Abstract
Adipocytes play pivotal roles in maintaining energy homeostasis by storing lipids in adipose tissue (AT), regulating the flux of lipids between AT and the circulation in response to the body's energy requirements and secreting a variety of hormones, cytokines and other factors. Proper AT development and function ensure overall metabolic health. Nuclear factor erythroid 2-related factor 1 (NFE2L1, also known as NRF1) belongs to the CNC-bZIP family and plays critical roles in regulating a wide range of essential cellular functions and varies stress responses in many cells and tissues. Human and rodent Nfe2l1 genes can be transcribed into multiple splice variants resulting in various protein isoforms, which may be further modified by a variety of post-translational mechanisms. While the long isoforms of NFE2L1 have been established as master regulators of cellular adaptive antioxidant response and proteasome homeostasis, the exact tissue distribution and physiological function of NFE2L1 isoforms, the short isoforms in particular, are still under intense investigation. With regard to key roles of NFE2L1 in adipocytes, emerging data indicates that deficiency of Nfe2l1 results in aberrant adipogenesis and impaired AT functioning. Intriguingly, a single nucleotide polymorphism (SNP) of the human NFE2L1 gene is associated with obesity. In this review, we summarize the most significant findings regarding the specific roles of the multiple isoforms of NFE2L1 in AT formation and function. We highlight that NFE2L1 plays a fundamental regulatory role in the expression of multiple genes that are crucial to AT metabolism and function and thus could be an important target to improve disease states involving aberrant adipose plasticity and lipid homeostasis.
Keywords: NFE2L1, Adipose, Adipocytes, Adipogenesis, Energy metabolism
Abbreviations
- aa
amino acids
- AD1/2
acidic domain 1/2
- adipoq-Cre
adiponectin-Cre
- ARE
antioxidant response element
- AT
adipose tissue
- BAC
brown adipocyte
- BAT
brown adipose tissue
- bZIP
basic leucine zipper
- β-TrCP
beta-transducin repeats-containing protein
- C/EBPs
CCAAT/enhancer-binding proteins
- CNC
cap ‘n’ collar
- CTD
C-terminal domain
- EBF2
transcription factor early B-cell factor-2
- ESCs
embryonic stem cells
- EpRE
electrophile responsive element
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- gWAT
gonadal white adipose tissue
- HRD1
ubiquitin ligase HMG-CoA reductase degradation 1
- iWAT
inguinal white adipose tissue
- KO
knock out
- LCRF1
Locus Control Region-Factor 1
- Maf
musculoaponeurotic fibrosarcoma oncogene
- Metrnl1
meteorin like factor 1
- MSCs
mesenchymal stem cells
- mTORC1
rapamycin complex 1
- Neh
Nrf2–ECH homology
- NFE2L1
Nuclear factor erythroid 2-related factor
- NST
asparagine/serine/threonine
- NTD
N-terminal domain
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PPARγ
peroxisome proliferator activated receptor-γ
- PRDM16
PR domain-containing 16
- SAT
subcutaneous adipose tissue
- SNP
single nucleotide polymorphism
- SR
serine-rich
- SREBP
sterol-regulatory element binding protein
- SVF
stromal vascular fraction
- TCF11
transcription factor 11
- UCP1
uncoupling protein-1
- UPR
unfolded protein response
- VAT
visceral adipose tissue
- WAT
white adipose tissue
1. Introduction
Adipose tissue (AT) is not only a site for calorie storage and release, but also a tissue of endocrine regulating physiological processes, such as glucose metabolism, appetite, angiogenesis, inflammatory responses, blood pressure and reproductive function [1]. The impairment of AT formation and/or dysfunction at the adipocyte level are associated with various metabolic syndromes. Thus, the formation and maintenance of healthy AT are essential for energy homeostasis and prevention of several metabolic disorders.
Nuclear factor erythroid 2-related factor 1 (NFE2L1, also known as NRF1), a transcription factor belonging to the cap ‘n’ collar-basic leucine zipper (CNC-bZIP) subfamily, plays a central role in transcriptional control of genes involved in antioxidant response, proteasome homeostasis, genetic stability, mitochondrial respiration, inflammation, metabolism and cell differentiation [[2], [3], [4], [5], [6]]. Accumulating evidence indicates that functional activity of NFE2L1 is finely tuned by a steady-state balance between production and the processing into distinct isoforms (called proteoforms) before concomitant turnover [[7], [8], [9], [10]]. Following translation of Nfe2l1, multiple topovectorial processes facilitate selective post-synthetic processing of NFE2L1 in different tempo-spatial subcellular locations from the endoplasmic reticulum (ER) to the nucleus. Various genetic loss and gain of function studies of NFE2L1 over the past decades have shown that NFE2L1 has profound roles in adipose biology involving adipogenesis, lipid and glucose metabolism, thermogenesis, oxidation-reduction and proteasome homeostasis [[11], [12], [13], [14]]. Besides, a single nucleotide polymorphism (SNP), rs3764400, occurring in the 5′-flanking region of the human NFE2L1 gene appears to be associated with obesity in humans [15,16]. Thus, the study of NFE2L1 function in AT is a scientifically attractive theme with bona fide significances in normal and abnormal function.
Herein, we review data derived from cell-based studies and gene knockout (KO) mice to help synthesize the current understanding of NFE2L1 and the molecular mechanisms underlying its regulation and actions in AT biology. Further, we attempt to shed light on the isoform-specific roles of NEF2L1 in adipogenesis and adipocyte function and provide a novel insight into prevention and treatment of metabolic disorders involving AT.
2. Basics of NFE2L1 genetics and molecular biology
NFE2L1 is a member of CNC-bZIP protein family that is characterized by a highly conserved CNC-domain and a bZIP domain required for dimerization and DNA binding. NFE2L1 acts as an obligate heterodimer in formation with small musculoaponeurotic fibrosarcoma oncogene (Maf) proteins, such as MafG, MafK and MafF, for binding to DNA [[17], [18], [19], [20]]. In addition, ATF/CREB proteins and c-Maf are potential partners of NFE2L1 as well [21]. NFE2L1 plays a central role in transcriptional control with genes harboring the NF-E2/AP1-like antioxidant response element (ARE)/electrophile responsive element (EpRE) site [2,17,19,22,23]. The downstream genes typically involve antioxidant response, proteasome homeostasis, genetic stability, mitochondrial respiration, inflammation, lipid metabolism and cell differentiation [[2], [3], [4], [5]].
2.1. DNA structure and transcripts of mouse Nfe2l1
Based on the newest version of Ensembl Genome Browser (http://asia. ensembl.org/index.html), the mouse Nfe2l1 gene maps to the distal end of chromosome 11 [24], spans 12,554 bp DNA, and is divided into 10 exons (Fig. 1) [24]. According to the database, alternative splicing and selective use of translation initiation codons give rise to 12 transcripts (splice variants), resulting in at least two long protein isoforms (L-NFE2L1) containing 741 and 742 amino acids (aa), as well as two short protein isoforms (S-NFE2L1) containing 453 and 583 aa [25,26]. To distinguish these isoforms, the terminology NFE2L1-742, -741, -583 and -453 are used throughout this review.
Fig. 1.
DNA structure and transcripts of mouse Nfe2l1. Schematic diagram of mouse Nfe2l1 genomic sequence (GeneID: ENSMUSG00000038615) and depiction of different isoforms of Nfe2l1 transcripts. Sequences are from the Ensembl GRCm38. p6 release101-August 2020 (www.ensemble.org). Solid black lines and dashed black lines represent introns and flanking 10 kb length to either side, respectively. White open boxes represent exons. Dark blue and light blue open boxes represent coding regions and untranslated regions, respectively. The numbers in parentheses under each isoform are the gene ID in Ensembl. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. The structural domains of mouse NFE2L1
Although the exact origin of different NFE2L1 isoforms is not conclusively known, the comparison of their structural domains helps elucidate or predict their functions, regulatory relationships and mechanisms of expression and activation.
