Graphical abstract
Keywords: Xylitol fatty acid esters, Solvent-free systems, Biocatalysis, Ultrasound-assistance, Biosurfactants
Highlights
-
•
Enzymatic esterification of free fatty acids with xylitol in solvent-free systems.
-
•
Enhanced biocatalytic synthesis of xylitol esters assisted by ultrasonic irradiation.
-
•
Ultrasonic agitation enhanced enzymatic synthesis of bio-based nonionic surfactants.
Abstract
A commercial immobilized lipase was successfully used for the synthesis of five xylityl acyl esters by means of the esterification of free fatty acids (caprylic, capric, lauric and myristic, respectively) with xylitol under solvent-free conditions. Ultrasound-assistance was shown to be a key tool to overcome the handicap imposed by both the mutual immiscibility of fatty acids and xylitol substrates, and the semisolid character of the initial reaction mixtures. In such semisolid systems, ultrasonic irradiation may enable the transport of substrate molecules to the enzyme catalytic-site, leading to the efficient synthesis of xylityl fatty ester (e.g. up to 95% yield after 90 min at 40 °C), with xylityl monoacyl ester and xylitol diacyl ester appearing as the main products (greater than 96%), assessed by HPLC and NMR analyses. The separation of products was carried out by heating and simple centrifugation of the reaction medium, which was possible due to different densities of the resulting fractions.
1. Introduction
Sugar fatty acid esters are nonionic amphiphilic molecules obtained from renewable resources, which show high emulsifying, stabilizing and detergency behavior [1], [2]. Other properties of sugar fatty esters include, their tasteless, odorless and non-toxicity nature, and the fact that they are non-irritable and biodegradable, making them highly appreciated for industrial applications in the food, cosmetic, and pharmaceutical industries [3]. In 2016, the market for natural surfactants (bio-based surfactants) was USD 12.89 billion, and is expected to reach USD 17.27 billion by 2022, due to their growing use in detergents, personal care products, and petroleum-based chemicals [4]. The industrial and medical interest of xylitol fatty acid esters as bio-surfactants, lies in their outstanding preservative and antimicrobial effectiveness in cosmetic products [5], [6], as well as in their use as drugs in the treatment of hemoglobin-related diseases and tumors [7].
Lipases have been widely reported as suitable catalysts for the synthesis of sugar esters in non-aqueous reaction media, whether by esterification or transesterification, although poor solubilization of the substrates is major problem. As can be seen in Table 1, reaction media based on polar organic solvents, or mixtures of them, have been widely used to carry out the biocatalytic esterification of polyol compounds with fatty acids (see Fig. 1). It should be noted that the use of non-natural vinyl acyl esters, as activated acyl donor substrates for the enzymatic synthesis of sugar esters has been seen to provide better results than those obtained by free fatty acid, although long reaction times were necessary to reach full conversion (i.e. up to 3 days reaction, see Entries 1–6; Table 1). Furthermore, it was reported how the increase in reaction time to improve the monoester product yield may also involve a decrease in the selectivity, because the same polyol molecule may be esterified by two-times (see Entries 4, 5; Table 1). Ionic liquids (ILs) are environmentally friendly alternatives to volatile organic solvents because of their very low vapor pressure and good thermal stability [8].
Table 1.
Overview of studies published on the lipase-catalysed synthesis of sugar fatty acid esters in volatile organic solvent and ionic liquids.
| Entry | Acyl acceptor | Acyl donor | Product | Solvent | Reaction conditions | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Glucose | Vinyl palmitate | Glucosyl palmitate | ACN, THF or Acetone | 45 °C, 72 h | 100 | [9] |
| 2 | Glucose | Vinyl Laurate | Mixture of glucosyl mono- and di-laurate | 2-methyl-2-butanol | 60 °C, 120 h | 96 (83)b | [10] |
| 3 | Xylitol | Butanoic anhydride | Xylityl 1,5-di-butyrate | ACN or THF | 35 °C, 12 h | 80 | [11] |
| 4 | Xylitol | Oleic acid | Mixture of Xylityl monoacyl-, diacyl- and triacyl esters | 2-Methyl-2-propanol/DMSO 80:20 (v/v) | 45 °C, 24 h | 100 (73) b | [12] |
| 5 | Xylitol | Decanoic acid Lauric acid Myristic acid |
Mixture of xylityl monoacyl- and diacyl esters | t-Butanol/Pyridine, 45:55 (v/v) |
45 °C, 20 h | 41 (30) b | [13] |
| 6 | Xylitola | Lauric acid Butiric acid |
Xyliltyl monoacyl ester | t-Butanol | 45 °C, 24 h | 86 | [14] |
| 7 | Glucose | Vinyl laurate Vinyl mirstate Palmitic acid |
Glucosyl monoacyl ester | [Bmim][BF4] / t-Butanol, 60:40 (v/v) [Bmim][PF6] / t-Butanol, 60:40 (v/v) |
60 °C, 72 h | 90 | [15] |
| 8 | Glucose Fructose Sucrose |
Vinyl laurate | Monolaurate of sugar | [Bmim][Tf2N]/[Bmim][TfO], 1:1 (v/v) | 40 °C, 12 h | 58 | [16] |
| 9 | Glucose | Decanoic acid Vinyl Decanoate |
Glycosyl monodecanoate | Decanoic acid/Menthol (1:1, mol/mol) DES | 50 °C, 24 h | 11 | [17] |
| 10 | Glucose | Vinyl Decanoate | Glycosyl monodecanoate | Choline chloride/Urea (1/1, mol/mol) DES | 50 °C, 24 h | < 1 | [18] |
| Assistance by ultrasonic cleaning bath | |||||||
| 11 | Fructose | Palmitic acid | Fructosyl palmitate | [Bmim][TfO]/[Omim][NTf2], 1:1 (v/v) | 60 °C, 14 h | 78 | [19] |
| 12 | Glucose | Lauric acid | Glucosyl monolaurate | [Bmim][TfO] | 50 °C, 6 h | 90 | [20] |
| 13 | Xylitola | Oleic acid | Xylityl monooleate | t-Butanol | 60 °C, 6 h | 60 | [21] |
Protected in 2, 3 and 4 hydroxyl group positions with p-toluene sulfonic acid; b Selectivity; ACN, Acetonitrile; THF, Tetrahydrofuran; DMSO, Dimethyl sulfoxide; [Bmim] 1-butyl-3-methylimidazolium; [Omim], 1-octyl-3-methylimidazolium [BF4], tetrafluoroborate; [PF6], hexafluorophosphate; [NTf2], Bis(trifluoromethylsulfonyl)imide; [TfO], triflate;
Fig. 1.
