effect of early versus late start of high-efficacy DMT was assessed by the delta-EDSS in EIT and ESC groups.
In addition, the effect sizes (ESs) were calculated to estimate the magnitude of effect (clinical or practical relevance) of the delta-EDSS between EIT and ESC groups (from very small to very large). 21 All statistical analyses were evaluated at two-tailed α = 0.05. Analyses was performed using R version 3.2.0 and SAS version 9.4 (SAS Institute Inc.).
Results
Longitudinal clinical data of 53,010 patients from 89 MS centers were available in the IMSR at the time of data extraction. After applying the inclusion and the exclusion criteria we retrieved 2702 RRMS patients from 62 Italian MS centers. In this cohort, 365 patients received a treatment classifiable as EIT approach, while a larger sample of 2337 patients was treated according to the ESC strategy. The flowchart which describes the patient selection procedure is reported in Figure 1. The PS matching procedure produced 363 pairs of patients (Figure 1).
Figure 1.
Flowchart of the patient selection procedure.
EDSS, Expanded Disability Status Scale; EIT, early intensive treatment; ESC, escalation approach; MS, multiple sclerosis; PS, propensity score; RR, relapsing–remitting; RRMS, relapsing–remitting MS.
The demographic and disease characteristics of eligible patients before and after the PS matching are shown in Table 1. Before the PS matching, a significant imbalance was found between EIT and ESC groups in terms of age at the first DMT prescription, time from disease onset to the first DMT prescription (disease duration), baseline EDSS and frequency of EDSS evaluations during the follow-up after the first DMT start. Patients of the EIT group were older at the first DMT [mean (SD) age years: 31.13 (10.06) versus 29.37 (9.22), SMD = 18.19], with a shorter disease duration at the first DMT [time in months: 12.69 (9.61) versus 14.04 (9.64), SMD = −14.10], more disabled [mean (SD) EDSS: 2.63 (1.60) versus 1.85 (1.26), SMD = 54.60] and had a lower number of EDSS evaluations during the follow-up from the first DMT [21.99 (15.71) versus 24.43 (16.75), SMD = 15.01] than patients in ESC group. After PS matching, no variable exhibited a significant imbalance, as is shown in Table 1. In fact, the SMD was within the interval of ± 10 for all the matching covariates.
Table 1.
Comparison of clinical and demographic features between ESC and EIT groups before and after PS matching.
| Variable | Before PS matching | After PS matching | ||||
|---|---|---|---|---|---|---|
| ESC n = 2337 |
EIT n = 365 |
SMD | ESC n = 363 |
EIT n = 363 |
SMD | |
| Female sex, n (%) | 1541 (65.94) | 240 (65.75) | −0.39 | 222 (61.16) | 240 (66.12) | 10.32 |
| Age at first DMT, mean (SD), years | 29.37 (9.22) | 31.13 (10.06) | 18.19 | 30.28 (9.26) | 31.04 (10.02) | 7.84 |
| Time to first DMT, mean (SD), months | 14.04 (9.64) | 12.69 (9.61) | −14.10 | 12.92 (9.74) | 12.73 (9.61) | −1.87 |
| EDSS at the DMT start, mean (SD) | 1.85 (1.26) | 2.63 (1.60) | 54.60 | 2.63 (1.54) | 2.61 (1.57) | −1.24 |
| Number of EDSS evaluations from the first DMT, mean (SD) | 24.43 (16.75) | 21.99 (15.71) | −15.01 | 22.24 (15.03) | 22.05 (15.72) | −1.24 |
| Number of patients with relapses in the last 2 years before DMT start, mean (SD) | 2007 (85.88) | 315 (86.30) | - | 308 (84.85) | 313 (86.23) | - |
| Onset type, mean (SD) | ||||||
| Monofocal | 1992 (85.24) | 315 (86.30) | 3.05 | 296 (81.54) | 314 (86.50) | 13.56 |
| Multifocal | 345 (14.76) | 50 (13.70) | - | 67 (18.46) | 49 (13.50) | - |
DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; EIT, early intensive treatment; ESC, escalation approach; N, number; PS, propensity score; SD, standard deviation; SMD, standardized mean difference.
The median [interquartile range (IQR)] follow-up time of the whole post-matched sample (n = 726) after the first visit was 8.5 (6.5–11.7) years.
