Abstract
Cutaneous squamous cell carcinoma (cSCC) accounts for 20% of skin cancers. At an advanced stage the prognosis is poor, making cSCC the second leading cause of death from skin cancer. In cases of metastatic or unresectable disease, anti-programmed cell death 1 (anti-PD1) treatment has shown promising results in a recent phase II study. Although anti-PD1 treatment now offers higher response rates, the responses remain inconsistent and may lead to therapeutic impasses. Preclinical data have suggested synergy between anti-epidermal growth factor receptor (anti-EGFR) and immunotherapy. We report the case of a patient with metastatic cSCC that proved refractory first to anti-EGFR/carboplatin and then to immunotherapy, but who showed a complete and durable response with cetuximab/pembrolizumab combination. This response could reflect synergy of the two treatments.
Keywords: anti-PD1, chemotherapy, combination immunotherapy, cutaneous squamous cell carcinoma, EGFR inhibitor
Introduction
Cutaneous squamous cell carcinoma (cSCC) accounts for 20% of skin cancers, making it the second most common skin cancer after basal cell carcinoma. 1 The annual incidence rate varies by region of the world. 2 In Europe, the reported age-standardized incidence of cSCC ranges from 15 to 77 per 100,000 individuals per year. 2 In Australia, cSCC incidence is higher (270 per 100,000 individuals per year). 3 The incidence is constantly increasing because of population ageing and sun exposure habits. 4 At an early stage, the prognosis is excellent, with a 90% 10-year survival rate, but when the disease is locally advanced or metastatic, the prognosis is poor with a median overall survival of 15.3 months from the start of first-line therapy, 5 making cSCC the second leading cause of death from skin cancer, after melanoma.
Over the last 2 years, immunotherapy has emerged as a standard of care in the management of advanced cSCC. Cemiplimab has shown promising results in a phase I and then a phase II study, with response rates between 43.6% and 50% and median progression-free survival and median overall survival not reached after 12 months of follow-up. 6 Similarly, pembrolizumab has achieved in a recent phase II study a 38.5% response rate, with excellent tolerability. 7
Although anti-PD1 (programmed cell death 1) treatment now offers higher response rates, the responses remain inconsistent and may lead to therapeutic impasses.
We present here the case of a patient with metastatic cSCC that proved refractory to first-line anti-EGFR (epidermal growth factor receptor)/carboplatin and then to immunotherapy, but who showed a complete response with a cetuximab/pembrolizumab combination.
Observation
In December 2016, a 65-year-old man presented with right subclavicular squamous cell carcinoma of the skin, which had been treated by surgery followed by adjuvant radiotherapy. One year later, a local recurrence was observed, with infiltration of the sternocleidomastoid muscle and associated right cervical adenopathy palpable on clinical examination.
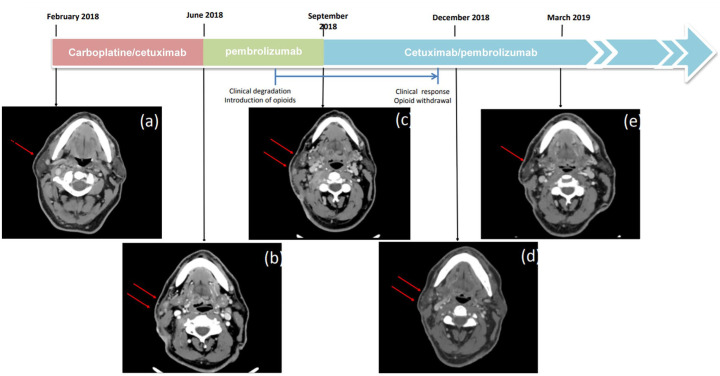
In February 2018, first-line systemic treatment was initiated with carboplatin/cetuximab. In June 2018, computed tomography (CT) scan performed after five cycles of treatment showed a mixed response, with 38% shrinkage of the right supraclavicular infiltration and emergence of a new right parotid lesion [Figure 1(a) and (b) and Figure 2(a)]. Considering tumour progression according to the RECIST 1:1 criteria, a therapeutic change with pembrolizumab was made. In July 2018, CT scan after three cycles of pembrolizumab showed a progression of cervical lymph nodes and the right parotid lesion. A new CT scan performed 2 months later (September 2018) confirmed progression 3 months after treatment initiation [Figure 1(c)]. Radiographic progression was associated with decreased performance status secondary to appearance of intense neuralgia following invasion of the brachial plexus, requiring introduction of opioids treatment [Figure 2(b)].