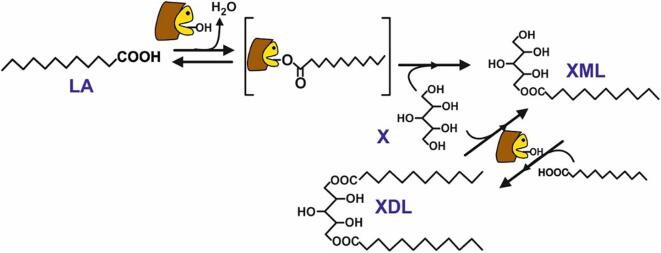
Schema of the reaction mechanism in the immobilized lipase-catalysed synthesis of xylityl monolaurate (XML) and xylityl dilaurate (XDL) by esterification of lauric acid (LA) with xylitol (X).
As reaction media for applied biocatalysis, water-immiscible ILs have been described as exceptional non-aqueous environments for lipase-catalysed ester synthesis both by transesterification reactions with vinyl esters (e.g. citronellyl butyrate) [22], as well as by esterification reactions with free carboxylic acids (e.g. geranyl acetate from geraniol and acetic acid) in the absence of volatile organic solvents [23]. However, the use of ionic liquids as reaction media for the enzymatic synthesis of sugar esters did not improve the results because of the long reaction times, and the necessity of further steps for product separation (see Entries 7, 8; Table 1). In the same way, the enzymatic synthesis of sugar esters in reaction media based on deep eutectic solvents (DESs), as another ecofriendly alternatives to organic solvents, resulted in low product yield (e.g. up to 11% after 24 h reaction), even when using vinyl esters as acyl donor (see Entries 9, 10; Table 1).
Ultrasonic irradiation has recently emerged as a sustainable tool for activating the enzymes, because of the increase in product yield and faster reaction rates under mild frequency conditions [24]. Furthermore, as ultrasound pulses often save energy and provide cleaner products with little or no by-products, the technique can also be considered as “green” [25]. The application of ultrasound agitation for the lipase-catalysed esterification of fructose, glucose, or xylitol in reaction media based on ILs or t-butanol improved the enzyme activity by shortening reaction times (see Entries 11–13; Table 1). Nevertheless, it should be noticed that sustainable approaches devoted to the synthesis of natural products cannot be based on the use of non-natural substrates, such are alkyl vinyl esters as acyl donors, or volatile organic solvents as reaction media, which should be regarded as a breakdown point in any attempt to develop sustainable biocatalytic processes.
In this context and in spite of the solid character of the substrates used, it has been reported that the biocatalytic synthesis of amphiphilic molecules can be carried out by the direct esterification of free fatty acids with the corresponding polyol in a solvent-free reaction medium (e.g. panthenyl monolaurate, etc.), [8], [26] which is the best sustainable approach for the synthesis of xylitol esters (see Fig. 1). However, the solid/semisolid character of the substrates mixture, together with their mutual immiscibility, are clearly handicaps that need to be overcome. Zang et al. [27] described the enzymatic synthesis of xylitol dicaprate ester using a direct esterification approach in the resulting semisolid substrate mixture, obtaining yield of up to 24% after 24 h under conventional stirring at 50 °C. By increasing the reaction temperature to 60 °C, Adnani et al. [28] carried out the esterification reaction of xylitol with capric acid in solvent-free conditions, and obtaining in an increase in product (mixture of mono-, di- and tricaprate xylitol esters) yield up to 74% after 29 h reaction.
This paper describes for the first time a sustainable process for the biocatalytic synthesis of four different xylitol fatty acid esters (caprylic, capric, lauric and myristic, respectively) using a direct esterification approach in solvent-free systems with ultrasound-assistance by using a cup-horn device at mild temperature (40 °C, see Fig. 2). The proposed biocatalytic approach in the cup-horn ultrasonic system was scaled up by factor 200 to demonstrate its suitability. Finally, the product esters can easily be separated by heating and centrifugation, due to the high melting point of the unreacted xylitol, demonstrating the suitability of the biocatalytic approach based on solvent-free media as a sustainable procedure for large-scale production
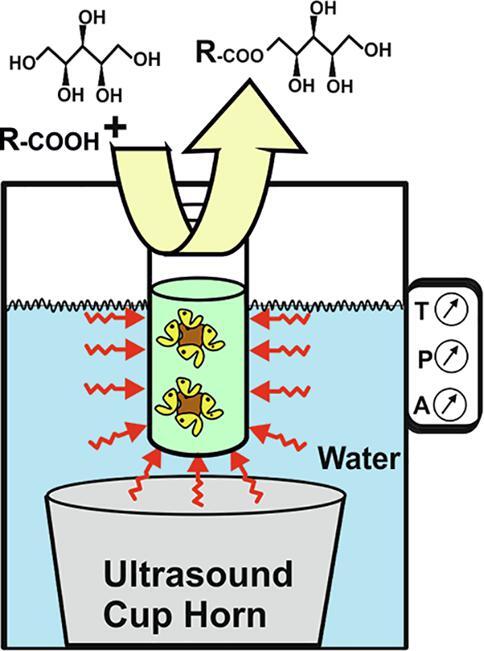
Fig. 2.
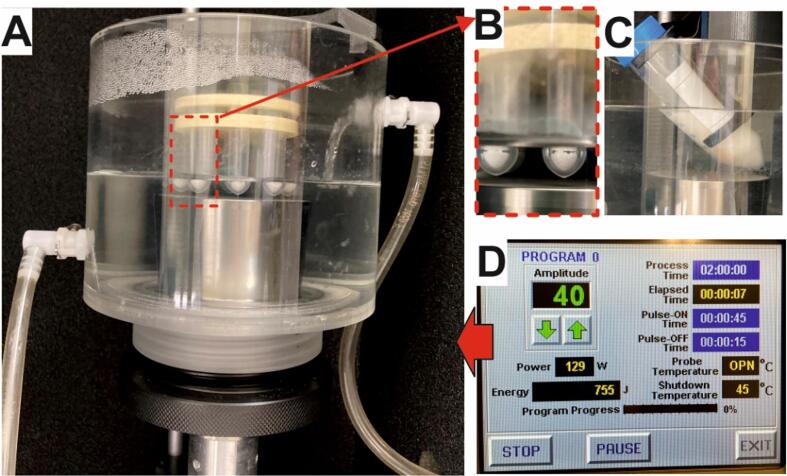
Experimental set-up for ultrasound-assisted biocatalytic synthesis of xylitol esters in solvent free systems using a cup horn device. Both the rack of 2-mL Eppendorf tubes (A, B), and the 50-mL tube (C) are placed 0.5 cm above the ultrasonic horn and at a selected water height, and maintained at a constant controlled temperature by the chiller. D. Display of the control unit of the Q-700 ultrasonic device.
