Abstract
Purpose
To study post-interventional findings in patients with dysthyroid optic neuropathy (DON) treated with teprotumumab.
Observations
In this multicenter observational Case series, patients with DON were treated with teprotumumab, an insulin-like growth factor I receptor inhibitor (10 mg/kg for the first infusion then 20 mg/kg for subsequent infusions, every three weeks for a total 8 infusions). This study included patients with acute and chronic thyroid eye disease (TED) with DON who had failed conventional therapies and were not candidates for surgical decompression. Data collected included best corrected visual acuity (BCVA), color vision, RAPD when present, and orbital CT or MRI. Proptosis, clinical activity score (CAS), Gorman diplopia score (GDS), and Humphrey visual fields (HVF) were also evaluated.
Ten patients (6 women, 4 men) with an average age 64 years old were included in this study. Mean follow up after completion of infusions was 15 weeks. Baseline visual acuity (VA) impairment ranged from hand motion (HM) to 20/25 in affected eyes. All patients had pre-treatment orbital CT or MRI that confirmed orbital apex compression. Seventy percent of patients had objective improvement in DON after 2 infusions of teprotumumab measured as significant improvement in visual acuity, resolution of RAPD, or both. After completion of treatment, affected eyes had a mean BCVA improvement of 0.87 logMAR (p=0.0207), proptosis reduction of 4.7 mm (p<0.00001), CAS improvement of 5.25 points (p<0.00001), and GDS improvement of 0.75 points (p=0.160). All 6 patients who presented with an RAPD had resolution or improvement of RAPD. All 7 patients who presented with color vision deficits had normalization or improvement of color vision.
Conclusions and Importance
Teprotumumab infusions resulted in medical decompression and objective resolution or improvement of dysthyroid optic neuropathy. Most patients had rapid improvement of visual acuity and reversal of RAPD. Post-infusion imaging demonstrated reduction in extraocular muscle size that correlated with improvement in visual dysfunction. However, patients who presented with longstanding severe visual loss had limited improvement. There was no recurrence of DON after completion of teprotumumab in our cohort.
Keywords: Thyroid eye disease (TED), Compressive optic neuropathy (CON), Dysthyroid optic neuropathy (DON), Teprotumumab, Insulin-like growth factor I receptor (IGF-IR), Medical decompression, Apical crowding, Relative afferent pupillary defect (RAPD), Optic disc edema, Optic atrophy
Highlights
-
•
Teprotumumab is effective for the treatment of dysthyroid optic neuropathy.
-
•
Most patients demonstrated rapid objective improvement after 2 infusions.
-
•
Visual acuity improved and relative afferent pupillary defect resolved after treatment.
-
•
Orbital imaging showed improvement of orbital apex crowding after treatment.
-
•
Patients with longstanding severe vision loss had limited improvement in visual acuity.
1. Introduction
Thyroid eye disease (TED) frequently results in volumetric expansion of the extraocular muscles and orbital fat. These factors may lead to dysthyroid optic neuropathy (DON) secondary to compression of the optic nerve, a rare but serious complication of TED that can lead to permanent vision loss if not treated in a timely manner.1, 2, 3, 4 In DON, orbital imaging may demonstrate expansion of orbital fat volume, as well as effacement of the normal orbital fat separating the optic nerve and extraocular muscles. Although the extraocular muscles are frequently enlarged, it is also common to detect orbits with high orbital fat volume and relatively normal extraocular muscles. In addition to apical compression, the optic nerve may also appear stretched (thinner and straightened with tenting of the globe posteriorly).5,6 DON occurs in 1–8% of patients with TED.7 DON can present with decreased visual acuity, relative afferent pupillary defect (RAPD) if unilateral or asymmetric, red desaturation, color vision abnormalities, visual field defects, and loss of nerve fiber layer on optical coherence tomography (OCT).8 DON is traditionally managed with a variety of modalities, often in combination, including oral or intravenous (IV) high-dose corticosteroids, orbital radiation, and urgent surgical decompression.8
Teprotumumab, a human monoclonal antibody directed against the insulin-like growth factor I receptor (IGF-IR), was approved by the U.S. Food and Drug Administration (FDA) for the treatment of TED on January 21st, 2020. Although patients with optic neuropathy were excluded from the two original clinical efficacy trials, the trial data showed significant reduction in proptosis, clinical activity score (CAS), diplopia, and improvement in quality-of-life score in the treatment arm compared to placebo.9, 10, 11 Furthermore, orbital imaging in the Phase 3 trial demonstrated a reduction in extraocular muscle size (including at the orbital apex) making teprotumumab a potential therapeutic option for patients with DON.11
The purpose of this Case series was to study post-interventional findings in patients with dysthyroid optic neuropathy (DON) treated with teprotumumab.
2. Methods
In this observational Case series, patients with DON were treated with teprotumumab (10 mg/kg for the first infusion then 20 mg/kg for subsequent infusions) every three weeks for a total of 8 infusions per the protocol in clinical trials.9,11 DON was defined as visual loss secondary to thyroid eye disease related compression and/or stretching of the optic nerve. Patients either had acute (9 months or less duration) progressive active TED or chronic (10 months or greater duration) active TED. Patients were treated with teprotumumab because they were poor surgical candidates and had failed other interventions including oral prednisone, IV methylprednisolone, orbital radiation, and/or surgical orbital decompression with progression of DON despite prior interventions.
Patient gender, ethnicity, baseline thyroid status, and smoking status were documented. A complete eye examination was performed. Data collected included best corrected visual acuity (BCVA), color vision, RAPD or disc edema when applicable, Humphrey visual field (HVF), and orbital imaging with evidence of apical crowding or optic nerve stretch. Proptosis (Hertel exophthalmometer), CAS and Gorman diplopia score (GDS) were also evaluated. Baseline thyroid function tests included thyroid-stimulating hormone (TSH) and thyroid stimulating immunoglobulin (TSI). The change in the data from baseline to most recent post-treatment visit is reported for all patients.
Institutional Review Board (IRB)/Ethics Committee approval was obtained. This study adheres to the tenets of the Declaration of Helsinki as amended in 2013 and the Health Insurance Portability and Accountability Act. Written informed consent was obtained from all patients for publication of this Case series and for use of all identifiable photographs.
3. Results
Participants included 6 women and 4 men, ages 36–84 years (mean age 64-years-old). There were four Caucasians, two Asians, two African Americans, one Hispanic, and one East Asian Indian. Two patients were prior tobacco smokers (cases 4, 5). TED duration ranged from several weeks to greater than 10 years (Table 1). Average follow up was 15 weeks (range 0–33 weeks) after the last infusion.
Table 1.
Patient demographics and clinical features.
| Case | Age | Sex | Tobacco use | TED Duration | Prior treatment | TSH | TSI |
|---|---|---|---|---|---|---|---|
| 1 | 76 | F | Never | 10 years | IV/oral steroids, surgical decompression OU | 0.07 | 509 |
| 2 | 84 | F | Never | 1 year | IV steroids | 0.01 | 483 |
| 3 | 62 | F | Never | 6 months | IV steroids | <0.0025 | 380 |
| 4 | 73 | F | Prior | 1 year | IV steroids, surgical decompression OD | 3.41 | 453 |
| 5 | 45 | M | Prior | 1.5 year | IV steroids, orbital radiation OD | 0.48 | 610 |
| 6 | 55 | F | Never | 2 months | IV/oral steorids, surgical decompression OD | 0.11 | 330 |
| 7 | 73 | F | Never | 2 months | Oral steroids | 0.88 | 408 |
| 8 | 57 | M | Never | 10 years | IV steroids, surgical decompression OU, orbital radiation OU | 0.68 | <89 |
| 9 | 40 | M | Never | 10 months | IV/oral steroids | 0.16 | 389 |
| 10 | 76 | M | Never | 5 years | IV/oral steroids, surgical decompression OD | 0.01 | 353 |
IV = intravenous, OD = right eye, OS = left eye, OU = both eyes, TED = thyroid eye disease, TSH = thyroid-stimulating hormone, TSI = thyroid stimulating immunoglobulin.
