Abstract
Introduction:
Allergic and nonallergic hypersensitivity reactions to iodinated contrast media (ICM) and gadolinium-based contrast media are classified as immediate or non-immediate hypersensitivity reactions (IHR and NIHR), respectively. Skin tests and provocation tests are recommended for the evaluation of hypersensitivity reactions to contrast agents; however provocations are not common in clinical practice.
Methods:
A MEDLINE search was conducted to investigate studies comprising both skin tests and provocation tests that evaluated hypersensitivity reactions to ICM.
Results:
Nineteen studies were identified that reported on skin tests, followed by provocations. In the case of IHR to ICM, 65/69 (94%) patients with a positive skin test for the culprit media tolerated a challenge with a skin-test-negative alternative ICM. In IHR to ICM with a negative skin test for the culprit media, provocations were positive in 3.2%–9.1% patients. In the case of a NIHR to ICM with a positive skin test, provocation with a skin-test-negative agent was tolerated in 75/105 (71%) of cases. In NIHR with a negative skin test for the culprit agent, re-exposure to the culprit or an alternative was positive in 0%–34.6% patients. Provocations with the same ICM in skin test positive patients with IHR or NIHR were positive for a majority of the patients, although such provocation tests were rarely performed. Data on hypersensitivity reactions, skin tests and provocations with gadolinium-based contrast media were limited; however, they exhibited a pattern similar to that observed in ICM.
Conclusion:
In both ICM and gadolinium-based contrast media, the risk of an immediate repeat reaction is low when skin tests are negative. In contrast, a provocation with a skin-test-positive contrast medium showed a high risk of an immediate repeat hypersensitivity reaction. Therefore, a thorough medical history is necessary, followed by skin tests. A provocation is recommended, for diagnostic work-up, when the diagnosis is uncertain.
Keywords: allergy, contrast, gadolinium contrast media, hypersensitivity reaction, immediate hypersensitivity reaction, iodinated contrast media, non-immediate hypersensitivity reaction, provocation, skin test
Introduction
Iodinated contrast media (ICM) and gadolinium-based contrast media are essential for radiographic imaging in current medical practice. ICM is annually used in over 75 million procedures worldwide. 1 Following the introduction of the MRI, gadolinium-based MR agents have been used. 2
Hypersensitivity reactions may occur in patients upon administration of the contrast media. The first large prospective survey in 1975 on ICM-induced hypersensitivity reactions showed an overall incidence of contrast reactions in 2.33%–5.65% patients. 3 Recent numbers vary from 1% to 12% and severe reactions, mainly anaphylaxis, comprise 0.01% to 0.2% of all reactions.4–6 Hypersensitivity reactions were more frequently observed with high osmolar contrast agents than with low osmolar contrast agents, that is approximately 15% versus 3% respectively. This has led to the reduced use of high osmolar agents over the years. 7 Iodixanol (Visipaque®) and iohexol (Omnipaque®) are both low osmolar ICMs and are commonly used in clinical practice nowadays.
Moreover, severe reactions are also less common with the use of nonionic ICM than with ionic ICM. 8
Further, hypersensitivity reactions against gadolinium are less common, with an estimated prevalence of 0.07%–2.4%. 9
Reactions observed during or after administration of ICM and gadolinium-based contrast media are clinically divided into three categories: hypersensitivity reactions, pharmacological toxicity and events unrelated to contrast media exposure, including other allergens other than the contrast media.2,8 The term hypersensitivity is used to describe objectively reproducible symptoms or signs initiated by exposure to a defined stimulus (i.e. contrast agent), at a dose normally tolerated in people. 10 Hypersensitivity reactions can be an allergic hypersensitivity or a non-allergic hypersensitivity reaction. 10 Furthermore, hypersensitivity reactions are classified as either immediate (IHR) or non-immediate reactions (NIHR). 8 Immediate reactions to ICM or gadolinium-based contrast media occur within 1 h; however they have been reported to occur up to 6 h after exposure and are based on either IgE-mediated or non-IgE-mediated hypersensitivity. 11 The latter is thought to occur due to direct activation of basophilic granulocytes and mast cells because of the hyperosomolar nature of older types of radiocontrast media, or via complement anaphylatoxins C3a and C5a. 12 Although most IHRs are non-allergic, in case of a severe IHR to ICM the patient is more likely to have an IgE-mediated reaction. 8 It is important to note that hypersensitivity reactions in ICM are not due to hypersensitivity to iodine but to the chemical structure of ICM. For instance, there is no cross-reactivity between ICM hypersensitivity reactions and shellfish or povidone-iodine allergies.13,14 Lastly, in contrast to popular belief, clonal mast cell disorders are not a risk factor for radiocontrast media hypersensitivity, as previously stated in the AAAAI Work Group Report last year. 15
Non-immediate hypersensitivity allergic reactions are T cell-mediated type IV hypersensitivity reactions.8,16 NIHR can develop after 1 h or even after 7 days and can persist for 1–7 days. 8 The frequency of NIHR ranges from 0.5% to 23% for ICM. 17 The NIHRs to gadolinium are probably not common as there are only a few published cases. These reactions usually present with maculopapular exanthema. 8 Although rare, NIHR such as DRESS, Stevens-Johnson syndrome and toxic epidermal necrolysis have been reported. 8
To evaluate whether there is an allergic hypersensitivity reaction, skin prick tests (SPT) and intradermal tests (IDT) including non-immediate reading (after 48 h) should be performed, between 1 and 6 months after the hypersensitivity reaction.18–22 Drug provocation tests are recommended in addition to skin tests for hypersensitivity reactions to radiocontrast agents. 23 However, radiocontrast agent provocations are recommended based on a risk benefit analysis 11 and are suggested to confirm the tolerability of radiocontrast media after a very severe reaction in case of a negative skin test. 24 Provocations are not common in clinical practice and practical guidelines are lacking.
The aim of this literature review is to evaluate the added value of a provocation test, in addition to skin tests, for the assessment of immediate and non-immediate hypersensitivity contrast reactions. We performed extensive literature review and data analysis and prepared a flow chart, to optimize the diagnostic evaluation of hypersensitivity reactions to iodine and gadolinium-based contrast media, including provocation tests.
