Abstract
Background and Objective
Obesity is a chronic disease associated with many serious comorbidities. Pharmacologic therapies are approved for the treatment of obesity; however, short‐term biomarkers to predict weight loss are not well understood. This study aimed to determine the ability of single‐meal energy intake (EI) to predict weight loss in participants with obesity treated with liraglutide.
Methods
In this randomized, double‐blind, placebo‐controlled study, participants received subcutaneous liraglutide (titrated to 3.0 mg/day) or placebo once daily, with inpatient assessments at baseline and weeks 3 and 6. The primary endpoint was change from baseline (CFB) in EI during consecutive ad libitum lunch meals at weeks 3 and 6. Secondary endpoints included CFB in 24‐ and 48‐h EI, weight, appetite scores, and gastric emptying measures.
Results
Sixty‐one participants were randomized (n = 32, liraglutide; n = 29, placebo). The least squares mean (LSM) difference (95% CI; p‐value) in CFB in EI during ad libitum lunch meals between the liraglutide and placebo groups was −236 (−322, −149; p < 0.0001) kcal at week 3 and –244 (−339, −148, p < 0.0001) kcal at week 6. The liraglutide group experienced significant weight loss at weeks 3 and 6, compared with placebo. Weight loss was significantly correlated with EI, but not with appetite score or gastric emptying.
Conclusions
EI during a single meal is a robust clinical predictor of weight changes in participants with obesity. Future clinical trials can utilize EI at a single meal as a predictor of weight loss.
Keywords: energy intake, glucagon‐like peptide‐1, obesity, weight loss
1. INTRODUCTION
Currently, there are four US Food and Drug Administration approved pharmacologic therapies for the long‐term treatment of obesity (orlistat, phentermine/topiramate, naltrexone/bupropion, and liraglutide). Liraglutide is a glucagon‐like peptide‐1 (GLP‐1) receptor (GLP‐1R) agonist approved at a dose of 3.0 mg as an adjunctive pharmacological treatment for chronic weight management in adults. 1 GLP‐1 is a neuroendocrine hormone predominantly released from the intestinal epithelium in response to food intake. 2 Activation of GLP‐1R stimulates insulin release, inhibits glucagon secretion in a glucose‐dependent manner, and delays gastric emptying. 3 , 4 GLP‐1 has been shown to increase satiety and suppress food intake in nondiabetic individuals with obesity. 5
To evaluate the effect of liraglutide on weight loss, previous studies have used energy intake (EI) as a clinical measure. 5 , 6 , 7 These studies, conducted in diabetic or nondiabetic adults with obesity, have yielded variable results regarding weight loss, EI, and the relationship between them. Two phase 1 studies evaluated the effect of liraglutide on food intake and appetite. 5 , 6 Participants in each of these studies experienced moderate weight loss that was associated with a decrease in food intake during ad libitum meals. In another study, liraglutide treatment was assessed in participants with type 2 diabetes. Significantly increased weight loss was observed in participants taking liraglutide compared to placebo, but EI was not significantly different. 7
Previous trials assessing the efficacy of weight‐loss interventions evaluated changes in body weight at relatively infrequent intervals, such as several months apart, or assessed efficacy based on the amount of body weight lost at the end of treatment. 8 , 9 Given the duration and cost of many long‐term weight‐loss efficacy trials, identifying biomarkers of short‐term weight loss would be valuable for providing an earlier indication of intervention efficacy.
The objectives of this study were to determine the ability of EI at a single meal to predict weight loss in individuals with obesity treated with the GLP‐1R agonist liraglutide and to determine if EI measured at a single meal, compared with 24‐ or 48‐h periods, is more closely correlated with a weight‐loss response. It was hypothesized that EI at a single meal would be a better predictor of weight loss with the GLP‐1R agonist liraglutide, compared with an appetite assessment via the visual analog scale (VAS) or gastric emptying measures.
2. METHODS
2.1. Participants
Male and female participants between 18 and 75 years of age with BMI between 30 and 40 kg/m2 were eligible for inclusion. Participants were excluded if they had a current or prior diagnosis of type 1 or type 2 diabetes mellitus or they had an HbA1c ≥6.5% at screening. At the screening visit, participants were asked about their body weight over the prior 12 weeks, and participants who reported a change in body weight of ≥5 kg were excluded from the study. Similarly, participants who reported the use of weight‐modifying medications, including prescription or over the counter medications, herbal supplements, or marijuana within 12 weeks of screening, were also excluded from the study.
2.2. Study design
This randomized, double‐blind, placebo‐controlled, two‐arm, parallel‐group, study assessed the effect of 6 weeks of liraglutide administration on EI in participants with obesity. The study spanned from 20 February 2017 until 16 January 2018, and included three outpatient visits (screening, run‐in, and follow‐up) and three inpatient visits to the Clinical Research Unit (CRU, AdventHealth Translational Research Institute) (week 0, week 3, and week 6) (Figure 1). After completing screening procedures to confirm eligibility, participants completed an outpatient run‐in visit prior to the initial inpatient visit to minimize the effect of clinical trial participation on baseline body weight. No significant differences in body weight were observed between the placebo run‐in visit and the baseline visit in either the liraglutide or the placebo groups, indicating that body weight was stable in the participants prior to randomization. After the run‐in visit, participants were admitted for the week 0 inpatient visit, during which they received baseline study assessments and their first dose of randomized, blinded study treatment. After randomization, participants were administered either liraglutide or placebo once daily for 6 weeks. Endpoints were evaluated at baseline and weeks 3 and 6. A follow‐up visit and end‐of‐study telephone call occurred approximately 10 and 31 days after the end of the randomized dosing period, respectively, to assess adverse events and to assess compliance with the contraceptive requirements of the study.
FIGURE 1.