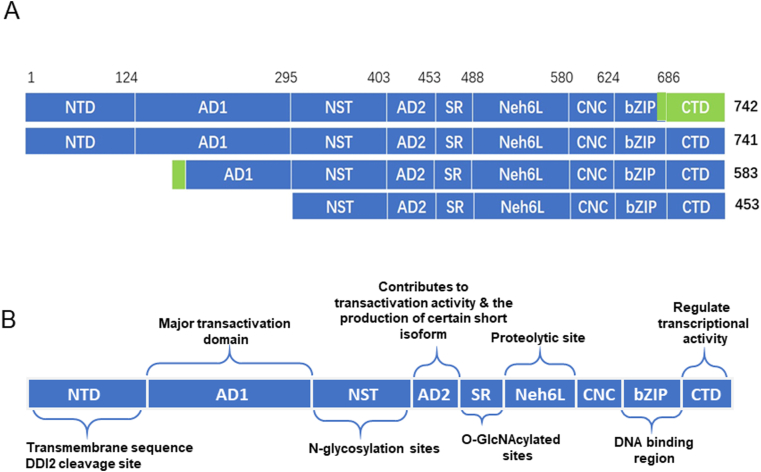
L-NFE2L1 contains nine discrete structural domains, which are NTD (N-terminal domain), AD1 (acidic domain 1), NST (asparagine/serine/threonine), AD2, SR (serine-rich), CNC, bZIP and Neh3L (Nrf2–ECH homology 3-like) and Neh6L, respectively. These domains dictate the selective post-synthetic processing of NFE2L1 to yield multiple polypeptide isoforms with distinctive and even opposing functions in regulating target gene expression (Fig. 2) [[27], [28], [29], [30], [31], [32], [33]]. The bZIP domain of NFE2L1 is located near the carboxyl terminus of the protein characterized by heptad repeats of leucine and hydrophobic residues within an amphipathic helical domain of 40 aa, and is preceded by 30 aa region which is rich in arginine and lysine residues [26]. N-terminal to the bZIP domain is a highly conserved 43 aa domain referred to as the “CNC” domain after the Drosophila cap-n collar protein, which is not present in JUN, FOS or other bZIP proteins. The CNC and bZIP enable NFE2L1 to bind DNA [34]. The Neh3L region located in the C-terminal domain (CTD) at the carboxyl-end of the NFE2L1 polypeptide negatively regulates NFE2L1. AD1 and AD2 near the N-terminus are separated by NST. The SR domain located near the CNC motif of NFE2L1 positively regulates NFE2L1 but all are negatively regulated by the Neh6L domain [29,32,35]. The NTD determines the membrane topology of NFE2L1 [27].
Fig. 2.
The structural domains of NFE2L1. (A) Schematic protein structures of different isoforms of mouse NFE2L1. (B) Domain structure and related function of L-NFE2L1. Abbreviations: NTD, N-terminal domain; AD1, acidic domain 1; NST, Asn/Ser/Thr-rich; AD2, acidic domain 2; SR, serine-repeat; Neh6L, Neh6-like; CNC, Cap ‘n’ collar; bZIP, basic-region zipper; CTD, C-terminal domain. Green boxes represent unique sequences different from other isoforms. All the sequences are from the National Center for Biotechnology (www.ncbi.nlm.nih.gov). . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.3. Protein isoforms of mouse NFE2L1
The single Nfe2l1 gene can be transcribed via alternative mRNA splicing with different initiation signals. Following translation, resulting proteins undergo post-translational processing to give rise to multiple proteoforms with different length and modifications such as phosphorylation and glycosylation [23,36]. Thus, different isoforms of NFE2L1 migrate distinctively on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) thereby displaying multiple bands with various molecular weights. Certainly, the variations of NFE2L1 on SDS-PAGE may also impacted by many other experimental factors, including the antibodies used, polyacrylamide concentration, pH value, buffer and detergent type [8,31,37]. Thus, given that NFE2L1 is produced and degraded in a complex way, it is easy to see confusion arising regarding the correct migratory pattern and nomenclature of different isoforms of NFE2L1. As shown in Table 1, we attempt to summarize the nomenclature of various isoforms of mouse NFE2L1.
Table 1.
The summary of the nomenclature of various isoforms of murine endogenous NFE2L1.
| Basis of nomenclature | Nomenclature | Position on SDS PAGE/NuPAGE (kDa) | Amino acid length (aa) | Synonyms | Details |
|---|---|---|---|---|---|
| Originally discovered and named | NFE2L1 [24] | 65 [24] | 741 [24,43] | Nrf1a [40], Nrf1α [2], Nrf-1 [60], Nrf1 [105], p120 Nrf1 [81] | A full-length isoform yielded by the first translation initiation signal within the main open reading frame of alternatively spliced mRNA transcripts [38] |
| Nrf1 [105] | 120 & 110 [105] | 741 [105] | NFE2L1 [24], Nrf1a [40], Nrf1α [2], Nrf-1 [60], p120 Nrf1 [81] | ||
| Nrf1a [40] | 120 & 110 [40,105] | 741 [105] | NFE2L1 [24], Nrf1α [2], Nrf-1 [60], Nrf1 [105], p120 Nrf1 [81] | ||
| P120 Nrf1 [45] | 120 [45] | 741 [6] | NFE2L1 [24], Nrf1a [40], Nrf1α [2], Nrf-1 [60], Nrf1 [105], | ||
| Nrf1b [40] | 95 [40], 100 [23] | 583 [40] | Nrf1△N [30,106], p95 Nrf1 [81] | A short isoform started from an alternative promoter site on Nfe2l1 [40] | |
| LCR-F1 [44] | 65 [35] | 453 [30] | Nrf1β [2], p65 Nrf1 [45] | A short isoform started from internal ATG codon of Nfe2l1 [26,35,61,107] | |
|
p65 Nrf1 [45] |
65 [45] |
453 [30] |
Nrf1β [2], LCR-F1 [44] |
Alternative translation initiation |
|
| α, β system [30] (Named by MW on NuPAGE) | Nrf1α | /120, 85, 65 [30] | 741 [30] | p120 Nrf1 [81] | Derived from modification and/or proteolytic processing of Nrf1 [30] |
| Nrf1β | /55 [28,29,35] | 453 [30] | p85 Nrf1 [81], LCR-F1 [44] | A short isoform started from in-frame translation and/or selective proteolysis [2,26,45]. | |
| Nrf1β2 | /46 [30,31] | 453 [30] | p46 Nrf1 [81] | A cleaved Nrf1β [30,31] | |
| Nrf1γ | /36 [[30], [31], [32]] | 325 [30] | p36 Nrf1 [81] | A potential in-frame translation or Nrf1β selective endoproteolytic processing [[30], [31], [32]]. | |
| Nrf1δ | /25 [[30], [31], [32]] | 194 [30] | p25 Nrf1 [81] | A potential in-frame translation or Nrf1β selective endoproteolytic processing [[30], [31], [32]]. |
Abbreviations: aa, amino acid; LCR-F1, Locus control region-factor 1; MW, molecular weight; NFE2L1, nuclear factor-E2-related factor 1; Nrf1, NF-E2 related factor 1; TCF11, transcription factor 11. The symbol ~ refers to approximate.
Based on the Ensembl database, four major isoforms of NFE2L1 in mice have been predicted. While the L-NFE2L1-742 is predicted to be different from L-NFE2L1-741 at the 640th to 742nd aa region, there are no published data to describe its distribution and exact function. The L-NFE2L1-741 (also termed Nrf1a or Nrf1α in literature) is derived from an alternatively spliced mRNA missing exon 4 that is presented in human transcription factor 11 (TCF11) [2,26,[38], [39], [40]]. This long isoform yields multiple bands on SDS-page with apparent MW of from 140 kDa to 25 kDa via selective post-translational processing [[30], [31], [32],40]. The S-NFE2L1-583 isoform (also known as Nrf1b) is generated through an alternate promoter and contains a unique N-terminus encoded by an alternate first exon, which has an apparent MW of approximately 100 kDa on SDS-page [[40], [41], [42]]. The S-NFE2L1-453 isoform, also known as locus control region-factor 1 (LCRF1), p65Nrf1 or Nrf1β, is encoded by an alternate transcript as documented in the Ensembl database or derived from internal ATG codon located in the transcripts encoding L-NFE2L1 [26,35]. It lacks the N-terminal AD1 with an apparent weight of 65 kDa on SDS page [23,30,35,43,44]. Although S-NFE2L1-453 exhibits a weak transactivation activity [32,42,44], it was thought to be a significant dominant negative regulator of ARE-driven gene transactivation against L-NFE2L1 and/or NFE2L2 [32,45]. In addition to the isoforms predicted by this database, other proteoforms have also been described [30,31]. Some smaller isoforms of NFE2L1, known as Nrf1γ (NFE2L1-325) and Nrf1δ (NFE2L1-194) of 36 kDa and 25 kDa, respectively, can be produced by the potential in-frame translation, as well as the selective endoproteolytic processing of longer NFE2L1 proteins [31,32]. They can function as dominant inhibitors to down-regulate expression of NF-E2/AP1-like ARE-driven genes [32].
However, our current understanding of the expression, function, and tissue distribution of these various isoforms of NFE2L1 is woefully incomplete and inconsistent. In mice, so far as is now known, NFE2L1-741 and -742 regulate cellular adaptive antioxidant response [5,6,46,47] and are versatile in their essential roles for proteasome homeostasis [6,7,9], obstruction of adipogenesis [11], negative regulation of macrophage activation and M1 polarization [4]. They can also protect bone marrow-derived mesenchymal stem cells (MSCs) against arsenite-induced cytotoxicity via suppression mtROS in addition to facilitating cellular arsenic efflux [6]. NFE2L1-583 is targeted to the nucleus where it activates ARE-genes [40]. In contrast, NFE2L1-453 is a dominant negative regulator of ARE-mediated transcription in mouse [45]. Moreover, Zhang et al. have identified differential expression profiles of distinct target genes regulated by NFE2L1-741, -453, -325 alone or in their cooperation, respectively [36]. NFE2L1-325 as a putative dominant-negative inhibitor is likely to interfere with the functional assembly of active transcription factors (NFE2L1-741, NFE2L1-453, and NFE2L2), leading to down-regulation of several key genes, some of which are up-regulated by NFE2L1-741 and -453 [27,29,31,32].
