Abstract
Objectives
To describe clinical characteristics, laboratory tests, radiological data and outcome of pediatric cases with SARS-CoV-2 infection complicated by neurological involvement.
Study design
A computerized search was conducted using PubMed. An article was considered eligible if it reported data on pediatric patient(s) with neurological involvement related to SARS-CoV-2 infection. We also described a case of an acute disseminated encephalomyelitis (ADEM) in a 5-year-old girl with SARS-CoV-2 infection: this case was also included in the systematic review.
Results
Forty-four articles reporting 59 cases of neurological manifestations in pediatric patients were included in our review. Most (32/59) cases occurred in the course of a multisystem inflammatory syndrome in children (MIS-C). Neurological disorders secondary to cerebrovascular involvement were reported in 10 cases: 4 children with an ischemic stroke, 3 with intracerebral hemorrhage, 1 with a cerebral sinus venous thrombosis, 1 with a subarachnoid hemorrhage, 1 with multiple diffuse microhemorrhages. Reversible splenial lesions were recognized in 9 cases, benign intracranial hypertension in 4 patients, meningoencephalitis in 4 cases, autoimmune encephalitis in 1 girl, cranial nerves impairment in 2 patients and transverse myelitis in 1 case. Five cases had Guillain-Barré syndrome (GBS) and two, including ours, had ADEM. Radiological investigations were performed in almost all cases (45/60): the most recurrent radiological finding was a signal change in the splenium of the corpus callosum. The presence of SARS-CoV-2 viral nucleic acid in the cerebrospinal fluid was proved only in 2 cases. The outcome was favorable in almost all, except in 5 cases.
Conclusions
Our research highlights the large range of neurological manifestations and their presumed pathogenic pathways associated with SARS-CoV-2 infection in children. Nervous system involvement could be isolated, developing during COVID-19 or after its recovery, or arise in the context of a MIS-C. The most reported neurological manifestations are cerebrovascular accidents, reversible splenial lesions, GBS, benign intracranial hypertension, meningoencephalitis; ADEM is also a possible complication, as we observed in our patient. Further studies are required to investigate all the neurological complications of SARS-CoV-2 infection and their underlying pathogenic mechanism.
Introduction
At the end of December 2019, many cases of atypical pneumonia of unknown origin were described in the city of Wuhan, China. In January 2020 a novel coronavirus, later called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was identified as the responsible of a new disease called coronavirus disease 2019 (COVID-19), declared pandemic by the World Health Organization (WHO) in March 2020.
As regards pediatric COVID-19 cases, unlike the clinical presentation of adult patients, a systematic review showed that the most commonly reported symptoms are fever, cough, pharyngitis and rhinorrhea; other frequent symptoms are headache, myalgia, rash, conjunctivitis, syncopal episodes and gastrointestinal manifestations such as vomiting, diarrhea, abdominal pain and difficulty in feeding [1–3].
In later April 2020, a novel syndrome in children and adolescents, termed multisystem inflammatory syndrome in children (MIS-C), related to SARS-CoV-2 infection was first described: initial reports surfaced in the United Kingdom and Italy [4, 5]. This condition, similar to Kawasaki disease and toxic shock syndrome, is characterized by persistent fever, a multisystem (≥ 2) organ involvement, elevation of inflammatory markers, link to SARS-CoV-2 (verified by polymerase chain reaction, serology or COVID-19 contact) and the exclusion of alternative diagnosis [6].
Regarding neurological involvement in COVID-19, severe neurological manifestations (encephalopathy, meningoencephalitis, stroke, seizure, Guillain-Barré syndrome, acute disseminated encephalomyelitis) have been reported mainly in adults [7, 8], while a few cases have been described in children. Two mechanisms were proposed to explain how SARS-CoV-2 may induce neurological damage: direct viral infection of nervous system through ACE2 receptors and inflammatory injury mediated by cytokines release [9]; in the latter case, neurological manifestations may be part of a MIS-C [10].
We describe here a case of acute disseminate encephalomyelitis (ADEM) related to SARS-CoV-2 infection in a pediatric patient and, with the aim of focus our attention on neurological manifestations of pediatric patients with SARS-CoV-2 infection, we performed a systematic review of the literature contextualizing our new case among all the cases retrieved in our search.
Case report
A 5-year-old girl presented with a 3-day history of fever, neck swelling and erythematous skin rash. In the previous days an antigen rapid swab test for SARS-CoV-2 was performed with a negative result and she was treated with antibiotic and anti-inflammatory therapy.
