Abstract
Polysaccharide Of Atractylodes Macrocephala Koidz (PAMK) has been proved to have anti-cancer, antitumor, anti-inflammation function and improve the immune level of the organism. The miRNA plays a very important role in regulating the immune function by negatively regulate the expression of target genes. To explore the molecular mechanism of PAMK active the lymphocytes, thirty 61-d-old geese were randomly divided into 4 groups (C, CTX, PAMK, PAMK+CTX). The thymus morphology, the level of serum granulocyte-macrophage colony-stimulating factor (GMC-SF), IL-1β, IL-3, IL-5, the relative mRNA expression of CD25, novel_mir2, CTLA4 and CD28 signal pathway were measured. Further more, the lymphocytes was extracted from thymus to measure the relative mRNA expression of CD28 signal pathway. The results showed that PAMK could significantly maintain normal cell morphology of thymus, alleviate the decrease level of GMC-SF, IL-1β, IL-5, IL-6, TGF-β, the increase level of IL-4, IL-10, and the decrease relative mRNA expression of novel_mir2, CD25 and CD28 signal pathway in thymus and lymphocytes induced by cyclophosphamide (CTX). In conclusion, PAMK alleviated the decreased T lymphocytes activation levels induced by CTX through novel_mir2/CTLA4/CD28/AP-1 signal pathway.
Key words: PAMK, CTX, novel_mir2, CTLA4, CD28 signal pathway
INTRODUCTION
Polysaccharide Of Atractylodes Macrocephala Koidz (PAMK) is one of the main components extracted from Atractylodes macrocephala, which has been used as Chinese traditional medicine for nearly 2000 years. At present, PAMK has been reported to relieve upper respiratory tract infection, enhance the immune function of the spleen, thymus and stomach, promote gastrointestinal peristalsis, maintain the balance of intestinal microbiota(Guo et al., 2012; Shi et al., 2012; Xu and Tian, 2015). In terms of immune function, PAMK could anti-cancer, anti-inflammatory, anti-antitumor, improve the level of cellular immunity and humoral immunity(Sun et al., 2015; Xu and Tian, 2015; Guo et al., 2019). Some researchers indicated that PAMK could increase the spleen and thymus index of mice and geese. Besides it also could promote the lymphocytes proliferation, regulate the balance of T/B cells and Th1/Th2 cells, maintain the balance of secretion of cytokines(Li et al., 2009; Guo et al., 2012; Liu et al., 2015). CTX, which is known as immunosuppressor, was often used to make immunosuppressive model, caused it could inhibit bone marrow hematopoietic and the lymphocytes promotion, decrease the secretion of cytokines, reduce the level of humoral immunity and cellular immunity(Guo et al., 2019; Ibrahim et al., 2019). Of all immune factors, we specially focus on the activation of T lymphocytes, which indicated the immune level of the organism. However, the mechanism that PAMK enhances cytokines and activates lymphocytes is still unknown. Modern research has confirmed that a novel polysaccharide obtained from Craterellus cornucopioides, Ganoderma lucidum polysaccharides could alleviate the immunosuppressive active of T lymphocytes caused by CTX through TLR4-MyD88-NF-κB, Ca2+/CaN/NFAT/IL-2 pathway(Yu et al., 2015; Guo et al., 2019) Thus, to investige the signal pathway that PAMK active the T lymphocytes would be necessary.
Small RNA (miRNA) refers to a single-stranded non-coding RNA with a length of about 20-24 bp, which regulates post-transcriptional gene expression by selectively degrading target gene mRNA or inhibiting translation, some miRNAs are specific to target gene regulation. Based on the previous research of the research group, we found a small RNA significantly differently expressed between the PAMK and PAMK+CTX groups: novel_mir2, and forecast it's target geen to be Cytotoxic T lymphocyte-associated antigen 4 (CTLA4). To explore whether PAMK active the T lymphocytes by novel_mir2/CTLA4/CD28 signal pathway, geese immunosuppressive model was constructed. The thymus histological structure, serum level of cytokines, relative mRNA expression of cytokines in thymus and novel_mir2/CD28/CTLA4 signal pathway in thymus and T lymphocytes, was measured by Hematoxylin-eosin staining (HE), Transmission Electron Microscope (TEM), scanning electron microscope (SEM), ELISA, and qPCR. That will give a conclusion that PAMK may alleviate decreased T lymphocytes activation levels induced by CTX through novel_mir2/CTLA4/CD28/AP-1 signal pathway.
MATERIALS AND METHODS
Experiment Grouping and Treatments
All geese were purchased form Guangdong Qingyuan Jinyufeng Goose Co., Ltd., which is a professional goose-breeding company. The geese were housed in specific pathogen-free environment and were enrolled in experiments at 1 day of age, with half of them male and half female and randomly divided into four groups (C; PAMK; CTX; PAMK+CTX; 9 geese/group). All geese were treated humanely, and the experiments received prior ethical approval in accordance with Zhongkai University of Agriculture and Engineering under the approved protocol number SRM-11.
