Abstract
We describe a case of a 10-year-old immunocompetent girl with a left renal abscess due to Group C Salmonella (Salmonella serovar Oranienburg). Percutaneous drainage of the abscess was done. She also received 2 weeks of intravenous ceftriaxone, followed by 4 weeks of oral co-trimoxazole with resolution seen on ultrasound. A review of pediatric Salmonella renal abscesses is also presented.
Keywords: renal, abscess, Salmonella, urinary tract infection, pediatric, children
Introduction
Renal and perinephric abscesses are uncommon in children. 1 A review done by a pediatric hospital in Taiwan reported only 45 cases over 10 years, while renal abscesses occurred in only 0.4% of pediatric urinary tract infections (UTI) in a Greek hospital.2,3 Renal abscesses can originate via ascending infections from the urinary tract, inflammation, and infection near the kidney or hematogenous spread. 4 Patients usually present with symptoms similar to UTI, such as dysuria, increase in urinary frequency, costovertebral angle pain tenderness, fever, and elevated inflammatory markers. 5 The diagnosis of renal abscess requires ultrasound or computed tomography (CT) imaging of the kidney, ureters, and bladder. 6 Common organisms associated with renal abscesses include Staphylococcus aureus and Gram-negative organisms such as Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis.4,6
Non-typhoidal Salmonella (NTS) and Salmonella typhi are Gram-negative bacilli which belong to the order Enterobacterales. 7 NTS infection typically presents in the form of gastroenteritis (GE). NTS are rarely isolated from the urinary tract with or without concomitant GE.8,9 Reports of renal abscesses caused by NTS or Salmonella typhi are rare, usually occurring in patients with urologic abnormalities, immunosuppression, or diabetes.10-12 Renal abscesses are generally managed with antibiotics, with concomitant surgical intervention in some cases.10-14
Here, we report a rare case of an immunocompetent 10-year-old girl with Salmonella renal abscess confirmed by imaging and fluid culture. We also conducted a literature review of the management of pediatric Salmonella renal abscesses.
Case Presentation
A 10-year-old girl without significant past medical history presented with 1 week of fever and intermittent vomiting with 1 day of abdominal pain and localized discomfort over the left hypochondrium. Two weeks prior to this presentation, she had 4 days of fever with diarrhea and vomiting which resolved spontaneously. There was no recent history of trauma, sick contact, or travel history, except for a superficial scratch (<2 cm open wound) caused by a household cat 3 months prior, which healed without intervention.
On physical examination during admission, she was alert and non-toxic, had costovertebral angle tenderness on the left side, but did not report any dysuria or increase in urinary frequency. She was febrile at 38.5°C, with other vitals being stable. Laboratory evaluation revealed leukocytosis and elevated inflammatory markers (Table 1). Urinalysis done was unremarkable and no microorganism was isolated from her urine culture. She had microcytic hypochromic anemia and was subsequently diagnosed to have alpha 2—thalassemia minor during this admission.
Table 1.
Our Patient’s Baseline Laboratory Results That were Taken on Admission.
| Laboratory parameters | Results (normal values) | Urine components | Results |
|---|---|---|---|
| Urea, serum (mmol/L) | 3.1 (2.6-6.8) | Specific gravity, urine | 1.005 (1.016-1.022) |
| Sodium, serum (mmol/L) | 139 (138-145) | RBC microscopy | 0/uL (0-2) |
| Potassium, serum (mmol/L) | 3.8 (3.4-4.7) | WBC microscopy | 0/uL (0-4) |
| Bicarbonate, serum (mmol/L) | 23 (14-23) | Epithelial cell microscopy | 0/uL |
| Chloride, serum (mmol/L) | 103 (98-107) | Casts, urine | Nil |
| Creatinine, serum (mmol/L) | 36 (27-54) | Crystals, urine | Nil |
| C-reactive protein (CRP) (mg/L) | 62.5 (0-5) | Glucose, urine | Nil |