Figure 1.
Tomography scan assessments of the right parotid lesion at different time points. (a) Before starting the first course of carboplatin/cetuximab. (b) After completing five cycles of carboplatin/cetuximab and before starting the first course of pembrolizumab. (c) After completing four cycles of pembrolizumab and before starting the first course of pembrolizumab/cetuximab. (c) Three months and (d) 2 years after introduction of pembrolizumab/cetuximab.
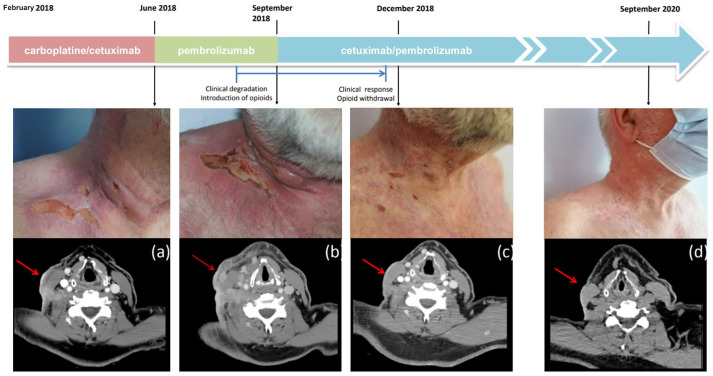
Figure 2.
Clinical and computed tomography assessments of the right supraclavicular lesion at different time points. (a) Before starting the first course of pembrolizumab. (b) After completing four cycles of pembrolizumab and before starting the first course of pembrolizumab/cetuximab. (c) Three months and (d) 2 years after introduction of pembrolizumab/cetuximab.
In September 2018, a combination treatment with cetuximab (500 mg/m2; day 1) and pembrolizumab (200 mg; day 7) every 3 weeks was initiated. Due to the lack of safety data for a combination of cetuximab and pembrolizumab, a sequential dosing regimen was chosen to monitor the occurrence of adverse events.
In December 2018, a partial radiological response was observed, associated with major clinical improvement [Figure 1(d) and Figure 2(c)]. In June 2019, a positron emission tomography scan showed no sign of metabolic activity, suggesting a complete response. After 15 cycles, cervical magnetic resonance imaging showed only a retractile fibrous laterocervical sequela with no progressive lesion. In November 2019, repeat imaging/clinical exam revealed persistence of complete response.
Because the patient developed Common Terminology Criteria for Adverse Events grade 3 folliculitis refractory to topical and systemic antibiotics, cetuximab was stopped after 19 cycles (14.3 months). In September 2020, a complete clinical and radiological response was still observed, allowing us to stop the immunotherapy [Figure 2(d)]. In January 2021, after 4 months off treatment, a complete clinical and radiological response is still observed.
Discussion
We report the observed case of a patient with metastatic cSCC who experienced a durable complete response to third-line treatment with combination anti-EGFR and anti-PD1 therapy.
There have been two previous reports concerning cSCC patients showing major responses to anti-EGFR/anti-PD1 combination therapy, but as the combination was initiated in anti-PD1 naïve patients, this could not reliably be interpreted as a potential synergistic effect.8,9
In our case, the successive failures of carboplatin/cetuximab combination therapy and of pembrolizumab monotherapy suggest that the response obtained with the cetuximab/pembrolizumab combination could reflect synergy of the two treatments.
The lack of response to pembrolizumab would suggest the presence of a primary resistance mechanism to PD1 therapy. Several mechanisms of primary resistance to anti-PD1 have been described. Innate resistance may be notably related to the absence or low expression of programmed death ligand 1 (PDL1) by the tumour or stromal cells, the absence of tumour-expressed antigen, the inability of tumour-specific T cells to infiltrate the tumour microenvironment or the presence of a PD1-independent pathway suppressing anti-tumour immune responses. 10
In our case, the addition of cetumixab appears to have overcome resistance to anti-PD1. In addition to inhibition of the EGFR receptor and downstream signalling pathways, anti-EGFR treatment can stimulate an anti-tumour immune response. By contributing to natural killer (NK) cell activation, cetuximab induces tumour cell death via an antibody-dependent cell-mediated cytotoxicity mechanism. Interestingly, NK cell activation by cetuximab leads to the release of tumour antigens, promoting the action of dendritic cells. 11 This stimulation, however, activates negative feedback controls via increased expression of PD1, PDL1 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). The observed synergy might thus be due to the lifting of these controls through blocking of the PD1/PDL1 or CTLA-4 pathway. 11 EGFR tyrosine kinase inhibitors (TKIs) have also demonstrated an ability to modulate the immune system. In a model of EGFR-mutated lung adenocarcinoma, Sugiyama et al. 12 found the EGFR inhibitor erlotinib to reduce infiltration of regulatory T-lymphocytes into the tumour microenvironment. In murine models, they also evidenced an association of EGFR TKI/anti-PD1 combination treatment with a better and prolonged tumour response.