On physical examination, the child was febrile (body temperature 39 °C); the skin was characterized by a maculopapular and not itchy rash on the face, neck, trunk and extremities, with palmoplantar involvement. A right laterocervical and painful lymphadenopathy, eyelid, hand and foot edema, red and fissuring lips and injected pharynx were present. The abdomen was painful and she complained of diarrhea. Cardiovascular, respiratory and neurological examinations were normal. Vital signs showed oxygen saturation 99%, heart rate 104 bpm, blood pressure 104/60 mmHg.
Blood tests revealed microcytic and hypochromic anemia, leukocytosis with lymphopenia, C-reactive protein (CRP) 20.55 mg/dL (normal value < 0.6), procalcitonin 4.5 ng/mL (normal value < 0.5), fibrinogen 649 mg/dL (normal range 200–400), D-dimer 2653 ng/mL (normal range < 500), ferritin 603 ng/mL (normal range 11–306), hyponatremia and hypoalbuminemia. Chest radiograph and abdomen ultrasound showed no abnormalities, while neck ultrasound revealed different oval-shape nodes with maximum diameter of 1.6 cm. Echocardiogram and electrocardiogram, performed to rule out Kawasaki disease, did not show pathological findings.
Two days after hospital admission, the girl became irritable; neck stiffness, muscular weakness and right Babinski sign were also found. In suspicion of viral encephalitis, she was treated with intravenous (IV) acyclovir 10 mg/kg three times a day. Brain MRI showed two lesions, one in the splenium of the corpus callosum and the other in the subcortical white matter of the left parietal lobe, that exhibit restricted diffusion without contrast enhancement (Figs. 1, 2 and 3).
Fig. 1.

MRI DWI: lesion in the left subcortical white matter
Fig. 2.

MRI DWI: lesion of the splenium of corpus callosum (transversal section)
Fig. 3.

MRI DWI: lesion of the splenium of corpus callosum (sagittal section)
Electroencephalogram (EEG) disclosed a generalized slowing of background activity. Cerebrospinal fluid (CSF) was tested: samples were acellular, with normal levels of proteins and glucose and no evidence of viral or bacterial infection (Escherichia coli, Streptococcus pneumoniae, Haemophilus influenzae, Klebsiella pneumoniae, Streptococcus agalactiae, Neisseria meningitidis, Lysteria monocytogenes, Adenovirus, Herpes simplex virus 1–2, Varicella Zoster virus, Citomegalovirus, Epstein-Barr virus, Enterovirus) on real-time polymerase chain reaction (RT-PCR). Tests for oligoclonal bands in CSF and serum neuronal autoantibodies (anti-NMDA, anti-VGCK, anti-AMPA) had negative results.
The molecular nasopharyngeal swab test for SARS-CoV-2 detected initially low viral load, while the second specimen was negative. A COVID-19 serology test, performed a week after the hospital admission, revealed IgG positive and IgM within grey-zone limits.
According to multi-organ involvement, neuroradiological findings, laboratory exams with elevated inflammatory parameters, temporal relationship with SARS-CoV-2 infection and exclusion of other causes, a diagnosis of ADEM in a patient with MIS-C was made; she started methylprednisolone 1 mg/kg/day IV and immunoglobulin 0.4 g/kg/day for 5 days IV, with a progressive resolution of the systemic hyperinflammatory state and improvement of neurological symptoms. Brain MRI, performed two weeks after the first one, demonstrated no abnormalities.
Literature search
A computerized search was performed using PubMed, combining the terms (neurolog* OR CNS OR nervous OR encephal*) AND (COVID OR SARS-CoV-2 OR coronavirus) AND (baby OR child* OR pediatr*) with English language filter, to identify studies on neurological manifestations in children with SARS-CoV-2 infection, published until December 31, 2020. Furthermore, references within the included articles were scanned for other relevant papers. The following data were evaluated for each case: age, sex, comorbidities, clinical features, radiological and other neurological investigations, laboratory test for confirmation of SARS-CoV-2 infection and outcome; we also assessed if neurological complication occurred in the course of a MIS-C. We excluded articles that reported only aggregate data and that revealed the presence of coinfection with other microbes. The selected articles were reviewed by two independent authors and judged on their relevant contribution to the subject of the study. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines were followed [11].