| Erythrocyte sedimentation rate (ESR) (mm/h) | 74 (3-15) | Protein, urine | 1+ |
| White blood cell count (WBC) (×109/L) | 18.23 (4-13.5) | Nitrite, urine | Nil |
| Neutrophil (%) | 55 | Leucocyte, urine | Nil |
| Neutrophil absolute (×109/L) | 10.03 (1.5-8) |
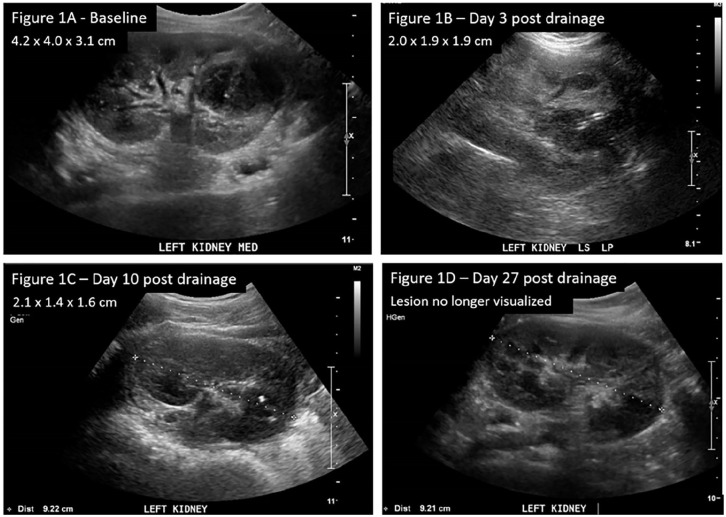
Due to the concerns of pyelonephritis or colitis, an ultrasound of the abdomen was ordered on the second day of admission. Imaging of both kidneys showed multiple cysts and a heterogeneous structure 4.2 cm × 4.0 cm × 3.1 cm (Figure 1A) extending from the left mid to the lower pole, consistent with a renal abscess, and much of it appeared liquefied. Her parents declined genetic testing for polycystic kidney disease and there was no known family history of kidney cysts.
Figure 1.
Ultrasound of kidneys, ureters, and bladder. (A) (Baseline) A heterogeneous structure noted in the left kidney extending from the mid to lower pole. This lesion is consistent with a renal abscess and much of it appears liquefied. (B) (Day 3 post drainage) Abscess decreased in size. (C) (Day 10 post drainage) Previously noted abscess smaller in size with catheter in situ. (D) (Day 27 post-drainage) Cystic lesion no longer visualized.
Intravenous (IV) gentamicin (5 mg/kg/day) was initiated on admission for concerns of pyelonephritis but was switched to IV ceftriaxone (100 mg/kg/day) after a renal abscess was detected, for better abscess penetration. Subsequently, an image-guided drainage of the renal abscess was done, where 15 mL of brown frank pus was aspirated and the drain was left in situ. The fluid culture revealed a group C Salmonella species (serotyped as Salmonella serovar Oranienburg by Singapore National Public Health Laboratory) that was susceptible to ampicillin, ceftriaxone, and co-trimoxazole. All other microbiological investigations including blood culture, repeat urine culture, acid-fast bacilli smear of the pus, Mycobacterium tuberculosis polymerase chain reaction, mycobacterial culture and fungal culture were unremarkable. A stool culture was not performed as her diarrhea resolved prior to admission. As group C Salmonella is an atypical cause of renal abscess, an immunological screen consisting of immunoglobulin levels and CD3, CD4, and CD8 T cells count was performed, which was unremarkable.
Serial kidney ultrasounds were done on day 3, day 10, and day 27 post-drainage. Her fever lysed by the second day post-drainage, and by day 3 post-drainage, the renal abscess decreased to 2.0 cm × 1.9 cm × 1.9 cm (Figure 1B). Her C-reactive protein normalized by day 10 post-drainage (4.1 mg/L), with a further decrease in renal abscess size to 2.1 cm × 1.4 cm × 1.6 cm (Figure 1C). Hence, the drain was removed. After receiving 2 weeks of intravenous ceftriaxone, she was discharged with another 4 weeks of oral co-trimoxazole (4 mg/kg/dose twice daily of trimethoprim component). By day 27 post-drainage, the abscess had resolved completely on the ultrasound scan (Figure 1D).
Discussion
Salmonella (NTS and typhoidal) infection commonly presents as gastroenteritis, invasive infection, or metastatic focal infection, but renal involvement, especially in the form of renal abscess, is rare.8-12,15 A retrospective review done in Spain found only 1.4% of all infections due to NTS presenting as UTI. 16 Characteristics of patients with NTS isolated from the urine are summarized in Table 2. Risk factors for renal abscess include previous urinary tract infection, urologic instrumentation or surgery, urinary tract obstruction, or an immunocompromized state. 17 To our knowledge, only 3 other cases of pediatric Salmonella (NTS and typhoidal) renal abscesses have been described,10,11,18 of which only 1 case had an existing congenital atrophy of the left kidney. 11 In a case series described by Paterson et al, 12 only 3 of 23 patients with NTS UTI had structural abnormalities, and none was immunosuppressed.
