Abstract
Inflammatory bowel disease (IBD) is a chronic disease of the intestinal tract that commonly presents with diarrhea. Clostridioides difficile infection (CDI) is one of the most common complications associated with IBD that lead to flare-ups of underlying IBD. The pathophysiology of CDI includes perturbations of the gut microbiota, which makes IBD a risk factor due to the gut microbial alterations that occur in IBD, predisposing patients CDI even in the absence of antibiotics. Superimposed CDI not only worsens IBD symptoms but also leads to adverse outcomes, including treatment failure and an increased risk of hospitalization, surgery, and mortality. Due to the overlapping symptoms and concerns with false-positive molecular tests for CDI, diagnosing CDI in patients with IBD remains a clinical challenge. It is crucial to have a high index of suspicion for CDI in patients who seem to be experiencing an exacerbation of IBD symptoms. Vancomycin and fidaxomicin are the first-line treatments for the management of CDI in IBD. Microbiota restoration therapies effectively prevent recurrent CDI in IBD patients. Immunosuppression for IBD in IBD patients with CDI should be managed individually, based on a thorough clinical assessment and after weighing the pros and cons of escalation of therapy. This review summarizes the epidemiology, pathophysiology, the diagnosis of CDI in IBD, and outlines the principles of management of both CDI and IBD in IBD patients with CDI.
Keywords: clostridioides difficile, infection, inflammatory bowel disease, ulcerative colitis, crohn’s disease, autoimmune disease
Introduction
Clostridioides difficile (C. difficile) is an anaerobic, gram-positive, spore-forming bacterium ubiquitously present in the environment. An intact gastrointestinal microbiota provides colonization resistance against the development of C. difficile infection (CDI), which is the clinical manifestation of active toxin production and inflammation due to presence of the C. difficile bacterium in the colon. Disturbances and changes in the gut microbial ecology permit an unchecked growth of C. difficile and leads to CDI. 1 These acute perturbations of the intestinal microbiota commonly occur with antibiotic therapy, which is universally known to predispose patients to CDI development. There are important known causes of chronic perturbations of the intestinal microbiota such as autoimmune and inflammatory diseases of the colon which predispose towards CDI.
Inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn’s disease, are chronic inflammatory disease of the intestinal tract typically characterized by abdominal pain, diarrhea and bleeding, systemic symptoms such as weight loss, fever, and fatigue, along with extra-intestinal manifestations including the skin, eyes, and joint disease. Causes implicated in the pathogenesis of IBD include genetic factors, abnormal intestinal immunity, and associations postulated with a disrupted gut microbiota. 2 Similar to antibiotic exposure, a result of disturbed microbiota is the colonization and the establishment of infection by pathobionts such as C. difficile. In fact, CDI is the most common complication and the leading cause of disease flare-up in patients with IBD. 3
Worsening of diarrhea that occurs due to an underlying flare of IBD can often be clinically indistinguishable from the presence of superimposed CDI. An IBD flare is managed with immunosuppression, while CDI is managed with antibiotics; the infection itself leads to IBD flares, making management challenging.4,5 Immunosuppressive therapy may increase the risk of complications from CDI and worsen clinical outcomes. Since these two conditions mandate different therapies, and CDI leads to adverse outcomes in IBD patients, it is imperative to identify CDI in a patient experiencing an IBD flare.
The burden of CDI in IBD
A retrospective study demonstrated that not only is the incidence of CDI higher in the IBD population as compared with the non-IBD population, but UC patients are at a particularly higher risk. 6 The association of CDI with UC is the result of colonic inflammation. A population-based retrospective cohort study determining the effect of CDI on health outcomes in patients with UC found that 9% of UC patients admitted to hospital had concomitant CDI and had an increased short- and long-term mortality. 7 An analysis of a national hospitalization database, the Nationwide Inpatient Sample (NIS), revealed an increase in the frequency of CDI complicating the hospital course of both Crohn’s disease (with 8/1000–12/1000 patient admissions from 1998 to 2004) and UC (with 24/1000–39/1000 patient admissions from 1998 to 2004). 8 A similar study analyzed patients with CDI and IBD using the National Hospital Discharge Survey (NHDS) database from 2005 to 2009. 9 The overall incidence of CDI was 369.8/10,000 IBD hospital admissions, where the incidence in UC admissions was 445.6/10,000 and was significantly higher than Crohn’s disease at 220.3/10,000 admissions (p < 0.0001).
A study evaluated the incidence of concurrent infections in patients presenting with an IBD flare over a 5-year time period. 5 Approximately 10% of IBD relapses were attributed to infections, of which half were due to CDI and the other half were due to organisms. Another study evaluated all patients admitted with an IBD flare for the presence of CDI from 2012 to 2014. 10 Stool testing for C. difficile was carried out using cultures, stool cytotoxicity assays, glutamate dehydrogenase, and the presence of toxins A or B. A total of 461 patients were hospitalized and 35 were CDI positive on admission, diagnosed by stool culture or stool cytotoxicity assays. Out of these, C. difficile toxin was detected in 12 (34%) patients, while C. difficile toxigenic strains without circulating toxin were detected in 23 (66%) patients. These data suggested IBD patients without the presence of toxins may have been colonized with C. difficile.