Tables 2 and 3 report the distribution of the DMTs in the two comparison groups. The patients in the ESC group started their treatment history with one of following DMTs: IFN beta 1a intramuscular (i.m.) (n = 72, 19.83%), IFN beta 1a subcutaneous (s.c.) (n = 162, 44.63%), IFN beta 1b s.c. (n = 71, 19.56%), GA (n = 33, 9.09%), azathioprine (n = 25, 6.89%). All of these patients escalated to a higher-efficacy DMT after a median (IQR) time of 6.3 (3.1–8.4) years, and remained exposed to these high efficacy DMTs for a median (IQR) time of 4.0 (1.0–5.7) years. These patients escalated to alemtuzumab (n = 6, 1.65%), fingolimod (n = 141, 38.84%), natalizumab (n = 156, 42.98%), mitoxantrone (n = 51, 14.05%), ocrelizumab (n = 8, 2.20%), cladribine (n = 1, 0.28%) Table 2. Patients of the EIT group were exposed to fingolimod (n = 64, 17.63%), natalizumab (n = 148, 40.77%), mitoxantrone (n = 141, 38.84%) and cladribine (n = 10, 2.75%); no one included in the study cohort received alemtuzumab and ocrelizumab. Applying the inclusion criteria and PS matching procedure, no patient of the ESC group with a treatment of dimethylfumarate or teriflunomide was selected, as no one received alemtuzumab or ocrelizumab in the EIT group of our study cohort. The median exposure time to these drugs was 7.2 (5.3–10.3) years in the EIT group.
Table 2.
Distribution of moderate-efficacy DMTs before the escalation and of high-efficacy DMTs after escalation in the ESC group after PS matching.
| First DMT (before the escalation) | Pts, n (%) |
|---|---|
| Interferon beta 1a i.m. | 72 (19.56) |
| Interferon beta 1a s.c. | 162 (44.63) |
| Interferon beta 1b s.c. | 71 (19.56) |
| Glatiramer acetate | 33 (9.09) |
| Azathioprine | 25 (6.89) |
| High-efficacy DMTs at the escalation | |
| Alemtuzumab | 6 (1.65) |
| Fingolimod | 141 (38.84) |
| Natalizumab | 156 (42.98) |
| Mitoxantrone | 51 (14.05) |
| Ocrelizumab | 8 (2.20) |
| Cladribine | 1 (0.28) |
DMT, disease-modifying therapy; ESC, escalation approach; i.m., intramuscular; n, number; PS, propensity score; Pts, patients; s.c., subcutaneous.
Table 3.
Distribution of high-efficacy DMTs in the EIT group after PS matching.
| First DMT | Pts, n (%) |
|---|---|
| Alemtuzumab | 0 (0.00) |
| Fingolimod | 64 (17.63) |
| Natalizumab | 148 (40.77) |
| Mitoxantrone | 141 (38.84) |
| Ocrelizumab | 0 (0.00) |
| Cladribine | 10 (2.75) |
DMT, disease-modifying therapy; EIT, early intensive treatment; PS, propensity score; Pts, patients.
Table 4 shows the estimated EDSS scores with relative confidence intervals at each follow-up year in the two compared groups, the number of patients in each group for whom an EDSS score was available and the mean estimated delta-EDSS score differences between the two groups. The estimated baseline EDSS with relative (95% confidence interval) value was 2.52 (2.33–2.71) in the ESC group and 2.45 (2.26–2.64) in the EIT group.
Table 4.
The estimated mean EDSS scores and the estimated mean delta-EDSS scores versus baseline in ESC and EIT groups at each follow-up year.