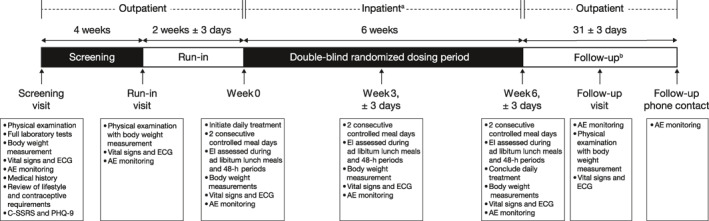
Study design. a Participants had inpatient visits at weeks 0, 3, and 6, which comprised 4 days and 3 nights each. b A follow‐up visit and phone call occurred at 10 ± 3 and 31 ± 3 days after the final dose, respectively. AE, adverse event; C‐SSRS, Columbia‐Suicide Severity Rating Scale; ECG, electrocardiogram; EI, energy intake; PHQ‐9, Patient Health Questionnaire‐9.
Each inpatient stay comprised 4 days and 3 nights, spanning over day −2, day −1, day 0, and day 1. For the first inpatient stay at week 0, participants were admitted on the day prior to food intake assessments (day −2), with food intake and appetite assessments occurring on day −1 and day 0. Acetaminophen administration and assessment of plasma acetaminophen levels occurred on day −1. Participants then received their first dose of randomized blinded study treatment on day −1 and were discharged from the clinical research unit on the day after the second EI assessment. [Corrections added on April 6, 2021 after first online publication: In the preceeding two sentences, the day numbers were changed from “day 1” to “day −1.”] The inpatient visits at weeks 3 and 6 followed the same structure, with the exception that all participants had been receiving treatment throughout.
2.3. Treatment
Liraglutide (Saxenda®, initiated at a dose of 0.6 mg/day and escalated by 0.6 mg/week up to a maximum of 3.0 mg/day) or placebo (0.9% w/v sodium chloride) were administered subcutaneously by pen or syringe injections, respectively. Volumes of placebo injections matched titrated volumes of liraglutide. Participants and site staff performing the study assessments were blinded to the study intervention. However, a pharmacist and a designated administrator were unblinded. Each dose was given by the unblinded administrator into a participant's abdomen, thigh, or upper arm. Participants used noise‐canceling headphones and blindfolds during treatment to preserve the blind.
For participants unable to tolerate dose‐escalation, titration was delayed by 1 week. Participants were permitted to progress to week 6 if they were able to tolerate a minimum liraglutide dose of 1.8 mg/day for at least 2 weeks prior to week 6.
2.4. Meals
All meals were prepared at the study site during inpatient visits, and menus were identical across visits. Participants were instructed to maintain normal diets and physical activity levels outside of inpatient visits. Daily calorie requirements for each participant were calculated from anthropometrics, age, ethnicity, and sex. 10 To assess changes in EI and the selection of macronutrients, the quantity of food presented exceeded the calculated calorie requirements for each participant by 30%. The total daily nutritional composition offered in inpatient meals was approximately 50% carbohydrates, 35% fat, and 15% protein.
On each inpatient visit day, participants were served a fixed 500 kilocalorie (kcal) breakfast to be consumed in its entirety within 20 min. Breakfasts were identical to minimize any variability in food intake assessment of the succeeding ad libitum lunch and dinner meals and any variability in the measurement of gastric emptying. The remainder of the daily calorie content was divided between lunch and dinner, with lunch providing 45% and dinner providing 55% of the remaining daily calories. Participants were allowed 30 min to complete ad libitum lunch and ad libitum dinner meals and were instructed to eat to satiety. Meals were consumed in private rooms of the CRU to minimize influence of environmental factors (i.e., food odors) on eating behaviors. The environment was also free of distractions, such as reading materials, television, cell phones, or computers.
2.5. Assessments of energy intake
For every ad libitum meal (lunch or dinner), each portion was weighed and recorded before and after each meal to measure the following EI parameters: total kcal consumed per day, total kcal of each macronutrient consumed per day, total kcal consumed per meal, and total kcal of each macronutrient consumed per meal. ProNutra™ nutrition software was used to calculate the total kcal and macronutrient content consumed, and also the weight of the portions not consumed, based on the weight of each meal. 11 These data were used to calculate the actual energy and macronutrient intake provided and consumed for each meal during inpatient visits. Mean EI was evaluated on consecutive days during inpatient visits. EI was calculated to the nearest 50 kcal as the difference between the total number of kcal provided versus remaining after meals.
2.6. Evaluations
Primary endpoint. Change from baseline (CFB) in mean EI (in kcal) during ad libitum lunch meals.
Secondary/exploratory endpoints. CFB in 48‐h EI (kcal), body weight, gastric emptying measures, and appetite and satiety scores (as measured by a VAS questionnaire).
Safety endpoints. Vital sign measurements, ECG monitoring, clinical laboratory testing, and reporting of adverse events (AEs).
Body weight was measured in the morning, under standardized conditions at all inpatient and outpatient visits at the clinical research unit. The same calibrated scale was used for each participant for all body weight measurements obtained at the study site. To measure gastric emptying, liquid acetaminophen (1.5 g) was administered orally to participants during breakfast. Blood samples for acetaminophen levels were taken from participants on the first morning of weeks 0, 3, and 6 as follows: fasting, and 30, 60, 90, 120, 180, and 300 min after acetaminophen ingestion. Gastric emptying was measured at breakfast and not measured at ad libitum lunch meals due to the variation in caloric intake between patients during ad libitum lunch meals. To score appetite, participants rated satiety, fullness, hunger, and prospective consumption using validated VAS questionnaires. 12 VAS questionnaires were administered immediately prior to the breakfast and lunch meals on the first full inpatient days of weeks 0, 3, and 6, and lunch meals only on the second inpatient days of weeks 0, 3, and 6. Questionnaires were administered at 30, 60, and 120 min following the start time of breakfast or lunch meals. The 30‐min postprandial appetite rating was also derived from VAS questionnaires.