Table 2.
Characteristics of Patients with Non-Typhoidal Salmonella Isolated in Urine Cultures Described in Various Case Series.
| Study | Time period | Number of patients with NTS in urine | Mean age in years (age range) | Number of patients ≤18 years old | Most common serovar (n, %) | Medical history/risk factors (n, %) |
|---|---|---|---|---|---|---|
| Allerberger et al 19 | 1979-1989 | 30 | 52.6 (3-81 years) | 3 | S. typhimurium (n = 8, 26.7%), S. Enteritidis (n = 5, 16.7%), S. Agona (n = 3, 10%), S. Blockley (n = 1, 3%), S. Bovismorbificans (n = 1, 3%), S. Derby (n = 1, 3%), S. Java (n = 1, 3%), S. Manhattan (n = 1, 3%), S. Muenchen (n = 1, 3%), S. Newport (n = 1, 3%), S. Ohio (n = 1, 3%), S. Panama (n = 1, 3%), S. Sandiego (n = 1, 3%), S. Schwarzengrund (n = 1, 3%), Serotype not investigated (n = 3, 10%) | Significant underlying disease (n = 24, 80%), urologic conditions (n = 23, 76.7%) |
| Ramos et al 16 | 1978-1992 | 28 | 56 (22-82 years) | Nil | S. Enteritidis (n = 16, 57.1%), S. Typhimurium (n = 5, 17.9%), S. Derby (n = 2, 7.1%), S. Blockley (n = 1, 3.6%), S. California (n = 1, 3.6%), S. Heidelberg (n = 1, 3.6%), S. Ohio (n = 1, 3.6%), S. Tilburg (n = 1, 3.6%) | Severely immunocompromized (n = 17, 60.7%), urologic conditions (n = 14, 50%) |
| Paterson et al 12 | 1995-1996 | 23 | 35 (1-89) | 7 | S. Birkenhead (n = 3, 13%), S. Muenchen (n = 2, 8.7%), S. Virchow (n = 2, 8.7%), Others not stated explicitly | None had organ transplant, HIV infection, immunosuppression. Urologic conditions (n = 3, 13%) |
| Tena et al 8 | 1990-2005 | 19 | 62.5 (3-92) | 2 | S. Enteritidis (n = 16, 84.2%), S. Typhimurium (n = 3, 15.8%) | Diabetes Mellitus (n = 8, 42.1%), immunosuppressant treatment (n = 7, 36.8%), urologic conditions (n = 8, 42.1%) |
Our patient had gastrointestinal symptoms 2 weeks prior that had resolved by the time of admission. This might have been the origin of her Group C Salmonella infection. In addition, her suspected underlying polycystic kidney disease may be a possible risk factor for renal infections due to possible outflow obstruction causing retrograde infection from the urinary bladder.20-22 Although she was incidentally diagnosed to have alpha 2—thalassemia minor, minor forms of the thalassemia trait as a predisposing factor for Salmonella infections are not as well described as underlying established hemoglobinopathies, such as sickle cell anemia, thalassemia with high iron load, or previous splenectomy.23-28 Generally, NTS as a renal pathogen is unusual, and an occult urologic problem or an immunodeficiency should be considered. 16
Most reported pediatric renal abscess cases were managed with antibiotic therapy as a mainstay, and in some cases, with concomitant abscess drainage (Table 3). Common antibiotic therapy for renal pathogens includes third-generation cephalosporins, co-trimoxazole, fluoroquinolones, and aminoglycosides.29,30 However, aminoglycosides are not ideal for renal abscesses due to the anaerobic and acidic pH environment of the abscesses. 31 Antimicrobial therapy for our patient was empirically switched from gentamicin to ceftriaxone when a renal abscess was found, which also provided adequate coverage for Group C Salmonella when it was subsequently identified. In the setting of renal abscesses, third generation cephalosporins can be considered as empiric therapy.
Table 3.