Methods
Review of the literature
A literature search was conducted using MEDLINE, which was finalised on 21st of December 2020. The title and abstract [tiab] were screened to identify studies addressing the value of the provocation test in addition to skin tests. The search terms used were as follows: (hypersensitivity [tiab] AND contrast [tiab] AND test [tiab]) OR (hypersensitivity [tiab] AND radiocontrast [tiab] AND test [tiab]). Further, the search was repeated using allergy instead of hypersensitivity.
Inclusion criteria
We included studies that described any type of skin tests as well as a provocation test or follow-up with a re-exposure to contrast media in the evaluation of an immediate hypersensitivity reaction (IHR) and/or a non-immediate hypersensitivity reaction (NIHR) for patients with a history of hypersensitivity reactions to contrast media.
Exclusion criteria
Research articles and reviews published in languages other than English were excluded. Studies reporting only skin tests without re-exposure to contrast media or a provocation test were excluded.
The titles and abstracts of the articles were screened by two independent reviewers (RB and SR) who applied the inclusion and exclusion criteria. Consensus was reached; therefore the opinion of a third reviewer was not required.
Analysis
Data from the included studies were assessed based on the results of the provocation tests or re-exposure of contrast media in relation to the skin tests (SPT, IDT, patch test) for contrast hypersensitivity. Provocation or re-exposure consisted of the (re-)introduction of the culprit or alternative radio-contrast agent in patients with a negative skin test, the introduction of a skin test negative radio-contrast agent in patients with positive skin tests or the reintroduction of a skin-test positive radio-contrast agent in patients with a positive skin test.
Hypersensitivity reactions were categorised as IHR and NIHR. The studies that included both IHR and NIHR were re-assessed and extracted as data for IHR and NIHR. The distinction between IHR and NIHR was based on the corresponding clinical presentation and results from the performed skin tests (SPT, IDT or patch test). Patch tests are generally performed to analyse non-immediate hypersensitivity reactions. Delayed readings were also reported for SPT and IDT which were then correlated with the clinical presentation.18,25,26
Studies were assessed based on the outcome of provocation or re-exposure to a radio-contrast agent.
The negative predictive value was calculated as follows:
the number of negative skin tests followed by a negative provocation/(number of negative skin tests followed by positive and negative provocations).
The positive predictive value was calculated as follows:
the number of positive skin tests followed by a positive provocation/(number of positive skin tests followed by positive and negative provocations).
Results
The literature search identified 508 studies, of which 19 studies were included, after screening the title and abstract, and applying the inclusion criteria (Figure 1). One study was excluded from the analysis as patients without previous contrast hypersensitivity reactions or previous exposure to contrast media were included. 27 Another study was excluded, as the results of the provocation data were combined for patients with a previous history of hypersensitivity reactions and controls without a previous contrast reaction. 28
Figure 1.
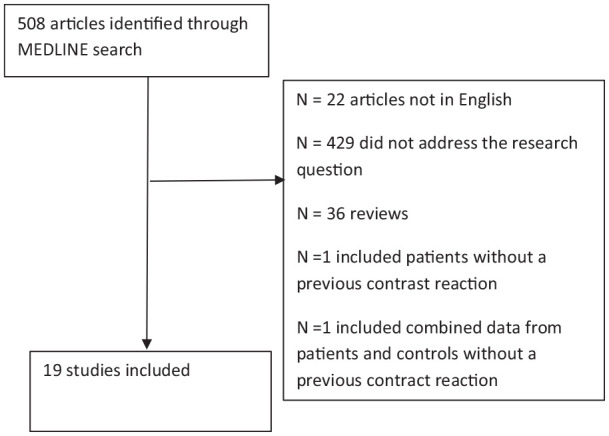
Flow-chart of study selection after application of search terms.
Fourteen studies reported on the outcomes of iodinated contrast-based reactions, four on gadolinium-based reactions and one published on both. Eleven out the 19 studies were retrospective studies. The results are shown in Tables 1 to 4.
Table 1.
Patient characteristics of included studies with ICM (n = 14 studies). Severity of symptoms was reported as mild, moderate or severe or grade I, II, III, IV, corresponding to increasing severity.
