Abstract
Epigenetics is defined as the study of changes in gene function that are mitotically or meiotically heritable and do not lead to a change in DNA sequence. Epigenetic modifications are important mechanisms that fine tune the expression of genes in response to extracellular signals and environmental changes. In vertebrates, crucial epigenetic reprogramming events occur during early embryogenesis and germ cell development. Chicken embryo, which develops external to the mother's body, can be easily manipulated in vivo and in vitro, and hence, it is an excellent model for performing epigenetic studies. Environmental factors such as temperature can affect the development of an embryo into the phenotype of an adult. A better understanding of the environmental impact on embryo development can be achieved by analyzing the direct effects of epigenetic modifications as well as their molecular background and their intergenerational and transgenerational inheritance. In this overview, the current possibility of epigenetic changes during chicken embryonic development and their effects on long-term postembryonic development are discussed.
Key words: chicken embryo, epigenetics, primordial germ cells, in ovo
INTRODUCTION
Waddington (1968) defined epigenetics as the branch of biology that studies the causal interactions between genes and their products that lead to phenotypic development. However, with the rapid growth of molecular genetics, the meaning of this concept has changed. Today, the generally accepted definition of epigenetics is the study of changes in gene function that are mitotically and/or meiotically heritable and that do not entail a change in DNA sequence (Wu and Morris, 2001). Epigenetics refers not only to heritable changes in gene activity and expression but also to long-term changes in the transcriptional potential of a cell. Thus, in addition to genetic information, cells also inherit information that is not encoded in DNA sequence. Patterns of gene expression are established during cell development and sustained when cells are mitotically dividing. Epigenetic regulation of gene expression occurs by the interaction of the external environment with the transcription and translation of information encoded in nucleic acids. Various external factors such as nutrition, sanitary conditions, stress, and climate lead to epigenetic changes, which may affect the phenotypic traits of an individual.
The epigenetic processes that alter gene expression patterns and transfer changes during cell division include DNA methylation or hydroxymethylation of CG dinucleotides, chemical modifications of histones, interaction of DNA with small RNAs, and different states of chromatin condensation (Guerrero-Bosagna et al., 2018). The DNA methylation process involves the addition of methyl residues to cytosines contained in the CpG islands and restricts the access of enzymes (including transcriptases) to DNA, thus inhibiting the transcription of genes from DNA to mRNA. This process is characterized by the stability of cytosine modification within the CpG dinucleotides (Shen and Waterland, 2007). MicroRNAs (miRNAs) are short (20-30 nt) RNA species involved in gene regulatory processes and are transcribed as parts of longer RNA molecules in the nucleus. After transportation to the cytoplasm, single-stranded miRNAs bind to their complementary sequences in mRNA and inhibit translation. Thus, miRNA promotes the silencing of the target genes. This binding is nonhomologous and enables individual miRNAs to regulate hundreds of target genes (Taganov et al., 2007). Histones are evolutionarily highly conserved basic proteins that noncovalently bind to the DNA helix and are major constituents of the chromosomes. The histone proteins present in the chromatin can undergo posttranslational modifications, which loosen the chromatin and facilitate DNA replication or transcription (Bannister and Kouzarides, 2011). Specifically, the N-terminal tails of core histones are reversibly acetylated, methylated, phosphorylated, or ubiquitinated, and these patterns are thought to contribute to the regulation of DNA transcription, thus extending the potential of the genetic code. This assumption is hypothesized to be a histone code, as a part of the epigenetic code (Carlberg and Molnar, 2018).
The epigenetic effects can be classified into 2 categories: (1) The intergenerational, context-dependent inheritance and (2) germline-dependent inheritance. The intergenerational (Perez and Lehner, 2019) “context-dependent” (Burggren, 2015) epigenetic inheritance affects the phenotype through a direct and continuous exposure to an environmental stressor within or across generations (between the mates and their immediate progeny). In the presence of a stressor, the phenotype remains modified.
In contrast, the transgenerational “germline-dependent” inheritance occurs when the germline of an organism is directly affected, and consequently, phenotypic modifications persist across generations even in the absence of the original causative agent (i.e., the environmental stressor). Thus, only the altered phenotypes occurring in the second (for male transmission) or third (for female transmission) generation following a trigger are considered as the transgenerational effects. For instance, an environmental stimulus can directly affect a gestating embryo or the already-formed oocytes within a gestating female fetus in mammals (Skinner, 2008).
CHICKEN EMBRYO MODEL
Domestic chicken (Gallus gallus domesticus) holds a very specific evolutionary position that bridges the mammals and vertebrates, which makes this species an important animal model for conducting essential studies in various disciplines of science, such as an important model for use in classical experimental embryology (Rashidi and Sottile, 2009), studies focusing on the molecular basis of cell development or cell–cell interactions (Weeke-Klimp et al., 2010), genomics (Cogburn et al., 2003), experimental medicine (Ribatti, 2012), immunology, behavior, reproduction (Burt and Pourquie, 2003; Li et al., 2011), and the relatively new field of epigenetics. The fascination with the chick as a model organism started a long time ago. Interestingly, the first documented developmental studies conducted by opening chicken eggs at different stages of development were performed by Aristotle as early as 350 BC.
In addition to the important evolutionary position of chicken, its embryos have several advantages over mammalian ones that make them powerful model organisms in general and in epigenetics in particular. First, compared to mammalian models, chicken embryos develop very fast. When the egg is laid, the avian embryo consists of a flat, two-layered blastoderm that lies on the surface of the yolk and therefore is readily accessible. Subsequent development occurs external to the mother's body. Within 2 to 3 d of laying, chick embryos undergo gastrulation, neurulation, and histogenesis and fold into three-dimensional (3D) animals with beating hearts, somites, and complex nervous systems, eventually completing their entire development by the time of hatching at 21 d (Hamburger and Hamilton, 1992). This rapid development is a huge advantage when designing experiments and collecting data on time. Moreover, incubation of chicken eggs can be terminated at any time, thereby providing embryos at the desired developmental stage for a particular experiment. Furthermore, chicken embryos are of sufficient size that enables to practically conduct several types of micromanipulation even at early stages. Because the chicken embryo is similar to the human embryo at the molecular, cellular, and anatomical levels, the chick embryo plays a crucial role in biomedical research (Kain et al., 2014).
Another important advantage of the chicken embryo over mammalian models is that it can be easily incubated and manipulated both in ovo and ex ovo at a very low cost without requiring any major infrastructure. After the egg is laid, subsequent development occurs external to the mother's body, where only two easily controllable parameters of incubation are essential to be met: temperature and humidity. Moreover, several culture methods, each specifically adapted to a particular developmental stage, can be used for avian embryos. For instance, ex ovo culture is the method of choice for experimental manipulation of whole embryos at the pregastrula stage through the neurula stage for cultures lasting up to 2 d. On the other hand, in ovo culture is usually applicable for whole embryos to observe them and manipulate their development starting from HH stage 16 to hatching (reviewed by Darnell and Schoenwolf, 2000). In ovo manipulations on chicken embryos can be performed at any specific embryonic stage through a small window in the eggshell, wherein closing the window and reincubating the egg ensure further development, which is more difficult to achieve in mammals. Furthermore, many well-established experimental methods, including tissue ablation, rotation, auto- and allografting, implantation of beads coated with growth factors or small molecules, tissue explant culture, and cell culture systems, are also available for the chicken embryo model (reviewed by Darnell and Schoenwolf, 2000; Belecky-Adams et al., 2008). Importantly, both in ovo and ex ovo chicken embryos are easy to visualize. In in ovo experiments, live optical imaging of the chick embryo can be simply and easily performed through a small window in the eggshell. Chicken embryos are semitransparent and thus ready for easy examination of internal tissues even under a microscope. Additionally, a wide variety of cell marking techniques is available for tracking cell movements and fates in chicken embryo in real time (Vergara and Canto-Soler, 2012).
Another advantage of chicken as a model organism is detailed knowledge about its genome. In 2004, the first draft sequence of the chicken genome was released along with bacterial artificial chromosome (BAC) libraries and a BAC-based physical map (Wallis et al., 2004) as well as single nucleotide polymorphisms (SNPs) database (International Chicken Polymorphism Map Consortium, 2004). Since then, the knowledge of the chicken genome and the quality of its assembly have been constantly improved. Currently, a variety of resources on the chicken genome and transcriptome, such as linkage maps, databases for quantitative trait loci (QTL) and gene expression ontology, and sequence assemblies, are available to the research community at the “Chicken Genome Resources” database created and curated by NCBI (reviewed by Stern, 2005). Interestingly, the sequencing of the chicken genome revealed that the number of chicken genes is very similar to that in humans. A high level of sequence conservation not only in the coding region but also in intronic and flanking noncoding regions was also detected between homologous genes in mammals and birds (reviewed by Stern, 2005; Burt, 2005).
