Abstract
Background:
Although traditionally memory has been viewed as a simple concept, converging and complementary evidence from patient studies and more recent neuroimaging research suggest that memory is a collection of mental abilities that use different neuroanatomical systems within the brain. Neurologic injury may cause damage to one or more of these memory systems.
Review Summary:
In this review a number of different memory systems are discussed, including their function, neuroanatomy, and the different disorders that disrupt them. Episodic memory, the most clinically relevant memory system, depends upon the hippocampus and other medial temporal lobe structures, the limbic system, and the frontal lobes. Several other kinds of memory are contrasted with episodic memory, including semantic memory, simple classic conditioning, procedural memory, priming, and working memory.
Conclusion:
Improved understanding of these different types of memory will aid the clinician in the diagnosis and treatment of the memory disorders of their patients. As more specific therapeutic strategies are developed for the treatment of diseases which cause memory dysfunction, this knowledge will become increasingly important.
Keywords: memory, Alzheimer disease, frontal lobes, false memory, memory distortions
Neurologists are increasingly referred patients with complaints of impaired memory. Memory dysfunction can be caused by a diverse group of neurologic disorders, including neurodegenerative diseases, strokes, tumors, head trauma, hypoxia, cardiac surgery, malnutrition, attention deficit hyperactivity disorder, depression, anxiety, and medication effects.1,2 Memory function can also be altered by normal aging.3 Although once thought to be a simple concept, we now consider memory to be a collection of mental abilities that use different systems and components within the brain. Memory research that began with neuropsychological studies of patients with focal brain lesions and now includes newer methods such as positron emission tomography, functional MRI, and event-related potentials has provided the rationale for a more refined and improved classification system.4 In this article, a number of different forms of memory will be reviewed, including the important anatomic structures for each, and the major neurologic disorders that disrupt them (Tables 1 and 2). A greater understanding of these memory systems will aid neurologists in their diagnosis and treatment of the memory disorders of their patients. This improved understanding will become increasingly important as new therapeutic interventions for memory disorders are developed.
TABLE 1.
Selected Memory Systems
| Memory System | Examples | Awareness | Length of Storage | Major Anatomical Structures |
|---|---|---|---|---|
| Episodic memory | Remembering a short story, what you had for dinner last night, and what you did on your last birthday | Explicit declarative | Minutes to years | Medial temporal lobe, anterior thalamic nucleus, mamillary body, fornix, prefrontal cortex |
| Semantic memory | Knowing who was the first President of the US, the color of a lion, and how a fork and comb are different | Explicit declarative | Minutes to years | Inferior lateral temporal lobes |
| Autonomic simple classical | Pavlov’s dog; a fear response conditioning | Implicit nondeclarative | Minutes to years | Amygdala and basolateral limbic system |
| Motoric simple classical conditioning | Eye-blink conditioning | Implicit nondeclarative | Minutes to months | Cerebellum |
| Procedural memory | Driving a standard transmission car, and learning the sequence of numbers on a touch-tone phone without trying | Implicit nondeclarative | Minutes to years | Basal ganglia, cerebellum, supplementary motor area |
| Perceptual priming | Word-stem completion: octopus→oct____ | Implicit nondeclarative | Minutes to days | Cortical sensory association areas (eg, extrastriate visual cortex for visual perceptual priming) |
| Conceptual priming | Word-stem completion: sea creatures→oct____ | Implicit nondeclarative | Minutes to days | Inferior prefrontal cortex |
| Working memory | Phonological: keeping a phone number “in your head” before dialing Spatial: mentally following a route, or rotating an object in your mind | Explicit declarative | Seconds to minutes; information actively rehearsed or manipulated | Phonological: prefrontal cortex, Broca’s area, Wernicke’s area Spatial: prefrontal cortex,visual association areas |
TABLE 2.
Selective Memory System Disruptions in Common Clinical Disorders
| Disease | Episodic Memory |
Semantic Memory |
Simple Classical Conditioning |
Procedural Memory |
Priming | Working Memory |
|---|---|---|---|---|---|---|
| Alzheimer disease | +++ | ++ | + | − | − perceptual + conceptual | ++ |
| Frontotemporal dementia | ++ | ++ | ? | − | ? | +++ |
| Semantic dementia | + | +++ | ? | ? | ? | − |
| Lewy body dementia | ++ | ? | ? | ? | ? | ++ |
| Stroke and vascular dementia | + | + | + | + | + | ++ |
| Parkinson disease | + | + | − | +++ | − | ++ |
| Huntington disease | + | + | − | +++ | − | +++ |
| Progressive supranuclear palsy | + | + | ? | ++ | ? | +++ |
| Korsakoff syndrome | +++ | − | + | − | + | |
| Multiple sclerosis | + | + | ? | − | ++ | |
| Transient global amnesia | +++ | ? | − | − | − | |
| Hypoxic-ischemic injury | ++ | − | − | − | + | |
| Head trauma | + | + | ? | ++ | ||
| Tumors | ± | ± | ± | ± | ± | ± |
| Depression | + | ± | ? | ++ | ? | ± |
| Anxiety | + | − | ± | − | ± | ± |
| Obsessive compulsive disorder | + | − | ± | ++ | ± | ++ |
| Attention deficit hyperactivity disorder | − | − | − | ? | ± | + |
+++ indicates early and severe impairment; ++, moderate impairment; +, mild impairment; ±, occasional impairment or impairment in some studies, but not others; −, no significant impairment; ?, unknown.
A memory system is a way for the brain to process information that will be available for use at a later time.5 Some systems are associated with conscious awareness (explicit) and can be consciously recalled (declarative), whereas others are typically unconscious (nondeclarative) and are instead expressed by a change in behavior (implicit).6 Memory can also be categorized in other ways, such as the nature of the material to be remembered, verbal7 versus visual.8
Episodic Memory
Episodic memory refers to the explicit and declarative memory system used to remember a particular episode of your life.