Results
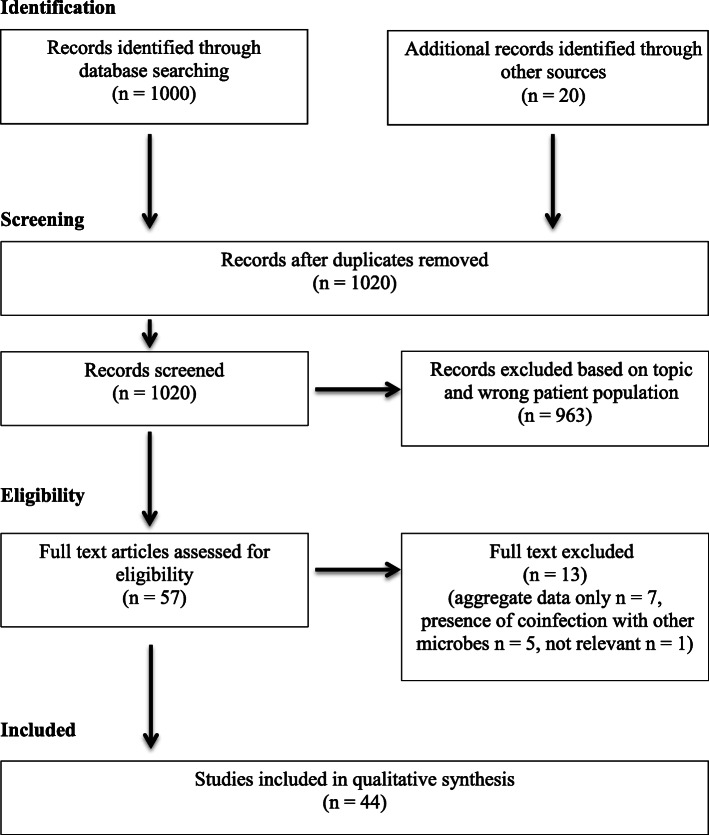
After an extensive search in PubMed, 1000 articles were identified, along with 20 additional records detected though hand-searching (Fig. 4). 1020 records were screened; 963 were excluded after title and abstract screening and 13 were excluded after full-text review. We selected 44 studies for inclusion [5, 12–54], reporting 59 cases of neurological manifestations in pediatric patients with SARS-CoV-2 infection. Most of the articles were single case reports, 10 were case series. Clinical and radiological features, diagnosis and outcome of 60 patients (including our new case) are systematically reported in Table 1.
Fig. 4.
PRISMA study flow diagram: flow diagram of study identification, screening, eligibility, and included studies
Table 1.
Reported cases of neurological involvement during SARS-CoV-2 infection in children
| Author/Country [Ref.] | Age/sex | Pre-existing medical conditions | Neurological symptoms | Respiratory symptoms | Other symptoms | Diagnosis of MIS-C | NP/CSF/Serology SARS-CoV-2 | Radiology and other neurological investigations | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| Abdel-Mannan et al./UK [12] 4 cases | 8 y/M | No | Headache, meningism, confusion, muscular weakness | No | Fever, rash, abdominal pain, emesis, shock | Yes | Pos/Neg/ND | CT: hypodensity of the splenium of the corpus callosum | Improved |
| 9 y/M | No | Headache, confusion, ataxia, dysarthria, muscular weakness | No | Fever, rash, emesis, shock | Yes | Pos/Neg/ND | MRI: signal changes of the genu and splenium of corpus callosum and bilateral centrum semiovale with restricted diffusion | Recovered | |
| 15 y/F | No | Confusion, dysarthria, dysphagia, muscular weakness | Yes | Fever, rash, emesis, shock | Yes | Pos/ND/Pos | MRI: signal changes in the splenium of corpus callosum and bilateral centrum semiovale with restricted diffusion | Improved | |
| 15 y/F | No | Headache, confusion, muscular weakness | Yes | Fever, rash, emesis, shock | Yes | Pos/ND/Pos | MRI: signal change in the splenium of corpus callosum with restricted diffusion | Recovered | |
| Abel et al./USA [13] | 3 y/M | No | Irritability, hypotonia, muscular weakness | Yes | Fever, rash, emesis | Yes | Neg/Neg/Pos |
MRI: restricted diffusion in the bilateral lateral thalamic nuclei EEG: moderate slow background activity |
Improved, under physiotherapy |
| Asif et al./UK [14] | 18 y/M | No | Headache, photophobia | No | Fever, cough and myalgia before neurological manifestations | No | Neg/ND/ND (previous diagnosis of COVID-19) | CT venogram: filling defects in the sigmoid and transverse sinuses bilaterally and in the straight and superior sagittal sinuses | Improved |
| Baccarella et al./US A[15]2 cases | 9 y/M | No | Headache, diplopia, right abducens nerve palsy | No | Fever, abdominal pain | Yes | Neg/Neg/Pos |
MRI: normal LP: elevated opening pressure |
Recovered |
| 6 y/M | No | Headache, diplopia, right abducens nerve palsy | No | NR | Yes | Pos/Neg/Pos | MRI: finding consistent with elevated intracranial pressure | Recovered | |
| Basirjafari et al./Iran [16] | 9 y/M | No | Headache, bilateral fixed mydriasis | Yes | Fever, abdominal pain | No | Pos/ND/ND | CT: hyperdensity at basal cisterns, interhemispheric and bilateralSylvian fissures suggesting of subarachnoid hemorrhage and reduction of white matter density (brain edema) | Died |
| Bektas et al./Turke y[17]2 cases | 10 y/M | No | Visual hallucinations, personality changes | Yes | Fever, diarrhea, rash, hands and feet edema | Yes | Neg/ND/Pos |
MRI: hyperintensity in the splenium of corpus callosum with restricted diffusion EEG: slowed background activity |
Recovered |
| 11 y/F | No | Personality changes | Yes | Fever, diarrhea, rash, conjunctivitis, hypotension | Yes | Neg/ND/Pos |
MRI: hyperintensity in the splenium of corpus callosum with restricted diffusion EEG: slowed background activity |
Recovered | |