Regarding the field of epigenetics, birds are a highly advantageous model over mammals, especially for studying transgenerational epigenetic inheritance (Guerrero-Bosagna et al., 2018). Chickens show an early sexual maturity, a high rate of egg production (300 eggs in a year), a shorter interval between generations, and a requirement of less floor space and less feed. Moreover, chicken eggs are available year-round, are inexpensive, and can be purchased in any specified quantity. Furthermore, by using semen diluent and artificial insemination, a virtually unlimited number of offspring can be obtained from one rooster. Similarly, the Japanese quail is a popular laboratory animal, which because of its small size and short generation interval (3 to 4 generations in a year) is particularly preferable for long-term, multigenerational genetic studies (Vali, 2008; Vitorino Carvalho et al., 2020).
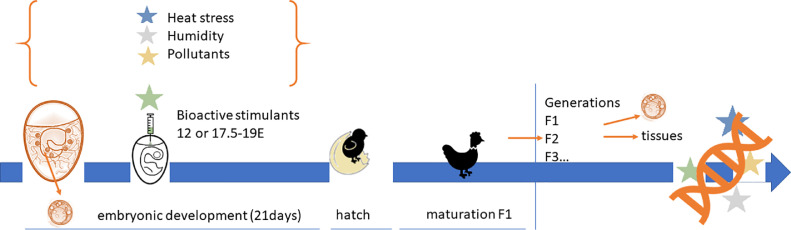
Another major advantage of chick embryos as a model organism in the epigenetic study is the fact that they develop external to the mother's organism; thus, the maternal effect is restricted to egg composition. Additionally, environmental factors such as the temperature of incubation and humidity can be strictly controlled to minimize the interindividual environmental variability (Guerrero-Bosagna et al., 2018). It is now evident that a portion of the variability of complex traits is affected by interactions with the environment through the epigenetic phenomenon (Feil and Fraga, 2012). An “epigenetic code” is established early in the development, and it enables cells to receive and remember environmentally induced signals to create a more stable state (Cavalli and Paro, 1998). As there is a constant interplay between genetic and epigenetic codes, environmentally modified epigenetic patterns result in differential expression of specific genes and consequently divergent phenotype formation, leading to interindividual phenotypic variation (Turan et al., 2010) (Figure 1). Generally, in animal production, a high level of interindividual environmental variation is not preferred by breeders and farmers. Particularly, the lower the interindividual genetic and environmental variability, the better is the prediction of animal performance and animal production results, because genetically and epigenetically similar birds under the same environmental conditions should yield products of comparable quality and quantity. Moreover, intraindividual variability, both genetic and epigenetic polymorphisms, were found to be crucial in the regulation of genetic resistance to diseases (Tian et al., 2013) and response to drug treatments (reviewed by Ivanov et al., 2012).
Figure 1.
An in ovo model for studying the mechanisms of potential inheritance of the epigenetic code
The chicken-based model of inheritance of the epigenetic code includes the analysis of PGCs in the embryo receiving an epigenetic impact. The environmental factors/stressors or in ovo application of bioactive compounds (pro/synbiotics, vitamins, and nutrients) may induce epigenetic alterations in genes during prenatal development of chicken. PGCs may transmit the changed epigenome to the consecutive generation(s). Therefore, the analysis of the transcriptome of PGCs and tissues prior to the epigenetic impact in the hatched chickens and in the next generation will allow to analyze heritability of the altered epigenome. PGCs: primordial germ cells; E: day of embryo incubation; F: filial generation.
The chicken embryo is also a powerful animal model to study processes of epigenetic reprogramming. In vertebrates, crucial epigenetic reprogramming events occur during early embryogenesis and germ cell development (Wrenzycki and Niemann, 2003). First, global DNA demethylation followed by de novo methylation occurs during the migration of primordial germ cells (PGCs) toward their final destination in the gonads (Lees-Murdock and Walsh, 2008). Second, successive events of resetting of methylation patterns and de novo methylation occur after fertilization (Reik et al., 2001). During these two periods, avian methylomes are more prone to be modulated by environmental stimuli than later in life (Feil and Fraga, 2012), thus constituting specific windows of methylome sensitivity to environmental factors (Guerrero-Bosagna et al., 2018). Therefore, environmental stimuli occurring in these two periods have a more profound effect on animal phenotype than similar factors later in life. Moreover, during the perinatal period, embryo growth is very intense and is achieved through somatic cell division, during which epigenetic patterns are transmitted mitotically. Consequently, it can be assumed that all cells and subsequent tissues that originate from the initial cell will manifest the same epigenome because the epigenome is programmed and maintained in this cell population during its further differentiation. Furthermore, as the epigenome fine tunes the gene expression, the environmental epigenetic marks in the epigenome can significantly affect the developmental process (Skinner, 2011). For instance, it was found that the exposure of the embryo to divergent stress factors during its epigenetic reprogramming period results in a range of abnormal developmental outcomes. In mammals, some classical examples include “large offspring syndrome” that results from the application of assisted reproductive technologies, particularly in ruminants (Behboodi et al., 1995). In mouse models, experiments using the agouti viable yellow (Avy) mouse revealed that embryo culture from the zygote to the blastocyst stage caused a 3- to 4-fold change in the rate of reprogramming of CpG methylation of the agouti gene when compared with that in embryos subjected to embryo transfer without culture. Consequently, induced persistent epigenetic changes resulted in an altered postnatal phenotype, i.e., yellow coat (Morgan et al., 2008). In birds, embryo exposure to abiotic factors has been found to influence embryonic development and adult phenotype. For instance, poultry embryos exposed to different temperatures at the end of egg incubation period can lead to their better adaptation to later climatic conditions (Renaudeau et al., 2012). Moreover, it was found that the exposure of fertile eggs to green monochromatic LED light during embryogenesis has a growth-promoting effect on adult turkeys and broiler chickens (Rozenboim et al., 2003; Zhang et al., 2012). Although epigenetic processes were not directly investigated in these studies, they are potential candidates for mediating the described mechanisms.
NEW EPIGENETIC RESEARCH POSSIBILITIES USING PGCS
Embryonic cells undergo two stages of development, the first of which occurs during embryogenesis to form PGCs. The molecular basis of this process is very well understood in two species – Drosophila (Dansereau and Lasko, 2008) and Caenorhabditis elegans (Mainpal et al. 2015). Research on PGCs is needed to define the genetic and environmental basis and to define the genes and their activity necessary for their formation and migration. In mammals, knowledge in this area is gained from research performed on mice (Saitou and Yamaji, 2012), because such research has not been conducted on a large scale in humans due to technical and ethical reasons. In most multicellular organisms, reproductive cells ensure the preservation of genetic and epigenetic information over several generations.
Epigenetic modifications of the genome, such as DNA methylation and chromatin modifications, are relatively stable in somatic cells; however, in Amniote embryos (including all vertebrates), PGCs are distinguished early in the development as progenitors of the gametes. In chickens, these cells originate from the epiblast (Eyal-Giladi et al., 1981). They are located at the center of the area pellucida at stage X of Hamburger Hamilton and translocated anteriorly to the germinal crescent (Tagami and Kagami, 1998). After vascular circulation is established, they circulate temporarily through the bloodstream and migrate interstitially into the gonadal anlage (Szczerba et al., 2019), where they accumulate as gonadal germ cells and differentiate into spermatogonia in males or into oogonia in females.
This specific developmental stage of a chicken embryo enables the PGCs to be easily isolated from the blood (Kuwana, 1993) and the gonads (Nakajima et al., 2011). The isolation of cells from early donor embryos, their in vitro culture, and injection into recipient embryos lead to new research opportunities. Fresh, frozen, or thawed donor PGCs are transferred from one embryo to another to form functional germ cells in the recipient embryo (Yasuda et al., 1992; Chojnacka-Puchta et al., 2015; Sawicka et al., 2015). Consequently, germline chimera can be produced by germ cell transfer into a blood vessel (Han et al., 2002) and used as a model in the interaction studies of the donor–recipient cells. Moreover, the development of a precise method for bird PGC isolation (Nakajima et al., 2011) and a method for RNA isolation from single cells obtained from an embryo can allow to perform early molecular analysis and detection of transcriptome changes during embryonic development (Dunislawska et al., 2020b).
PGCs are potentially important in understanding the epigenetic regulatory mechanisms occurring in vertebrates, including humans. PGCs have their DNA methylation patterns precisely erased and de novo reestablished by the time of formation of a germline lineage. PGCs allow to study the complexity of DNA imprinting and to understand the mechanisms of a proper cell programming at the epigenetic level. The development of PGCs depends on a precise interplay of transcription factors (involving BMP4 and BLIMP1) that determine their normal differentiation into functional gametes. Thus, epigenetic reprogramming of PGCs is particularly significant for sex differentiation and to achieve adequate chromatin conditions for further embryo development (Cantão et al., 2017).