2. Materials and methods
2.1. Chemicals
Immobilized Candida antarctica lipase B (Novozym® 435) was a gift from Novozymes S.A. (Spain). Xylitol (98% purity), capric acid (99% purity), caprylic acid (98% purity), lauric acid (98% purity), myristic acid (99% purity), molecular sieves 13x (MS13x, 270 mg H2O/g adsorption capacity), solvents and other chemicals were from Sigma-Aldrich-Fluka (Madrid, Spain).
2.2. Ultrasound-assisted biocatalytic synthesis of xylitol esters in solvent free systems
Into 2-mL Eppendorf tubes, 0.25, 0.5, or 1 mmol of free fatty acid (FFA), i.e. capric acid, lauric acid, myristic acid or oleic acid, were combined with the adequate amount of xylitol to finally achieve 4:1, 2:1, 1:1 or 1:2 xylitol/fatty acid molar ratios. The final reaction mixtures were completed by addition of a dehydrating agent (MS13x molecular sieves, 25 mg/mmol FFA). The reaction tubes were pretreated by indirect sonication for 15 min (45 s/min pulses, 10–100% amplitude) at a controlled temperature (40 °C) by using a Q-700 ultrasonic processor (20 kHz frequency, 700 W maximum power) equipped with a 431C2 cup horn (Qsonica LLC, USA) and a recirculating chiller device (mod 4905 Qsonica LLC, USA) for temperature control (45 °C maximum temperature), leading to the homogenization of solid/semisolid reaction mixtures (see Fig. 2). The control unit of the device permits several parameters, such as process time, pulse-on time, pulse-off time, temperature (°C) and amplitude (%), to be selected.
The reaction was started by addition of the immobilized enzyme (Novozym 435, 50 mg/mmol FFA and maintained under ultrasound irradiation for 90 min in the selected conditions. During the course of the reaction, the control unit of the device displays the ultrasonic power (W) used for maintaining the selected amplitude (%), as well as the ultrasonic energy (J) supplied by the device. For time-course profiles, the system was stopped at a selected time, and the full content of a reaction test-tube was used for analysis. For this, the test-tube was first placed into an ice-bath to stop the reaction, and the reaction mixture was then fully dissolved in 1 mL dimethylsulphoxide (DMSO), and finally centrifuged at 14,000 rpm for 10 min at 10 °C, before analysis by HPLC.
To carry out 200-fold scaled-up reactions, an accessory adaptor for 50 mL tubes was used (see Supplementary Data, S.D.). Lauric acid (25 or 50 mmol), xylitol (25 or 50 mmol) and dehydrating agent (MS13x molecular sieves, 10 mg/mmol lauric acid) were placed in a 50 mL polypropylene tube, and pre-treated by ultrasonic irradiation for 15 min (45 s/min pulses, 40% amplitude) at 40 °C using the Q-700 device. The reaction was started by adding the immobilized enzyme (10 mg/mmol lauric acid), and by irradiating with the Q-700 system (90 min, 45 s/min pulses, 40% amplitude) at 40 °C. To obtain time-course profiles, aliquots (40 μL) were taken at regular intervals, dissolved in 960 μL acetonitrile/methanol/1% acetic acid in H2O (75/15/10, v/v/v) mixture, and centrifuged at 14,000 rpm for 10 min at 10 °C. The sample solution was then analyzed by HPLC. At the end of the reaction, products were separated from the mixture by centrifugation at 50 °C, which resulted in the following three phases: a liquid upper-phase containing the xylitol ester products, a middle-phase containing the immobilized enzyme, and a solid bottom-phase of unreacted xylitol. The upper phase containing the reaction product mixture was directly collected for further HPLC-MS and 1H and 13C NMR analyses.
2.3. HPLC-ELSD analysis
A Shimadzu LC-20 HPLC instrument, coupled to an evaporative light scattering detector (ELSD-LT II, Shimadzu), and equipped with a RP-C18 column (LiChrospher, Merck, 250 mm × 5 μm), was used to analyze the reaction samples. The elution protocol was carried out using acetonitrile (A), methanol (B) and 1% v/v acetic acid in water (C), as mobile phases, through two isocratic steps: 0–5 min (A, 45%; B, 30%; C, 25%); 6–30 min (A, 75%; B, 15%; C, 10%) at 50 °C. Peak retention times (min) were as follows: xylitol 2.4; caprylic acid, 3.3; xylityl monocaprylate 2.6; xylitol dicaprylate 12.6; capric acid, 4.7; xylityl monocaprate, 3.2; xylitol dicaprate, 14.2; lauric acid, 7.6; xylitol monolaurate, 5.1; xylityl dilaurate, 17.1; myristic acid, 11.9; xylityl monomyristate, 8.1; xylityl dimyristate, 27.0 (see S.D. for details of the HPLC analysis).
2.4. HPLC-MS analysis for the identification of xylitol esters.
The HPLC-MS analyses were performed in a HPLC-DAD Agilent 1200 instrument coupled with an ESI TOF detector Agilent 6220 (Agilent, USA). Separation was achieved with a RP-C18 column (250 mm × 5 μm) and elution in isocratic mode by using a mobile phase: 75% acetonitrile: 15% methanol: 10% water (75:15:10 v/v), following the identification of peaks by UV detection at 205 nm. As representative example, the esterification reaction of xylitol with lauric acid was selected. The total ion signals were obtained by scanning the range of mass corresponding to m/z values ranging from 100 to 1000 in negative ion mode. Each peak of polyol ester was identified by comparison of the mass spectra with those in a computer library (NIST Library). Xylitol, retention time (rt, min): 2.3, positive ion (m/z): 115.9, 151.0; lauric acid, rt: 4.3, positive ion (m/z): 199.2; xylityl monolaurate, rt: 3.2, positive ion (m/z): 115.9, 151.0, 199.2, 265.1, 333.2; xylityl dilaurate, rt: 7.3, positive ion (m/z): 115.9, 151.0, 199.2, 265.1, 515.4.