What about the clinical data? A phase II trial evaluating pembrolizumab/cetuximab combination treatment in patients with recurrent or metastatic head and neck squamous cell carcinoma yielded encouraging results, with an overall response rate of 45% and a 14.9-month median duration of response (NCT03082534). 13
We cannot completely rule out the possibility of pseudoprogression, that is, an increase in tumour burden followed by a decrease, on pembrolizumab alone, although this phenomenon is quite rare (less than 10% of cases). 14 In a retrospective multicentre study of immunotherapy applied to non-small cell lung cancer, Fujimoto et al. 15 observed pseudoprogression in 14 out of 542 patients (3%). Among these 14 patients, the median time between anti-PD1 initiation and confirmation of a response was 2.4 months, and in most cases a response was noted within 3 months of treatment. The median time between the first progressive disease and confirmation of a response was 1.3 months. In our case, clear progression was observed at 6 weeks and confirmed clinically and radiologically at 3 months. In addition, the worsening of clinical symptoms required the introduction of opioid therapy, and therefore we considered it inappropriate to continue anti-PD1 monotherapy.
Regarding treatment-related toxicity, our patient suffered from grade III folliculitis, which is characteristic of cetuximab; withdrawal of this drug after 6 weeks resulted in complete resolution of this toxicity. Our patient showed no adverse events related to pembrolizumab, nor did we observe any cross-toxicity between anti-EGFR and anti-PD1. Nonetheless, one case is not enough to draw conclusions from, and more studies are needed to corroborate our findings. Contrary to the combination EGFR TKI and anti-PD1, which is associated with a risk of pneumopathy, the combination cetuximab and anti-PD1 is well tolerated13,16 The most common grade III adverse event reported was oral mucositis. 13
Conclusion
With a likely synergistic effect on the immune system, anti-EGFR/anti-PD1 combination treatment could be a promising therapeutic option for locally advanced or metastatic cSCC.
Acknowledgments
The authors acknowledge the patient for his participation.
Footnotes
Author contributions: The authors have contributed to this work equally.
Availability of data and material: Available upon request to the corresponding author.
Conflict of interest statement: CH reports non-financial support from Bristol-Myers Squibb, non-financial support from AstraZeneca, non-financial support from Pierre Fabre Medicament, non-financial support from AbbVie, outside the submitted work; PJ reports personal fees and non-financial support from Novartis, non-financial support from Pierre Fabre, non-financial support from Mylan Medical SAS, personal fees and non-financial support from Boehringer Ingelheim France, outside the submitted work. ED reports personal fees and non-financial support from BMS, non-financial support from Pierre Fabre, non-financial support from AbbVie, non-financial support from MSD France, non-financial support from Mylan Medical SAS, personal fees and non-financial support from Pierre Fabre Medicament, personal fees from Merck Serono, non-financial support from Janssen-Cilag, non-financial support from Lilly France SAS, personal fees from Novartis, outside the submitted work. AG reports personal fees and non-financial support from BMS, personal fees and non-financial support from Pierre Fabre, non-financial support from AbbVie, non-financial support from MSD France, non-financial support from Mylan Medical SAS, non-financial support from Pierre Fabre Medicament, non-financial support from Roche, non-financial support from LEO Pharma, non-financial support from Amgen SAS, non-financial support from Janssen-Cilag, non-financial support from Takeda France. LM reports personal fees and non-financial support from Novartis, personal fees and non-financial support from MSD, personal fees and non-financial support from Pierre Fabre, personal fees and non-financial support from BMS, non-financial support from Lilly France SAS, non-financial support from Celgene SAS, non-financial support from PFIZER SAS, non-financial support from Janssen-Cilag, outside the submitted work.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval and consent to participate: Need for approval was waived.
Patient consent for publication: All patients provided written consent for study participation.