Thus far, many studies have investigated and described the epigenetic reprogramming of PGCs, especially in mice, which are considered as a model for humans due to similar epigenetic pattern. DNA methylation is an essential epigenetic control mechanism observed in vertebrates. During embryonic development, DNA methylation acts as a significant epigenetic barrier that guides and restricts cell differentiation, and thus prevents regression into an undifferentiated state. However, for sexual reproduction and the adoption of the hypomethylated PGC epigenome and the preimplantation embryo, DNA methylation marks should be removed (Messerschmidt et al., 2014).
The signals from the postgenital tissues induce a unique network of gene regulation in germline-competent cells for PGC specification. In addition, this network initiates a comprehensive reset of the epigenome, including global DNA demethylation and chromatin reorganization (Leitch et al., 2013). In mice, PGCs are significantly methylated before colonization of the gonads, which corresponds to the pattern in somatic cells, including the normal methylation pattern associated with the imprinted genes. Clearly, PGCs are not exempt from the extensive genomic de novo methylation that begins in the inner cells of the blastocyst cell mass. Analyses also show that methylation imprints are initially inherited and maintained in PGCs. Rapid demethylation occurs when PGCs enter the gonad. This rapid reprogramming is selective and affects only single and nonimprinted genes (Leitch et al., 2013). Dynamic changes in DNA methylation occur during the early developmental stages of reproductive sex cells. PGCs require a precise imprinting with proper epigenetic marks according to the sex, which ensures that the gestating embryo genes are programmed properly in a switch on/switch off mode. However, all the consequences of this epigenetic reprogramming are still not well understood (Maatouk et al., 2006). In mammals, DNA methylation reprogramming of PGCs enables monoallelic expression of imprinting genes, maintains retrotransposons in the inactive state, inactivates one of the X chromosomes, and suppresses gene expression.
Still, some fundamental questions remain to be answered: to identify molecular basis for germline competence, to identify the interplay of transcription factors in the maintenance of the germ cell fate, and to identify the epigenetic factors associated with PGC reprogramming. In vitro conditions for long-term maintenance and differentiation of human PGCs to the advanced gonadal stages are problematic and require further development (Tang et al., 2016). In chicken PGCs, however, these in vitro goals can be relatively easily reached. For example, Ji et al. (2018) successfully generated haploid spermatids from chicken PGCs. Thus far, several studies have been conducted to identify relationships between PGC epigenetics and cell fates. In this regard, the following scientific problems were reported: competence for a germline (mice and human; Tang et al., 2016), gamete generation (mice; Ohta et al., 2017), and consequences of PGC reprogramming (mice; Niemitz, 2013). Tang et al. (2016) identified the epigenetic marks associated with transition from naïve embryonic cells in programming of the human germ line - the accumulation of posttranslational monomethylation and acetylation of histones. Ohta et al. (2017) described the epigenetic mechanism required for PGCs to gain competence for female germ cell fate, which is manifested by demethylation in PGCs and induction of oocyte differentiation by BMP (bone morphogenetic protein) and RA (retinoic acid).
Very less data have been published on the role of methylation in chicken PGCs. By using expression microarray, 1.5-fold change in the expression of 261 PGC transcripts was demonstrated compared to that in embryonic chicken fibroblasts (Jang et al., 2013). In addition, 203 differentially methylated regions were detected within imprinting and X-linked homologous regions between male PGCs and female PGCs. These regions may be directly or indirectly associated with gene expression during early embryonic development, and the epigenetic difference between mammals and birds could be evolutionarily conserved.
An investigation by He et al. (2018) provided some interesting insights into the regulation patterns of DNA methylation during the differentiation of germline stem cells, especially into male germ cells. Three types of chick germ cells—embryonic stem cells, PGCs, and spermatogonial stem cells—were used in a study of epigenetic regulation mechanisms during spermatogenesis. The results showed that PGCs exhibited a higher level of genome-wide methylation than embryonic stem cells and spermatogonial stem cells during the differentiation of chick germ cells. The authors of the study concluded that multiple epigenetic events, including DNA methylation, histone modifications, and occurrence of noncoding RNAs, may act synergistically instead of a single regulation mode during embryonic development. Moreover, universal gene markers and unique chicken markers were discovered for identifying male germline stem cells.
Jiang et al. (2021) provided novel evidence for the regulation of PGC development by long-chain noncoding RNAs (lncRNAs), where histone acetylation increases the levels (upregulates expression) of lncPGCR. In another chicken study, Zuo et al. (2019) showed that the epigenetic posttranslational histone ubiquitination on Smad5 could be involved in reprogramming the recruitment of RA in promoting the differentiation of spermatogonial stem cells and inhibition of the BMP4 signal transduction (promoting the PGC state).
The study of Dunislawska et al. (2020b) was the first step toward understanding the epigenetic regulation of gene expression in gonadal PGCs during embryonic development of White Leghorn. Transcriptome dynamics of PGCs were analyzed at three key developmental stages: 4.5, 8, and 12 d of embryo incubation. A significant negative regulation of gene expression was demonstrated, which may be related to DNA methylation. On the basis of these data, a comparative transcriptome analysis of White Leghorn and Green-legged Partridgelike (GP) chicken gPGCs was performed. Differences between gPGCs of both genotypes were detected on d 8 of embryonic development. Epigenetic analysis involved measuring the level of global methylation and individual genes that were silenced. Global methylation analysis showed changes on d 8 of embryonic development. Methylation analysis of genes revealed the influence of sex and breed. The results suggest faster development of GP embryos than White Leghorn (WL). This allows to consider that changes can be determined by genetic and environmental factors (Dunislawska et al., 2021, unpublished data).
EPIGENETIC MODIFICATIONS DURING EMBRYONIC DEVELOPMENT
The environmental effect on epigenetic changes during embryonic development has been well documented (Li et al., 2016b). As mentioned earlier, the influence of maternal factors on the embryonic development of chicken is limited only to egg composition. In contrast, external factors such as temperature, humidity, and others (Grochowska et al., 2019) are controlled for a large number of, sometimes even several hundred thousand, embryos incubated under uniform environmental conditions. Thus, it is feasible to conduct studies on epigenetic modifications during embryonic development by using a bird model, especially studies on environmental factors with a large group or cohort.
Temperature Influence
Environmental factors such as temperature can affect embryo development into an adult phenotype. Epigenetic processes play an important role in mediating the thermal tolerance mechanisms. Numerous studies have reported the application of thermal manipulation for eggs during incubation to improve the performance, physiology, and metabolism of chickens as well as the regulation of gene expression (Loyau et al., 2015). Loyau et al. (2016) analyzed the long-term modification of physiological regulators induced by thermal manipulation (TM) during embryogenesis and the subsequent effect of heat challenge on the pectoralis major muscle of 34-d-old broilers. In the experimental conditions, TM broilers exhibited a greatly modified profile of overall muscle expression compared to controls, which was believed to be related to epigenetic modifications and active RNA splicing.
David et al. (2019) provided evidence of numerous molecular rearrangements on the DNA packaging histone protein H3, within the genes of TM broiler chickens, prenatally (at E7-16 d). They performed whole genome chromatin immunoprecipitation sequencing in hypothalamus and muscle tissue samples from 35-d-old chickens by using antibodies specific to H3K4me3 and H3K27me3 epigenetic marks. Importantly, the neurodevelopmental functions in the hypothalamus were mainly impacted by histone mark changes induced by TM. This finding confirms the important role of neurogenesis in adaptation to heat stress later in life.
Al-Zghoul et al. (2019) investigated the effects of embryonic TM on the levels of mRNA expression and the total antioxidant capacity of genes associated with heat-induced oxidative stress (NOX4, GpX2, SOD2, catalase, and AvUCP) in two breeds of broiler chicken. The results of the study suggested that embryonic TM has a long-lasting impact on the thermotolerance acquisition in chicken breeds. Embryonic TM may indeed improve thermotolerance acquisition in broiler chickens as shown by the mRNA expression levels of the genes associated with the redox pathway.
Al-Zghoul and Saleh (2020) performed a trial with broiler chickens (E8-16) subjected to TM that were challenged with a chronic heat stress later in life. The authors studied mRNA levels of selected proinflammatory cytokines, toll-like receptors, heat shock proteins, and antioxidant enzymes in the jejunal mucosae. They found reduced expression of antioxidant enzymes in heat-stressed chickens and significant changes in the mRNA levels of heat shock factors, potentially resulting from epigenetic rearrangements in TM embryos. In another important study on methylome, Corbett et al. (2020) identified changes in cardiac DNA methylation profiles that were associated with increased eggshell temperature (38.9°C from E8 onward) and varying CO2 incubation levels. First, the authors confirmed that the higher incubation temperature negatively affected the heart weight at hatch. The subsequent bisulfite sequencing and epigenome-wide association study revealed changes in cardiac methylation signatures that proved epigenetic impact of the eggshell temperature on heart development. Twenty-three CpG sites were identified, whose methylation was significantly associated with the heart weight, while the annotation of differentially methylated genes showed enrichment for heart-specific developmental processes.
