Highlights
-
•
Breast cancer recurrences deep to pectoralis are very rare after mastectomy.
-
•
Post-mastectomy chest wall radiation should exclude deep chest wall structures.
-
•
Radiation after immediate implant reconstruction increases complications.
-
•
Post-mastectomy radiation should target subcutaneous tissues and pectoralis major.
Keywords: Breast cancer, Post-mastectomy radiation, Local recurrence, Immediate breast reconstruction, Breast implant, Contracture, Chest wall, Mastectomy, Radiation therapy
Abstract
Introduction
Most studies report post-mastectomy local recurrences as chest wall recurrences without clarifying whether the recurrence is in the subcutaneous tissue, muscle or underlying rib. Post-mastectomy chest wall radiation is recommended in patients at increased risk of locoregional recurrence. Chest wall radiation-related fibrosis has become an important clinical consideration in the era of immediate implant-based breast reconstruction. In patients with commonly performed subpectoral implant-based reconstruction, the pectoralis major becomes relocated anterior to the implant and just deep to skin, therefore raising the question of value in radiating deep chest wall structures. This study assessed the rate of recurrence in each anatomical region of chest wall in post-mastectomy patients.
Methods
A comprehensive breast cancer database of 4287 patients at a single regional cancer center from 2006 to 2018 was retrospectively analyzed to identify 1571 mastectomy patients. Recurrences were classified as local skin/subcutaneous, pectoralis muscle (pectoralis major), deep chest wall (pectoralis minor, intercostal muscle or rib) or regional axillary recurrence.
Results
A total of 26 patients with locoregional recurrence were identified. Most recurrences were in the skin/subcutaneous level. Of 1571 mastectomy patients, only one patient developed a local recurrence posterior to pectoralis major. Our literature search and meta-analysis revealed that local recurrences post-mastectomy are much more likely to be in subcutaneous tissues/pectoralis major versus deeper chest wall.
Conclusion
A reduced clinical target volume which encompasses skin/subcutaneous and pectoralis muscle layers without treating deep chest wall may be more appropriate to reduce radiation-associated toxicity since avoiding circumferential radiation of an implant may prevent capsular contracture without compromising treatment benefit.
Introduction
Patients with a primary breast cancer diagnosis require complete surgical excision of the affected area, with either breast conserving surgery or mastectomy, depending on the extent of disease. Patients eligible for breast conserving surgery also require breast radiation to minimize the risk of local in-breast recurrence. More recent data has demonstrated that post-mastectomy radiotherapy (PMRT) can also reduce rates of both local and distant metastatic recurrence in patients who are lymph node positive [1], [2]. A joint guideline published by the American Society of Clinical Oncology (ASCO), American Society for Radiation Oncology (ASTRO), and Society of Surgical Oncology (SSO) was updated in 2016 to reflect this standard [3]. Prior guidelines recommended regional PMRT to the chest wall and nodal basins in patients who have four or more positive lymph nodes, however newer guidelines suggest PMRT with minimum mandatory target volumes to the chest wall be considered in order to reduce locoregional recurrence in those with 1 to 3 positive lymph nodes [3], [4].
Most published studies report post-mastectomy local recurrences as “chest wall” recurrences with no differentiation between subcutaneous tissue, muscle (pectoralis major), and underlying deep chest wall structures (pectoralis minor/serratus anterior/intercostal muscle/rib). PMRT delivered to the deep chest wall can result in radiotherapy-associated toxicities like cardiac disease, radiation pneumonitis, lymphedema, and pulmonary fibrosis [5], [6]. Therefore, stratification of chest wall recurrences by soft tissue layer or depth of involvement within the chest may help identify the regions at risk of recurrence and likely to benefit from treatment. Modifying dose volumes to avoid significant radiation dose to intercostal muscles, ribs, and lung may reduce treatment related toxicity. This would align with a recent ESTRO consensus guideline for post-mastectomy radiation, particularly in patients who have immediate subpectoral implant-based breast reconstruction since the implant displaces the subcutaneous tissues and pectoralis muscle away from the deep chest wall structures [7].
We assessed the rates of recurrence in each anatomical layer of the chest wall in patients following mastectomy in order to provide evidence for radiation planning to effectively treat ‘at-risk’ areas while avoiding toxicity resulting from unnecessary treatment. Avoiding radiation-induced fibrosis could minimize the risk of contracture that negatively impacts cosmesis in patients who have undergone reconstructive surgery as well as reducing the significant proportion of these patients requiring revision surgeries to address complications related to radiation. We then performed a systematic review of the literature and meta-analysis to contrast our recurrence rates with published post-mastectomy recurrence rates.
Methods
Quantification of chest wall recurrences
A prospectively collected comprehensive breast cancer database of patients treated in London, Ontario, initiated in 2006, was retrospectively analyzed to identify all patients who had undergone mastectomy between 2006 and 2018 inclusively in order to capture patients who experienced locoregional chest wall recurrence. Our study period ended in 2018 to ensure that selected patients had completed their treatment and all of their data had been collected. Patients who presented concurrently with local and distant disease were included, however those who first presented with distant recurrence prior to any presentation of local disease recurrence were excluded given their metastatic status. Electronic medical records and computed tomography imaging of patients were re-analyzed to stratify chest wall recurrences into three levels: (1) skin/subcutaneous recurrence, (2) pectoralis major muscle recurrence, or (3) deep chest wall recurrence. Patients with regional axillary and clavicular nodal recurrences were also captured.