| Bhatta et al./USA [18] | 11 y/M | No | Seizure | No | No | No | Pos/NR/ND | CT: normal | Recovered |
| Burr et al./USA [19] | 23 m/F | No | Irritability, hyperkinetic movements of head, arms and legs | No | Fever | No | Pos/Neg/Pos |
MRI: normal NMDAR-IgG positivity |
Recovered |
| Chiotos et al./US A[20]4 cases | 14 y/F | No | Headache | Yes | Fever, rash, diarrhea | Yes | Neg/ND/Pos | ND | Recovered |
| 12 y/M | No | Altered mental status, irritability | Yes | Fever, fissured lips, abdominal pain, diarrhea, shock | Yes | Neg/ND/Pos | ND | Recovered | |
| 5 y/F | No | Altered mental status, irritability, nuchal rigidity | No | Fever, conjunctivitis, shock | Yes | Neg/ND/Pos | ND | Recovered | |
| 5 y/F | No | Irritability, nuchal rigidity | No | Fever, rash, conjunctivitis, fissured lips, swollen hands, emesis, diarrhea, shock | Yes | Pos/ND/Pos | CT: diffuse cerebral edema | Recovered | |
| Curtis et al./India [21] | 8 y/M | No | Muscolar weakness, paralysis and paresthesia of the lower limbs | No | No | No | Pos/Neg/Pos |
MRI: enhancement of the posterior nerve roots from T11 to cauda equine LP: albuminocytologic dissociation |
Improved |
| de Miranda Henriques-Souza et al./Brazil [22] | 12 y/F | No | Headache, muscular weakness, tetraplegia | Yes | Fever, rash | Yes | Pos/Neg/ND | MRI: bilateral and symmetric areas of restricted diffusion involving the subcortical and deep white matter. Extensive cervical myelopathy | Improved |
| De Paulis et al./Brazil [23] | 4 y/F | No | Confusion, lethargy | Yes | Fever, emesis, rash, palpebrae, hands and feet edema, cracked lips, shock | Yes | Neg/Neg/Pos |
CT: normal LP: pleiocytosis and elevated protein |
Improved |
| Emami et al./Iran [24] | 2.9 y/M | Allergy to cow milk | Seizure, altered mental status, dysarthria | No | Fever | No | Pos/ND/NR |
MRI: right occipital mass andintracerebral hemorrhage EEG: generalized slowing (pathology of the mass: normal brain tissue with dilated vessels and haemorrhage) |
Recovered |
| Enner et al./USA [25] | 14 y/F | No | Seizure and central apnea | Yes | Fever, nasal congestion, myalgia | No | Pos/Neg/ND |
MRI: normal EEG: epileptiform abnormalities |
Improved |
| Frank et al./Brazil [26] | 15 y/M | No | Ascending weakness froma the lower to the upper limbs, headache | No | Fever | No | Pos/Neg/Pos |
MRI: normal Electroneurography: acute motor axonal neuropathy |
Improved, under physiotherapy |
| Gaur et al./ U K[27]2 cases | 12 y/M | NR | Headache, lethargy | No | Fever, diarrhea, conjunctivitis, shock | Yes | Neg/ND/Pos | MRI: hyperintensity in the splenium of corpus callosum with restricted diffusion | Recovered |
| 9 y/M | NR | Lethargy, ataxia, dysarthria | No | Fever | No | Neg/ND/NDPos broncho-alveolar lavage | MRI: hyperintensity in the splenium of corpus callosum and in the deep cerebral white matter with restricted diffusion | Recovered | |
| Gulko et al./USA [28] | 13 y/F | No | Headache, muscular weakness, speech difficulty | No | No | No | Pos/ND/ND |
CT: left frontal hypodensity concerning for ischemic infarct. MRI: hyperintensity with restricted diffusion in the left frontal, parietal and temporal lobes; stenosis of the left middle cerebral artery |
Improved |
| Kaur et al./Mexico [29] | 3 y/F | No | Quadriparesis and paresthesia | Yes | Neurogenic respiratory failure | No | Pos/Neg/ND | MRI: swelling of the cervical spinal cord involving most of the transverse aspect of the spinal cord, extending from the lower medulla to the midthoracic level | Quadriparesis |
| Khalifa et al./Saudi Arabia [30] | 11 y/M | No | Muscular weakness, hypotonia, paresthesia in the lower limbs | Yes | Fever and cough before neurological manifestations | No | Pos/NR/ND |
MRI: cauda equina nerve root enhancement LP: albuminocytologic dissociation |
Recovered |
| Kim et al./USA [31] | 7 y/M | No | Headache, emesis | No | Fever, abdominal pain | Yes | Pos/Neg/ND |
CT: diffuse cerebral edema EEG: generalized voltage attenuation |
Died |