Analysis populations. Participants who completed baseline assessments of EI, received at least one dose of randomized treatment, completed at least one post‐baseline measurement, and were able to tolerate a liraglutide dose of at least 1.8 mg/day were included in the full analysis set (FAS). Participants who received at least one dose of randomized treatment were included in the safety analysis set.
2.7. Statistical analyses
Sample size was determined based on the primary endpoint (change in mean EI from baseline to week 6 for ad libitum lunch meals) and an assumed drop‐out rate of 15%. For a between‐group comparison, 25 completers in each arm provided approximately 85% power to detect a true mean change from baseline in EI equal to 200 kcal, assuming type 1 error equal to 5% (one‐sided).
For all continuous endpoints, linear mixed‐effect model repeated measures (MMRMs) with fixed effects for treatment, time, baseline value of the endpoint of interest, and a random effect for participants, were used to analyze the CFB in each endpoint. Estimates of CFB were determined by calculating the least squares mean (LSM) and a 95% confidence interval for the LSMs at weeks 3 and 6 for liraglutide versus placebo for each endpoint. Mean EI was determined as the mean of the measurements taken at two consecutive ad libitum lunch meals. Mean baseline EI was calculated using the week 0 inpatient assessment.
For secondary and exploratory endpoints related to EI, analyses of CFB in EI during individual ad libitum lunch meals, 24‐h EI, and 48‐h EI were also performed using linear MMRMs, as described above. Baseline EI was calculated from individual lunch meals for the first and second lunch meals during the week 0 visit. For 24‐ and 48‐h EI endpoints, total EI during each of the individual inpatient days and both inpatient days together, respectively, was calculated. Baseline 24‐ and 48‐h EI was determined from the week 0 visit.
Baseline body weight was calculated as the average of measurements taken over 3 days of the week 0 visit, prior to receiving study medication. Postbaseline weights were calculated as the average of measurements taken over 3 days of the week 3 visit and 3 days of the week 6 visit.
For gastric emptying, area under the concentration–time profiles from 0 to 60 min (AUC0–60 min) and from 0 to 300 min (AUC0–300 min) were calculated for acetaminophen using a linear/log trapezoidal method.
Appetite score was calculated as the average of four individual VAS scores (satiety + fullness + [100 – prospective food consumption] + [100 – hunger])/4. Mean AUC rating from 30 to 120 min (AUC30–120 min) and the 30‐min postprandial appetite rating were calculated using a linear/log trapezoidal method, and LSM differences between participants treated with liraglutide versus placebo were determined using an MMRM. Appetite scores for ad libitum lunch meals were averaged for each respective visit.
Pearson correlation coefficients were calculated to determine the linearity of relationships between CFB in body weight and CFB in mean EI during ad libitum lunch, 48‐h EI, CFB in appetite score (both AUC30–120 min and the 30‐min postprandial appetite rating), and CFB in gastric emptying measures (AUC0–300 min).
2.8. Safety analysis
AEs were determined by direct observation or spontaneous reporting from participants from screening through the last telephone contact. Laboratory tests, vital signs, and electrocardiograms were also monitored.
The study was approved by the Florida Hospital Institutional Review Board and was conducted in accordance with the Declaration of Helsinki.
3. RESULTS
3.1. Participants
After completing screening procedures, a total of 61 participants were randomized to blinded study treatment (n = 32, liraglutide; n = 29, placebo). One participant randomized to the placebo group withdrew from the study prior to receiving the blinded study medication.
Of the 60 participants who received the study medication, 56 participants (n = 30, liraglutide; n = 26, placebo) were included in the FAS, and 54 completed the study (n = 28, liraglutide; n = 26 placebo), with six participants discontinuing during the treatment phase (Figure 2). Reasons for discontinuation were AEs (n = 4, liraglutide) and other reasons (n = 2, placebo). The FAS comprised 18 male and 38 female participants, with an average (standard deviation [SD]) age of 45.4 (11.9) years, body weight of all participants was 94.8 (12.9) kg, and BMI of 34.5 (2.8) kg/m2. Overall, baseline demographics were comparable among participants randomized to liraglutide or placebo (Table 1).
FIGURE 2.
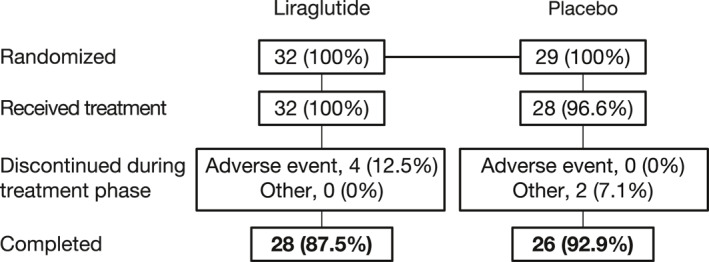
Participant disposition
TABLE 1.
Participant characteristics
| Demographic characteristics of full analysis set | |||
|---|---|---|---|
| Liraglutide (n = 30) | Placebo (n = 26) | Total (n = 56) | |
| Age, years, mean (SD) | 43.0 (10.88) | 48.1 (12.63) | 45.4 (11.89) |
| Female, n (%) | 22 (73.3) | 16 (61.5) | 38 (67.9) |
| Race, n (%) | |||
| White | 21 (70.0) | 19 (73.1) | 40 (71.4) |
| Black | 9 (30.0) | 6 (23.1) | 15 (26.8) |
| Asian | 0 (0.0) | 1 (3.8) | 1 (1.8) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 8 (26.7) | 9 (34.6) | 17 (30.4) |
| Not Hispanic or Latino | 22 (73.3) | 17 (65.4) | 39 (69.6) |
| Height, cm, mean (SD) | 165.67 (9.16) | 166.57 (9.04) | 166.09 (9.04) |
| Weight, kg, mean (SD) | 93.55 (12.81) | 96.30 (13.04) | 94.82 (12.87) |
| BMI, kg/m2, mean (SD) | 34.18 (2.62) | 34.77 (2.92) | 34.45 (2.76) |
BMI, body mass index; SD, standard deviation.
