Abstract
Prebiotics may modify the biological processes in the chickens' gastrointestinal tract to improve poultry performance and health. Prebiotics are natural feed additives that offer many economic advantages by decreasing mortality rates, increasing growth rates, and improving birds' feed efficiency. Prebiotic action potentially affects the degradation of indigestible dietary compounds, the synthesis of nitrogen components and vitamins, and simplifies the removal of undesirable elements in the diet. Prebiotics could also induce desirable gut microbiome modifications and affect host metabolism and immune health. It is worth mentioning that gut bacteria metabolize the prebiotic compounds into organic compounds that the host can subsequently use. It is important to limit the concept of prebiotics to compounds that influence the metabolism of resident microorganisms. Any medicinal component or feed ingredient beneficial to the intestinal microecosystem can be considered a prebiotic. In this review, the impacts of prebiotics on the gut microbiome and physiological structure are discussed, emphasizing the poultry's growth performance. The current review will highlight the knowledge gaps in this area and future research directions.
Key words: microbiome, organic poultry, performance, poultry gastrointestinal tract, prebiotics
INTRODUCTION
The balance of bacteria within the various segments of the gastrointestinal tract (GIT) is known as gut health. Prebiotics are nondigestible dietary ingredients that promote beneficial bacteria such as Lactobacillus and bifidobacteria, which improve gut health and boost host health (Kleessen et al., 2001). Gibson and Roberfroid (1995) described the requirements and properties of the constituents to be categorized as prebiotics and indicated that these components must be 1) a selective constituent that promotes the metabolic activity or growth of one or more beneficial bacteria; 2) able to modify microbiota in the direction of a healthy state; 3) able to induce systemic or luminal beneficial influences on the host; 4) neither absorbed nor hydrolyzed in the upper part of the GIT.
Nondigestible carbohydrates meet these conditions; they are utilized by endogenous colonic bacteria and are not absorbed in the upper GIT part. Later, Gibson et al. (2004) revised the prebiotics definition as “selectively fermented ingredients that allow specific changes, both in the composition and activity in the gastrointestinal microbiota which confers beneficial effects on host well-being and health”. Inulin and transgalactooligosaccharide were considered the only constituents that wholly met previous requirements to be considered prebiotics (Roberfroid, 2007). More recently, resistant starch, mannan-oligosaccharides (MOS), and lactulose have been considered prebiotics (Boler and Fahey, 2012; Ricke, 2015; Hutkins et al., 2016).
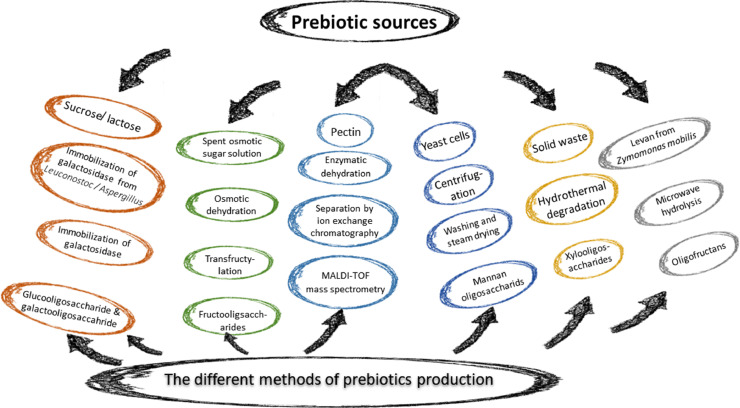
The different sources of prebiotics available in the market are summarized in Figure 1.
Figure 1.
Different sources of prebiotics.
Prebiotics were first utilized to manipulate the microbiome's genetic structure through dietary feed supplements (Gibson and Roberfroid, 1995). Manipulation of the bacterial composition can occur by the introduction of bacteria into the large intestine, which goes through a series of fermentation reactions and affects gut physiology. The mechanism of pathogen inhibition in the microbiome includes the competition for nutrients and binding locations on the intestinal epithelium, creating short-chain fatty acids (SCFAs) and decreasing the pH (Rolfe, 2000; Ricke, 2003). The present review highlights the practical use of prebiotics and their potential impacts on broiler chicks and laying hens' performance.
HISTORICAL PERSPECTIVES OF PREBIOTICS FOR THE IMPROVEMENT OF POULTRY PRODUCTION
Since the 1950s, antibiotic growth promoters (AGPs) have been used in the poultry sector to improve performance, control the GIT microbiota, and limit the resistance of pathogenic bacteria (Jones and Ricke, 2003; Ricke, 2015,2018). Reducing the incidence of intestinal disease-associated pathogens is essential not only due to public health concerns, but it is believed that animal productivity and mortality are directly related (Castanon 2007; Gaggìa et al., 2010).
Dibner and Richards (2005) indicated that the population of antibiotic-resistant bacteria might increase with the continued use of AGPs in the agricultural sector (Dibner and Richards, 2005). Therefore, interest has grown in developing alternative approaches to maintain or enhance farm animals' growth performance (Bachaya et al., 2015).
The broiler production system depends on a high growth rate and a low marketing age at the lowest possible cost. Feed additives such as prebiotics play a vital role in boosting the growth to rapidly market the bird (Kim et al., 2019). De Maesschalck et al. (2015) showed xylooligosaccharides (XOS) with prebiotic properties significantly increased the ileum villus length and the populations of intestinal microbiota (e.g., Clostridium spp. in the ceca and lactobacilli species in the colon) of broiler chicks. Since the GIT is highly colonized, microbial composition and corresponding microbial physiology are critical (Apajalahti and Kettunen, 2006). Thus, the possibility of enhancing the production of SCFAs by selected prebiotics has been identified in several investigations (Donalson et al., 2008a; Ricke, 2015).
Prebiotics have a positive influence on the laying rate of hens. According to Chen et al. (2005), supplementation of oligofructose-type prebiotic (1.0%) and inulin (1.0%) in the layer's diet increased egg production by 13.35 and 10.73%, respectively, and increased the egg weight by 12.50 and 10.96%, respectively compared to the control.
Chen and Chen (2004) suggested a beneficial effect of oligofructose and inulin in layer hens because they are directly correlated with the minerals’ absorption rate. Supplementation of these compounds significantly enhanced eggshell strength, eggshell weight, total ash, serum calcium levels, tibia phosphorus, and calcium levels (Chen and Chen, 2004).
USE OF PREBIOTICS IN DIETS OF BROILERS AND LAYING HENS
The poultry industry's development owes its success to the parallel growth of per capita consumption (FAO, 2017). From 2003 to 2013, broiler meat and table eggs’ global production grew by 4.1% and 2.3% annually, respectively. Nutritional management is a crucial element to reach high production goals (Callaway and Ricke 2012). Higher production results from increased feed intake, improved absorption, and digestion of nutrients, along with proper balance in the quantitative and qualitative microbial load in the animals' gut (Huyghebaert et al., 2011). The potential modulations of microbial load with new supplements, such as prebiotics, have become an attractive proposition.
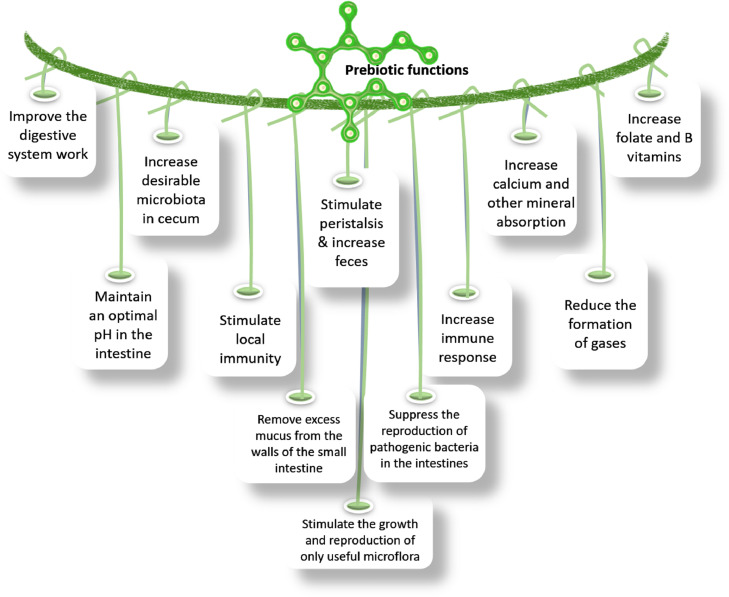
The different functions of prebiotics in poultry nutrition are highlighted in Figure 2.
Figure 2.
Important functions of prebiotics in poultry nutrition.
Prebiotics were added to poultry diets as prophylaxis against enteric diseases (Elgeddawy et al., 2020). For instance, in laying hens, the dietary supplementation of probiotics (such as Clostridium butyricum or a combination of Pediococcus acidilactici and Saccharomyces boulardii) may improve laying performance, feed conversion, egg quality, eggshell strength, and the gut health of laying hens (Xiang et al., 2019). For broiler nutrition, probiotic species such as Lactobacillus, Candida, Bacillus, Enterococcus, Saccharomyces, Aspergillus, Streptococcus, and Bifidobacterium have potential beneficial impacts on the modulation of microflora in the intestinal tract while inhibiting pathogenic bacteria (Higgins et al., 2007). These beneficial microorganisms can improve broiler performance (Ashayerizadeh et al., 2009), intestinal morphometry (Chichlowski et al., 2007), immunomodulation (Mathivanan and Kalaiarasi, 2007), sensory characteristics of dressed meat (Pelicano et al., 2003), and enhance the microbiological quality of meat (Kabir et al., 2005).