All patients had pre-treatment orbital CT or MRI that confirmed orbital apex compression and/or stretching of the optic nerve with tenting of the globe. All patients had diagnostic findings supporting diagnosis of DON with decline in visual acuity (VA), presence of RAPD (when asymmetric), decreased color vision, and/or visual field defects on HVF. All patients had evidence of active inflammation with CAS greater than 3.
Seventy percent of patients had objective improvement after 2 infusions including either significant improvement in visual acuity, resolution of RAPD, or both. Six patients had an RAPD prior to treatment with teprotumumab; all had resolution (n=5) or improvement (n=1) of RAPD after one to two infusions of teprotumumab. Three patients did not have an RAPD due to symmetric bilateral involvement. All seven patients who had color vision deficits had rapid normalization (n=6) or improvement (n=1) of color vision after 2 infusions. Three patients presented with severe vision loss due to longstanding compressive optic neuropathy and had minimal objective improvement from pre-treatment VA of hand motion (HM) (Table 2).
Table 2.
Compressive optic neuropathy response to teprotumumab.
| Case | Laterality of CON | RAPD | Resolution of RAPD | VA OD/OS Pre Infusion | VA OD/OS Post Infusion | Color vision deficit | Normalization of color vision |
|---|---|---|---|---|---|---|---|
| 1 | OU | No | n/a | HM | 20/800 | n/aa | n/a |
| HM | 20/800 | ||||||
| 2 | OU | No | n/a | HM | 20/800 | n/aa | n/a |
| HM | 20/800 | ||||||
| 3 | OU | No | n/a | 20/200 | 20/50 | OU | Yes |
| 20/200 | 20/30 | ||||||
| 4 | OS | No | n/a | 20/30 | 20/20 | OS | Yes |
| 20/30 | 20/20 | ||||||
| 5 | OU (OS > OD) | OS | Yes | 20/40 | 20/20 | OS | Yes |
| 20/125 | 20/20 | ||||||
| 6 | OS | OS | Yes | 20/25 | 20/25 | OS | Yes |
| 20/100 | 20/25 | ||||||
| 7 | OD | OD | Yes | 20/25 | 20/20 | n/ab | n/a |
| 20/25 | 20/20 | ||||||
| 8 | OU (OS > OD) | OS | Yes | 20/100 | 20/25 | OU | Yes |
| 20/60 | 20/30 | ||||||
| 9 | OS | OS | Yes | 20/30 | 20/20 | OS | Yes |
| 20/40 | 20/20 | ||||||
| 10 | OU (OS > OD) | OS | Noc | 20/100 | 20/70 | OD | Nod |
| HM | 20/200 |
CON = compressive optic neuropathy, HM = hand motion, OD = right eye, OS = left eye, OU = both eyes, RAPD = relative afferent pupillary defect, VA = visual acuity.
VA too poor to evaluate color vision.
Congenital color blindness.
Improvement of RAPD.
Improvement of color vision from 0/11 to 3/11 OU.
Visual acuity ranged from hand motion (HM) to 20/25 in affected eyes at baseline. Snellen VA was converted to logMAR units for analysis. Average improvement of visual acuity was 0.64 (±0.58) logMAR (p=0.0190) in all eyes and 0.87 (±0.60) logMAR (p=0.0207) in affected eyes. Average reduction in proptosis at last follow up was 4.8 (±2.7) mm in all eyes (p<0.00001) and 4.7 (±2.7) mm in eyes affected by DON (p<0.00001). Average reduction in CAS was 5.25 (±1.2) (p<0.00001) and average reduction in GDS was 0.75 (±1.1) (p=0.160) (Table 3). Only one patient (Case 8) who completed 8 infusions per protocol had only 1 mm reduction in proptosis; all others had at least 2 mm of proptosis reduction. Fifty percent of patients received post-infusion orbital CT or MRI that confirmed improvement in orbital crowding.
Table 3.
Therapeutic Response: Proptosis reduction, clinical activity score, diplopia.
|
CASE |
# of infusions received |
Proptosis Reduction (mm) |
CAS Improvement |
GDS Improvement |
|
|---|---|---|---|---|---|
| OD | OS | ||||
| 1 | 8 | 3 | 2 | 6 | n/a |
| 2 | 8 | 3 | 3 | 4 | n/a |
| 3 | 8 | 4 | 5 | 4 | 2 |
| 4 | 8 | 8 | 6 | 5 | 0 |
| 5 | 8 | 3 | 6 | 5 | 0 |
| 6 | 8 | 3.5 | 3.5 | 4 | 0 |
| 7 | 8 | 7 | 7 | 7 | 1 |
| 8 | 8 | 1 | 1 | 4 | 0 |
| 9 | 8 | 5 | 4 | 7 | 0 |
| 10 | 8 | 3 | 3 | 6 | 3 |
CAS = clinical activity score, GDS = Gorman diplopia score, OD = right eye, OS = left eye.
Case 2 has had a delayed treatment schedule due to concerns regarding the novel coronavirus 2019-nCoV (COVID-19). Case 4 has had limited follow up testing due to COVID-19 concerns and limitations. Three patients had severe vision loss that precluded pre-treatment HVF and OCT (cases 1, 2, 10).
All 7 patients who responded had additional clinical improvement with resolution of optic neuropathy findings and no recurrence of acute DON after completion of teprotumumab. No patients needed subsequent urgent decompression or additional treatments for DON after completion of teprotumumab. Sixty percent of patients had complete resolution of DON findings defined as return of VA to pre-DON baseline (if known) or to 20/25 (if unknown) and resolution of RAPD, without need for subsequent urgent surgical decompression. The three patients who did not have significant improvement in vision presented with severe vision loss (HM), had longstanding progressive vision loss over one year or longer and had evidence of optic nerve stretch on orbital imaging.
4. Case summaries
Case 1: A 76-year-old Caucasian woman with Graves disease, alcoholism, opioid addiction, type 2 diabetes and hypertension presented with chronic, active, progressive TED with worsening vision loss and proptosis. She first noticed bulging, redness and swelling of her eyes over 10 years ago and slowly progressive visual loss over 2–3 years. She was poorly compliant on methimazole (TSH: 0.07/TSI: 509). Prior therapeutics included oral and IV corticosteroids, bilateral orbital decompression and right tarsorrhaphy. On presentation, her visual acuity was HM OU, Hertel: 30 mm right eye (OD), 30 mm left eye (OS), CAS: 6. Color vision, GDS and HVF could not be assessed due to severe vision loss. Corneal opacification and neovascularization obscured view of the optic nerves. Orbital CT confirmed TED related apical compression of both optic nerves. After 2 infusions, the patient had improvement in VA to 20/800 both eyes (OU), red desaturation markedly improved, and CAS improved to 3. After 8 infusions of teprotumumab, she retained her improvement in visual acuity from HM OU to 20/800 OU, CAS: 2 and proptosis improved by 3 mm OD and 2 mm OS. Sixteen weeks after completion of therapy, VA remained 20/800 OU, CAS 0, proptosis improved by 3 mm OD and 2 mm OS, no diplopia. Despite persistent DON, she reports significant improvement in quality of life due to improved vision and comfort.