3.2. Assessment of energy intake
3.2.1. Mean EI during the ad libitum lunch meals
The mean EI (±SD) at baseline during ad libitum lunch meals was similar between the liraglutide (935 ± 295 kcal) and placebo (948 ± 253 kcal) groups. However, at weeks 3 and 6, the mean EI was significantly decreased in participants receiving liraglutide compared to those receiving placebo (Figure 3). The mean difference (95% CI; p‐value) in the CFB between the liraglutide and placebo groups was −236 (−322, −149; p < 0.0001) kcal at week 3 and –244 (−339, −148, p < 0.0001) kcal at week 6.
FIGURE 3.
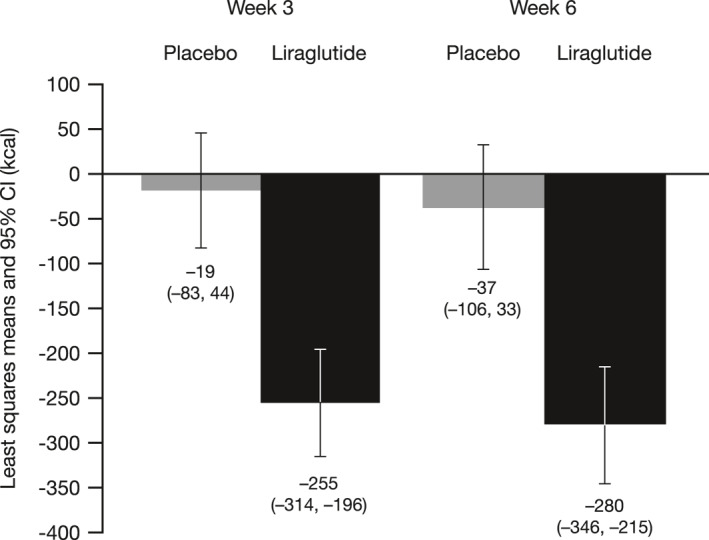
Change from baseline (CFB) in mean EI during ad libitum lunch meals—full analysis set. Baseline was defined as the mean EI during two ad libitum lunches at baseline. Placebo n = 26 at week 3, n = 26 at week 6; liraglutide n = 30 at week 3, n = 28 at week 6. Energy intake is presented as the least squares mean of two lunches during week 3, and two lunches during week 6. A linear mixed effect model repeated measures (MRMM) with fixed effects for treatment, visit, a treatment‐by‐time interaction, a baseline value of EI, and a random effect for subject, was used to analyze the CFB. An unstructured matrix was used to model the covariance structure. CI, confidence interval; EI, energy intake MMRM, mixed‐effect model repeated measure.
The mean EI during ad libitum lunch meals on individual testing days was also similar between the groups at baseline; the mean (±SD) EI values during the first and second ad libitum lunches during week 0, respectively, were 864 ± 289 kcal and 1006 ± 318 kcal for liraglutide and 896 ± 271 kcal and 999 ± 277 kcal for placebo. The LSM difference (95% CI; p‐value) in CFB in mean EI between the liraglutide and placebo groups during the first ad libitum lunch was –221 (−323, −119; p = 0.0001) kcal at week 3 and –267 (−374, −160; p < 0.0001) kcal at week 6 (Figure S1). During the second ad libitum lunch meals of weeks 3 and 6, the LSM difference (95% CI; p‐value) in the CFB between the liraglutide and placebo groups was −256 (−358, −155; p < 0.0001) kcal and −226 (−328, −124; p = 0.0001) kcal, respectively (Figure S1).
3.2.2. 48‐h EI
The mean (±SD) 48‐h EI was similar between treatment groups at baseline (5253 ± 1237 kcal and 5451 ± 1150 kcal for liraglutide and placebo, respectively). Liraglutide was superior to placebo in reducing 48‐h EI during weeks 3 and 6. The LSM (95% CI; p‐value) CFB in 48‐h EI between the liraglutide and placebo groups at weeks 3 and 6 were −860 (–1196, −523; p < 0.0001) and −929 (−1317, −540; p < 0.0001) kcal, respectively.
3.2.3. Comparison of EI assessments
A forest plot (Figure 4) shows the placebo‐adjusted LSM CFB at weeks 3 and 6 for EI variables, including mean (of day 1 and day 2) EI at lunch, EI at lunch for individual testing days, 24‐h EI for individual testing days, and 48‐h EI. Liraglutide resulted in significantly lower mean EI compared to placebo, at both weeks 3 and 6, for all outcomes investigated.
FIGURE 4.
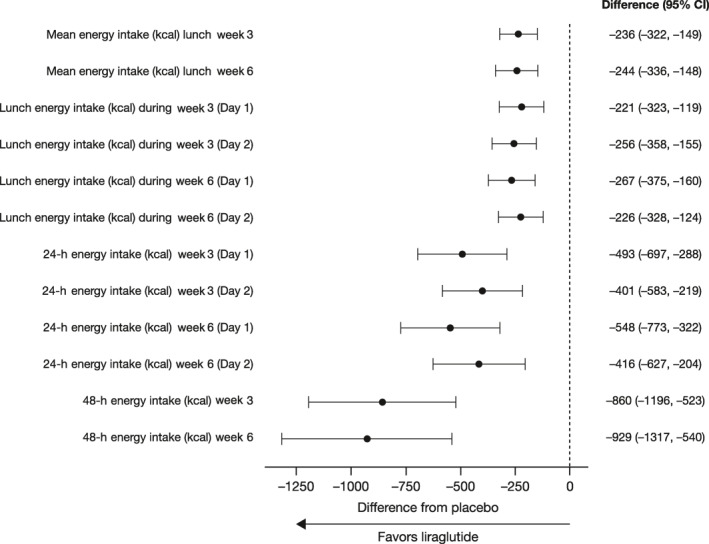
A forest plot of the placebo‐adjusted least squares mean change from baseline at weeks 3 and 6 for EI outcomes. CI, confidence interval; EI, energy intake.