| Lin et al./USA [32] | 13 y/F | No | Dizziness, gait instability, auditory hallucinations | Yes | Fever, diarrhea, emesis, hypotension | Yes | Pos/Neg/Pos |
MRI: hyperintensity in the splenium of corpus callosum with restricted diffusion EEG: slow background activity |
Recovered |
| Lorenz et al./Germany [33] | 40 w/F | No | Lethargy, hyperexcitable | Yes | Fever | No | Pos/Neg/ND | US: normal | Recovered |
| Manji et al./Tanzania [34] | 12 y/M | No | Progressive paresis, bilateral facial nerve paresis | Yes | Fever and cough before neurological manifestations | No | Pos/ND/ND | ND | Died |
| McAbee et al./ USA [35] | 11 y/M | No | Seizure | No | Fever | No | Pos/Neg/ND |
CT: normal EEG: intermittent frontal delta activity LP: pleiocytosis |
Recovered |
| Mirzaee et al./Iran [36] | 12 y/M | No | Seizure, dysarthria, hemiparesis | No | No | No | Pos/Pos/ND | MRI: acute infarction with narrowing of the left middle cerebral artery | Improved, under rehabilitation |
| Moreno-Galarraga et al./Spain [37] | 2 m/F | No | Headache, seizure | No | Diarrhea Flu-like symptoms before neurological manifestations | No | Pos/NR/ND |
MRI: normal LP: normal |
Recovered |
| Natarajan et al./India [38] | 13 y/F | No | Headache, irritability, seizure | No | Fever | No | Pos/Neg/ND |
MRI: normal LP: pleiocytosis |
Recovered |
| Paybast et al./Iran [39] | 14 y/F | NR | Progressive paresthesia, muscular weakness, headache, dizziness | No | Flu-like symptoms before neurological manifestations | No | Pos/ND/ND | LP: albuminocytologic dissociation | Improved |
| Raj et al./India [40] | 2 y/M | No | Seizure | No | Fever, diarrhea, hypotension | Yes | Pos/Neg/Neg | ND | Recovered |
| 15 m/M | No | Seizure | No | Fever, rash, conjunctivitis, cheilitis | Yes | NR/ND/NR(COVID-19 contact) | ND | Recovered | |
| 8 m/M | NR | Seizure | No | Fever | No | Pos/ND/ND | ND | Recovered | |
| Regev et al./Israel [41] | 16 y/M | No | Headache, nuchal rigidity | No | Fever, abdominal pain, rash, conjunctivitis, pharyngitis, shock | Yes | Pos/ND/Pos | MRI: multiple low attenuating small lesionsin the subcortical white matter, internal and external capsule and in the anterior and posterior part of the corpus callosum, suggesting microhemorrhages | Recovered |
| Roussel et al./France [42] | 6 y/F | Sickle cell disease, cerebral vasculopathy, HSCT | Impairment of V-VII-IX cranial nerves | Yes | No | No | Pos/Neg/ND | MRI: cranial nerves enhancement (left hypoglossal nerve and bilateral facial nerves) | Improved |
| Saeed et al./Iran [43] | 3 y/M | No | Seizure | No | Fever, hypotension | Yes | Pos/Neg/ND |
CT: cerebral edema MRI: intracerebral hemorrhage in the right occipital lobe |
Recovered |
| Savić et al./Kuwait [44] | 13 y/F | No | Altered mental status, right side weakness | No | No | No | Pos/ND/ND |
CT: left side frontoparietal intracerebral hematoma with intraventricular extension CT angiography: pseudoaneurysm of the frontoparietal branch of the left middle cerebral artery |
Not improved |
| Schupper et al./USA [45] | 5 y/M | No | Right mydriasis | Yes | Fever, abdominal pain, shock | Yes | NR/NR/Pos | CT: a right middle cerebral artery infarction, cerebral edema and diffuse contralateral subarachnoid hemorrhage | Died |
| Seth et al./India [46] | 15 y/M | NR | Headache, emesis, photophobia | No | Fever before neurological manifestations | No | Pos/Neg/ND |
MRI: normal LP: elevated opening pressure and pleiocytosis |
Recovered |
| Shenker et al./USA [47] | 12 y/M | NR | Seizure | No | Fever, rash, conjunctivitis, neck swelling, cracked lips, hypotension | Yes | Pos/Neg/ND |
MRI: normal EEG: focal epilepsy arising in the central region |
Recovered |
| Swarz et al./USA [48] | 9 y/M | No | Seizure | No | Fever, emesis | No | Pos/ND/ND |
MRI: normal EEG: delta activity in the right hemisphere |
Recovered |
| Theophanous et al./USA [49] | 6 y/M | Prematurity, chromosome 17 and 19 deletions, submucosal palate cleft, atrial and ventricular septal defects, agammaglobulinemia with hyper-IgM, hypospadias, asthma, OSAS, gastrostomy | Right facial nerve palsy | No | No | No | Pos/ND/ND | ND | Recovered |
