Abstract
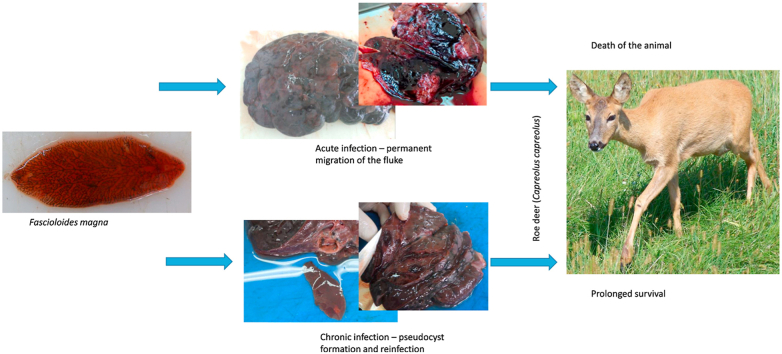
Fascioloidosis is an allochthonous parasitic disease in Europe caused by the digenean trematode Fascioloides magna. The final hosts of F. magna in Europe are defined as definitive, aberrant and dead-end. Roe deer are aberrant hosts in which juvenile flukes permanently migrate through the liver parenchyma. Failure in pseudocysts formation leads to the death of both the host and the parasite. In this paper we present gross and histological findings of F. magna infection in 34 roe deer. The special emphasis is on the pseudocyst formation accompanied with new fluke's migratory channels observed in 7 animals, suggesting reinfection and prolonged survival of roe deer. No F. magna eggs were recovered from the faeces of the infected animals. These findings indicate that pseudocyst formation is essential for roe deer survival, but also point out the potential beginning of adaptation processes in roe deer, altering otherwise acute and fatal disease into a chronic one.
Keywords: Roe deer, Fascioloides magna, Host-parasite interaction, Chronic infection, Adaptation
Graphical abstract
Highlights
-
•
Allochthonous parasite.
-
•
Roe deer and F. magna in some parts of Europe form relatively new host-parasite association.
-
•
F. magna as a high risk for local survival of roe deer.
-
•
Pseudocyst formation and reinfection as indicators of chronic infection and prolonged survival of infected roe deer.
1. Introduction
Parasitism is defined as a relationship between two organisms in which the parasite uses the host as its habitat and source of nutrients at the same time (Dogiel, 1964). According to some parasitologists, the definition of this relationship must include the potential of a parasite to cause harmful effects to the host, when it can also be called a pathogen (Crofton, 1971). While the definition of parasitism is mostly oriented towards the parasite, the host-parasite interaction defines the relationship between the host and a parasite, in which first one tries to remove the parasite from the organism, while the other one tries to avoid the host's immune responses, and thereby continue to live in it. This is a dynamic process/coevolution, which in part may be compared to the predator-prey coevolution. Since digenean trematode Fascioloides magna is an allochthonous parasite in Europe, its interaction with both intermediate and final hosts are extremely important to monitor.
Roe deer are Laurasiatherian mammal from the order Cetartiodactyla and the family Cervidae (Hu et al., 2012). They reach the weight of approximately 25 kg (Janicki et al., 2007). Roe deer were the most abundant game species in Croatia, however, populations in eastern Slavonia, Baranja and Posavina have dramatically decreased over the last decade (data available from the Croatian Central Hunting Database, https://sle.mps.hr). The reasons for this are probably multifactorial, but Fascioloides magna stands among them and is designated as a high-risk factor. Roe deer are characterized as aberrant hosts for F. magna in Europe and is the only cervid in this group of hosts. In aberrant hosts the pseudocysts fail to develop in the liver parenchyma, leading to the constant migration of the juvenile fluke in the liver, causing excessive tissue damage and severe hemorrhages, and often the death of the animal (Králová-Hromadová et al., 2016). This massive tissue damages, often seen also in lungs and spleen, are caused even by a low intensity of infection (Conboy and Stromberg, 1991; Králová-Hromadová et al., 2016).