Pathogenesis
A diverse gut microbiota provides resistance against colonization and infection by opportunistic non-commensal microorganisms. A disruption of the normal gut flora can predispose patients to the establishment of CDI. The most implicated risk factor for CDI development is the use of antibiotics. 11 Other risk factors include immunosuppression, chemotherapy, acid reduction therapy, prior C. difficile infection, hospitalization, stays in a long-term care facility, and surgeries.12,13
The pathophysiology of CDI tends to differ for patients with IBD than those without. 3 Patients with concomitant CDI and IBD are mostly younger and more often have community-acquired CDI; in contrast, patients without IBD are likely to be older, with hospital-acquired CDI and more likely have antibiotic exposure. In addition, patients with IBD have persistent disturbances in their gut microbial environment, which puts them at a higher risk of recurrence. Patients without IBD are likely to discontinue antibiotics that potentially put them at a risk of CDI, correlating with a lower chance of CDI recurrence compared with those with IBD. 3
The colitis that results with IBD is associated with a disrupted gut microbial environment predisposing to CDI. Patients with IBD acquire C. difficile more commonly from the community compared with healthcare settings by exposure to spores in the environment. 6 These C. difficile spores survive the acidic contents of the stomach and reach the intestine. In the intestine, they are converted into vegetative forms under favorable conditions; chiefly, these are an appropriate body temperature and the presence of primary bile acids. 14 The vegetative forms subsequently produce two major protein exotoxins, toxin A and toxin B. These two toxins are the main virulence factors of C. difficile that cause direct damage to the colonic epithelial cells, causing a loss of epithelial integrity and eventually leading to diarrhea. 15 In patients with IBD, the diarrhea that results due to CDI worsens the underlying IBD and leads to a flare-up. The treatment of CDI with antibiotics further disturbs the gut microbial environment, potentially propagating the cycle of recurrent CDI.
Data also suggest that the type of therapy used for the management of IBD may influence the risk of CDI. A study reported that the prevalence of CDI in IBD patients receiving azathioprine was significantly higher, and that patients on combined-therapy with a biologic agent and an immunomodulator showed no CDI. 16 Similarly, another study demonstrated that the use of immunomodulators such as azathioprine and 6-mercaptopurine, or mesalamine, was associated with the development of CDI. The maintenance of immunosuppression was independently associated with the emergence of CDI in IBD patients. 12 All IBD patients who present with diarrhea should be tested for CDI, irrespective of IBD medications used.
Clinical features and outcomes
Toxigenic strains of C. difficile can produce a wide range of outcomes, from asymptomatic carriage and mild diarrhea to life-threatening colitis and even death. 17 A typical presentation of CDI includes an increase in stool frequency, watery consistency, abdominal pain, nausea, and fever. Patients with IBD and CDI may have atypical manifestations, such as bloody stools; in particular, this is seen in younger individuals sometimes without a history of antibiotics or exposure to hospital facilities. 12 The classical endoscopic or histological findings of CDI, including pseudo-membranes, are uncommon in most IBD patients with CDI.12,18 Patients with IBD and CDI with pseudo-membranes most commonly present with fever, but their clinical outcome is similar to those IBD patients without pseudo-membranes. 18
Studies have investigated the correlation between CDI and IBD activity demonstrated conflicting findings. Weber et al. 19 evaluated 64 patients with relapsed IBD and 9% tested positive for CDI. Some studies have observed a correlation between CDI and IBD disease activity. In a study of 50 UC patients, 24% patients with acute disease had CDI, compared with 4% of quiescent patients. 20 In addition, 66% of the C. difficile positive patients had severe IBD, 33% had moderate IBD, and none had mild IBD. Patients with IBD who have CDI have worse long-term clinical outcomes (including UC-related hospitalizations, failure of immunosuppression, emergency visits, and the need for colectomy) compared with CDI negative patients at 1 year after the initial admission.6,8,12,21–23
Data from the NIS from 1993 to 2003 for all IBD patients with CDI demonstrated that the case fatality for UC patients with C. difficile was 8.5% and was especially high (⩾25%) in patients who underwent routine intestinal surgery. 24 Patients with both CDI and IBD have a longer hospital length of stay by 3 days than those with IBD but without CDI. 8 Patients with IBD and CDI more frequently require colectomy and other gastrointestinal surgeries.8,12 A study included patients from two hospitals over a span of 13 years to establish clinical and laboratory predictors of poor outcomes (colectomy or death within 180 days) of CDI in patients with IBD. 25 Older age, low serum albumin (<3 g/dl), low hemoglobin (<9 g/dl), and high serum creatinine (>1.5 g/dl) were associated with an increased risk of these adverse outcome. 25 The type of IBD and prior hospitalization related to IBD were not associated with colectomy or with CDI-related death. 25 An overall high mortality rate of 15.2% was noted in the 180-day follow up period. Another retrospective cohort study demonstrated that IBD patients were 33% more likely to experience a recurrence of CDI. 23 Within this cohort, the use of certain drugs including antibiotics, 5-aminosalicylic acid, and biologics such as infliximab, as well as non-ileal Crohn’s disease were found to be predictors of recurrent CDI.