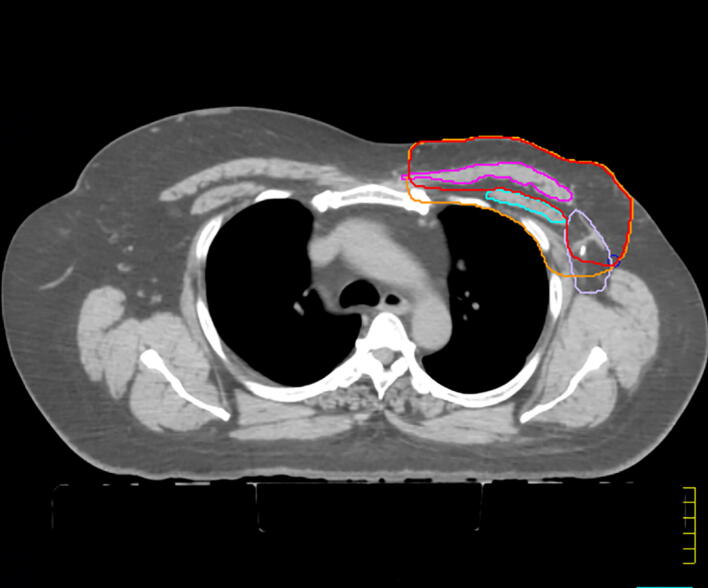
Skin/subcutaneous recurrences were defined as recurrences in the skin, subcutaneous tissues, or retromammary fat layers anterior to but not including the pectoralis major or serratus anterior muscle. Pectoralis muscle recurrence included any recurrence which involved the pectoralis major specifically, including any invasion through the pectoralis major fascia. Deep chest wall recurrences included recurrences posterior to pectoralis muscle, including pectoralis minor, serratus anterior (if lateral), intercostal muscle or rib. In the case that a recurrence was located in more than one level at diagnosis, the most posterior layer affected was recorded. Patients presenting with concurrent axillary and chest wall recurrences were coded according to the deepest level of their local chest wall recurrence. Fig. 1 illustrates the anatomic classification of chest wall recurrences used for this study on a CT simulation image. The classification of each local recurrence was completed by both a surgical oncologist and radiation oncologist with extensive knowledge of breast recurrence and clinical interpretation of radiological imaging.
Fig. 1.
Stratification of chest wall recurrence locations with proposed contours post-mastectomy without reconstruction. Contours: Red = proposed chest wall contours for post-mastectomy; Orange = current chest wall contour; Blue = pectoralis minor; Pink = pectoralis major; Purple = axilla level 1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Additional patient details were collected for descriptive statistics including age at time of mastectomy, cancer staging at time of mastectomy, radiotherapy, estrogen receptor (ER)/progesterone receptor (PR)/human epidermal growth factor receptor 2 (HER2) status, time to recurrence and recurrence size.
Systematic review and meta-analysis
A systematic review was performed to identify articles which described the location of chest wall recurrences in patients with breast cancer following mastectomy or breast surgery. A literature search was conducted using Pubmed, EMBASE and Cochrane to capture articles discussing “chest wall” and “recurrence” and “mastectomy” and “radiation”. Search results included original articles and literature reviews that were screened for relevancy. Published reviews were also read to search for reference of other relevant articles [8]. Articles were included for complete analysis if they contained data on chest wall recurrence location with similar stratification criteria described earlier: skin/subcutaneous recurrence, pectoralis muscle recurrence and deep chest wall recurrence. Statistical analysis was completed using Comprehensive Meta-Analysis (Version 3) by Biostat. A Mantel-Haenszel random-effects analysis model was used to generate risk ratio (RR) and accompanying 95% confidence intervals (CI).
Results
Quantification of chest wall recurrences
The London Breast Cancer Database identified 4287 women treated for breast cancer from 2006 to 2018, 1571 of whom underwent a mastectomy (36.6%). Of those undergoing mastectomy, 26 patients were reported as having had a locoregional recurrence over a median follow-up of 6.78 years and were included for further stratification and analysis (1.7% locoregional recurrence rate among mastectomy patients). Among all 1571 mastectomy patients, 137 underwent immediate breast reconstruction (8.7%), which represented only patients with Stage 0-II disease and included both autologous and implant-based reconstruction with a ratio of 1:4. While 28.5% of reconstructed patients received radiation, no reconstructed patients were among those who recurred. Overall, 39.5% of all mastectomy patients received adjuvant chest wall radiation (all patients presenting with locally advanced/stage III and 35% of stage II patients). Patients were stratified based on risk: average risk was defined as early-stage node positive, close margins; while high risk was defined as advanced stage (T3-T4, N2-N3), young age defined as <50 yrs, triple negative disease, positive margins. A radiation total dose of 50 Gy was delivered in 25 fractions (Monday to Friday) to chest wall for average risk stage II and delivered to chest wall and regional nodes for high-risk stage II and stage III patients according to institutional guidelines. Patient characteristics did not appear to be very different when contrasting patients who recurred locally to those who did not (see Table 1). For example, 46.2% of patients who recurred had also received adjuvant chest wall radiation (versus 39.5% of overall mastectomy cohort). Table 1 outlines the tumor and clinical characteristics for the patients who presented with a locoregional recurrence. The average age of patients with recurrence was 58.0 ± 16.4 years with most patients staged as T2 at time of mastectomy. Average time from surgery to recurrence was 1.9 ± 1.6 years with average recurrence size of 2.9 ± 2.4 cm in largest dimension. While tumor characteristics are presented in Table 1, the number of patients in this locoregional recurrence cohort are too few to make any meaningful analyses based on histological subtype.