In this paper we present findings on livers of 34 roe deer infected with F. magna, with special emphasis on a chronic infection.
2. Material and methods
Sampling for this study was performed during the regular hunting management operations. Liver and fecal samples of 227 roe deer were collected from the area of the Vukovarsko-srijemska County (VSC, n = 8), the Sisačko-moslavačka County (SMC, n = 22), the Brodsko-posavska County (BPC, n = 31) and Bjelovarsko-bilogorska County (BBC, n = 165). Samples were collected by hunters and transported along with an information sheet (sex, estimated age, and location) to the Veterinary Faculty for further analysis. The age of the animals was estimated by game wardens using dental wear and the general appearance of the live animal as criteria (Wagenknecht, 1984).
The livers were examined macroscopically for fibrin deposits, shape, size, irregular formations, translucency of the Glisson's capsule, traces of iron-porphyrin, and the overall appearance of the hepatic lymph nodes. Each liver was photographed from both sides and cut into approx. 2 cm thick slices. Each slice was thoroughly examined from both sides. Gross lesions and flukes were analyzed and categorized according to their features as: the flukes' migratory path, pseudocysts, degrading pseudocysts, juvenile and adult flukes. Livers containing iron-porphyrin staining and juvenile flukes with their migratory paths were categorized as acutely infected. In the case when both juvenile and adult flukes, migratory paths and any kind of pseudocyst (active or degraded) were detected, livers were regarded as chronically infected. Tissue samples were collected, stored in 10% buffered formalin, sectioned and stained routinely using H&E. Fecal samples were analyzed by the standard flotation method (Zajac and Conboy, 2012) using ZnSO4 of specific gravity 1.30.
3. Results
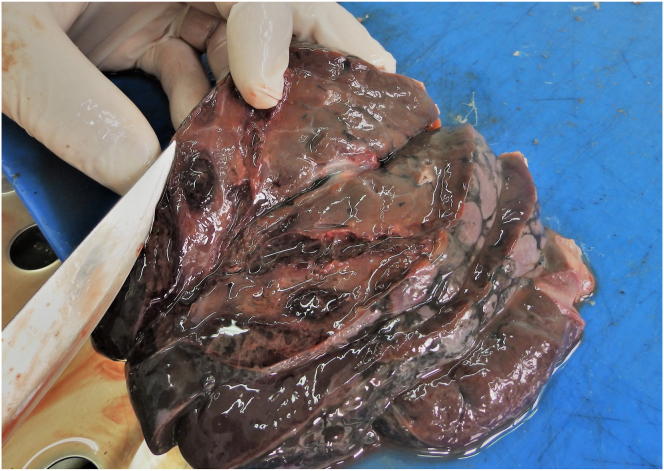
Out of the 227 roe deer samples analyzed, 34 were positive for F. magna infection (P = 14.97%). According to the County, the highest prevalence was determined in the SMC (P = 22.72%), followed by the BBC (P = 14.02%) and BPC (P = 12.90%). None of the animals was positive in VSC sample. In the case of infected animals, the diaphragmatic surface of the livers was covered by various amount of fibrinous deposits. In case of excessive fibrin deposition, the diaphragm was attached to the liver surface. The liver surface was irregular, mainly containing dark stained protruding nodules (Fig. 1). Glisson's capsule was opaque and traces of iron-porphyrin were visible. On the cut surface of the livers, the flukes' migratory paths were detected, followed by excessive hemorrhages (Fig. 2). These migratory paths were usually determined as single, and only four animals had several migratory channels. Pseudocysts were found in 14 animals, representing 41.17% of the positive animals. There was an important finding of the flukes' migratory paths, juvenile flukes and active pseudocysts with sexually mature flukes, or degrading pseudocysts in the livers of 7 animals, which suggests the longer survival of the infected roe deer (Fig. 3, Fig. 4). In these livers fluke migratory paths were not accompanied by excessive hemorrhages (Fig. 4). Proportions of each type of lesion or fluke developmental stages are presented in the Table (number of infected livers was used as denominator) (Table 1). Histological analysis revealed numerous F. magna eggs in the liver samples indicating sexual maturation of the flukes (Fig. 5). However, all fecal samples were negative for F. magna eggs.