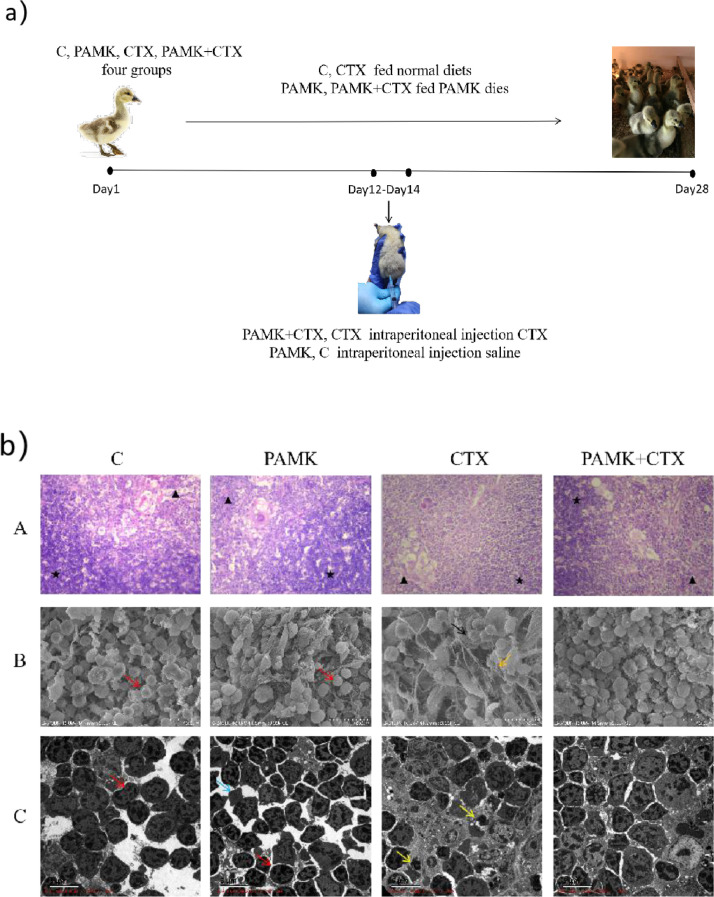
PAMK (purity 95 %) was purchased from Yanglingciyuan Biotechnology company (Xi'an, China). The four groups of geese had free access to food and water. The C and CTX groups were fed normal diets, whereas groups PAMK and CTX+PAMK were fed the normal diet supplemented with PAMK at a dose of 400 mg(kg body weight). In addition, C and PAMK were injected with 0.5 mL saline, while CTX and CTX+PAMK groups were injected with CTX (Jiangsu Hengrui Pharmaceutical, China) at 40 mg(kg body weight) per day at 12 to 14 days of age (Figure 1a). Thymus and blood were collected at 28 d of age. All the samples were placed in liquid nitrogen immediately and stored at -80 °C until analysis.
Figure 1.
(a) Schematic outlines of the experimental approaches tested in geese; (b) Effects of PAMK on histology, ultramicroscopic morphology of geese thymus treated with CTX. A. HE staining of the thymus (400 × ); B. Scanning electron microscopy (SEM; 3000 × ) of the thymus. C. Transmission electron microscopy (TEM; 3000 × ) of the thymus. ★ indicates cortical, ▲ indicates medullary, the red arrow points to normal lymphocytes, the black arrow points to wrinkled lymphocytes, the yellow arrow points to apoptotic lymphocytes, the orange arrow points to connective tissue, the blue arrow points to red blood cells.
Thymus Histology and Ultramicroscopic Morphology Observation
HE staining: We serially sectioned the paraffin-fixed blocks into 5-6-μm-thick coronal slices. For routine histological examination, the paraffin sections were stained with HE. HE-stained slices were analyzed under a Nikon fluorescence microscope (Nikon, Tokyo, Japan).
TEM: We divided each thymus into small blocks of 1 mm3 and then fixed with 2.5 % glutaraldehyde at 4 °C. Ultrathin slices with 50-70 nm thickness were prepared and stained with uranyl acetate (22400, EMS, USA) and lead citrate (19314, TED PELLA, USA) following the conventional protocol. The samples were examined under a transmission electron microscope (JEM-1400, Japan).
SEM: The thymus were fixed with 2.5 % glutaraldehyde for >2 h and observed under a scanning electron microscope (Hitachi S3000N, Tokyo, Japan).
Assays of Cytokines in Serum
The blood of geese was collected and centrifuged to separate serum. The levels of IL-3 (XY-ELA0093), IL-5 (XY-ELA0095), IL-1β (XY-ELA1071), and GM-CSF (XY-ELA8641) were measured by using ELISA kits (Shanghai Xinyu Technology Company, Shanghai, China).
Quantitative Reserve-Transcription PCR Analysis
Total RNA was extracted from the thymus with TRIzol reagent (Thermo Fisher, USA) and reverse-transcribed was performed by the Reverse Transcription Kit (Takara, Japan) and ImPro-IITM Reverse Transcription Systerm (promega, USA). The primers (Table 1) of related genes were designed by using Primer Premier 5.0 software (Premier Biosoft International, USA). An ABI PRISM 7500 detection system (Applied Biosystems, Foster City, CA) was used to measure the relative mRNA expression of genes related to the T lymphocytes activation.
Table 1.
Primer sequences for qPCR.