Diagnosis
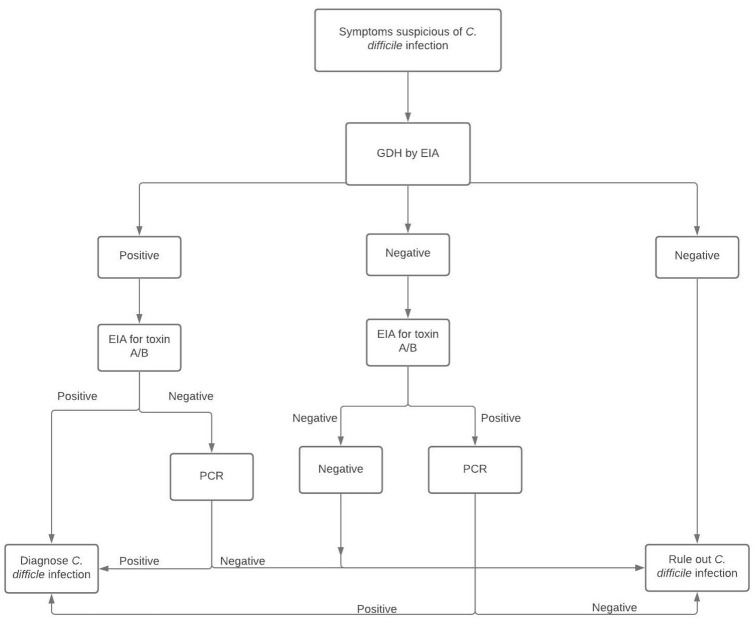
Due to the presence of overlapping symptoms in CDI and IBD, it is crucial to have a high index of suspicion for CDI in patients experiencing an IBD flare-up. C. difficile infection and IBD can present with diarrhea as a prominent symptom, and also with abdominal pain and fever. Bloody diarrhea is a more common symptom of IBD compared with CDI. Nonetheless, diagnosis on the basis of symptoms alone is not reliable. Diagnostic tests for CDI are chiefly composed of nucleic acid amplification tests (NAATs) or enzyme immunoassays (EIAs). A positive stool test for C. difficile by nucleic acid amplification does not diagnose CDI due to possible symptomless carriage of toxigenic strains of C. difficile. This can potentially lead to over diagnosis with the very sensitive PCR assays. In addition, toxin-based assays such as EIAs have a low sensitivity and may lead to under-diagnosis. Due to these diagnostic challenges, a 2-step testing approach is preferred 3 (Figure 1). The first step includes testing for glutamate dehydrogenase (GDH) with an EIA, which is highly sensitive but not specific. 3 A negative GDH rules out CDI. If the GDH is positive, it is followed up by an EIA for C. difficile toxin; this is highly specific but not sensitive and helps to confirm the diagnosis of CDI (Figure 1).
Figure 1.
The 2-step approach for diagnosing CDI – (i) GDH by EIA (ii) EIA for toxin A/B. A negative GDH test can rule out CDI but if there is a high index of suspicion, it should be followed by EIA for toxins. A negative test rules out CDI, but a positive test should be followed by PCR which can confirm the diagnosis. In contrast, a positive GDH test mandates EIA for toxins A/B. A positive toxin test confirms the diagnosis, while a negative test requires PCR for diagnosis.
CDI, Clostridioides difficile infection; EIA, enzyme immunoassay; GDH, glutamate dehydrogenase enzyme; PCR, polymerase chain reaction.