2.5. Nuclear magnetic Resonance spectroscopy
Nuclear Magnetic Resonance spectroscopy was performed using a Bruker Avance 600 MHz spectrometer equipped with triple gradient TXI (1H/13C/15N) and broad band (13C) probes. Samples (10 μL)were dissolved in 500 μL of acetone-δ6 and analyzed by NMR at room temperature (see S.I.). The assignment of the protons was achieved by COSY and NOESY homonuclear experiments, while those of the carbons were performed on the basis of the 1H,13C HSQC spectra. . Xylityl monolaurate: 1H NMR δ (ppm), 4.15 (C-1H); 3.96 (C-2H); 3.64 (C-3H); 3.62 (C-4H); 3,77 (C-5H); 2.28–2.33 (C-2′H); 1.58 (C-3′H); 1.25–1.33 (C-(4′-11′)H); 0.88 (C-12′H3). 13C NMR δ (ppm) 173.86 (C1′=O); 66.32 (C-1); 71.45 (C-2); 71.73 (C-3); 64.22 (C-4); 73.70 (C-5); 34.69 (C2′); 25.77 (C3′); 32.71 (C-(4′-10′)); 23.43 (C-11′)14.37 (C-12′). Xylityl dilaurate: 1H NMR δ (ppm), 4.16 (C-1H); 3.97 (C-2H); 3.64 (C-3H); 3.62 (C-4H); 3.77 (C-5H); 2.28–2.33 (C-2′H); 1.58 (C-3′H); 1.25–1.33 (C-(4′-11′)H); 0.88 (C-12′H3). 13C NMR δ (ppm) 173.81 (C1′=O); 66.17 (C-1); 71.25 (C-2); 71.73 (C-3); 64.33 (C-4); 73.80 (C-5); 34.74 (C2′); 25.75 (C3′); 32.71 (C(4′-10′)); 23.43 (C-11′) 14.37 (C-12′). (see S.D. for detailed RMN analysis).
3. Results and discussion
3.1. Biocatalytic synthesis of xylitol lauric acid esters in solvent-free conditions under ultrasonic irradiation.
The immobilized lipase-catalysed synthesis of xylityl laurate esters by direct esterification of xylitol (94.5 °C m.p.) with lauric acid (43.8 °C m.p.) was carried out in a solvent-free system. When the reaction was performed under mechanical or magnetic stirring, no synthetic activity was observed after 8 h, after increasing the temperature up to 60 °C. Both the mutual immiscibility between the different fatty acids and xylitol substrates and the semisolid character of the initial reaction mixture, might have contributed to the poor mass-transfer of these substrates from the bulk medium to the enzyme active site, resulting in no product being synthesized.
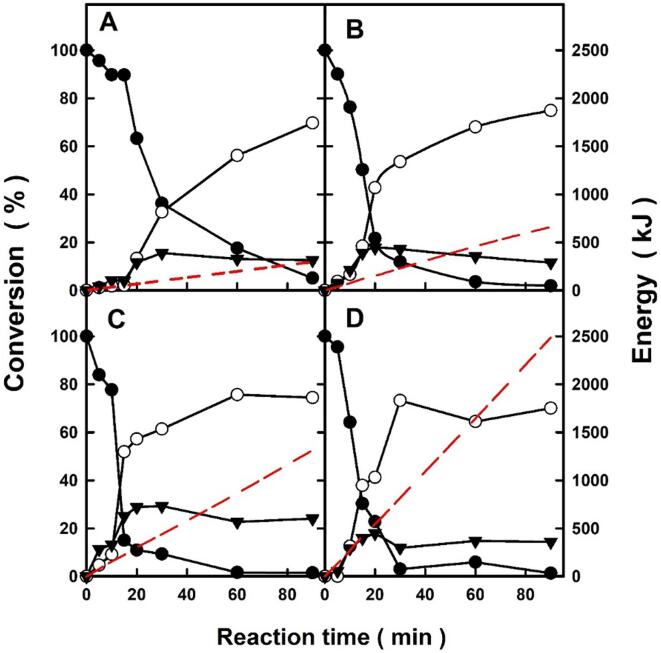
To overcome this limitation, the suitability of ultrasound-assistance for this biocatalytic reaction in solvent-free systems was studied. Ultrasound is an acoustic wave with a frequency of ≥ 20 kHz that needs a medium to propagate, both the ultrasonic power /amplitude and the energy supplied being key parameters for enhancing the rate of chemical reactions [29]. Fig. 3 depicts the time-course profiles for the biocatalytic synthesis of both the xylityl monolaurate and xylityl dilaurate, obtained by esterification of xylitol with lauric acid (1:1 mol/mol ratio) as a function of the ultrasonic amplitude (%)/power supplied at 40 °C. As can be seen, the immobilized lipase was able to catalyze the synthesis of both xylityl monolaurate and xylityl dilaurate ester products by direct esterification. Although the temperature of the reaction medium (40 °C maximum, a limit imposed by the instrument) was lower than the melting points of both substrates, ultrasonic agitation was seen to be an efficient tool for favoring mass-transfer phenomena around the immobilized enzyme particle, thus enabling enzyme catalysis for the successfully synthesis of the ester products in all cases.
Fig. 3.
Time-course profiles for the Novozym 435-catalysed synthesis of xylityl monolaurate (○), and xylityl dilaurate (▾) using xylitol and lauric acid (●) as substrates at 1:1 (mol/mol) ratio, as well as the energy supplied (red dashed line) under different ultrasound power conditions (A, 10% amplitude, 56 ± 4 W; B, 40% amplitude, 130 ± 5 W; C, 70% amplitude, 256 ± 4 W; and D, 100% amplitude, 460 ± 10 W) at 40 °C (see experimental section). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Ultrasound and enzymes exhibited a synergistic effect for lowering the diffusion-limiting barrier between enzymes and substrates, which enhances the mass transfer within the reaction vessel and accelerates the reaction rate [30]. Taking into account that the mechanism of lipases-catalysed esterification involves a covalently bound acyl-enzyme intermediate (see Fig. 1), the synthetic product is only obtained by deacylation of this intermediate through the nucleophilic attack of a hydroxyl group of the xylitol molecule. Thus, in this semisolid reaction system, ultrasonic irradiation could melt lauric acid and favor its transfer to the active site of lipase to form the acyl intermediate, while water is released as by-product molecule from the enzyme microenvironment, and is retained by the undissolved hygroscopic xylitol, avoiding the reversible hydrolytic pathway. Both ultrasound-promoted mass-transfer phenomena are probably related with the rapidness of the lipase-catalyzes synthesis of xylityl laurates, because free lauric acid is fully converted after 90 min of reaction.
Additionally, it should be noted that all the time-course profiles showed an initial step with a slow reaction rate, which then increases exponentially. During this slow period, the synthesis of xylityl dilaurate was favored over the synthesis of monoester, probably because the xylitol mass transfer was lower than that of lauric acid. Furthermore, the surfactant power of the mono- and diester products would favor the solubilization of undissolved xylitol, increasing the synthesis of the xylityl monolaurate product, not only by esterification, but also by transesterification of the diester product with xylitol. Also, it should be emphasized that the maximum content of both the monoester and the diester products was practically the same for all the assayed ultrasound powers (c.a. 70% xylityl monolaurate, 30% xylityl dilaurate, as determined by NMR analysis, see S.D.), although the rate of reaction synthesis increases with the supplied ultrasound power supplied. In this context, ultrasonic agitation was seen to accelerate lipase-catalysed isobutyl propionate synthesis by esterification in solvent-free systems, obtaining c.a. 100% yield after 200 min reaction, which was related with the acoustic cavitation [31]. Rufino et al. [21] described an improvement in the biocatalytic synthesis of xylityl monooleate by direct esterification of oleic acid with chemically-protected xylitol in t-butanol medium and with ultrasound assistance. However, only a maximum yield of 60% product was obtained after 7 h reaction using an ultrasonic cleaning bath (20 kHz, 85 W). The applied power affects the amplitude, and should be able to supply the pressure needed for cavitation. However, a drastic increase in power may disrupt bubble dynamics as it contributes to bubbles growing abnormally during expansion, which may result in poor cavitation [29]. Although a cavitational horn has been described as a more suitable ultrasonic device than an ultrasonic bath for lipase- catalysed reactions, it should be taken into account that the high power and frequency are undesirable for long enzyme exposure times, which may cause the enzyme deactivation [24].