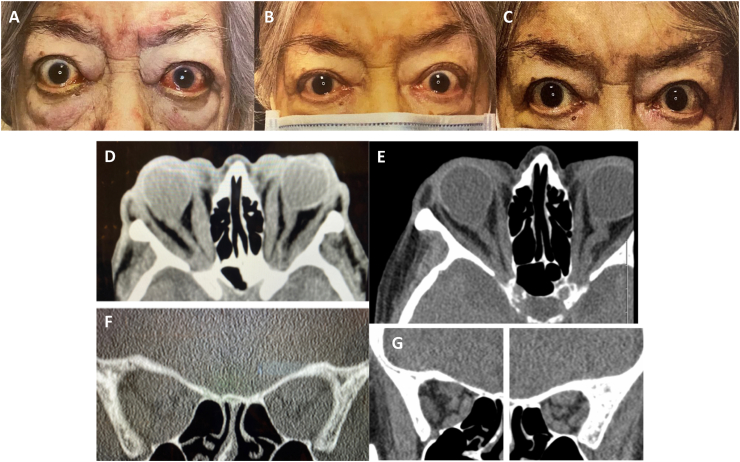
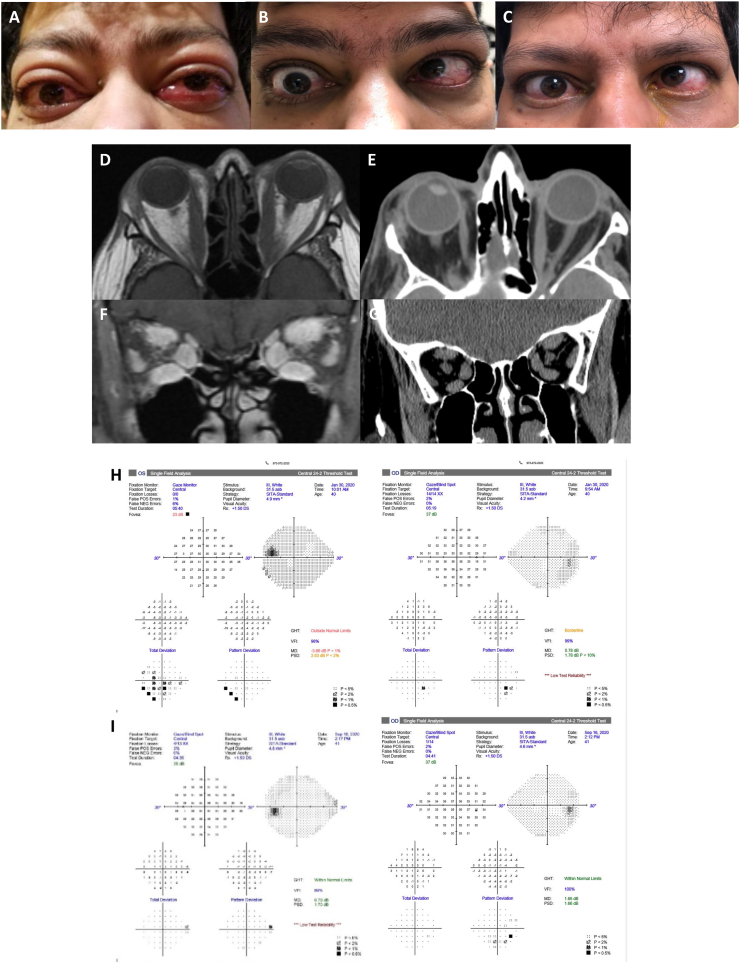
Case 2: An 84-year-old Hispanic woman with dementia, hypertension and asthma presented to an emergency room with palpitations, panic attack and blindness (HM OU) due to severe hyperthyroidism and TED. She had proptosis and slowly progressive vision loss for approximately one year due to chronic, active, progressive TED. Her TSH was 0.01 and TSI was 483. Prior therapeutics included 500 mg of IV methylprednisolone daily for 2 days followed by 60 mg of oral prednisone that was poorly tolerated and had no impact on vision or comfort. After high-dose steroids, her VA remained at HM OU and CAS 4. Poor vision precluded ability to assess formal color vision, GDS and HVF. The patient had severe proptosis (Hertel of 28 mm OD, 29 mm OS) and bilateral optic nerve pallor on examination. CT orbital imaging confirmed TED related bilateral apical compression and an optic nerve stretch with globe tenting. She was a poor surgical candidate and could not tolerate high dose oral or intravenous corticosteroids. After one infusion of teprotumumab, she had subjective resolution of eye pain and improvement in inflammatory signs. After 2 infusions, CAS improved to 1 and proptosis improved by 3 mm OD and 3 mm OS. After 4 infusions, she had improvement of vision to 20/800 OD and 20/800 OS allowing her to function better at home. Her post-infusion 5 CT scan demonstrated improved extraocular muscle size, less stretch on her optic nerve but persistent tenting of her globe. After 6 infusions, vision was 20/800 OU, no additional change in proptosis and all pain was gone (CAS 0). Several infusions were delayed due to COVID-19 concerns. Three weeks after completion of infusions, VA remained at 20/800 OU, CAS 0, with a total proptosis reduction of 3 mm OU from baseline, and no diplopia. Despite her persistent DON, she reported improvement in quality of life with much improved comfort, brightness and color saturation. Fig. 1.
Fig. 1.
Case 2 with bilateral proptosis with symmetric longstanding, severe loss of vision (HM OU). External photos (A) pre-treatment, (B) after 3 infusions (week 8), and (C) after 5 infusions with improvement in visual acuity, color vision, pain, proptosis, conjunctival injection and chemosis. Axial and coronal CT (D, F) pre-treatment and (E, G) after infusion 5 showing tenting of the globe and apical compression of the optic nerve. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
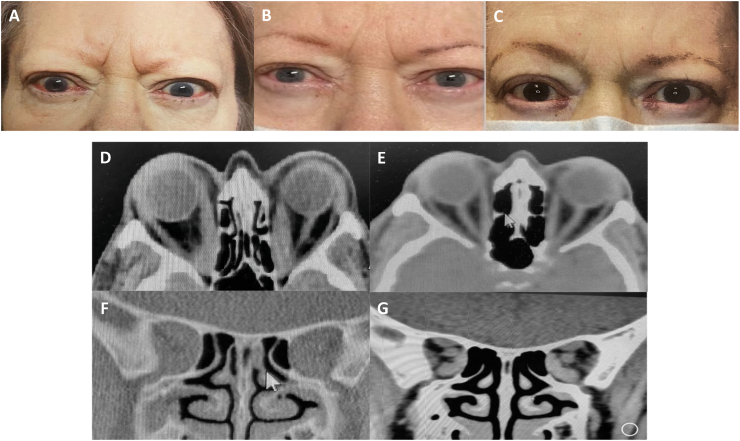
Case 3: A 62-year-old Caucasian woman with Graves disease, type 2 diabetes, and hypertension presented with a 6 month history of bulging eyes, redness, swelling, pain and blurred vision due to acute, active, progressive TED. She was poorly compliant with her medications including methimazole (TSH was <0.0025 and TSI was 380). She failed 6 days of methylprednisolone 500 mg IV. Her visual acuity decreased to 20/200 OU, color vision was 0/11 OU and proptosis worsened (Hertel: 30 mm OD, 29 mm OS), CAS:4, and GDS:2. Formal visual fields were not performed due to concerns about transmission of COVID-19 and staffing issues. CT imaging confirmed bilateral TED related apical optic nerve compression. She was started on teprotumumab infusions and after the second infusion her VA improved to 20/60 OD and 20/40 OS. She received a total of 8 infusions with sustained improved VA, color vision (11/11 OU), reduction of proptosis by 4 mm OD and 5 OS, CAS: 0, and GDS: 1. Post-infusion 6, orbital CT demonstrated markedly improved extraocular muscle size with relief of apical compression. Twenty-two weeks after completion of infusions, VA was 20/50 OD and 20/30 OS, CAS: 0, with a total reduction of proptosis of 4 mm OD and 5 mm OS from baseline, and resolution of diplopia with GDS 0. She is now able to read and work on the computer without difficulty but is pursuing surgery for her visually significant cataracts. Fig. 2.