Table 1.
Patient and primary tumor characteristics at time of primary surgery for all patients who underwent mastectomy (n = 1571) as well as those who ultimately recurred post-mastectomy (n = 26).
| Characteristic | All Mastectomy Patients (n = 1571) | Patients with Chest Wall Recurrence (n = 26) |
|---|---|---|
| Age (years)* - Mean ± SD | 53 ± 13.0 | 58.0 ± 16.4 |
| Tumor stage* | ||
| T0 | 11 (0.8%) | 1 (3.8%) |
| T1 | 494 (31.4%) | 8 (30.8%) |
| T2 | 546 (34.8%) | 11 (42.3%) |
| T3 | 90 (5.7%) | 6 (23.1%) |
| T4 | 114 (37%) | 0 |
| Regional Lymph Nodes (N Stage) | ||
| N0 | 798 (50.8%) | 9 (34.6%) |
| N1 | 330 (21%) | 10 (38.4%) |
| N2 | 107 (6.8%) | 3 (11.5%) |
| N3 | 42 (2.7%) | 4 (15.4%) |
| Radiotherapy | ||
| Yes | 620 (39.5%) | 12 (46.2%) |
| No | 971 (60.5%) | 14 (53.8%) |
| Radiotherapy Boost to Chest Wall** | ||
| Yes | 395 (63.7%) | 11 (91.7%) |
| No | 225 (36.3%) | 1 (8.3%) |
| Radiotherapy Bolus to Mastectomy Flap** | ||
| Yes | 223 (36%) | 4 (33.3%) |
| No | 397 (64%) | 8 (66.7%) |
| Radiotherapy to Axilla** | ||
| Yes | 431 (69.5%) | 5 (41.7%) |
| No | 189 (30.5%) | 7 (58.3%) |
| Chemotherapy | ||
| Yes | 724 (46.1%) | 3 (11.5%) |
| No | 847 (53.9% | 23 (88.5%) |
| Molecular Subtype Classification | ||
| Luminal A | 766 (48.8%) | 10 (38.5%) |
| Luminal B | 352 (21.8%) | 6 (23.1%) |
| Triple Negative | 157 (10.0%) | 6 (23.1%) |
| HER2/neu+ | 417 (26.5%) | 4 (15.4%) |
| Clinical factors | ||
| Size of recurrence (cm) – Mean ± SD | n/a | 2.9 ± 2.4 |
| Time to recurrence (years)*** – Mean ± SD | n/a | 1.9 ± 1.6 |
*At time of mastectomy.
**Calculated as a percentage of the patients who received radiation.
**From date of mastectomy.
Table 2 details the stratification of recurrence locations. Of the 26 patients identified, the majority (78%) had recurrences confined to the skin/subcutaneous or pectoralis muscle levels. Five patients (19%) presented with axillary recurrence alone. These locoregional recurrences represents 1.7% of all mastectomy patients in this database. Only one of these patients (representing 3.8% of all locoregional recurrences and 0.06% of all mastectomy patients) presented with a deep chest wall recurrence.
Table 2.
Anatomic Site of All Locoregional Recurrences (n = 26).
| Level | Patients |
|---|---|
| Skin/subcutaneous (+/− axillary recurrence) | 12 (46.2%) |
| Pectoralis muscle (+/− axillary recurrence) | 8 (30.8%) |
| Chest wall (pectoralis minor, intercostal muscle, rib) | 1 (3.8%) |
| Axillary (+/− Clavicular) Nodal recurrence only | 5 (19.2%) |
Systematic review results
Our search (Pubmed, Embase and Cochrane) conducted in 2021 yielded 544 accessible publications. Limiting the accessible publications to those in English and involving human subjects yielded 95 relevant publications, of which 7 were duplicates. Full manuscripts could not be obtained or did not specify the location of recurrences in 74 publications. A total of 14 relevant articles were identified and were included in our meta-analysis [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22].
Langstein et al. was limited to immediate breast reconstruction patient, and they stratified the recurrences into two levels: skin/subcutaneous and “chest wall” (defined as recurrence to any of pectoralis major muscle, skeletal or intercostal muscle involvement) and as a result were excluded from our review as they had clearly included pectoralis and deep chest wall recurrences together [23]. We did note however that the majority of patients in that study (71.8%) experienced recurrences to the skin/subcutaneous layers [23].