3.2. Influence of the molar ratio and nature of fatty acid substrate on the enzymatic esterification of xylitol under ultrasound-assistance
Taking into account that the mechanism of lipase-catalysed esterification reactions occurs via an acyl-enzyme intermediate, which is deacylated by the attack of xylitol (nucleophile) as acyl acceptor (see Fig. 1), both the fatty acid/xylitol molar ratio, and the nature of the fatty acid, are key parameters that must be borne in mind for any further application of the process.
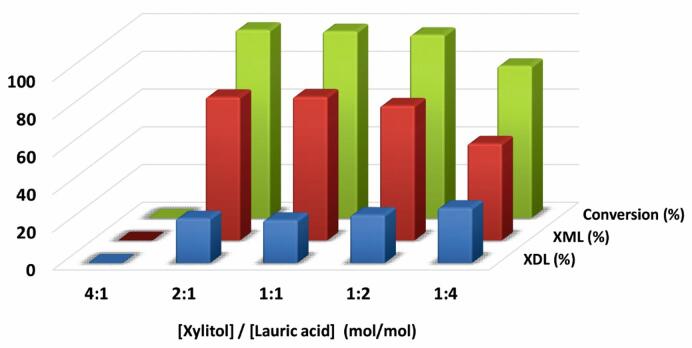
As representative examples, reaction mixtures based on 4/1, 2/1, 1/1, 1/2 and 1/4, xylitol/lauric acid molar ratio, respectively, were tested as solvent-free reaction media for the Novozym 435-catalysed the synthesis of xylitol lauric acid esters with ultrasonic agitation at 40 °C (see Fig. 4). All the reaction systems were assayed with the same overall mass-substrate content (0.36 g) to ensure the same ultrasound irradiation occurred in each reaction vial (i.e. 30 min, 70% ultrasound amplitude). As can be seen, no reaction was observed at the highest xylitol content assayed, which could be related with the predominance of the solid character of this polyol in the reaction conditions, but also with its non-miscibility with lauric acid. This nearly-solid system was clearly unable to permit enzymatic reaction in spite of ultrasound irradiation. However, the semisolid systems resulting from a higher lauric acid content were shown as suitable reaction media. The best results were obtained when a 1:1 or 1:2 (mol/mol) xylitol/lauric acid ratio was assayed, (i.e. 70% xylityl monolaurate, and 30% xylityl dilaurate yields, respectively). For the highest lauric acid content (1:4 mol/mol xylitol/lauric acid), the products yields were reduced, although the synthesis of the diester product improved with respect to that obtained in other reaction media with a lower lauric acid content.
Fig. 4.
Influence of the xylitol/lauric molar ratio on the biocatalytic synthesis of xylityl monolaurate (XML) and xylityl dilaurate (XDL) after 30 min of reaction in solvent-free systems under ultrasound assistance (70% ultrasound amplitude) at 40 °C.
In addition, the effect of the aliphatic chain of the acyl donor (capric, caprylic, lauric or myristic acids, respectively) on the lipase- catalysed synthesis of xylitol fatty acid esters under ultrasound assistance in a cup horn device was studied for 1:1 and 1:2 (mol/mol) xylitol:fatty acid ratios at 40 °C (see Table 2). As can be seen, all the assayed fatty acids were seen to be suitable acyl donors for the esterification reaction, the overall fatty acid conversion to ester products improving with increasing alkyl chain length, except in the case of myristic acid, probably due to its higher melting point which results in a nearly-solid system during operation. Table 1 also shows how a lower alkyl chain length of the acyl donor provided a higher yield of xylityl monoacyl ester product than that of the diester, which could be attributed to an improved mass-transfer phenomenon resulting from the lower fatty acid melting point. As the viscosity of the medium increases with increasing FFA chain length, the ability of the monoester product to exit from the enzyme microenvironment seems to be diminished, favoring lipase-catalysed a second esterification in the same xylitol molecule. Analogous results were obtained for both 1:1 and 1:2 (mol/mol) xylitol/fatty acid ratios. Similarly, it was described how the acyl donor with the longest alkyl chain provided the highest conversion rate in the biocatalytic synthesis of fatty acid glucose esters from different fatty acyl donors [9]. At this point, it should be noted that Candida antarctica lipase B (CALB) contains a flexible lid that undergoes a large conformational change when transforming hydrophobic substrates [32]. It was also reported that ultrasonic irradiation might favor the substrate interaction with the active site of the enzyme by flipping the conformation to an active open state [24].
Table 2.
Effect of the length of fatty acid chain on the biocatalytic synthesis of xylityl monoacyl (XME) and xylityl diacyl (XDE) esters after 90 min of reaction in solvent-free systems under ultrasound assistance (70% ultrasound amplitude) at 40 °C.
| Entry | FFA | Xylitol: FFA molar ratio | XME (%) | XDE (%) | Conv. (%) |
|---|---|---|---|---|---|
| 1 | Caprylic cida | 1:1 | 45.1 | 2.0 | 47.2 |
| 2 | Caprylic acida | 1:2 | 56.1 | 5.4 | 61.5 |
| 3 | Capric acidb | 1:1 | 77.0 | 7.0 | 83.9 |
| 4 | Capric acidb | 1:2 | 68.7 | 9.7 | 78.4 |
| 5 | Lauric acidc | 1:1 | 72.8 | 24.0 | 96.8 |
| 6 | Lauric acidc | 1:2 | 63.8 | 23.3 | 87.1 |
| 7 | Myristic acidd | 1:1 | 39.00 | 9.22 | 48.20 |
| 7 | Myristic acidd | 1:2 | 31.63 | 9.65 | 41.28 |
Melting points: a, 16.7 °C; b, 31.6 °C; c, 43.8 °C; d, 54.4 °C.