Fig. 2.
Case 3 with bilateral proptosis, pain, decreased visual acuity and color vision, conjunctival injection, chemosis and pain. External photos (A) pre-treatment, (B) after 2 infusions (week 8), and (C) two weeks after 6 infusions (week 20) with improvement of all symptoms and signs. Axial and coronal orbital CT (D, F) pre-treatment and (E, G) after 6 infusions (week 20) demonstrating reduction in EOM especially the medial rectus at the orbital apex. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Case 4: A 73-year-old Caucasian woman with Graves disease, hypertension and chronic lung disease presented with a one-year history of bulging eyes and progressive blurred vision due to chronic, active, progressive TED. She was previously treated with RAI and was on levothyroxine (TSH was 3.41 and TSI 453). Her prior TED interventions included IV methylprednisolone and decompression OD for optic neuropathy. Eight months after right decompression, she presented with blurred vision OS. On presentation, VA was 20/30 OU, color vision 10/10 OD, 7/10 OS, CAS: 5, GDS: 2. On exam there was left optic nerve edema. HVF showed an inferior paracentral visual defect OS. Orbital CT confirmed TED related apical compression OS. She was a poor surgical candidate due to progression of her severe lung disease. After 6 infusions, VA improved to 20/25 OS (remained 20/30 OD), color vision 9/10 OS, CAS: 1, GDS:1, proptosis improved by 8 mm OD and 6 mm OS and the optic nerve edema resolved OS. Due to COVID-19-related limitations, imaging and photography were not performed. Sixteen weeks after completion of infusions, VA continued to improve to 20/20 OU, CAS improved to 0, and there was a total reduction in proptosis of 9 mm OD and 10 mm OS from baseline, but diplopia was stable with GDS 2.
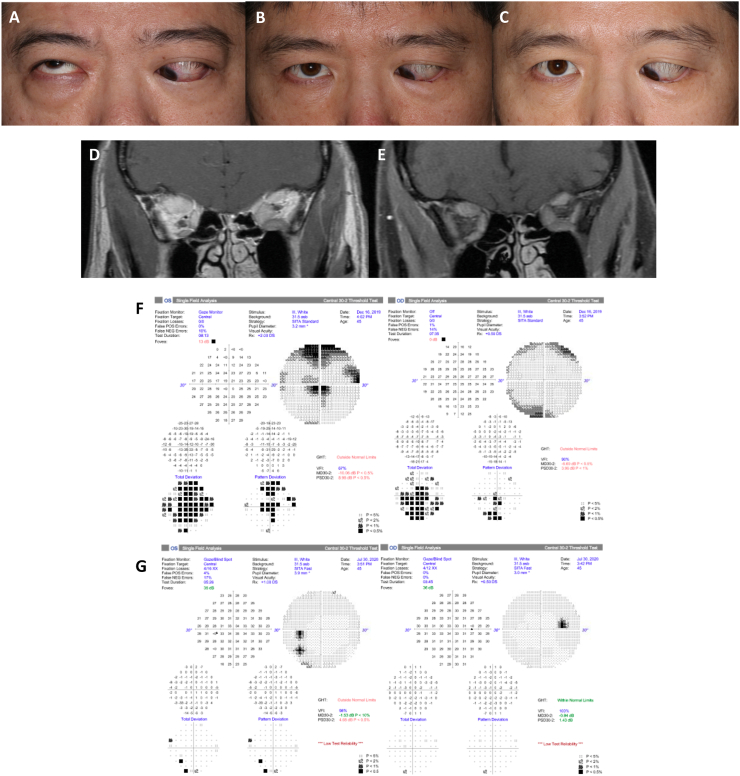
Case 5: A 45-year-old Asian man with Graves disease, hypertension, hyperlipidemia and prediabetic hyperglycemia presented with 17 months of chronic, active, progressive thyroid eye disease refractory to intravenous steroids and right orbital radiation. He was medicated with methimazole (TSH 0.48/TSI 610). The patient had worsening visual acuity to 20/40 OD and 20/125 OS, left RAPD, color vision 15/15 OD and 12/15 OS, CAS: 5, GDS: 3, restricted eye movements, and severe hypotropia OS. HVF showed inferior and central visual defects OD and superior arcuate and central visual defects OS. Orbital MRI confirmed TED related bilateral apical compression (OS » OD). Corticosteroids were contraindicated due to recent myocardial infarction and the patient was not a surgical candidate due to anticoagulation for recent coronary stents. After two infusions of teprotumumab, the patient's VA improved to 20/20 OD and 20/30 OS, RAPD resolved, decrease of CAS: 2, stable GDS: 3, and reduction of proptosis of 2 mm OD and 4 mm OS. Post-infusion 3 MRI showed decreased extraocular muscle size and improved orbital apex crowding. After the patient received 8 infusions, VA was stable, there was further decrease of CAS: 0, stable GDS: 3, and total reduction of proptosis to 3 mm OD and 6 mm OS. Thirty weeks after completion of infusions, VA further improved to 20/20 OU, CAS increased to 1, and there was a total reduction in proptosis of 3.5 OD and 7mm OS from baseline, but diplopia was unchanged with GDS 3. Fig. 3.
Fig. 3.
Case 5 external photos (A) pre-treatment, (B) after infusion 4 (week 12), and (C) after infusion 8 (week 25). (D) Pre-treatment non-contrast coronal MRI shows apical crowding with left greater than right extraocular muscle enlargement. (E) Post-infusion 3 coronal MRI shows improvement of apical crowding with decreased size of extraocular muscles. (F) Pre-treatment HVF shows global visual field deficit of both eyes. (G) Post infusion 8 HVF shows significant improvement in global visual field deficits. Pre-treatment external photo and HVF and pre- and post-treatment MRI previously published in OPRS. Permission from publisher obtained for reuse.
Case 6: A 55-year-old Asian woman presented to the emergency room with acute, active and rapidly progressive TED consisting of acute rapidly progressing blurry vision (right greater than left), pain with extraocular movements, and diplopia. Thyroid function tests were consistent with hyperthyroidism. VA was 20/200 OD and 20/20 OS with right RAPD, restriction in all gazes, color vision 0/13 OD and 5/13 OS, and CAS: 7. The orbital CT confirmed TED related apical compression bilaterally. She was admitted and treated with high-dose IV methylprednisolone (1g daily x3 days). She had minimal improvement in VA with persistent color vision deficits and right RAPD prompting an emergent right medial wall and orbital floor decompression. Postoperatively, there was improvement in VA to 20/25 OD, color vision 14/14 OD and 13/14 OS, and proptosis, and resolution of right RAPD. She was started on IV methylprednisolone (500 mg weekly) but developed uncontrolled blood glucose, confusion and progressive TED through therapy. Proptosis increased OS, and she had worsening VA 20/100 OS (OD stable at 20/25), new left RAPD, worsening color vision 3/14 OS (OD stable at 14/14), CAS: 4, and GDS: 4, TSH was 0.11 and TSI was 330. HVF showed a new central scotoma OS. Surgery was deferred due to COVID-19 surgery restrictions and she was started on teprotumumab infusions. After the second infusion her VA improved from 20/100 to 20/30 OS (OD stable at 20/25) with resolution of RAPD OS, normalization of color vision OS, CAS: 2, GDS: 3, and proptosis improved by 3 mm OD and 2.5 mm OS. The visual field defect also resolved on repeat HVF. After infusion 3, orbital CT showed improvement in optic nerve compression. She has received 8 infusions with sustained VA improvement, CAS:1, GDS:2, and total reduction in proptosis of 3.5 mm OU at last follow up. After 8 infusions, VA further improved to 20/25 OU, CAS 0.5, and there was a total proptosis reduction of 3.5 mm OD and 4.5 mm OS from baseline, but there was no change in diplopia with GDS 3. Fig. 4.