Although our knowledge about prebiotic effects and their role in animal performance has increased, research is required on their mechanisms of action in the animals' metabolism and physiology.
MECHANISMS OF ACTION OF PREBIOTICS IN POULTRY NUTRITION
Prebiotics are metabolized through commensal microorganisms, leading to host health benefits (Gibson et al., 2017). Most of the prebiotic impact occurs in the lower parts of the GIT, particularly the birds' ceca, with some microbial hydrolysis that could occur in the upper sections, such as the crop (Ricke, 2018).
Prebiotics and probiotics could stabilize and control the multiplication of pathogenic microflora in the GIT based on the competitive exclusion (CE) mechanism (Dankowiakowska et al., 2013). The CE mechanism reduces pathogenic bacterial colonization of the intestinal epithelium by preventing bacterial toxins, enhancing the immune system's local activity, and the intestinal epithelium nutrition (Schneitz, 2006).
Prebiotics provide energy and a carbon source for microorganisms, which generally live in the colon, where bacterial fermentation processes occur for some nutrients (Dankowiakowska et al., 2013). Prebiotics affect the proliferation of bifidobacteria species, reduce the growth of detrimental microorganisms, remove harmful toxic metabolites and enzymes, enhance animals and birds’ performance, exhibit hypocholestermic effect, and lower blood pressure, and prevent carcinogenesis processes (Mateova et al., 2008). Also, prebiotics affects the synthesis of vitamins such as folic acid, nicotinic acid, B1, B2, B6, and B12 (Kannan et al., 2005; Pilarski et al., 2005).
Prebiotics act as growth substrates to improve the activity of beneficial bacteria such as butyrate-producing clostridia and bifidobacteria (Scott et al., 2015; Patrascu et al., 2017). The commensal microorganisms' genome controls the enzymes they produce as they have a different preference for, and can consume, the prebiotics (Wilson and Whelan, 2017). Earlier, the influence of dietary prebiotics supplementation was determined by monitoring the increase in the population of Bifidobacterium spp. and Lactobacillus spp. as a standard technique (Gibson and Roberfroid, 1995). However, recent advanced sequencing techniques have revealed that, via a cross-feeding process, prebiotics modify a more comprehensive range of microorganisms (Gibson et al., 2017).
Prebiotics are metabolized via bacteria into organic molecules that the host can use later, but antibiotics are not. It is important to limit the concept of prebiotics to compounds that influence the metabolism of resident microorganisms. Furthermore, any medicinal component or feed ingredient beneficial to the intestinal microecosystem can be considered a prebiotic (Bindels et al., 2015). Prebiotics need to be resistant to gastrointestinal absorption, enzymatic hydrolysis, and gastric acidity and must be selectively metabolized by beneficial commensal bacteria. Their fermentation should lead to systemic or local benefits to the host (Gibson and Roberfroid, 1995).
According to Angelakis (2017), inulin-derived fructooligosaccharide (FOS) and inulin products fulfill all the livestock's prebiotic classification criteria. Candidate prebiotics includes MOS, XOS, maltooligosaccharides, galactooligosaccharides (GOS), glycol-oligosaccharides, pectins, gluco-oligosaccharides, lactose, and its derivatives (lactosucrose and lactulose) (EFSA, 2017). The underlying mechanisms to boost poultry's performance depend mainly on prebiotic-mediated alterations in the microorganisms living within the GIT (Rinttilä and Apajalahti, 2013; Ricke et al., 2020).
Valcheva and Dieleman (2016) indicated that prebiotics’ most apparent impact is manipulating the GIT microbiota, enriching the resident microbial groups, using these components as energy sources for fermentative processes. The production of SCFAs in the intestinal host by prebiotic fermentation provides energy for epithelial cells and reduces luminal pH. Additionally, balanced bacterial populations confer protective, trophic, and metabolic functions to the host through a wide range of products accessible to the host's cell, influencing physiological processes of the host (Valcheva and Dieleman, 2016).
SOME PREBIOTICS USED TO IMPROVE POULTRY PRODUCTION
Fructooligosaccharides
FOS can act as substrates for specific intestinal microorganisms and are not hydrolyzed in the upper GIT; thus, they are suitable as prebiotics (Hartemink et al., 1997; Kumar et al., 2019). FOS is fermented by bifidobacteria and Lactobacillus species, which could boost the host's gut health (Ricke, 2015,2018). FOS may increase lactic acid and SCFA concentration and inhibits the growth of pathogens such as Clostridium perfringens, one of the major causes of high mortality in poultry production (Ricke, 2015; Kumar et al., 2019). Also, colonization of Salmonella species in broilers was reduced by 19% with the addition of FOS in the drinking water (Oyarzabal and Conner, 1996).
Bailey et al. (1991) showed that enterobacteria, such as Escherichia coli and Salmonella, do not use FOS as a carbon source. A decrease in Salmonella colonization by 42% was observed when FOS was combined with a cell culture medium. A population decline of 2 log of Salmonella typhimurium was detected in vitro by FOS addition to the feed (Donalson et al., 2007; Ricke, 2015,2018), which agrees with Bailey et al.’s (1991) findings.
Shang and Kim (2017) demonstrated a positive effect of FOS on the broilers’ body weight gain (BWG) and feed efficiency. Treatment with a prebiotic increased layers’ production of cecal lactic acid and SCFAs (Donalson et al., 2008a). On the other hand, there was no significant influence in laying hens attributed to the microbiome's minimal utilization ability, fast fermentation of similar substrates, or a reduced prebiotic intake (Donalson et al., 2008b).
Donalson et al. (2008b) reported that hens fed diets containing FOS had a significantly lower Salmonella population in the liver and ovaries. Conversely, reduced Salmonella contamination in broilers’ cecal contents and carcasses was also reported (Waldroup et al., 1993). The improvement in feed conversion ratio (FCR) was positively associated with the increased enzymatic activity of protease, amylase, and leucine aminopeptidase (Xu et al., 2003; Ricke, 2015,2018; Micciche et al., 2018; Kim et al., 2019). However, the adverse influence of FOS was revealed by Ten Bruggencate et al. (2003), claiming that these components may encourage some harmful bacterial growth. Rapid fermentation of FOS can cause excessive SCFAs production that potentially prompts the colonic mucosal inflammation or damage due to decreased pH, thus reducing resistance to intestinal pathogens. If many beneficial bacteria are already present in the host, prebiotics may not enhance health and growth (Ten Buggencate et al., 2004). Wu et al. (1999) suggested that the optimal FOS levels may range between 2.5 and 5.0 g/kg diet, and they suggested these levels could improve BWG and feed efficiencies. On the contrary, increased FOS supplementation (10 g/kg) caused diarrhea, leading to reduced productive performance (Wu et al., 1999). These findings convincingly indicate that optimum FOS levels may enhance the birds’ productive performance and health, particularly under stressful conditions.
Galactooligosaccharides
The prebiotic GOS are synthesized from lactose by glycosyl transfer of the D-galactosyl unit to the D-galactose moiety of lactose, and the catalysis of the hydrolysis of β-galactosides via β-galactosidase (Ricke, 2015,2018; Micciche et al., 2018; Kim et al., 2019).
Tzortzis et al. (2005) demonstrated that GOS not only enhanced the growth rate of bifidobacteria species by 0.9 log but also increased the production of acetic and lactic acid in the proximal colon of pigs by 3 and 4-fold, respectively, compared with the negative control. This study was of particular interest because the impact of prebiotics on bacterial species level was identified, compared to other conventional studies of prebiotics where only the amount of specific bacterial genera was investigated (Tzortzis et al., 2005). For instance, Ruiz-Moyano et al. (2013) revealed a variation in the favored substrates among Bifidobacterium subspecies. Bifidobacterium longum subsp. infantis and B. bifidum can utilize human milk oligosaccharides while B. longum subsp. longum and B. breve cannot. The GOS possess antiadhesive properties; they inhibited the adherence of enteropathogenic E. coli to Caco-2 cells and HEP-2 cells by 40-70% and 65%, respectively (Shoaf et al., 2006). Among the many prebiotics studied by Shoaf et al. (2006), GOS exhibited the highest adhesion inhibition of E. coli to cells. This inhibition might be due to structural variances among oligosaccharides.
Chemical or enzymatic modification of GOS has been recommended to produce prebiotics with a similar action to human breast milk oligosaccharides and to have strong antiadherence activity against Helicobacter pylori, Campylobacter jejuni, and E. coli (de Araújo and Giugliano, 2000). GOS significantly increased the total population of anaerobic bacteria in broilers, including bifidobacteria and lactobacilli, by 1.32 and 0.53 log units, respectively (Jung et al., 2008).