| Follow-up year | Estimated mean EDSS (95% CI) (n pts) | Estimated mean delta-EDSS scores (95% CI) versus baseline | p-value | Effect size | |
|---|---|---|---|---|---|
| ESC group | EIT group | ||||
| Baseline | 2.52 (2.33–2.71) (363) | 2.45 (2.26–2.64) (363) | 0.25 | ||
| 1 year | 2.53 (2.33–2.73) (250) | 2.36 (2.16–2.56) (265) | 0.10 (0.01–0.19) | 0.0286 | 0.13 |
| 2 years | 2.68 (2.48–2.88) (249) | 2.39 (2.19–2.59) (265) | 0.22 (0.08–0.36) | 0.0026 | 0.18 |
| 3 years | 2.81 (2.61–3.01) (234) | 2.53 (2.33–2.73) (258) | 0.21 (0.03–0.39) | 0.0193 | 0.13 |
| 4 years | 2.96 (2.76–3.16) (255) | 2.57 (2.37–2.77) (252) | 0.32 (0.12–0.52) | 0.0022 | 0.22 |
| 5 years | 3.09 (2.89–3.29) (238) | 2.72 (2.52–2.92) (239) | 0.30 (0.07–0.53) | 0.0095 | 0.26 |
| 6 years | 3.24 (3.04–3.44) (218) | 2.89 (2.69–3.09) (220) | 0.28 (0.03–0.53) | 0.026 | 0.21 |
| 7 years | 3.35 (3.15–3.55) (192) | 3.00 (2.79–3.21) (182) | 0.27 (0.00–0.54) | 0.0465 | 0.25 |
| 8 years | 3.59 (3.38–3.80) (169) | 2.88 (2.66–3.10) (144) | 0.64 (0.35–0.93) | <0.0001 | 0.33 |
| 9 years | 3.76 (3.54–3.98) (137) | 2.97 (2.74–3.20) (120) | 0.72 (0.40–1.04) | <0.0001 | 0.38 |
| 10 years | 3.81 (3.58–4.04) (118) | 3.07 (2.81–3.33) (86) | 0.67 (0.31–1.03) | 0.0003 | 0.36 |
CI, confidence interval; EDSS, Expanded Disability Status Scale; EIT, early intensive treatment; ESC, escalation approach; pts, patients.
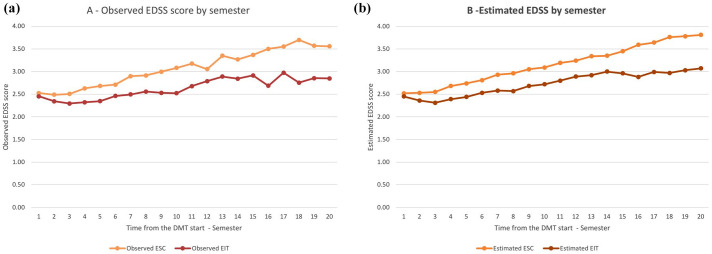
Mean estimated delta-EDSSs at each 12-month period were all significantly (p < 0.02) higher in the ESC group compared with the EIT group. In particular, the mean estimated delta-EDSS differences between the two groups tended to increase from 0.1 (0.01–0.19, p = 0.03) at 1 year, to 0.30 (0.07–0.53, p = 0.009) and to 0.64 (0.35–0.93, p < 0.001) at 5 and 8 years respectively, while at 10 years (the last year of study observation) it was 0.67 (0.31–1.03, p = 0.0003). The maximum mean delta-EDSS difference was 0.72 (0.40–1.04, p < 0.001) measured at 9 years. The ES for each of the yearly comparisons of the mean estimated delta-EDSS was little to moderate, but it increased over time up to 0.36 at year 10 of follow-up (Table 4). A graphical representation of the disability trajectories of the two compared groups over the 10 year follow-up is shown in Figure 2. Figure 2(a) shows the comparison between the disability trajectories of ESC and EIT groups built through the observed EDSS scores at each time-point; in Figure 2(b), the disability trajectories are instead built through the estimated EDSS scores at each time-point. Both the observed and estimated curves have a similar pattern over time and confirm the high goodness of fit of the model.
Figure 2.
Comparison of disability trajectories of the observed (a) and of the estimated (b) EDSS scores by semester between the ESC and EIT groups.
EIT, early intensive treatment; EDSS, Expanded Disability Status Scale; ESC, escalation approach.