Treatment
The management of ongoing CDI in a patient with IBD
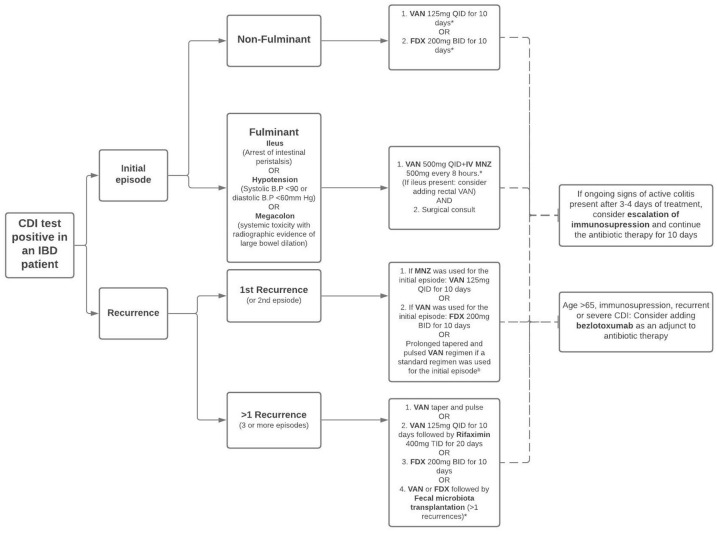
The management of CDI is approached based on severity and the number of recurrences. The infection is classified as non-severe, severe, or fulminant, based on clinical and laboratory parameters. 26 A non-severe initial episode is defined by leukocytosis with a white blood cell count of ⩽15,000 cells/ml and a serum creatinine level <1.5 mg/dl. In contrast, an initial severe episode is defined by leukocytosis with a white blood cell count of ⩾15,000 cells/ml or a serum creatinine level >1.5 mg/dl. A fulminant episode includes the presence of hypotension or shock, ileus, or megacolon. 26
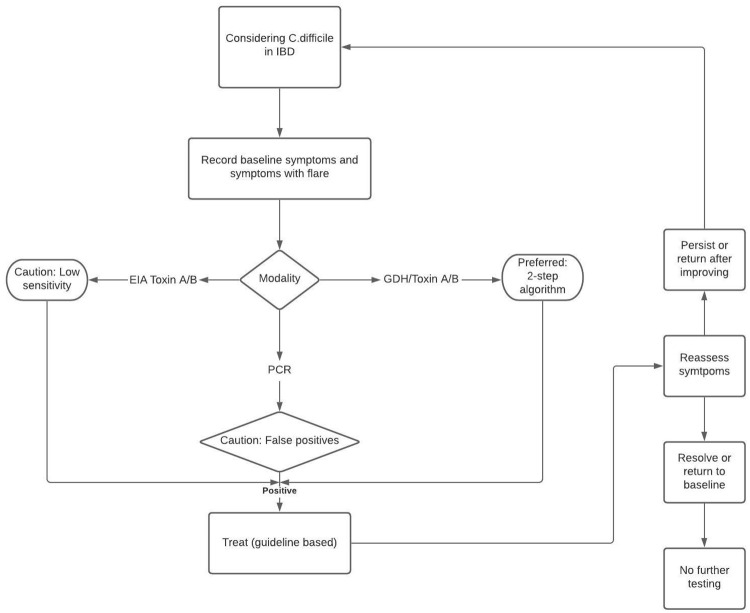
Patients who present with symptoms of an IBD flare-up should undergo testing for CDI. Positive lab results should be followed by guideline-based treatment with a frequent reassessment of symptoms (see Figure 2 for the broad management of symptoms and Figure 3 for treatment guidelines for CDI in IBD patients).3,26 As an acute CDI episode in IBD patients is associated with increased colectomy rates and lengthy hospital stays, IBD should be considered as a CDI severity marker. These patients, especially those positive for severity indicators (severe abdominal pain, profuse diarrhea, and markedly increased leukocyte count) should be aggressively managed as inpatients.12,27 Broadly speaking, there are three treatment options available for CDI treatment; pharmacotherapy, fecal microbiota transplantation, and surgery.
Figure 2.
Broad approach to management of CDI in patients with IBD.
CDI, Clostridioides difficile infection; IBD, irritable bowel disease.
Figure 3.
Guideline based treatment of CDI in IBD patients.
aFor patients who have delayed response to 10-day treatment, the therapy duration should be increased to 14 days.
bVancomycin taper regimen: VAN 125mg QID for 10–14 days, two times per day for a week, once per day for a week, and then every 2 or 3 days for 2–8 weeks.
*Strong strength of recommendation.
BID, 2 times a day; CDI, Clostridioides difficile infection; FDX, fidaxomicin; IBD, irritable bowel disease; MNZ, metronidazole; QID, 4 times a day; TID, 3 times a day; VAN, vancomycin.
An initial episode of CDI in an IBD patient can be treated with vancomycin (an extended regimen may be considered) or fidaxomicin for 10 days. Metronidazole is no longer recommended for CDI treatment, especially in patients of IBD.3,26 A retrospective observational study in patients with IBD showed that the patients with UC treated with vancomycin for non-severe CDI had shorter hospital stays and less frequent readmissions as compared with metronidazole therapy [length of stay, 6.38 days versus 13.62 days (p = 0.02); 30-day readmissions 0% versus 31% (p = 0.04)]. 28
Another study investigating the relationship between the duration of antibiotic treatment and recurrence rates of CDI in IBD patients showed that long duration (21–42 days) vancomycin is associated with significantly lower recurrence rates as compared with short duration (10–14 days) vancomycin (1.8% versus 11.7%; p = 0.043). 29 Fidaxomicin is used as an alternative to vancomycin for the treatment of CDI. There are limited data on fidaxomicin as a therapeutic option of CDI in IBD patients. A retrospective study measuring the efficacy of fidaxomicin in the treatment of CDI in IBD patients showed resolution of diarrhea in 81.8% patients (n = 22), with a 30-day recurrence of 19% (n = 21). 30
A first recurrence is treated with fidaxomicin for 10 days (if vancomycin was used for the initial episode), or with a prolonged pulsed and tapered regimen of vancomycin. Studies also suggest that fidaxomicin extended regimens may be beneficial as well (Figure 3). Recent data evaluating the outcomes of fidaxomicin extended regimen versus standard vancomycin in patients with CDI showed that extended fidaxomicin was superior to standard vancomycin for sustained clinical cure of CDI. 31 Although fidaxomicin appears to be the better drug with statistical significance, its cost continues to be an ongoing challenge, with cost-effectiveness analyses demonstrating a benefit for fidaxomicin. 32 Patient assistance programs have been established in order to help cover the cost of fidaxomicin for eligible individuals. For further recurrences, prolonged vancomycin regimens or vancomycin followed by rifaximin can be used. In addition, fidaxomicin for 10 days or vancomycin or fidaxomicin followed by FMT are recommended treatment options (Figure 3).