Hatching studies on the level of DNA methylation and histones in chickens subjected to heat stress indicate that epigenetic features differ depending on the environment in which the chickens reside during the hatching period (Kisliouk and Meiri, 2009). It has been proved that the expression of the BDNF gene, which is the key regulator of thermal tolerance in the hippocampus and hypothalamus, differs between control group individuals and individuals accustomed to higher temperatures in the early stages after hatching. Changes in the methylation level of CpG sites and histone modifications in the BDNF gene promoter were observed during the acquisition of thermal tolerance on the third day after hatching (Kisliouk and Meiri, 2009; Kisliouk et al., 2011).
In Ovo Feeding and Stimulation
Another important research on epigenetic changes during embryo development is related to the modification of egg composition and its effect on methylation and gene expression in the offspring. Prenatal malnutrition caused, for example, by partial protein removal from eggs leads to long-term changes in chicken liver transcriptome (Willems et al., 2016). The effect of this malnutrition on embryo development was evaluated at the level of gene expression. Significant changes were observed in the expression of genes involved in the processes related to embryonic development and reproductive system development and functioning. In addition, molecular pathways such as metabolism of amino acids and carbohydrates and protein synthesis were changed. Three key genes—UBC, NR3C1, and ELAVL1—which interact with many other regulated genes were identified (Willems et al., 2016). Sun et al. (2018) demonstrated the role of in ovo zinc injection in enhancing the embryonic development in eggs from Zn-deficient hens through epigenetic and antioxidant mechanisms, and they concluded that organic Zn was more effective than inorganic Zn in enhancing DNA methylation and H3K9 acetylation in the liver MT4 promoter.
The methylation process is also influenced by many diet components such as selenium, folic acid, flavonoids, and probiotic bacteria (Jaenisch and Bird, 2003). Liu et al. (2016) showed that an injection of 150 μg folic acid on the 11th embryonic day of incubation could upregulate the expression of insulin-like growth factor 2 by modulating DNA hypomethylation and improving chromatin accessibility in the gene promoter region, thereby facilitating embryonic growth and organ development in broilers.
Similarly, vitamin C treatment by in ovo injection is believed to interfere with the epigenome reprogramming process in chicken embryos, regulate embryonic development, and improve the performance of broiler chickens. Zhu et al. (2019) found that in ovo injection of vitamin C at an embryonic age of 15 d promoted the expression of enzymes related to DNA methylation and reduced the expression of those related to DNA demethylation in the spleen of broilers.
In ovo administration of betaine was shown to regulate the hepatic metabolism of cholesterol in newly hatched chickens by epigenetic mechanisms such as DNA and histone methylation. Betaine administration led to changes in the methylation pattern and cholesterol homeostasis in chickens, which was reflected by prolonged deregulation of cholesterol metabolism in adult chickens (Hu et al., 2015).
Our many years of research shows the same trend for in ovo stimulation of the chicken embryo microbiome and its long-term postembryonic effect (Siwek et al., 2018). It was shown that in ovo administration of bioactive substances such as prebiotics or synbiotics on the 12th day of chicken embryo incubation directly affected the microbiota composition (Villaluenga et al., 2004; Pilarski et al., 2005; Bednarczyk et al., 2011, 2016; Dunislawska et al., 2017) and indirectly improved physiological (Brudnicki et al., 2015; Pruszynska-Oszmalek et al., 2015; Miśta et al., 2017; Kolodziejski et al., 2018; Stadnicka et al., 2020; Zhang et al., 2020), immunological (Madej et al., 2015; Madej and Bednarczyk, 2016; Stefaniak et al., 2019, 2020), and intestinal development (Bogucka et al., 2016, 2017; Sobolewska et al., 2017) and the performance traits of chickens (Bednarczyk et al., 2016; Maiorano et al., 2017; Tavaniello et al., 2019, 2020).
We and other research groups analyzed the molecular mechanisms that determine the phenotypic effects (Hu et al., 2015; Płowiec et al., 2015; Li et al., 2016a; Slawinska et al., 2016, 2019; Dunislawska et al., 2017, 2019, 2020a; Berrocoso et al., 2017; Kolodziejski et al., 2018; Zhu et al., 2019; Pietrzak et al., 2020; Zhang et al., 2020). To date, we have shown that in ovo-administered synbiotics significantly affect the regulation of gene expression of cytokines (IL-1β, IL-6, IL-18, IL-12p40, IFN-γ, and IFN-β) in the spleen and cecal tonsils of adult chickens. The signatures of immune-related gene expression were observed to depend on synbiotic composition, the genotype and age of chicken, and the type of tissue (Slawinska et al., 2014; Płowiec et al., 2015; Dunislawska et al., 2017). To further determine the molecular pathways underlying the immunomodulatory effects of the in ovo-delivered synbiotics, we analyzed the transcriptomic modulation in the spleen and cecal tonsils of adult chickens (Slawinska et al., 2016). The results showed that synbiotics triggered the expression of those genes that induce immune responses such as T lymphocyte activation, lymphocyte differentiation, cytokine production, and lymphocyte proliferation. In addition, a study reported that 5 mg doses of chitooligosaccharide delivered in ovo seemed to modulate the expression of genes related to intestinal immune responses in broiler chickens (Zhang et al., 2020). Similarly, Berrocoso et al. (2017) showed that the expression levels of CD3 and chB6, which are T cell and B cell marker genes, respectively, were significantly enhanced by in ovo injection of 4.5 mg of the prebiotic raffinose. These findings indicate that molecular tools are useful in predicting the effect of prebiotics and synbiotics administered in ovo on the immune functions of adult individuals.
In ovo administration of synbiotics during embryonic development has been shown to affect the methylation profile of individual immunological and metabolic genes in the liver. This effect is correlated with the silencing of gene expression (Dunislawska et al., 2020a). Epigenetic changes have also been proven to depend on the genotype and the substance administered in ovo. The administration of a prebiotic exerts a significant influence on the level of expression and methylation of individual genes in the spleen, while the administration of an exogenous dose of a probiotic does not show a significant effect. This finding suggests that the administration of an external dose of bacteria into the egg is not a strong environmental signal, as is the prebiotic itself. This sheds new light on the epigenetic nature of microbiota programming in poultry during embryonic development and host-microbiome interactions (Dunislawska et al., 2021, unpublished data).
Immunological Processes Underlying the Susceptibility of Chickens to Infection
DNA methylation is associated with the silencing of transcription, which leads to the etiology and pathogenesis of some diseases (Liu et al., 2016). Epigenetic mechanisms are involved in the immunological processes underlying the susceptibility of chickens to Salmonella infection (Gou et al., 2012) and to Marek's disease (Luo et al., 2012a, 2012b). In the study of the molecular mechanisms underlying the susceptibility to Salmonella infection, chickens were injected with Salmonella enteritidis. The birds susceptible to infection died within 5 d of injection. The number of bacteria in susceptible chickens was found to be significantly higher than that in resistant chickens, and the expression of TLR4, TLR2-1, and TLR21 (the TLR signaling pathways constitute the first line of defense against S. enteritidis infection) was significantly reduced in leukocytes of chickens susceptible to infection as compared to that in resistant chickens. DNA methylation in the TLR4 and TLR21 gene promoter region and in the CpG islands of the TLR2-1 gene was significantly higher in susceptible chickens than in resistant chickens (Gou et al., 2012).
Research on Marek's disease focuses mainly on the genetic differences between resistant and susceptible chickens. The epigenetic nature of this disease was examined on the basis of histone modification of the whole genome of three lines of chickens resistant and susceptible to Marek's virus. Tri-methylation in the enrichment of histone H3 Lys4 was found to positively correlate with the expression of genes encoding proteins as well as miRNA genes, while tri-methylation at histone H3 Lys27 showed a negative correlation. By identifying line-specific histone modifications in Marek's disease, the unique islands of H3K4me3 were found to be activated in disease-resistant chicken genes that are associated with immune response and cell adhesion. On the basis of these studies, it was found that internal epigenetic mechanisms may play a key role in resistance and susceptibility to Marek's disease (Luo et al., 2012a). Marek's virus can also induce changes in the expression level of all three genes in the DNMT methyltransferase family (DNMT1, DNMT3A, and DNMT3B). It has also been shown that some candidate genes have a higher level of promoter methylation in chickens susceptible to Marek's disease than in chickens resistant to the disease. Hypermethylated genes are involved mainly in the organization of cellular components and immunological processes and can play a key role in susceptibility to the disease by deregulating these genes (Luo et al., 2012b)
CONCLUSIONS
The phenotype of an individual is the result of complex interactions between genotype and current, past, and ancestral environment, leading to a lifelong remodeling of its epigenome (Jammes et al., 2011). Therefore, it is evident that genomic tools cannot explain the full heritable variation in the production traits of animals. Consideration of epigenetic regulation markers in animal selection could improve the phenotype prediction and enable a better evaluation of individual genitors.