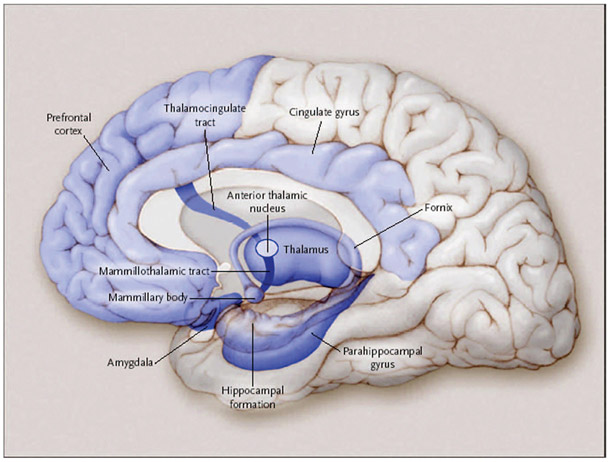
Episodic memory refers to the explicit and declarative memory system used to remember a particular episode of your life, such as sharing a meal with a friend. This memory system is dependent upon the medial temporal lobes (including the hippocampus), as episodic memory has been largely defined by what patients with medial temporal lobe lesions cannot remember relative to healthy individuals. Other critical structures in the episodic memory system (some of which are associated with a circuit described by Papez in 19379) include the basal forebrain with the medial septum and diagonal band of Broca, the retrosplenial cortex, the presubiculum, the fornix, mammillary bodies, the mammillothalamic tract, and the anterior nucleus of the thalamus (Fig. 1).2 A lesion in any one of these structures may cause the impairment that is characteristic of dysfunction of the episodic memory system.
FIGURE 1.
Episodic memory. The medial temporal lobes, including the hippocampus and parahippocampus, form the core of the episodic memory system. Other brain regions are also necessary for episodic memory to function correctly. In addition to being involved in episodic memory, the amygdala is also important for the autonomic conditioning. (Adapted with permission from Budson and Price, New England Journal of Medicine, 2005).
Memory loss due to dysfunction of the episodic memory system generally follows a pattern known as Ribot’s law, which states that events just prior to an ictus are most vulnerable to decay, whereas remote memories are more resistant. Thus, dysfunction of the episodic memory system typically causes greatest disruption in the ability to learn new information (anterograde amnesia) and moderate disruption in the ability to recall recently learned information (retrograde amnesia), whereas the ability to recall remotely learned information is generally intact (Fig. 2).10
FIGURE 2.

Ribot’s law.
The core of the episodic memory system is the medial temporal lobe and hippocampus.
The core of the episodic memory system is the medial temporal lobe and hippocampus. It is worth examining these structures more closely. The medial temporal lobes may seem simple but they are actually neuroanatomically complex structures with multiple regions and subregions. In Figure 3 you can see the medial temporal lobe structures, including the parahippocampal gyrus, presubiculum, subiculum, and hippocampus proper, including its subregions.
FIGURE 3.
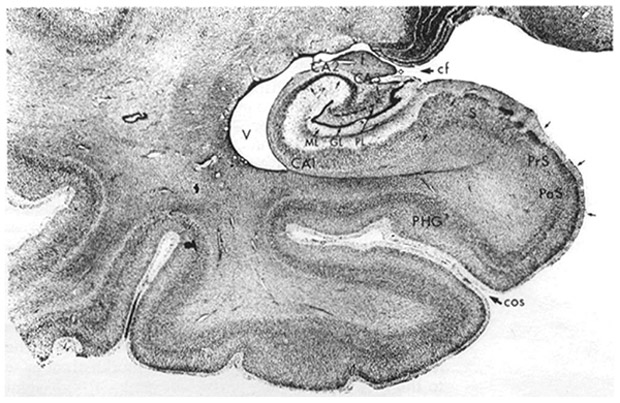
Detailed anatomy of the medial temporal lobe. PHG, indicates parahippocampal gyrus; Pr, presubiculum; v, ventricle; S, subiculum. CA1, CA2, and CA3 are subregions of the hippocampus, and ML, GL, and PL are different regions of the dentate gyrus of the hippocampus. (Adapted with permission from Martin JH. Neuroanatomy: Text and Atlas. New York, NY.: Elsevier, 1989, p. 391; permission granted by Elsevier).
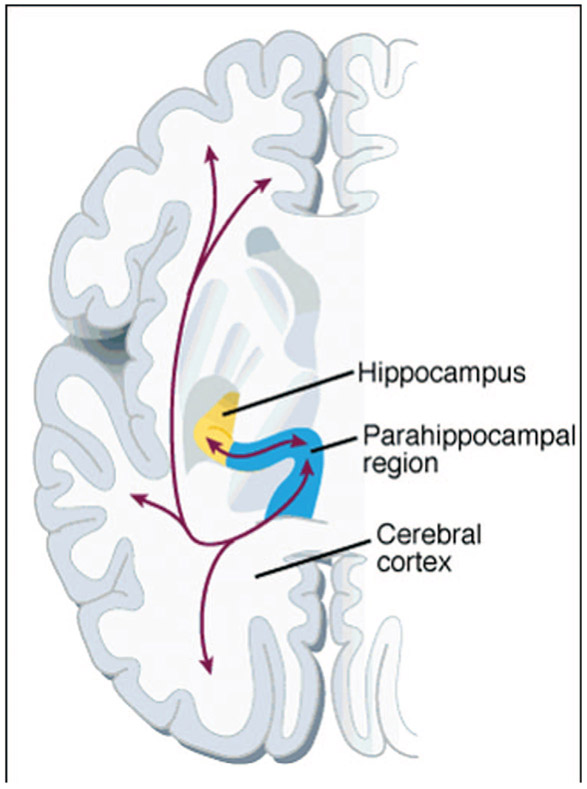
Although we do not completely understand how the medial temporal lobes store and retrieve memories, our current understanding from cognitive neuroscience is as follows. An individual experiences an episode of their life, such as having breakfast that morning. The cortically distributed patterns of neural activity representing the sights, sounds, smells, tastes, emotions, and thoughts during that episode are transferred first to the parahippocampal region and then to the hippocampus proper (Fig. 4). After being transferred to the entorhinal cortex, the information is processed in the dentate gyrus, and then transferred to the CA3 region where it is further processed (Fig. 5). It is in this CA3 region where the critically important hippocampal index is assigned, allowing the memory to be stored in a unique way so that it can later be recalled.
FIGURE 4.
Areas of the cerebral cortex, including sensory areas, are connected bidirectionally to the parahippocampal region, which is in turn bidirectionally connected to the hippocampus. (Adapted with permission from Eichenbaum, 1997;permission granted by Science Magazine).
FIGURE 5.