| cDNA | Primer sequences | cDNA | Primer sequences |
|---|---|---|---|
| IL4-F | GGCATCTACCTCAACTTGCT | CD86-F | GGATTATGGATGAGGGACAGT |
| IL4-R | CTCTTTCGCTACTCGTTGGA | CD86-R | CCCTGTGGGTAGCTGTGTTA |
| IL-6-F | AGACTTCCATCCAGTTGCCT | PIK3K-F | GGACCTCTGTCTGCTACCAT |
| IL-6-R | CATTTCCACGATTTCCCAGAG | PIK3K-R | TTCCTTCAGCCACTGGTTTA |
| IL10-F | ATCATGACATGGACCCGGTA | VAV2-F | GAAGTGGATGGAGCAGTTTG |
| IL10-R | ATTGCTCCATGACAGTTGCT | VAV2-R | GGAGGAATGATTTCCAAGCA |
| TGF-β-F | CATCACAGAGACAGGAACCT | JNK-1F | ACAGGGGATAGTATGTGCAGC |
| TGF-β-R | CTTTCACATCACCACTGGAA | JNK-1R | CCAGGGATTTTTGCGGTGTG |
| CTLA4-F | CCCTAGCCGAAACAATGTG | AP-1-F | CTTCTACGCGTCGGACTGG |
| CTLA4-R | GCTCCATCTTGCAGACATAA | AP-1-R | AGACGAAGGTGGAGGTGTAG |
| CD28-F | TCCCCACAGTGCATTTAAC | novel_mir2-F | ACACTCCAGCTGGGTTAGTGCGCAGTAAGCTAG |
| CD28-R | CAAGCAGTTTGTACCACGTT | novel_mir2-R | CTCAACTGGTGTCGTGGA |
| CD25-F | ATCTGGACACCCCTCAACAT | β-actin-F | GCACCCAGCACGATGAAAAT |
| CD25-R | TGAACTGGATGCTGTAGGAA | β-actin-R | GACAATGGAGGGTCCGGATT |
| CTLA4-WT-F | CCGCTCGAGAACAGGGAGAGGGAGCCTGTTTCCTAGC | CTLA4-WT-R | ATAAGAATGCGGCCGCATCGCAAAGAATTAAAATGGTAGAGC |
| mutCTLA4F | GCGTCCCCATCAGATGATATGAAAGTTAACCTTGATGATATCTGAGCCAGT | mutCTLA4R | GTTAACTTTCATATCATCTGATGGGGACGCAATGCCAGAGAAAGGTGCTGA |
| U6-F | CTCGCTTCGGCAGCACA | U6-R | AACGCTTCACGAATTTGC |
Double Luciferase Reporter Gene to Validation of Target Gene
We constructed new vectors based on psiCHECK-2 plasmids. Inserting the target response elements (CTLA4 3’UTR) into psiCHECK-2 and using XhoI/NotI to double digestion. The target fragment was ligated with the vector with T4 ligase, transformed, and the plasmid was digested to identify positive clones and sequenced. Mutating the target locus to a new vector, which named psiCHECK-2-CTLA4-plasmids. Mutate the binding site of novel-mir-2, repeating the above steps and rename it mut-psiCHECK-2-CTLA4-plasmids. The 293T cells were co-transfected with the mut-psiCHECK-2-CTLA4-plasmids, psiCHECK-2-CTLA4-plasmids, and novel-mir-2-mimic, novel-mir-2-inhibitor using Lipofectamine 2000 (Thermo Fisher Scientific, USA). The mut-psiCHECK-2-CTLA4-plasmids sequences were shown in Table 2, the red sequence represents the mutation sequence. At 24 h after transfection, luciferase activity was assessed using the DualLuciferase Reporter Assay System (Promega, USA). The activity of Renilla luciferase was normalized to the activity of firefly luciferase (Renilla LUC/firefly LUC).
Table 2.
The sequence of mut-psiCHECK-CTLA4-plasmids.
| CCTGGCGTGCTGAGACGAGCAGTAATTCTAGGCGATCGCTCGAGAACAGGGAGAGGGAGCCTGTTTCCTAGCTGGGATGGAGAATCTGCTAACTGACTTAAGTCAAAGAAATAAAATTTATCTTATTACAAAGGTGTATGGGAAAGAGAGATAATATTCCTTGTAACTTCTACGGTTTGGAGGAGAATAAATATAGTTTATGCATTAATATTTCAAGGTTAACCCTGTTACATAAAAATGGCTACATTAGGCCATTGATTCTTTGAGTTGTATTGTACCAATATGTAGTCTATATATATATGCATATATATATGTATAGCATTAAGTGCTATTGCTGTTGCAGCAGAACATCTTATTGTTAGATTGTGCCATATATACTGATCAACTGAGATCCAAACACTATTAAGACAGAAGCTCGTGTGTCATTGCAAGGCTAATCCTACAGGTCTGGGAGGCAGAGGCAGAGGAGCACACTAATGTACAAAAGAATGTTGACTTGTTTCTGTGCAGTGCTATTTATTGGTTCCATTTCTTGCATTAGTGCTTCGCGCTTCAACAGAGGCATCACCATAGGCTGGCAGTGATGATACTGCTGCTCTAAAAGCCTGATCTCAGCACCTTTCTCTGGCATTGCGTCCCCATCAGATGATATGAAAGTTAACCTTGATGATATCTGAGCCAGTGCTTGGAGAGAAAAGCTGCCCAGCTGGAGACCTCTTAAGCTGTTGCTGGGAAGATGTTCCAGCTCTGCTGGGGCCTCACATTTTAGAAATGCTAAATTAGCATCTATCCAAAAGGCAGAAGTATGTTGCTGTGTTTAACTGAATCTATTTTCCATGAAAATACCAACAGTGTATAATCTTGTTTATTAGTTATGTACCATTCAATAAAAACCTTGAAGCTTTGCCCAAATTAAAGTTCATTTCTTATTCACCATTCTCAGAAGCAACAGGAAGTGGCTACATGTTAGAAAGCAGTGATGACAAGATGAACCTTTCCCCTCTGACCCTGTGTTTTGCCTTTCTGAGGAGCTTGGGTGCTCATGCCAACACTTACTTTCTCATCCCTCCTCCTCAGGGGTCACCATTAAAATCTGCCAAGTCTTCTATTTGTTTAGTTAGAGCTGCTGTGATGTGGTTCGCAGATGCTACCAGTCACCTTTACACTTCCAGCAAAGTTACTTGCTTGCAGAAGGTAACAGTAACGACTTTGGTAGCATCTCCTGTATCTGTTTATACAAAAAGCTAGTTCTAGTTTCAAATCCTGTTTTCTTTGCACCTCCTTGACACTCTCACTCACTTAATGCTACCTGACACACAAAGCTTTTAGAGCTTAAAACCATTTGAGAATTCTTAGCTCTACCATTTTAATTCTTTGCGATGCGGCCGCTGGCCGCAATAAAATATCTTTATTTTCATTACATCTGTGTGTTGTTTTAATGG |
Culture and Transfection of Thymus Lymphocytes
The geese thymus were aseptically extracted and separated into single lymphocytes with lymphocyte separation solution (MPbio, USA). Lymphocytes were adjusted to a density of 3 × 106 cells/mL, and cultured in a 12-well plate (Corning Incorporated, USA). After 4 h of culture, transfection reagent NC mimic, NC inhibior, novel-mir-2 mimic novel_mir2 inhibitor (RiboBio Co., LTD, Guangzhou, China) (Table 3) was added, and cells were collected 24 h later for relative mRNA expression detection.