Fig. 4.
Case 6 external photos (A) pre-treatment, (B) after infusion 2 (week 4), and (C) after infusion 8 (week 25). (D) Pre-treatment non-contrast coronal CT shows symmetric enlargement of the bilateral extraocular muscles, most notably involving the inferior, middle, and superior recti muscles. (E) Post-infusion 3 non-contrast coronal CT shows surgical decompression changes, improved but persistent right greater than left extraocular muscle enlargement and improved orbital apex crowding. (F) Pre-treatment HVF shows inferior and paracentral visual field deficit of left eye. (G) Post infusion 8 HVF shows global improvement of visual field deficits.
Case 7: A 73-year-old Caucasian man with Graves disease and a three-month history of TED presented with bilateral periorbital edema and worsening vision in the right eye for about one month. He was on methimazole (TSH: 0.88/TSI: 409). VA was 20/60 OD and 20/30 OS with right RAPD, color vision 1/16 OU (patient colorblind), CAS: 7, and GDS: 1. He had bilateral, symmetric limitation of abduction and supraduction and right optic disc edema. Orbital CT imaging confirmed TED related bilateral apical compression. HVF showed nonspecific paracentral defects. Prior to initiation of teprotumumab, he was placed on high-dose (80 mg daily) oral prednisone with improvement in his vision to 20/25 OD and 20/25 OS and improvement of periorbital edema, however, the patient continued to have right-sided disc edema, and therefore, he was started on teprotumumab. The patient noticed further subjective improvement of VA after 2 doses of teprotumumab. After 3 infusions, VA improved to 20/20 OU. After 8 infusions, the patient continues to have normal vision 20/20 OU, no RAPD, CAS:0, GDS:1, and reduction of proptosis by 7 mm OD and 7 mm OS. Congenital color vision deficit remained unchanged as expected.
Case 8: A 57-year-old African American man with a 10-year history of Graves disease presented with active, progressive thyroid eye disease resulting in bilateral globe subluxation anterior to the eyelids and blurred vision due to chronic TED. He had urgent bilateral surgical decompression for his severe proptosis with Hertel's of 35 mm OU. His Graves disease was treated with methimazole to a euthyroid level (TSH 1.85 and TSI <89). He continued to have pain and decreased vision due to optic nerve dysfunction. He was treated with IV methylprednisolone 1 g per day for 3 days followed by 60 mg of prednisone for six weeks, and bilateral external beam radiation with improvement in diplopia and pain. After surgical decompression, IV steroids, and bilateral orbital radiation, he continued to have signs and symptoms of bilateral optic neuropathy (left worse than right) with VA 20/100 OD and 20/60 OS, persistent left RAPD, impaired color vision 1/11 OD and 1/11 OS, CAS: 4, GDS: 2 and proptosis with Hertel 30 mm OD and 30 mm OS at 105. OCT and clinical examination revealed mild optic atrophy OU. Orbital CT confirmed TED related apical compression and severe proptosis causing the optic nerve to be on stretch. He was started on teprotumumab with subsequent improvement in vision, comfort, and diplopia. After 1 infusion, CAS improved to 1. After 2 infusions, VA improved to 20/25 OD and 20/40 OS and the left RAPD resolved. He has received a total of 8 infusions and has sustained VA 20/25 OD and 20/40 OS, color vision 11/11 OU, CAS: 0, GDS: 2, and improvement in proptosis of 1 mm OU. Twenty-two weeks after completion of infusions, VA further improved to 20/25 OD and 20/30 OS, CAS 0, reduction in proptosis was stable at 1 mm OU, and diplopia remained unchanged with GDS 2.
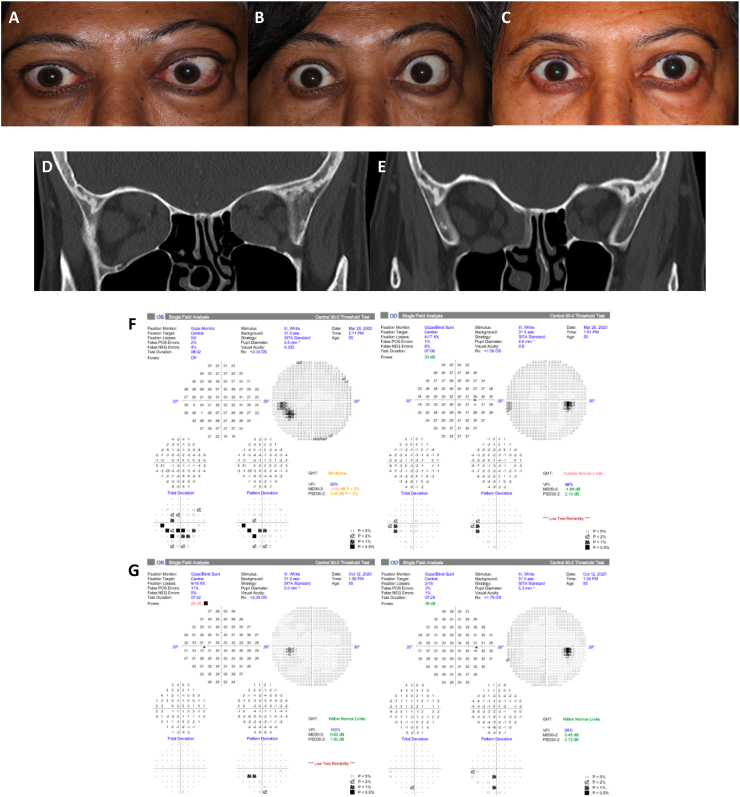
Case 9: A 40-year-old Indian man with Graves disease presented with acute, progressive, active TED causing tearing and palpitations for several months. He was medicated with methimazole, propranolol and a variety of topical ocular medications. The patient had progressive orbital pain and severe orbital asymmetric restrictive strabismus that progressed for 2–3 months despite treatment with 6 g of intravenous methylprednisolone and oral steroids 60 mg daily. Four months after IV steroids, VA decreased to 20/30 OD and 20/40 OS, with new left RAPD, color vision 6/6 OD and 5/6 OS, CAS: 7, and GDS: 3. Proptosis worsened (Hertel 27 mm OD, 26 mm OS) and motility worsened with restriction in all gazes and a large angle left hypertropia in primary gaze. HVF showed paracentral depression OS. MRI confirmed TED related bilateral apical compression. He was maintained on oral prednisone until institution of teprotumumab which was started 10 months into his TED presentation. The patient had an immediate subjective and durable response within one week of his first dose. After 2 infusions, VA improved to 20/20 OD and 20/20 OS, with resolution of left RAPD, and color vision returned to normal OU. After 8 infusions, VA remained 20/20 OU with normal color vision OU, CAS: 0, and GDS: 3. Proptosis improved by 5 mm OD and 3 mm OS. Post infusion HVF were normal with resolution of visual defect OS. Restrictive strabismus remained similar but ocular misalignment progressed resulting in a persistent large left hypertropia (25-20 prism diopters) and new large angle esotropia (40+ prism diopters) with persistent diplopia. Post-infusion CT scan showed dramatic improvement in EOM size and resolution of apical compression. However, the patient developed paradoxical worsening of strabismus after treatment, developing severe restriction and underwent strabismus surgery with partial improvement. Thirty-three weeks after completion of infusions, VA was stable at 20/20 OU, CAS 0, there was additional reduction in proptosis of 7 mm OD and 9 mm OS from baseline, but there was no improvement in diplopia with GDS 3. Fig. 5.
Fig. 5.