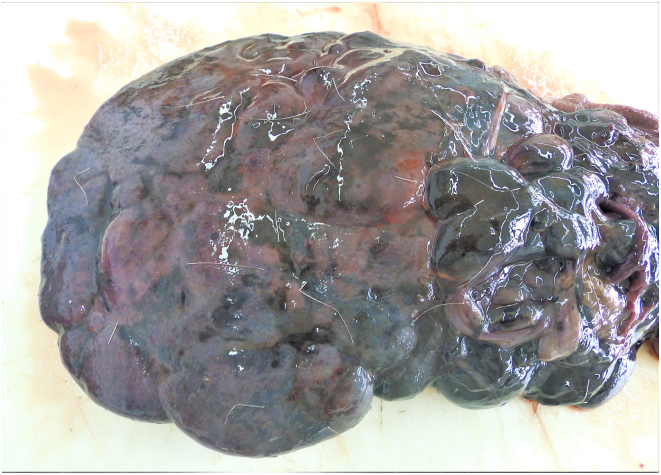
Fig. 1.
Acutely infected roe deer liver. Irregular liver surface with opaque Glissons capsule, traces of dark pigment and nodular protrusions (hemorrhages).
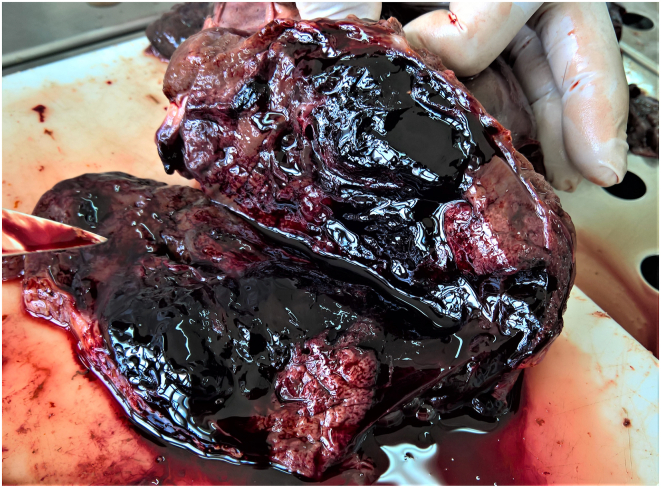
Fig. 2.
Flukes' migratory paths with excessive hemorrhages.
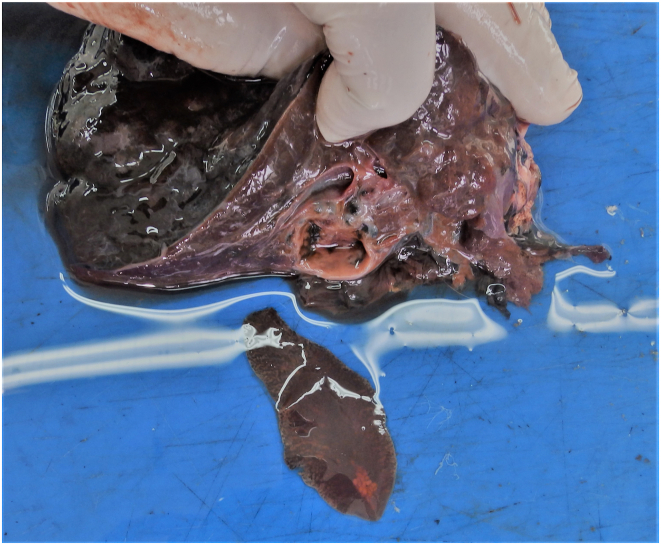
Fig. 3.
Cut surface of roe deer liver. Note the pseudocyst and numerous iron-porphyrin traces in the liver parenchyma and fluke extracted from the pseudocyst.
Fig. 4.
Same liver as in Fig. 3. Left part of the liver contains new migratory channels. Note less pronounced hemorrhages and clean migratory channels, as well as traces of iron-porphyrin on surface of the livers.