Discussion
In this multicenter, observational, retrospective cohort study with prospectively acquired data, we evaluated the long-term disability trajectories in naïve RRMS patients who commenced their first therapy within 13 months from disease onset with high-efficacy DMTs according to the EIT strategy or with moderate-efficacy DMTs followed by escalation to high-efficacy DMTs according to the ESC approach. Applying a PS-based matching, we compared RRMS patients with an active disease (⩾1 relapse before the treatment start) and with a mild-to-moderate baseline EDSS score before the start of the first DMT [mean (SD) EDSS 2.63 (1.54) in the ESC group versus 2.61 (1.57) in the EIT group]. In our study, the EIT algorithm was strongly associated with lower disability progression, expressed by the increase of the 12-month EDSS score. This effect not only persisted but continued to increase over time despite all patients in the ESC group being escalated to a higher-efficacy DMT. In fact, over the entire duration of the observation period our results demonstrated that the yearly mean delta-EDSS remained significantly higher in patients in the ESC group compared with those receiving high-efficacy DMTs from the beginning of their therapy history. This could be due to a more potent effect on disability progression of the high-efficacy DMTs in the early years of the disease or alternatively to the longer exposure to them. 22 Although subgroup analyses and open-label extensions of RCTs on alemtuzumab, 10 cladribine, 23 natalizumab 24 and fingolimod 6 have suggested that they are more efficacious in terms of disease activity control and disability worsening in the case of earlier commencement, and data from observational studies have demonstrated that switching to a more potent agent can control the breakthrough disease in the case of an inadequate response to the first-line DMTs,25,26 no RCT has directly compared so far the impact of an early versus a delayed use of high-efficacy DMTs on the long-term MS disability outcomes.
Four recent real-world retrospective studies compared the ESC and the EIT strategies, suggesting an association of the latter strategy with a lower hazard of clinical relapses, a lower mean change in EDSS score at 5 years, a higher median time to sustained accumulation of disability and a lower risk of conversion to secondary progressive MS (SPMS), compared with initial treatment with first-line agents.12,13,22,27
In a real-life setting, a recent UK cohort study including 592 RRMS patients 13 reported 5-year change in EDSS score significantly lower in patients treated with an EIT first-line treatment strategy than in those who started their treatment history with a moderate-efficacy DMT. Median time to sustained accumulation of disability was longer for the EIT than for ESC group, but no difference was found between the ESC group who escalated to high-efficacy DMT as second-line treatment and EIT group. However, in that cohort 60% of those who escalated to high-efficacy DMTs were observed to develop disability accumulation while still receiving initial moderate-efficacy treatment before escalation.
In a cohort of 615 RRMS from MSBase registry, 12 235 patients with initial treatment with fingolimod, alemtuzumab or natalizumab, started after 6.5 years from onset, were associated with a lower risk of conversion to SPMS than PS-matched patients (n = 380) with initial treatment with GA or IFN beta started after 5.1 years from onset, in a median follow-up of 5.8 years. However, in the same paper, the authors reported that the probability of conversion to SPMS was lower when GA or IFN beta was started within 5 years of disease onset versus later, and when GA or IFN beta were escalated to fingolimod, alemtuzumab or natalizumab within 5 years versus later, in a median follow-up of 5.3 years. 12
In a retrospective international observational study on data from the MSBase registry and the Swedish MS registry, 22 213 patients who commenced high efficacy therapy (rituximab, ocrelizumab, mitoxantrone, alemtuzumab or natalizumab) early (0–2 years from onset) were PS matched to 253 who commenced high efficacy therapy later (4–6 years from onset) in the disease course. In the sixth year after disease onset, the mean EDSS score was significantly lower in the early group compared with late start group [2.2 (SD 1.6) versus 2.9 (SD 1.8), p < 0.0001]. This difference, evaluated by using a linear mixed-effects model, persisted throughout each year of follow-up until the 10th year after disease onset. It is noteworthy that patients in both groups could be treated with other DMTs before they were treated with high-efficacy therapy, and notably this study evaluated only 4 years of follow-up (from the sixth to the 10th year).
Finally, a Danish observational study proved a lower probability of a 6-month confirmed EDSS score worsening and of a first relapse over a follow-up of 4 years for 194 patients starting their therapy with high-efficacy DMTs compared with 194 PS matched patients starting with medium-efficacy DMTs. 27
Considering these evidences, the RRMS patients of our cohort early treated with stronger therapies experienced a slower disability progression versus the patients switching to them after a suboptimal response to moderately efficacious DMTs.
Unlike previous studies, we compared two groups of naïve RRMS patients starting their first DMT, early in the disease (within 13 months from the disease onset). Moreover, in our cohort all the patients in the ESC escalated to a high efficacy DMT during the follow-up. Another relevant methodologic aspect of our study is the longer follow-up period, including patients with a minimum follow-up of 5 years and obtaining a sample of subjects with a median follow-up of 8.5 (6.5–11.7) years.