3.3. Body weight change
Relative to placebo, liraglutide treatment produced significantly greater reductions in body weight, with mean differences (95% CI; p‐value) in CFB of −2.03 kg (−2.65, −1.41; p < 0.001) at week 3 and –3.85 kg (−4.71, −2.99; p < 0.001) at week 6 (Figure 5). On average, the placebo group did not experience weight loss at either week 3 or week 6.
FIGURE 5.
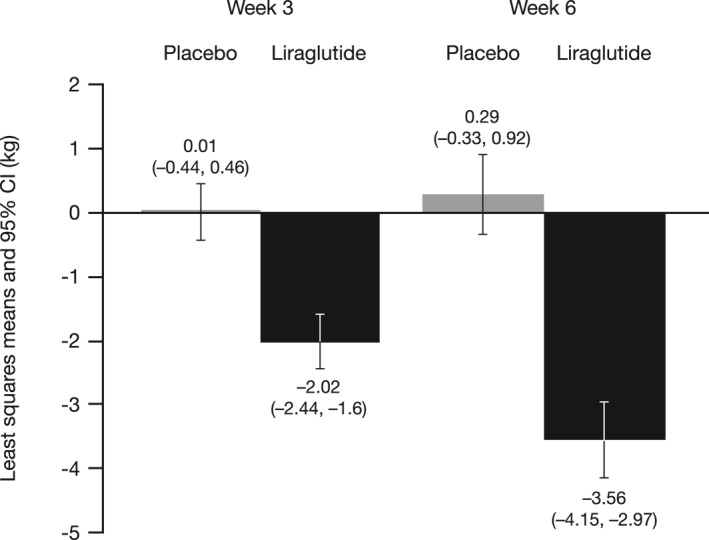
Change from baseline (CFB) in body weight. Baseline was calculated as the mean of three baseline values. Placebo n = 26 at week 3 and n = 26 at week 6; liraglutide n = 30 at week 3 and n = 28 at week 6. Weight loss is presented as the least squares mean values for each visit. A linear MRMM with fixed effects for treatment, visit, a treatment‐by‐time interaction, a baseline value of measurement, and a random effect for subject, was used to analyze the CFB. An unstructured matrix was used to model the covariance structure. MMRM, mixed‐effect model repeated measure.
3.4. Gastric emptying
The LSM CFB in plasma acetaminophen AUC0–60 min and AUC0–300 min at weeks 3 and 6 was increased in the liraglutide group, compared with the placebo group (Figure S2), with statistically significant differences between the groups at week 6 (AUC0–60 min, p = 0.0348; AUC0–300 min, p = 0.0152). The increase in plasma acetaminophen AUC observed in the liraglutide group at week 6, relative to placebo, indicated that liraglutide caused a significant delay in gastric emptying when administered over 6 weeks.
3.5. Appetite score
Mean appetite scores (AUC30–120 min and the 30‐min postprandial rating) were calculated for ad libitum lunch meals during weeks 3 and 6. Statistically significant increases in appetite VAS scores (indicating decreased appetite) were observed in the liraglutide group at weeks 3 and 6, as compared with placebo (Table S1). Participants receiving liraglutide also exhibited significant differences in AUC30–120 min for prospective food consumption and hunger subscores at weeks 3 and 6, and in the 30‐min postprandial rating for prospective food consumption (weeks 3 and 6) and hunger (week 3 only), compared with placebo. Although satiety and fullness appeared to increase with liraglutide relative to placebo, the differences between the groups did not reach statistical significance.
3.6. Weight loss correlations with energy intake, gastric emptying measures, and appetite score
Weight loss was significantly correlated with EI, but not with gastric emptying measures or appetite scores (Figure 6). CFB in body weight was linearly correlated with mean CFB in EI during ad libitum lunch meals in the liraglutide group at week 6 (r = 0.437; p = 0.0200) and in the placebo group at week 3 (r = 0.694; p < 0.001) and week 6 (r = 0.571; p = 0.0023) (Figure 6A). Interestingly, CFB in body weight at week 6 was also significantly correlated with CFB in EI during lunches at week 3 in both the liraglutide group (r = 0.40; p = 0.0332) and the placebo group (r = 0.56; p = 0.0027) (Figure 7). Similar to correlations observed with CFB in ad libitum lunch EI, CFB in body weight was linearly correlated with mean CFB in EI over 48 h in the liraglutide group at week 6 (r = 0.428; p = 0.0231) and in the placebo group at week 3 (r = 0.702; p < 0.001) and week 6 (r = 0.658; p = 0.0003) (Figure 6B). CFB in body weight was not significantly correlated with CFB in EI during ad libitum lunch meals (r = 0.33; p = 0.0753) or over 48 h (r = 0.28; p < 0.1288) in the liraglutide group at week 3 (Figure 6A,B, respectively). CFB in body weight was not significantly correlated (p > 0.05) with CFB in appetite score AUC30–120 min (Figure 6C), 30‐min postprandial rating (Figure 6D), or gastric emptying (AUC0–300 min) (Figure 6E).
FIGURE 6.