In all studies, the majority of recurrences were found in the skin/subcutaneous layers, followed by pectoralis muscle, with very few located within the deep chest wall. Table 3 outlines recurrence locations for each study. Only Gerber et al. [12], Gilliland et al. [13] and Wang et al. [22] reported recurrences in the deep chest wall. Wang et al. did not clearly differentiate their subcutaneous and pectoralis recurrences and these are reported together in Table 3 to reflect this, which did not impact our analysis.
Table 3.
Stratified chest wall recurrences in the literature.
| Author | Recurrence location |
|||
|---|---|---|---|---|
| Skin/Subcutaneous | Pectoralis muscle | Deep chest wall | Total | |
| Chang et al. [9] | 22 (75.9%)* | 7 (24.1%)* | 0 (0%)* | 29* |
| Cont et al. [10] | 14 (200%) | 0 | 0 | 14 |
| Farras et al. [11] | 8 (80%) | 2 (20%) | 0 | 10 |
| Gerber et al. [12] | 4 (66.7%) | 0 | 2 (33.3%) | 6 |
| Gilliland et al. [13] | 50 (80%) | 0 | 10 (20%) | 60 |
| Johnson et al. [14] | 7 (100%) | 0 | 0 | 7 |
| Meretoja et al. [15] | 8 (100%) | 0 | 0 | 8 |
| Noone et al. [16] | 12 (75.0%) | 4 (25.0%) | 0 (0%) | 16 |
| Pifer et al. [17] | 11 (64.7%) | 6 (35.3%) | 0 (0%) | 17 |
| Slavin et al. [18] | 17 (100%) | 0 (0%) | 0 (0%) | 17 |
| Sood et al. [19] | 6 (100%) | 0 | 0 | 6 |
| Stanec et al. [20] | 15 (100%) | 0 | 0 | 15 |
| Uriburu et al. [21] | 3 (100%) | 0 | 0 | 3 |
| Wang et al. [22] | 96 (82.8%)** | 20 (17.2%) | 116 | |
* Stratified by tumors (n = 29) across 25 patients.
** Includes both skin/subcutaneous and pectoralis muscle recurrences.
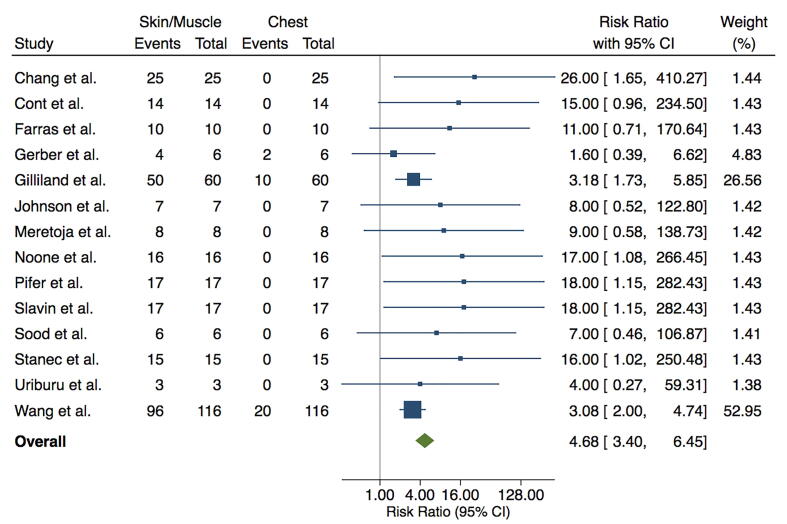
The Forest plot in Fig. 2 illustrates the summary risk ratio (RR) for recurrence location. Summary RR of reviewed studies was 4.68 (95% CI [3.4, 6.45]), indicating almost a 5-fold greater risk of recurrence to subcutaneous or pectoralis muscle levels compared to deep chest wall. Recurrences to deep chest wall occurred significantly less often than those to skin/subcutaneous and pectoralis muscle.
Fig. 2.
Risk ratio of chest wall recurrences in the literature. Shown are forest plots calculated using Mantel-Haenszel (MH) random effects model, illustrating risk ratio with confidence interval, Z-value and p-value.
Discussion
To our knowledge, this study is the first to report post-mastectomy chest wall recurrences from a large institutional database with a meta-analysis of all similar publications to date. It is also the largest study to date to report chest wall recurrences based on detailed anatomic location. The American Joint Committee on Cancer (AJCC) 8th edition supports our description of the chest wall (termed ‘deep chest wall’) as “including ribs, intercostal muscles, and serratus anterior muscle, but not the pectoralis muscles” [24]. Historically, distinguishing recurrence location from within the subcutaneous, pectoralis or chest wall structures did not seem clinical relevant since the structures lay adjacent to one another in post-mastectomy patients without reconstruction. In the current era of immediate implant-based reconstruction, this study showing such a low risk of deep chest wall recurrence provides an opportunity to decrease implant reconstruction failure, capsular contracture requiring reoperation, cardiac toxicity, and lung toxicity by excluding the deep chest wall from the radiation field using a smaller clinical target volume (CTV).