Characteristics and Management of Pediatric Patients with Salmonella Renal Abscesses.
| Study | Age (years)/sex | Past medical history | Important labs | Salmonella serotype | Sensitivity | Source of isolate(s) | Abscess size (cm) | Antibiotic therapy | Invasive intervention | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Rus and Kersnik Levart 10 | 14/male | None | CRP (30 mg/L). ESR (74 mm/h). WBC (15.8 × 109/L). Blood urea nitrogen (44.9 mmol/L). Serum creatinine (425 µmol/L) | S. Enteritidis | [S] ampicillin, ceftriaxone, co-trimoxazole, ciprofloxacin | Urine culture | First scan – size not stated but imaging showed multiple renal abscesses. Second scan (during relapse) – multiple renal abscesses seen (largest 3.3 cm). | First course: Intravenous ceftriaxone for 12 days, followed by oral co-trimoxazole for 9 days. 2nd course: Intravenous ceftriaxone for 4 weeks, followed by oral ciprofloxacin for 2 weeks. | No | Initially cured but had relapse 13 days after initial treatment. Developed arterial hypertension after 6-month follow-up |
| Kaur et al 11 | 10/male | Congenital atrophy of left kidney | WBC (16.5 × 109/L). Blood urea (11.8 mmol/L). Serum creatinine (61.9 µmol/L) | S. Typhi | [S] ceftriaxone, cefoperazone-sulbactam, co-trimoxazole, [R] ciprofloxacin | Fluid culture | 4.2 cm × 3.1 cm | Intravenous cefoperazone-sulbactam 1 g TDS for 4 days, followed by 7 days of oral cefixime 200 mg BD. | Aspiration | Cure |
| D’Cruz et al 18 | 17/male | None | WBC (3.6 ×109/L) | S. Paratyphi A | [S] ceftriaxone, cefoperazone-sulbactam, ofloxacin, amikacin, gentamicin | Fluid culture | 2.5 × 2.6 cm | Amikacin and ofloxacin for 2 weeks, followed by 2 weeks of ofloxacin monotherapy | Aspiration | Cure |
| Our case | 10/female | Nil | CRP (62.5 mg/L). ESR (74 mm/h). WBC (18.23 × 109/L). Blood urea (3.1 mmol/L). Serum creatinine (23 µmol/L) | Group C Salmonella (Salmonella serovar Oranienburg) | [S] ampicillin, ceftriaxone, co-trimoxazole | Fluid culture | 4.2 × 4.0 × 3.1 cm | Intravenous ceftriaxone 100 mg/kg/day for 2 weeks, followed by 4 weeks of oral co-trimoxazole (8 mg/kg/day of trimethoprim twice daily) | Drainage | Cure |
[S], sensitive; [R], resistant.
The optimal criteria and timing for surgical intervention remains unknown. 6 Broad-spectrum parenteral antibiotic coverage and surgical drainage had been recommended due to paucity of evidence regarding safe and effective treatment with antibiotics alone in pediatrics, as compared to adult studies. 4 However, several recent case series on pediatric renal abscesses report success with conservative antibiotic treatment for smaller abscesses (ie, ≤3 cm in size).1,32,33 Our patient’s renal abscess measured 4.2 cm × 4.0 cm × 3.1 cm. The prompt resolution of our patient’s clinical symptoms on day 3 of admission after initiation of appropriate antibiotics and drainage suggests that surgical intervention is likely to be helpful, particularly if done early and in the setting of a relatively large renal abscess.
The optimal duration of antibiotic treatment for renal abscesses remains to be determined. In the treatment of acute lobar nephronia, which may represent early stage of development of renal abscess, 3 weeks of treatment had superior outcomes compared to 2 weeks.34,35 The need for prolonged antibiotic therapy was also suggested by Rus and Kersnik Levart 10 in a case of a previously healthy 14-year old with a NTS renal abscess complicated by acute renal failure who had a recurrence of infection. The patient was initially conservatively treated with 12 days of intravenous ceftriaxone followed by 9 days of co-trimoxazole. Although there was clinical response with normalization of laboratory parameters and a subsequent negative urine culture at the end of the first course, there was a recurrence 13 days after the completion of the initial treatment. He then completed 4 additional weeks of intravenous ceftriaxone followed by 2 weeks of ciprofloxacin. Both his kidneys showed signs of chronic pyelonephritis on ultrasound 3 years later. The authors then concluded that at least 6 weeks of antibiotic treatment should be considered.
Here, we took a more conservative approach by performing serial ultrasound imaging to ascertain radiological improvement before the child was discharged with oral antibiotics to complete the total 6-week course, to ensure therapeutic success. As there was complete resolution of the renal abscess in our patient by day 27 post-drainage, this suggests that a minimum of 4 weeks of antibiotics (inclusive of at least 2 weeks intravenous) is required, even with early surgical intervention.