Table 3.
Information of novel_mir2.
| miRNA id | novel_mir2 |
|---|---|
| Chromosome | NW_013185761 |
| Strand | 2801038 |
| Sequence(mature) | UUAGUGCGCAGUAAGCUAGGGUGU |
| Sequence(star) | CGCUAGCUGCUCUGCACUAACU |
| Start | 1 |
| End | 2800980 |
| Sequence(precursor) | UUAGUGCGCAGUAAGCUAGGGUGUGAAUUGACAGCACGCUAGCUGCUCUGCACUAACU |
| Novel-mir-2-mimic | UGUGGGAUCGAAUGACGCGUGAUU |
| Novel-mir-2-inhibitor | AAUCACGCGUCAUUCGAUCCCACA |
Statistical Analysis
The data were analysed using SPSS for Windows (version 18, SPSS Inc, Chicago, IL, USA) and expressed as the means ± standard deviations (SD). All the qPCR assays were repeated in triplicate, and the relative expression levels were measured in terms of threshold cycle (Ct) values and normalized via the formula 2−∆∆Ct. Differences between groups were compared using one-way analysis of variance (ANOVA) followed by Tukey's honestly significant difference test. P < 0.05 was considered statistically significant.
RESULTS
PAMK Alleviated CTX-induced Thymus Damage
In HE staining (Figure 1b) A), CTX could make thymocytes disorderly arranged, irregular morphology, fewer thymocytes and unclear boundaries of cortical medulla, while PAMK could alleviate this phenomenon. In SEM (Figure 1b) B), CTX could make lymphocytes shrink and connective tissue increase, while PAMK could restore cell morphology and reduce connective tissue hyperplasia. In TEM(Figure 1b) C), CTX group chromatin borders, nuclei disappear, and apoptotic microsomes appear. PAMK could restore normal cell morphology and significantly reduced the number of apoptotic microsomes.
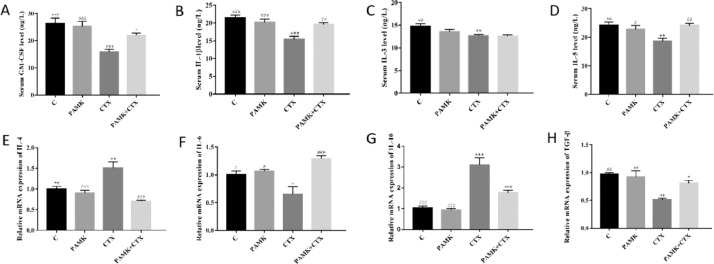
PAMK Alleviated the Decrease of Serum Cytokines Induced by CTX
Blood was centrifuged to obtain the serum (Figure 2 A-D). The results showed that the levels of serum GM-CSF, IL-1β, IL-3, IL-5 in CTX group were significantly decreased when it compared with C group, which means that CTX induced an immunosuppressive model successfully constructed. Compared with the CTX group, the level of GM-CSF, IL-1β, IL-5 was significantly increased and IL-3 had increase trend in PAMK+CTX group, which indicated that PAMK could alleviate the decrease of cytokines induced by CTX. The levels of all cytokines in PAMK group has no significant difference when it compared with C group. All the results indicated that PAMK could alleviate the decrease of serum cytokines induced by CTX.
Figure 2.
Effects of PAMK on level of serum cytokines and relative mRNA expression of cytokines in geese thymus treated with CTX. Serum levels of (A) GM-CSF (B) IL-1β (C) IL-3 (D) IL-5. Relative mRNA expression of (E) IL-4; (F) IL-6; (G) IL-10; (H) TGF-β; Data are expressed as the means ± SD, n = 9 *P < 0.05, **P < 0.01, ***P < 0.001, compared with C group; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with CTX group.
PAMK Alleviated the Disorder of Relative mRNA Expression of Cytokines in Thymus Induced by CTX
Total RNA was extracted from thymus to measure the relative mRNA expression of cytokines (Figure 2 E-H). The results showed that the relative mRNA expression of IL-4 and IL-10 in CTX group was increased significantly and the relative mRNA expression of IL-6 and TGF-β was significant decreased when it compared with C group. However all the relative mRNA expression of cytokines in the PAMK+CTX group were significant difference compared with CTX group, which means that PAMK alleviated the imbalance relative mRNA expression of cytokines caused by CTX, and made the relative mRNA expression back to normal level. The relative mRNA expression of IL-4, IL-6, IL-10 and TGF-β in PAMK group showed no difference compared with C group. Thus PAMK could alleviate the disorder of relative mRNA expression of cytokines in thymus induced by CTX.