There are several reasons why birds are an excellent model in epigenetic studies. An embryo of chicken or any bird develops external to the mother's body and can be easily manipulated in vivo and in vitro, thus serving as an excellent model for epigenetic studies. However, there is a lack of precise knowledge on the dynamics of the epigenetic reprogramming of PGCs and early embryo in birds. A new technique for isolating viable avian PGCs combined with a method for RNA isolation from a single cell could represent a unique approach for such studies. This opens up interesting research opportunities, given that in chicken, as in other vertebrates, important epigenetic reprogramming and DNA methylome reprogramming events in particular occur during gametogenesis and early embryo development.
Moreover, in ovo embryo stimulation with various bioactive substances, a technique that has been intensively developed in recent years, provides new evidence for the epigenetic impact of environmental factors on embryonic and postembryonic development of a chicken. Currently, research on epigenetics is driven by a massive amount of up-to-date information obtained through next-generation sequencing (NGS) methods. This applies particularly to the modern NGS assay-based multiomics approach such as whole-genome or reduced representation bisulfite sequencing, total RNA sequencing, and chromatin immunoprecipitation sequencing, which is used to identify epigenetic marks such as DNA methylation and histone modifications and to analyze the regulation of gene expression by noncoding RNAs. This approach enables to effectively analyze the biological processes, including epigenetic mechanisms, compared to that performed using a single omics tool. In the current postgenomic era, the development of the state-of-the-art high-throughput NGS assays will facilitate the identification of epigenetic signatures in multiple layers of genome organization (histone modifications, DNA methylation, and transcription of noncoding RNAs), thereby shifting the scope of experiments from a single gene or transcript to the level of whole genome, transcriptome, and methylome.
To date, different developmental epigenetic patterns have been studied in various chicken types (Hu et al., 2013; Tian et al., 2013; Bélteky et al., 2018; Dunislawska et al., 2021, unpublished data); these studies have proved that the chicken transcriptome could be reprogrammed by manipulation of different environmental factors during early embryogenesis. In particular, in ovo treatment with carefully selected bioactives proved to be effective in modulating gene expression changes in a range of chicken tissues, and some of these changes had epigenetic characteristics.
One of the fundamental questions when studying epigenetics in a poultry breeding context is whether and how epigenetic marks can be inherited through the germ line and whether selection can act on this variation directly. We believe that further research will fill this knowledge gap and lead to the identification of new epigenetic markers to improve the accuracy of selection in terms of current and new traits, including disease resistance.
ACKNOWLEDGMENTS
This study was supported by the National Science Center (NSC) in Cracow, Poland (Grant Nos. UMO-2017/25/N/NZ9/01822 and UMO-2017/27/B/NZ9/01510).
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- Al-Zghoul M.B., Mohammad Saleh K.M. Effects of thermal manipulation of eggs on the response of jejunal mucosae to posthatch chronic heat stress in broiler chickens. Poult. Sci. 2020;99:2727–2735. doi: 10.1016/j.psj.2019.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Zghoul M.B., Sukker H., Ababneh M.M. Effect of thermal manipulation of broilers embryos on the response toheat-induced oxidative stress. Poult. Sci. 2019;98:991–1001. doi: 10.3382/ps/pey379. [DOI] [PubMed] [Google Scholar]
- Bannister A.J., Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarczyk M., Stadnicka K., Kozłowska I., Abiuso C., Tavaniello S., Dankowiakowska A., Sławińska A., Maiorano G. Influence of different prebiotics and mode of their administration on broiler chicken performance. Animal. 2016:1271–1279. doi: 10.1017/S1751731116000173. [DOI] [PubMed] [Google Scholar]
- Bednarczyk M., Urbanowski M., Gulewicz P., Kasperczyk K., Maiorano G., Szwaczkowski T. Field and in vitro study on prebiotic effect of raffinose family oligosaccharides in chickens. Bull. Vet. Inst. Pulawy. 2011;55:465–469. [Google Scholar]
- Belecky-Adams T., T. Haynes, J. M. Wilson, and K. Del Rio-Tsonis. 2008. The Chick as A Model for Retina Development and Regeneration. Pages 102–119 in Animal Models in Eye Research. Elsevier Publisher, Amsterdam, Netherlands.
- Bélteky J., Agnvall B., Bektic L., Höglund A., Jensen P., Guerrero Bosagn a C. Epigenetics and early domestication: differences in hypothalamic DNA methylation between red junglefowl divergently selected for high or low fear of humans. Genet Sel Evol. 2018;50:13. doi: 10.1186/s12711-018-0384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrocoso J.D., Kida R., Singh A.K., Kim Y.S., Jha R. Effect of in ovo injection of raffinose on growth performance and gut health parameters of broiler chicken. Poult. Sci. 2017;96:1573–1580. doi: 10.3382/ps/pew430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogucka J., Dankowiakowska A., Elminowska-Wenda G., Sobolewska A., Jankowski J., Szpinda M., Bednarczyk M. Performance and small intestine morphology and ultrastructure of male broilers injected in ovo with bioactive substances. Ann. Anim. Sci. 2017;17:179–195. [Google Scholar]
- Bogucka J., Dankowiakowska A., Elminowska-wenda G., Sobolewska A., Szczerba A., Bednarczyk M. Effects of prebiotics and synbiotics delivered in ovo on broiler small intestine histomorphology during the first days after hatching. Folia Biol. (Cracow) 2016;64:131–143. doi: 10.3409/fb64_3.131. [DOI] [PubMed] [Google Scholar]
- Brudnicki A., Szymeczko R., Bednarczyk M., Głowińska B., Pietruszyńska D. Assessment of the effect of alpha-galactosides on yolk sac resorption rate in broiler chickens. J. Anim. Plant Sci. 2015;25:387–391. [Google Scholar]
- Burggren W.W. Dynamics of epigenetic phenomena: intergenerational and intragenerational phenotype “washout. J. Exp. Biol. 2015;218:80–87. doi: 10.1242/jeb.107318. [DOI] [PubMed] [Google Scholar]
- Behboodi E., Anderson G.B., BonDurant R.H., Cargill S.L., Kreuscher B.R., Medrano J.F., Murray J.D. Birth of large calves that developed from in vitro-derived bovine embryos. Theriogenology. 1995;44:227–232. doi: 10.1016/0093-691x(95)00172-5. [DOI] [PubMed] [Google Scholar]
- Burt D.W. Chicken genome: Current status and future opportunities. Genome Res. 2005;15:1692–1698. doi: 10.1101/gr.4141805. [DOI] [PubMed] [Google Scholar]
- Burt D., Pourquie O. Chicken genome - Science nuggets to come soon. Science. 2003;300:1669. doi: 10.1126/science.1086231. [DOI] [PubMed] [Google Scholar]
- Cavalli G., Paro R. The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell. 1998;93:505–518. doi: 10.1016/s0092-8674(00)81181-2. [DOI] [PubMed] [Google Scholar]
- Cantão I.H., Tesser R.B., Stumpp T. An initial investigation of an alternative model to study rat primordial germ cell epigenetic reprogramming. Biol. Proced. 2017;19:9. doi: 10.1186/s12575-017-0058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlberg C., Molnár F. Human Epigenomics. Springer; Singapore: 2018. The Histone Code. [Google Scholar]
- Chojnacka-Puchta L., Sawicka D., Lakota P., Plucienniczak G., Bednarczyk M., Plucienniczak A. Obtaining chicken primordial germ cells used for gene transfer: in vitro and in vivo results. J. Appl. Genet. 2015;56:493–504. doi: 10.1007/s13353-015-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogburn L.A., Wang X., Carre W., Rejto L., Porter T.E., Aggrey S.E., Simon J. Systems-wide chicken DNA microarrays, gene expression profiling, and discovery of functional genes. Poult. Sci. 2003;82:939–951. doi: 10.1093/ps/82.6.939. [DOI] [PubMed] [Google Scholar]