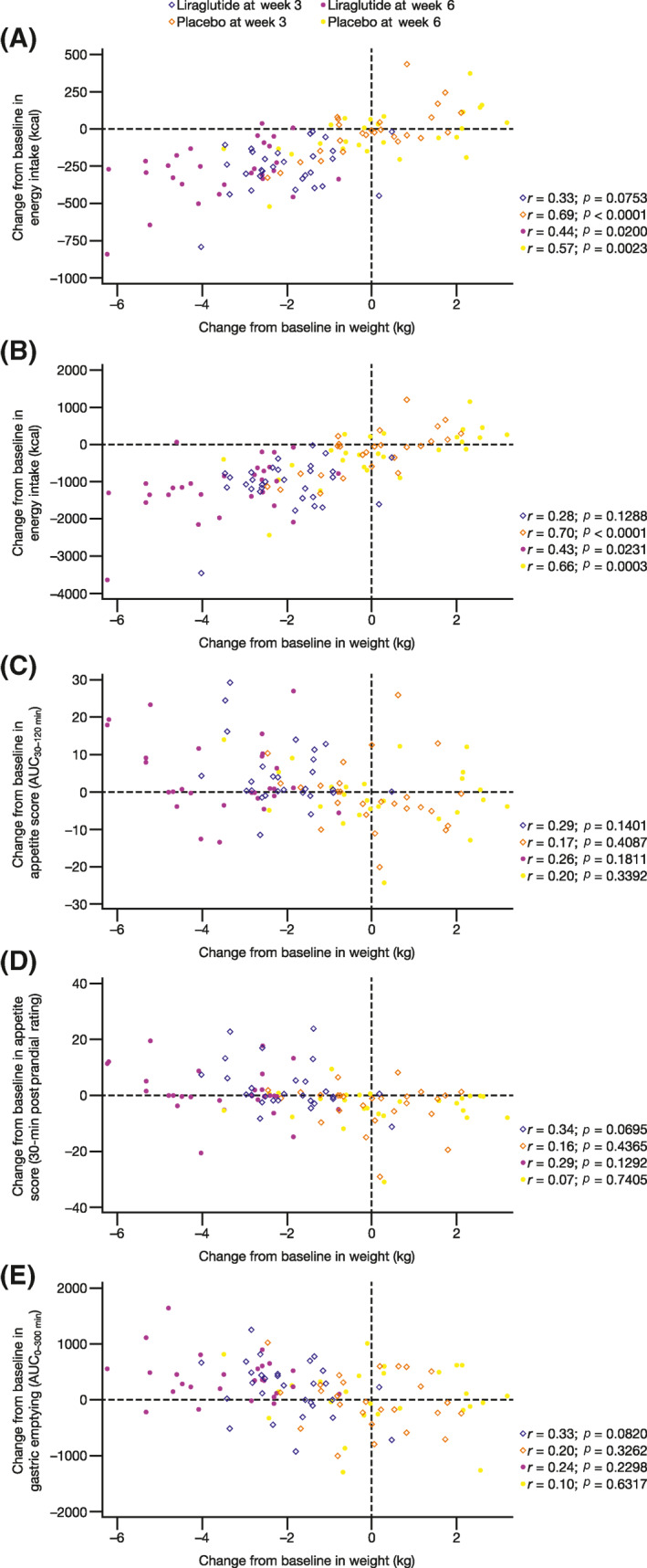
Correlation analyses of the change from baseline (CFB) in body weight with (A) the CFB in mean EI at lunch, (B) 48‐h EI, (C) the CFB in appetite score (AUC30–120 min), (D) the CFB in appetite score (the 30‐min postprandial rating), and (E) the CFB in gastric emptying (AUC0–300 min). Body weight was calculated as the mean of the measurements during weeks 0, 3, and 6. Baseline values were calculated as the mean measurements during baseline. AUC30–120 min, area under the curve from 30 to 120 min; AUC0–300 min, area under the curve from 0 to 300 min; EI, energy intake.
FIGURE 7.
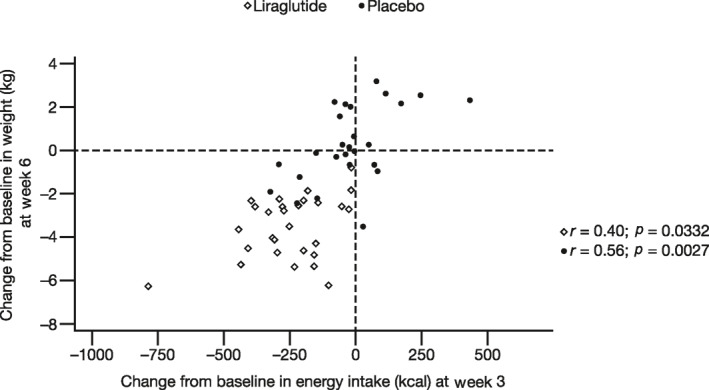
Correlation analyses of the change from baseline (CFB) in body weight at week 6 with the CFB in mean EI at lunch at week 3. Body weight was calculated as the mean of the measurements during weeks 0, 3, and 6. Baseline values were calculated as the mean measurements during baseline. EI, energy intake.
3.7. Safety
Table 2 presents a summary of the AEs. The most frequently reported treatment‐emergent AEs (TEAEs) were nausea, diarrhea, headache, constipation, and injection‐site bruising. Four participants treated with liraglutide discontinued due to treatment‐related AEs. Two participants discontinued due to vomiting (while receiving 1.2 mg liraglutide), one due to abdominal pain (while receiving 1.2 mg liraglutide), and one due to nausea, headache, and anxiety (while receiving 0.6 mg liraglutide). All AEs were mild or moderate in intensity and resolved by the last follow‐up.
TABLE 2.