Yeast
Yeast cultures (YC) were introduced into animal feed as an alternative approach to feed supplements after antibiotics were banned. Eckles and Williams (1925) were the first to introduce the concept of YC as a feed additive, using Saccharomyces cerevisiae as a growth promoter for animals. In the gut, YC has tremendous potential for improving digestive function by modifying microbiota; therefore, commercial YC usage became a common animal production approach (Beev et al., 2007), including poultry (Shen et al., 2009).
The YC's mode of action in the animals' GIT remains unclear (Beev et al., 2007). However, Stanley et al. (2004) demonstrated that supplementation of YC improve commensal microorganisms’ growth and decrease pathogenic microorganisms’ growth. Gao et al. (2008) suggested that MOS (a component of the yeast cell wall) enhances the host's immunity and promotes beneficial bacterial growth.
YC supplementation enhanced phytate phosphorus utilization and nutrient digestibility in poultry (Shen et al., 2009) and improved broilers’ intestinal mucosal development (Ricke 2015, 2018; Micciche et al., 2018; Kim et al., 2019). A study that investigated the impacts of different YC levels on broilers’ immunomodulatory functions and growth performance was carried out by Gao et al. (2008). They demonstrated that dietary supplementation of YC improved FCR and the average daily gain of broilers. Gao et al. (2008) also reported that broilers fed YC (2.5 g/kg diet) had high digestibility of calcium and phosphorus and elevated villi height to crypt depth ratios in the intestine, compared to the control. Additionally, immunomodulatory functions against Newcastle disease were also improved. It was concluded that an optimal level of YC is essential for dietary supplementation in broilers to yield beneficial effects (Gao et al. 2008).
Mannanoligosaccharides
MOS are prebiotic constituents produced from the yeast cell wall, associated with boosting the broiler chicks’ growth performance (Hooge, 2004; Rosen, 2007). However, a significant performance improvement was only observed in young birds, as they have a smaller population of gut microbiota. Older birds did not have substantial gains due to their complex indigenous microbiota population. The GIT of newly hatched chicks stabilizes by 2 wk of age (Rosen, 2007).
MOS decreased the Salmonella population in broiler chicks' intestines by 25 fold (4.01 vs. 5.40 log cfu/g) than the control group (Spring et al., 2000). Zaghini et al. (2005) showed that MOS-treated hens produced smaller eggs in the second and third wk compared to the negative control and birds treated with 2.5 ppm aflatoxin B1 (AFB1), agreed with the findings of Rizzi et al. (2003). A low level of aflatoxin present in the birds’ liver revealed MOS's capability to degrade aflatoxins (Zaghini et al., 2005). They also reported the shell thickness was negatively affected when both AFB1 and MOS were used; thus, it was concluded that MOS positively affected egg quality traits (Zaghini et al., 2005).
Supplementation of MOS has shown that livestock remains healthier by increasing immunoglobulin-A production (Ricke, 2015, 2018). Similar to FOS, the administration of MOS has revealed some inconsistencies in animals’ performance responses. Pelicano et al. (2004) and Stanczuk et al. (2005) reported that no significant impacts of MOS supplementation were found in the BWG of broiler chickens and turkeys, while Sims et al. (2004) found improvement in live body weight of turkeys. Hooge (2004) showed that MOS inclusion in broilers’ diets significantly boosted body weight and FCR and reduced mortality compared to the nonsupplemented diet. The inclusion of MOS in diets as a prebiotic could increase broilers’ growth rate (Rehman et al., 2020). However, when comparing the antibiotics to MOS, there were no significant differences in birds’ body weight and FCR (Ricke, 2015, 2018; Micciche et al., 2018; Kim et al., 2019).
As a feed additive, MOS has no effect on the apparent metabolizable energy value (Yang et al., 2007). MOS supplementation in broilers’ diets increased BWG but did not affect gut function and morphology potentially due to variations in rearing conditions, diets, and species (Yang et al., 2007).
Nondigestible Carbohydrates and Dietary Fiber Sources
Chemically defined prebiotics such as lactulose, inulin, oligofructose, gluten, and fructans are a few other compounds that are also believed to possess prebiotics-like properties. These include milk oligosaccharides, pectin, and resistant starch (Hutkins et al., 2016). The effect of resistant starch on some SCFAs, such as butyrate, was documented and confirmed by Bird et al. (2010).
Bird et al. (2010) found that the dietary intake of resistant starch levels in the population at low risk of diet-related intestine ailments was high compared with people at high risk of ailments. In addition, consumption of resistant starch augmented SCFA concentration (especially butyric acid) in the large intestine and reduced colonocyte proliferation (Dronamraju et al., 2009).
The feed is withdrawn to modify the birds’ behavior to promote molting before a second laying cycle. However, this method affects hens' health, making them susceptible to infection by Salmonella enteritidis. Mixing alfalfa with layer hens’ feed induced the molt but inhibited the S. enteritidis growth due to its high fiber, high protein, well-balanced amino acids, antioxidants, and vitamins. Kim et al. (2005) observed that a premolt treatment of 100% alfalfa significantly increased egg production compared with 90% alfalfa-treated birds. The feed withdrawal group's shell weight was heavier when fed 90% rather than 100% alfalfa at the end of the 2nd laying cycle. Therefore, alfalfa-based feeding could serve as an alternative method to prompt the molt in birds to avoid adverse changes in the microbiome and immunity of birds, making them more susceptible to the infection by S. enteritidis (Ricke et al., 2013; Teng and Kim, 2018; Swaggerty et al., 2019).
Evaluating the impacts of prebiotics is necessary because there are different types and mechanisms of prebiotics. Compositional analysis of the microbiota, pathogen quantification, and identifying specific microorganisms should also be considered.
IMPACTS OF PREBIOTICS ON THE GUT STRUCTURE OF BIRDS
An Insight into the Microbiota Composition of Birds' GIT
Microorganisms fill birds' GIT, where different parts are colonized by specialized microbiota adapted to the host physiology, nutrient availability, and physicochemical conditions (Borda-Molina et al., 2016). Although fungi, viruses, and archaea have been identified, bacteria are the primary colonizer (Wei et al., 2013). However, microbial communities are strongly interconnected among organs of the GIT, affecting the microbiota in the upper and lower section (Stanley et al., 2014).
Furthermore, the microbial communities exhibit wide variations in genome content, which affects their roles within the overall ecosystem. The crop and gizzard, where the feed is temporally stored, fermented and milled, are highly dominated by lactic acid-producing bacteria belonging to Lactobacillus species (Borda-Molina et al., 2016). The duodenum and ileum, where most of the absorption and enzymatic digestion of nutrients takes place, are primarily colonized by Lactobacillus species and, to a lesser degree, by species of Enterococcus, Streptococcus, and Clostridium (Stanley et al., 2014; Borda-Molina et al., 2016; Kumar et al., 2019).
The cecum has the longest transit period (12–20 h) of the digestive tract. It is where fermentation of complex undigested components including cellulose and other polysaccharides takes place in 2 blind pouches (Pan and Yu, 2014). The most densely colonized organ is the cecum, and its bacterial diversity is much higher than in the upper digestive tract of birds (Stanley et al., 2014). It contains more than 2,300 operational taxonomic units (95% sequence identity) (Danzeisen et al., 2011), with the most abundant bacterial families belonging to Lactobacilaceae, Peptoccaceae, Erysipelotrichaceae, Anaeroplasmataceae, Lachnospiraceae, and Ruminococcaceae (Borda-Molina et al., 2016). However, a large proportion has been identified belonging to Coriobacteriaceae and Bifidobacteriaceae (Apajalahti and Vienola, 2016). The mapping of the cecum's microbial community assemblage continues, making it the target organ to assess responses related to feeding practices of poultry (Rinttilä and Apajalahti, 2013; Stanley et al., 2014).
Major alterations occur in the taxonomical composition of the GIT during the lifespan of broilers and laying hens (Oakley et al., 2014; Videnska et al., 2014; Ranjitkar et al., 2016). Despite this dynamic microbiota succession, Ranjitkar et al. (2016) recommended that microorganisms are formed in broiler chicks after 22 d of age. In contrast, Videnska et al. (2014) claimed extensive successional changes occur during the hens' lifespan, with a stable microbiota composition from 210 d of age. The age-related microbiota changes could be strongly affected by dietary differences, signifying that it would be appropriate to investigate these influences on microbiota in the intestine at various ages of poultry (Oakley et al., 2014).
The diet is the primary influence on the microbial profile and its encoded functions, affecting it toward the preferred trend (Rehman et al., 2020). While the diet has been formulated for the poultry's nutritional needs, its capacity to influence metabolically active microorganisms has been overlooked (Apajalahti and Vienola, 2016). Owing to microbial species having several nutrient preferences for growth and maintenance, the digestive system's microbial profile, especially the cecum, is generally considered a reflection of the feed ingested and the nutrients absorbed in the small intestine (Pan and Yu, 2014).
Influence of Prebiotics on Composition and Physiology of the Gut
Microorganisms produce the SCFAs during complex sugar molecules fermentation (Cummings and Macfarlane, 1991), which can modify physical villi properties in the GIT, leading to an increase in the absorption of minerals and other molecules (Xu et al., 2003). Additionally, Lactobacillus and Bifidobacterium present in the GIT can limit pathogen colonization through lactic acid production (van der Wielen et al., 2000). The use of prebiotics as feed additives to hasten the complexity of the GIT microbiota in the newly hatched bird aims to decrease exposure to intestinal pathogens (Nisbet, 2002).