One of the newer therapies approved by the FDA for recurrent CDI is bezlotoxumab (a monoclonal antibody against the C. difficile toxin B). 33 Although its efficacy has not been studied separately on patients with IBD, phase III clinical trials have shown that bezlotoxumab alone, when used as an adjunct with the standard of care antibiotic therapy leads to a significant decrease in the recurrence rate of CDI as compared with the placebo group (MODIFY I: 17% versus 28%; p < 0.001; MODIFY II: 16% versus 26%; p < 0.001). 34 A post hoc analysis showed that the most significant risk reduction from bezlotoxumab was seen in the high-risk patient groups (age ⩾65 years, severe CDI, immunocompromised state, and prior episode of CDI with the hypervirulent B1/NAP1/027). 35 A post hoc analysis in the IBD sub-population from phase III clinical trials showed a trend of reduction in recurrent CDI in the bezlotoxumab group compared with placebo [27.2% absolute reduction with 95% confidence interval (CI) of −57.9 to 9.6]. 36
Fecal microbiota transplantation is emerging as a preferred treatment for multiple, recurrent CDIs in patients with underlying IBD. A longitudinal retrospective cohort study conducted on 134 patients (46 with IBD and 88 without IBD) showed no difference in CDI recurrence in the two groups at 2 months (22.5% versus 17.9%; p = 0.63) and 6 months (38.7% versus 36.5%; p > 0.99). 37 Another retrospective cohort study comparing CDI in IBD and non IBD patients showed no significant difference in their response rate to FMT (87% versus 75%; p = 0.13), CDI relapse rate (17% versus 25%; p = 0.38), or re-infection rates (14% versus 20% p = 0.46). 38
A prospective, multicenter cohort study evaluating IBD and the safety outcomes of FMT showed that the transplantation was safe and well-tolerated, with 67.3% (n = 49) of patients showing improvement in IBD post-FMT (measured by Harvey-Bradshaw Index score and Mayo score). Overall, 30.6% of the patients showed no change in their IBD status, while one patient showed a de novo flare of IBD. 39 One prospective study conducted on 35 IBD patients suffering from recurrent CDI showed negative CDI testing in 97% of patients post-FMT. Side effects included IBD flares requiring immunosuppression escalation in 54% of patients, surgery (diverting ileostomy or total proctocolectomy) in 6% of patients, and perianal abscess/fistulas in 9% of patients. 40
Another study carried out with 56 patients showed 85.7% efficacy of FMT in treating recurrent CDI in patients suffering from IBD (measured as the disappearance of toxin B on stool PCR). A flare of underlying IBD was seen in 57.1% of patients with UC, compared with 10.7% seen in Crohn’s disease (p = 0.002). Flares were more frequent in patients who had either an underlying primary sclerosing cholangitis associated with IBD, or a moderate or higher severity score on endoscopy. 41 Another multicenter study showed a 79% (n = 67) cure rate of CDI with the initial dose of FMT, which improved to 90% after repeat FMTs. Overall IBD outcomes at 3rd-month post-FMT were improved in 46.3% of patients, while 35.8% showed no change, and 17.9% showed worsening of IBD. 42
At the same time, studies require caution for the use of FMT for IBD. One study found that FMT is slightly less effective in resolving recurrent CDI in IBD patients, and that more than 25% of patients with IBD had a flare-up of their disease after FMT. 43 One case report described a flare-up of UC following FMT in a patient who had been quiescent for more than 20 years. 44 A similar case report described the development of arthralgia and erythema nodosa in a patient with Crohn’s disease who was treated with FMT for CDI. 45 One study reported bacteremia after FMT in a patient with Crohn’s disease; the authors suggested that this was likely due to the altered intestinal permeability related to Crohn’s disease. 46 Another study described two patients developing extended spectrum beta-lactamase producing Escherichia coli bacteremia after FMT conducted for research indications in non-IBD patients and both these cases were associated with the same stool donor. 47 Patients with IBD who had CDI did not develop this infection, despite receiving stool from the same donor. 47 These data are balanced by studies that do not show adverse events from FMT in IBD patients.39,48 With a rare chance of adverse events from FMT, overall, FMT appears to be safe and effective for managing recurrent CDI in IBD patients with a small chance of an IBD flare-up.