2.4. The regulation of NFE2L1
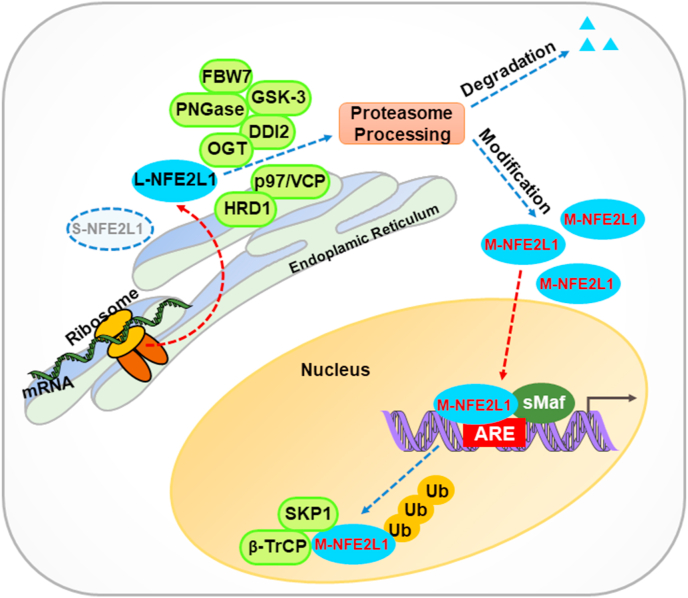
In order to act against a variety of cellular stresses, NFE2L1 is tightly regulated at several levels through distinct mechanisms. As shown in Fig. 3, after translation, L-NFE2L1 is inserted into the ER via the uncleavable NHB1 sequence [28]. Subsequently, the NHB2-connecting portions of the nascent polypeptide are translocated into the ER lumen, where the NST domain is N-glycosylated so as to become an inactive NFE2L1 glycoprotein [33]. When NFE2L1-dependent stress response is required, the ER-protected transactivation domains of L-NFE2L1 are dynamically retro-translocated through an as yet unknown mechanism and repositioned from the luminal side of ER membranes into the cyto/nucleo-plasmic side, whereupon it is deglycosylated to yield an active isoform with transactivation activity [31]. It has been reported that the AAA protein p97/VCP may provide the power to drive this dynamic retrotranslocation [8,9,37] and the subsequent endoproteolytic cleavage is theoretically catalyzed by an unidentified cytosolic protease, which may be the 26S proteasome. Before being translocated into the nucleus, the isoforms may undergo other post-translational modifications in the cyto/nucleo-plasmic compartments, such as N-deglycosylation, O-GlcNAcylation, de-GlcNAcylation, phosphorylation. A variety of enzymes are identified as function in this process, including PNGase [48], O-Linked N-acetylglucosamine transferase [49,50], protein DDI1 homolog 2 [51,52], beta-transducin repeats-containing protein (β-TrCP) [10], ubiquitin ligase HMG-CoA reductase degradation 1 (HRD1) [10], F-box and WD repeat domain containing 7 [53], Casein kinase Ⅱ [54] and glycogen sysnthase kinase 3 [55].
Fig. 3.
Proposed activation pathway of L-NFE2L1. After translation, L-NFE2L1 is inserted into the ER and its NST domain is N-glycosylated so as to become an inactive NFE2L1 glycoprotein. When required for induction by biological cues, the ER-protected transactivation domains of NFE2L1 are dynamically retrotranslocated via the AAA protein p97/VCP and repositioned from the luminal side of ER membranes into the cyto/nucleo-plasmic side, whereupon it undergoes various post-translational modifications, such as N-deglycosylation, O-GlcNAcylation, de-GlcNAcylation and phosphorylation to yield active isoforms (M-NFE2L1). In this process, diverse enzymes like PNGase, O-Linked N-acetylglucosamine transferase (OGT), DDI2, beta-transducin repeats-containing protein (β-TrCP) and ubiquitin ligase HMG-CoA reductase degradation 1 (HRD1) are involved in. There are two distinct (β-TrCP- and HRD1-dependent) degradation mechanisms regulating NFE2L1 protein levels. In the cytoplasm, NFE2L1 is degraded and suppressed by the ER-associated degradation ubiquitin ligase HRD1 and VCP. NFE2L1 is also degraded in the nucleus via β-TrCP-mediated degradation. In cells with insufficient proteasome capacity, active NFE2L1 accumulates and then migrates to the nucleus, where it heterodimerizes with cofactors (small Maf proteins) to bind ARE to induce gene transcription. In contrast, complete proteasomal processing of NFE2L1 may lead to decreased NFE2L1 protein levels and transcriptional activity. FBW7, F-box and WD repeat domain containing 7; GSK3, glycogen sysnthase kinase 3; SKP-1, S-phase kinase-associated protein 1.
The ubiquitinated L-NFE2L1 is normally degraded rapidly by proteasome. However, when proteasome activity is partially inhibited, the active forms of L-NFE2L1 are able to enter the nucleus to activate ARE-dependent gene expression [8]. β-TrCP and HRD1-dependent degradation are the two distinct mechanisms regulating L-NFE2L1 transcriptional activity. In the nucleus, β-TrCP promotes the degradation of L-NFE2L1 by catalyzing its polyubiquitination. Meanwhile, in the cytoplasm, L-NFE2L1 is normally degraded and suppressed by the ER-associated degradation (ERAD) ubiquitin ligase HRD1 and VCP [10]. It is noteworthy that there are paradoxical effects of proteasomal inhibition on L-NFE2L1. While it is activated via ‘bounce-back’ response to lower doses of proteasomal inhibitors, it can also be suppressed by higher levels of the same agents [56,57].
Collectively, the dynamic membrane topological organization of the L-NFE2L1 biomolecule determines how selective post-translational modifications control its ability to regulate target genes. Since S-NFE2L1 does not contain an ER-associated domain, regulation may bypass the mechanism just described. Thus, where the short isoforms come from and how they are adjusted remains undetermined. Further study is needed to determine the mechanisms underlying differential transcriptional expression of the Nfe2l1 gene and the subsequent post-transcriptional processing of gene products into multiple mRNA transcripts in different pathophysiological conditions.
2.5. The multifaceted roles of NFE2L1
In adult tissues, high levels of Nfe2l1 transcript are detected in the heart, kidney, skeletal muscle, fat and brain, while lower levels are seen in the liver, pancreas, and other organs. During development in the mouse, NFE2L1 is widely expressed and plays critical roles in many physiological processes [22]. To explore the physiological roles of NFE2L1, global and conditional knockout (KO) strategies targeting the Nfe2l1 gene in mice have been used. However, the global Nfe2l1-KO in mice is unfortunately an embryonic lethal gene loss, indicating the importance of this gene in the development and/or function of key aspects for survival. Three independent lines of mice carrying Nfe2l1flox/flox allele have been developed by the lab of Yamamoto, Chan and Hotamisligil, respectively [12,58,59]. Although there are differences in the strategic details to silence the function of the gene, the 10th exon of Nfe2l1 gene, the common region coding L-NFE2L1 and S-NFE2L1 is the consistent deletion target. Thus, the resulting conditional KO mice lose the expression and function of all the isoforms of NFE2L1. To acquire cell-specific Nfe2l1-KO mice, Nfe2l1flox/flox mice are crossed with the mice expressing Cre recombinase gene specifically in certain types of cells. To verify the phenotype, the mice with Nfe2l1 flox/flox and/or Cre-positive mice are generally used as controls.
Table 2 is a summary on the effects of Nfe2l1 ablation constitutively or cell-specifically. Global loss of Nfe2l1 in mice results in embryonic lethality due to anemia from impaired fetal liver erythropoiesis and severe oxidative stress indicating that this gene is indispensable for normal development, healthy growth, and defense from oxidative stress [[60], [61], [62], [63]]. Hepatocyte-specific KO of Nfe2l1 [Nfe2l1(L)-KO] in mice leads to development of steatosis, impaired glucose metabolism, inflammation and liver tumorigenesis [16,58]. The hepatocytes of Nfe2l1(L)-KO mice are characterized by both elevated oxidative and ER stress [59,64]. In addition, NFE2L1 was identified as a regulator of the Lipin1 and peroxisome proliferator-activated receptor γ coactivator 1β (Pgc1β) genes, providing new insights into NFE2L1 function in hepatic lipid metabolism [65]. CamK2-Cre-derived neuron-specific Nfe2l1-KO mice develop age-dependent forebrain atrophy as a result of progressive neurodegeneration [66]. In addition, accumulation of ubiquitinated proteins and apoptosis occur in the brain. In contrast, loss of Nfe2l1 in neurons generated using Nestin-Cre, which is expressed in neuronal and glial precursor cells, results in motor ataxia and neurodegeneration, as well as chromatolysis in the spinal cord [67]. Kim and colleagues disrupted Nfe2l1 specifically in osteoblasts [Nfe2l1(O)–KO] using Col1α2-Cre mice. The resulting Nfe2l1(O)–KO mice show decreased bone size, trabecular bone, peak bone mass and mechanical strength, indicating that NFE2L1 is involved in regulating osterix expression, osteoblast differentiation, and bone formation [68]. To investigate the role of NFE2L1 in regulating pancreatic β-cell in vivo, we developed a line of mice where β-cell NFE2L1 was targeted using Ins2-Cre mice. It was found that deficiency of Nfe2l1 in pancreatic β-cells disrupts glucose metabolism and ATP production in these cells leading to impaired insulin secretion, severe fasting hyperinsulinemia and glucose intolerance, suggesting that NFE2L1 plays a key role in β-cell physiology.