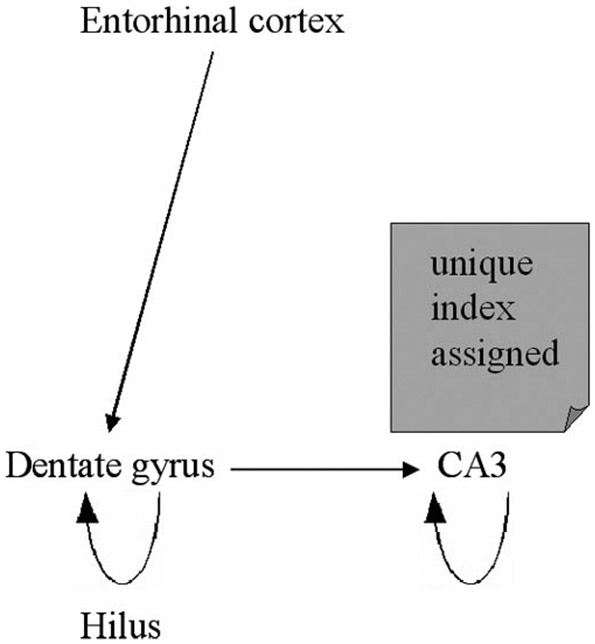
Schematic representation of encoding in the medial temporal lobe.
Typically memories are retrieved when a cue from the environment matches a part of the stored memory. Continuing our breakfast example, years later the individual might now bite into a little cake that tastes remarkably like the one that he had previously at breakfast. This sensory cue is transferred from the cortex to the parahippocampal region and to the hippocampus (Fig. 4). After the cue is transferred from the entorhinal cortex it now goes directly to the CA3 region where the original hippocampal index is retrieved (Fig. 6). When found, the hippocampal index may be used to retrieve much of the original pattern of the neural activity representing the original episode stored in memory. This retrieved pattern of activity may then be transferred to the CA1 region, the subiculum, the entorhinal cortex, and then back to the cortex—recreating all sights, sounds, smells, tastes, emotions, and thoughts of the original memory episode (Fig. 4).
FIGURE 6.
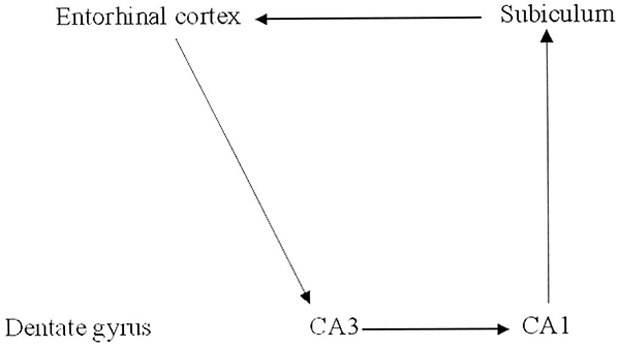
Schematic representation of retrieval in the medial temporal lobe.
The hippocampus remains critical for memory retrieval until a process known as consolidation occurs.
The hippocampus remains critical for memory retrieval until a process known as consolidation occurs. Much research still needs to be done to better understand consolidation, but one thought is that once a memory is consolidated the distributed pattern of cortical neural activity is directly linked together, such that when a cue is encountered, the memory may be retrieved directly from cortical-cortical connections, without the need for the hippocampus. And, although there are many details that need to be learned, there is much data suggesting that sleep is critical for consolidation to occur.11,12
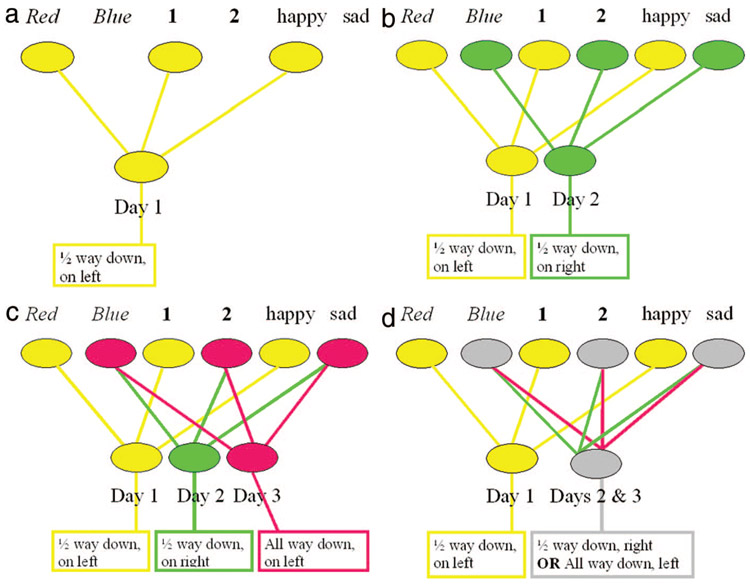
In looking at the cognitive neuroscience model just presented of how memories are stored and retrieved, it is the CA3 region of the hippocampus that seems most critical—it is this region in which the hippocampal index is formed, and in which pattern matching of cues and memories occurs. Although we do not exactly know how the CA3 region is involved in these activities, a number of models using neural networks have been proposed. A simplified example of such a model is as follows. In this example, we will show how an individual can find their car when they park in a parking garage over 3 successive days—and also why it is sometimes difficult for an individual to find their car. In this mythical parking garage, there are 2 areas, Red and Blue, and 2 levels, 1st and 2nd. Two affective states, happy and sad, are also shown to represent not only emotions but other contextual details that may differ between one day and the next. On the first day, the car is parked in the Red area, on the 1st floor, and it was a good day. From this distributed pattern of neural activity a hippocampal index can be formed that helps one remember that the car was parked in the Red area, on the 1st floor, and half-way down the aisle on the left (Fig. 7a). On the second day, the car is parked in the Blue area, on the 2nd floor, and it was not such as good day. Nonetheless, this distinct pattern of neural activity allows a unique hippocampal index to form that enables one to remember that the car was parked in the Blue area, on the 2nd floor, and half-way down the aisle on the right (Fig. 7b). On the third day, the car is also parked in the Blue area, on the 2nd floor, and it again was not such a good day (Fig. 7c). Although one might wish that a hippocampal index will form to enable one to remember that the car was parked in the Blue area, on the 2nd floor, and all the way down the aisle on the left—there is a problem. When there are completely overlapping patterns of neural activity, a separate hippocampal index cannot form. Instead, there is a single hippocampal index that forms for both days 2 and 3 (Fig. 7d). This hippocampal index is strengthened for the common aspects of the 2 memories: parking in the Blue area, on the 2nd floor. But, this index will also contain divergent aspects of the memory: half-way down the aisle on the right and all the way down the aisle on the left. Thus, on day 3, it will be easy to remember that the car is parked in the Blue area on the 2nd floor, but it will be difficult to remember if it is parked half-way down the aisle on the right or all the way down the aisle on the left.