Managing IBD in patients with IBD and CDI
On the one hand, CDI could lead to a flare-up of underlying IBD; on the other hand, the use of immunosuppressive medications for the management of IBD could worsen CDI. Prospective or randomized controlled data to guide a clinical decision of escalating, de-escalating, or even withholding immunosuppressive therapy is lacking. Retrospective studies have revealed conflicting data on the matter. A multicenter retrospective cohort analyzed IBD patients complicated with CDI and found that immunosuppressive therapy escalation (with corticosteroids or biologic agents) within 90 days of CDI was not associated with worse clinical outcomes. 49 Another retrospective cohort illustrated that within 3 months of admission, there was a 12% rate of adverse outcomes of in-hospital megacolon, bowel perforation, shock, respiratory failure, colectomy, or death in patients managed with antibiotics and immunosuppression (including prednisone, thiopurines, methotrexate, cyclosporine, tacrolimus, or biologics of any kind). 50 These adverse outcomes were not observed in patients who were managed with antibiotics alone. One study demonstrated that escalating corticosteroids for patients with IBD and CDI had a higher risk of downstream colon surgery, but adverse outcomes did not differ with changing the dosage of non-steroid immunosuppression regimens, such as azathioprine, 6-mercaptopurine, methotrexate, and biologics. 51 Another retrospective cohort aimed to evaluate the effect of early corticosteroid use on the outcomes of IBD patients complicated by CDI. 52 More than 70% of patients received corticosteroids within 48 h of admission and there was no observed difference in the colectomy rates. However, the length of hospital stay was reduced in patients who did not receive early corticosteroids.
Despite a lack of data, withholding immunosuppressive therapy to treat IBD patients complicated with CDI is not advisable. A decision to modify management in such patients should only be made after thorough clinical assessment and weighing up the risks and benefits. If there are ongoing signs of active colitis 3–5 days after the initiation of antibiotic therapy for CDI management, escalation of immunosuppression is recommended.
If CDI is detected in an IBD patient presenting with a flare, antibiotics such as vancomycin or fidaxomicin should be initiated for treatment. The patient’s status must be evaluated for the next 3–4 days. If the patient continues to experience signs of colitis without any improvement, escalation of immunosuppression should be considered. In addition, the evaluation for other causes such as cytomegalovirus by endoscopy is recommended. However, if there is improvement in symptoms, the patient’s ongoing immunosuppression regimen can be continued and the antibiotic course can be completed over 10 days.
Conclusions
C difficile infection is one of the most common complications in patients with IBD. To initiate appropriate therapy, it is imperative to differentiate CDI from a flare-up of IBD. The diagnosis of CDI is recommended by a two-step approach. CDI can be treated with antibiotics such as vancomycin or fidaxomicin and recurrence prevention strategies have to be implemented. FMT can be considered in patients with recurrent CDI. The decision to escalate or de-escalate immunosuppression therapy for IBD patients with superimposed CDI is made on an individual basis and must entail a thorough clinical assessment with evaluation of risks and benefits.
Footnotes
Conflict of interest statement: The authors declare that there is no relevant conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sahil Khanna  https://orcid.org/0000-0002-7619-8338
https://orcid.org/0000-0002-7619-8338
Contributor Information
Kanika Sehgal, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Devvrat Yadav, Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA.
Sahil Khanna, Division of Gastroenterology and Hepatology, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA.
References
- 1. Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol 2016; 14: 609–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsuoka K, Kobayashi T, Ueno F, et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J Gastroenterol 2018; 53: 305–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khanna S, Shin A, Kelly CP. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the clinical practice updates committee of the AGA institute. Clin Gastroenterol Hepatol 2017; 15: 166–174. [DOI] [PubMed] [Google Scholar]
- 4. Bolton RP, Sherriff RJ, Read AE. Clostridium difficile associated diarrhoea: a role in inflammatory bowel disease? Lancet 1980; 1: 383–384. [DOI] [PubMed] [Google Scholar]
- 5. Mylonaki M, Langmead L, Pantes A, et al. Enteric infection in relapse of inflammatory bowel disease: importance of microbiological examination of stool. Eur J Gastroenterol Hepatol 2004; 16: 775–778. [DOI] [PubMed] [Google Scholar]
- 6. Rodemann JF, Dubberke ER, Reske KA, et al. Incidence of Clostridium difficile infection in inflammatory bowel disease. Clin Gastroenterol Hepatol 2007; 5: 339–344. [DOI] [PubMed] [Google Scholar]