Table 2.
Major phenotype of Nfe2l1 deficiency in specific tissue/cell.
| Target tissue/cell | Major phenotype | Genotype of mice/cells |
|---|---|---|
| Global | Embryonic lethality [60,61], | Nfe2l1-null |
| Oxidative stress ↑ [62,63], | ||
| Genetic instability [80] | ||
| Liver | Tumorigenesis ↑ [58,108] | Nfe2l1flox/flox; Alb-Cre+ |
| Lipid and amino acid metabolism ↓ [65] | ||
| Mitochondrial respiratory function ↓ [65] | ||
| Proteasome activity ↓ ER stress ↑ [64] | ||
| Macrophage | oxidative stress↑ [4] | /Nfe2l1-KD RAW264.7 cells |
| macrophage M1 polarization↑ [4] | ||
| Pancreatic β-cell | oxidative stress ↑ [16] | Nfe2l1flox/flox; Ins2-Cre+ |
| insulin secretion ↓ [16] | /Nfe2l1-KD MIN6 β-cells, | |
| tumorigenicity, aggressiveness, chemoresistance ↑ [109] | ||
| Osteoblast | osteoblast differentiation↓ [68] | Nfe2l1flox/flox; Col1α2-Cre+ |
| Neuronal cell | Proteasome activity ↓ [66,110] | Nfe2l1flox/flox; Camk2-Cre+ |
| Neurodegeneration [66,67,110]. | Nfe2l1flox/flox; Nestin-Cre+ | |
| Motor ataxia [67] | ||
| Chromatolysis [67] | ||
| Adipocyte | Lipolysis ↓ [13] | Nfe2l1flox/flox; Adipoq-Cre+ |
| Adipogenesis ↑ [11] adipocyte hypertrophy [13,14,98] | /Nfe2l1-KD 3T3-L1 cells | |
| inflammation [13,14,98] | ||
| Brown adipocyte | ER stress ↑ inflammation | Nfe2l1flox/flox; Ucp1-Cre+ |
| whitening [12] |
Overall, NFE2L1 is ubiquitously expressed producing various protein isoforms. NFE2L1 can be induced by stress signals from a broad spectrum of stimuli and plays an important role in regulating a range of cellular functions including antioxidant defense [60,63,[69], [70], [71]], proteasome homeostasis [7,8,64,66,72,73], inflammation [42,74], regeneration, metabolism [16,58,65,75], cellular differentiation [[76], [77], [78], [79]], maintenance of chromosomal stability and genomic integrity [80]. However, the underlying mechanisms by which these unique functions of various NFE2L1 isoforms are tightly regulated at various levels are not well elucidated [81]. Further research is needed to determine exactly how the proteoforms originate, their distribution and how each isoform of NFE2L1 contributes to its unique role in regulating expression of ARE-driven cytoprotective genes against various pathophysiological stresses.
3. Adipocytes
Adipose tissue (AT), which is primarily composed of adipocytes surrounded by fibroblasts, preadipocytes, endothelial cells, nerves and immune cells, is crucial for maintaining energy and metabolic homeostasis. AT can also secrete a variety of hormones, cytokines and other factors [82,83]. There are two main types of adipocytes with distinct functions. White adipocytes (WAC) are characterized by large unilocular lipid droplets and best appreciated as repositories of lipid storage by two primary metabolic activities, lipogenesis and lipolysis. In addition, WAC can also secrete a variety of adipokines associated with satiety regulation, whole-body insulin sensitivity, and so on [15,[82], [83], [84]]. These cells make up the bulk of white adipose tissue (WAT) mass in mice, and populate visceral depots of AT (VAT) in the abdomen like gonadal WAT (gWAT) as well as subcutaneous depots (SAT) like inguinal WAT (iWAT). By contrast, brown adipocytes (BAC) contain large numbers of mitochondria, which account for their brown color upon visualization. They are specialized in dissipating stored chemical energy from fats in the form of heat through the actions of uncoupling protein-1 (UCP1), a brown adipose tissue (BAT)-specific protein located within the mitochondria [[85], [86], [87]]. UCP1 catalyzes a proton leak across the inner mitochondrial membrane, thus ‘‘uncoupling’’ fuel oxidation from ATP synthesis. BAT has anatomically distinct positions in depots between the scapulae, around the neck and within the chest cavity. In addition, beige adipocytes exhibiting properties of both white and brown adipocytes have been discovered. These adipocytes have abundant mitochondria, are thermogenic like BAC but are located predominately in classical subcutaneous WAT depots in mice.
Despite the functional and locational differences between white and brown adipocytes, the two cell types share many common differentiation features. All adipocytes differentiate from MSCs in a process known as adipogenesis, which is thought to occur in two stages: commitment of MSCs to preadipocytes and terminal differentiation of preadipocytes towards mature adipocytes, involving a complex integration of cytoarchitecture, the micro-environment, signaling pathways and transcription regulators. Indeed, a core adipogenic transcriptional cascade is well characterized. Upstream components of this pathway include CCAAT/enhancer-binding proteins (C/EBPs) transcription factors that work sequentially to activate peroxisome proliferator activated receptor γ (PPARγ), the master regulator of adipocyte differentiation [[88], [89], [90], [91], [92]]. However, C/EBPα may be dispensable for maintaining certain aspects of adipocyte identity once these cells have been established. For brown adipogenesis, the core program is adjoined by unique transcriptional co-regulators, including PR domain-containing 16 (PRDM16) and the transcription factor early B-cell factor-2 (EBF2), that physically interact with PPARγ to direct BAC-specific target gene expression. By contrast, PRDM16 does not affect white adipogenesis.
Changes in AT amounts usually results from either hypertrophy or hyperplasia of adipocytes but the former is the major expansion factor in adulthood [93]. This is particularly true of WAT expansion and adipocytes. Local inflammation in response to adipocyte hypertrophy is essential for WAT expansion and subsequent remodeling [94]. However, in case of excessive adipocyte hypertrophy, such as in severe obesity, the inflammatory insult becomes persistent and may disturb energy homeostasis [95,96]. Metabolic fluxes are the primary determinants of adipocyte hypertrophy, and thus a shift in balance between influx and efflux of lipid may affect the function and fate of adipocytes. There is no doubt that in this disrupted homeostasis, lipolysis is the main contributor to lipid efflux, while lipogenesis mainly guarantees the influx.
4. Unraveling the emerging roles of NFE2L1 in AT
Given that AT functions as a key regulator of energy balance and nutritional homeostasis [97], it is worth figuring the significance of NFE2L1 in AT. To understand the biological function of NFE2L1 in AT, BAC-specific (Nfe2l1ΔBAT) [12] and adipocyte-specific Nfe2l1-KO mice [Nfe2l1(f)-KO] [13] have been developed by crossing Nfe2l1 flox/flox mice with Ucp1-Cre and adiponectin-Cre (adipoq-Cre) mice, respectively. The data from these distinct models demonstrated that NFE2L1 plays crucial roles in adipose biology involving adipogenesis, lipid and glucose metabolism, thermogenesis, antioxidant response and proteasome homeostasis.
4.1. NFE2L1 in adipogenesis
Our group used stromal vascular fraction (SVF) cells isolated from WAT of Nfe2l1(f)-KO mice and 3T3-L1 preadipocytes with altered levels of various isoforms of NFE2L1 to study their roles in adipogenesis, a process that preadipocytes differentiate into mature adipocytes. We found that deletion of either long or all isoforms of NFE2L1 in the cells results in augmented adipogenesis. The interesting cellular phenotype from SVF cells had gender- and depot-dependent differences. Conversely, overexpression of L-NFE2L1-741, but not S-NFE2L1, in 3T3-L1 cells led to attenuated adipogenesis [11]. Mechanistic studies suggest that L-NFE2L1 might dominate over S-NFE2L1s and negatively regulates the expression of PPARγ2 by directly binding to the promoter region of Pparg2 gene to repress its transcription [11]. However, we did not verify the exact roles of S-NFE2L1s in Pparg2 expression and adipogenesis. In addition, the molecular details for the suppression of Pparg2 expression by L-NFE2L1 and potential binding partners of L-NFE2L1 in the complex process remain vague.