FIGURE 7.
A neural network model. See text for details.
Over the last 10 years, it has become increasingly clear that in addition to the medial temporal lobes and Papez’s circuit, the frontal lobes are also important for episodic memory.
Over the last 10 years, it has become increasingly clear that in addition to the medial temporal lobes and Papez’s circuit, the frontal lobes are also important for episodic memory.13,14 Whereas the medial temporal lobes are critical for the retention of information, the frontal lobes are important for the acquisition, registration, or encoding of information7; retrieval of information without contextual and other cues15; recollection of the source of information16; and assessment of the temporal sequence and recency of events.17 Also, notable is that the left medial temporal and left frontal lobes are most active when a person is learning words,7 and that the right medial temporal and right frontal lobes are most active when learning visual scenes.8
One important reason why the frontal lobes are highly involved in episodic memory is that they enable the individual to focus their attention on the information to be remembered and to engage the medial temporal lobes. Dysfunction of the frontal lobes may cause a variety of memory problems, including distortions of episodic memory and false memories, such as when information becomes associated with the wrong context18 or incorrect specific details.19 Extreme memory distortions are often synonymous with confabulations, which occur when “memories” are created to be consistent with current information,18 such as “remembering” that someone broke into the house and rearranged household items.
A clinically useful analogy can be used to help conceptualize the dysfunction in episodic memory that occurs due to damage to the medial temporal lobes (and Papez’s circuit) versus damage to the frontal lobes.20,21 The frontal lobes are analogous to the “file clerk” of the episodic memory system, the medial temporal lobes to the “recent memory file cabinet,” and other cortical regions to the “remote memory file cabinet” (Table 3). Thus, if the frontal lobes are impaired, it is difficult—but not impossible—to get information in and out of storage. For example, getting information into storage may require stronger encoding, and getting information out of storage may require stronger cues from the environment. Additionally, when the frontal lobes are impaired the information stored in memory may be distorted due to “improper filing” that leads to an inaccurate source, context, or sequence. If, on the other hand, the medial temporal lobes are impaired, it may be impossible for recent information to be stored. This will often lead the patient to ask for the same information again and again—perhaps 20 times in an hour. Older information that has been consolidated over months to years is likely stored in other cortical regions and will therefore be available for retrieval even when the medial temporal lobes or Papez’s circuit are damaged. To illustrate this analogy we can compare the episodic memory dysfunction attributable to Alzheimer disease versus depression. Patients with Alzheimer disease have a dysfunctional “recent memory file cabinet,” whereas patients with depression have a dysfunctional “file clerk.”
TABLE 3.
A Filing Analogy of Episodic Memory
| Brain Structure | Analogy |
|---|---|
| Frontal lobes | File clerk |
| Medial temporal lobes | Recent memory files |
| Other cortical regions | Remote memory files |
The time course of the patient’s episodic memory deficit is often extremely helpful in distinguishing different disorders. Disorders of episodic memory may be transient, such as those due to concussion, seizure, or transient global amnesia. Static disorders, including traumatic brain injury, hypoxic or ischemic injury, single strokes, surgical lesions, and encephalitis, are typically maximal at onset, improve, and then become stable. (Note that in static disorders the onset may last several days, and the period of improvement may last 2 years or more.) Degenerative diseases, such as Alzheimer disease,22 dementia with Lewy bodies, and frontotemporal dementia, begin insidiously and progress gradually. Disorders that affect multiple brain regions, such as multiple sclerosis and vascular dementia, generally progress in a stepwise manner. Some disorders of memory can have a more complicated and variable time course, including memory dysfunction attributable to tumors, hypoglycemia, medications, and Korsakoff syndrome.
When a disorder of episodic memory is suspected due to inability to remember recent information and experiences accurately, further evaluation is warranted. A detailed history of the memory dysfunction should be taken, with particular emphasis on the time course of the memory disorder. Speaking with a caregiver or other informant is usually critical, since the patient with memory dysfunction will invariably not remember important aspects of the history. A history of other cognitive deficits (such as deficits in attention, language, visuospatial, and executive function) should be obtained. Medical and neurologic examinations should be performed, searching for signs of systemic illness, focal neurologic injury, and neurodegenerative disorders.
Brief cognitive testing may be performed by asking the patient to remember several words or a short story, or by using tools such as Mini-Mental State Examination,23 the Blessed Dementia Scale,24 the Three Words-Three Shapes memory test,2 the word list memory test of the Consortium to Establish a Registry for Alzheimer disease,25 the Drilled Word Span Test,2 and the Seven-Minute Screen.26 To help distinguish episodic memory dysfunction attributable to impairment of the frontal lobes versus impairment of the medial temporal lobes, difficulties in the encoding and retrieval of information should be contrasted with a primary failure of storage. When information cannot be remembered even when multiple rehearsals have maximized encoding, and retrieval demands have been minimized with the use of a multiple-choice recognition test, a primary failure of storage is present. In complex cases, a formal neuropsychological evaluation should be obtained.
The history, examination, and cognitive testing will suggest a differential diagnosis, which in turn will determine which laboratory and imaging studies are indicated. Treatment depends upon the specific disorder identified. Cholinesterase inhibitors have been approved by the Food and Drug Administration (FDA) to treat Alzheimer disease27 and Parkinson disease dementia28; these medications have also been used to treat vascular dementia29 and dementia with Lewy bodies.30 Memantine has been approved to treat Alzheimer disease, with or without concomitant treatment with cholinesterase inhibitors.31
Semantic Memory
Semantic memory refers to our store of conceptual and factual knowledge that is not related to any specific memory.