- 7. Murthy SK, Steinhart AH, Tinmouth J, et al. Impact of Clostridium difficile colitis on 5-year health outcomes in patients with ulcerative colitis. Aliment Pharmacol Ther 2012; 36: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 8. Ananthakrishnan AN, McGinley EL, Binion DG. Excess hospitalisation burden associated with Clostridium difficile in patients with inflammatory bowel disease. Gut 2008; 57: 205–210. [DOI] [PubMed] [Google Scholar]
- 9. Saffouri G, Gupta A, Loftus EV, Jr, et al. The incidence and outcomes from Clostridium difficile infection in hospitalized adults with inflammatory bowel disease. Scand J Gastroenterol 2017; 52: 1240–1247. [DOI] [PubMed] [Google Scholar]
- 10. Sokol H, Lalande V, Landman C, et al. Clostridium difficile infection in acute flares of inflammatory bowel disease: a prospective study. Dig Liver Dis 2017; 49: 643–646. [DOI] [PubMed] [Google Scholar]
- 11. Khanna S, Pardi DS. Clinical implications of antibiotic impact on gastrointestinal microbiota and Clostridium difficile infection. Expert Rev Gastroenterol Hepatol 2016; 10: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 12. Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol 2007; 5: 345–351. [DOI] [PubMed] [Google Scholar]
- 13. Balram B, Battat R, Al-Khoury A, et al. Risk factors associated with Clostridium difficile infection in inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis 2019; 13: 27–38. [DOI] [PubMed] [Google Scholar]
- 14. Allegretti JR, Kearney S, Li N, et al. Recurrent Clostridium difficile infection associates with distinct bile acid and microbiome profiles. Aliment Pharmacol Ther 2016; 43: 1142–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hunt JJ, Ballard JD. Variations in virulence and molecular biology among emerging strains of Clostridium difficile. Microbiol Mol Biol Rev 2013; 77: 567–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viazis N, Pontas C, Karmiris K, et al. Prevalence of Clostridium difficile infection among hospitalized inflammatory bowel disease patients in Greece. Eur J Gastroenterol Hepatol 2019; 31: 773–776. [DOI] [PubMed] [Google Scholar]
- 17. Kelly CP, LaMont JT. Clostridium difficile–more difficult than ever. N Engl J Med 2008; 359: 1932–1940. [DOI] [PubMed] [Google Scholar]
- 18. Ben-Horin S, Margalit M, Bossuyt P, et al. Prevalence and clinical impact of endoscopic pseudomembranes in patients with inflammatory bowel disease and Clostridium difficile infection. J Crohns Colitis 2010; 4: 194–198. [DOI] [PubMed] [Google Scholar]
- 19. Weber P, Koch M, Heizmann WR, et al. Microbic superinfection in relapse of inflammatory bowel disease. J Clin Gastroenterol 1992; 14: 302–308. [DOI] [PubMed] [Google Scholar]
- 20. Kochhar R, Ayyagari A, Goenka MK, et al. Role of infectious agents in exacerbations of ulcerative colitis in India. A study of Clostridium difficile. J Clin Gastroenterol 1993; 16: 26–30. [DOI] [PubMed] [Google Scholar]
- 21. Jodorkovsky D, Young Y, Abreu MT. Clinical outcomes of patients with ulcerative colitis and co-existing Clostridium difficile infection. Dig Dis Sci 2010; 55: 415–420. [DOI] [PubMed] [Google Scholar]
- 22. Palacios Argueta P, Salazar M, Attar B, et al. 90-day specific readmission for Clostridium difficile infection after hospitalization with an inflammatory bowel disease flare: outcomes and risk factors. Inflamm Bowel Dis 2021; 27: 530–537. [DOI] [PubMed] [Google Scholar]
- 23. Razik R, Rumman A, Bahreini Z, et al. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the RECIDIVISM study. Am J Gastroenterol 2016; 111: 1141–1146. [DOI] [PubMed] [Google Scholar]
- 24. Ricciardi R, Ogilvie JW, Jr, Roberts PL, et al. Epidemiology of Clostridium difficile colitis in hospitalized patients with inflammatory bowel diseases. Dis Colon Rectum 2009; 52: 40–45. [DOI] [PubMed] [Google Scholar]
- 25. Ananthakrishnan AN, Guzman-Perez R, Gainer V, et al. Predictors of severe outcomes associated with Clostridium difficile infection in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2012; 35: 789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66: e1–e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jen MH, Saxena S, Bottle A, et al. Increased health burden associated with Clostridium difficile diarrhoea in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2011; 33: 1322–1331. [DOI] [PubMed] [Google Scholar]
- 28. Horton HA, Dezfoli S, Berel D, et al. Antibiotics for treatment of Clostridium difficile infection in hospitalized patients with inflammatory bowel disease. Antimicrob Agents Chemother 2014; 58: 5054–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lei DK, Ollech JE, Andersen M, et al. Long-duration oral vancomycin to treat clostridioides difficile in patients with inflammatory bowel disease is associated with a low rate of recurrence. Am J Gastroenterol 2019; 114: 1904–1908. [DOI] [PubMed] [Google Scholar]
- 30. Vehreschild M, Taori S, Goldenberg SD, et al. Fidaxomicin for the treatment of Clostridium difficile infection (CDI) in at-risk patients with inflammatory bowel disease, fulminant CDI, renal impairment or hepatic impairment: a retrospective study of routine clinical use (ANEMONE). Eur J Clin Microbiol Infect Dis 2018; 37: 2097–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guery B, Menichetti F, Anttila VJ, et al. Extended-pulsed fidaxomicin versus vancomycin for Clostridium difficile infection in patients 60 years and older (EXTEND): a randomised, controlled, open-label, phase 3b/4 trial. Lancet Infect Dis 2018; 18: 296–307. [DOI] [PubMed] [Google Scholar]