4.2. NFE2L1 in white adipocyte
Our recent studies showed that Nfe2l1(f)-KO mice express reduced levels of multiple lipolytic genes in adipocytes, leading to adipocyte hypertrophy followed by severe inflammation, pyroptosis and insulin resistance, implicating an important role of NFE2L1 in adipocyte biology [13]. Moreover, the diminished WAT mass induced by Nfe2l1 deficiency specifically in adiponectin-expressing cells is age-, gender- and depot-dependent [98]. In line with our findings, a Ph.D. thesis from Dr. Chan's lab also described a similar phenotype of reduced inguinal WAT mass in the adipocyte-specific Nfe2l1-KO (Nrf1 FatKO) mice they developed [99]. To further clarify the regulatory role of NFE2L1 in lipid metabolism of adipocytes and verify the mechanisms underlying the phenotype of Nfe2l1(f)-KO mice, we used protracted rosiglitazone (RGZ) treatment to create an extremely positive lipid content balance in Nfe2l1(f)-KO mice. While three weeks of RGZ treatment significantly down-regulated mRNA levels of a group of inflammation-related genes in WAT of adult Nfe2l1-Floxed control mice, the adipose phenotype of Nfe2l1(f)-KO mice was aggravated showing further increased inflammation and macrophage infiltration, enhanced transcript expression related to inflammation and pyroptosis in WAT [14]. In addition, the effect of CL316243, a β3 adrenergic agonist that promotes lipolysis via a post-translational mechanism, on adipose inflammation in juvenile Nfe2l1(f)-KO mice was also studied [98]. In contrast to adult mice, 4 weeks old juvenile Nfe2l1(f)-KO mice displayed a normal fat distribution but reduced fasting plasma glycerol levels and elevated adipocyte hypertrophy and macrophage infiltration in inguinal and gonadal WAT. In addition, Nfe2l1(f)-KO mice had decreased expression of multiple lipolytic genes and reduced lipolytic activity in WAT. While 7 days of CL316243 treatment showed no significant effect on adipose inflammation in Nfe2l1-Floxed control mice, the same treatment dramatically alleviated macrophage infiltration and mRNA expression of inflammation and pyroptosis-related genes in WAT of Nfe2l1(f)-KO mice. The adipose tissue phenotypes of Nfe2l1(f)-KO mice and Nfe2l1-Floxed control mice under different treatment conditions were summarized in Table 3 [13,14,98].
Table 3.
The adipose tissue phenotypes of Nfe2l1(f)-KO mice and Nfe2l1-Floxed control mice under different treatment conditions.
| Genotype | Phenotype | Treatment |
||
|---|---|---|---|---|
| Veh | RGZ | CL316243 | ||
| Nfe2l1-Floxed | Lipolysis | – | – | ↑ |
| Lipogenesis | – | ↑ | – | |
| Hypertrophy | – | – | ↓ | |
| Inflammation |
– |
↓ |
– |
|
| Nfe2l1(f)-KO | Lipolysis | ↓ | ↓ | ↑ |
| Lipogenesis | – | ↑ | – | |
| Hypertrophy | ↑↑ | ↑↑↑ | ↑ | |
| Inflammation | ↑↑ | ↑↑↑ | ↑ | |
↑, increase; ↓, reduce; —, comparable.
Overall, our findings from Nfe2l1(f)-KO mice strongly indicate that NFE2L1 plays a fundamental regulatory role in the expression of a variety of lipolytic genes and thereby regulates lipid flux, WAT plasticity and global energy homeostasis (Fig. 4).
Fig. 4.
NFE2L1 regulates lipid flux and WAT plasticity. The hypertrophy and hyperplasia of adipocytes affect the physiology of WAT. Metabolic fluxes are the primary determinants of adipocyte hypertrophy, in which lipolysis is the main contributor to lipid efflux, while lipogenesis mainly impacts the influx. Once the balance between influx and efflux of lipid is disrupted, adipocytes will more readily go beyond their maximum volume limits and reach a critical death size, which induces local inflammation. Hyperplasia of adipocytes can provide additional capacity for storage in response to excess lipid which is primarily regulated by PPARγ. The expression of key lipolytic genes is, at least in part, dependent on NFE2L1. Adipocyte-specific deficiency of Nfe2l1 results in reduced lipolytic activity and uncoupled lipid metabolism precipitating adipocyte hypertrophy and inflammation. Rosiglitazone (RGZ)-induced elevation of de novo lipogenesis worsens adipocyte hypertrophy, inflammation and pyroptosis. CL316243, a β3 adrenergic agonist, promotes lipolysis via a post-translational mechanism improving the activity of lipolytic enzymes. Follow CL316243 treatment, this phenotype is improved.
4.3. NFE2L1 in brown adipocyte
In mice, Nfe2l1 mRNA expression is more enriched in BAT than that in WAT. Cold adaptation leads to markedly higher Nfe2l1 expression in BAT and to a minor extent also in iWAT [12]. Based on the findings, Bartelt et al. carried out a series of experiments to reveal a novel guardian role of NFE2L1 in BAC function providing increased proteometabolic quality control for adapting to cold or obesity [12].
Under thermogenic conditions, Nfe2l1ΔBAT mice displayed lowered BAT-mediated whole-body respiration and energy expenditure. BAT also became progressively discolored and lipid-laden with ER stress, inflammation, diminished mitochondrial function, and limited proteasome subunit mRNA expression [12]. Moreover, a few findings to some extent ruled out the possibility that the BAT phenotypes of Nfe2l1ΔBAT mice are simply a reflection of a defect in the general integrity of the tissue induced by loss of Nfe2l1. Specifically, changes did not occur in UCP1 protein levels or in mRNA levels of critical BAC regulators such as Ucp1, Ppargc1a, Ppargc1b, Pparα or Pparγ in BAT of Nfe2l1ΔBAT mice, either at 30 °C or at 22 °C. Chemical inhibition of proteasome or Nfe2l1 deficiency did not cause reduced protein levels of components of the respiratory chain in isolated mitochondria per se. Lipolysis in BAT and WAT explants remained unchanged by Nfe2l1 deletion.
Activated BAC has robust metabolic activity. Thus, a powerful proteometabolic quality control is needed to maintain the normal operation of the cells. ER is an adaptive organelle for defending homeostasis where enzymes couple nutrient-derived cues, substrates, or stress signals with coordinated changes in protein synthesis, folding, and secretion as well as lipid metabolism and trafficking [[100], [101], [102]]. In this process, the unfolded protein response (UPR) and the sterol response element binding protein 2 (SREBP2) sterol-sensing complex are critical, which engage metabolic and inflammatory outputs to restore homeostasis. NFE2L1 is an ER membrane sensor, which co-evolves with SREBP2 as a Yin-Yang counterbalance to stabilize ER-resident metabolic activity [103] and the downstream target genes encoding certain component of proteasome. Therefore, NFE2L1 may influences BAC profoundly through multiple pathways, including ER and oxidative stress, proteasome homeostasis, mitochondrial physiology under the states of normal or high activity.
At present, the role of NFE2L1 in AT has rarely been systematically explored. The AT phenotypes induced by Nfe2l1 deletion vary greatly among rather few studies available (Table 4). In addition, because of complex interactions among many pathways altered by Nfe2l1 ablation and the limited understanding on the transcription factor, it is still difficult for us to make clear explanations on the divergences in the key findings from Nfe2l1ΔBAT, Nrf1 FatKO and Nfe2l1(f)-KO mice. Considering the central role of AT in metabolic health and disease and the critical roles of NFE2L1 in many basic cellular functions in BAC and WAC, the physiological and pathophysiological roles of NFE2L1 warrant further investigations beyond the scope of the studies published.
Table 4.
Comparison of three lines of adipocyte-specific Nfe2l1-KO mice.
| Name | Nrf1 FatKO mice [99] | Nfe2l1(f)-KO mice [13] | Nfe2l1ΔBAT mice [12] | |
|---|---|---|---|---|
| Source of Nfe2l1-floxed mice | University of California, Irvine, USA | Tohoku University, Japan | Harvard T.H. Chan School, USA | |
| Strain of Cre transgenic mice | Adipoq-Cre | Adipoq-Cre | Ucp1-Cre | |
| General | Body weight | Male↓; Female — | Male —; Female ND | ND |
| Glucose tolerance | ↑ | ↓ | ||
| WAT | iWAT mass | ↓ | Male ↓; Female ↓↓ | |
| gWAT mass | ND | Male—; Female ↓ | ||
| Morphology | Lipid droplet size ↓ | Hypertrophy, CLS | ||
| Inflammation | ND | ↑ | ↑ | |
| Lipolysis | ND | ↓ | – | |
| Mitochondrial Function | ND | – | ↓ | |
| Stress | ND | ND | ER stress | |
| Molecular alterations | BAC markers ↑ | Lipolytic genes ↓ | ND | |
| Adipogenic genes ↓(iWAT) ↑(gWAT) | ||||
| Inflammatory response genes ↑ | ||||
| Pyroptosis-related genes ↑ | ||||
| BAT | Mass | ↑ | ↓ | ND |
| Morphology | Whitening | Whitening | Whitening | |
| Inflammation | ND | ↑↑ | ↑ | |
| Lipolysis | ↓↓ | – | ||
| Mitochondrial function | ↓ | ↓ | ||
| Stress | ND | ER stress | ||
| Thermogenesis | ↓ | ↓ | ||
| Molecular alterations | Adipogenic genes ↓ | |||
| Pgc1a ↑ | Lipolytic genes ↓ | Proteasome subunit genes↓ | ||
| Metrnl1 ↑ | Thermogenic gene ↓ | Stress genes↑ | ||
| Inflammatory response genes ↑ | ||||
Abbreviations: CLS, crown-like structures; ER, endoplasmic reticulum; ND, no detection. ↑, increase; ↓, reduce; —, comparable.