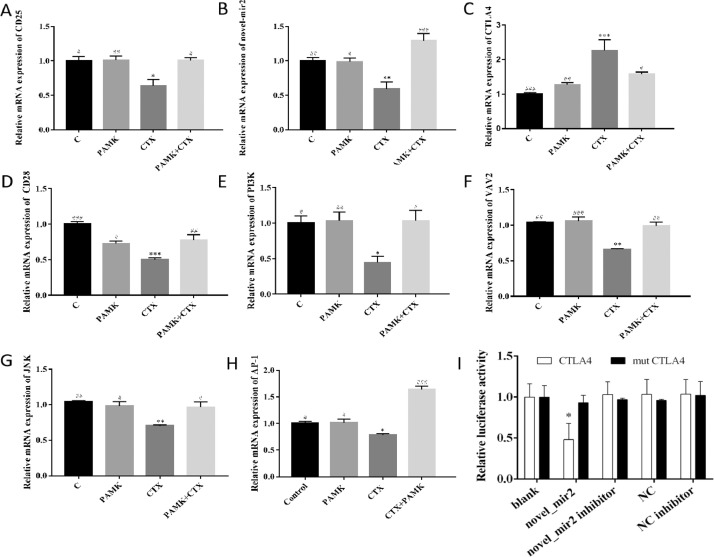
PAMK Alleviated the Decrease of Relative mRNA Expression of CD25, novel_mir2, and CD28 Signal Pathway in Thymus Treated by CTX
Total RNA was extracted from thymus to measure the relative mRNA expression of CD25, novel_mir2,CTLA4 and CD28 signal pathway (Figure 3 A-H). Compared with C group, the relative mRNA expression of CD25, novel_mir2, CD28, PI3K, VAV2, JNK, AP-1 in CTX group were significantly decreased. As the target gene of novel_mir2, the relative mRNA expression of CTLA4 showed the reverse results. In CTX group, the relative mRNA expression of CTLA4 was significantly increased compared with C group. In PAMK+CTX group, the level of CD25, novel_mir2, CD28, PI3K, VAV2, JNK, AP-1 and CTLA4 showed significantly increased and decreased respectively when it compared with CTX group, which indicated that PAMK could alleviate the decrease relative mRNA expression of CD25, novel_mir2, CD28, PI3K, VAV2, JNK, AP-1 and the increase of CTLA4. The results also showed that the relative mRNA expression of CD25, novel_mir2, CTLA4 and CD28 signal pathway in PAMK has no significant difference compared with C group.
Figure 3.
Effects of PAMK on the relative mRNA expression of CD25, novel_mir2, CTLA4 and CD28 signal pathway of geese thymus treated with CTX and dual luciferase reporter gene validation novel_mir2 targets CTLA4. Relative mRNA expression of (A) CD25; (B) novel_mir2; (C) CTLA4. (D) CD28; (E) PI3K; (F) VAV2; (G)JNK; (H)AP-1. Data are expressed as the means ± SD, n = 9 *P < 0.05, **P < 0.01, ***P < 0.001, compared with C group; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with CTX group. (I) comparison of relative fluorescence values between wild-type and mutant plasmids. * P < 0.05, compared with mutCTLA4.
Dual Luciferase Reporter Gene Validation novel_mir2 Targets CTLA4
When novel_mir2 was co-transfected with wild-type CTLA4, the R/F value was significantly lower than the blank group and NC group. It showed that novel_mir2 interacted with wild-type CTLA4. The double fluorescence detection results are shown in Figure 3 I. There is no significant difference in the R/F values of novel_mir2 compared with the blank group and the NC group, indicating that the predicted binding site mutations after novel_mir2 cannot be combined with CTLA4 interaction. The results indicated that novel_mir2 targets CTLA4.
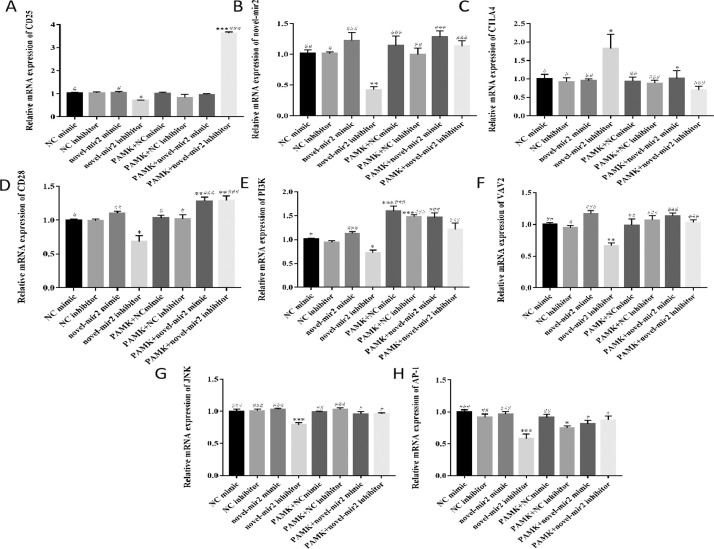
PAMK Alleviated the Decrease of Relative mRNA Expression of CD25, novel_mir2, and CD28 Signal Pathway of Lymphocytes in Thymus induced by novel_mir2 Inhibitor
Total RNA was extracted from lymphocytes to measure the relative mRNA expression of CD25, novel_mir2, CTLA4 and CD28 signal pathway. From the Figure 4 A-H, the results indicated that the relative mRNA expression of CD25, novel_mir2 CTLA4, CD28, PI3K, VAV2, JNK, AP-1 in NC mimic and NC inhibitor has no significant difference. The relative mRNA expression of CD25, novel_mir2, CTLA4, CD28, PI3K, VAV2, JNK, AP-1 in novel_mir2 mimic group and novel_mir2 inhibitor group showed significant difference. When compared with NC mimic group, the relative mRNA expression of CD25, novel_mir2, CD28, PI3K, VAV2, JNK, AP-1 showed significant decrease in novel_mir2 inhibitor group but it showed significant increase in PAMK+novel_mir2 inhibitor group when it compared with novel_mir2 inhibitor group. However, the relative mRNA expression of CTLA4 showed the opposite results. All the results indicated that PAMK could alleviate the the reduced of relative mRNA expression of CD25, novel_mir2 CD28, PI3K, VAV2, JNK, AP-1 and the increased of CTLA4 induced by novel_mir2 inhibitor.