CE treatment administered to newly hatched chicks serves as the classic example for reducing pathogenic microorganisms and increasing the population of beneficial microorganisms in the intestine (Nurmi and Rantala, 1973). For CE, beneficial microorganisms are introduced in the GIT of the host, which excludes the colonization of pathogenic microorganisms (Nisbet, 2002). Some investigations revealed that prebiotics have modes of action that help the host's gut microbes in an entirely different approach than the CE technique (Patterson and Burkholder, 2003). While CE methods present a single microorganism or exogenous microbial population to the host (Mead, 2000; Abd El-Hack et al., 2018), prebiotics indirectly affect the microbiota by increasing the concentration of beneficial indigenous microorganisms (Gibson and Roberfroid, 1995).
Prebiotics are known to increase the absorption rate of minerals (Scholz-Ahrens and Schrezenmeir, 2002), boosting immune function and preventing colon cancer from developing (Geier et al., 2006). The most important impact of prebiotics is the selection for augmented lactobacilli and bifidobacteria populations. These bacteria are considered an indication of a healthy microbiota due to their inhibition of putrefactive proteolytic bacterial growth. Scholz-Ahrens and Schrezenmeir (2002) showed that inulin-type fructans increased SCFAs production and villus height, enhancing the absorption surface (Xu et al., 2003). The SCFAs production is important due to their diverse effects on the host's gut microbiota (Ricke, 2003).
By inducing the immune response through sparing glutamine that serves the lymphatic tissues as a preferred substrate (Ouwehand et al., 2005) while decreasing pH in the GIT lumen to inhibit pathogenic microbes, production of mucin was induced to improve the morphology of the colon (Barcelo et al., 2000). Oligofructoses could improve the solubility of the minerals, inducing the transportation rate of phosphorus and calcium (Scholz-Ahrens and Schrezenmeir, 2002).
Prebiotics play another beneficial role; to prevent the colonization of infectious bacteria by competing for receptor places on epithelial cells of the intestine (Gibson et al., 2005). Buddington et al. (2002) revealed antiadhesive properties related to prebiotics in E. coli. A distinct group of prebiotics are discussed in this current review, including MOS, GOS, FOS, and nondigestible oligosaccharides. FOS are linear chains of fructose units found in plants such as garlic, chicory, onion, banana, artichoke, and asparagus (Sabater-Molina et al., 2009). GOS is synthesized from lactose using β-galactosidase. Finally, MOS is produced from the cell wall of yeast.
Prebiotics-Mediated Effects on the Immune System and Intestinal Morphology
Intestinal cell proliferation, increased villi height, the villi:crypt ratio, and the intestinal epithelial barrier are all promoted by strengthening tight-junctions by prebiotic fermentation into SCFAs (especially butyric acid) (Swaggerty et al., 2019). Enhancements in the morphology of the GIT increased feed utilization and produce a protective barrier against intestinal infections by improving the integrity of epithelial cells, reducing endotoxin permeability and the risk of pathogen invasion (Teng and Kim, 2018; Swaggerty et al., 2019).
Dietary prebiotics develop a balanced gut microbiota that protects the host from the formation of intestinal pathogens. Common bacterial communities colonize the intestinal mucous membrane and form a dense layer of bacteria that covers the mucosal surface. This bacterial layer inhabits various niches that block attachment points and the colonization by intestinal pathogens via CE (Nurmi et al., 1992). Also, bactericidal and bacteriostatic substances are produced that control pathogenic populations.
Investigations have shown that broiler chicks’ lactic acid, and other SCFAs created by the commensal bacteria, prevent the growth of S. typhimurium, C. perfringens, and E. coli through decreased pH and the bactericidal influence of the undissociated form of SCFAs (Bodie et al., 2019; Kumar et al. 2019). Different strains of bacteria isolated from the chickens’ gut can create bacteriocins with inhibitory effects against Listeria monocytogenes, Campylobacter jejuni, and S. enteritidis (Micciche et al., 2018). Also, resident bacteria boost mucosal defense mechanisms, inducing mucus production and the number of goblet cells (Gaggìa et al., 2010). Umesaki (2014) indicated that microorganisms act as antigenic promoters for the maturation of lymphoid tissues associated with the GIT and increase the immunoglobulin producing cells and intraepithelial lymphocytes.
Prebiotics and Nutritional Benefits of the Host
Nutritional benefits of the host from supplementation of prebiotics are correlated to their fermentation into SCFAs in the lower gut (propionic, butyric, acetic, and lactic acids). SCFAs improve protein and mineral availability because they decrease the intestine's pH and promote nutrient solubility. Also, in mature birds, SCFAs are absorbed by passive diffusion across the cecum's epithelial cells, providing up to 11% of dietary metabolizable energy (Annison et al., 1968). Microbial alterations mediated by prebiotics affect nitrogen compounds and vitamin synthesis, and indigestible nutrients degradation, and facilitate removal of undesirable dietary elements.
The broiler cecum microbiome encodes up to 10% of the genes for amino acids and protein metabolism and 5% for the synthesis of cofactors and vitamins that can be used via the host or microbial metabolism itself (Danzeisen et al., 2011; Pan and Yu, 2014). Metagenomic analysis of broilers detected genes encoding cellulase, arabinoxylanse, hemicellulase, and lactase activity. These contributed to the activity of protease and amylase, and aid the microbial digestion of indigestible dietary constituents for the production of SCFAs (Xu et al., 2003). Microbial action could reduce antinutritional factors, such as mycotoxins and saponins, and increase the nutritional value of feedstuffs.
NUTRITIONAL FACTORS AFFECTING THE EFFICACY OF PREBIOTICS
One of the factors affecting prebiotics is the type of pill used in diet formulations. The commensal microbial profile of birds fed with a corn-based diet mainly differs in the community composition and diversity to barley, rice, or wheat diets (Hammons et al., 2010). Thus, the resident GIT bacteria can ferment prebiotics and use them and their metabolites as a growth substrate, negatively affecting the range of action mediated by prebiotics.
In general, cereals containing high levels of nonstarch polysaccharides (NSP; indigestible and water-soluble), such as barley, rice, or wheat, favor the proliferation of E. coli or C. perfringens, whereas those cereals lower in NSP do not (Kumar et al. 2019). Even minor differences in the type of cereal grain can affect enteric bacteria at the strain level (Hammons et al., 2010). They also reported that the standard corn-soybean diet, with or without wheat middling, affects the populations of Lactobacillus agilis.
Thus, to suitably assess the prebiotics’ usefulness and efficacy in the context of poultry nutrition, it must be examined with several cereal matrixes. The effectiveness of prebiotics can be affected by other feed supplements. Combining these supplements with probiotics (direct-fed microorganisms) in the diet, and converting them into synbiotics, gives positive results beyond those achieved via prebiotics alone (Awad et al., 2009).
When probiotics are combined with prebiotics in the feed, they may benefit the host by enhancing the survival of beneficial microorganisms in the gut by acting as their substrate (Gibson and Roberfroid, 1995). The most effective probiotic implantations, combined with the stimulating influence of prebiotics on the resident bacteria, contribute to maintaining homeostasis of the intestine and the host's general health status. Awad et al. (2009) stated that combining prebiotics with probiotics in poultry diets improved the GIT microbial flora and the growth performance characteristics.
Prebiotics’ efficacy could be maximized by combining them with natural antimicrobials or organic acids that decrease the potentially pathogenic microorganisms in the GIT (Kong et al., 2010). Thus, prebiotics could be selectively fermented and used as substrates for beneficial commensal bacteria that confer many benefits to the host (Bozkurt et al., 2009; Taherpour et al., 2012). These indications suggest that it is useful to evaluate combinations of prebiotics with other dietary supplements to identify potential synergism.
The intestinal microbiome of birds is greatly affected by diet. Any dietary constituents which escape host digestion and absorption may act as a substrate for enteric bacterial growth. For instance, wheat and barley stems are rich in water-soluble indigestible NSP. These diets favor the growth and proliferation of C. perfringens with subsequent necrotic enteritis of young chicks, whereas diets containing low levels of NSP (like corn-based diets) do not predispose the birds (Annett et al., 2002; Jia et al., 2009). The increased levels of NSP may result in a decline in the passage rate of digesta, nutrient digestibility, and an increase in digesta viscosity, favoring C. perfringens (Timbermont et al., 2011) and other bacterial types as compared with corn or wheat-based diets (Shakouri et al., 2009).
As there is little variation in dietary cereal composition, this affects the intestines’ bacterial strains, so the source and dietary protein level may impact the gut microbiome. Unlike soybean meal, fermented cottonseed meal as a protein source could increase and decrease the population of lactobacilli and cecal coliforms in broilers, respectively (Sun et al., 2013).
It was also reported that diets containing high levels of animal protein (such as fishmeal) resulted in C. perfringens proliferation in the poultry hind-gut, which may predispose chickens to necrotic enteritis (Drew et al., 2004). It has been reported that broiler chicks fed a diet rich in animal fat (such as the mixture of tallow and lard) resulted in C. perfringens proliferation in the ileum (Knarreborg et al., 2002).