| Author | N | F/M | Age (median, range) or mean ± SD | IHR | NIHR | Unknown type of reaction | Symptoms/severity IHR: events | Symptoms/severity NIHR: (number of reactions) |
|---|---|---|---|---|---|---|---|---|
| Vernassiere et al.53 | 15 | 11/4 | 55.4 (37–78) | NA | 15 | – | NA | MPE: 5 |
| Macular rash: 5 | ||||||||
| Pruritus: 1 | ||||||||
| Pompholyx: 1 | ||||||||
| Erythema/edema: 3 | ||||||||
| Seitz et al. 25 | 32 | 17/15 | 48 (24–71) | NA | 32 | – | NA | Exanthema gr.I: 7 |
| Exanthema gr. II: 20 | ||||||||
| Exanthema gr. III: 5 | ||||||||
| Caimmi et al.54 | 120 | 75/45 | 56 (45–65) | 101/120 | 17 | 2 | Gr. I: 42 | Mild: 1 |
| Gr. II: 34 | Moderate: 16 | |||||||
| Gr. III: 20 | ||||||||
| Gr. IV: 5 | ||||||||
| Torres et al. 29 | 161 | 79/82 | 58.5 (IQR 48.85–66.5) | NA | 161 | – | NA | Mild: 16 |
| Moderate:143 | ||||||||
| Severe: 2 | ||||||||
| Salas et al. 30 | 90 | 63/27 | 54.50 ± 27 | 90 | NA | – | Gr. I: 69 | NA |
| Gr. II: 18 | ||||||||
| Gr. III: 3 | ||||||||
| Prieto-Garcia et al.55 | 106 | 64/42 | 56.7 ± 16.9 | 106 | NA | – | Gr. I: 66 | NA |
| Gr. II: 29 | ||||||||
| Gr. III: 11 | ||||||||
| Ahn et al. 21 | 23 | 13/10 | 48.6 ± 14.8 | 17 | 6 | – | Anaphylaxis: 10 | MPE: 23 |
| Urticarial: 7 | ||||||||
| Della-Torre et al. 31 | 36 | 27/9 | 58 (22–75) | 19 | 17 | – | Gr. I: 12 | Mild 16 |
| Gr. II: 3 | Moderate: 1 | |||||||
| Gr. III: 4 | ||||||||
| Sese et al.56 | 37 | 24/13 | 49.3 | 37 | NA | – | Gr. I: 26 | NA |
| Gr. II: 4 | ||||||||
| Gr. III: 7 | ||||||||
| Schrijvers et al. 18 | 597 | 406/191 | 60 (13–92) | 423 | 118 | 56 | Gr. I: 122 | Not severe: 109 |
| Gr. II: 104 | Severe: 9 | |||||||
| Gr. III + IV: 100 | ||||||||
| Trautmann et al. 26 | 45 | 30/15 | 55-58 (20–80) | 11/32 | 13 | – | Mild: 20 | MPE: 11 |
| Mod: 7 | Systemic: 1 | |||||||
| Severe: 5, | FDE: 1 | |||||||
| Kwon et al. 32 | 69 | 40/29 | 58.8 ± 10.9 | 69 | NA | – | Mild: 25 | NA |
| Mod: 5 | ||||||||
| Severe: 39 | ||||||||
| Meucci et al. 33 | 98 | 53/45 | 65.6 (23–90) | 82 | 16 | – | Gr. I: 47 | Mild: 15 |
| Gr. II:24 | Moderate: 1 | |||||||
| Gr. III: 10 | ||||||||
| Gr. IV: 0 | ||||||||
| Dona et al. 34 | 101 | 52/49 | 62 (IQR 49–69) | 12 | 89 | – | Gr. I: 7 | Maculopapular exanthema: 60 |
| Gr. II: 2 | ||||||||
| Gr.III:3 | Delayed urticaria:29 | |||||||
| Gr. IV:0 |
NA: not applicable; F: female; M: male; Gr: grade; MPE: maculopapular exanthema; FDE: fixed drug eruption; IQR: interquartile range.
Table 4.
Provocation/re-exposure to gadolinium contrast media (n = 4 studies).
| Study | Study design | N | Immediate reactions |
Non-immediate reaction |
Culprit skin test negative |
Culprit skin test positive |
Premedication administered prior to
provocation or re-exposure (%) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Positive SPT or IDT (%) | Positive patch test/SPT and/or IDT (%) | Positive provocation test with culprit or skin test negative alternative GCM (%) | Negative predictive value | Positive provocation with skin test negative GCM (%) | Negative predictive value | Positive provocation with skin test positive GCM | Positive predictive value | ||||
| Chiriac et al. 35 | P | 27 | 5/26 (19.2) | 0/1 (0) | 0/10 (0) | 1 | 0/1 (0) | 1 | – | NA | 3/6 (50) |
| Moulin et al. 36 | P | 1 | 1/1 | – | – | NA | 0/1 (0) | 1 | – | NA | NR |
| Kolenda et al. 37 | R | 33 | 19/33 (57.6) | – | 0/9 (0) | 1 | 0/11 (0) | 1 | – | NA | NR |
| Seta et al. 38 | R, I | 14 | 3/12 (25) | 0/1 (0) | 2/11 (18.2) | 0.82 | 0/1 (0) | 1 | – | NA | NR |
N: number of patients; P: prospective; R: retrospective; I: intervention; NA: not applicable; NR: not reported.
Repeating the search using the term ‘allergy’ instead of ‘hypersensitivity’ did not reveal any other study that fulfilled the inclusion criteria.
Iodinated contrast media
Seven studies included skin tests and provocation/re-exposure to ICM for both IHR and NIHR to ICM, four studies assessed IHR for ICM and three studies assessed NIHR for ICM (Table 1).
Standard pre-medication before provocation was administered in two studies.21,31 In another study pre-medication was used in a subgroup consisting of patients, including those with mast cell disorders or chronic urticaria who had negative skin tests, 18 Table 2.
Table 2.
Provocation/re-exposure of ICM in patients with an immediate hypersensitivity reaction (IHR) (n = 11 studies).
| Author | Study design | N (IHR) | Direct positive SPT or IDT (%) | Culprit skin test negative |
Culprit skin test positive |
Premedication administered prior to provocation or re-exposure | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive provocation test or re-exposure reaction to culprit or skin test negative alternative ICM (%) | Negative predictive value | Positive provocation test or exposure reaction to skin test negative ICM (%) | Negative predictive value | Positive provocation test or exposure reaction to skin test positive ICM (%) | Positive predictive value | |||||
| Caimmi et al.54 | R | 101 | 17/101 (16.8) | 1/23 (4.4) | 0.96 | 0/1 (0) | 1 | – | NA | None |
| Salas et al. 33 | P, I | 90 | 5/90 (5.6) | 3/74 (4.1) | 0.96 | 2/4 (50) a | 0.5 | – | NA | None |
| Prieto-Garcia et al.55 | P, I | 106 | 11/106 (10.4) | – | NA | 0/7 (0) | 1 | – | NA | None |
| Ahn et al. 21 | P | 17 | 11/17 (64.7) | – | NA | 0/2 (0) | 1 | 2/2 (100) | 1 | In all |
| Della-Torre et al. 31 | R | 19 | 7/19 (36.8) | 1/12 (8.3) | 0.92 | 0/7 (0) | 1 | – | NA | In all |
| Sesé et al.56 | R,I | 37 | 5/37 (13.5) | 1/31 (3.2) | 0.97 | 0/5 (0) | 1 | – | NA | None |
| Schrijvers et al. 18 | R | 423 | 56/423 (13.2) | 8/159 (5.3) b | 0.95 | 0/9 c (0) | 1 | – | NA | In 31/150 |
| Trautmann et al. 26 | R | 32 | 11/32 (34.4) | – | NA | 0/10 (0) | 1 | – | NA | None |
| Kwon et al. 32 | R | 69 | 38/69 (55.0 ) | 2/22 (9.1) | 0.91 | 0/11 (0) | 1 | 4/5 (80) | 0.8 | None |
| Meucci et al. 33 | R | 82 | 7/82 (8.5) | 3/75 (4) | 0.96 | 0/7 (0) | 1 | – | NA | None |
| Dona et al. 34 | P | 12 | 6/12 (50) | 6/6 (100) d | 0 | 2/6 (33) | 0.67 | – | NA | None |
N: number of patients; P: prospective; R: retrospective; I: intervention; NA: not applicable.