| Tiwari et al./India [50] | 9 y/F | No | Headache, right hemiplegia, right facial nerve palsy | Yes | Fever, conjunctivitis, emesis | Yes | Pos/Neg/Pos |
CT: multifocal hypodensities in the genu and body of corpus callosum, left basal ganglia and bilateral thalami suggestive of infarcts CT angiography: multifocal stenosis of both intracranial internal carotid arteries, right middle cerebral artery, both A2 segments of the anterior cerebral arteries and M2/M3segments of both middle cerebral arteries |
Improved, under rehabilitation |
| Verdoni et al./Ital y[5]5 cases | 7 y/M | No | Meningism | Yes | Fever, conjunctivitis, changes in lips and oral cavity, diarrhea | Yes | Pos/ND/Pos | ND | Recovered |
| 7.7 y/F | Congenital adrenal hyperplasia | Meningism | No | Fever, conjunctivitis, changes in lips and oral cavity, diarrhea | Yes | Neg/ND/Pos | ND | Recovered | |
| 5 y/M | No | Meningism | No | Fever, rash, conjunctivitis hands and feet anomalies | Yes | Neg/ND/Pos | ND | Recovered | |
| 5.5 y/M | No | Meningism | No | Fever, rash, conjunctivitis hands and feet anomalies | Yes | Neg/ND/Pos | ND | Recovered | |
| 5.5 y/M | No | Drowsiness | Yes | Fever, rash, conjunctivitis hands and feet anomalies, diarrhea | Yes | Neg/ND/Pos | ND | Recovered | |
| Verkuil et al./USA [51] | 14 y/F | No | Headache, right abducens nerve palsy | Yes | Fever, diarrhea, rash, shock | Yes | Neg/ND/Pos |
MRI: finding consistent with elevated intracranial pressure LP: elevated opening pressure |
Recovered |
| Vivanti et al./Francea [52] | 3 d/M | Prematurity | Irritability, opisthotonos | No | Feeding difficulty | No | Pos/Neg/ND | MRI: hyperintensity of the periventricular and subcortical frontal and parietal white matter | Improved |
| Yousefi et al./Iran [53] | 9 y/F | NR | Headache, diplopia, photophobia, meningism | No | Fever | No | Neg/Pos/ND | LP: pleiocytosis, elevated protein, decreased glucose | Recovered |
| Zombori et al./UK [54] | 17 y/F | Cornelia de Lange syndrome | Seizure | Yes | Fever | Yes | Pos/ND/ND | MRI: multifocal cortical, cerebellar and thalamic swelling areas EEG: bilateral independent periodic lateralized epileptiform discharges | Improved, under rehabilitation |
| Our case | 5 y/F | No | Irritability, nuchal rigidity | No | Fever, rash, diarrhea, neck swelling | Yes | Pos/ND/Pos | MRI: two lesions, one in the splenium of the corpus callosum and the other in the subcortical white matter of the left parietal lobe, with restricted diffusion | Recovered |
Abbreviations: y years; m months; w weeks; d days; F female; M male; NP nasopharyngeal; CSF cerebrospinal fluid; MRI magnetic resonance imaging; CT computerized tomography; US ultrasound; EEG electroencephalogram; LP lumbar puncture; Pos positive; Neg negative; NR not reported; ND not done; HSCT hematopoietic stem-cell transplantation; OSAS obstructive sleep apnea syndrome
atransplacental transmission of SARS-CoV-2 infection
There were 35 boys and 25 girls. The median age was 9 years. All children had no comorbidity, except 7 patients with no reported data and 6 patients with underlying conditions: a 3-year-old male with allergy to cow milk [24], a 6-year-old girl with sickle cell disease, complicated by cerebral vasculopathy, who underwent hematopoietic stem cell transplantation [42], a 6-year-old male with history of prematurity, chromosome 17 and 19 deletions, submucosal cleft palate, atrial and ventricular septal defects, immune deficit, hypospadias, asthma, obstructive sleep apnea syndrome and gastrostomy [49], a female with congenital adrenal hyperplasia [5], a male born preterm [52] and a 17-year-old female with Cornelia de Lange syndrome [54]. Four children were under 1 year old: one case of transplacental transmission of SARS-CoV-2 was demonstrated in a neonate born to a mother infected in the last trimester [52].