CHALLENGES ASSOCIATED WITH PREBIOTICS AS IT RELATES TO BIRDS GI HEALTH AND PERFORMANCE
Despite the current use of prebiotics and probiotics as alternatives to antibiotics in mammalian and poultry feeds, few researchers have dealt with their limitations and adverse effects on the birds’ GIT. For example, in humans, probiotics could be responsible for harmful effects in susceptible individuals: deleterious metabolic actions, excessive immune stimulation, gene transfer, and systemic infections. There are limited data on “bacteremia” in humans where there is a separation of probiotic microorganisms from infections resulting from cancer, skin lesions, and chronic illness (Shishehchian et al., 2001).
Some limitations to be considered in mammalian nutrition are the following; firstly, prebiotic must be resistant to stomach acidic pH, not be absorbed in the GIT, and not hydrolyzed by mammalian enzymes. Secondly, it can be fermented by intestinal microbiota. Thirdly, the activity and/or growth of the enteric bacteria can be selectively boosted via these compounds, and this process enhances the host's health (Gibson et al., 2010). In poultry, prebiotics can generally be considered an alternative to AGPs in poultry nutrition. Nevertheless, there is still further research under more standardized conditions needed to evaluate the right dosage and the exact mechanism of actions (Abd El-Aziz et al., 2020; Elgeddawy et al., 2020; El-Shall et al., 2020).
CONCLUSIONS
The inclusion of AGPs in the poultry diet can inhibit enteric pathogens’ growth, reduce disease incidence and promote bird growth. As antibiotic resistance has grown, the use of most AGPs has been banned in the European Union. This ban has increased interest in alternative growth promoters and feed supplements in poultry production. Prebiotics in various forms offer a promising strategy to modify the GIT microbiota and benefit the bird in multiple ways; however, further investigations must further understand the mechanisms associated with prebiotic effects on the avian GIT to optimize their dosage and beneficial impact. The development of next-generation sequencing technology has opened the horizon for identifying the gut microbial populations responding to prebiotic administration. However, the gut microbial composition change might not always be detectable; therefore, other approaches such as metabolomics and transcriptomics are often required to gain an in-depth understanding of the GIT microbial and host functional responses. More research is needed on possible limitations (such as their resistance to stomach acidic pH and their fermentation by intestinal microbiota) and adverse effects on the GIT of birds.
AUTHOR CONTRIBUTION
All authors were equally contributed in writing this review article. All authors reviewed and approved the final version of the manuscript.
ACKNOWLEDGMENT
This work was financially supported by “The National Key Research and Development Program of China (2018YFE0112700)” and “The Science and Technology Key Projects of Zhejiang Province, China (2019C02005)”.
No ethical approval was required as this is a review article.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- El-Aziz Abd, H. A., El-Kasrawy N.I., Abd El-Hack M.E., Kamel S.Z., Mahrous U.E., El-Deeb E.M., Abo Ghanima M.M. Growth, immunity, relative gene expression, carcass traits and economic efficiency of two rabbit breeds fed prebiotic supplemented diets. Anim. Biotech. 2020:1–12. doi: 10.1080/10495398.2020.1800485. [DOI] [PubMed] [Google Scholar]
- Abd El-Hack M.E, Alagawany M, Arif M., Emam M., Saeed M., Arain M.A., Siyal F.A., Patra A., Elnesr S.S., Khan R.U. The uses of microbial phytase as a feed additive in poultry nutrition–a review. Ann. Anim. Sci. 2018;18:639–658. [Google Scholar]
- Angelakis E. Weight gain by gut microbiota manipulation in productive animals. Microb. Pathog. 2017;106:162–170. doi: 10.1016/j.micpath.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Annett C.B., Viste J.R., Chirino-Trejo M., Classen H.L., Middleton D.M., Simko E. Necrotic enteritis: effect of barley, wheat and corn diets on proliferation of Clostridium perfringens type A. Avian Pathol. 2002;31:598–601. doi: 10.1080/0307945021000024544. [DOI] [PubMed] [Google Scholar]
- Annison E.F., Hill K.J., Kenworthy R. Volatile fatty acids in the digestive tract of the fowl. Br. J. Nut. 1968;22:207–216. doi: 10.1079/bjn19680026. [DOI] [PubMed] [Google Scholar]
- Apajalahti, J., and A. Kettunen. 2006. Microbes of the chicken gastrointestinal tract. Pages 124–137 in Avian Gut Function in Health and Disease. Poultry Science Symposium Series, vol. 28. G. Perry, ed. CAB International, Oxon, UK.
- Apajalahti J., Vienola. K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Tech. 2016;221:303–323. [Google Scholar]
- Ashayerizadeh A., Dabiri N., Ashayerizadeh O., Mirzadeh K.H., Roshanfekr H., Mamooee M. Effect of dietary antibiotic, probiotic and prebiotic as growth promoters, on growth performance, carcass characteristics and hematological indices of broiler chickens. Pak. J. Biol. Sci. 2009;12:52–57. doi: 10.3923/pjbs.2009.52.57. [DOI] [PubMed] [Google Scholar]
- Awad W.A., Ghareeb K., Abdel-Raheem S., Böhm J. Effects of dietaryinclusion of probiotic and synbiotic on growth performance, organ weights, and intestinal histomorphology of broiler chickens. Poult. Sci. 2009;88:49–56. doi: 10.3382/ps.2008-00244. [DOI] [PubMed] [Google Scholar]
- Bachaya H.A., Abbas R.Z., Raza M.A., Iqbal Z., Rehman T.U., Baber W., Hussain R. Existence of coccidiosis and associated risk factors in broiler chickens in Southern Punjab, Pakistan. Pak. Vet. J. 2015;35:81–84. [Google Scholar]
- Bailey J.S., Blankenship L.C., Cox N.A. Effect of fructooligosaccharide on Salmonella colonization of the chicken intestine. Poult. Sci. 1991;70:2433–2438. doi: 10.3382/ps.0702433. [DOI] [PubMed] [Google Scholar]
- Barcelo A., Claustre J., Moro F., Chayvialle J.A., Cuber J.C., Plaisancié P. Mucin secretion is modulated by luminal factors in the isolated vascularly perfused rat colon. Gut. 2000;46:218–224. doi: 10.1136/gut.46.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beev G., Todorova P., Tchobanova S. Yeast cultures in ruminant nutrition. Bulgarian J. Agri. Sci. 2007;13:357–374. [Google Scholar]
- Bindels L.B., Delzenne N.M., Cani P.D., Walter J. Towards a morecomprehensive concept for prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015;12:303–310. doi: 10.1038/nrgastro.2015.47. [DOI] [PubMed] [Google Scholar]
- Bird A., Conlon M., Christophersen C., Topping D. Resistant starch, largebowel fermentation and a broader perspective of prebiotics and probiotics. Benef. Microbes. 2010;1:423–431. doi: 10.3920/BM2010.0041. [DOI] [PubMed] [Google Scholar]
- Bodie A.R., Micciche A.C., Atungulu G.G., Rothrock M.J., Jr, Ricke S.C. Current trends of rice milling byproducts for agricultural applications and alternative food production systems. Front. Sustain. Food Syst. 2019;3:47. [Google Scholar]
- Boler, B. M. V., and G. C. Fahey Jr. 2012. Prebiotics of plant and microbial origin. Pages 13–26 in Direct-Fed Microbials and Prebiotics for Animals. T. Callaway and S. Ricke, eds. Springer, New York, NY.