Undoubtedly, it is necessary to strengthen these results with further studies, also in order to examine the data about the long-term safety since the concerns of severe adverse events of the higher-efficacy DMTs are remarkable. Two prospective, multicenter, randomized, pragmatic trials comparing early high-efficacy therapy with an escalation approach are ongoing: the TRaditional versus Early Aggressive Therapy for MS (TREAT-MS, ClinicalTrials.gov no. NCT03500328) and the Determining the Effectiveness of Early Intensive versus Escalation Approaches for the Treatment of RRMS (DELIVER-MS, ClinicalTrials.gov no. NCT03535298).
Some limitations of this study deserve discussion. First, as in many observational studies, also in our cohort the number of patients with available information decreases over time. After 10 years of follow-up EDSS score data were available for only 118 patients in the ESC group and 86 patients in the EIT group. However, the statistical model used allowed to include all patients and most importantly to assign the estimated EDSS score at each date-point, based on “within correlation” for each patient. Second, although the baseline MRI features are considered a crucial prognostic factor to guide the first treatment choice, we could not include MRI as covariate for the patient PS-matching procedure because of the lack of a systematic MRI acquisition and protocol analysis. However, it is important to consider that current clinical and MRI monitoring are mainly focused on revealing disease activity, so they are not sensitive to detect disability accumulation (the main outcome of our study) and, consequently, they are not responsive enough to trigger escalation.
Third, we used the EDSS changes over time as main outcome, therefore all the limitations of the EDSS apply to our study: the EDSS relies deeply on lower limb function, its sensitivity being relatively low relative to upper limb function and cognitive changes in advanced MS.
The real challenge for neurologists is to identify patients with poor prognosis factors 28 and choose for them an early intensive treatment in order to ensure the most efficacious therapy and the best clinical outcomes.
Conclusions
The results of our real-world study indicate that the long-term disability trajectories are more favorable with an EIT strategy than with moderate-efficacy DMTs followed by escalation to high-efficacy DMTs. Although further studies are necessary, especially to establish the long-term safety risks of the EIT approach, these findings may drive the treatment decisions of physicians, in particular in the cases of naïve patients with poor prognosis factors at the onset of disease.
Footnotes
Conflict of interest statement: All the authors report no competing interest related to this specific project. The authors report no conflicts of interest with respect to the contents of the current study, but note that the patients in the study were treated with a number of disease modifying drugs and that authors Pietro Iaffaldano, Giuseppe Lucisano, Francesca Caputo, Damiano Paolicelli, Francesco Patti, Mauro Zaffaroni, Vincenzo Brescia Morra, Carlo Pozzilli, Giovanna De Luca, Matilde Inglese, Giuseppe Salemi, Giorgia Teresa Maniscalco, Eleonora Cocco, Patrizia Sola, Giacomo Lus, Antonella Conte, Maria Pia Amato, Franco Granella, Claudio Gasperini, Paolo Bellantonio, Rocco Totaro, Marco Rovaris, Marco Salvetti, Valentina Liliana Adriana Torri Clerici, Roberto Bergamaschi, Davide Maimone, Elio Scarpini, Marco Capobianco, Giancarlo Comi, Massimo Filippi and Maria Trojano report having received advisory board membership, speaker’s honoraria, travel support, research grants, consulting fees, or clinical trial support from the manufacturers of those drugs, including Actelion, Allergan, Almirall, Bayer Schering, Biogen, Celgene, Excemed, Genzyme, Forward Pharma, Ipsen, Medday, Merck, Merz, Mylan, Novartis, Sanofi, Roche, Teva, and their local affiliates.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs: Iaffaldano P  https://orcid.org/0000-0003-2308-1731
https://orcid.org/0000-0003-2308-1731
Paolicelli D  https://orcid.org/0000-0002-8645-1763
https://orcid.org/0000-0002-8645-1763
Patti F  https://orcid.org/0000-0002-6923-0846
https://orcid.org/0000-0002-6923-0846
Pozzilli C  https://orcid.org/0000-0002-6360-4798
https://orcid.org/0000-0002-6360-4798
Contributor Information
Pietro Iaffaldano, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
Giuseppe Lucisano, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari Aldo Moro, Bari, Italy; CORESEARCH, Pescara, Italy.
Francesca Caputo, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
Damiano Paolicelli, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari Aldo Moro, Bari, Italy.
Francesco Patti, Dipartimento di Scienze Mediche e Chirurgiche e Tecnologie Avanzate, GF Ingrassia, Sez. Neuroscienze, Centro Sclerosi Multipla, Università di Catania, Catania, Italy.