As regards neurological symptoms, the most commonly reported were headache in 2/3 of cases, altered mental status (from irritability and confusion to lethargy) in 32% of cases, seizure in 14/60 patients, muscular weakness in 14/60 children and meningism in 10/60.
Concerning neurological manifestations, we recognized acute cerebrovascular accidents in 10 children (4 cases of ischemic stroke, 3 cases of intracerebral hemorrhage, a subarchnoid hemorrhage, a case of multiple diffuse microhemorrhages, a cerebral sinus venous thrombosis), reversible splenial lesions in 9 cases, GBS in 5 persons, benign intracranial hypertension or pseudotumor cerebri in 4 patients, meningoencephalitis in 4 cases, autoimmune encephalitis in 1 girl, ADEM in 2 children (including ours), cranial nerves impairment in 2 patients and transverse myelitis in 1 case. Furthermore we found one report of severe encephalopathy with bilateral thalamic lesions and one article of fatal cerebral edema.
Fever was recorded in 75% of cases, while respiratory symptoms were present in 23/60 children. Six patients had flu-like symptoms before the onset of neurological complications. More than half of patients (55%) showed neurological complications in the course of a MIS-C, associated with a multisystem organ involvement (especially mucocutaneous, gastrointestinal and cardiac).
Radiological investigations (CT, MRI and/or ultrasound) were performed in almost all cases (45/60): the most recurrent radiological finding was a signal change in the splenium of the corpus callosum (12/60).
The diagnosis of SARS-CoV-2 infection was made according to the presence of SARS-CoV-2 viral nucleic acid in the nasopharyngeal swab in 29 cases and positive serology in 15 children; both nasopharyngeal swab and serology were positive in 11 patients. The presence of SARS-CoV-2 viral nucleic acid in the CSF was proved only in 2 cases (associated with a positive nasopharyngeal swab in 1 case). The outcome was favorable in almost all cases; 5 children died.
Discussion
We described a case of ADEM in a pediatric patient with MIS-C related to SARS-CoV-2 infection. The diagnosis of ADEM was established according to the consensus criteria of the International Pediatric Multiple Sclerosis Study Group in 2013: a polyfocal, clinical central nervous system (CNS) event with a presumed inflammatory demyelinating cause; an encephalopathy that cannot be explained by fever; no new clinical and MRI findings emerging 3 months or more after the onset; abnormal brain MRI during the acute phase [55]. The close temporal relationship between encephalopathy and SARS-CoV-2 infection in our patient allowed us to consider the novel coronavirus as the trigger of the immune-mediated response against CNS, as already reported for other human coronavirus [56]. Furthermore our patient fulfilled the criteria for the diagnosis of MIS-C: she presented fever, mucocutaneous involvement, lymphadenopathy, diarrhea and neurological symptoms associated with elevated inflammatory markers and the presence of antibodies against SARS-CoV-2; unfortunately, the search for the novel coronavirus in the CSF was not performed, because a validated test was not available.
As recommended by American College of Rheumatology (ACR) [6], the first-tier agents for MIS-C treatment are IV immunoglobulin (typically 1–2 g/kg) and/or low to high doses of glucocorticoids (from 1 to 2 mg/kg/day to a bolus of 20–30 mg/kg/day for 3 days); acute treatment approach for pediatric ADEM is high-dose IV glucocorticoids for 3 or 5 days (either 10–30 mg/kg/day methylprednisolone or 1 mg/kg/day dexamethasone) followed by an oral steroid tapering or IV immunoglobulin at a total dose of 1–2 g/kg, administered either as a single dose or divided in 5 days (usually 400 mg/kg/day) [57]. Our girl was treated with glucocorticoids and immunoglobulin with a complete recovery; the outcome was favorable.