Semantic memory refers to our store of conceptual and factual knowledge that is not related to any specific memory, such as the color of broccoli or what a fork is used for. Like episodic memory, semantic memory, is an explicit and declarative memory. Evidence that semantic memory and episodic memory are separate memory systems has come from both neuroimaging studies4 and the fact that previously acquired semantic memory is spared in patients who have severe impairment of the episodic memory system, such as with disruption of Papez’s circuit or surgical removal of the medial temporal lobes.32
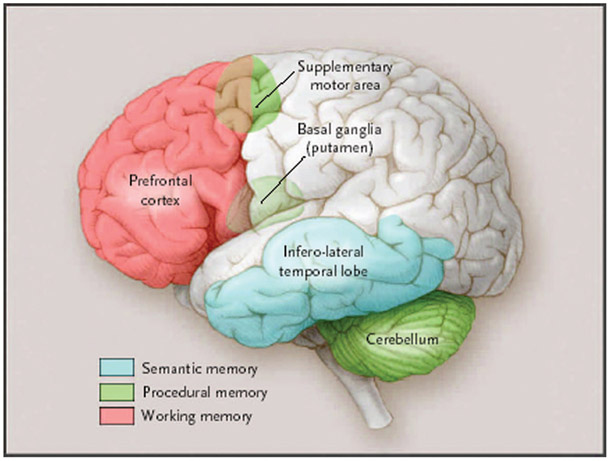
In its broadest sense, semantic memory includes all our knowledge of the world not related to any specific episodic memory. It could therefore be argued that semantic memory resides in multiple cortical areas throughout the brain. For example, there is evidence that visual images are stored in nearby visual association areas.33 A more restrictive view of semantic memory justified in light of the naming and categorization tasks by which it is usually tested, however, localizes semantic memory to the anterior and inferolateral temporal lobes (Fig. 8).34,35
FIGURE 8.
Semantic, procedural, and working memory. The anterior and inferolateral temporal lobes are important in the naming and categorization tasks by which semantic memory is typically assessed. However, in the broadest sense, semantic memory may reside in multiple and diverse cortical areas that are related to various types of knowledge. The basal ganglia, cerebellum, and supplementary motor area are critical for procedural memory. The prefrontal cortex is active in virtually all working memory tasks; other cortical and subcortical brain regions will also be active, depending on the type and complexity of the working memory task. In addition to being involved in procedural memory, the cerebellum is also important for the motoric conditioning. (Adapted with permission from Budson and Price, New England Journal of Medicine, 2005).
The most common clinical disorder disrupting semantic memory is Alzheimer disease. This disruption may be due to pathology in the anterior and inferolateral temporal lobes36 or to pathology in the frontal cortex,37 leading to poor activation and retrieval of semantic information.38 Supporting the idea that 2 separate memory systems are impaired in Alzheimer disease, episodic and semantic memory decline independently of each other in this disorder.39
Almost any disorder that can disrupt the anterior and inferolateral temporal lobes may cause impairment of semantic memory.
Almost any disorder that can disrupt the anterior and inferolateral temporal lobes may cause impairment of semantic memory, including traumatic brain injury, stroke, surgical lesions, encephalitis, and tumors (Table 2). Patients with semantic dementia (the temporal variant of frontotemporal dementia) exhibit deficits in all functions of semantic memory, such as naming, single-word comprehension, and impaired general knowledge (such as the color of common items). Other aspects of cognition, however, are relatively preserved, including components of speech, perceptual and nonverbal problem-solving skills, and episodic memory.40
Although naming difficulties (particularly with proper nouns) are common in healthy older adults, naming difficulties may also be a sign of a disorder of semantic memory. When a disorder of semantic memory is suspected, the evaluation should include the same components as the evaluation for episodic memory disorders. One of the first aspects of the history and cognitive examination that should be ascertained is whether the problem is solely one of difficulty in recalling people’s names and other proper nouns (common in healthy older adults) or to a true loss of semantic information. Patients with mild dysfunction of semantic memory may show only reduced generation of words in a semantic category (for example, the number of grocery items that can be generated in 1 minute), whereas patients with a more severe impairment of semantic memory usually show a 2-way naming deficit: they are unable to name an item when it is described, and they are also unable to describe an item when it is named. General knowledge is also impoverished in these more severely affected patients. Treatment will depend upon the specific disorder identified.
Simple Classic Conditioning
Simple classic conditioning involves the pairing of 2 stimuli—an unconditioned stimulus and a conditioned stimulus. When paired together repeatedly, the response can then be elicited by the conditioned stimulus alone (Table 1). Think of the famous case of Pavlov’s dog: the meat (the unconditioned stimulus) is paired with the bell (the conditioned stimulus). After a number of pairings, the response—salivation—is elicited by the bell (the conditioned stimulus) alone.41 This form of memory is nondeclarative and implicit because conscious awareness (although often present) is not necessary for the learning to take place. Two types of conditioning are that of an autonomic conditioned response (such as a fear response), and a motoric conditioned response (such as an eyeblink). The amygdala, related structures, and connections in the basolateral limbic system (including the dorsomedial thalamic nuclei, subcallosal area, and the stria terminalis) are important for autonomic conditioning (Fig. 1).42 In motoric conditioning, the cerebellum appears to play the most important role (Fig. 8).43,44
Although disruption of this form of memory rarely comes to clinical attention, patients have been described with selective impairment of simple classic conditioning. In one study, 3 patients are reported. The first, who had selective bilateral amygdala damage, had no difficulty with episodic memory (remembering a new list of items) but could not acquire a classic conditioning autonomic response. The second patient, who had selective bilateral hippocampal damage, showed episodic memory dysfunction (being unable to remember the list of items) but did acquire the classic conditioning. The third, who had bilateral damage to both amygdala and hippocampi, showed impairment in episodic memory and also did not acquire the classic conditioning.45 Other patients who have disruption in the amygdala, thalamus, or cerebellum may also show impairments of one or more types of simple classic conditioning, including those with Alzheimer disease (impaired autonomic conditioning due primarily to pathology in amygdala46) and those with damage to the cerebellum or its connections (impaired motor conditioning47).
Procedural Memory
Procedural memory refers to the ability to learn cognitive and behavioral skills and algorithms that operate at an automatic, unconscious level.