One case was excluded in which iobitridol was provocated, but a basophil activation test was performed instead of a skin test.
Two cases in the group of patients with IHR and with negative skin tests and a positive reaction upon provocation were excluded due to the provocation with an unknown ICM.
Two cases in the group of patients with IHR and positive skin tests and with a positive reaction upon provocation were excluded due to the provocation with an unknown ICM.
Patients with a proven ICM allergy, based on clinical history, skin tests and drug provocation tests, were evaluated for an allergy to another ICM.
Most studies included more women than men. The median and mean age of the patients was between 48 and 62 years. Severe reactions for IHR and NIHR were not reported frequently (Table 1). Most studies used the Ring and Messmer grading scale for IHR: grade 1, generalised (muco)cutaneous symptoms; grade 2, mild systemic manifestations; grade 3, life-threating systemic reactions including shock and grade 4, cardiac or respiratory arrest (Table 1).
Immediate hypersensitivity reactions to ICM
Skin prick tests or intradermal tests were positive in 5.6%–64.7% patients with IHR (Table 2). In the case of a negative skin test, provocation with the culprit or a negatively tested alternative was positive in 3.2%–9.1% patients (Table 2). However, in one study with six skin-test-negative patients and confirmed immediate hypersensitivity to another ICM all provocation tests were positive for the alternative. 34 More-over in the 11 studies performed, 65 of 69 (94%) patients with a positive skin test with the culprit ICM tolerated a challenge with a skin-test-negative alternative ICM. Two out of four patients in one study 30 as well as two out of six patients in another study, 34 with positive skin tests for the culprit media and challenged with a negative-skin-test alternative ICM, experienced symptoms during provocation, Table 2. Dona et al. 34 reported that the symptoms were similar to those recorded earlier, however they were milder .
Provocations with the culprit ICM are rarely performed in cases with a positive skin test. Only two studies addressed this issue and positive provocation tests were seen in 4/5 and 2/2 patients respectively.21,32
Non-immediate hypersensitivity reactions to ICM
Skin prick tests, intradermal tests or patch tests were positive in 16.9%–53.3% of patients with NIHR (Table 3). In case of a negative skin test for the culprit or alternative ICM, provocation with the tested ICM was positive in 0%–34.6% of cases and in 50/50 (100%) patients challenged with the skin-test negative culprit with a proven non-immediate type allergic sensitivity to another ICM (Table 3). In case of a NIHR with a positive skin test, provocation with an alternative skin test negative agent was tolerated in 75/105 (71%) of cases Table 3. Provocation with the culprit was rarely performed when the skin test was positive, resulting in a hypersensitivity response in two out of three patients.18,33
Table 3.
Provocation/re-exposure of ICM in patients with a non-immediate hypersensitivity reaction (NIHR) (n = 10 studies).
| Study | Study design | N (NIHR) | (Delayed) Positive SPT and/or IDT or patch test (%) | Culprit skin test negative |
Culprit skin test positive |
Premedication administered prior to
provocation or re-exposure |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive provocation test or re-exposure reaction to culprit or skin test negative alternative ICM (%) | Negative predictive value | Positive provocation test or exposure reaction to skin test negative ICM (%) | Negative predictive value | Positive provocation test or exposure reaction to skin test positive ICM (%) | Positive predictive value | |||||
| Vernassiere et al.53 | R, I | 15 | 8/15 (53.3) | 2/7 (28.6) | 0.71 | 3/8 (37.5) | 0.625 | – | NA | None |
| Seitz et al. 25 | R, I | 32 | 6/32 (18.8) | – | NA | 0/4 (0) | 1 | – | NA | None |
| Caimmi et al.54 | R | 17 | 3/17 (42.9) | 1/4 (25) | 0.75 | – | NA | – | NA | None |
| Torres et al. 29 | P, I | 161 | 34/161 (21.1) | 44/127 (34.6) | 0.65 | 11/34 (32.4) | 0.68 | – | NA | None |
| Ahn et al. 21 | P | 6 | 3/6 (50) | – | NA | 0/1 (0) | 1 | – | NA | In all |
| Della-Torre et al. 31 | R | 17 | 5/17 (29.4) | 0/12 (0) | 1 | 0/5 (0) | 1 | – | NA | In all |
| Schrijvers et al. 18 | R | 118 | 20/118 (16.9) | 5/37(12.8) a | 0.86 | 0/4 a (0) | 1 | 1/2 a (50) | 0.5 | In 20.7% |
| Trautmann et al. 26 | R | 13 | 13/13 (100) | – | NA | 0/8 (0) | 1 | – | NA | None |
| Meucci et al. 33 | R | 16 | 3/16 (18.8 ) | 4/13 (30.8) | 0.69 | 2/2 (100) | 0 | 1/1 (100) | 1 | None |
| Dona et al. 34 | P | 89 | 39/89 (43.8) | 50/50 (100) b | 0 | 14/39 (35.9) | 0.64 | – | NA | None |
N: number of patients; P: prospective; R: retrospective; NA: not applicable.
Five patients had a positive skin test. One with NIHR and positive skin tests and with a positive reaction upon provocation was excluded due to the provocation with an unknown ICM.
Patients with a proven ICM allergy, based on clinical history, skin tests and drug provocation tests, were evaluated for an allergy to other ICM.