5. Conclusion and perspectives
Accumulating data indicates that NFE2L1 plays crucial roles in adipogenesis and adipose function. We have endeavored to summarize current understandings on NFE2L1 in adipocytes and AT which are mainly obtained from Nfe2l1ΔBAT and Nfe2l1(f)-KO mice and 3T3-L1 cells. First, NFE2L1 regulates adipogenesis in an isoform-specific manner. Of particular, L-NFE2L1 serves as a distinct negative regulator of Pparγ2 and adipogenesis. Second, NFE2L1 is a fundamental adaptive regulator of BAT thermogenic function and may act as a general cellular guardian of BAC activity. Third, NFE2L1-dependent lipolytic activity is crucial for WAT plasticity and lipid homeostasis. Because the gene knockout strategy used in the in vivo studies targets the exon 10 of the Nfe2l1 gene, a shared sequence across all the isoforms of Nfe2l1 transcripts, it is difficult, if not impossible, to determine the distinct roles of individual isoform of NFE2L1 in these models. While overexpressing individual isoform of NFE2L1 or in combinations in various cell models, such as 3T3-L1 cells, could provide informative data on the distinct roles of individual isoform of NFE2L1, it is challenging to verify their roles at AT or whole-body levels. To truly assess the isoform-specific roles of NFE2L1 in AT, conditional isoform-specific overexpressing in vivo models would be helpful.
Human and mouse Nfe2l1 genes contain multiple nucleotide polymorphisms that might affect the expression and/or function of different isoforms of NFE2L1. In addition, Nfe2l1 gene can be transcribed into multiple splice variants resulting in various protein isoforms, which may be further modified by a variety of post-translational mechanisms. While the L-NFE2L1 has been discovered as a key regulator of cellular adaptive antioxidant response, proteasome homeostasis, macrophage polarization [4] and adipogenesis [11], the exact tissue distribution and physiological function of various isoforms of NFE2L1, the short isoforms in particular, are still under investigation. Together with the findings on the linkage between gene polymorphisms of human NFE2L1 and obesity [[15], [16]] and the crucial role of NFE2L1 in adipose function, it is time to begin to pay more attention to the under-appreciated CNC-bZIP protein for its important and novel roles regulating adipocyte function and various metabolic disorders.
Declaration of competing interest
The authors declare that they have no conflict of interest. All authors approved the final manuscript.
Acknowledgements
This research was supported in part by the National Natural Science Foundation of China: 81830099 (J.P.), 82020108027 (J.P.), 81400839 (Y.C.), 82022063 (Y.X.), 81402661 (Y.H.), 81573106 (J.P.), 82073513 (H.W.), 82003500 (Y.B.) and 81402635 (J.F.); National Key R&D Program of China : 2018YFC1311600 (J.P.); and Liaoning Key Research and Development Guidance Plan 2019JH8/10300012 (J.P.). China Postdoctoral Management Foundation YJ20190263 (Y.B). The authors have no conflicting financial interests.
Contributor Information
Yanyan Chen, Email: chenyanyan_cmu@163.com.
Jingbo Pi, Email: jbpi@cmu.edu.cn, jingbopi@163.com.
References
- 1.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol. Scand. 2005;184(4):285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Xiang Y. Molecular and cellular basis for the unique functioning of Nrf1, an indispensable transcription factor for maintaining cell homoeostasis and organ integrity. Biochem. J. 2016;473(8):961–1000. doi: 10.1042/BJ20151182. [DOI] [PubMed] [Google Scholar]
- 3.Cui Q. Deficiency of long isoforms of Nfe2l1 sensitizes MIN6 pancreatic beta cells to arsenite-induced cytotoxicity. Toxicol. Appl. Pharmacol. 2017;329:67–74. doi: 10.1016/j.taap.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Wang H. Silencing of long isoforms of nuclear factor erythroid 2 like 1 primes macrophages towards M1 polarization. Free Radic. Biol. Med. 2018;117:37–44. doi: 10.1016/j.freeradbiomed.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Zhao R. Long isoforms of NRF1 contribute to arsenic-induced antioxidant response in human keratinocytes. Environ. Health Perspect. 2011;119(1):56–62. doi: 10.1289/ehp.1002304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lou B. Long-isoform NRF1 protects against arsenic cytotoxicity in mouse bone marrow-derived mesenchymal stem cells by suppressing mitochondrial ROS and facilitating arsenic efflux. Toxicol. Appl. Pharmacol. 2020;407:115251. doi: 10.1016/j.taap.2020.115251. [DOI] [PubMed] [Google Scholar]
- 7.Radhakrishnan S.K. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell. 2010;38(1):17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sha Z., Goldberg A.L. Proteasome-mediated processing of Nrf1 is essential for coordinate induction of all proteasome subunits and p97. Curr. Biol. 2014;24(14):1573–1583. doi: 10.1016/j.cub.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steffen J. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol. Cell. 2010;40(1):147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchiya Y. Dual regulation of the transcriptional activity of Nrf1 by beta-TrCP- and Hrd1-dependent degradation mechanisms. Mol. Cell Biol. 2011;31(22):4500–4512. doi: 10.1128/MCB.05663-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue P. Long isoforms of NRF1 negatively regulate adipogenesis via suppression of PPARgamma expression. Redox Biol. 2020;30:101414. doi: 10.1016/j.redox.2019.101414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartelt A. Brown adipose tissue thermogenic adaptation requires Nrf1-mediated proteasomal activity. Nat. Med. 2018;24(3):292–303. doi: 10.1038/nm.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou Y. Adipocyte-specific deficiency of Nfe2l1 disrupts plasticity of white adipose tissues and metabolic homeostasis in mice. Biochem. Biophys. Res. Commun. 2018;503(1):264–270. doi: 10.1016/j.bbrc.2018.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Ren S. Protracted rosiglitazone treatment exacerbates inflammation in white adipose tissues of adipocyte-specific Nfe2l1 knockout mice. Food Chem. Toxicol. 2020;146:111836. doi: 10.1016/j.fct.2020.111836. [DOI] [PubMed] [Google Scholar]
- 15.Speliotes E.K. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010;42(11):937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirotsu Y. Transcription factor NF-E2-related factor 1 impairs glucose metabolism in mice. Gene Cell. 2014;19(8):650–665. doi: 10.1111/gtc.12165. [DOI] [PubMed] [Google Scholar]
- 17.Itoh K. Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell Biol. 1995;15(8):4184–4193. doi: 10.1128/mcb.15.8.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnsen O. Small Maf proteins interact with the human transcription factor TCF11/Nrf1/LCR-F1. Nucleic Acids Res. 1996;24(21):4289–4297. doi: 10.1093/nar/24.21.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnsen O. Interaction of the CNC-bZIP factor TCF11/LCR-F1/Nrf1 with MafG: binding-site selection and regulation of transcription. Nucleic Acids Res. 1998;26(2):512–520. doi: 10.1093/nar/26.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marini M.G. hMAF, a small human transcription factor that heterodimerizes specifically with Nrf1 and Nrf2. J. Biol. Chem. 1997;272(26):16490–16497. doi: 10.1074/jbc.272.26.16490. [DOI] [PubMed] [Google Scholar]
- 21.Newman J.R.S., Keating A.E. Comprehensive identification of human bZIP interactions with coiled-coil arrays. Science. 2003;300(5628):2097. doi: 10.1126/science.1084648. [DOI] [PubMed] [Google Scholar]
- 22.Murphy P., Kolsto A. Expression of the bZIP transcription factor TCF11 and its potential dimerization partners during development. Mech. Dev. 2000;97(1–2):141–148. doi: 10.1016/s0925-4773(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim H.M., Han J.W., Chan J.Y. Nuclear factor erythroid-2 like 1 (NFE2L1): structure, function and regulation. Gene. 2016;584(1):17–25. doi: 10.1016/j.gene.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scambler P. Cloning and mapping of murine Nfe2l1. Genomics. 1995;25(3):716–719. doi: 10.1016/0888-7543(95)80015-e. [DOI] [PubMed] [Google Scholar]