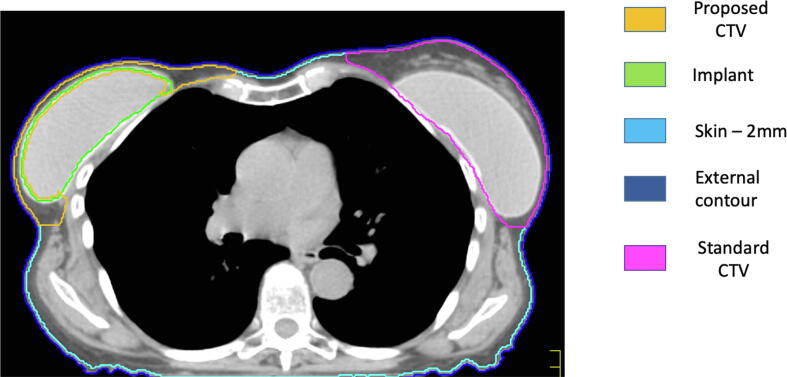
Our finding of a 0.06% post-mastectomy recurrence rate to the deep chest wall aligns with publications in the literature that report most recurrences are within the skin and subcutaneous levels with rare reports of local recurrences in the deep chest wall structures specifically (see Table 3). Our meta-analysis provides the relative risk of recurrence by tissue level (Fig. 3), and it supports the findings of a recent systematic review that virtually all local post-mastectomy recurrences occur in the subcutaneous tissues and pectoralis muscle [8]. Our findings align with the ESTRO consensus guideline for radiotherapy in post-mastectomy patients with immediate subpectoral breast reconstruction [7], where the pectoralis and subcutaneous tissues lie anterior to the implant and can thus be treated while excluding structures deep to implant to avoid delivering radiation circumferentially around the implant. This approach has been pilot-tested in Milan, by the team at European Institute of Oncology, with the HALFMOON TomoTherapy (Helical ALtered Fractionation for iMplant partial OmissiON) approach, using photon-based Tomotherapy. Similar isodose distributions can be achieved with linear accelerator equipped to deliver intensity modulated radiotherapy [25]. An ongoing trial by the Danish Breast Cancer Cooperative Group called the DBCG RT Recon Trial (NCT03730922) is evaluating delayed-immediate versus delayed breast reconstruction using adjuvant locoregional radiotherapy with a CTV excluding the deep chest wall structures to provide information on patient satisfaction and complications such as rates of capsular contracture [26].
Fig. 3.
Stratification of chest and proposed contours post-mastectomy with immediate reconstruction. Right Breast: Yellow contour demonstrates the CTV based on this manuscript with deep chest wall exclusion; Green contour represents implant. Left Breast: Pink contour demonstrates CTV based on prior approaches including implant (Green contour) and deep chest wall within the CTV. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
For patients undergoing mastectomy, immediate implant-based reconstruction is a surgical technique used to provide immediate reconstruction that does not require the surgical morbidity and recovery time seen with autologous tissue flap reconstruction techniques. As such it is the most common type of immediate reconstruction used to improve the quality of life and cosmetic outcomes of these patients. Typically, implants are placed anterior to the pectoralis minor, intercostal muscle and ribs, and posteriorly to the pectoralis major muscle. This technique effectively lifts the skin/subcutaneous tissue and pectoralis major from the deep chest wall. This differs from reconstruction using autologous tissue, which resembles pre-pectoral implant reconstruction in that it is placed anterior to the pectoralis major, therefore radiating the subcutaneous tissues and pectoralis would necessitate including the reconstruction within the radiated field. Autologous reconstruction uses perfused tissues which tolerate radiotherapy better than prosthetic implants, but the surgical complexity and recovery from an autologous reconstruction is much more significant. Thus, while it remains a reconstruction option for many patients, the vast majority of patients undergo immediate breast reconstruction using subpectoral implants. It is in these patients that the radiation target volume could potentially be limited to the anterior structures most commonly affected by local recurrence (subcutaneous tissue and pectoralis major muscle) while satisfying constraints to exclude most of the implant and the deep chest wall structures (pectoralis minor, serratus, intercostal muscles and ribs). Pre-pectoral implant-based reconstruction, where the implant is anteriorly covered by an acellular dermal matrix or mesh and skin flaps, is a much less commonly used method of immediate breast reconstruction. In these patients, the pectoralis lies deep to the implant and excluding it in the target volume may place this patient at increased risk of recurrence. None of the patients in our study received pre-pectoral reconstruction and thus our data was unable to address radiotherapy implications for this form of reconstruction.
In exploring post-mastectomy chest wall radiation guidelines, it is clear that there remains a lack of international consensus regarding which structures should be included when contouring the chest wall for post-mastectomy radiotherapy in breast cancer [7], [26], [27], [28], [29]. As illustrated in Fig. 3, current Radiation Therapy Oncology Group (RTOG) guidelines dictate the inclusion of the breast and chest wall following lumpectomy in locally advanced cases, and the ‘chest wall’ following mastectomy [28]. In this guideline, the posterior border of chest wall structures includes treatment of pectoralis muscles, chest wall muscles (intercostal), and ribs [28]. With very few cases of deep chest wall failure in intercostal muscle and ribs shown in this cohort and subsequent meta-analysis (Fig. 2), radiotherapy to these structures should be reconsidered as it may lead to overtreatment.