Case 9 external photos (A) pre-treatment, (B) after infusion 2, and (C) after 8 infusions (week 26). He had proptosis, decreased visual acuity and color vision, dysmotility, conjunctival injection and chemosis OS > OD. All of his signs and symptoms improved except for dysmotility. (D, F) Axial and coronal MRI before and (E, G) CT after 2 infusions (week 8). (H) Pre-treatment HVF shows left paracentral defect in left inferotemporal region. (I) Post-infusion 8 (week 26) HVF shows resolution of visual field defect after treatment with teprotumumab. Visual field testing of the right eye remained normal. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Case 10: A 76-year-old African American man with a 5-year history of Graves disease, 20-month history of chronic, active, progressive TED and hypertension presented with a 1-year history of progressive bilateral visual loss. The patient had received both IV steroids and oral prednisone over the course of 2019 (records of dosing were unavailable). He continued to have progressive bilateral vision loss and underwent orbital surgical decompression OD in December. The decompression resulted in reduced proptosis but worsened visual acuity and diplopia (>50 PD esotropia). The patient was reluctant to undergo decompression of the left eye. Eight months later, he pursued a second opinion for bilateral visual loss, proptosis, dysmotility and pain. His TSH <0.01 and TSI 353. His vision was CF at 3 feet OD and hand motion OS but with pinhole corrected to 20/100 OD, color vision 0/11 OU, RAPD OS, CAS: 6 and GDS: 3. He had bilateral mild temporal atrophy on clinical examination. Prior to treatment he was unable to cooperate with HVF or OCT. After the first infusion of teprotumumab, VA improved to 20/80 OD and 20/400 OS, RAPD improved, CAS:1, GDS: 3, and proptosis improved by 1mm OD and 2mm OS. After infusion 2, he had complete resolution of pain and improved motility. After infusion 3, VA improved to 20/70 OD, with additional improvement in proptosis (3 mm from baseline OD, 3 mm from baseline OS) and color vision OD 3/11. Five weeks after completion of infusions, VA further improved to 20/70 OD and 20/200 OS, CAS improved to 0, there was a reduction in proptosis of 3 mm OU from baseline, and resolution of diplopia with GDS 0. He has 3+ nuclear sclerosis cataracts that will be addressed.
5. Discussion
Prior to teprotumumab, therapeutic options for DON included corticosteroids, external beam orbital radiation, surgical decompression, or a combination of these interventions.8 Despite the widespread use of these modalities over decades for TED, the literature is mostly comprised of retrospective studies, Case reports and small case series which now guide practice patterns in the treatment of DON.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 There has only been one prospective, randomized controlled trial (RCT) to compare corticosteroids versus corticosteroids and surgical decompression for DON.12 In this study, Wakelkamp et al. reported similar initial outcomes after medical decompression and surgical decompression, and found that immediate surgical decompression did not result in better visual outcomes nor did it preclude the use of corticosteroids.12 Complete recovery of central visual acuity, color vision and improvement of apical crowding on imaging has been shown to be more likely to occur with the combination of steroids and orbital radiation or surgical decompression rather than steroid monotherapy.12, 13, 14, 15 External beam radiation has also been used for decades for the treatment of active TED including patients with DON.30, 31, 32, 33, 34, 35, 36, 37 Radiation therapy, like corticosteroids, dampens the inflammatory cascade in active TED.35
Corticosteroids, radiation, and surgical decompression are not without untoward effects. Prolonged oral corticosteroids result in weight gain, mood alterations, fluctuations of glucose, osteopenia, and gastric distress. High-dose pulsed IV corticosteroids are usually better tolerated than longer duration oral courses, but pose significant risks including fulminant hepatic failure, cardiac arrhythmias, and myocardial infarction.8,38 These complications are rare but can be fatal. Orbital radiation can result in worsening of diabetic retinopathy and dry eye syndrome.39,40 Surgical decompression may cause or worsen diplopia, ocular motility, facial anesthesia and can rarely lead to vision loss. Some patients are not surgical candidates or have contraindications to high-dose corticosteroids due to recent stroke, myocardial infarction, severe pulmonary disease, or hepatic failure. In our series, many of the patients had failed traditional therapies or were poor surgical candidates, therefore, teprotumumab was initiated.
Teprotumumab binds to IGF-IR and inhibits its activation and signaling, thereby reducing inflammation and preventing muscle and fat tissue remodeling, which can lead to orbital apex compression. Teprotumumab was recently evaluated in both Phase 2 and Phase 3 multicenter, double-blinded, placebo-controlled trials in patients with active, moderate-to-severe TED.9,11 All patients had recent onset of ocular/periorbital symptoms (<9 months), no prior treatment, CAS greater or equal to 4, and thyroid function tests above or below normal. Patients with optic neuropathy were excluded in all phases of prospective, placebo-controlled clinical trials as treatment of reversible and permanent vision loss with a placebo would be unethical.9,11 Both trials randomized patients to receive either placebo or active drug administered intravenously once every 3 weeks for a total of 8 infusions.9,11 Trial data shows a significantly greater proportion of patients had reduction in proptosis, CAS, diplopia, and improvement in quality-of-life scores in the treatment arm compared to placebo.9,11 In the Phase 3 clinical trial, orbital imaging was performed on six subjects; all showed a significant reduction in extraocular muscle volume, orbital fat volume, or both.11 Trial data also demonstrates that patients tolerated teprotumumab well. The more common side-effects included muscle spasms, alopecia, nausea and fatigue.9,11
We believe the reduction in extraocular muscle size, including at the orbital apex, seen in the Phase 3 trial supports the use of teprotumumab in patients with DON. While optic neuropathy was one of the exclusion criteria for initial landmark clinical trials, our initial Case series shows promise in the treatment of DON with teprotumumab. Additionally, Sears et al. recently reported successful medical decompression of DON with teprotumumab in a patient who presented with progressive vision loss secondary to DON that was refractory to IV corticosteroids and orbital radiation. The patient presented with vision loss, RAPD, visual field defects on HVF and optic nerve compression on orbital imaging, but was a poor corticosteroid and surgical candidate similar to several of the patients presented in this case series.41 This patient had significant improvement in VA, proptosis, and apical crowding, and had resolution of RAPD after treatment with teprotumumab.
Seventy percent of our patients with DON had a rapid response to teprotumumab and showed a clinical response after 2 infusions with objective improvement of DON findings. All patients with documented color vision loss had normalization (n=6) or improvement (n=1) of color vision (except one patient with congenital color blindness). Ninety percent of patients had reduction of proptosis by at least 2 mm, with an average reduction of 4.7 mm in affected eyes. Additionally, in patients in whom post-treatment orbital imaging was possible, there was notable decrease in the size of the extraocular muscles and improvement of apical crowding. All of our patients failed prior interventions including IV and/or oral steroids, orbital radiation, surgical decompression, or a combination of these interventions. Despite treatment resistant disease prior to teprotumumab, seven patients had successful resolution or significant improvement of DON after teprotumumab therapy alone and did not require additional treatment for DON after completion of teprotumumab with 15 week mean follow up.