- Borda-Molina D., Vital M., Sommerfeld V., Rodehutscord M., Camarinha-Silva A. Insights intro broilers' gut microbiota fed with phosphorus, calcium, and phytase supplemented diets. Front. Microbiol. 2016;7:2033. doi: 10.3389/fmicb.2016.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt M., Küçükyılmaz K., Çatlı A.U., Çınar M. The effect of single or combined dietary supplementation of prebiotics, organic acids and probiotics on performance and slaughter characteristics of broilers. S. Afr. J. Anim. Sci. 2009;39:197–205. [Google Scholar]
- Buddington K.K., Donahoo J.B., Buddington R.K. Dietary oligofructoseand inulin protect mice from enteric and systemic pathogens and tumor inducers. J. Nutr. 2002;132:472–477. doi: 10.1093/jn/132.3.472. [DOI] [PubMed] [Google Scholar]
- Callaway T.R., Ricke. S.C. Science and Mechanisms of Action. Springer-Verlag; New York: 2012. Direct-fed microbials and prebiotics for animals; p. 206. [Google Scholar]
- Castanon J. History of the use of antibiotic as growth promoters in Europeanpoultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Chen Y., Chen. T. Mineral utilization in layers as influenced by dietaryoligofructose and inulin. Int. J Poult. Sci. 2004;3:442–445. [Google Scholar]
- Chen Y., Nakthong C., Chen T. Improvement of laying hen performance by dietary prebiotic chicory oligofructose and inulin. Int. J. Poult. Sci. 2005;4:103–108. [Google Scholar]
- Chichlowski M., Croom W.J., Edens F.W., McBride B.W., Qiu R., Chiang C.C., Daniel L.R., Havenstein G.B., Koci M.D. Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, primalac, and salinomycin. Poult. Sci. 2007;86:1121–1132. doi: 10.1093/ps/86.6.1121. [DOI] [PubMed] [Google Scholar]
- Cummings J., Macfarlane G. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991;70:443–459. doi: 10.1111/j.1365-2672.1991.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Dankowiakowska A., Kozłowska I., Bednarczyk M. Probiotics, prebiotics and snybiotics in poultry–mode of action, limitation, and achievements. J. Cent. Euro. Agri. 2013;14:467–478. [Google Scholar]
- Danzeisen J.L., Kim H.B., Isaacson R.E., Tu Z.J., Johnson T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS One. 2011;6:e27949. doi: 10.1371/journal.pone.0027949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Araújo A.N., Giugliano. L.G. Human milk fractions inhibit the adherence of diffusely adherent Escherichia coli (DAEC) and enteroaggregative E. coli (EAEC) to HeLa cells. FEMS Microbiol. Lett. 2000;184:91–94. doi: 10.1111/j.1574-6968.2000.tb08996.x. [DOI] [PubMed] [Google Scholar]
- De Maesschalck C., Eeckhaut V., Maertens L., De Lange L., Marchal L., Nezer C., Baere S.De, Croubels S., Daube G., Dewulf J., F. Haesebrouck, R.Ducatelle, B.Taminau, and F.Van Immerseel. Effects of xylo-oligosaccharides on broiler chicken performance and microbiota. Appl. Environ. Microbiol. 2015;81:5880–5888. doi: 10.1128/AEM.01616-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J., Richards J. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Donalson, L., W. Kim, V. Chalova, P. Herrera, J. McReynolds, V. Gotcheva, D.Vidanović, C. Woodward, L. Kubena, and Nisbet. 2008a. In vitro fermentation response of laying hen cecal bacteria to combinations of fructooligosaccharide prebiotics with alfalfa or a layer ration. Poult. Sci. 87:1263–1275. [DOI] [PubMed]
- Donalson L., McReynolds J., Kim W., Chalova V., Woodward C., Kubena L., Nisbet D., Ricke S. The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, Salmonella enteritidis infection, and intestinal shedding in laying hens. Poult. Sci. 2008;87:1253–1262. doi: 10.3382/ps.2007-00166. [DOI] [PubMed] [Google Scholar]
- Donalson L., Kim W.K., Chalova V., Herrera P., Woodward C., McReynolds J., L.Kubena D.Nisbet, Ricke S. In vitro anaerobic incubation of Salmonella enterica serotype typhimurium and laying hen cecal bacteria in poultry feed substrates and a fructooligosaccharide prebiotic. Anaerobe. 2007;13:208–214. doi: 10.1016/j.anaerobe.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Drew M.D., Syed N.A., Goldade B.G., Laarveld B., Van Kessel A.G. Effects of dietary protein source and level on intestinal populations of Clostridium perfringens in broiler chickens. Poult. Sci. 2004;83:414–420. doi: 10.1093/ps/83.3.414. [DOI] [PubMed] [Google Scholar]
- Dronamraju S.S., Coxhead J.M., Kelly S.B., Burn J., Mathers J.C. Cellkinetics and gene expression changes in colorectal cancer patients given resistant starch: A randomized controlled trial. Gut. 2009;58:413–420. doi: 10.1136/gut.2008.162933. [DOI] [PubMed] [Google Scholar]
- Eckles C., Williams. V. Yeast as a supplementary feed for lactating cows. J. Dairy Sci. 1925;8:89–93. [Google Scholar]
- EFSA EFSA (Panel on Biological Hazards – BIOHAZ) and EMA (Committee for Medicinal Products for Veterinary Use – CVMP), Joint Scientific Opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA) EFSA J. 2017;15:e04666. doi: 10.2903/j.efsa.2017.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgeddawy S.A., Shaheen H.M., El-Sayed Y.S., Abd Elaziz M., Darwish A., Samak D., Elnesr S.S. Effects of the dietary inclusion of a probiotic or prebiotic on florfenicol pharmacokinetic profile in broiler chicken. J. Anim. Physiol. Anim. Nutr. 2020;104:549–557. doi: 10.1111/jpn.13317. [DOI] [PubMed] [Google Scholar]
- El-Shall N.A., Awad A.M., Abd El-Hack M.E., Naiel M.A., Othman S.I., Allam A.A., Sedeik M.E. The simultaneous administration of a probiotic or prebiotic with live Salmonella vaccine improves growth performance and reduces fecal shedding of the bacterium in Salmonella-challenged broilers. Animals. 2020;10:70. doi: 10.3390/ani10010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO., 2017. Food and Agriculture Organization of the Unite Nations. http://www.fao.org/faostat/en/#data/QL.
- Gaggìa F., Mattarelli P., Biavati B. Probiotics and prebiotics in animalfeeding for safe food production. Int. J. Food Microbiol. 2010;141:S15–S28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Gao J., Zhang H., Yu S., Wu S., Yoon I., Quigley J., Gao Y., Qi G. Effects of yeast culture in broiler diets on performance and immunomodulatory functions. Poult. Sci. 2008;87:1377–1384. doi: 10.3382/ps.2007-00418. [DOI] [PubMed] [Google Scholar]
- Geier M.S., Butler R.N., Howarth G.S. Probiotics, prebiotics and synbiotics: A role in chemoprevention for colorectal cancer? Cancer Biol. Ther. 2006;5:1265–1269. doi: 10.4161/cbt.5.10.3296. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid. M.B. Dietary modulation of the human colonicmicrobiota: Introducing the concept of prebiotics. J. Nutr. 1995;125:1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Probert H.M., Van Loo J., Rastall R.A., Roberfroid M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Scott K.P., Rastall R.A., Tuohy K.M., Hotchkiss A., Dubert-Ferrandon A., Gareau M., Murphy E.F., Saulnier D., Loh G., Macfarlane S., Delzenne N., Ringel Y., Kozianowski G., Dickmann R., Lenoir-Wijnkoop I., Walker C., Buddington R. Dietary prebiotics: current status and new definition. Food Sci. Technol. Bull. Funct. Foods. 2010;7:1–19. [Google Scholar]
- Gibson G., McCartney A., Rastall R. Prebiotics and resistance togastrointestinal infections. Br. J. Nutr. 2005;93:S31–S34. doi: 10.1079/bjn20041343. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Hutkins R., Sanders M.E., Precott S.L., Reimer R.A., Salminen S.J., Scott K., Stanton C., Swanson K.S., Cani P.D., Verbeke K., Reid G. Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017;14:491–502. doi: 10.1038/nrgastro.2017.75. [DOI] [PubMed] [Google Scholar]
- Hammons S., Oh P.L., Martínez I., Clark K., Schlegel V.L., Sitorius E., Scheideler S.E., Walter J.A. Small variation in diet influences the Lactobacillus strain composition in the crop of broiler chickens. Syst. Appl. Microbiol. 2010;33:275–281. doi: 10.1016/j.syapm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Hartemink R., Van Laere K., Rombouts F. Growth of enterobacteria onfructo-oligosaccharides. J. Appl. Microbiol. 1997;83:367–374. doi: 10.1046/j.1365-2672.1997.00239.x. [DOI] [PubMed] [Google Scholar]
- Higgins J.P., Higgins S.E., Vicente J.L., Wolfenden A.D., Tellez G., Hargis B.M. Temporal effects of lactic acid bacteria probiotic culture on Salmonella in neonatal broilers. Poult. Sci. 2007;86:1662–1666. doi: 10.1093/ps/86.8.1662. [DOI] [PubMed] [Google Scholar]
- Hooge D.M. Meta-analysis of broiler chicken pen trials evaluating dietarymannan oligosaccharide, 1993-2003. Int. J. Poult. Sci. 2004;3:163–174. [Google Scholar]
- Hutkins R.W., Krumbeck J.A., Bindels L.B., Cani P.D., Fahey G., Goh Y.J., Hamaker B., Martens E.C., Mills D.A., Rastal R.A., Vaughan E., Sanders M.E. Prebiotics: why definitions matter. Curr. Opin. Biotechnol. 2016;37:1–7. doi: 10.1016/j.copbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyghebaert G., Ducatelle R., Immerseel F.V. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011;187:182–188. doi: 10.1016/j.tvjl.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Jia W., Slominski B.A., Bruce H.L., Blank G., Crow G., Jones O. Effects of diet type and enzyme addition on growth performance and gut health of broiler chickens during subclinical Clostridium perfringens challenge. Poult. Sci. 2009;88:132–140. doi: 10.3382/ps.2008-00204. [DOI] [PubMed] [Google Scholar]