- Corbett R.J., Te Pas M., van den Brand H., Groenen M., Crooijmans R., Ernst C.W., Madsen O. Genome-wide assessment of DNA methylation in chicken cardiac tissue exposed to different incubation temperatures and CO2 levels. Front. Genet. 2020;11 doi: 10.3389/fgene.2020.558189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell D.K., Schoenwolf G.C. The chick embryo as a model system for analyzing mechanisms of development. Methods Mol. Biol. 2000;135:25–29. doi: 10.1385/1-59259-685-1:25. [DOI] [PubMed] [Google Scholar]
- Dansereau D.A., Lasko P. The development of germline stem cells in Drosophila. Methods Mol Biol. 2008;450:3–26. doi: 10.1007/978-1-60327-214-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S.A., Carvalho A.V., Gimonnet C., Brionne A., Hennequet-Antier C., Piégu B., Crochet S., Couroussé N., Bordeau T., Bigot Y., Collin A., Coustham V. Thermal manipulation during embryogenesis impacts H3K4me3 and H3K27me3 histone marks in chicken hypothalamus. Front. Genet. 2019;10:1207. doi: 10.3389/fgene.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunislawska A., Slawinska A., Siwek M. Hepatic DNA methylation in response to early stimulation of microbiota with lactobacillus synbiotics in broiler chickens. Genes. 2020;11:579. doi: 10.3390/genes11050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunislawska A., Slawinska A., Stadnicka K., Bednarczyk M., Gulewicz P., Jozefiak D., Siwek M. Synbiotics for broiler chickens—in vitro design and evaluation of the influence on host and selected microbiota populations following in ovo delivery. PLoS One. 2017;12 doi: 10.1371/journal.pone.0168587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunislawska A., Slawinska A., Bednarczyk M., Siwek M. Transcriptome modulation by in ovo delivered lactobacillus synbiotics in a range of chicken tissues. Gene. 2019;698:27–33. doi: 10.1016/j.gene.2019.02.068. [DOI] [PubMed] [Google Scholar]
- Dunislawska A., Szczerba A., Siwek M., Bednarczyk M. Dynamics of the transcriptome during chicken embryo development based on primordial germ cells. BMC Res. Notes. 2020;13:441. doi: 10.1186/s13104-020-05286-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyal-Giladi H., Ginsburg M., Farbarov A. Avian primordial germ cells are of epiblastic origin. J. Embryol. Exp. Morphol. Vol. 1981;65:139–147. [PubMed] [Google Scholar]
- Feil R., Fraga M.F. Epigenetics and the environment: Emerging patterns and implications. Nat. Rev. Genet. 2000;13:97–109. doi: 10.1038/nrg3142. [DOI] [PubMed] [Google Scholar]
- Gou Z., Liu R., Zhao G., Zheng M., Li P., Wang H., Zhu Y., Chen J., Wen J. Epigenetic modification of TLRs in leukocytes is associated with increased susceptibility to Salmonella enteritidis in chickens. PLoS One. 2012;7:e33627. doi: 10.1371/journal.pone.0033627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochowska E., Kinal A., Sobek Z., Siatkowski I., Bednarczyk M. Field study on the factors affecting egg weight loss, early embryonic mortality, hatchability, and chick mortality with the use of classification tree technique. Poult. Sci. 2019;98:3626–3636. doi: 10.3382/ps/pez180. [DOI] [PubMed] [Google Scholar]
- Guerrero-Bosagna C., Morisson M., Liaubet L., Rodenburg T.B., de Haas E.N., Košťál Ľ., Pitel F. Transgenerational epigenetic inheritance in birds. Environ. Epigenetics. 2018;4:1–8. doi: 10.1093/eep/dvy008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. Dev. Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Han J.Y., Park T.S., Hong Y.H., Jeong D.K., Kim J.N., Kim K.D., Lim J.M. Production of germline chimeras by transfer of chicken gonadal primordial germ cells maintained in vitro for an extended period. Theriogenology. 2002;58:1531–1539. doi: 10.1016/s0093-691x(02)01061-0. [DOI] [PubMed] [Google Scholar]
- He Y., Zuo Q., Edwards J., Zhao K., Lei J., Cai W., Nie Q., Li B., Song J. DNA methylation and regulatory elements during chicken germline stem cell differentiation. Stem Cell Rep. 2018;10:1793–1806. doi: 10.1016/j.stemcr.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Sun Q., Li X., Wang M., Cai D., Li X., Zhao R. In ovo injection of betaine affects hepatic cholesterol metabolism through epigenetic gene regulation in newly hatched chicks. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Xu H., Li Z., Zheng X., Jia X., Nie Q., Zhang X. Comparison of the genome-wide DNA methylation profiles between fast-growing and slow-growing broilers. PLoS One. 2013;8:e56411. doi: 10.1371/journal.pone.0056411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov M., Kacevska M., Ingelman-Sundberg M. Epigenomics and interindividual differences in drug response. Clin. Pharmacol. Ther. 2012;92:727–736. doi: 10.1038/clpt.2012.152. [DOI] [PubMed] [Google Scholar]
- Jammes H., Junien C., Chavatte-Palmer P. Epigenetic control of development and expression of quantitative traits. Reprod Fertil Dev. 2011;23:64–74. doi: 10.1071/RD10259. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jang H.J., Seo H.W., Lee B.R., Yoo M., Womack J.E., Han J.Y. Gene expression and DNA methylation status of chicken primordial germ cells. Mol. Biotechnol. 2013;54:177–186. doi: 10.1007/s12033-012-9560-5. [DOI] [PubMed] [Google Scholar]
- Ji M., Tang S., Pei W., Ning M., Ma Y., Li X., Guan W. Generation of haploid spermatids from chicken embryonal primordial germ cells. Int. J. Mol. Med. 2018;42:53–60. doi: 10.3892/ijmm.2018.3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Chen C., Cheng S., Yuan X., Jin J., Zhang C., Sun X., Song J., Zuo Q., Zhang Y., Chen G., Bichun L. Long noncoding RNA LncPGCR mediated by TCF7L2 regulates primordial germ cell formation in chickens. Animals. 2021;11:292. doi: 10.3390/ani11020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain K.H., Miller J.W.I., Jones-Paris C.R., Thomason R.T., Lewis J.D., Bader D.M., Barnett J.V., Zijlstra A. The chick embryo as an expanding experimental model for cancer and cardiovascular research. Dev. Dyn. 2014;243:216–228. doi: 10.1002/dvdy.24093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisliouk T., Meiri N. A critical role for dynamic changes in histone H3 methylation at the Bdnf promoter during postnatal thermotolerance acquisition. Eur. J. Neurosci. 2009;30:1909–1922. doi: 10.1111/j.1460-9568.2009.06957.x. [DOI] [PubMed] [Google Scholar]
- Kisliouk T., Yosefi S., Meiri N. MiR-138 inhibits EZH2 methyltransferase expression and methylation of histone H3 at lysine 27, and affects thermotolerance acquisition. Eur. J. Neurosci. 2011;33:224–235. doi: 10.1111/j.1460-9568.2010.07493.x. [DOI] [PubMed] [Google Scholar]
- Kolodziejski P.A., Sassek M., Chalupka D., Leciejewska N., Nogowski L., Mackowiak P., Jozefiak D., Stadnicka K., Siwek M., Bednarczyk M., Szwaczkowski T., Pruszynska-Oszmalek E. GLP1 and GIP are involved in the action of synbiotics in broiler chickens. J. Anim. Sci. Biotechnol. 2018;9:13. doi: 10.1186/s40104-017-0227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwana T. Migration of avian primordial germ cells toward the gonadal anlage. Dev. Growth Differ. 1993;35:237–243. doi: 10.1111/j.1440-169X.1993.00237.x. [DOI] [PubMed] [Google Scholar]
- Lees-Murdock D.J., Walsh C.P. DNA methylation reprogramming in the germ line. Epigenetics. 2008;3:5–13. doi: 10.4161/epi.3.1.5553. [DOI] [PubMed] [Google Scholar]
- Li Q., Li N., Hu X., Li J., Du Z., Chen L., Yin G., Duan J., Zhang H., Zhao Y., Wang J., Li N. Genome-Wide Mapping of DNA Methylation in Chicken (P-A Defossez, Ed.) PLoS One. 2011;6:e19428. doi: 10.1371/journal.pone.0019428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhi L., Shen J., Li S., Yao J., Yang X. Effect of in ovo folic acid injection on hepatic IGF2 expression and embryo growth of broilers. J Anim Sci Biotechnol. 2016;7:40. doi: 10.1186/s40104-016-0099-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch H.G., Tang W.W.C., Surani M.A. 2013. Primordial germ-cell development and epigenetic reprogramming in mammals. Pages 149-187 in Current Topics in Developmental Biology. Academic Press, Burlington, VT. [DOI] [PubMed] [Google Scholar]
- Li S., Zhi L., Liu Y., Shen J., Liu L., Yao J., Yang X. Effect of in ovo feeding of folic acid on the folate metabolism, immune function and epigenetic modification of immune effector molecules of broiler. Br. J. Nutr. 2016;115:411–421. doi: 10.1017/S0007114515004511. [DOI] [PubMed] [Google Scholar]