Summary of AEs (safety analysis set)
| Liraglutide (n = 32) | Placebo (n = 28) | |
|---|---|---|
| Total AEs (treatment‐related) | 177 (100) | 52 (15) |
| Participants with AEs (treatment‐related) | ||
| Overall | 31 (28) | 20 (11) |
| SAEs | 0 | 0 |
| Leading to dose reduction/temporary discontinuation | 1 (1) | 1 (0) |
| Leading to permanent discontinuation | 4 (4) | 0 |
| AEs occurring in >5% of participants, n (%) | ||
| Abdominal distension | 4 (12.5) | 0 (0) |
| Upper abdominal pain | 6 (18.8) | 1 (3.6) |
| Constipation | 9 (28.1) | 3 (10.7) |
| Diarrhea | 11 (34.4) | 4 (14.3) |
| Dyspepsia | 7 (21.9) | 2 (7.1) |
| Nausea | 15 (46.9) | 1 (3.6) |
| Vomiting | 6 (18.8) | 0 (0) |
| Fatigue | 5 (15.6) | 0 (0) |
| Injection‐site bruising | 7 (21.9) | 4 (14.3) |
| Injection‐site erythema | 4 (12.5) | 0 (0) |
| Contusion | 1 (3.1) | 4 (14.3) |
| Decreased appetite | 8 (25.0) | 2 (7.1) |
| Back pain | 3 (9.4) | 1 (3.6) |
| Dizziness | 6 (18.8) | 1 (3.6) |
| Headache | 11 (34.4) | 3 (10.7) |
| Nasal congestion | 4 (12.5) | 0 (0) |
AE, adverse event; SAE, serious adverse event.
4. DISCUSSION
This study examined the ability of EI, as a clinical endpoint, to predict body‐weight loss in participants with obesity and compared its utility versus other clinical endpoints, including appetite score and gastric emptying measures. Participants receiving liraglutide had significantly reduced EI during ad libitum lunch meals and throughout the 24‐ and 48‐h observation periods, compared with participants receiving placebo. In addition, significantly greater weight loss was observed at weeks 3 and 6 in participants treated with liraglutide, compared with placebo. A significant correlation between CFB in EI and CFB in body weight was observed in the liraglutide group at week 6, but not at week 3, which could be due to the fact that participants were still titrating to the maximum dose of liraglutide. In contrast, the correlation between CFB in EI and CFB in body weight was significant in the placebo group at both weeks 3 and 6, which included both participants who lost and who gained weight during the study duration (note that, on average, the placebo group did not exhibit weight loss). Interestingly, CFB in body weight at week 6 was significantly correlated with CFB in EI during lunches at week 3 for both the liraglutide and placebo groups, indicating that lower EI at week 3 preceded and predicted the weight loss that occurred at week 6. The strength of the correlation in both situations of body weight loss and body weight gain supports the study hypothesis that changes in EI may serve as a short‐term biomarker for changes in body weight.
In the current study, reductions in EI during ad libitum lunch meals in patients receiving liraglutide were between 235 and 245 kcal. These reductions were greater than those previously reported in similar populations. For example, no significant reduction in EI, but significant weight loss, was reported in participants with diabetes treated with liraglutide, compared with placebo. 7 In contrast, in a phase 1 study, calorie intake during an ad libitum lunch was reduced by approximately 140 kcal, compared with placebo, in participants with obesity receiving liraglutide 3.0 mg daily for 5 weeks. 5 Furthermore, in participants with type 2 diabetes, liraglutide 1.8 mg daily reduced calorie intake during an ad libitum lunch by approximately 200 kcal. 6 Differences in EI between the current and previous studies could be attributed to study setting (CRU vs. clinic) and design (parallel‐group vs. crossover). The extended length of inpatient visits within highly controlled CRU environments (e.g., reduced noise/interruptions and clean air) may have also contributed to decreased variability in EI during ad libitum lunch meals.
In addition to EI, significant differences in appetite score (weeks 3 and 6) and gastric emptying measures (week 6) were reported between participants treated with liraglutide and those receiving placebo. Gastric emptying results from the current study are more robust than those of a previous study, conducted in a similar patient population, which showed significant reductions in AUC0–60 min, but not in AUC0–300 min in patients treated with liraglutide 3.0 mg versus placebo. 5 Despite significant reductions in appetite and gastric emptying, correlation analyses identified EI as the only parameter that was significantly correlated with weight loss at either week 3 or 6. Weight loss in this study was significantly correlated with reduced EI both during ad libitum lunch meals and over 48 h. This result indicates that EI is a better predictor of weight loss in clinical settings compared with appetite scoring or gastric emptying. Additionally, reduced EI could predict weight loss from a single meal.
Previously published literature has suggested that short‐term fluctuations in EI have little effect on body weight, 13 and single‐meal EI is not a reliable clinical measure to predict weight loss. 6 In contrast, persistent changes in EI are linked to more substantial long‐term weight changes. 13 Participants in the current study exhibited reduced EI during each ad libitum lunch meal during both weeks 3 and 6, which was consistent with the observed weight loss seen over the 6 weeks. Additionally, weight reduction was numerically higher at week 6 compared to week 3 in participants treated with liraglutide, which was indicative of positive drug effects during the portions of the study performed outside of the CRU. This is consistent with previous literature, which indicates that maximum reduction in EI is consistent with sustained reductions in EI at steady‐state. 14 The results of the current study also support those of a previous study demonstrating that the primary mechanism of action for liraglutide was via EI pathways and not due to energy expenditure. 15
Limitations of this study included the assessment of GI AEs via participant self‐report and the use of plasma acetaminophen levels to assess gastric emptying. Self‐report of GI AEs may not provide an accurate assessment of GI symptoms, compared with validated questionnaires that have been used in assessing functional GI disorders. As part of the informed consent process, participants were made aware of the possible side effects of liraglutide, per the Saxenda® label, which may have also impacted their adverse event reporting. 16 In addition, the limitations of the use of plasma acetaminophen levels in the assessment of gastric emptying is also acknowledged, as it may not reflect differences in gastric emptying with varying food content and caloric intake. 17 Previous inconsistencies in effects of liraglutide over time on gastric emptying may have been related to the methodologies used for gastric emptying. When assessed by scintigraphy, delays in gastric emptying with liraglutide administration were observed at 5 weeks, with persistence at longer duration of dosing. 18 In addition, other methods to provide food, including computerized vending machines, have been utilized in studies assessing ad libitum EI, and have resulted in reproducible assessments. 19 This study also had several strengths. The longitudinal nature and the collection of data over two consecutive lunch meals were unique aspects of this study, as compared with previous trials. Moreover, the robust study design allowed for highly controlled inpatient visits to better determine the predictors of weight loss in the study population.