Table 1.
Proportions of different lesions or fluke developmental stage. Proportions were calculated from infected livers, calculation were made based on number of appearances of each finding.
| Type of lesions or fluke | Fluke migratory paths | Juvenile flukes | Adult flukes | Newly formed pseudocyst | Pseudocyst | Degraded pseudocyst |
|---|---|---|---|---|---|---|
| Proportions | 70.58 | 55.58 | 32.35 | 8.82 | 47.05 | 26.47 |
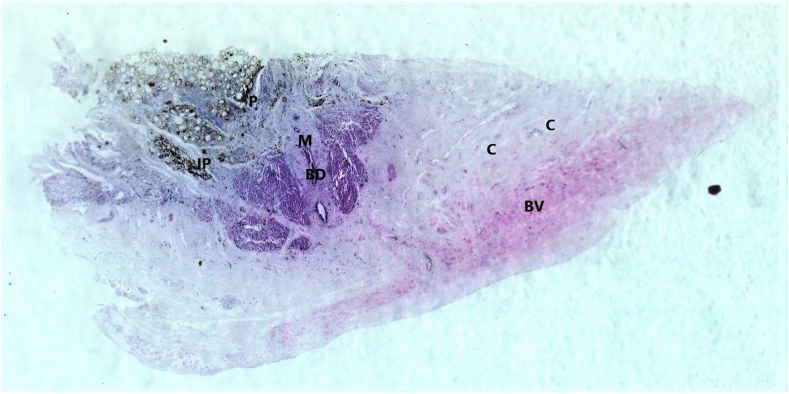
Fig. 5.
H&E liver pseudocyst fragment (Sony alpha 6000). The center of the cyst containing numerous F. magna eggs, traces of free (P) and phagocytosed intracellular iron-porphyrin (IP) embedded in thick collagenous tissue (C), surrounded by numerous irregular bile ducts (B), new blood vessels (BV) and hypertrophied smooth muscle bundles. Magnification 2x.
4. Discussion
The large American liver fluke Fascioloides magna was described for the first time by the Italian veterinarian Roberto Bassi, and was named Distomum magnum. It was described based on the gross lesions observed in the liver of a wapiti deer from the Royal Park La Mandria, near the city of Turin in Italy (Bassi, 1875). It took some time before it was finally renamed as Fascioloides magna (Ward, 1917). Since its arrival to Europe, F. magna has established host-parasite associations with three types of wild final hosts, definitive (red and fallow deer), aberrant (roe deer, mouflon) and dead-end (wild boar) (Králová-Hromadová et al., 2016). According to Králová-Hromadová et al. (2016) in the case of aberrant hosts, juvenile F. magna penetrates the intestinal wall and travels through the abdominal cavity searching for the liver. In this type of hosts, the course of events may vary between individuals from the outset. The unrestricted migration of the fluke through the liver, lungs and peritoneal cavity is typical, causing excessive tissue damage and frequently the death of the host (Conboy and Stromberg, 1991; Králová-Hromadová et al., 2016). If the fluke manages to reach the liver, it will continue to migrate through the parenchyma, avoiding the host's immune responses. This will result in permanent migration with consequent failure of pseudocyst formation, heavy tissue damage, and excessive hemorrhages (Králová-Hromadová et al., 2016). Contrary to the nature of infection development, the fate of the infection is almost certainly the death of both the host and the parasite.