Procedural memory refers to the ability to learn cognitive and behavioral skills and algorithms that operate at an automatic, unconscious level. Procedural memory is nondeclarative and implicit. Examples include learning to ride a bike or play the piano (Table 1). Because procedural memory is spared in patients who have severe deficits of the episodic memory system (such as those who have undergone surgical removal of the medial temporal lobes), it is clear that the procedural memory system is separate and distinct from the episodic memory system.32,47
Functional imaging research has shown that a number of brain regions involved in procedural memory become active as a new task is learned, including the supplementary motor area, basal ganglia, and cerebellum (Fig. 8).48 Convergent evidence comes from studies of patients with damage to the basal ganglia or cerebellum who show impairment in learning procedural skills.49 Because the basal ganglia and cerebellum are relatively spared in early Alzheimer disease, despite their episodic memory deficit these patients show normal acquisition and maintenance of their procedural memory skills.
Parkinson disease is the most common disorder disrupting procedural memory. Patients in the early stages of Huntington chorea and olivopontocerebellar degeneration also show impaired procedural memory while performing nearly normally on episodic memory tests.47,50 Other causes of damage to the basal ganglia or cerebellum including tumors, strokes, and hemorrhages may also disrupt procedural memory. Patients with major depression also show impairment in procedural memory tasks, perhaps because depression involves dysfunction of the basal ganglia.51
Disruption of procedural memory should be suspected when patients show evidence of either substantial difficulties in learning new skills (compared with their baseline) or the loss of previously learned skills. For example, patients may lose the ability to perform automatic, skilled movements, such as writing, swinging a tennis racket, or playing a musical instrument. Although these patients may be able to relearn the fundamentals of these skills, explicit thinking becomes required for their performance. As a result, patients with damage to the procedural memory system lose the automatic effortlessness of simple motor tasks that healthy individuals take for granted. The evaluation of disorders of procedural memory is similar to that of disorders of episodic memory; treatment depends upon the specific disease process. Lastly, it is worth noting that patients whose episodic memory has been devastated by a static disorder, such as encephalitis, have had successful rehabilitation by using procedural memory (and other nondeclarative forms of memory) to learn new skills.52
Priming
Priming occurs when a prior encounter with a particular item changes the response to the current item (Table 1). Because this phenomenon occurs even if the individual does not consciously remember encountering the prior item, priming is another example of an implicit and nondeclarative form of memory. Priming is often divided into perceptual priming, which is modality specific (eg, auditory, visual) and does not benefit from elaborate encoding when materials are being learned, versus conceptual priming, which is not modality specific and shows enhancement with increased encoding.
Perceptual priming depends upon a perceptual representation system, involved in processing information regarding the form and structure of items but not their meanings.53 Converging evidence suggests that posterior cortical regions involved in processing of sensory information are important for perceptual priming. A patient with bilateral occipital lobe lesions demonstrated normal episodic memory and conceptual priming while failing to show perceptual priming.54 Neuroimaging studies of visual perceptual priming using PET and fMRI show changes in activation of visual peristriate cortex.55 By contrast, neuroimaging studies of conceptual priming typically show changes in left prefrontal regions.55 Most studies have shown that patients with early degenerative diseases that do not affect the sensory association cortices, such as Alzheimer, Parkinson, and Huntington diseases, demonstrate normal perceptual priming.56 For conceptual priming, however, many studies have found these groups to be impaired.47
Working Memory
Working memory refers to the ability to temporarily maintain and manipulate information that one needs to keep in mind.
Bringing together the traditional fields of attention, concentration, and short-term memory, working memory refers to the ability to temporarily maintain and manipulate information that one needs to keep in mind. Requiring active and conscious participation, working memory is an explicit and declarative memory system. Working memory has traditionally been divided into 3 components: one that processes phonologic information (eg, keeping a phone number “in your head”), one that processes spatial information (eg, mentally following a route), and an executive system that allocates attentional resources.57
Studies have demonstrated that working memory involves a network of cortical and subcortical areas, which differ depending on the particular task.58 Participation of the prefrontal cortex, however, is involved in virtually all working memory tasks (Fig. 8).13 The network of cortical and subcortical areas typically includes posterior brain regions (eg, visual association areas) that are linked with prefrontal regions to form a circuit. Research suggests that spatial working memory tends to involve more regions on the right side, and phonologic working memory tends to involve more regions on the left side of the brain. Bilateral brain activation is observed, however, in more difficult working memory tasks, regardless of the nature of the material being processed.1 Additionally, an increase in the number of brain regions activated in prefrontal cortex is observed as the complexity of the task increases.59
Because working memory depends upon networks that include frontal and parietal cortical regions as well as subcortical structures, most neurodegenerative diseases impair working memory. Studies have demonstrated that working memory may be impaired in patients with Alzheimer disease, Parkinson disease, Huntington chorea, and dementia with Lewy bodies, as well as less common disorders such as progressive supranuclear palsy (Table 2).60,61 In addition to these neurodegenerative diseases, almost any disease process that disrupts the frontal lobes or their connections to posterior cortical regions and subcortical structures can interfere with working memory. Such processes include tumors, strokes, multiple sclerosis, head injury, and others.62,63 Because it involves the silent rehearsal of verbal information, almost any type of aphasia may impair phonologic working memory. Disorders that diminish attentional resources, including attention deficit hyperactivity disorder, obsessive compulsive disorder, depression, and schizophrenia, can also impair working memory.64-66
Disorders of working memory may present in several different ways. Often the patient will exhibit an inability to concentrate or pay attention. Impairment in performing a new task with multistep instructions is frequently seen. Interestingly, a disorder of working memory may also present as a problem with episodic memory, because information must first be “kept in mind” by working memory in order for episodic memory to encode it.13 Such cases will therefore show a primary impairment in encoding.
The evaluation of disorders of working memory is similar to that of disorders of episodic memory. Treatment depends upon the underlying cause. Stimulants, approved by the FDA for the treatment of attention deficit hyperactivity disorder,67,68 will often be helpful in disorders of working memory.
CONCLUSION
Although traditionally, memory has been viewed as a simple concept, converging and complementary evidence from patient studies and more recent neuroimaging research suggest that memory is composed of separate and distinct systems. Improved understanding of these different types of memory will aid the clinician in the diagnosis and treatment of the memory disorders of their patients. As more specific therapeutic strategies are developed for the treatment of diseases that cause memory dysfunction, this knowledge will become increasingly important.
ACKNOWLEDGMENTS
This work was supported by National Institute on Aging grant P30 AG13846. This material is also the result of work supported with resources and the use of facilities at the Edith Nourse Rogers Memorial Veterans Hospital in Bedford, MA.