Figure 4.
Effects of PAMK on the relative mRNA expression of CD25, novel_mir2, CTLA4 and CD28 signal pathway of lymphocytes in thymus treated with different transfection reagents. Relative mRNA expression of (A) CD25; (B) novel_mir2; (C) CTLA4. (D) CD28; (E) PI3K; (F) VAV2; (G)JNK; (H)AP-1. Data are expressed as the means ± SD, n = 9 *P < 0.05, **P < 0.01, ***P < 0.001, compared with NC mimic group; #P < 0.05, ##P < 0.01, ###P < 0.001, compared with novel_mir2 inhibitor group.
DISCUSSION
Novel_mir2 is a newly discovered miRNA located on chromosome NW_013185761. CTX is often used to generate immunosuppressive models to research the function of polysaccharide. From the results of HE, TEM and SEM in our research, it indicated that CTX could make the thymocytes sparse and irregular, and had many apoptotic bodies, but PAMK could alleviate this phenomenon. The cell morphology and tightness in PAMK+CTX group showed no difference compared with C group, which indicated that PAMK could maintain the morphology stable of thymocytes and have protect effect not toxic effect. These findings were consistent with previous research(Fu et al., 2018; Meng et al., 2019; Garrett-Thomson et al., 2020). These results suggested that PAMK could alleviate the morphological structure of immunosuppression geese thymocytes induced by CTX at the macro level.
Cytokines play a very important role in infection, inflammation, and cancer (Fu et al., 2018; Fan et al., 2019; Guo et al., 2019; Meng et al., 2019). Increased level of IL-3, IL-5, IL-1β, GM-CSF could help active the lymphocytes and promote lymphocytes proliferation(Kumar et al., 2020). IL-3, IL-5, and GM-CSF are produced primarily by activated T cells, promote myeloid cell proliferation and differentiation, and play a role in a variety of human diseases, particularly cancer and inflammation( Koh et al., 2010). In our research, we indicated that PAMK could active the lymphocytes and promote the serum cytokines level of IL-3, IL-5, GM-CSF, IL-6, TGF-β, which could activate the proliferation and differentiation of bone marrow precursor cells, promote the activation and proliferation of T, B cells, improve the level of cellular and humoral immunity of the body, and alleviate the lymphocytes immunosuppression caused by CTX. IL-4 and IL-10 are produced by mast cells, basophils and mononuclear macrophages, respectively, and can inhibit the activation and secretion of IL-2, IFN-γ by T cells(Silva et al., 2020). The results in our research found that the relative mRNA expression level of IL-4 and IL-10 in PAMK+CTX group was significant decrease when compared with CTX group, which indicated that PAMK could alleviate the imbalance of cytokines caused by CTX and maintain the balance of cytokines secretion levels. Similar to our research, Zhu and Zhou found that Codonopsis pilosulaA Polysaccharide and Lycium barbarum polysaccharide could increase the serum secretion level of TGF-β, IL-6, TNF-α, sIgA, enhance intestinal flora, increase CD4/CD8 T lymphocytes ratio to enhance the body immunity level(Zou et al., 2019; Zhu et al., 2020) Ying and zhou indicated that polysaccharide could alleviate the immunosuppression induced by CTX through increase the serum secretion level of IL-2, IL-3, IL-6, TGF-β(Wang et al., 2018; Ying et al., 2020). Taken together, these findings confirm that PAMK can alleviate the imbalance level of cytokines induced by CTX.
T lymphocytes are the important immune cells, which take part in cellular immunity. The early hallmark for T cell activation is CD25(Files et al., 2020) In our research, we found that the relative mRNA expression of CD25 in PAMK+CTX group significant increase compared with CTX group, which indicated that PAMK could active T lymphocytes. Our research found that the relative expression of novel_mir2 and CTLA4 showed the opposite trend in thymus. The double luciferase reporter gene showed that the novel_mir2 target CTLA4. Some researchers indicated that activation of CD28 can activate downstream PI3K and PI3K products regulate the activity of VAV2, which activates the JNK/SAPK pathway through RAC1. When JNK/SAPK is activated, it active AP-1(Samstag et al., 2020). In our research, we suggested that the relative mRNA expression of CD28, PI3K, VAV2, JNK and AP-1 showed significant up regulated in PAMK+CTX group when compared with CTX group, which was very important for exploring the molecular mechanisms of PAMK. Thus, we indicated that PAMK could activate T lymphocytes by CD28/PI3K/VAV2/JNK/AP-1 signal pathway. To confirm our conclusion, we validated them at the cellular level. From the results in the cellular experience, we found that NC mimic and NC inhibitor showed no significant difference, which means the transfection reagent had no effect on the experiment. We also found that PAMK+NC mimic group and PAMK+NC inhibitor group showed no significant difference when compared with NC mimic and NC inhibitor, which indicated that PAMK does not have a significant effect on relative mRNA expression of CD25, novel_mir2 and CD28 signal pathway and can maintain the body at normal immune levels. However, the relative mRNA expression level of CD28 signal pathway significant increase in PAMK+novel_mir2 inhibitor group when compared with novel_mir2 inhibitor group, which suggests that PAMK can increase the relative mRNA expression of novel_mir2, which in turn targets CTLA4, promote CD28 activation and activates the CD28 signaling pathway. Some research indicated that miR-181a, miR-21, miR-34c-5p could involve in T cell activation and regulation of downstream TCR pathway(Li et al., 2007; Loffler et al., 2007). In addition to cell activation, miRNA could regulate the activity of lymphocytes by AP-1 and TLR4/NF-κB signal pathway (Liu et al., 2014; Li et al., 2017). However, whether miRNA is involved in the regulation of PAMK to cellular immunity has not been reported. In our research, we suggest that novel_mir2 participate the regulation of PAMK and play an important role in the activation of CD28 signal pathway.