As stated before, host-parasite interactions are dynamic and it can be expected that both sides will try to adapt to this situation. It is just a question of how long it will take and what casualties it will cause. Unfortunately, this coevolution is rarely studied in wild, natural conditions and mainly relies on microparasite infections and small hosts with short generation time (Feis et al., 2016). Using the example of a small vertebrate model, three-spined sticklebacks (Gasterosteus aculeatus), Eizaguirre et al. (2012) have already demonstrated the rapid adaptive evolution of the MHC genes in the case of a G2 fish. On a large wild model, red deer (Cervus elaphus), Bujanić (2019) has demonstrated the presence of the specific allele of the exon 2 of the DRB loci of the MHC class II genes, with a potential role in resistance against the F. magna infection. These findings suggest that the potential adaptation of the host to a relatively new parasite might be more rapid than generally expected. Anyhow, it is difficult to estimate the expected time frame of such coevolution, but it can be studied if parasites are taken from different regions (Feis et al., 2016). In Europe F. magna represents a good wild model to analyze host-parasite coevolution since it is an allochthonous parasite, making it possible to mimic experimental conditions in the wild through case-control studies. Furthermore, its spread over Europe can be easily monitored due to excessive tissue damages that it causes, its large size and already observed reduction in population size of aberrant hosts. As stated before, F. magna is present in Europe for some 150 years, but its spread over Croatia started approximately 20 years ago, and is still an ongoing process. Also, import of F. magna to Europe have probably induced not only adaptations of hosts as speculated in this article, but also adaptations of the fluke to new intermediate and final hosts. This is supported by the fact that spatial variations in interactions between parasites and hosts (especially new hosts) is a major force in the coevolutionary process (see in Dybdahl and Storfer, 2003). Potential adaptation that would enable the survival of the F. magna in the dead-end host was described by Konjević et al. (2017) in the case of wild boar. In that particular case they found thin-walled pseudocysts in a wild boar (which is characteristic for definitive hosts), containing up to 7 cm long, sexually mature flukes with numerous eggs.
Similar to our findings, pseudocyst formation in roe deer was also described by Demiaszkiewicz et al. (2018) in Poland. They described one positive roe deer (out of 20 examined), which had 5 pseudocysts, of which three contained intact flukes, eggs and cell detritus. In our study, we found 14 animals with pseudocysts, 7 of which had both pseudocysts containing sexually mature flukes and migratory juvenile flukes at the same time. This indicates that these animals had survived the initial infection and were re-infected. Following a case described by Demiaszkiewicz et al. (2018), our findings on a larger sample indicate potential adaptation trend in roe deer, rather than accidental finding. If placed within a time frame, this new moment occurs 18 years after the first discovery of F. magna in Croatia (Marinculić et al., 2002). In the case of SMC and BBC roe deer, this period is even shorter (up to 10 years). Calculating a prevalence of infection in roe deer is of less importance since still large proportions of infected animals will die due to the acute infection, and will be probably lost due to dense vegetation, rapid degradation or predation/scavenging.
Regarding the impact of environmental factors that should be considered here (Ashby et al., 2019), it is noteworthy to recall that roe deer are, unlike red deer, territorial animals that live mainly in small groups. The characteristics of each territory (type of soil, possible flooding, the existence of micro depressions, the presence of intermediate hosts, etc.) are major risk factors for F. magna infection, as seen in the case of red deer (Konjević et al., 2018). Animals that live in drier habitats will have a higher chance to remain uninfected, and therefore to survive. Another environmental risk factor is the size of the infected red deer population. As a typical, definitive host, red deer shed parasite eggs into the environment, exerting higher environmental pressure and increasing the risk of infection in roe deer.
Declaration of competing interest
The authors declare no competing financial or non-financial interests. We have no conflicts of interest to report.
Acknowledgements
The study was fully supported by the Croatian Science Foundation grant No. 8963, ‘‘Host-parasite interaction: relationship between three types of hosts and Fascioloides magna infection’‘.