- Li S., Zhu Y., Zhi L., Han X., Shen J., Liu Y., Yao J., Yang X. DNA methylation variation trends during the embryonic development of chicken. PLoS One. 2016;11 doi: 10.1371/journal.pone.0159230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyau T., Hennequet-Antier C., Coustham V., Berri C., Leduc M., Crochet S., Sannier M., Duclos M.J., Mignon-Grasteau S., Tesseraud S., Brionne A., Métayer-Coustard S., Moroldo M., Lecardonnel J., Martin P., Lagarrigue S., Yahav S., Collin A. Thermal manipulation of the chicken embryo triggers differential gene expression in response to a later heat challenge. BMC Genomics. 2016;17:329. doi: 10.1186/s12864-016-2661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyau T., Bedrani L., Berri C., Métayer-Coustard S., Praud C., Coustham V., Mignon-Grasteau S., Duclos M.J., Tesseraud S., Rideau N., Hennequet-Antier C., Everaert N., Yahav S., Collin A. Cyclic variations in incubation conditions induce adaptive responses to later heat exposure in chickens: a review. Animal. 2015;9:76–85. doi: 10.1017/S1751731114001931. [DOI] [PubMed] [Google Scholar]
- Luo J., Mitra A., Tian F., Chang S., Zhang H., Cui K., Yu Y., Zhao K., Song J. Histone methylation analysis and pathway predictions in chickens after MDV infection. PLoS One. 2012;7:e41849. doi: 10.1371/journal.pone.0041849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J., Yu Y., Chang S., Tian F., Zhang H., Song J. DNA methylation fluctuation induced by virus infection differs between MD-resistant and -susceptible chickens. Front. Genet. 2012;3:20. doi: 10.3389/fgene.2012.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan H.D., Jin X.L., Li A., Whitelaw E., O'Neill C. The culture of zygotes to the blastocyst stage changes the postnatal expression of an epigentically labile allele, agouti viable yellow, in mice. Biol. Reprod. 2008;79:618–623. doi: 10.1095/biolreprod.108.068213. [DOI] [PubMed] [Google Scholar]
- Maatouk D.M., Kellam L.D., Mann M.R.W., Lei H., Li E., Bartolomei M.S., Resnick J.L. DNA methylation is a primary mechanism for silencing postmigratory primordial germ cell genes in both germ cell and somatic cell lineages. Development. 2006;133:3411–3418. doi: 10.1242/dev.02500. [DOI] [PubMed] [Google Scholar]
- Madej J.P., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on the morphology and specific immune cell composition in the gut-associated lymphoid tissue. Poult. Sci. 2016;95:19–29. doi: 10.3382/ps/pev291. [DOI] [PubMed] [Google Scholar]
- Madej J.P., Stefaniak T., Bednarczyk M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chickens. Poult. Sci. 2015;94:1209–1219. doi: 10.3382/ps/pev076. [DOI] [PubMed] [Google Scholar]
- Mainpal R., Nance J., Yanowitz J.L. A germ cell determinant reveals parallel pathways for germ line development in Caenohabditis elegans. Development. 2015;142:3571–3581. doi: 10.1242/dev.125732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiorano G., Stadnicka K., Tavaniello S., Abiuso C., Bogucka J., Bednarczyk M. In ovo validation model to assess the efficacy of commercial prebiotics on broiler performance and oxidative stability of meat. Poult. Sci. 2017;96:511–518. doi: 10.3382/ps/pew311. [DOI] [PubMed] [Google Scholar]
- Messerschmidt D.M., Knowles B.B., Solter D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014;28:812–828. doi: 10.1101/gad.234294.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miśta D., Króliczewska B., Pecka-Kiełb E., Kapuśniak V., Zawadzki W., Graczyk S., Kowalczyk A., Łukaszewicz E., Bednarczyk M. Effect of in ovo injected prebiotics and synbiotics on the caecal fermentation and intestinal morphology of broiler chickens. Anim. Prod. Sci. 2017;57:1884–1892. [Google Scholar]
- Nakajima Y., Minematsu T., Naito M., Tajima A. A new method for isolating viable gonadal germ cells from 7-day-old chick embryos. J. Poult. Sci. 2011;48:106–111. [Google Scholar]
- Niemitz E. Primordial germ cell reprogramming. Nat Genet. 2013;45:123. [Google Scholar]
- Ohta H.M.H., Nagaoka S., Nakaki F., Sasaki K., Hayashi K., Yabuta Y., Nakamura T., Yamamoto T., Saitou M. Bone morphogenetic protein and retinoic acid synergistically specify female germ-cell fate in mice. EMBO J. 2017;36:3100–3119. doi: 10.15252/embj.201796875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez M.F., Lehner B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 2019;21:143–151. doi: 10.1038/s41556-018-0242-9. [DOI] [PubMed] [Google Scholar]
- Pietrzak E., Dunislawska A., Siwek M., Zampiga M., Sirri F., Meluzzi A., Tavaniello S., Maiorano G., Slawinska A. Splenic gene expression signatures in slow-growing chickens stimulated in ovo with galactooligosaccharides and challenged with heat. Animals. 2020;10:474. doi: 10.3390/ani10030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilarski R., Bednarczyk M., Lisowski M., Rutkowski A., Bernacki Z., Wardeńska M., Gulewicz K. Assessment of the effect of alpha-galactosides injected during embryogenesis on selected chicken traits. Folia Biol. (Cracow). 2005;53:13–20. doi: 10.3409/1734916054663474. [DOI] [PubMed] [Google Scholar]
- Płowiec A., Sławińska A., Siwek M.Z., Bednarczyk M.F. Effect of in ovo administration of inulin and Lactococcus lactis on immune-related gene expression in broiler chickens. Am. J. Vet. Res. 2015;76:975–982. doi: 10.2460/ajvr.76.11.975. [DOI] [PubMed] [Google Scholar]
- Pruszynska-Oszmalek E., Kolodziejski P.A., Stadnicka K., Sassek M., Chalupka D., Kuston B., Nogowski L., Mackowiak P., Maiorano G., Jankowski J., Bednarczyk M. In ovo injection of prebiotics and synbiotics affects the digestive potency of the pancreas in growing chickens. Poult. Sci. 2015;94:1909–1916. doi: 10.3382/ps/pev162. [DOI] [PubMed] [Google Scholar]
- Rashidi H., Sottile V. The chick embryo: hatching a model for contemporary biomedical research. BioEssays. 2009;31:459–465. doi: 10.1002/bies.200800168. [DOI] [PubMed] [Google Scholar]
- Reik W., Dean W., Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- Renaudeau D., Collin A., Yahav S., De Basilio V., Gourdine J.L., Collier R.J. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal. 2012;5:707–728. doi: 10.1017/S1751731111002448. [DOI] [PubMed] [Google Scholar]
- Rozenboim I., Huisinga R., Halevy O., El Halawani M.E. Effect of embryonic photostimulation on the posthatch growth of Turkey poults. Poult. Sci. 2003;82:1181–1187. doi: 10.1093/ps/82.7.1181. [DOI] [PubMed] [Google Scholar]
- Ribatti D. Chicken chorioallantoic membrane angiogenesis model. Methods Mol. Biol. 2012;843:47–57. doi: 10.1007/978-1-61779-523-7_5. [DOI] [PubMed] [Google Scholar]
- Skinner M.K. Role of epigenetics in developmental biology and transgenerational inheritance. Birth Defects Res. Part C - Embryo Today Rev. 2011;93:51–55. doi: 10.1002/bdrc.20199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C.D. The Chick: A Great Model System Becomes Even Greater. Dev. Cell. 2005;8:9–17. doi: 10.1016/j.devcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Saitou M., Yamaji M. Primordial germ cells in mice. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicka D., Chojnacka-Puchta L., Zielinski M., Plucienniczak G., Plucienniczak A., Bednarczyk M. Flow cytometric analysis of apoptosis in cryoconserved chicken primordial germ cells. Cell. Mol. Biol. Lett. 2015;20:143–159. doi: 10.1515/cmble-2015-0005. [DOI] [PubMed] [Google Scholar]
- Shen L., Waterland R.A. Methods of DNA methylation analysis. Curr. Opin. Clin. Nutr. Metab. Care. 2007;10:576–581. doi: 10.1097/MCO.0b013e3282bf6f43. [DOI] [PubMed] [Google Scholar]
- Siwek M., Slawinska A., Stadnicka K., Bogucka J., Dunislawska A., Bednarczyk M. Prebiotics and synbiotics – in ovo delivery for improved lifespan condition in chicken. BMC Vet. Res. 2018;14:402. doi: 10.1186/s12917-018-1738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M.K. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod. Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A., Dunislawska A., Plowiec A., Radomska M., Lachmanska J., Siwek M., Tavaniello S., Maiorano G. Modulation of microbial communities and mucosal gene expression in chicken intestines after galactooligosaccharides delivery In Ovo (S-B Wu, Ed.) PLoS One. 2019;14 doi: 10.1371/journal.pone.0212318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A., Plowiec A., Siwek M., Jaroszewski M., Bednarczyk M. Long-term transcriptomic effects of prebiotics and synbiotics delivered in ovo in broiler chickens. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska A., Siwek M.Z., Bednarczyk M.F. Effects of synbiotics injected in ovo on regulation of immune-related gene expression in adult chickens. Am. J. Vet. Res. 2014;75:997–1003. doi: 10.2460/ajvr.75.11.997. [DOI] [PubMed] [Google Scholar]