- 25.Caterina J.J. Cloning and functional characterization of LCR-F1: a bZIP transcription factor that activates erythroid-specific, human globin gene expression. Nucleic Acids Res. 1994;(12):12. doi: 10.1093/nar/22.12.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan J.Y., Han X.L., Kan Y.W. Cloning of Nrf1, an NF-E2-related transcription factor, by genetic selection in yeast. Proc. Natl. Acad. Sci. U. S. A. 1993;90(23):11371–11375. doi: 10.1073/pnas.90.23.11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y. Transcription factor Nrf1 is topologically repartitioned across membranes to enable target gene transactivation through its acidic glucose-responsive domains. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0093458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y. The NHB1 (N-terminal homology box 1) sequence in transcription factor Nrf1 is required to anchor it to the endoplasmic reticulum and also to enable its asparagine-glycosylation. Biochem. J. 2007;408(2):161–172. doi: 10.1042/BJ20070761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., Lucocq J.M., Hayes J.D. The Nrf1 CNC/bZIP protein is a nuclear envelope-bound transcription factor that is activated by t-butyl hydroquinone but not by endoplasmic reticulum stressors. Biochem. J. 2009;418(2):293–310. doi: 10.1042/BJ20081575. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y., Hayes J.D. The membrane-topogenic vectorial behaviour of Nrf1 controls its post-translational modification and transactivation activity. Sci. Rep. 2013;3:2006. doi: 10.1038/srep02006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y. The selective post-translational processing of transcription factor Nrf1 yields distinct isoforms that dictate its ability to differentially regulate gene expression. Sci. Rep. 2015;5(1):12983. doi: 10.1038/srep12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y. The C-terminal domain of Nrf1 negatively regulates the full-length CNC-bZIP factor and its shorter isoform LCR-F1/Nrf1beta; both are also inhibited by the small dominant-negative Nrf1gamma/delta isoforms that down-regulate ARE-battery gene expression. PloS One. 2014;9(10) doi: 10.1371/journal.pone.0109159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Hayes J.D. Identification of topological determinants in the N-terminal domain of transcription factor Nrf1 that control its orientation in the endoplasmic reticulum membrane. Biochem. J. 2010;430(3):497–510. doi: 10.1042/BJ20100471. [DOI] [PubMed] [Google Scholar]
- 34.Bean T.L., Ney P.A. Multiple regions of p45 NF-E2 are required for β-globin gene expression in erythroid cells. Nucleic Acids Res. 1997;(12):12. doi: 10.1093/nar/25.12.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Husberg C. Two domains of the human bZIP transcription factor TCF11 are necessary for transactivation. J. Biol. Chem. 2001;276(21):17641–17652. doi: 10.1074/jbc.M007951200. [DOI] [PubMed] [Google Scholar]
- 36.Wang M. Distinct isoforms of Nrf1 diversely regulate different subsets of its cognate target genes. Sci. Rep. 2019;9(1):2960. doi: 10.1038/s41598-019-39536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radhakrishnan S.K., den Besten W., Deshaies R.J. p97-dependent retrotranslocation and proteolytic processing govern formation of active Nrf1 upon proteasome inhibition. Elife. 2014;3 doi: 10.7554/eLife.01856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luna L. Structural organization and mapping of the human TCF11 gene. Genomics. 1995;27(2):237–244. doi: 10.1006/geno.1995.1037. [DOI] [PubMed] [Google Scholar]
- 39.Luna L. Molecular cloning of a putative novel human bZIP transcription factor on chromosome 17q22. Genomics. 1994;22(3):553–562. doi: 10.1006/geno.1994.1428. [DOI] [PubMed] [Google Scholar]
- 40.Kwong E.K. Characterization of Nrf1b, a novel isoform of the nuclear factor-erythroid-2 related transcription factor-1 that activates antioxidant response element-regulated genes. PloS One. 2012;7(10) doi: 10.1371/journal.pone.0048404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Novotny V. Nrf1 in a complex with fosB, c-jun, junD and ATF2 forms the AP1 component at the TNF alpha promoter in stimulated mast cells. Nucleic Acids Res. 1998;26(23):5480–5485. doi: 10.1093/nar/26.23.5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prieschl E.E. A novel splice variant of the transcription factor Nrf1 interacts with the TNFalpha promoter and stimulates transcription. Nucleic Acids Res. 1998;26(10):2291–2297. doi: 10.1093/nar/26.10.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y. Negative regulation of the Nrf1 transcription factor by its N-terminal domain is independent of Keap1: nrf1, but not Nrf2, is targeted to the endoplasmic reticulum. Biochem. J. 2006;399(3):373–385. doi: 10.1042/BJ20060725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caterina John J., Donze D., Sun Chiao-Wang, Ciavatta Dominic J., Townes Tim M. Cloning and functional characterization of LOR-Fi: a bZIP transcription factor that activates erythroid-specific, human globin gene expression. Nucleic Acids Res. 1994;22:2383–2391. doi: 10.1093/nar/22.12.2383. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W., Kwok A.M., Chan J.Y. The p65 isoform of Nrf1 is a dominant negative inhibitor of ARE-mediated transcription. J. Biol. Chem. 2007;282(34):24670–24678. doi: 10.1074/jbc.M700159200. [DOI] [PubMed] [Google Scholar]
- 46.Biswas M., Chan J.Y. Role of Nrf1 in antioxidant response element-mediated gene expression and beyond. Toxicol. Appl. Pharmacol. 2010;244(1):16–20. doi: 10.1016/j.taap.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao R. Cross-regulations among NRFs and KEAP1 and effects of their silencing on arsenic-induced antioxidant response and cytotoxicity in human keratinocytes. Environ. Health Perspect. 2012;120(4):583–589. doi: 10.1289/ehp.1104580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tomlin F.M. Inhibition of NGLY1 inactivates the transcription factor Nrf1 and potentiates proteasome inhibitor cytotoxicity. ACS Cent. Sci. 2017;3(11):1143–1155. doi: 10.1021/acscentsci.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekine H. O-GlcNAcylation signal mediates proteasome inhibitor resistance in cancer cells by stabilizing NRF1. Mol. Cell Biol. 2018;38(17) doi: 10.1128/MCB.00252-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han J.W. Nuclear factor-erythroid-2 related transcription factor-1 (Nrf1) is regulated by O-GlcNAc transferase. Free Radic. Biol. Med. 2017;110:196–205. doi: 10.1016/j.freeradbiomed.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 51.Lehrbach N.J., Ruvkun G. Vol. 5. Elife; 2016. (Proteasome Dysfunction Triggers Activation of SKN-1A/Nrf1 by the Aspartic Protease DDI-1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koizumi S. Vol. 5. Elife; 2016. (The Aspartyl Protease DDI2 Activates Nrf1 to Compensate for Proteasome Dysfunction). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biswas M. The Fbw7 tumor suppressor regulates nuclear factor E2-related factor 1 transcription factor turnover through proteasome-mediated proteolysis. J. Biol. Chem. 2011;286(45):39282–39289. doi: 10.1074/jbc.M111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsuchiya Y. The casein kinase 2-nrf1 axis controls the clearance of ubiquitinated proteins by regulating proteasome gene expression. Mol. Cell Biol. 2013;33(17):3461–3472. doi: 10.1128/MCB.01271-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas M. Glycogen synthase kinase 3 regulates expression of nuclear factor-erythroid-2 related transcription factor-1 (Nrf1) and inhibits pro-survival function of Nrf1. Exp. Cell Res. 2013;319(13):1922–1931. doi: 10.1016/j.yexcr.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Titus B.K. Hyperoxic regulation of mitochondrial cell death via Trx2. Free Radic. Biol. Med. 2011;51:S17. [Google Scholar]
- 57.Xiang Y. Mechanisms controlling the multistage post-translational processing of endogenous Nrf1α/TCF11 proteins to yield distinct isoforms within the coupled positive and negative feedback circuits. Toxicol. Appl. Pharmacol. 2018;360:212–235. doi: 10.1016/j.taap.2018.09.036. [DOI] [PubMed] [Google Scholar]
- 58.Xu Z. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc. Natl. Acad. Sci. U. S. A. 2005;102(11):4120–4125. doi: 10.1073/pnas.0500660102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohtsuji M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J. Biol. Chem. 2008;283(48):33554–33562. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan J.Y. Targeted disruption of the ubiquitous CNC-bZIP transcription factor, Nrf-1, results in anemia and embryonic lethality in mice. EMBO J. 1998;17(6):1779–1787. doi: 10.1093/emboj/17.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farmer S.C. The bZIP transcription factor LCR-F1 is essential for mesoderm formation in mouse development. Genes Dev. 1997;11(6):786–798. doi: 10.1101/gad.11.6.786. [DOI] [PubMed] [Google Scholar]
- 62.Kwong M. The CNC basic leucine zipper factor, Nrf1, is essential for cell survival in response to oxidative stress-inducing agents. ROLE for Nrf1 IN gamma -gcsL and gss expression IN mouse fibroblasts. J. Biol. Chem. 1999;274:37491–37498. doi: 10.1074/jbc.274.52.37491. [DOI] [PubMed] [Google Scholar]