REFERENCES
- 1.Newman SD, Carpenter PA, Varma S, et al. Frontal and parietal participation in problem solving in the Tower of London: fMRI and computational modeling of planning and high-level perception. Neuropsychologia. 2003;41:1668–1682. [DOI] [PubMed] [Google Scholar]
- 2.Mesulam MM. Principles of Behavioral and Cognitive Neurology. 2nd ed. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 3.Ally BA, Waring JD, Beth EH, et al. Aging memory for pictures: using high-density event-related potentials to understand the effect of aging on the picture superiority effect. Neuropsychologia. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schacter DL, Wagner AD, Buckner RL. Memory systems of 1999. In: Tulving E, Craik FIM, eds. The Oxford Handbook of Memory. New York: Oxford University Press; 2000:627–643. [Google Scholar]
- 5.Schacter DL, Tulving E. What are the memory systems of 1994? In: Schacter DL, Tulving E, eds. Memory Systems 1994. Cambridge, Massachusetts: MIT Press; 1994:1–38. [Google Scholar]
- 6.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. [DOI] [PubMed] [Google Scholar]
- 7.Wagner AD, Schacter DL, Rotte M, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. [DOI] [PubMed] [Google Scholar]
- 8.Brewer JB, Zhao Z, Desmond JE, et al. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. [DOI] [PubMed] [Google Scholar]
- 9.Papez JW. A proposed mechanism of emotion. Arch Neurol Psychiatry 1937;3:725–743. [Google Scholar]
- 10.Ribot T Les Maladies de la Mémoire. Paris: Félix Alcan; 1881. [Google Scholar]
- 11.Stickgold R Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. [DOI] [PubMed] [Google Scholar]
- 12.Stickgold R Neuroscience: a memory boost while you sleep. Nature. 2006;444:559–560. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher PC, Henson RNA. Frontal lobes and human memory: insights from functional neuroimaging. Brain. 2001;124:849–881. [DOI] [PubMed] [Google Scholar]
- 14.Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Reviews: Neuroscience. 2003;4:637–648. [DOI] [PubMed] [Google Scholar]
- 15.Petrides M The mid-ventrolateral prefrontal cortex and active mnemonic retrieval. Neurobiol Learn Mem. 2002;78:528–538. [DOI] [PubMed] [Google Scholar]
- 16.Johnson MK, Kounios J, Nolde SF. Electrophysiological brain activity and memory source monitoring. Neuroreport. 1997;8:1317–1320. [DOI] [PubMed] [Google Scholar]
- 17.Kopelman MD, Stanhope N, Kingsley D. Temporal and spatial context memory in patients with focal frontal, temporal lobe, and diencephalic lesions. Neuropsychologia. 1997;35:1533–1545. [DOI] [PubMed] [Google Scholar]
- 18.Johnson MK, O’Connor M, Cantor J. Confabulation, memory deficits, and frontal dysfunction. Brain Cogn. 1997;34:189–206. [DOI] [PubMed] [Google Scholar]
- 19.Budson AE, Sullivan AL, Mayer E, et al. Suppression of false recognition in Alzheimer’s disease and in patients with frontal lobe lesions. Brain. 2002;125:2750–2765. [DOI] [PubMed] [Google Scholar]
- 20.Budson AE, Price BH. Memory: Clinical disorders. In: Fullerlove G, ed. Encyclopedia of Life Sciences. Vol. 11. London, England: Macmillan Publishers Ltd, Nature Publishing Group; 2002:529–536. [Google Scholar]
- 21.Budson AE, Price BH. Memory dysfunction. N Engl J Med. 2005;352:692–699. [DOI] [PubMed] [Google Scholar]
- 22.Solomon PR, Budson AE. Alzheimer’s Disease. Clin Symp. 2003;54:1–44. [Google Scholar]
- 23.Folstein MF, Folstein SE, McHugh PR. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 24.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. [DOI] [PubMed] [Google Scholar]
- 25.Welsh KA, Butters N, Hughes JP, et al. Detection and staging of dementia in Alzheimer’s disease. Use of the neuropsychological measures developed for the Consortium to Establish a Registry for Alzheimer’s Disease. Arch Neurol. 1992;49:448–452. [DOI] [PubMed] [Google Scholar]
- 26.Solomon PR, Hirschoff A, Kelly B, et al. A 7 minute neurocognitive screening battery highly sensitive to Alzheimer’s disease. Arch Neurol. 1998;55:349–355. [DOI] [PubMed] [Google Scholar]
- 27.Winblad B, Engedal K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57:489–495. [DOI] [PubMed] [Google Scholar]
- 28.Emre M, Aarsland D, Albanese A, et al. Rivastigmine for dementia associated with Parkinson’s disease. N Engl J Med. 2004;351:2509–2518. [DOI] [PubMed] [Google Scholar]
- 29.Moretti R, Torre P, Antonello RM, et al. Use of galantamine to treat vascular dementia. Lancet. 2002;360:1512–1513. [DOI] [PubMed] [Google Scholar]
- 30.McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356:2031–2036. [DOI] [PubMed] [Google Scholar]
- 31.Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291:317–324. [DOI] [PubMed] [Google Scholar]
- 32.Corkin S Lasting consequences of bilateral medial temporal lobectomy: clinical course and experimental findings in H. M. Semin Neurol. 1984;4:249–259. [Google Scholar]
- 33.Vaidya CJ, Zhao M, Desmond JE, et al. Evidence for cortical encoding specificity in episodic memory: memory-induced re-activation of picture processing areas. Neuropsychologia. 2002;40:2136–2143. [DOI] [PubMed] [Google Scholar]
- 34.Damasio H, Grabowski TJ, Tranel D, et al. A neural basis for lexical retrieval. Nature. 1996;380:499–505. [DOI] [PubMed] [Google Scholar]
- 35.Perani D, Cappa SF, Schnur T, et al. The neural correlates of verb and noun processing. A PET study. Brain. 1999;122:2337–2344. [DOI] [PubMed] [Google Scholar]
- 36.Price JL, Morris JC. Tangles and plaques in nondemented aging and “pre-clinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. [DOI] [PubMed] [Google Scholar]
- 37.Lidstrom AM, Bogdanovic N, Hesse C, et al. Clusterin (apolipoprotein J) protein levels are increased in hippocampus and in frontal cortex in Alzheimer’s disease. Exp Neurol. 1998;154:511–521. [DOI] [PubMed] [Google Scholar]