- Sobolewska A., Bogucka J., Dankowiakowska A., Elminowska-Wenda G., Stadnicka K., Bednarczyk M. The impact of synbiotic administration through in ovo technology on the microstructure of a broiler chicken small intestine tissue on the 1st and 42nd day of rearing. J. Anim. Sci. Biotechnol. 2017;8:61. doi: 10.1186/s40104-017-0193-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnicka K., Bogucka J., Stanek M., Graczyk R., Krajewski K., Maiorano G., Bednarczyk M. Injection of raffinose family oligosaccharides at 12 days of egg incubation modulates the gut development and resistance to opportunistic pathogens in broiler chickens. Animals. 2020;10:592. doi: 10.3390/ani10040592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak T., Madej J.P., Graczyk S., Siwek M., Łukaszewicz E., Kowalczyk A., Sieńczyk M., Bednarczyk M. Selected prebiotics and synbiotics administered in ovo can modify innate immunity in chicken broilers. BMC Vet. Res. 2019;15:105. doi: 10.1186/s12917-019-1850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefaniak T., Madej J.P., Graczyk S., Siwek M., Łukaszewicz E., Kowalczyk A., Sieńczyk M., Maiorano G., Bednarczyk M. Impact of prebiotics and synbiotics administered in ovo on the immune response against experimental antigens in chicken broilers. Animals. 2020;10:643. doi: 10.3390/ani10040643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Lu L., Liao X., Zhang L., Lin X., Luo X., Ma Q. Effect of in ovo zinc injection on the embryonic development and epigenetics-related indices of zinc-deprived broiler breeder eggs. Biol. Trace Elem. Res. 2018;185:456–464. doi: 10.1007/s12011-018-1260-y. [DOI] [PubMed] [Google Scholar]
- Szczerba A., Kuwana T., Bednarczyk M. The developmental changes in the extra-embryonic vascular system in the circulating phase of primordial germ cells in aves. Folia Biol. (Cracow) 2019;67:79–84. [Google Scholar]
- Tagami T., Kagami H. Developmental origin of avian primordial germ cells and its unique differentiation in the gonads of mixed-sex chimeras. Mol. Reprod. Dev. 1998;50:370–376. doi: 10.1002/(SICI)1098-2795(199807)50:3<370::AID-MRD14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Taganov K.D., Boldin M.P., Baltimore D. MicroRNAs and immunity: tiny players in a big field. Immunity. 2007;26:133–137. doi: 10.1016/j.immuni.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Tang W., Kobayashi T., Irie N., Dietmann S., Surani M.A. Specification and epigenetic programming of the human germ line. Nat Rev Genet. 2016;17:585–600. doi: 10.1038/nrg.2016.88. [DOI] [PubMed] [Google Scholar]
- Tavaniello S., Slawinska A., Prioriello D., Petrecca V., Bertocchi M., Zampiga M., Salvatori G., Maiorano G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020;99:612–619. doi: 10.3382/ps/pez556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavaniello S., Mucci R., Stadnicka K., Acaye O., Bednarczyk M., Maiorano G. Effect of in ovo administration of different synbiotics on carcass and meat quality traits in broiler chickens. Poult. Sci. 2019;98:464–472. doi: 10.3382/ps/pey330. [DOI] [PubMed] [Google Scholar]
- Tian F., Zhan F., VanderKraat s N.D., Hiken J.F., Edwards J.R., Zhang H., Zhao K., Song J. DNMT gene expression and methylome in Marek's disease resistant and susceptible chickens prior to and following infection by MDV. Epigenetics. 2013;8:85–98. doi: 10.4161/epi.24361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan N., Katari S., Coutifaris C., Sapienza C. Explaining inter-individual variability in phenotype: Is epigenetics up to the challenge? Epigenetics. 2010;5:16–19. doi: 10.4161/epi.5.1.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara M.N., Canto-Soler M.V. Rediscovering the chick embryo as a model to study retinal development. Neural Dev. 2012;7:1–19. doi: 10.1186/1749-8104-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vali N. The Japanese quail: a review. Int. J. Poult. Sci. 2008;7:925–931. [Google Scholar]
- Villaluenga C.M., Wardeńska M., Pilarski R., Bednarczyk M., Gulewicz K. Utilization of the chicken embryo model for assessment of biological activity of different oligosaccharides. Folia Biol. (Cracow). 2004;52:135–142. doi: 10.3409/1734916044527502. [DOI] [PubMed] [Google Scholar]
- Vitorino Carvalho A., Hennequet-Antier C., Crochet S., Bordeau T., Couroussé N., Cailleau-Audouin E., Chartrin P., Darras V.M., Zerjal T., Collin A., Coustham V. Embryonic thermal manipulation has short and long-term effects on the development and the physiology of the Japanese quail. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddington, C. H. 1968. The basic ideas of biology. Pages 1–32 in Towards a Theoretical Biology. Edinburgh University Press, Edinburgh, Scotland.
- Wallis J.W., Aerts J., Groenen M.A., Crooijmans R.P., Layman D., Graves T.A., Scheer D.E., Kremitzki C., Fedele M.J., Mudd N.K., Cardenas M., Higginbotham J., Carter J., McGrane R., Gaige T., Mead K., Walker J., Albracht D., Davito J., Yang S.P., Leong S., Chinwalla A., Sekhon M., Wylie K., Dodgson J., Romanov M.N., Cheng H., de Jong P.J., Osoegawa K., Nefedov M., Zhang H., McPherson J.D., Krzywinski M., Schein J., Hillier L., Mardis E.R., Wilson R.K., Warren W.C. A physical map of the chicken genome. Nature. 2004;432:761–764. doi: 10.1038/nature03030. [DOI] [PubMed] [Google Scholar]
- Weeke-Klimp A., Bax N.A.M., Bellu A.R., Winter E.M., Vrolijk J., Plantinga J., Maas S., Brinker M., Mahtab E.A.F., Gittenberger-de Groot A.C., van Luyn M.J.A., Harmsen M.C., Lie-Venema H. Epicardium-derived cells enhance proliferation, cellular maturation and alignment of cardiomyocytes. J. Mol. Cell. Cardiol. 2010;49:606–616. doi: 10.1016/j.yjmcc.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Willems E., Guerrero-Bosagna C., Decuypere E., Janssens S., Buyse J., Buys N., Jensen P., Everaert N. Differential expression of genes and DNA methylation associated with prenatal protein undernutrition by Albumen Removal in an avian model. Sci. Rep. 2016;6 doi: 10.1038/srep20837. 20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenzycki C., Niemann H. Epigenetic reprogramming in early embryonic development: effects of in-vitro production and somatic nuclear transfer. Reprod. Biomed. Online. 2003;7:649–656. doi: 10.1016/s1472-6483(10)62087-1. [DOI] [PubMed] [Google Scholar]
- Wu Ct., Morris J.R. Genes, genetics, and epigenetics: a correspondence. Science. 2001;293:1103–1105. doi: 10.1126/science.293.5532.1103. [DOI] [PubMed] [Google Scholar]
- Yasuda Y., Tajima A., Fujimoto T., Kuwana T. A method to obtain avian germ-line chimaeras using isolated primordial germ cells. J. Reprod. Fertil. 1992;96:521–528. doi: 10.1530/jrf.0.0960521. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang H.J., Qiao X., Yue H.Y., Wu S.G., Yao J.H., Qi G.H. Effect of monochromatic light stimuli during embryogenesis on muscular growth, chemical composition, and meat quality of breast muscle in male broilers. Poult. Sci. 2012;91:1026–1031. doi: 10.3382/ps.2011-01899. [DOI] [PubMed] [Google Scholar]
- Zhang J., Cai K., Mishra R., Jha R. In ovo supplementation of chitooligosaccharide and chlorella polysaccharide affects cecal microbial community, metabolic pathways, and fermentation metabolites in broiler chickens. Poult. Sci. 2020;99:4776–4785. doi: 10.1016/j.psj.2020.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.F., Li S.Z., Sun Q.Z., Yang X.J. Effect of in ovo feeding of vitamin C on antioxidation and immune function of broiler chickens. Animal. 2019;13:1927–1933. doi: 10.1017/S1751731118003531. [DOI] [PubMed] [Google Scholar]
- Zuo Q., Jin J., Jin K., Sun C., Song J., Yani Z., Chen G., Li B. Distinct roles of retinoic acid and BMP4 pathways in the formation of chicken primordial germ cells and spermatogonial stem cells. Food Funct. 2019;10:7152–7163. doi: 10.1039/c9fo01485c. [DOI] [PubMed] [Google Scholar]