- 63.Leung L. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J. Biol. Chem. 2003;278(48):48021–48029. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- 64.Lee C.S., Ho D.V., Chan J.Y. Nuclear factor-erythroid 2-related factor 1 regulates expression of proteasome genes in hepatocytes and protects against endoplasmic reticulum stress and steatosis in mice. FEBS J. 2013;280(15):3609–3620. doi: 10.1111/febs.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hirotsu Y. NF-E2-related factor 1 (Nrf1) serves as a novel regulator of hepatic lipid metabolism through regulation of the Lipin1 and PGC-1beta genes. Mol. Cell Biol. 2012;32(14):2760–2770. doi: 10.1128/MCB.06706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee C.S. Loss of nuclear factor E2-related factor 1 in the brain leads to dysregulation of proteasome gene expression and neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 2011;108(20):8408–8413. doi: 10.1073/pnas.1019209108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kobayashi A. Central nervous system-specific deletion of transcription factor Nrf1 causes progressive motor neuronal dysfunction. Gene Cell. 2011;16(6):692–703. doi: 10.1111/j.1365-2443.2011.01522.x. [DOI] [PubMed] [Google Scholar]
- 68.Kim J. Targeted disruption of nuclear factor erythroid-derived 2-like 1 in osteoblasts reduces bone size and bone formation in mice. Physiol. Genom. 2010;40(2):100–110. doi: 10.1152/physiolgenomics.00105.2009. [DOI] [PubMed] [Google Scholar]
- 69.Myhrstad M.C.W. TCF11/Nrf1 overexpression increases the intracellular glutathione level and can transactivate the gamma-glutamylcysteine synthetase (GCS) heavy subunit promoter. Biochim. Biophys. Acta Gene Struct. Expr. 2001;1517(2):212–219. doi: 10.1016/s0167-4781(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 70.Yang H. Nrf1 and Nrf2 regulate rat glutamate-cysteine ligase catalytic subunit transcription indirectly via NF-kappaB and AP-1. Mol. Cell Biol. 2005;25(14):5933–5946. doi: 10.1128/MCB.25.14.5933-5946.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. U. S. A. 1996;93(25):14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ho D.V., Chan J.Y. Induction of Herpud1 expression by ER stress is regulated by Nrf1. FEBS Lett. 2015;589(5):615–620. doi: 10.1016/j.febslet.2015.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groll M. Molecular machines for protein degradation. Chembiochem. 2005;6(2):222–256. doi: 10.1002/cbic.200400313. [DOI] [PubMed] [Google Scholar]
- 74.Berg D.T. Negative regulation of inducible nitric-oxide synthase expression mediated through transforming growth factor-beta-dependent modulation of transcription factor TCF11. J. Biol. Chem. 2007;282(51):36837–36844. doi: 10.1074/jbc.M706909200. [DOI] [PubMed] [Google Scholar]
- 75.Tsujita T. Transcription factor Nrf1 negatively regulates the cystine/glutamate transporter and lipid-metabolizing enzymes. Mol. Cell Biol. 2014;34(20):3800–3816. doi: 10.1128/MCB.00110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xing W. Nuclear factor-E2-related factor-1 mediates ascorbic acid induction of osterix expression via interaction with antioxidant-responsive element in bone cells. J. Biol. Chem. 2007;282(30):22052–22061. doi: 10.1074/jbc.M702614200. [DOI] [PubMed] [Google Scholar]
- 77.Inoue K., Imai Y. Identification of novel transcription factors in osteoclast differentiation using genome‐wide analysis of open chromatin determined by DNase‐seq. J. Bone Miner. Res. 2014;29(8):1823–1832. doi: 10.1002/jbmr.2229. [DOI] [PubMed] [Google Scholar]
- 78.Narayanan K. The CCAAT enhancer-binding protein (C/EBP)beta and Nrf1 interact to regulate dentin sialophosphoprotein (DSPP) gene expression during odontoblast differentiation. J. Biol. Chem. 2004;279(44):45423–45432. doi: 10.1074/jbc.M405031200. [DOI] [PubMed] [Google Scholar]
- 79.Jacob A., Zhang Y., George A. Transcriptional regulation of dentin matrix protein 1 (DMP1) in odontoblasts and osteoblasts. Connect. Tissue Res. 2014;55(Suppl 1):107–112. doi: 10.3109/03008207.2014.923850. S1. [DOI] [PubMed] [Google Scholar]
- 80.Oh D.H. Deficiency in the nuclear-related factor erythroid 2 transcription factor (Nrf1) leads to genetic instability. FEBS J. 2012;279(22):4121–4130. doi: 10.1111/febs.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bugno M. Changing gears in Nrf1 research, from mechanisms of regulation to its role in disease and prevention. Biochim. Biophys. Acta. 2015;1849(10):1260–1276. doi: 10.1016/j.bbagrm.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156(1–2):20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomou T. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450–455. doi: 10.1038/nature21365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Proenca A.R. New concepts in white adipose tissue physiology. Braz. J. Med. Biol. Res. 2014;47(3):192–205. doi: 10.1590/1414-431X20132911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoneshiro T. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity. 2011;19(1):13–16. doi: 10.1038/oby.2010.105. [DOI] [PubMed] [Google Scholar]
- 86.Hankir M.K., Klingenspor M. Brown adipocyte glucose metabolism: a heated subject. EMBO Rep. 2018;19(9) doi: 10.15252/embr.201846404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Heeren J., Scheja L. Brown adipose tissue and lipid metabolism. Curr. Opin. Lipidol. 2018;29(3):180–185. doi: 10.1097/MOL.0000000000000504. [DOI] [PubMed] [Google Scholar]
- 88.Lowell B.B. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell. 1999;99(3):239–242. doi: 10.1016/s0092-8674(00)81654-2. [DOI] [PubMed] [Google Scholar]
- 89.Lehrke M., Lazar M.A. The many faces of PPARgamma. Cell. 2005;123(6):993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 90.Moseti D., Regassa A., Kim W.K. Molecular regulation of adipogenesis and potential anti-adipogenic bioactive molecules. Int. J. Mol. Sci. 2016;17(1):124. doi: 10.3390/ijms17010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tontonoz P., Hu E., Spiegelman B.M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79(7):1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 92.Lefterova M.I., Lazar M.A. New developments in adipogenesis. Trends Endocrinol. Metabol. 2009;20(3):107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 93.Choe S.S. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wernstedt Asterholm I. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metabol. 2014;20(1):103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Virtue S., Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim. Biophys. Acta. 2010;1801(3):338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 96.Giordano A., Frontini A., Cinti S. Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 2016;15(6):405–424. doi: 10.1038/nrd.2016.31. [DOI] [PubMed] [Google Scholar]
- 97.Berry D.C. The developmental origins of adipose tissue. Development. 2013;140(19):3939–3949. doi: 10.1242/dev.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z. CL316243 treatment mitigates the inflammation in white adipose tissues of juvenile adipocyte-specific Nfe2l1 knockout mice. Free Radic. Biol. Med. 2021;165:289–298. doi: 10.1016/j.freeradbiomed.2021.01.043. [DOI] [PubMed] [Google Scholar]
- 99.Schneider K.S. University of California; eScholarship: 2016. Functional Analysis of Nrf1 and Nrf2 Transcription Factors in Adipose Tissue. [Google Scholar]
- 100.Fu S., Watkins S.M., Hotamisligil G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metabol. 2012;15(5):623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 101.Goldstein J.L., DeBose-Boyd R.A., Brown M.S. Protein sensors for membrane sterols. Cell. 2006;124(1):35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 102.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8(7):519. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 103.Widenmaier S.B. NRF1 is an ER membrane sensor that is central to cholesterol homeostasis. Cell. 2017;171(5):1094–1109 e15. doi: 10.1016/j.cell.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Wang W., Chan J.Y. Nrf1 is targeted to the endoplasmic reticulum membrane by an N-terminal transmembrane domain. Inhibition of nuclear translocation and transacting function. J. Biol. Chem. 2006;281(28):19676–19687. doi: 10.1074/jbc.M602802200. [DOI] [PubMed] [Google Scholar]
- 106.Zhang Y. Molecular and Cellular Control of the Nrf1 Transcription Factor: an Integral Membrane Glycoprotein. the first ed. ed. Vdm Verlag Dr Muller Publishing House; 2009. [Google Scholar]
- 107.Murphy P., Kolstø A. Expression of the bZIP transcription factor TCF11 and its potential dimerization partners during development. Mech. Dev. 2000;97(1):141–148. doi: 10.1016/s0925-4773(00)00413-5. [DOI] [PubMed] [Google Scholar]
- 108.Maurizio P., Novo E. Nrf1 gene expression in the liver: a single gene linking oxidative stress to NAFLD, NASH and hepatic tumours. J. Hepatol. 2005;43(6):1096–1097. doi: 10.1016/j.jhep.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Fu J. Nfe2l1-silenced insulinoma cells acquire aggressiveness and chemoresistance. Endocr. Relat. Canc. 2018;25(3):185–200. doi: 10.1530/ERC-17-0458. [DOI] [PubMed] [Google Scholar]
- 110.Taniguchi H. Possible roles of the transcription factor Nrf1 (NFE2L1) in neural homeostasis by regulating the gene expression of deubiquitinating enzymes. Biochem. Biophys. Res. Commun. 2017;484(1):176–183. doi: 10.1016/j.bbrc.2017.01.038. [DOI] [PubMed] [Google Scholar]