In conclusion, PAMK could activate T lymphocytes in thymus through novel_mir2/CTLA4/CD28 signal pathway, maintain the balance of cytokines production in the body, promote T lymphocytes activation and proliferation, restore the thymus cells morphology to alleviate the immune suppression caused by CTX thereby restore the organism back to the normal immunity level.
AUTHOR CONTRIBUTIONS
WL and SX contributed to the hypothesis generation, experimental design, data interpretation, and manuscript preparation. XX and DX conducted the experiments. YH, NC and YT contributed to the data interpretation.
ACKNOWLEDGEMENTS
The authors thank the members of the College of Animal Science & Technology, Guangdong Province Key Laboratory of Waterfowl Healthy Breeding for their help in collecting, the samples. The authors also acknowledge the support of College of Veterinary Medicine, Northeast Agricultural University.
FUNDING
This work was supported by GuangDong Basic and Applied Basic Research Foundation 2019A1515110106); National Key Technologies R & D Program of China (2016YFD0500510); National Natural Science Foundation of China (32072730); Science and Technology Planning Project of Guangzhou (201904010076). This funding had no impact on our study design or collection, analysis, and interpretation of the data. Further support was provided solely from institutional and/or departmental sources.
CONFLICTS OF INTEREST
All authors read and approved the final manuscript. The authors declare that there are no conflicts of interest.
DATA AVAILABILITY STATEMENT
All data generated or analyzed in this study are included in this published article.
Footnotes
Appropriate scientific section: Molecular and Cellular Biology
REFERENCES
- Fan S., Li J., Bai B. Purification, structural elucidation and in vivo immunity-enhancing activity of polysaccharides from quinoa (Chenopodium quinoa Willd.) seeds. Biosci. Biotechnol. Biochem. 2019;83:2334–2344. doi: 10.1080/09168451.2019.1650635. [DOI] [PubMed] [Google Scholar]
- Files J.K., Boppana S., Perez M.D., Sarkar S., Lowman K.E., Qin K., Sterrett S., Carlin E., Bansal A., Sabbaj S., Long D.M., Kutsch O., Kobie J., Goepfert P., Erdmann N. Sustained cellular immune dysregulation in individuals recovering from SARS-CoV-2 infection. J. Clin. Invest. 2021;131 doi: 10.1172/JCI140491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y.P., Feng B., Zhu Z.K., Feng X., Chen S.F., Li L.X., Yin Z.Q., Huang C., Chen X.F., Zhang B.Z., Jia R.Y., Song X., Lv C., Yue G.Z., Ye G., Liang X.X., He C.L., Yin L.Z., Zou Y.F. The polysaccharides from codonopsis pilosula modulates the immunity and intestinal microbiota of cyclophosphamide-treated immunosuppressed mice. Molecules. 2018;23:1801–1814. doi: 10.3390/molecules23071801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett-Thomson S.C., Massimi A., Fedorov E.V., Bonanno J.B., Scandiuzzi L., Hillerich B., Seidel R.R., Love J.D., Garforth S.J., Guha C., Almo S.C. Mechanistic dissection of the PD-L1:B7-1 co-inhibitory immune complex. Plos One. 2020;15 doi: 10.1371/journal.pone.0233578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Sun Y.L., Wang A.H., Xu C.E., Zhang M.Y. Effect of polysaccharides extract of rhizoma atractylodis macrocephalae on thymus, spleen and cardiac indexes, caspase-3 activity ratio, Smac/DIABLO and HtrA2/Omi protein and mRNA expression levels in aged rats. Mol. Biol. Rep. 2012;39:9285–9290. doi: 10.1007/s11033-012-1677-x. [DOI] [PubMed] [Google Scholar]
- Guo M.Z., Meng M., Feng C.C., Wang X., Wang C.L. A novel polysaccharide obtained from Craterellus cornucopioides enhances immunomodulatory activity in immunosuppressive mice models via regulation of the TLR4-NF-kappaB pathway. Food Funct. 2019;10:4792–4801. doi: 10.1039/c9fo00201d. [DOI] [PubMed] [Google Scholar]
- Ibrahim H.M., Mohammed-Geba K., Tawfic A.A., El-Magd M.A. Camel milk exosomes modulate cyclophosphamide-induced oxidative stress and immuno-toxicity in rats. Food Funct. 2019;10:7523–7532. doi: 10.1039/c9fo01914f. [DOI] [PubMed] [Google Scholar]
- Koh Y.I., Shim J.U., Lee J.H., Chung I.J., Min J.J., Rhee J.H., Lee H.C., Chung D.H., Wi J.O. Natural killer T cells are dispensable in the development of allergen-induced airway hyperresponsiveness, inflammation and remodelling in a mouse model of chronic asthma. Clin. Exp. Immunol. 2010;161:159–170. doi: 10.1111/j.1365-2249.2010.04151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Rani L., Mhaske S.T., Pote S.T., Behera S., Mishra G.C., Wani M.R. IL-3 receptor expression on activated human Th cells is regulated by IL-4, and IL-3 synergizes with IL-4 to enhance Th2 cell differentiation. J Immunol. 2020;204:819–831. doi: 10.4049/jimmunol.1801629. [DOI] [PubMed] [Google Scholar]