- Jones F., Ricke. S. Observations on the history of the development ofantimicrobials and their use in poultry feeds. Poult. Sci. 2003;82:613–617. doi: 10.1093/ps/82.4.613. [DOI] [PubMed] [Google Scholar]
- Jung S., Houde R., Baurhoo B., Zhao X., Lee B. Effects of galactooligosaccharides and a bifidobacteria lactis-based probiotic strain on the growth performance and fecal microflora of broiler chickens. Poult. Sci. 2008;87:1694–1699. doi: 10.3382/ps.2007-00489. [DOI] [PubMed] [Google Scholar]
- Kabir S.M.L., Rahman M.M., Rahman M.B. Potentiation of probiotics in promoting microbiological meat quality of broilers. J. Bangladesh Soc. Agric. Sci. Technol. 2005;2:93–96. [Google Scholar]
- Kannan M., Karunakaran R., Balakrishnan V., Prabhakar T.G. Influence of prebiotics supplementation on lipid profile of broilers. Int. J. Polut. Sci. 2005;4:994–997. [Google Scholar]
- Kim S.A., Jang M.J., Kim S.Y., Yang Y., Pavlidis H.O., Ricke S.C. Potential for prebiotics as feed additives to limit foodborne Campylobacter establishment in the poultry gastrointestinal tract. Front. Microbiol. 2019;10:91. doi: 10.3389/fmicb.2019.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W., Donalson L., Herrera P., Kubena L., Nisbet D., Ricke S. Comparisons of molting diets on skeletal quality and eggshell parameters in hens at the end of the second egg-laying cycle. Poult. Sci. 2005;84:522–527. doi: 10.1093/ps/84.4.522. [DOI] [PubMed] [Google Scholar]
- Kleessen B., Hartmann L., Blaut M. Oligofructose and long-chain inulin:influence on the gut microbial ecology of rats associated with a human faecal flora. Br. J. Nutr. 2001;86:291–300. doi: 10.1079/bjn2001403. [DOI] [PubMed] [Google Scholar]
- Knarreborg A., Simon M.A., Engberg R.M., Jensen B.B., Tannock G.W. Effects of dietary fat source and subtherapeutic levels of antibiotic on the bacterial community in the ileum of broiler chickens at various ages. Appl. Environ. Microbiol. 2002;68:5918–5924. doi: 10.1128/AEM.68.12.5918-5924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Kumar S., Shang Y., Kim W.K. Insight into dynamics of gut microbial community of broilers fed ith fructooligosaccharides supplemented low calcium and phosphorus diets. Front. Vet. Sci. 2019;6:95. doi: 10.3389/fvets.2019.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateova S., Saly J., Tuckova M., Koscova J., Nemcova R., Gaalova M., Baranova D. Effect of preobiotics, prebiotics and herb oil on performance and metabolic parameters of broiler chickens. Medycyna Weterynaryja. 2008;64:294–297. [Google Scholar]
- Mathivanan R, Kalaiarasi. K. Panchagavya and Andrographis paniculata as alternative to antibiotic growth promoters on haematological, serum biochemical parameters and immune status of broilers. J. Poult. Sci. 2007;44:198–204. [Google Scholar]
- Mead G. Prospects for ‘competitive exclusion’ treatment to control Salmonellasand other foodborne pathogens in poultry. Vet. J. 2000;159:111–123. doi: 10.1053/tvjl.1999.0423. [DOI] [PubMed] [Google Scholar]
- Micciche A.C., Foley S.L., Pavlidis H.O., McIntyre D.R., Ricke S.C. A review of prebiotics against Salmonella in poultry: current and future potential for microbiome research application. Front. Vet. Sci. 2018;5:191. doi: 10.3389/fvets.2018.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet D. Defined competitive exclusion cultures in the prevention of enteropathogen colonisation in poultry and swine. Antonie Van Leeuwenhoek. 2002;81:481–486. doi: 10.1023/a:1020541603877. [DOI] [PubMed] [Google Scholar]
- Nurmi E., Rantala. M. New aspects of Salmonella infection in broilerproduction. Nature. 1973;241:210–211. doi: 10.1038/241210a0. [DOI] [PubMed] [Google Scholar]
- Nurmi E., Nuotio L., Schneitz C. The competitive exclision concept: development and future. Int. J. Food Microbiol. 1992;15:237–240. doi: 10.1016/0168-1605(92)90054-7. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Buhr R.J., Ritz C.W., Kiepper B.H., Berrang M.E., Seal B.S., Cox N.A. Successional changes in the chicken cecal microbiome during 42 days of growth are independent of organic acid feed additives. BMC Vet. Res. 2014;10:282. doi: 10.1186/s12917-014-0282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwehand A.C., Derrien M., de Vos W., Tiihonen K., Rautonen N. Prebiotics and other microbial substrates for gut functionality. Curr. Opin. Biotechnol. 2005;16:212–217. doi: 10.1016/j.copbio.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Oyarzabal O.A., Conner. D.E. Application of direct-fed microbial bacteria and fructooligosaccharides for Salmonella control in broilers during feed withdrawal. Poult. Sci. 1996;75:186–190. doi: 10.3382/ps.0750186. [DOI] [PubMed] [Google Scholar]
- Pan D., Yu. Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrascu O., Béguet-Crespel F., Marinelli L., Chatilier E.Le, Abraham A.L., Leclerc M., Klopp C., Terrapon N., Henrissat B., Blotitière H.M., Doré J., BéraMaillet C. A fibrolitic potential in the human ileum mucosal microbiota revealed by functional metagenomic. Sci. Rep. 2017;7:40248. doi: 10.1038/srep40248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J.A., Burkholder. K.M. Application of prebiotics and probiotics in poultry production. Poult. Sci. 2003;82:627–631. doi: 10.1093/ps/82.4.627. [DOI] [PubMed] [Google Scholar]
- Pelicano E.R.L., de Souza P.A., de Souza H.B.A., Oba A., Norkus E.A., Kodawara L.M., de Lima T.M.A. Effect of different probiotics on broiler carcass and meat quality. Br. J. Poult. Sci. 2003;5:207–214. [Google Scholar]
- Pelicano E.R.L., De Souza P., De Souza H., Leonel F., Zeola N., Boiago M. Productive traits of broiler chickens fed diets containing different growth promoters. Rev. Bras. Cienc. Avic. 2004;6:177–182. [Google Scholar]
- Pilarski R., Bednarczyk M., Lisowski M., Rutkowski A., Bernacki Z., Wardeńska M., Gulewicz K. Assessment of the effect of α-galactosides injected during embryogenesis on selected chicken traits. Folia Biol. 2005;53:13–20. doi: 10.3409/1734916054663474. [DOI] [PubMed] [Google Scholar]
- Ranjitkar S., Lawley B., Tannock G., Engberg R.M. Bacterial succession in the broiler gastrointestinal tract. Appl. Environ. Microbiol. 2016;82:2399–2410. doi: 10.1128/AEM.02549-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman A., Arif M., Sajjad N., Al-Ghadi M.Q., Alagawany M., Abd El-Hack M.E., Alhimaidi A.R., Elnesr S.S., Almutairi B.O., Amran R.A., Hussein E.O.S., Swelum A.A. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult. Sci. 2020;99:6946–6953. doi: 10.1016/j.psj.2020.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricke S.C. Perspectives on the use of organic acids and short chain fatty acids as antimicrobials. Poult. Sci. 2003;82:632–639. doi: 10.1093/ps/82.4.632. [DOI] [PubMed] [Google Scholar]
- Ricke S.C. Potential of fructooligosaccharide prebiotics in alternative and nonconventional poultry production systems. Poult. Sci. 2015;94:1411–1418. doi: 10.3382/ps/pev049. [DOI] [PubMed] [Google Scholar]
- Ricke S.C. Impact of prebiotics on poultry production and food safety. Yale J. Biol. Med. 2018;91:151–159. [PMC free article] [PubMed] [Google Scholar]
- Ricke S.C., Dunkley C., Durant J. A review on development of novelstrategies for controlling Salmonella enteritidis colonization in laying hens: fiber-based molt diets. Poult. Sci. 2013;92:502–525. doi: 10.3382/ps.2012-02763. [DOI] [PubMed] [Google Scholar]
- Ricke S.C., Lee S.I., Kim S.A., Park S.H., Shi Z. Prebiotics and the poultry gastrointestinal tract microbiome. Poult. Sci. 2020;99:670–677. doi: 10.1016/j.psj.2019.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinttilä T., Apajalahti. J. Intestinal microbiota and metabolites—implications for broiler chicken health and performance. J. Appl. Poult. Res. 2013;22:647–658. [Google Scholar]
- Rizzi L., Simioli M., Roncada P., Zaghini A. Aflatoxin B1 and clinoptilolite in feed for laying hens: effects on egg quality, mycotoxin residues in livers, and hepatic mixed function oxygenase activities. J. Food Prot. 2003;66:860–865. doi: 10.4315/0362-028x-66.5.860. [DOI] [PubMed] [Google Scholar]
- Roberfroid M. Prebiotics: the concept revisited. J. Nutr. 2007;137:830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- Rolfe R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000;130:396S–402S. doi: 10.1093/jn/130.2.396S. [DOI] [PubMed] [Google Scholar]
- Rosen G. Holo-analysis of the efficacy of Bio-Mos® in broiler nutrition. Br. Poult. Sci. 2007;48:21–26. doi: 10.1080/00071660601050755. [DOI] [PubMed] [Google Scholar]
- Ruiz-Moyano S., Totten S.M., Garrido D.A., Smilowitz J.T., German J.B., B.Lebrilla C., Mills D.A. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl. Environ. Microbiol. 2013;79:6040–6049. doi: 10.1128/AEM.01843-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabater-Molina M., Larqué E., Torrella F., Zamora S. Dietaryfructooligosaccharides and potential benefits on health. J. Physiol. Biochem. 2009;65:315–328. doi: 10.1007/BF03180584. [DOI] [PubMed] [Google Scholar]
- Schneitz, C. 2006. Competitive exclusion in poultry production. Pages 294–310 in Avian Gut Function in Health and Disease. Poultry Science Symposium Series, vol. 28. G. Perry, ed. CAB International, Oxon, UK.