Mauro Zaffaroni, Multiple Sclerosis Center, Hospital of Gallarate, ASST della Valle Olona, Gallarate (Varese), Italy.
Vincenzo Brescia Morra, Department of Neuroscience (NSRO), Multiple Sclerosis Clinical Care and Research Center, Federico II University, Naples, Italy.
Carlo Pozzilli, Department of Human Neuroscience, Multiple Sclerosis Center, S. Andrea Hospital, Rome, Italy.
Giovanna De Luca, Centro Sclerosi Multipla, Clinica Neurologica, Policlinico SS. Annunziata, Abruzzo, Chieti, Italy.
Matilde Inglese, Dipartimento Di Neuroscienze, Riabilitazione, Oftalmologia, Genetica E Scienze Materno – Infantili (DINOGMI), Universita’ di Genova, Genova, Liguria, Italy; Ospedale Policlinico San Martino, IRCCS, Genova, Italy.
Giuseppe Salemi, Department of Biomedicine, Neuroscience and Advanced Diagnostics, University of Palermo, Palermo, Sicilia, Italy.
Giorgia Teresa Maniscalco, Neurological Clinic and Multiple Sclerosis Center, A Cardarelli Hospital, Naples, Italy.
Eleonora Cocco, Department of Medical Science and Public health, Centro Sclerosi Multipla, University of Cagliari, Italy.
Patrizia Sola, Azienda Ospedaliera Universitaria di Modena/OCB, UO Neurologia, Italy.
Giacomo Lus, Multiple Sclerosis Center, II Division of Neurology, Department of Clinical and Experimental Medicine, Second University of Naples, Caserta, Campania, Italy.
Antonella Conte, Department of Human Neurosciences, Sapienza University of Rome, Italy; IRCCS Istituto Neurologico Mediterraneo (INM) Neuromed, Pozzilli, Italy.
Maria Pia Amato, Department NEUROFARBA, University of Florence, Firenze, Italy; IRCCS Fondazione Don Carlo Gnocchi, Florence, Italy.
Franco Granella, Unit of Neurosciences, Department of Medicine and Surgery, University of Parma, Parma, Emilia-Romagna, Italy.
Claudio Gasperini, Centro Sclerosi Multipla – Azienda Ospedaliera S. Camillo Forlanini, Rome, Italy.
Paolo Bellantonio, UOC di Neurologia, IRCCS Neuromed, Pozzilli (IS), Italy.
Rocco Totaro, Centro Malattie Demielinizzanti - Clinica Neurologica, Ospedale San Salvatore, L’Aquila, Abruzzo, Italy.
Marco Rovaris, Multiple Sclerosis Center, IRCCS Fondazione don Carlo Gnocchi ONLUS, Milan, Italy.
Marco Salvetti, IRCCS Istituto Neurologico Mediterraneo (INM) Neuromed, Pozzilli, Italy; CENTERS Centro Neurologico Terapie Sperimentali – Sapienza University, S. Andrea Hospital, Roma, Lazio, Italy.
Valentina Liliana Adriana Torri Clerici, Fondazione IRCCS Istituto Neurologico “C. Besta” U.O. Neuroimmunologia e Malattie Neuromuscolari, Italy.
Roberto Bergamaschi, IRCCS Mondino Foundation, Pavia, Lombardia, Italy.
Davide Maimone, Centro Sclerosi Multipla – UOC di Neurologia – ARNAS Garibaldi, Catania, Sicilia, Italy.
Elio Scarpini, Centro Sclerosi Multipla – UOSD Malattie Neurodegenerative – IRCCS Ospedale Maggiore Policlinico, Università Milano, Milano, Lombardia, Italy.
Marco Capobianco, Struttura Complessa Ospedaliera Neurologia & CRESM (Centro di Riferimento Regionale per la SM) – AOU San Luigi, Orbassano (Torino), Italy.
Giancarlo Comi, Institute of Experimental Neurology, IRCCS San Raffaele Hospital, Milan, Italy.
Massimo Filippi, Dipartimento di Neurologia, Neurofisiologia e Neuroriabilitazione, San Raffaele Scientific Institute, Vita-Salute San Raffaele University, Milan, Italy.
Maria Trojano, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University of Bari “Aldo Moro” Bari, Piazza G. Cesare, 11, Bari, 70124, Italy.