Our series included 7 patients with chronic active TED greater than 9 months duration, with mean duration of disease of 3 years in all patients. This differs from the Phase 2 and Phase 3 clinical trials which only included patients with active TED of <9 months. Three patients in our study with longstanding severe vision loss (HM) had minimal to no improvement in DON despite proptosis reduction of at least 2 mm and decreased apical compression on orbital imaging. We suspect this is due to long-standing optic nerve compression and/or stretch associated with optic nerve atrophy. These patients had previously failed prior conventional DON therapies including IV corticosteroids and surgical decompression and remained stable after completion of teprotumumab with sustained reduction in proptosis and clinical activity scores that remained 0, therefore, no further treatment was initiated after completion of teprotumumab. Ozzello et al. also reported successful medical decompression with teprotumumab of a patient with chronic, stable TED with low CAS who was a poor corticosteroid, radiation, and surgical candidate.42 However, similar to our patients with longstanding severe vision loss, despite medical decompression with EOM reduction at the orbital apex on the post-treatment CT scan, there was lack of improvement in chronic vision loss suggesting that treatment needs to precede significant optic atrophy.42
Our study has several limitations. This is an observational study with a small sample size of 10 patients. There is limited follow up data due to recent completion of infusions. Patients were followed at different institutions, therefore, follow-up timepoints varied. Additionally, as a result of state shutdowns, staff quarantines and furloughs, and concerns about patient safety during the COVID-19 pandemic, patients had fewer in-person follow-up visits and less testing. Many studies were deferred, specifically HVF, OCT, and orbital imaging due to COVID-19 concerns.
It is worth noting that the medical therapy with teprotumumab for DON was very useful during the COVID-19 pandemic as other options such as surgery were not available at some institutions due to extended surgery shutdowns. Further, teprotumumab, based on its targeted mechanism of action, may not result in the global immunosuppression that is typically associated with corticosteroids. We recognize that additional follow-up is needed, and prospective studies are warranted to identify the patient population that this treatment would be most effective in, as well as how many infusions will provide a sustainable resolution of optic nerve compression.
6. Conclusion
In our Case series, teprotumumab worked rapidly, often after 2 infusions, and effectively to achieve visually significant improvement in most patients with dysthyroid optic neuropathy, including patients with active TED >9 months. Teprotumumab was effective in patients who had failed other interventions including high-dose corticosteroids, orbital radiation, and surgical decompression; and was also effective in patients who were poor surgical candidates. Teprotumumab significantly improved subjective and objective findings in all patients. All patients reported subjective improvements in visual acuity, color vision and comfort. Patients with long-standing compressive optic neuropathy with optic atrophy and/or globe tenting on CT imaging were less likely to achieve significant improvement in vision in at least one eye. CAS improved in all patients and proptosis improved by at least 2 mm in 90% of patients. Teprotumumab was safe and effective for the majority of patients in this small case series of DON. Teprotumumab should be considered in patients with DON prior to significant optic atrophy, especially patients who are poor surgical candidates and those who have failed prior therapeutic interventions.
Patient consent
Informed consent to publish photos for this Case series has been obtained from all patients in writing.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
Andrea L Kossler, Kimberly Cockerham and Raymond Douglas are consultants for Horizon Therapeutics. The following authors have no financial disclosure: CMS, YW, LAB, RT, PSS.
Acknowledgements
None.
References
- 1.Bartalena L., Wiersinga W.M., Pinchera A. Graves' ophthalmopathy: state of the art and perspectives. J Endocrinol Invest. 2004 Mar;27(3):295–301. doi: 10.1007/BF03345280. [DOI] [PubMed] [Google Scholar]
- 2.Khong J.J., Finch S., De Silva C. Risk factors for Graves' orbitopathy; the Australian thyroid-associated orbitopathy research (ATOR) study. J Clin Endocrinol Metab. 2016 Jul;101(7):2711–2720. doi: 10.1210/jc.2015-4294. [DOI] [PubMed] [Google Scholar]
- 3.Neigel J.M., Rootman J., Belkin R.I. Dysthyroid optic neuropathy. The crowded orbital apex syndrome. Ophthalmology. 1988 Nov;95(11):1515–1521. doi: 10.1016/s0161-6420(88)32978-7. [DOI] [PubMed] [Google Scholar]
- 4.Saeed P., Tavakoli Rad S., Bisschop P.H.L.T. Dysthyroid optic neuropathy. Ophthalmic Plast Reconstr Surg. 2018 Jul/Aug;34(4S Suppl 1):S60–S67. doi: 10.1097/IOP.0000000000001146. [DOI] [PubMed] [Google Scholar]
- 5.Kennerdell J.S., Rosenbaum A.E., El-Hoshy M.H. Apical optic nerve compression of dysthyroid optic neuropathy on computed tomography. Arch Ophthalmol. 1981 May;99(5):807–809. doi: 10.1001/archopht.1981.03930010807002. [DOI] [PubMed] [Google Scholar]
- 6.Weis E., Heran M.K., Jhamb A. Quantitative computed tomographic predictors of compressive optic neuropathy in patients with thyroid orbitopathy: a volumetric analysis. Ophthalmology. 2012 Oct;119(10):2174–2178. doi: 10.1016/j.ophtha.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 7.Rutkowska-Hinc B., Maj E., Jabłońska A., Milczarek-Banach J., Bednarczuk T., Miśkiewicz P. Prevalence of radiological signs of dysthyroid optic neuropathy in magnetic resonance imaging in patients with active, moderate-to-severe, and very severe Graves orbitopathy. Eur Thyroid J. 2018 Mar;7(2):88–94. doi: 10.1159/000486828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blandford A.D., Zhang D., Chundury R.V., Perry J.D. Dysthyroid optic neuropathy: update on pathogenesis, diagnosis, and management. Expet Rev Ophthalmol. 2017;12(2):111–121. doi: 10.1080/17469899.2017.1276444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith T.J., Kahaly G.J., Ezra D.G. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017 May 4;376(18):1748–1761. doi: 10.1056/NEJMoa1614949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Douglas R.S. Teprotumumab, an insulin-like growth factor-1 receptor antagonist antibody, in the treatment of active thyroid eye disease: a focus on proptosis. Eye. 2019 Feb;33(2):183–190. doi: 10.1038/s41433-018-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douglas R.S., Kahaly G.J., Patel A. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020 Jan 23;382(4):341–352. doi: 10.1056/NEJMoa1910434. [DOI] [PubMed] [Google Scholar]
- 12.Wakelkamp I.M., Baldeschi L., Saeed P., Mourits M.P., Prummel M.F., Wiersinga W.M. Surgical or medical decompression as a first-line treatment of optic neuropathy in Graves' ophthalmopathy? A randomized controlled trial. Clin Endocrinol. 2005 Sep;63(3):323–328. doi: 10.1111/j.1365-2265.2005.02345.x. [DOI] [PubMed] [Google Scholar]