Conclusions
In conclusion, renal abscesses in immunocompetent children caused by NTS are rare. A prolonged course of antibiotic therapy of at least 4 weeks with concomitant surgical intervention may be required for the treatment of a large renal abscess.
Footnotes
Author Contributions: CWMP: Contributed to the conception, acquisition, and analysis of data; drafted manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
XFVS: Contributed to the conception, acquisition, and analysis of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
CYC: Contributed to the acquisition and analysis of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
IG: Contributed to the acquisition and analysis of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
MM: Contributed to the acquisition and analysis of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
KN: Contributed to the acquisition and analysis of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
KQK: Contributed to the acquisition and analysis of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
NWHT: Contributed to the conception, acquisition, and analysis of data; critically revised manuscript; gave final approval; agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Ethical Approval and Informed Consent: Relevant data and material that was required for the write-up was obtained and included in the manuscript. Patient’s consent to participate and for publication was obtained. Centralised Institutional Review Board approval is not required for case reports.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Cedric Wei Ming Poh  https://orcid.org/0000-0002-6828-8392
https://orcid.org/0000-0002-6828-8392
References
- 1. Linder BJ, Granberg CF. Pediatric renal abscesses: a contemporary series. J Pediatr Urol. 2016;12:99. [DOI] [PubMed] [Google Scholar]
- 2. Cheng CH, Tsai MH, Su LH, et al. Renal abscess in children: a 10-year clinical and radiologic experience in a tertiary medical center. Pediatr Infect Dis J. 2008;27:1025-1027. [DOI] [PubMed] [Google Scholar]
- 3. Bitsori M, Raissaki M, Maraki S, et al. Acute focal bacterial nephritis, pyonephrosis and renal abscess in children. Pediatr Nephrol. 2015;30:1987-1993. [DOI] [PubMed] [Google Scholar]
- 4. Angel C, Shu T, Green J, et al. Renal and peri-renal abscesses in children: proposed physio-pathologic mechanisms and treatment algorithm. Pediatr Surg Int. 2003;19:35-39. [DOI] [PubMed] [Google Scholar]
- 5. Chen CY, Kuo HT, Chang YJ, et al. Clinical assessment of children with renal abscesses presenting to the pediatric emergency department. BMC Pediatr. 2016;16:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rubilotta E, Balzarro M, Lacola V, et al. Current clinical management of renal and perinephric abscesses: a literature review. Urologia. 2014;81:144-147. [DOI] [PubMed] [Google Scholar]
- 7. American Academy of Pediatrics. [Salmonella infections.] In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2018 Report of the Committee on Infectious Diseases. 31st ed. American Academy of Pediatrics; 2018:711-718. [Google Scholar]
- 8. Tena D, González-Praetorius A, Bisquert J. Urinary tract infection due to non-typhoidal Salmonella: report of 19 cases. J Infect. 2007;54:245-249. [DOI] [PubMed] [Google Scholar]
- 9. Gorelik Y, Paul M, Geffen Y, et al. Urinary tract infections due to nontyphoidal Salmonella. Am J Med Sci. 2017;353:529-532. [DOI] [PubMed] [Google Scholar]
- 10. Rus RR, Kersnik Levart T. Acute pyelonephritis with renal abscesses and acute renal failure after Salmonella infection. Acta Paediatr. 2010;99:470-473. [DOI] [PubMed] [Google Scholar]
- 11. Kaur A, Sarma S, Kumar N, et al. Renal abscess caused by Salmonella typhi. J Lab Physicians. 2015;7:121-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Paterson DL, Harrison MW, Robson JM. Clinical spectrum of urinary tract infections due to nontyphoidal Salmonella species. Clin Infect Dis. 1997;25:754. [DOI] [PubMed] [Google Scholar]
- 13. Olson ES, Asmussen T, Vicca AF, et al. A case report of renal abscess caused by Salmonella virchow phage type 1 associated with a papillary renal cell carcinoma. J Infect. 1999;38:56-57. [DOI] [PubMed] [Google Scholar]