- 32. Rajasingham R, Enns EA, Khoruts A, et al. Cost-effectiveness of treatment regimens for clostridioides difficile infection: an evaluation of the 2018 infectious diseases society of America guidelines. Clin Infect Dis 2020; 70: 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bartlett JG. Bezlotoxumab - A new agent for Clostridium difficile infection. N Engl J Med 2017; 376: 381–382. [DOI] [PubMed] [Google Scholar]
- 34. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection. N Engl J Med 2017; 376: 305–317. [DOI] [PubMed] [Google Scholar]
- 35. Gerding DN, Kelly CP, Rahav G, et al. Bezlotoxumab for prevention of recurrent Clostridium difficile infection in patients at increased risk for recurrence. Clin Infect Dis 2018; 67: 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kelly CP, Wilcox MH, Glerup H, et al. Bezlotoxumab for Clostridium difficile infection complicating inflammatory bowel disease. Gastroenterology 2018; 155: 1270–1271. [DOI] [PubMed] [Google Scholar]
- 37. Hirten RP, Grinspan A, Fu SC, et al. Microbial engraftment and efficacy of fecal microbiota transplant for Clostridium difficile in patients with and without inflammatory bowel disease. Inflamm Bowel Dis 2019; 25: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meighani A, Hart BR, Bourgi K, et al. Outcomes of fecal microbiota transplantation for Clostridium difficile infection in patients with inflammatory bowel disease. Dig Dis Sci 2017; 62: 2870–2875. [DOI] [PubMed] [Google Scholar]
- 39. Allegretti JR, Kelly CR, Grinspan A, et al. Inflammatory bowel disease outcomes following fecal microbiota transplantation for recurrent C. difficile infection. Inflamm Bowel Dis. Epub ahead of print 6 November 2020. DOI: 10.1093/ibd/izaa283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chin SM, Sauk J, Mahabamunuge J, et al. Fecal microbiota transplantation for recurrent Clostridium difficile infection in patients with inflammatory bowel disease: a single-center experience. Clin Gastroenterol Hepatol 2017; 15: 597–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Newman KM, Rank KM, Vaughn BP, et al. Treatment of recurrent Clostridium difficile infection using fecal microbiota transplantation in patients with inflammatory bowel disease. Gut Microbes 2017; 8: 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fischer M, Kao D, Kelly C, et al. Fecal microbiota transplantation is safe and efficacious for recurrent or refractory Clostridium difficile infection in patients with inflammatory bowel disease. Inflamm Bowel Dis 2016; 22: 2402–2409. [DOI] [PubMed] [Google Scholar]
- 43. Khoruts A, Rank KM, Newman KM, et al. Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol 2016; 14: 1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Leon LM, Watson JB, Kelly CR. Transient flare of ulcerative colitis after fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol 2013; 11: 1036–1038. [DOI] [PubMed] [Google Scholar]
- 45. Teich N, Weber M, Stallmach A. First occurrence of severe extraintestinal manifestations of Crohn’s disease following faecal microbiota transplantation. J Crohns Colitis 2016; 10: 1254–1255. [DOI] [PubMed] [Google Scholar]
- 46. Quera R, Espinoza R, Estay C, et al. Bacteremia as an adverse event of fecal microbiota transplantation in a patient with Crohn’s disease and recurrent Clostridium difficile infection. J Crohns Colitis 2014; 8: 252–253. [DOI] [PubMed] [Google Scholar]
- 47. DeFilipp Z, Bloom PP, Torres Soto M, et al. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N Engl J Med 2019; 381: 2043–2050. [DOI] [PubMed] [Google Scholar]
- 48. Tariq R, Disbrow MB, Dibaise JK, et al. Efficacy of fecal microbiota transplantation for recurrent C. difficile infection in inflammatory bowel disease. Inflamm Bowel Dis 2020; 26: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 49. Lukin DJ, Lawlor G, Hudesman DP, et al. Escalation of immunosuppressive therapy for inflammatory bowel disease is not associated with adverse outcomes after infection with Clostridium difficile. Inflamm Bowel Dis 2019; 25: 775–781. [DOI] [PubMed] [Google Scholar]
- 50. Ben-Horin S, Margalit M, Bossuyt P, et al. Combination immunomodulator and antibiotic treatment in patients with inflammatory bowel disease and Clostridium difficile infection. Clin Gastroenterol Hepatol 2009; 7: 981–987. [DOI] [PubMed] [Google Scholar]
- 51. Solanky D, Pardi DS, Loftus EV, et al. Colon surgery risk with corticosteroids versus immunomodulators or biologics in inflammatory bowel disease patients with Clostridium difficile infection. Inflamm Bowel Dis 2019; 25: 610–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bar-Yoseph H, Daoud H, Ben Hur D, et al. Does early corticosteroid therapy affect prognosis in IBD patients hospitalized with clostridioides difficile infection? Int J Colorectal Dis 2020; 35: 513–519. [DOI] [PubMed] [Google Scholar]