Additionally, it should be underlined that xylityl triacyl esters were not detected in any of the assayed conditions, as determined by NMR analysis (see S.D.), although the synthesis of these polyester products has previously been reported when CALB-catalysed the esterification of xylitol with fatty acids in organic solvents under magnetic stirring.
3.3. Operational stability of Novozym 435-catalysed synthesis of xylityl laurate esters in solvent-free systems with ultrasound-assistance.
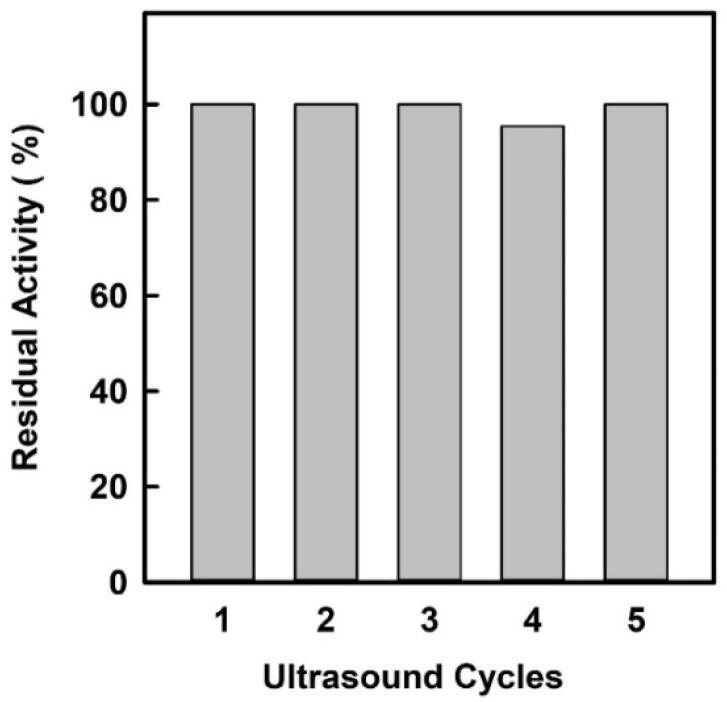
In an attempt to ascertain the suitability of ultrasonic agitation in the proposed biocatalytic approach for xylitol ester synthesis, the possible reuse of the enzyme was studied in 2-mL test tubes. However, due to the difficulty involved in separating the immobilized enzyme particles from the resulting semisolid reaction media for reuse, an alternative approach was assayed for a 1:2 (mol/mol) xylitol: lauric acid conditions (see Table 2, entry 6). Firstly, the mixture of lauric acid and immobilized enzyme particles was incubated under ultrasound for different cycles of 1 h (45 s/min pulses, 40% ultrasound amplitude) at 40 °C. After the desired number of cycles of ultrasonic stress, the corresponding amount of xylitol was added to the immobilized enzyme/lauric acid mixture, and the catalytic reaction was allowed to proceed for 60 min. As can be seen in Fig. 5, the residual activity of the Novozym 435 biocatalyst in this xylitol-lauric acid system was unchanged by the cycles of ultrasonic stress, demonstrating the excellent suitability of using this irradiation tool for carrying out xylitol ester synthesis in solvent-free conditions. It has been reported that Novozym 435 has a notable degree of reusability for more than 6–9 cycles of ultrasound-assisted esterification or transesterification or hydrolysis processes, also for production of biodiesel [24], although no data were found for the synthesis of xylitol fatty acid esters.
Fig. 5.
Operational stability of Novozym 435- catalysed synthesis of xylitol lauric acid esters by direct esterification of xylitol with lauric acid (1:2, mol/mol ratio) in solvent-free conditions and under ultrasound assistance (40% amplitude). Each cycle involved a 1 h (45 s/min pulses, 40% amplitude, 130 ± 5 W) ultrasonic pretreatment of the immobilized enzyme/lauric acid mixture at 40 °C. The synthetic process was started by adding the xylitol substrate, then allowed to proceed for 60 min under the same ultrasonic and temperature conditions.
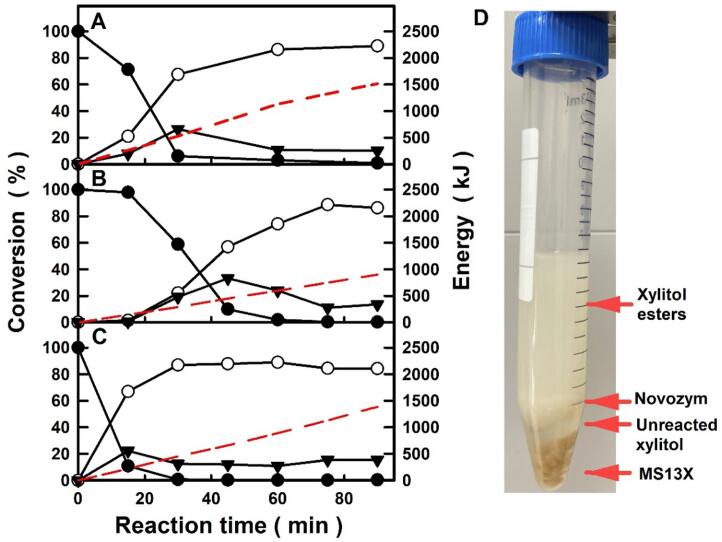
Additionally, the practical suitability of the biocatalytic process for producing xylitol lauric acid esters using an esterification approach in a solvent-free system was assessed by scaling up the reaction 100 and 200-fold under two different ultrasound amplitudes. As can be seen in Fig. 6A, the time-course profile of the biocatalytic reaction for a 100-fold scale-up (50 mmol, 8.8 g overall mass substrate) led to a slightly faster reaction than that observed for 2-mL test-tubes (see Fig. 3C), obtaining a 67% xylityl monolaurate yield after 30 min reaction, which increased to 89% at 90 min. By decreasing the ultrasound amplitude to 40% for the same reaction system (8.8 g overall mass-substrate, Fig. 6B), the reaction proceeds more slowly, leading only to 22% xylityl monolaurate yield at 30 min, although a similar product yield was obtained at the end of the reaction (90 min), as a result of increased mass-transfer limitations which slowed the biocatalytic process. However, when the reaction media was scaled up to 200-fold (100 mmol, 17.6 g overall mass-substrates) under the same ultrasonic irradiation (40% amplitude, see Fig. 6C), the faster biocatalytic reaction gave a 67% xylityl monolaurate yield after the first 15 min of the reaction, while the highest yield (87%) was obtained after 30 min. Taking into account that the biocatalytic process was carried out at 40 °C, a temperature near to the melting point of lauric acid, the increase in the overall mass-substrate of the reaction system involves an increase in the liquid (fused) substrate fraction in the medium at initial stages, favoring the ultrasonic agitation, and hence a better homogenization of the mixture. In all cases depicted in Fig. 6, the time-course of xylityl dilaurate showed a bell-shape profile that could be attributed to the reversible interconversion of xylityl monolaurate to xylityl dilaurate catalysed by the immobilized enzyme (see Fig. 1). Thus, the time necessary to achieve the maximum diester concentration could be directly related with mass-transfer phenomena, since the maximum is reached after short reaction times when the reaction medium is better homogenized (i.e. 15 min, see Fig. 6C), and viceversa (i.e. 45 min, Fig. 6B). Reaction products were easily separated as an upper liquid phase by centrifugation at 50 °C, while the immobilized enzyme, unreacted xylitol and the molecular sieves appeared in separated fractions (see Fig. 6D).