References
- 1. Trojano M, Tintore M, Montalban X, et al. Treatment decisions in multiple sclerosis-insights from real-world observational studies. Nat Rev Neurol 2017; 13: 105–118. [DOI] [PubMed] [Google Scholar]
- 2. Fernández O, Delvecchio M, Edan G, et al. Survey of diagnostic and treatment practices for multiple sclerosis in Europe. Eur J Neurol 2017; 24: 516–522. [DOI] [PubMed] [Google Scholar]
- 3. Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 2006; 354: 911–923. [DOI] [PubMed] [Google Scholar]
- 4. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 5. Edan G, Comi G, Le Page E, et al. Mitoxantrone prior to interferon beta-1b in aggressive relapsing multiple sclerosis: a 3-year randomised trial. J Neurol Neurosurg Psychiatry 2011; 82: 1344–1350. [DOI] [PubMed] [Google Scholar]
- 6. Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010; 362: 402–415. [DOI] [PubMed] [Google Scholar]
- 7. Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017; 376: 221–234. [DOI] [PubMed] [Google Scholar]
- 8. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 2017; 16: 271–281. [DOI] [PubMed] [Google Scholar]
- 9. Kalincik T, Jokubaitis V, Spelman T, et al. Cladribine versus fingolimod, natalizumab and interferon β for multiple sclerosis. Mult Scler J 2018; 24: 1617–1626. [DOI] [PubMed] [Google Scholar]
- 10. Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 2011; 10: 338–348. [DOI] [PubMed] [Google Scholar]
- 11. Merkel B, Butzkueven H, Traboulsee AL, et al. Timing of high-efficacy therapy in relapsing-remitting multiple sclerosis: a systematic review. Autoimmun Rev 2017; 16: 658–665. [DOI] [PubMed] [Google Scholar]
- 12. Brown JWL, Coles A, Horakova D, et al. Association of initial disease-modifying therapy with later conversion to secondary progressive multiple sclerosis. JAMA 2019; 321: 175–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harding K, Williams O, Willis M, et al. Clinical outcomes of escalation vs early intensive disease-modifying therapy in patients with multiple sclerosis. JAMA Neurol 2019; 76: 536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Prosperini L, Mancinelli CR, Solaro CM, et al. Induction versus escalation in multiple sclerosis: a 10-year real world study. Neurotherapeutics 2020; 17: 994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Trojano M, Bergamaschi R, Amato MP, et al. The Italian multiple sclerosis register. Neurol Sci 2019; 40: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014; 83: 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler 2018; 24: 96–120. [DOI] [PubMed] [Google Scholar]
- 18. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis. Neurology 2018; 90: 777–788. [DOI] [PubMed] [Google Scholar]
- 19. Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med 2007; 26: 734–753. [DOI] [PubMed] [Google Scholar]
- 20. Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. New York, NY: Oxford University Press, 2009, pp.1–644. [Google Scholar]
- 21. Cohen J. A power primer. Psychol Bull 1992; 112: 155–159. [DOI] [PubMed] [Google Scholar]
- 22. He A, Merkel B, Brown JWL, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol 2020; 19: 307–316. [DOI] [PubMed] [Google Scholar]
- 23. Giovannoni G, Cook S, Rammohan K, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol 2011; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- 24. Hutchinson M, Kappos L, Calabresi PA, et al. The efficacy of natalizumab in patients with relapsing multiple sclerosis: subgroup analyses of AFFIRM and SENTINEL. J Neurol 2009; 256: 405–415. [DOI] [PubMed] [Google Scholar]
- 25. Kalincik T, Horakova D, Spelman T, et al. Switch to natalizumab versus fingolimod in active relapsing-remitting multiple sclerosis. Ann Neurol 2015; 77: 425–435. [DOI] [PubMed] [Google Scholar]
- 26. Baroncini D, Ghezzi A, Annovazzi PO, et al. Natalizumab versus fingolimod in patients with relapsing-remitting multiple sclerosis non-responding to first-line injectable therapies. Mult Scler 2016; 22: 1315–1326. [DOI] [PubMed] [Google Scholar]
- 27. Buron MD, Chalmer TA, Sellebjerg F, et al. Initial high-efficacy disease-modifying therapy in multiple sclerosis: a nationwide cohort study. Neurology 2020; 95: e1041–e1051. [DOI] [PubMed] [Google Scholar]
- 28. Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol 2019; 15: 287–300. [DOI] [PubMed] [Google Scholar]