- Scholz-Ahrens K.E., Schrezenmeir. J. Inulin, oligofructose and mineralmetabolism-experimental data and mechanism. Br. J. Nut. 2002;87:S179–S186. doi: 10.1079/BJNBJN/2002535. [DOI] [PubMed] [Google Scholar]
- Scott K.P., Antonie J.M., Midlvedt T., van Hemert S. Manipulating the gut microbiota to maintain health and treat disease. Microb. Ecol. Health Dis. 2015;26:25877. doi: 10.3402/mehd.v26.25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakouri M.D., Iji P.A., Mikkelsen L.L., Cowieson A.J. Intestinal function and gut microflora of broiler chickens as influenced by cereal grains and microbial enzyme supplementation. J. Anim. Physiol. Anim. Nutr. 2009;93:647–658. doi: 10.1111/j.1439-0396.2008.00852.x. [DOI] [PubMed] [Google Scholar]
- Shang Y., Kim. W.K. Roles of fructooligosaccharides and phytase in broiler chickens: review. Int. J. Poult. Sci. 2017;16:16–22. [Google Scholar]
- Shen Y.B., Piao X.S., Kim S.W., Wang L., Liu P., Yoon I., Zhen Y.G. Effects of yeast culture supplementation on growth performance, intestinal health, and immune response of nursery pigs. J. Anim. Sci. 2009;87:2614–2624. doi: 10.2527/jas.2008-1512. [DOI] [PubMed] [Google Scholar]
- Shishehchian F., Yusoff F.M., Shariff M. The effects of commercial bacterial products on macrobenthos community in shrimp culture ponds. Aquac. Int. 2001;9:429–436. [Google Scholar]
- Shoaf K., Mulvey G.L., Armstrong G.D., Hutkins R.W. Prebioticgalactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006;74:6920–6928. doi: 10.1128/IAI.01030-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims M., Dawson K., Newman K., Spring P., Hoogell D. Effects of dietary mannan oligosaccharide, bacitracin methylene disalicylate, or both on the live performance and intestinal microbiology of turkeys. Poult. Sci. 2004;83:1148–1154. doi: 10.1093/ps/83.7.1148. [DOI] [PubMed] [Google Scholar]
- Spring P., Wenk C., Dawson K., Newman K. The effects of dietarymannaoligosaccharides on cecal parameters and the concentrations of enteric bacteria in the ceca of Salmonella-challenged broiler chicks. Poult. Sci. 2000;79:205–211. doi: 10.1093/ps/79.2.205. [DOI] [PubMed] [Google Scholar]
- Stanczuk J., Zdunczyk Z., Juskiewicz J., Jankowski J. Indices of response of young turkeys to diets containing mannanoligosaccharide or inulin. Vet. ir Zootech. 2005;31:98–101. [Google Scholar]
- Stanley D., Hughes R.J., Moore R.J. Microbiota of the chicken gastrointestinal tract: influence on health, productivity and disease. Appl. Microbiol. Biotechnol. 2014;98:4301–4310. doi: 10.1007/s00253-014-5646-2. [DOI] [PubMed] [Google Scholar]
- Stanley V., Gray C., Daley M., Krueger W., Sefton A. An alternative toantibiotic-based drugs in feed for enhancing performance of broilers grown on Eimeria spp.- infected litter. Poult. Sci. 2004;83:39–44. doi: 10.1093/ps/83.1.39. [DOI] [PubMed] [Google Scholar]
- Sun H., Tang J.W., Yao X.H., Wu Y.F., Wang X., Feng J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop. Anim. Health Prod. 2013;45:987–993. doi: 10.1007/s11250-012-0322-y. [DOI] [PubMed] [Google Scholar]
- Swaggerty, C. L., T. R. Callaway, M. H. Kogut, A. Piva, and E. Grilli. 2019. Modulation of the immune response to improve health and reduce foodborne pathogens in poultry. Microorganisms. 7:65. [DOI] [PMC free article] [PubMed]
- Taherpour K., Moravej H., Taheri H.R., Shivazad M. Effect of dietaryinclusion of probiotic, prebiotic and butyric acid glycerides on resistance against coccidiosis in broiler chickens. Jpn. Poult. Sci. Assoc. 2012;49:57–61. [Google Scholar]
- Ten Bruggencate S.J., Bovee-Oudenhoven I.M., Lettink-Wissink M.L., Van der Meer R. Dietary fructo-oligosaccharides dose-dependently increase translocation of Salmonella in rats. J. Nutr. 2003;133:2313–2318. doi: 10.1093/jn/133.7.2313. [DOI] [PubMed] [Google Scholar]
- Ten Bruggencate S.J., Bovee-Oudenhoven I., Lettink-Wissink M., Katan M., Van Der Meer R. Dietary fructo-oligosaccharides and inulin decrease resistance of rats to Salmonella: protective role of calcium. Gut. 2004;53:530–535. doi: 10.1136/gut.2003.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng P.Y., Kim. W.K. Review: roles of prebiotics in intestinal ecosystem of broilers. Front. Vet. Sci. 2018;5:245. doi: 10.3389/fvets.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Van Immerseel F. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Tzortzis G., Goulas A.K., Gee J.M., Gibson G.R. A novelgalactooligosaccharide mixture increases the bifidobacterial population numbers in a continuous in vitro fermentation system and in the proximal colonic contents of pigs in vivo. J. Nutr. 2005;135:1726–1731. doi: 10.1093/jn/135.7.1726. [DOI] [PubMed] [Google Scholar]
- Umesaki Y. Use of gnotobiotic mice to identify and characterize key microbesresponsible for the development of the intestinal immune system. Proc. J. Acad. Ser. B. Phys Biol. Sci. 2014;90:313–332. doi: 10.2183/pjab.90.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcheva R., Dieleman. L.A. Prebiotics: Definition and protective mechanisms. Best Pract. Res. Clin. Gastroenterol. 2016;30:27–37. doi: 10.1016/j.bpg.2016.02.008. [DOI] [PubMed] [Google Scholar]
- van der Wielen P.W., Biesterveld S., Notermans S., Hofstra H., Urlings B.A., Knapen F.van. Role of volatile fatty acids in development of the cecal microflora in broiler chickens during growth. Appl. Environ. Microbiol. 2000;66:2536–2540. doi: 10.1128/aem.66.6.2536-2540.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videnska P., Sedlar K., Lukac M., Faldynova M., Gerzova L., Cejkova D., Sisak F., Rychlik I. Succession and replacement of bacterial populations in the caecum of egg laying hens over their whole life. PLoS One. 2014;9 doi: 10.1371/journal.pone.0115142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldroup A., Skinner J., Hierholzer R., Waldroup P. An evaluation offructooligosaccharide in diets for broiler chickens and effects on salmonellae contamination of carcasses. Poult. Sci. 1993;72:643–650. doi: 10.3382/ps.0720643. [DOI] [PubMed] [Google Scholar]
- Wei S., Morrison M., Yu Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013;92:671–683. doi: 10.3382/ps.2012-02822. [DOI] [PubMed] [Google Scholar]
- Wilson B., Whelan K. Prebiotic inulin-type fructans and galactooligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J. Gastroenterol. Hepatol. 2017;32:64–68. doi: 10.1111/jgh.13700. [DOI] [PubMed] [Google Scholar]
- Wu T., Dai X., Wu L. Effect of fructooligsaccharide on the broiler production. Acta Agri. Zhejiangensis. 1999;11:85–87. [Google Scholar]
- Xiang Q., Wang C., Zhang H., Lai W., Wei H., Peng J. Effects of different probiotics on laying performance, egg quality, oxidative status, and gut health in laying hens. Animals. 2019;9:1110. doi: 10.3390/ani9121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.R., Hu C.H., Xia M.S., Zhan X.A., Wang M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora andmorphology of male broilers. Poult. Sci. 2003;82:1030–1036. doi: 10.1093/ps/82.6.1030. [DOI] [PubMed] [Google Scholar]
- Yang Y., Iji P., Kocher A., Mikkelsen L., Choct M. Effects ofmannanoligosaccharide on growth performance, the development of gut microflora, and gut function of broiler chickens raised on new litter. J. Appl. Poult. Res. 2007;16:280–288. [Google Scholar]
- Zaghini A., Martelli G., Roncada P., Simioli M., Rizzi L. Mannanoligosaccharides and aflatoxin B1 in feed for laying hens: effects on egg quality, aflatoxins B1 and M1 residues in eggs, and aflatoxin B1 levels in liver. Poult. Sci. 2005;84:825–832. doi: 10.1093/ps/84.6.825. [DOI] [PubMed] [Google Scholar]