Fig. 6.
Time-course profiles for the Novozym 435-catalysed synthesis of xylityl monolaurate (○), and xylityl dilaurate (▾) by using xylitol and lauric acid (●) as substrates (1:1, mol/mol ratio), and the energy supplied (red dashed line), at large scale (A, B, 8.8 g; C, 17.6 g overall mass substrates) under different ultrasound power conditions (A, 70% amplitude, 295 ± 5 W; B, 40% amplitude, 168 ± 4 W; C, 40% amplitude, 230 ± 5 W). D. Representative example of the reaction medium after the biocatalytic process under ultrasound assistance, followed by centrifugation at 50 °C (See experimental section and S.D.). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
These results show the excellent synergy obtained by combining the immobilized lipase and the ultrasonic agitation for the rapid biocatalytic synthesis of xylitol fatty acid esters in a solvent-free medium under mild conditions. The excellent suitability of ultrasonic irradiation to accelerate the biocatalytic synthesis of xylityl monolaurate in solvent-free conditions is clearly demonstrated, pointing to its potential as a straightforward and sustainable approach for the production this non-ionic surfactant of growing industrial interest.
4. Conclusions
The development of biocatalytic processes of industrial interest in solvent-free media is a key goal in sustainable chemistry, because of their high selectivity for transformations, and the possibility of obtaining pure products directly. However, in some cases, the limitations imposed by the mutual immiscibility of substrates, and their solid character under the reaction conditions are clear handicaps. Although xylitol and fatty acids are substrates that show these characteristics, ultrasonic irradiation was seen to be an efficient tool to enable the selective synthesis of xylityl monoacyl and xylityl diacyl esters by immobilized lipase-catalysed direct esterification in solvent-free conditions. The suitability of ultrasonic agitation was evident from the speed of the biocatalytic process, as well as by the preservation of the enzyme activity for long operation times. Ultrasound technology provides excellent reaction yields and selectivity for the proposed biocatalytic transformation in solvent-free conditions, and is also suitable for scaling up because of the ease with which the unreacted xylitol can be separated. Further studies related with the design of the process (e.g. reaction time, temperature, step-wise addition of fatty acid, etc.) might improve the selectivity of the reaction to obtain the pure monoester product.
The combination of enzymes and sustainable approaches, based on solvent-free reaction media containing natural substrates, and the application of enabling technologies, such as ultrasonic agitation, provides synergic opportunities and opens up new paths to promote the development of sustainable chemical processes of industrial interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was partially supported by RTI2018-098233-B-C21 (MICINN-FEDER), and 20790/PI/18 (Fundación SENECA-CARM).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2021.105606.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References:
- 1.Banat I.M., Carboué Q., Saucedo-Castañeda G., de Jesús Cázares-Marinero J. Biosurfactants: The green generation of speciality chemicals and potential production using solid-state fermentation (SSF) technology. Bioresourc. Technol. 2021;320:124222. doi: 10.1016/j.biortech.2020.124222. [DOI] [PubMed] [Google Scholar]
- 2.Khan N.R., Rathod V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process Biochem. 2015;50(11):1793–1806. doi: 10.1016/j.procbio.2015.07.014. [DOI] [Google Scholar]
- 3.Market and Market.. Natural Surfactants Market (Bio-based Surfactants) by Product Type (Anionic, Nonionic, Cationic, and Amphoteric), Application (Detergents, Personal Care, Industrial & Institutional cleaning, and Oilfield Chemicals), and Region - Global Forecast to 2022, (2018). https://www.marketsandmarkets.com/Market-Reports/natural-surfactant-market-25221394.html.
- 4.Amaral L.F.B., Camilo N.S., Pereda M.D.C.V., Levy C.E., Moriel P., Mazzola P.G. Evaluation of antimicrobial effectiveness of C-8 xylitol monoester as an alternative preservative for cosmetic products. Int. J. Cosmetic Sci. 2011;33:391–397. doi: 10.1111/j.1468-2494.2010.00633.x. [DOI] [PubMed] [Google Scholar]
- 5.Silveira J.E.P.S., Pereda M.C.V., Nogueira C., Dieamant G., Cesar C.K.M., Assanome K.M., Silva M.S., Torello C.O., Queiroz M.L.S., Eberlin S. Preliminary safety assessment of C-8 xylitol monoester and xylitol phosphate esters. Int. J. Cosmetic Sci. 2016;38(1):41–51. doi: 10.1111/ics.2016.38.issue-110.1111/ics.12262. [DOI] [PubMed] [Google Scholar]
- 6.Pouillart P., Douillet O., Scappini B., Gozzini A., Santini V., Grossi A., Pagliai G., Strippoli P., Rigacci L., Ronco G., Villa P. Regioselective synthesis and biological profiling of butyric and phenylalkylcarboxylic esters derivated from D-mannose and xylitol: influence of alkyl chain length on acute toxicity. Eur. J. Pharm. Sci. 1999;7:93–106. doi: 10.1016/S0928-0987(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 7.Villa R., Alvarez E., Porcar R., Garcia-Verdugo E., Luis S.V., Lozano P. Ionic liquids as an enabling tool to integrate reaction and separation processes. Green Chem. 2019;21(24):6527–6544. doi: 10.1039/C9GC02553G. [DOI] [Google Scholar]
- 8.Arcens D., Grau E., Grelier S., Cramail H., Peruch F. Impact of fatty acid structure on CALB-catalyzed esterification of glucose. Eur. J. Lipid Sci. Tehcnol. 2020;122(4):1900294. doi: 10.1002/ejlt.v122.410.1002/ejlt.201900294. [DOI] [Google Scholar]
- 9.Martinez‐Garcia M., Dejonghe W., Cauwenberghs L., Maesen M., Vanbroekhoven K., Satyawali Y. Enzymatic synthesis of glucose- and xylose laurate esters using different acyl donors, higher substrate concentrations, and membrane assisted solvent recovery. Eur. J. Lipid. Sci. Technol. 2021;123(2):2000225. doi: 10.1002/ejlt.202000225. [DOI] [Google Scholar]