Vargo et al. have proposed modifying the RTOG guidelines with a reduced CTV to exclude the deep chest wall structures while encompassing both skin/subcutaneous and pectoralis muscle layers which may better balance treatment with potential heart- and lung-related toxicity from radiotherapy [27]. Their proposal however articulates that the posterior aspect of the radiation field should be at the anterior rib/deep chest wall structures, and this should be further clarified to define the posterior aspect of the radiation field as the posterior aspect of pectoralis major. While anatomically this clarification does not change which structures are radiated, achieving the same goal of radiating anterior soft tissues plus pectoralis major, it would provide guidance for dosimetry contouring in immediate implant-based reconstruction patients.
In contrast to RTOG, European Society for Radiotherapy and Oncology (ESTRO) clinical target volumes (CTV) include skin/subcutaneous levels with pectoralis major, but not deep chest wall structures (pectoralis minor, intercostal muscles and rib) [7]. This ESTRO guideline allows for treatment to reduce the risk of recurrences in skin/subcutaneous and pectoralis major levels which make up the vast majority of cases, while avoiding treatment to deep chest wall structures and permitting the contouring to exclude the implant and structures deep to it. This guideline supports our proposed revisions to the RTOG contouring guidelines (see Fig. 3). The Danish Breast Cancer Cooperative Group developed a similar guideline [29] although they describe the deep aspect of chest wall radiation as including pectoralis minor, which would therefore mandate circumferential implant radiation and dose to deep chest wall and may not be necessary given the paucity of recurrences reported to occur within the pectoralis minor.
The availability of acellular dermal matrix products has allowed for significant increases in one-stage immediate implant-based reconstruction [31], however concern regarding whether patients may require post-mastectomy chest wall radiation based on final pathological nodal status has led to conservative recommendations against immediate reconstruction in patients who may require radiation [32], despite improved cosmetic outcome and quality of life among patients who undergo immediate reconstruction at the time of their mastectomy [33].
There are conflicting reports of complication rates in patients with immediate breast reconstruction who undergo adjuvant chest wall radiation [34], [35], but one meta-analysis that reviewed fifteen controlled trials with 5314 patients demonstrated a 5-fold increase in capsular contracture to 32.6% with radiation (which causes significant pain and is managed by re-operation), and a doubling in complete reconstruction failure to 17.3% when radiation was delivered [36]. We propose that much of the implant-related complications seen with adjuvant chest wall radiation may be attributed to circumferential radiation of the entire implant capsule, leading to fibrosis and contracture around the implant. Radiating only the anterior capsule of the implant (which would target the skin, subcutaneous tissues and pectoralis major) should avoid or minimize these complications by excluding the deep chest wall from radiation dosage based on a historical notion of the ‘chest wall’. This hypothesis will be informed by the DBCG RT Recon Trial findings once the study is completed [30].
Limitations to this study exist. Chest wall recurrences reported as secondary data are difficult to capture using traditional search strategies using Medical Subject Headings (MeSH), therefore despite a thorough literature review, it remains possible that not all relevant articles detailing chest wall recurrences were captured. While our institutional database with 4287 patients had a median follow-up of 6.7 years, and a mean time to recurrence of 1.9 + 1.6 years, a longer follow-up period may capture more patients who experience recurrence. We were unable in this retrospective review to evaluate whether local post-mastectomy recurrences in patients who received radiation were related to inadequate volume coverage. If feasible in the future, it would be very interesting to evaluate this in a multi-centered setting. Finally, while chest wall recurrences that were synchronous with distant metastases were included, our choice to exclude metachronous chest wall recurrences identified after distant metastases were diagnosed may be reductive, since even in the metastatic setting chest wall recurrences can present a challenge in management can dramatically impact on quality of life. Our findings should be validated in larger national administrative databases to confirm the rarity of deep chest wall recurrences. All future studies reporting chest wall recurrences should stratify their locations based on criteria used in this article and others to aid in data collection, analyses and clinical decision-making. This study supports the ESTRO consensus guideline recommendations to avoid post-mastectomy radiation to deep chest wall structures in early-stage breast cancer, which we feel may lead to fewer complications for patients with implant-based reconstruction. A contouring atlas should be developed to provide guidance in standardizing post-mastectomy radiotherapy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Arciero C, Thompson P, Meisel JL, Taylor CE, Torres MA, Wood WC. Multidisciplinary Approaches to Chest Wall Recurrences of Breast Cancer. Oncology (Williston Park) 2018;32(8):392-396,417. [PubMed]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378(9804):1707–1716. doi:10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed]
- 3.Recht A., Comen E.A., Fine R.E., Fleming G.F., Hardenbergh P.H., Ho A.Y. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Pract Radiat Oncol. 2016;6(6):e219–e234. doi: 10.1016/j.prro.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Breast Cancer. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Published 2019. Accessed May 21, 2019.