ORCID iD: Philippe Jamme  https://orcid.org/0000-0002-8188-9918
https://orcid.org/0000-0002-8188-9918
Contributor Information
Candice Hober, Service de Dermatologie, Hôpital C. Huriez, CHU de Lille, Lille, Hauts-de-France, France.
Philippe Jamme, Service de Dermatologie, Hôpital C. Huriez, CHU de Lille, Lille, Hauts-de-France, France.
Eve Desmedt, Service de Dermatologie, Hôpital C. Huriez, CHU de Lille, Lille, Hauts-de-France, France.
Anna Greliak, Service de Dermatologie, Hôpital C. Huriez, CHU de Lille, Lille, Hauts-de-France, France.
Laurent Mortier, Department of Dermatology, Hopital Claude Huriez, CHU of Lille, rue Michel Polonowski, Lille, Hauts-de-France, 59000, France.
References
- 1. Garcovich S, Colloca G, Sollena P, et al. Skin cancer epidemics in the elderly as an emerging issue in geriatric oncology. Aging Dis 2017; 8: 643–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: part 1. Epidemiology, diagnostics and prevention. Eur J Cancer 2020; 128: 60–82. [DOI] [PubMed] [Google Scholar]
- 3. Staples MP, Elwood M, Burton RC, et al. Non-melanoma skin cancer in Australia: the 2002 national survey and trends since 1985. Med J Aust 2006; 184: 6–10. [DOI] [PubMed] [Google Scholar]
- 4. Gellrich FF, Hüning S, Beissert S, et al. Medical treatment of advanced cutaneous squamous-cell carcinoma. J Eur Acad Dermatol Venereol 2019; 33(Suppl. 8): 38–43. [DOI] [PubMed] [Google Scholar]
- 5. Cowey CL, Robert NJ, Davies K, et al. Treatment patterns and outcomes among patients with advanced cutaneous squamous cell carcinoma (CSCC) in a US community oncology setting. J Clin Oncol 2019; 37: e21033. [Google Scholar]
- 6. Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med 2018; 379: 341–351. [DOI] [PubMed] [Google Scholar]
- 7. Maubec E, Boubaya M, Petrow P, et al. Pembrolizumab as first-line therapy in patients with unresectable cutaneous squamous cell carcinoma (cSCC): phase 2 results from CARSKIN. J Clin Oncol 2019; 37: 9547. [DOI] [PubMed] [Google Scholar]
- 8. Chen A, Ali N, Boasberg P, et al. Clinical remission of cutaneous squamous cell carcinoma of the auricle with cetuximab and nivolumab. J Clin Med 2018; 7: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Richetta AG, Sernicola A, Lampitelli S, et al. Case report: cetuximab and nivolumab use in advanced cutaneous squamous cell carcinoma resistant to chemotherapy. F1000Res 2019; 8: 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shergold AL, Millar R, Nibbs RJB. Understanding and overcoming the resistance of cancer to PD-1/PD-L1 blockade. Pharmacol Res 2019; 145: 104258. [DOI] [PubMed] [Google Scholar]
- 11. Ferris RL, Lenz H-J, Trotta AM, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev 2018; 63: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sugiyama E, Togashi Y, Takeuchi Y, et al. Blockade of EGFR improves responsiveness to PD-1 blockade in EGFR-mutated non-small cell lung cancer. Sci Immunol 2020; 5: eaav3937. [DOI] [PubMed] [Google Scholar]
- 13. Assuntina G Sacco, MD Ruifeng Chen, MS Prof Francis P Worden MD, et al. Pembrolizumab plus cetuximab in patients with recurrent or metastatic head and neck squamous cell carcinoma: an open-label, multi-arm, non-randomised, multicentre, phase 2 trial. The Lancet Oncology 2021. DOI: 10.1016/S1470-2045(21)00136-4 [DOI] [PubMed]
- 14. Jia W, Gao Q, Han A, et al. The potential mechanism, recognition and clinical significance of tumor pseudoprogression after immunotherapy. Cancer Biol Med 2019; 16: 655–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fujimoto D, Yoshioka H, Kataoka Y, et al. Pseudoprogression in previously treated patients with non–small cell lung cancer who received nivolumab monotherapy. J Thorac Oncol 2019; 14: 468–474. [DOI] [PubMed] [Google Scholar]
- 16. Schoenfeld AJ, Arbour KC, Rizvi H, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol 2019; 30: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]