- 10.Misra S., Raghuwanshi S., Gupta P., Saxena R.K. Efficient 1–5 regioselective acylation of primary hydroxyl groups of fermentative derived xylitol catalyzed by an immobilized Pseudomonas aeruginosa lipase. Biotechnol Bioproc. Eng. 2012;17(2):398–406. doi: 10.1007/s12257-011-0491-y. [DOI] [Google Scholar]
- 11.Castillo E., Pezzotti F., Navarro A., López-Munguía A. Lipase-catalyzed synthesis of xylitol monoesters: Solvent engineering approach. J. Biotechnol. 2003;102(3):251–259. doi: 10.1016/S0168-1656(03)00050-6. [DOI] [PubMed] [Google Scholar]
- 12.Cramer J.F., Dueholm M.S., Nielsen S.B., Pedersen D.S., Wimmer R., Pedersen L.H. Controlling the degree of esterification in lipase catalysed synthesis of xylitol fatty acid esters. Enzyme Microb. Technol. 2007;41(3):346–352. doi: 10.1016/j.enzmictec.2007.03.001. [DOI] [Google Scholar]
- 13.Rufino A.R., Biaggio F.C., Santos J.C., de Castro H.F. Chemoenzymatic synthesis: a strategy to obtain xylitol monoesters. J. Chem. Technol. Biotechnol. 2009;84(7):957–960. doi: 10.1002/jctb.2117. [DOI] [Google Scholar]
- 14.Ganske F., Bornscheuer U.T. Optimization of lipase-catalyzed glucose fatty acid ester synthesis in a two-phase system containing ionic liquids and t-BuOH. J. Mol. Cat. B Enzym. 2005;36(1-6):40–42. doi: 10.1016/j.molcatb.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Shin D.W., Mai N.L., Bae S.W., Koo Y.M. Enhanced lipase-catalyzed synthesis of sugar fatty acid esters using supersaturated sugar solution in ionic liquids. Enzyme Microb. Technol. 2019;126:18–23. doi: 10.1016/j.enzmictec.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Hollenbach R., Ochsenreither K., Syldatk C. Enzymatic synthesis of glucose monodecanoate in a hydrophobic deep eutectic solvent. Int. J. Mol. Sci. 2020;21:4342. doi: 10.3390/ijms21124342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollenbach R., Bindereif B., van der Schaaf U.S., Ochsenreither K., Syldatk C. Optimization of glycolipid synthesis in hydrophilic deep eutectic solvents. Front. Biotechnol. Bioeng. 2020;8:382. doi: 10.3389/fbioe.2020.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha S.H., Hiep N.M., Koo Y.-M. Enhanced production of fructose palmitate by lipase-catalyzed esterification in ionic liquids. Biotechnol. Bioproc. Eng. 2010;15(1):126–130. doi: 10.1007/s12257-009-3091-3. [DOI] [Google Scholar]
- 19.Lee S.H., Nguyen H.M., Koo Y.-M., Ha S.H. Ultrasound-enhanced lipase activity in the synthesis of sugar ester using ionic liquids. Process Biochem. 2008;43(9):1009–1012. doi: 10.1016/j.procbio.2008.05.001. [DOI] [Google Scholar]
- 20.Rufino A.R., Biaggio F.C., Santos J.C., de Castro H.F. Screening of lipases for the synthesis of xylitol monoesters by chemoenzymatic esterification and the potential of microwave and ultrasound irradiations to enhance the reaction rate. Int. J. Biol. Macromol. 2010;47(1):5–9. doi: 10.1016/j.ijbiomac.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Lozano P., Bernal J.M., Nieto S., Gomez C., Garcia-Verdugo E., Luis S.V. Active biopolymers in green non-conventional media: a sustainable tool for developing clean chemical processes. Chem. Commun. 2015;51(98):17361–17374. doi: 10.1039/C5CC07600E. [DOI] [PubMed] [Google Scholar]
- 22.Lozano P., Bernal J.M., Navarro A. A clean enzymatic process for producing flavour esters by direct esterification in switchable ionic liquid/solid phases. Green Chem. 2012;14:3026–3033. doi: 10.1039/c2gc36081k. [DOI] [Google Scholar]
- 23.Bansode S.R., Rathod V.K. An investigation of lipase catalysed sonochemical synthesis: A review. Ultrason. Sonochem. 2017;38:503–529. doi: 10.1016/j.ultsonch.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 24.Cintas P. Ultrasound and green chemistry - Further comments. Ultrason. Sonochem. 2016;28:257–258. doi: 10.1016/j.ultsonch.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Lozano P., Alvarez E., Nieto S., Villa R., Bernal J.M., Donaire A. Biocatalytic synthesis of panthenyl monoacyl esters in ionic liquids and deep eutectic solvents. Green Chem. 2019;21(12):3353–3361. doi: 10.1039/C9GC01076A. [DOI] [Google Scholar]
- 26.Zhang X., Nie K.L., Wang M., Liu L., Li K.F., Wang F., Tan T.W., Deng L. Site-specific xylitol dicaprate ester synthesized by lipase from Candida sp 99–125 with solvent-free system. J. Mol Cat. B Enzym. 2013;89:61–66. doi: 10.1016/j.molcatb.2012.12.006. [DOI] [Google Scholar]
- 27.Adnani A., Basri M., Chaibakhsh N., Ahangar H.A., Salleh A.B., Rahman R.N.Z.R.A., Abdul Rahman M.B. Chemometric analysis of lipase-catalyzed synthesis of xylitol esters in a solvent-free system. Carbohydr. Res. 2011;346(4):472–479. doi: 10.1016/j.carres.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 28.Pokhrel N., Vabbina P.K., Pala N. Sonochemistry: Science and Engineering. Ultrason. Sonochem. 2016;29:104–128. doi: 10.1016/j.ultsonch.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 29.Wang D.L., Yan L.F., Ma X.B., Wang W.J., Zou M.M., Zhong J.J., Ding T., Ye X.Q., Liu D.H. Ultrasound promotes enzymatic reactions by acting on different targets: Enzymes, substrates and enzymatic reaction systems. Int. J. Biol. Macromol. 2018;119:453–461. doi: 10.1016/j.ijbiomac.2018.07.133. [DOI] [PubMed] [Google Scholar]
- 30.Jaiswal K.S., Rathod V.K. Acoustic cavitation promoted lipase catalysed synthesis of isobutyl propionate in solvent free system: Optimization and kinetic studies. Ultrason. Sonochem. 2018;40:727–735. doi: 10.1016/j.ultsonch.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Luan B.Q., Zhou R.H. A novel self-activation mechanism of Candida antarctica lipase B. Phys. Chem. Chem. Phys. 2017;19:15709–15714. doi: 10.1016/10.1039/c7cp02198d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.