- 13.Miśkiewicz P., Rutkowska B., Jabłońska A. Complete recovery of visual acuity as the main goal of treatment in patients with dysthyroid optic neuropathy. Endokrynol Pol. 2016;67(2):166–173. doi: 10.5603/EP.a2016.0018. [DOI] [PubMed] [Google Scholar]
- 14.Garip Kuebler A., Wiecha C., Reznicek L. Evaluation of medical and surgical decompression in patients with dysthyroid optic neuropathy. Eye. 2020 Sep;34(9):1702–1709. doi: 10.1038/s41433-020-0897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shams P.N., Ma R., Pickles T., Rootman J., Dolman P.J. Reduced risk of compressive optic neuropathy using orbital radiotherapy in patients with active thyroid eye disease. Am J Ophthalmol. 2014 Jun;157(6):1299–1305. doi: 10.1016/j.ajo.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 16.Day R.M., Carroll F.D. Corticosteroids in the treatment of optic nerve involvement associated with thyroid dysfunction. Arch Ophthalmol. 1968 Mar;79(3):279–282. doi: 10.1001/archopht.1968.03850040281009. [DOI] [PubMed] [Google Scholar]
- 17.Trobe J.D., Glaser J.S., Laflamme P. Dysthyroid optic neuropathy. Clinical profile and rationale for management. Arch Ophthalmol. 1978 Jul;96(7):1199–1209. doi: 10.1001/archopht.1978.03910060033007. [DOI] [PubMed] [Google Scholar]
- 18.Panzo G.J., Tomsak R.L. A retrospective review of 26 cases of dysthyroid optic neuropathy. Am J Ophthalmol. 1983 Aug;96(2):190–194. doi: 10.1016/s0002-9394(14)77786-4. [DOI] [PubMed] [Google Scholar]
- 19.Guy J.R., Fagien S., Donovan J.P., Rubin M.L. Methylprednisolone pulse therapy in severe dysthyroid optic neuropathy. Ophthalmology. 1989 Jul;96(7):1048–1052. doi: 10.1016/s0161-6420(89)32784-9. discussion 1052-3. [DOI] [PubMed] [Google Scholar]
- 20.Ph Mourits M., Kalmann R., Sasim I.V. Methylprednisolone pulse therapy for patients with dysthyroid optic neuropathy. Orbit. 2001 Dec;20(4):275–280. doi: 10.1076/orbi.20.4.275.2612. [DOI] [PubMed] [Google Scholar]
- 21.Hart R.H., Kendall-Taylor P., Crombie A., Perros P. Early response to intravenous glucocorticoids for severe thyroid-associated ophthalmopathy predicts treatment outcome. J Ocul Pharmacol Therapeut. 2005 Aug;21(4):328–336. doi: 10.1089/jop.2005.21.328. [DOI] [PubMed] [Google Scholar]
- 22.Ben Simon G.J., Syed H.M., Syed H. Clinical manifestations and treatment outcome of optic neuropathy in thyroid-related orbitopathy. Ophthalmic Surg Laser Imag. 2006 Jul-Aug;37(4):284–290. doi: 10.3928/15428877-20060701-04. [DOI] [PubMed] [Google Scholar]
- 23.Jeon C., Shin J.H., Woo K.I., Kim Y.D. Clinical profile and visual outcomes after treatment in patients with dysthyroid optic neuropathy. Kor J Ophthalmol. 2012 Apr;26(2):73–79. doi: 10.3341/kjo.2012.26.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Currò N., Covelli D., Vannucchi G. Therapeutic outcomes of high-dose intravenous steroids in the treatment of dysthyroid optic neuropathy. Thyroid. 2014 May;24(5):897–905. doi: 10.1089/thy.2013.0445. [DOI] [PubMed] [Google Scholar]
- 25.Sundar G., Chiam N., Lun K., Koh V. Survey of common practices among oculofacial surgeons in the Asia-Pacific region: graves' orbitopathy. Orbit. 2014 Oct;33(5):319–325. doi: 10.3109/01676830.2014.938168. [DOI] [PubMed] [Google Scholar]
- 26.Kauh C.Y., Gupta S., Douglas R.S. Compressive optic neuropathy and repeat orbital decompression: a case series. Ophthalmic Plast Reconstr Surg. 2015 Sep-Oct;31(5):385–390. doi: 10.1097/IOP.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartalena L., Baldeschi L., Boboridis K. European group on Graves' orbitopathy (EUGOGO). The 2016 European thyroid association/European group on Graves' orbitopathy guidelines for the management of Graves' orbitopathy. Eur Thyroid J. 2016 Mar;5(1):9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong Y., Dickinson J., Perros P. A British Ophthalmological Surveillance Unit (BOSU) study into dysthyroid optic neuropathy in the United Kingdom. Eye. 2018 Oct;32(10):1555–1562. doi: 10.1038/s41433-018-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wen Y., Yan J.H. The effect of intravenous high-dose glucocorticoids and orbital decompression surgery on sight-threatening thyroid-associated ophthalmopathy. Int J Ophthalmol. 2019 Nov 18;12(11):1737–1745. doi: 10.18240/ijo.2019.11.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donaldson S.S., Bagshaw M.A., Kriss J.P. Supervoltage orbital radiotherapy for Graves' ophthalmopathy. J Clin Endocrinol Metab. 1973 Aug;37(2):276–285. doi: 10.1210/jcem-37-2-276. [DOI] [PubMed] [Google Scholar]
- 31.Lloyd W.C., 3rd, Leone C.R., Jr. Supervoltage orbital radiotherapy in 36 cases of Graves' disease. Am J Ophthalmol. 1992 Apr 15;113(4):374–380. doi: 10.1016/s0002-9394(14)76158-6. [DOI] [PubMed] [Google Scholar]
- 32.Gorman C.A., Garrity J.A., Fatourechi V. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for Graves' ophthalmopathy. Ophthalmology. 2001 Sep;108(9):1523–1534. doi: 10.1016/s0161-6420(01)00632-7. [DOI] [PubMed] [Google Scholar]
- 33.Bartalena L., Marcocci C., Tanda M.L. Orbital radiotherapy for Graves' ophthalmopathy. Thyroid. 2002 Mar;12(3):245–250. doi: 10.1089/105072502753600223. [DOI] [PubMed] [Google Scholar]
- 34.Sánchez-Orgaz M., Grabowska A., Royo-Oreja A., Asencio-Durán M., Romero-Martín R., Arbizu-Duralde A. Optic neuropathy following orbital irradiation for Graves' ophthalmopathy: a Case report and literature review. Orbit. 2012 Feb;31(1):30–33. doi: 10.3109/01676830.2011.603458. [DOI] [PubMed] [Google Scholar]
- 35.Godfrey K.J., Kazim M. Radiotherapy for active thyroid eye disease. Ophthalmic Plast Reconstr Surg. 2018 Jul/Aug;34(4S Suppl 1):S98–S104. doi: 10.1097/IOP.0000000000001074. [DOI] [PubMed] [Google Scholar]
- 36.Gold K.G., Scofield S., Isaacson S.R., Stewart M.W., Kazim M. Orbital radiotherapy combined with corticosteroid treatment for thyroid eye disease-compressive optic neuropathy. Ophthalmic Plast Reconstr Surg. 2018 Mar/Apr;34(2):172–177. doi: 10.1097/IOP.0000000000001003. [DOI] [PubMed] [Google Scholar]
- 37.Gorman C.A., Garrity J.A., Fatourechi V. A prospective, randomized, double-blind, placebo-controlled study of orbital radiotherapy for Graves' ophthalmopathy. Ophthalmology. 2020 Apr;127(4S):S160–S171. doi: 10.1016/j.ophtha.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 38.Le Moli R., Baldeschi L., Saeed P., Regensburg N., Mourits M.P., Wiersinga W.M. Determinants of liver damage associated with intravenous methylprednisolone pulse therapy in Graves' ophthalmopathy. Thyroid. 2007 Apr;17(4):357–362. doi: 10.1089/thy.2006.0267. [DOI] [PubMed] [Google Scholar]
- 39.Wakelkamp I.M., Tan H., Saeed P. Orbital irradiation for Graves' ophthalmopathy: is it safe? A long-term follow-up study. Ophthalmology. 2004 Aug;111(8):1557–1562. doi: 10.1016/j.ophtha.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 40.Nygaard B., Specht L. Transitory blindness after retrobulbar irradiation of Graves' ophthalmopathy. Lancet. 1998 Mar 7;351(9104):725–726. doi: 10.1016/S0140-6736(05)78494-4. [DOI] [PubMed] [Google Scholar]
- 41.Sears C.M., Azad A.D., Dosiou C., Kossler A.L. Teprotumumab for dysthyroid optic neuropathy: early response to therapy. Ophthalmic Plast Reconstr Surg. 2021;37(3):S157–S160. doi: 10.1097/IOP.0000000000001831. PMID: 32976335. [DOI] [PubMed] [Google Scholar]
- 42.Ozzello D.J., Kikkawa D.O., Korn B.S. Early experience with teprotumumab for chronic thyroid eye disease. Am J Ophthalmol Case Rep. 2020 May 15;19:100744. doi: 10.1016/j.ajoc.2020.100744. [DOI] [PMC free article] [PubMed] [Google Scholar]