References
- Ashby B., Iritani R., Best A., White A., Boots M. Understanding the role of eco-evolutionary feedbacks in host-parasite coevolution. J. Theor. Biol. 2019;464:115–125. doi: 10.1016/j.jtbi.2018.12.031. [DOI] [PubMed] [Google Scholar]
- Bassi R. Sulla cachessia ittero-verminosa, o marciaia dei cervi, causata dal Distoma magnum. Med. Vet. 1875;4:497–515. [Google Scholar]
- Bujanić M. University of Zagreb, The Faculty of Veterinary Medicine; Zagreb, Croatia: 2019. Variability of the Genes of Major Histocompatibility Complex in Red Deer (Cervus elaphus L.) in Relation to Fascioloides Magna Infection. PhD Thesis. [Google Scholar]
- Conboy G.A., Stromberg B.E. Hematology and clinical pathology of experimental Fascioloides magna infection in cattle and Guinea pigs. Vet. Parasitol. 1991;40:241–255. doi: 10.1016/0304-4017(91)90104-4. [DOI] [PubMed] [Google Scholar]
- Crofton H.D. A quantitative approach to parasitism. Parasitology. 1971;62:179–193. [Google Scholar]
- Demiaszkiewicz A.W., Kowalczyk R., Filip K.J., Pyziel A.M. Fascioloides magna (Bassi, 1875) pasożytem sarny w Borach Zielonogórskich. Med. Weter. 2018;74:257–260. doi: 10.21521/mw.6037. [DOI] [Google Scholar]
- Dogiel V.A. Oliver and Boyd; London: 1964. General Parasitology. [Google Scholar]
- Dybdahl M.F., Storfer A. Parasite local adaptation: red queen versus suicide king. Trends Ecol. Evol. 2003;18:523–530. doi: 10.1016/S0169-5347(03)00223-4. [DOI] [Google Scholar]
- Eizaguirre C., Lenz T.L., Kalbe M., Milinski M. Rapid and adaptive evolution of MHC genes under parasite selection in experimental vertebrate populations. Nat. Commun. 2012;3:621. doi: 10.1038/ncomms1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feis M.E., Anouk Goedknegt M., Thieltges D.W., Buschbaum C., Wegner K.M. Biological invasions and host–parasite coevolution: different coevolutionary trajectories along separate parasite invasion fronts. Zoology. 2016;119:366–374. doi: 10.1016/j.zool.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Hu J.-Y., Zhang Y.-P., Yu L. Summary of laurasiatheria (mammalia) phylogeny. Zool. Res. 2012;33(E5–6):E65–E74. doi: 10.3724/SP.J.1141.2012.E05-06E65. [DOI] [PubMed] [Google Scholar]
- Janicki Z., Slavica A., Konjević D., Severin K. University of Zagreb, The Faculty of Veterinary Medicine; Zagreb, Croatia: 2007. Zoologija Divljači (In Croatian) [Google Scholar]
- Konjević D., Bujanić M., Erman V., Gudan Kurilj A., Živičnjak T., Severin K., Tomić S., Martinković F. New data on wild boar (Sus scrofa L.) a dead-end host for large American liver fluke (Fascioloides magna) Helminthologia. 2017;54:77–80. doi: 10.1515/helm-2017-0006. [DOI] [Google Scholar]
- Konjević D., Janicki Z., Calmels P., Stojčević Jan D., Marinculić A., Šimunović M., Pavlak M., Krapinec K., Poljak Z. Evaluation of factors affecting the efficacy of treatment against Fascioloides magna in wild red deer population. Vet. Ital. 2018;54:33–39. doi: 10.12834/VetIt.970.5051.1. [DOI] [PubMed] [Google Scholar]
- Králová-Hromadová I., Juhásová L., Bazsalovicsová E. Springer International Publishing AG; Switzerland: 2016. The Giant Liver Fluke, Fascioloides Magna: Past, Present and Future Research. [Google Scholar]
- Marinculić A., Džakula N., Janicki Z., Hardy Z., Lučinger S., Živičnjak T. Appearance of American liver fluke (Fascioloides magna, Bassi, 1875) in Croatia. Vet. arhiv. 2002;72:319–325. [Google Scholar]
- Wagenknecht E. Verlag J. Neumann - Neudamm; Melsungen, Germany: 1984. Alters Bestimmung des Erlegten Wildes. [Google Scholar]
- Ward H.B. On the structure and classification of North American parasitic worms. J. Parasitol. 1917;4:1–12. [Google Scholar]
- Zajac A.M., Conboy G.A. eighth ed. John Willey & sons, Ltd.; USA: 2012. Veterinary Clinical Parasitology. [Google Scholar]