Gadolinium based contrast media
In gadolinium-based contrast media, one case report and three case series reported a positive skin test in 19.2%–57.6% of patients that had an IHR (Table 4). Limited information was available for NIHR.
In the case of IHR with positive skin tests, a provocation was performed with an alternative gadolinium-based contrast medium and a negative skin test: all provocations were negative.35–38
In the case of a negative skin test, provocations with the culprit or alternative ICM were negative in all the studies with the exception of one study that reported two positive provocation results in 11 cases, one with an immediate and one with a non-immediate hypersensitivity reaction (Table 4). However, the severity of the response was not mentioned. 38
One study reported on patients that had hypersensitivity reactions to ICM or gadolinium-based contrast media. 39 Of the 10 patients with an IHR and three with a NIHR, none showed a positive skin test. Of those, one patient with a previous NIHR response to ICM, tolerated the gadolinium-based contrast medium.
Discussion
Radiocontrast media provocation tests are recommended in addition to skin tests, although these are not common in clinical practice. A recent study suggests a provocation based on a risk benefit analysis. 11 In another recently published algorithm on contrast reactions, a negative skin test radiocontrast provocation is only recommended as a confirmation test for tolerability in case of very severe reactions. 24 However, a radiocontrast provocation could also be useful to differentiate an allergic hypersensitivity reaction from non-allergic hypersensitivity reactions and in case of less severe reactions.
In the studies in this review, provocations were mostly performed when a computerized tomography (CT) scan or MRI was needed and re-exposure to the contrast agent was indicated. The results of this review indicated that when the skin test was positive, provocation testing with the same agent as the culprit agent was positive in most cases, also reflected by the high positive predictive value of the skin test. Because these DPTs were positive in most cases, positive skin tests may represent/indicate a true allergy. A skin test can be positive in patients without previous exposure to contrast media, even if provocation with this particular contrast medium afterwards is well tolerated. 27 This illustrates that the clinical symptoms should be compatible with a hypersensitivity reaction for accurate interpretation of the skin test results, and a provocation can be of additional value in cases where the history of the patient and results of the skin test do not match.
In case of IHR and NIHR to ICM, the majority of patients (94% and 71% respectively) with a positive skin test for the culprit tolerated a challenge with a skin-test-negative alternative ICM. In case of a negative skin test for the culprit, provocations were mostly negative, as reflected by the high negative predictive value of the skin test, although the number of positive provocations was higher in the NIHR group. This variation can be explained by the number of patients in the studies, the inclusion of patients with varying numbers of allergic and non-allergic hypersensitivities and time and type of skin test performed. The risk of a NIHR, despite a provocation with a negative skin test ICM, is probably more around the 34.6% as reported in the study with the highest number of patients. 29
A previous reaction to ICM is the most common risk factor for IHR.4,8,40,41 Although cross-reactivity is relatively low for ICM in immediate hypersensitivity reactions, 1 the risk of a hypersensitivity reaction is higher in patients with a confirmed allergic ICM hypersensitivity, even with a negative skin test. 34
Higher degrees of cross reactivity, ranging from 32% to 75%, with skin tests for ICM were seen with NIHR.22,42 Therefore it is recommended to perform an additional provocation, in case of a proven allergy, even when the skin test for the alternative contrast agent is negative.
Other risk factors for IHR are asthma, use of beta-blockers, old age and cardiovascular diseases.4,8,41,43–46 Reported predisposing factors for NIHR are previous CM-induced hypersensitivity reactions, atopy, interleukin-2 treatment, serum creatinine level >2 mg/dL and a history of drug and contact allergy.8,47,48
Data on hypersensitivity reactions, skin tests and provocation tests for gadolinium-based contrast media were scarce; however they showed a similar pattern to the hypersensitivity reactions in ICM. There is no cross-reactivity between ICM and gadolinium contrast media. 49
When an allergic hypersensitivity reaction, evaluated by skin tests and a provocation, is excluded, it is generally considered as a non-allergic hypersensitivity reaction, or a reaction not related to the contrast media itself. Apart from hypersensitivity reactions, common side effects occurring immediately after administration of ICM include flushing, vomiting and occasionally dyspnea. Assessing the concentration of tryptase between 0.5 and 3 h after the onset of symptoms may help to determine the cause of the reaction. 50 An elevated tryptase level is indicative of a mast cell-mediated reaction and increases the probability of an IgE-mediated allergic reaction. 51 Although a high tryptase is associated with more severe immediate hypersensitivity reactions, a normal tryptase level does not exclude an IHR. 50 After 1–2 days, a plasma tryptase level can be drawn for baseline analysis. 52
Based on this review and other studies from the literature on this topic, the following diagnostic approach for the evaluation of a hypersensitivity reaction to contrast media is proposed:
In case of an immediate hypersensitivity reaction, the serum tryptase level should be measured between 15 and 180 min after the reaction. An increase in tryptase, particularly when confirmed with a positive skin test is suggestive of an IgE-mediated hypersensitivity reaction. A repeat measurement of tryptase at least 1 or 2 days later is useful to confirm normal baseline values. Otherwise, a thorough medical history is necessary, followed by an undiluted skin prick test and if negative subsequently an intradermal test with a dilution of 1:10 in case of iodine contrast media. In additional, an undiluted IDT can be performed in case of a non-immediate type hypersensitivity reaction for optimal sensitivity.18,29,34
For gadolinium-based hypersensitivity reactions, IDT including dilutions of 1:1000, 1:100 and 1:10 are recommended.35,37
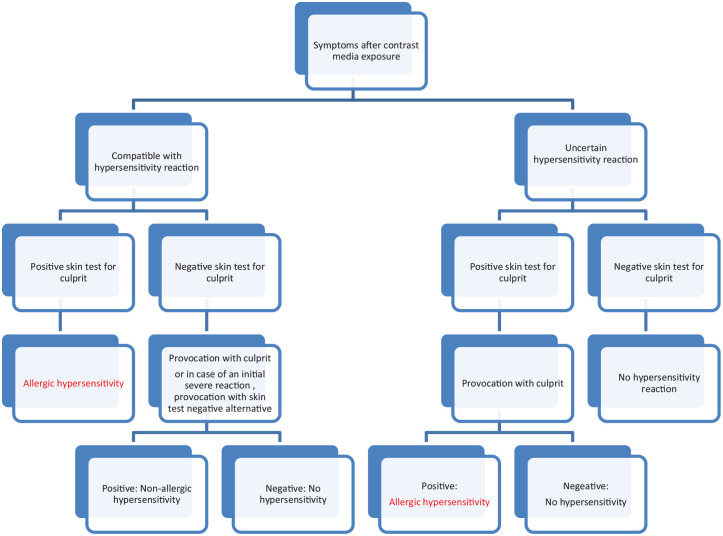
Patients with a positive skin test and the history of hypersensitivity reaction are classified as allergic (Figure 2).