- 38.Balota DA, Watson JM, Duchek JM, et al. Cross-modal semantic and homographic priming in healthy young, healthy old, and in Alzheimer’s disease individuals. J Int Neuropsychol Soc. 1999;5:626–640. [DOI] [PubMed] [Google Scholar]
- 39.Green JD, Hodges JR. Identification of famous faces and famous names in early Alzheimer’s disease. Relationship to anterograde episodic and general semantic memory. Brain. 1996;119:111–128. [DOI] [PubMed] [Google Scholar]
- 40.Hodges JR. Frontotemporal dementia (Pick’s disease): clinical features and assessment. Neurology. 2001;56:6–10. [DOI] [PubMed] [Google Scholar]
- 41.Pavlov IP. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. London: Oxford University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markowitsch HJ. Anatomical basis of memory disorders. In: Gazzaniga MS, ed. The Cognitive Neurosciences. Cambridge, Massachusetts: MIT Press, 1995:765–779. [Google Scholar]
- 43.Dimitrova A, Weber J, Maschke M, et al. Eyeblink-related areas in human cerebellum as shown by fMRI. Hum Brain Mapp. 2002;1:100–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Solomon PR, Stowe GT, Pendlbeury WW. Disrupted eyelid conditioning in a patient with damage to cerebellar afferents. Behav Neurosci. 1989;103:898–902. [DOI] [PubMed] [Google Scholar]
- 45.Bechara A, Tranel D, Damasio H, et al. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:115–118. [DOI] [PubMed] [Google Scholar]
- 46.Chu CC, Tranel D, Damasio AR, et al. The autonomic-related cortex: pathology in Alzheimer’s disease. Cereb Cortex. 1997;7:86–95. [DOI] [PubMed] [Google Scholar]
- 47.Heindel WC, Salmon DP, Shults CW, et al. Neuropsychological evidence for multiple implicit memory systems: a comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. J Neurosci. 1989;9:582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Daselaar SM, Rombouts SA, Veltman DJ, et al. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiol Aging. 2003;24:1013–1019. [DOI] [PubMed] [Google Scholar]
- 49.Exner C, Koschack J, Irle E. The differential role of premotor frontal cortex and basal ganglia in motor sequence learning: evidence from focal basal ganglia lesions. Learn Mem. 2002;9:376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salmon DP, Lineweaver TT, Heindel WC. Nondeclarative memory in neurodegenerative disease. In: Troster AI, ed. Memory in Neurodegenerative Disease: Biological, Cognitive, and Clinical Perspectives. Cambridge, UK: Cambridge University Press, 1998:210–225. [Google Scholar]
- 51.Sabe L, Jason L, Juejati M, et al. Dissociation between declarative and procedural learning in dementia and depression. J Clin Exp Neuropsychol. 1995;17:841–848. [DOI] [PubMed] [Google Scholar]
- 52.Glisky EL, Schacter DL. Extending the limits of complex learning in organic amnesia: computer training in a vocation domain. Neuropsychologia. 1989;27:173–178. [DOI] [PubMed] [Google Scholar]
- 53.Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;24:301–306. [DOI] [PubMed] [Google Scholar]
- 54.Keane MM, Gabrieli JD, Mapstone HC, et al. Double dissociation of memory capacities after bilateral occipital-lobe or medial temporal-lobe lesions. Brain. 1995;118:1129–1148. [DOI] [PubMed] [Google Scholar]
- 55.Schacter DL, Buckner RL. Priming and the Brain. Neuron. 1998;20:185–195. [DOI] [PubMed] [Google Scholar]
- 56.Koivisto M, Portin R, Rinne JO. Perceptual priming in Alzheimer’s and Parkinson’s diseases. Neuropsychologia. 1996;34:449–457. [DOI] [PubMed] [Google Scholar]
- 57.Baddeley AD. Recent developments in working memory. Cur Opin Neurobiol. 1998;8:234–238. [DOI] [PubMed] [Google Scholar]
- 58.Rowe JB, Toni I, Josephs O, et al. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;2:1656–1660. [DOI] [PubMed] [Google Scholar]
- 59.Jaeggi SM, Seewer R, Nirkko AC, et al. Does excessive memory load attenuate activation in the prefrontal cortex? Load-dependent processing in single and dual tasks: functional magnetic resonance imaging study. Neuroimage. 2003;1:210–225. [DOI] [PubMed] [Google Scholar]
- 60.Calderon J, Perry RJ, Erzinclioglu SW, et al. Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2001;70:157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gotham AM, Brown RG, Marsden CD. ‘Frontal’ cognitive functions in patients with Parkinson’s disease ‘on’ and ‘off’ levodopa. Brain. 1988;111:299–321. [DOI] [PubMed] [Google Scholar]
- 62.Kubat-Silman AK, Dagenbach D, Absher JR. Patterns of impaired verbal, spatial, and object working memory after thalamic lesions. Brain Cogn. 2002;50:178–193. [DOI] [PubMed] [Google Scholar]
- 63.Sfagos C, Papageorgiou CC, Kosma KK, et al. Working memory deficits in multiple sclerosis: a controlled study with auditory P600 correlates. J Neurol Neurosurg Psychiatry. 2003;4:1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Egeland J, Sundet K, Rund BR, et al. Sensitivity and specificity of memory dysfunction in schizophrenia: a comparison with major depression. J Clin Exp Neuropsychol. 2003;25:79–93. [DOI] [PubMed] [Google Scholar]
- 65.Klingberg T, Forssberg H, Westerberg H. Training of working memory in children with ADHD. J Clin Exp Neuropsychol. 2002;24:781–791. [DOI] [PubMed] [Google Scholar]
- 66.Purcell R, Maruff P, Kyrios M, et al. Cognitive deficits in obsessive-compulsive disorder on tests of frontal-striatal function. Biol Psychiatry. 1998;43:348–357. [DOI] [PubMed] [Google Scholar]
- 67.Elia J, Ambrosini PJ, Rapoport JL. Treatment of attention-deficit-hyperactivity disorder. N Engl J Med. 1999;340:780–788. [DOI] [PubMed] [Google Scholar]
- 68.Mehta MA, Goodyer IM, Sahakian BJ. Methylphenidate improves working memory and set-shifting in AD/HD: relationships to baseline memory capacity. J Child Psychol Psychiatry. 2004;45:293–305. [DOI] [PubMed] [Google Scholar]