- 14. Mokadem S, Nouioui MA, Kalai S, et al. Non typhoidal Salmonella Pyonephrosis in an asymptomatic immunocompetent patient. Case Rep Urol. 2019;2019:4198275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ochoa T, Santisteban-Ponce J. [Salmonella.] In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ, eds. Textbook of Pediatric Infectious Diseases. 8th ed. Elsevier Saunders; 2019:1066-1081. [Google Scholar]
- 16. Ramos JM, Aguado JM, García-Corbeira P, et al. Clinical spectrum of urinary tract infections due on nontyphoidal Salmonella species. Clin Infect Dis. 1996;23:388-390. [DOI] [PubMed] [Google Scholar]
- 17. Sobel J, Brown P. [Urinary tract infection.] In: Bennett JE, Dolin R, Blaser MJ, eds. Principles and Practice of Infectious Diseases. 9th ed. Elsevier Saunders; 2020:962-989. [Google Scholar]
- 18. D’Cruz S, Kochhar S, Chauhan S, et al. Isolation of Salmonella paratyphi A from renal abscess. Indian J Pathol Microbiol. 2009;52:117-119. [DOI] [PubMed] [Google Scholar]
- 19. Allerberger FJ, Dierich MP, Ebner A, et al. Urinary tract infection caused by nontyphoidal Salmonella: report of 30 cases. Urol Int. 1992;48:395-400. [DOI] [PubMed] [Google Scholar]
- 20. Schwab SJ, Bander SJ, Klahr S. Renal infection in autosomal dominant polycystic kidney disease. Am J Med. 1987;82:714-718. [DOI] [PubMed] [Google Scholar]
- 21. Sweet R, Keane WF. Perinephric abscess in patients with polycystic kidney disease undergoing chronic hemodialysis. Nephron. 1979;23:237-240. [DOI] [PubMed] [Google Scholar]
- 22. Tang RY, Cheong BM. Multiple bilateral renal abscesses in a previously healthy young patient. Med J Malaysia. 2017;72:250-251. [PubMed] [Google Scholar]
- 23. Krumme B, Conca W, Balle C, et al. Salmonella arthritis in a patient with severe lupus nephritis and thalassemia minor. Nephron. 1995;71:465-467. [DOI] [PubMed] [Google Scholar]
- 24. Behr MA, McDonald J. Salmonella neck abscess in a patient with beta-thalassemia major: case report and review. Clin Infect Dis. 1996;23:404-405. [DOI] [PubMed] [Google Scholar]
- 25. Rayan F, Mukundan C, Shukla DD. A case of relapsing Salmonella osteomyelitis in a thalassaemia trait patient. J Orthop Traumatol. 2009;10:31-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee CM, Lee MS, Yang TL, et al. Clinical features and risk factors associated with bacteremia of nontyphoidal salmonellosis in pediatric patients, 2010–2018. J Formos Med Assoc. 2020;120:196-203. [DOI] [PubMed] [Google Scholar]
- 27. Adeyokunnu AA, Hendrickse RG. Salmonella osteomyelitis in childhood. A report of 63 cases seen in Nigerian children of whom 57 had sickle cell anaemia. Arch Dis Child. 1980;55:175-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stephanie S, Schmalzle SA. Salmonella enterica serovar Typhi osteomyelitis in a young adult with sickle cell and thalassemia traits: a possible association. ID Cases. 2018;15:e00478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wagner C, Sauermann R, Joukhadar C. Principles of antibiotic penetration into abscess fluid. Pharmacology. 2006;78:1-10. [DOI] [PubMed] [Google Scholar]
- 30. Bamberger DM. Outcome of medical treatment of bacterial abscesses without therapeutic drainage: review of cases reported in the literature. Clin Infect Dis. 1996;23:592-603. [DOI] [PubMed] [Google Scholar]
- 31. Schlessinger D. Failure of aminoglycoside antibiotics to kill anaerobic, low-pH, and resistant cultures. Clin Microbiol Rev. 1988;1:54-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Seguias L, Srinivasan K, Mehta A. Pediatric renal abscess: a 10-year single-center retrospective analysis. Hosp Pediatr. 2012;2:161-166. [DOI] [PubMed] [Google Scholar]
- 33. Comploj E, Cassar W, Farina A, et al. Conservative management of paediatric renal abscess. J Pediatr Urol. 2013;9:1214-1217. [DOI] [PubMed] [Google Scholar]
- 34. Cheng CH, Tsau YK, Lin TY. Effective duration of antimicrobial therapy for the treatment of acute lobar nephronia. Pediatrics. 2006;117:e84-e89. [DOI] [PubMed] [Google Scholar]
- 35. Kaplan S L. [Renal abscess.] In: Cherry JD, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez PJ, eds. Textbook of Pediatric Infectious Diseases. 8th ed. Elsevier Saunders; 2019:408-411. [Google Scholar]