Figure 2.
Proposed flow-chart for assessment of presumed contrast hypersensitivity.
However, when the skin test is negative, a provocation with the alternative media should be performed.
If the provocation is positive, particularly when accompanied by a low tryptase, a non-allergic hypersensitivity can be diagnosed. Other causes that should be considered are the use of disinfectants, medication during procedures and (co-)morbidities. In case of a severe immediate reaction to an ICM based on its osmolarity, it is recommended to switch to an ICM with lower osmolarity. Furthermore, in case of a positive skin test but a negative provocation, no hypersensitivity is diagnosed and the ICM can be used in the future (Figure 2).
In patients with a positive skin test, but without a (typical) hypersensitivity reaction, a provocation with the suspected drug should be performed, particularly because patients can have a positive skin test without a previous reaction to the contrast media.27,28 In case of a negative provocation, the patient has no hypersensitivity to the contrast media, while in case of a positive provocation, an allergy is confirmed.
Ideally, no premedication is used during the provocation to enable reliable observation of the type of reaction. Figure 2 represents a proposed flowchart for the assessment of a presumed allergic reaction to contrast media.
This review has several limitations. Studies were either retrospective (10 studies) or prospective and mainly included case series and small cohorts, which can limit the interpretation of the results. Data were summarised and were not pooled in a meta-analysis, because of the heterogeneity in the studies. Furthermore practical guidelines are lacking. None of the studies included details about the provocations such as information on dosage. Moreover, the included studies may have been biased by the use of pre-medication. Four studies reported on the use of premedication (Tables 2–4).18,21,31,35 This may decrease the severity of the reaction and therefore influence the results of the provocation test. However, details about the specific premedication were lacking in three of the studies.
Despite these limitations, this review makes a novel contribution to the literature by the proposed flow-chart which could encourage the implementation of provocation as part of the diagnostic work-up in hypersensitivity reactions to contrast media. This proposed flow-chart should be further validated in the future, prior to implementation in practical guidelines.
Conclusion
In case of IHR and NIHR to ICM, the majority of patients with a positive skin test for the culprit tolerated a challenge with a skin-test-negative alternative ICM. In case of a negative skin test for the culprit ICM, provocations were mostly negative, although the number of positive provocations was higher in the NIHR group. Data on hypersensitivity reactions, skin tests and provocations with gadolinium-based contrast media were limited; however, they exhibited a pattern similar to that observed in ICM. In summary, a thorough medical history is necessary, followed by skin tests. A provocation is recommended for diagnostic work-up, when the diagnosis is uncertain.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Saskia M Rombach  https://orcid.org/0000-0003-1356-7033
https://orcid.org/0000-0003-1356-7033
Data accessibility statement: All data in this review article can be retrieved by accessing the original publications, as cited in the references.
References
- 1. Christiansen C. (2005) X-ray contrast media–an overview. Toxicology 209(2): 185–187. [DOI] [PubMed] [Google Scholar]
- 2. Jung JW, Kang HR, Kim MH, et al. (2012) Immediate hypersensitivity reaction to gadolinium-based MR contrast media. Radiology 264(2): 414–422. [DOI] [PubMed] [Google Scholar]
- 3. Shehadi WH. (1975) Adverse reactions to intravascularly administered contrast-media - comprehensive study based on a prospective survey. American Journal of Roentgenology 124(1): 145–152. [DOI] [PubMed] [Google Scholar]
- 4. Bottinor W, Polkampally P, Jovin I. (2013) Adverse reactions to iodinated contrast media. The International Journal of Angiology 22(3): 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bush WH, Swanson DP. (1991) Acute reactions to intravascular contrast media: Types, risk factors, recognition, and specific treatment. American Journal of Roentgenology 157(6): 1153–1161. [DOI] [PubMed] [Google Scholar]
- 6. Katayama H, Yamaguchi K, Kozuka T, et al. (1990) Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 175(3): 621–628. [DOI] [PubMed] [Google Scholar]
- 7. Pasternak JJ, Williamson EE. (2012) Clinical pharmacology, uses, and adverse reactions of iodinated contrast agents: A primer for the non-radiologist. Mayo Clinic Proceedings 87(4): 390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brockow K, Christiansen C, Kanny G, et al. (2005) Management of hypersensitivity reactions to iodinated contrast media. Allergy 60(2): 150–158. [DOI] [PubMed] [Google Scholar]
- 9. Ryoo CH, Choi YH, Cheon JE, et al. (2019) Preventive effect of changing contrast media in patients with a prior mild immediate hypersensitivity reaction to gadolinium-based contrast agent. Investigative Radiology 54(10): 633–637. [DOI] [PubMed] [Google Scholar]
- 10. Johansson SG, Bieber T, Dahl R, et al. (2004) Revised nomenclature for allergy for global use: Report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. The Journal of Allergy and Clinical Immunology 113(5): 832–836. [DOI] [PubMed] [Google Scholar]