- 5.Yi A., Kim H.H., Shin H.J., Huh M.O., Do A.S., Seo B.K. Radiation-induced complications after breast cancer radiation therapy: a pictorial review of multimodality imaging findings. Korean J Radiol. 2009;10(5):496–507. doi: 10.3348/kjr.2009.10.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown L.C., Mutter R.W., Halyard M.Y. Benefits, risks, and safety of external beam radiation therapy for breast cancer. Int J Womens Health. 2015;7:449–458. doi: 10.2147/IJWH.S55552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaidar-Person O, Vrou Offersen B, Hol S, Arenas M, Aristei C, Bourgier C, et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol. 2019;137:159-166. doi: 10.1016/j.radonc.2019.04.010. Epub 2019 May 17. PMID:31108277. [DOI] [PubMed]
- 8.Kaidar-Person O., Poortmans P., Offersen B.V., Siesling S., Sklair-Levy M., Meattini I. Spatial location of local recurrences after mastectomy: a systematic review. Breast Cancer Res Treat. 2020;183(2):263–273. doi: 10.1007/s10549-020-05774-4. [DOI] [PubMed] [Google Scholar]
- 9.Chang J.S., Lee J., Chun M., Shin K.H., Park W., Lee J.H. Mapping patterns of locoregional recurrence following contemporary treatment with radiation therapy for breast cancer: a multi-institutional validation study of the ESTRO consensus guideline on clinical target volume. Radiother Oncol. 2018;126(1):139–147. doi: 10.1016/j.radonc.2017.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Cont N.T., Maggiorotto F., Martincich L., Rivolin A., Kubatzki F., Sgandurra P. Primary tumor location predicts the site of local relapse after nipple-areola complex (NAC) sparing mastectomy. Br Can Res Treat. 2017;165(1):85–95. doi: 10.1007/s10549-017-4312-7. [DOI] [PubMed] [Google Scholar]
- 11.Farras Roca J.A., Dao T.H., Lantieri L., Lepage C., Bosc R., Meyblum E. Ipsilateral breast cancer recurrence after Deep Inferior Epigastric Perforator (DIEP) flap reconstruction: Incidence and radiological presentation. Diag Interv Imag. 2016;97(2):203–209. doi: 10.1016/j.diii.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 12.Gerber B., Krause A., Reimer T., Muller H., Kiichenmeister I., Makovitzky J. Skin-sparing mastectomy with conservation of the nipple-areolar complex and autologous reconstruction is an oncologically safe procedure. Ann Surg. 2003;238:120–127. doi: 10.1097/01.SLA.0000077922.38307.cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilliland MD, Barton RM, Copeland 3rd EM. The implications of local recurrence of breast cancer as the first site of therapeutic failure. Ann Surg 1983;197(3):284–287. https://www.ncbi.nlm.nih.gov/pubmed/6830336. [DOI] [PMC free article] [PubMed]
- 14.Johnson C.H., vanHeerden J.A., Donohue J.H., Martin K., Jr, Jackson I.T., Ilstrup D.M. Oncological aspects of immediate breast reconstruction following mastectomy for malignancy. Arch Surg. 1989;124:819–824. doi: 10.1001/archsurg.1989.01410070073015. [DOI] [PubMed] [Google Scholar]
- 15.Meretoja IJ, Rasia S, vonSmitten KAJ, Asko-Seljavaara SL, Kuokkanen HOM, Jahkola TA. Late results of skin-sparing mastectomy followed by immediate breast reconstruction. BJS 2007;94:1220–1225. [DOI] [PubMed]
- 16.Noone R.B., Frazier T.G., Noone G.C., Blanchet N.P., Murphy J.B., Rose D. Recurrence of breast carcinoma following immediate reconstruction: a 13-year review. Plast Reconstr Surg. 1994;93(1):96–106. [PubMed] [Google Scholar]
- 17.Pifer P.M., Bice R.P., Jacobson G.M., Lupinacci K., Beriwal S., Hazard H.W. The lack of consensus of international contouring guidelines for the dorsal border of the chest wall clinical target volume: what is the Impact on Organs at Risk and Relationships to Patterns of Recurrence in the Modern Era? Advancesradonc. 2019;4(1):35–42. doi: 10.1016/j.adro.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slavin S.A., Love S.M., Goldwyn R.M. Recurrent breast cancer following immediate reconstruction with myocutaneous flaps. Plast Reconstr Surg. 1994;93(6):1191–1204. doi: 10.1097/00006534-199405000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Sood S., Elder E., French J. Nipple-sparing mastectomy with implant reconstruction: the Westmead experience. ANZ J Surg. 2015;85(5):363–367. doi: 10.1111/ans.2015.85.issue-510.1111/ans.12641. [DOI] [PubMed] [Google Scholar]
- 20.Stanec Z., Zic R., Budi S., Stanec S., Milanovic R., Zlatko Z. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg. 2014;73(5):485–491. doi: 10.1097/SAP.0b013e31827a3oe6. [DOI] [PubMed] [Google Scholar]
- 21.Uriburu J.L., Vuoto H.D., Cogorno L., Isetta J.A., Candas G., Imach G.C. Local recurrence of breast cancer after skin-sparing mastectomy following core needle biopsy: case reports and review of the literature. Breast J. 2006;12(3):194–198. doi: 10.1111/j.1075-122X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang L-W, Li L, Zhang H-Y, Chen Y-Y, Zhong Y-H. Patterns of chest wall recurrence and suggestions on the clinical target volume of breast cancer: a retrospective analysis of 121 postmastectomy patients. Cancer Manage Res 2020:12 5909–5918. doi: 72.136.8.130/dovepress.com.2021.03.018. [DOI] [PMC free article] [PubMed]
- 23.Langstein H.N., Cheng M.-H., Singletary E.S., Robb G.L., Hoy E., Smith T.L. Breast cancer recurrence after immediate reconstruction: patterns and significance. Plast Reconstr Surg. 2003;111(2):712–720. doi: 10.1097/01.PRS.0000041441.42563.95. [DOI] [PubMed] [Google Scholar]
- 24.American Joint Committee on Cancer, 8th Edition. https://cancerstaging.org/references-tools/deskreferences/Documents/AJCC%20Breast%20Cancer%20Staging%20System.pdf. Accessed on November 18, 2019.