- Li Q.J., Chau J., Ebert P.J., Sylvester G., Min H., Liu G., Braich R., Manoharan M., Soutschek J., Skare P., Klein L.O., Davis M.M., Chen C.Z. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Li R., Sakwiwatkul K., Yutao L., Hu S. Enhancement of the immune responses to vaccination against foot-and-mouth disease in mice by oral administration of an extract made from Rhizoma Atractylodis Macrocephalae (RAM) Vaccine. 2009;27:2094–2098. doi: 10.1016/j.vaccine.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Li Y., Li S., Li R., Xu J., Jin P., Chen L., Ma F. Genome-wide miRNA screening reveals miR-310 family members negatively regulate the immune response in Drosophila melanogaster via co-targeting Drosomycin. Dev. Comp. Immunol. 2017;68:34–45. doi: 10.1016/j.dci.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Liu J., Chen X., Yue C., Hou R., Chen J., Lu Y., Li X., Li R., Liu C., Gao Z., Li E., Li Y., Wang H., Yan Y., Li H., Hu Y. Effect of selenylation modification on immune-enhancing activity of Atractylodes macrocephala polysaccharide. Int. J. Biol. Macromol. 2015;72:1435–1440. doi: 10.1016/j.ijbiomac.2014.10.022. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhou G., Deng X., Yu Q., Hu Y., Sun H., Wang Z., Chen H., Jia C., Wang D. Analysis of miRNA expression profiling in human macrophages responding to Mycobacterium infection: induction of the immune regulator miR-146a. J. Infect. 2014;68:553–561. doi: 10.1016/j.jinf.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Loffler D., Brocke-Heidrich K., Pfeifer G., Stocsits C., Hackermuller J., Kretzschmar A.K., Burger R., Gramatzki M., Blumert C., Bauer K., Cvijic H., Ullmann A.K., Stadler P.F., Horn F. Interleukin-6 dependent survival of multiple myeloma cells involves the Stat3-mediated induction of microRNA-21 through a highly conserved enhancer. Blood. 2007;110:1330–1333. doi: 10.1182/blood-2007-03-081133. [DOI] [PubMed] [Google Scholar]
- Meng M., Guo M., Feng C., Wang R., Cheng D., Wang C. Water-soluble polysaccharides from Grifola Frondosa fruiting bodies protect against immunosuppression in cyclophosphamide-induced mice via JAK2/STAT3/SOCS signal transduction pathways. Food Funct. 2019;10:4998–5007. doi: 10.1039/c8fo02062k. [DOI] [PubMed] [Google Scholar]
- Samstag Y., Bogert N.V., Wabnitz G.H., Din S., Therre M., Leuschner F., Katus H.A., Konstandin M.H. Reactive oxidative species-modulated Ca(2+) release regulates beta2 integrin activation on CD4(+) CD28(null) T cells of acute coronary syndrome patients. J. Immunol. 2020;205:2276–2286. doi: 10.4049/jimmunol.2000327. [DOI] [PubMed] [Google Scholar]
- Shi Y.Y., Guan S.H., Tang R.N., Tao S.J., Guo D.A. Simultaneous determination of four sesquiterpenoids in Atractylodes Macrocephala Rhizoma by GC-FID: optimisation of an ultrasound-assisted extraction by central composite design. Phytochem. Anal. 2012;23:408–414. doi: 10.1002/pca.1373. [DOI] [PubMed] [Google Scholar]
- Silva F., de Oliveira E.E., Ambrosio M., Ayupe M.C., de Souza V.P., Menegati L.M., Reis D., Machado M.A., Macedo G.C., Ferreira A.P. Disodium cromoglycate treatment reduces TH2 immune response and immunohistopathological features in a murine model of Eosinophilic Esophagitis. Int. Immunopharmacol. 2020;83 doi: 10.1016/j.intimp.2020.106422. [DOI] [PubMed] [Google Scholar]
- Sun W., Meng K., Qi C., Yang X., Wang Y., Fan W., Yan Z., Zhao X., Liu J. Immune-enhancing activity of polysaccharides isolated from Atractylodis macrocephalae Koidz. Carbohydr. Polym. 2015;126:91–96. doi: 10.1016/j.carbpol.2015.03.034. [DOI] [PubMed] [Google Scholar]
- Wang D., Li Q., Qu Y., Wang M., Li L., Liu Y., Li Y. The investigation of immunomodulatory activities of Gloeostereum incaratum polysaccharides in cyclophosphamide-induced immunosuppression mice. Exp. Ther. Med. 2018;15:3633–3638. doi: 10.3892/etm.2018.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Tian Y. Selenium and polysaccharides of atractylodes macrocephala koidz play different roles in improving the immune response induced by heat stress in chickens. Biol. Trace. Elem. Res. 2015;168:235–241. doi: 10.1007/s12011-015-0351-2. [DOI] [PubMed] [Google Scholar]
- Ying M., Yu Q., Zheng B., Wang H., Wang J., Chen S., Nie S., Xie M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020;235 doi: 10.1016/j.carbpol.2020.115957. [DOI] [PubMed] [Google Scholar]
- Yu Q., Nie S.P., Wang J.Q., Huang D.F., Li W.J., Xie M.Y. Signaling pathway involved in the immunomodulatory effect of Ganoderma atrum polysaccharide in spleen lymphocytes. J. Agric. Food Chem. 2015;63:2734–2740. doi: 10.1021/acs.jafc.5b00028. [DOI] [PubMed] [Google Scholar]
- Zhu W., Zhou S., Liu J., McLean R., Chu W. Prebiotic, immuno-stimulating and gut microbiota-modulating effects of Lycium barbarum polysaccharide. Biomed. Pharmacother. 2020;121 doi: 10.1016/j.biopha.2019.109591. [DOI] [PubMed] [Google Scholar]
- Zou Y.F., Zhang Y.Y., Fu Y.P., Inngjerdingen K.T., Paulsen B.S., Feng B., Zhu Z.K., Li L.X., Jia R.Y., Huang C., Song X., Lv C., Ye G., Liang X.X., He C.L., Yin L.Z., Yin Z.Q. A polysaccharide isolated from codonopsis pilosula with immunomodulation effects both in vitro and in vivo. Molecules. 2019;24:3632–3645. doi: 10.3390/molecules24203632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are included in this published article.