- 11. Torres MJ, Trautmann A, Bohm I, et al. (2020) Practice parameters for diagnosing and managing iodinated contrast media hypersensitivity. Allergy. Epub ahead of print 10 November 2020. DOI: 10.1111/all.14656. [DOI] [PubMed] [Google Scholar]
- 12. Stellato C, deCrescenzo G, Patella V, et al. (1996) Human basophil mast cell releasability .11. Heterogeneity of the effects of contrast media on mediator release. The Journal of Allergy and Clinical Immunology 97(3): 838–850. [DOI] [PubMed] [Google Scholar]
- 13. Dewachter P, Trechot P, Mouton-Faivre C. (2005) Allergie a l’iode: le point sur la question. Annales Francaises d Anesthesie et de Reanimation 24(1): 40–52. [DOI] [PubMed] [Google Scholar]
- 14. Beaty AD, Lieberman PL, Slavin RG. (2008) Seafood allergy and radiocontrast media: Are physicians propagating a myth? The American Journal of Medicine 121(2): 158 e1-4. [DOI] [PubMed] [Google Scholar]
- 15. Carter MC, Metcalfe DD, Matito A, et al. (2019) Adverse reactions to drugs and biologics in patients with clonal mast cell disorders: A Work Group Report of the Mast Cells Disorder Committee, American Academy of Allergy, Asthma & Immunology. The Journal of Allergy and Clinical Immunology 143(3): 880–893. [DOI] [PubMed] [Google Scholar]
- 16. Lerch M, Keller M, Britschgi M, et al. (2007) Cross-reactivity patterns of T cells specific for iodinated contrast media. The Journal of Allergy and Clinical Immunology 119(6): 1529–1536. [DOI] [PubMed] [Google Scholar]
- 17. Webb JAW, Stacul F, Thomsen HS, et al.; ESUR CMSC (2003) Late adverse reactions to intravascular iodinated contrast media. European Radiology 13(1): 181–184. [DOI] [PubMed] [Google Scholar]
- 18. Schrijvers R, Breynaert C, Ahmedali Y, et al. (2018) Skin testing for suspected iodinated contrast media hypersensitivity. Journal of Allergy and Clinical Immunology-In Practice 6(4): 1246–1254. [DOI] [PubMed] [Google Scholar]
- 19. Brockow K, Romano A, Aberer W, et al. (2009) Skin testing in patients with hypersensitivity reactions to iodinated contrast media - a European multicenter study. Allergy 64(2): 234–241. [DOI] [PubMed] [Google Scholar]
- 20. Trcka J, Schmidt C, Seitz CS, et al. (2008) Anaphylaxis to iodinated contrast material: Nonallergic hypersensitivity or IgE-mediated allergy? American Journal of Roentgenology 190(3): 666–670. [DOI] [PubMed] [Google Scholar]
- 21. Ahn YH, Koh YI, Kim JH, et al. (2015) The potential utility of iodinated contrast media (ICM) skin testing in patients with ICM hypersensitivity. Journal of Korean Medical Science 30(3): 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hasdenteufel F, Waton J, Cordebar V, et al. (2011) Delayed hypersensitivity reactions caused by iodixanol: An assessment of cross-reactivity in 22 patients. The Journal of Allergy and Clinical Immunology 128(6): 1356–1357. [DOI] [PubMed] [Google Scholar]
- 23. Rerkpattanapipat T, Chiriac AM, Demoly P. (2011) Drug provocation tests in hypersensitivity drug reactions. Current Opinion in Allergy and Clinical Immunology 11(4): 299–304. [DOI] [PubMed] [Google Scholar]
- 24. Brockow K. (2020) Medical algorithm: Diagnosis and treatment of radiocontrast media hypersensitivity. Allergy 75(5): 1278–1280. [DOI] [PubMed] [Google Scholar]
- 25. Seitz CS, Pfeuffer P, Raith P, et al. (2009) Radiocontrast media-associated exanthema: Identification of cross-reactivity and tolerability by allergologic testing. European Journal of Radiology 72(1): 167–171. [DOI] [PubMed] [Google Scholar]
- 26. Trautmann A, Brockow K, Behle V, et al. (2019) Radiocontrast media hypersensitivity: Skin testing differentiates allergy from nonallergic reactions and identifies a safe alternative as proven by intravenous provocation. Journal of Allergy and Clinical Immunology-In Practice 7(7): 2218–224. [DOI] [PubMed] [Google Scholar]
- 27. Lee JH, Kwon OY, Park SY, et al. (2020) Validation of the prescreening intradermal skin test for predicting hypersensitivity to iodinated contrast media: A prospective study with ICM challenge. The Journal of Allergy & Clinical Immunology in Practice 8(1): 267–272. [DOI] [PubMed] [Google Scholar]
- 28. Soyyigit S, Goksel O, Aydin O, et al. (2016) What is the clinical value of negative predictive values of skin tests to iodinated contrast media? Allergy & Asthma Proceedings 37(6): 482–488. [DOI] [PubMed] [Google Scholar]
- 29. Torres MJ, Gomez F, Dona I, et al. (2012) Diagnostic evaluation of patients with nonimmediate cutaneous hypersensitivity reactions to iodinated contrast media. Allergy 67(7): 929–935. [DOI] [PubMed] [Google Scholar]
- 30. Salas M, Gomez F, Fernandez TD, et al. (2013) Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy 68(9): 1203–1206. [DOI] [PubMed] [Google Scholar]
- 31. Della-Torre E, Berti A, Yacoub MR, et al. (2015) Proposal of a skin tests based approach for the prevention of recurrent hypersensitivity reactions to iodinated contrast media. European Annals of Allergy and Clinical Immunology 47(3): 77–85. [PubMed] [Google Scholar]
- 32. Kwon OY, Lee JH, Park SY, et al. (2019) Novel strategy for the prevention of recurrent hypersensitivity reactions to radiocontrast media based on skin testing. The Journal of Allergy & Clinical Immunology in Practice 7(8): 2707–2713. [DOI] [PubMed] [Google Scholar]
- 33. Meucci E, Radice A, Fassio F, et al. (2020) Diagnostic approach to hypersensitivity reactions to iodinated contrast media: A single-center experience on 98 patients. European Annals of Allergy and Clinical Immunology 52(5): 220–229. [DOI] [PubMed] [Google Scholar]