- 25.Leonardi M.C., Spoto R., Miglietta E., Trivellato S., La Rocca E., Luraschi R. HALFMOON TomoTherapy (Helical ALtered Fractionation for iMplant partial OmissiON): implant-sparing post-mastectomy radiotherapy reshaping the clinical target volume in the reconstructed breast. J Can Res Clin Oncol. 2019;145(7):1887–1896. doi: 10.1007/s00432-019-02938-8. [DOI] [PubMed] [Google Scholar]
- 26.Vargo J.A., Beriwal S. RTOG chest wall contouring guidelines for post-mastectomy radiation therapy: is it evidence-based? Int J Radiat Oncol Biol Phys. 2015;93(2):266–267. doi: 10.1016/j.ijrobp.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Vargo J.A., Beriwal S. In reply to Chang et al.: contouring guidelines for post-mastectomy radiotherapy a cry for international consensus. Radiother Oncol. 2017;123(3):483–484. doi: 10.1016/j.radonc.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 28.RTOG Foundation. Breast Cancer Atlas. https://www.rtog.org/corelab/contouringatlases/breastcanceratlas.aspx. Accessed March 20, 2019.
- 29.Nielsen M.H., Berg M., Pedersen A.N., Andersen K., Glavicic V., Jakobsen E.H. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol. 2013;52(4):703–710. doi: 10.3109/0284186X.2013.765064. [DOI] [PubMed] [Google Scholar]
- 30.National Library of Medicine (U.S.) (2018, November –). The DBCG RT Recon Trial: Delayed-immediate versus delayed breast reconstruction in early breast cancer patients treated with mastectomy and adjuvant loco-regional radiation therapy. A multicenter randomized clinical trial. Identifier NCT03730922. https://clinicaltrials.gov/ct2/show/NCT03730922.
- 31.Doherty C., Pearce S., Baxter N., Knowles S., Ross D., McClure J.A. Trends in immediate breast reconstruction and radiation after mastectomy: a population study. Breast J. 2020;26(3):446–453. doi: 10.1111/tbj.v26.310.1111/tbj.13500. [DOI] [PubMed] [Google Scholar]
- 32.Zhong T, Spithoff K, Kellett S, Boyd K, Brackstone M, Hanrahan R, et al. Breast cancer reconstruction surgery (immediate and delayed) across Ontario: Patient indications and appropriate surgical options. Toronto (ON). Cancer Care Ontario. Program in Evidence-Based Care Series No.: 17-10, 2016.
- 33.Eltahir Y., Werners L.L.C.H., Dreise M.M., van Emmichoven I.A.Z., Jansen L., Werker P.M.N. Quality-of-life outcomes between mastectomy alone and breast reconstruction: comparison of patient-reported BREAST-Q and other health-related quality-of-life measures. Plastic Recon Surg. 2013;132(2):201e–209e. doi: 10.1097/PRS.0b013e31829586a7. [DOI] [PubMed] [Google Scholar]
- 34.Momoh A.O., Ahmed R., Kelley B.P., Aliu O., Kidwell K.M., Kozlow J.H. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21(1):118–124. doi: 10.1245/s10434-013-3284-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berbers J., van Baardwijk A., Houben R., Heuts E., Smidt M., Keymeulen K. “Reconstruction: before or after postmastectomy radiotherapy?” a systematic review of the literature. Eur J Cancer. 2014;50(16):2752–2762. doi: 10.1016/j.ejca.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Pu Y, Mao T-C, Zhang Y-M, Wang SI, Fan D-L. The role of postmastectomy radiation therapy in patients with immediate prosthetic breast reconstruction: a meta-analysis. Medicine 2018;97:6(e9548). http://dx.doi.org/10.1097/MD.0000000000009548. [DOI] [PMC free article] [PubMed]