5. CONCLUSION
In conclusion, the results of this study indicate that EI during ad libitum lunch meals is a significant predictor of changes in weight over 6 weeks. Additionally, EI was found to be a more accurate predictor of changes in body weight compared with appetite scoring or gastric emptying measures. As such, single‐meal EI is a robust clinical endpoint for predicting weight loss in clinical studies.
CONFLICT OF INTEREST
Aditi R. Saxena and Anindita Banerjee are full‐time employees and stockholders of Pfizer Inc. Karen D. Corbin and Stephanie A. Parsons have no conflicts of interest to report. Steven R. Smith reports personal fees from Eisai outside of the submitted work.
AUTHOR CONTRIBUTIONS
Aditi R. Saxena, Steven R. Smith, Anindita Banerjee, Stephanie A. Parsons, and Karen D. Corbin were involved in the conception and design of the study/analyses. Steven R. Smith was involved in participant recruitment and/or data acquisition. Aditi R. Saxena and Anindita Banerjee performed the data and statistical analyses. Aditi R. Saxena, Steven R. Smith, Anindita Banerjee, and Karen D. Corbin interpreted the data. Revisions of the manuscript for important intellectual content were drafted by Aditi R. Saxena, Steven R. Smith, Anindita Banerjee, Stephanie A. Parsons, and Karen D. Corbin. Final approval for publication was given by Aditi R. Saxena, Steven R. Smith, Anindita Banerjee, Stephanie A. Parsons, and Karen D. Corbin.
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the study participants and the study staff at TRI. The authors also thank Kazuhiro Kanmuri for his contributions to the protocol, and Jill Sutt for her contributions to study conduct.
Medical writing support, under the direction of the authors, was provided by Eric Comeau, Ph.D., CMC Connect, McCann Health Medical Communications and was funded by Pfizer Inc, New York, New York, USA, in accordance with Good Publication Practice (GPP3) guidelines.
Funding was provided by Pfizer Inc.
DATA AVAILABILITY STATEMENT
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., the development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- 1. Novo Nordisk Inc . SAXENDA (Liraglutide) Prescribing Information. https://www.novo‐pi.com/saxenda.pdf. Accessed June 5, 2019. [Google Scholar]
- 2. Holst JJ. The physiology of glucagon‐like peptide 1. Physiol Rev. 2007;87:1409‐1439. [DOI] [PubMed] [Google Scholar]
- 3. Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon‐like peptide‐1 7‐36: a physiological incretin in man. Lancet. 1987;2:1300‐1304. [DOI] [PubMed] [Google Scholar]
- 4. Nauck MA, Niedereichholz U, Ettler R, et al. Glucagon‐like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997;273:E981‐E988. [DOI] [PubMed] [Google Scholar]
- 5. van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once‐daily GLP‐1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non‐diabetic adults. Int. J. Obes. 2014;38:784‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Flint A, Kapitza C, Zdravkovic M. The once‐daily human GLP‐1 analogue liraglutide impacts appetite and energy intake in patients with type 2 diabetes after short‐term treatment. Diabetes Obes Metab. 2013;15:958‐962. [DOI] [PubMed] [Google Scholar]
- 7. Horowitz M, Flint A, Jones KL, et al. Effect of the once‐daily human GLP‐1 analogue liraglutide on appetite, energy intake, energy expenditure and gastric emptying in type 2 diabetes. Diabetes Res. Clin. Pract. 2012;97:258‐266. [DOI] [PubMed] [Google Scholar]
- 8. Appel LJ, Clark JM, Yeh HC, et al. Comparative effectiveness of weight‐loss interventions in clinical practice. N Engl J Med. 2011;365:1959‐1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wadden TA, Volger S, Sarwer DB, et al. A two‐year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011;365:1969‐1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lam YY, Redman LM, Smith SR, et al. Determinants of sedentary 24‐h energy expenditure: equations for energy prescription and adjustment in a respiratory chamber. Am J Clin Nutr. 2014;99:834‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. VioCare Inc . ProNutra™. https://www.viocare.com/pronutra.html. Accessed 25 February 2019 [Google Scholar]
- 12. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38‐48. [DOI] [PubMed] [Google Scholar]
- 13. Chow CC, Hall KD. Short and long‐term energy intake patterns and their implications for human body weight regulation. Physiol Behav. 2014;134:60‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall KD, Sanghvi A, Gobel B. Proportional feedback control of energy intake during obesity pharmacotherapy. Obesity. 2017;25:2088‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harder H, Nielsen L, Tu DT, Astrup A. The effect of liraglutide, a long‐acting glucagon‐like peptide 1 derivative, on glycemic control, body composition, and 24‐h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004;27:1915‐1921. [DOI] [PubMed] [Google Scholar]
- 16. Rayner CK, Jones KL, Wu T, Horowitz M. Gut feelings about diabetes and GLP‐1 receptor agonists: lessons to be learnt from studies in functional gastrointestinal disorders. Diabetes Obes Metab. 2017;19:309‐312. [DOI] [PubMed] [Google Scholar]
- 17. Bartholome R, Salden B, Vrolijk MF, et al. Paracetamol as a post prandial marker for gastric emptying, A food‐drug interaction on absorption. PLoS One. 2015;10:e0136618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Halawi H, Khemani D, Eckert D, et al. Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo‐controlled pilot trial. Lancet Gastroenterol Hepatol. 2017;2:890‐899. [DOI] [PubMed] [Google Scholar]
- 19. Venti CA, Votruba SB, Franks PW, Krakoff J, Salbe AD. Reproducibility of ad libitum energy intake with the use of a computerized vending machine system. Am J Clin Nutr. 2010;91:343‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Upon request, and subject to certain criteria, conditions, and exceptions (see https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines, and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e., the development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary, and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.