- 34. Dona I, Bogas G, Salas M, et al. (2020) Hypersensitivity reactions to multiple iodinated contrast media. Frontiers in Pharmacology 11: 575437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chiriac AM, Audurier Y, Bousquet PJ, et al. (2011) Clinical value of negative skin tests to gadolinium contrast agents. Allergy 66(11): 1504–1506. [DOI] [PubMed] [Google Scholar]
- 36. Moulin C, Said BB, Berard F. (2011) Tolerability of gadobenate dimeglumine in a patient with reported allergy to gadoterate meglumine. American Journal of Roentgenology 197(6): W1163. [DOI] [PubMed] [Google Scholar]
- 37. Kolenda C, Dubost R, Hacard F, et al. (2017) Evaluation of basophil activation test in the management of immediate hypersensitivity reactions to gadolinium-based contrast agents: A five-year experience. The Journal of Allergy & Clinical Immunology in Practice 5(3): 846–849. [DOI] [PubMed] [Google Scholar]
- 38. Seta V, Gaouar H, Badaoui A, et al. (2019) Low-dose provocation and skin tests in patients with hypersensitivity to gadolinium-based contrast agents. Clinical and Experimental Allergy 49(5): 724–728. [DOI] [PubMed] [Google Scholar]
- 39. Tepetam FM, Ciftaslan N, Oruc O, et al. (2016) Should patients with risk factors be tested for hypersensitivity to contrast media: A prospective study. La Radiologia medica 121(8): 660–666. [DOI] [PubMed] [Google Scholar]
- 40. Kobayashi D, Takahashi O, Ueda T, et al. (2013) Risk factors for adverse reactions from contrast agents for computed tomography. BMC Medical Informatics and Decision Making 13: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Morzycki A, Bhatia A, Murphy KJ. (2017) Adverse reactions to contrast material: A Canadian update. Canadian Association of Radiologists Journal 68(2): 187–193. [DOI] [PubMed] [Google Scholar]
- 42. Kanny G, Pichler W, Morisset M, et al. (2005) T cell-mediated reactions to iodinated contrast media: Evaluation by skin and lymphocyte activation tests. The Journal of Allergy and Clinical Immunology 115(1): 179–185. [DOI] [PubMed] [Google Scholar]
- 43. Lang DM, Alpern MB, Visintainer PF, et al. (1993) Elevated risk of anaphylactoid reaction from radiographic contrast media is associated with both beta-blocker exposure and cardiovascular disorders. Archives of Internal Medicine 153(17): 2033–2040. [PubMed] [Google Scholar]
- 44. Pradubpongsa P, Dhana N, Jongjarearnprasert K, et al. (2013) Adverse reactions to iodinated contrast media: Prevalence, risk factors and outcome-the results of a 3-year period. Asian Pacific Journal of Allergy and Immunology 31(4): 299–306. [DOI] [PubMed] [Google Scholar]
- 45. Leder R. (1997) How well does a history of seafood allergy predict the likelihood of an adverse reaction to i.v. contrast material? American Journal of Roentgenology 169(3): 906–907. [DOI] [PubMed] [Google Scholar]
- 46. Sanchez-Borges M, Aberer W, Brockow K, et al. (2019) Controversies in drug allergy: Radiographic contrast media. Journal of Allergy and Clinical Immunology-In Practice 7(1): 61–65. [DOI] [PubMed] [Google Scholar]
- 47. Hosoya T, Yamaguchi K, Akutsu T, et al. (2000) Delayed adverse reactions to iodinated contrast media and their risk factors. Radiation Medicine 18(1): 39–45. [PubMed] [Google Scholar]
- 48. Egbert RE, De Cecco CN, Schoepf UJ, et al. (2014) Delayed adverse reactions to the parenteral administration of iodinated contrast media. American Journal of Roentgenology 203(6): 1163–1170. [DOI] [PubMed] [Google Scholar]
- 49. Saleh L, Juneman E, Movahed MR. (2011) The use of gadolinium in patients with contrast allergy or renal failure requiring coronary angiography, coronary intervention, or vascular procedure. Catheterization and Cardiovascular Interventions 78(5): 747–754. [DOI] [PubMed] [Google Scholar]
- 50. Dewachter P, Laroche D, Mouton-Faivre C, et al. (2011) Immediate reactions following iodinated contrast media injection: A study of 38 cases. European Journal of Radiology 77(3): 495–501. [DOI] [PubMed] [Google Scholar]
- 51. Laroche D, Aimone-Gastin I, Dubois F, et al. (1998) Mechanisms of severe, immediate reactions to iodinated contrast material. Radiology 209(1): 183–190. [DOI] [PubMed] [Google Scholar]
- 52. Laroche D, Vergnaud MC, Sillard B, et al. (1991) Biochemical markers of anaphylactoid reactions to drugs - comparison of plasma histamine and tryptase. Anesthesiology 75(6): 945–949. [DOI] [PubMed] [Google Scholar]
- 53. Vernassiere C, Trechot P, Commun N, et al. (2004). Low negative predictive value of skin tests in investigating delayed reactions to radio-contrast media. Contact Dermatitis 50(6): 359–366. [DOI] [PubMed] [Google Scholar]
- 54. Caimmi S, Benyahia B, Suau D, et al. (2010) Clinical value of negative skin tests to iodinated contrast media. Clinical & Experimental Allergy (40): 805–810. [DOI] [PubMed] [Google Scholar]
- 55. Prieto-García A, Tomás M, Pineda R, et al. (2013) Skin test-positive immediate hypersensitivity reaction to iodinated contrast media: The role of controlled challenge testing. Journal of Investigational Allergology & Clinical Immunology 23(3): 183–189. [PubMed] [Google Scholar]
- 56. Sesé L, Gaouar H, Autegarden J-E, et al. (2016) Immediate hypersensitivity to iodinated contrast media: Diagnostic accuracy of skin tests and intravenous provocation test with low dose. Clinical & Experimental Allergy 46(3): 472–478. [DOI] [PubMed] [Google Scholar]