Afterwards, we have conducted a systematic review of the neurological complications during SARS-CoV-2 infection in pediatrics. Headache, irritability, drowsiness and seizure are the most frequent symptoms, that could be signs of different neurological conditions or neuroimaging abnormal findings: ischemic stroke, cerebral hemorrhage, benign intracranial hypertension, encephalitis, GBS, ADEM, splenial lesions. Furthermore, we observed that neurological investigations, especially radiological examinations, were not performed in all patients, especially in those with mild symptoms; in these cases, it is not clear what neurological condition is associated to SARS-CoV-2 infection.
The clinical observations summarized above suggest that SARS-CoV-2 could be responsible for many neurological manifestations, which can be divided into three different scenarios, related to the presumed pathophysiologic mechanism:
Neurological involvement during COVID-19;
Neurological involvement that arises after the recovery from COVID-19;
Neurological involvement during MIS-C.
The first condition could be caused by direct invasion of CNS by the virus through hematogenous dissemination or neuronal retrograde dissemination. In hematogenous dissemination, the virus can pass to the bloodstream and then enters the brain by either infecting endothelial cells of the blood-brain barrier or epithelial cells of the blood-CSF barrier in the choroid plexus, though the binding between spike protein and ACE2 receptor; furthermore, coronavirus can infect leukocytes, that disseminate towards other tissues and cross the blood-brain barrier to access the CNS (the so-called Trojan horse mechanism) [58]. In neuronal retrograde dissemination, the virus can gain access to CNS though the infection of olfactory neurons, using retrograde axonal transport [58]. This pathophysiologic mechanism could explain how SARS-CoV-2 can induce encephalitis and vasculitis leading to cerebrovascular accidents; the detection of the virus in the CSF samples using RT-PCR is an important sign of its neurotropism.
The second condition could be related to a post-infectious immune-mediated mechanism: SARS-CoV-2 might induce an autoimmune response after a latent period following the infection illness [59], correlated to the hypothesis of “molecular mimicry” between microbial and self-antigens. For example, GBS is characterized by ascending paralysis, occurring after the resolution of COVID-19 symptoms (fever and cough): it is caused by a cross-reaction against gangliosid-components of the peripheral nerves [60].
The third condition, the most recurrent observed in this review, could be explained though indirect mechanism caused by the novel coronavirus: the cytokine storm, characterized by high levels of tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β, IL-6, IL-12, and interferon gamma (INFγ) [59]. The integrity of the blood-brain barrier may be disrupted by cytokine-driven injury without CNS direct invasion by the virus [59]. Moreover, the hyperinflammatory state can lead to a pro-coagulable state: initial vasculitis causes the disruption of vascular integrity, the exposure of thrombogenic basement membrane and, finally, the activation of the clotting cascade [9]. Children with MIS-C exhibit alteration of inflammatory biomarkers (procalcitonin, CRP, fibrinogen, ferritin, D-dimer, IL-6), that suggest a possible involvement of the immune system in the pathogenesis of this syndrome [6]. Many observational studies about clinical characteristics of patients with MIS-C have reported the presence of neurological involvement: children could complain of headache, confusion, altered mental status, stiff neck or meningism [10, 61–65]. In the course of MIS-C, neurological complications, such as ADEM (our case), pseudotumor cerebri [15, 46, 51], cerebral edema [20, 31], seizure [40, 47], cerebral stroke [45, 50] and cytotoxic lesions of the corpus callosum [13, 17, 27, 32] have been described and included in this review. During hyperinflammatory state, the corpus callosum, especially the splenium, is highly vulnerable to excess of cytokines and glutamate release from astrocytes because of its high concentration of cytokines and glutamate receptors: this higher density leads to a tendency of cytotoxic edema of the corpus callosum when cytokine storm occurs [66]. Despite the great variability of neurological manifestations, from mild to severe ones, the prognosis is favorable in the majority of cases.
This systematic review has several limitations due to the quality of the selected studies (all articles are case reports or case series and do not represent the full population) and the potential impact of publication bias.
Conclusions
Our research highlights the large range of neurological manifestations and their presumed pathogenic pathways associated with SARS-CoV-2 infection in children. CNS involvement could be isolated, developing during COVID-19 or after its recovery, or arise in the course of a MIS-C. The most reported neurological manifestations are cerebrovascular accidents, reversible splenial lesions, GBS, benign intracranial hypertension, encephalitis, cranial nerves impairment, transverse myelitis; ADEM is also a possible complication, as we observed in our patient. Outcome is good in almost all cases. Further studies are required to investigate all the neurological complications of SARS-CoV-2 infection and their underlying pathogenic mechanism.
Acknowledgements
Not applicable.
Authors’ contributions
CC, AC, GAR wrote the paper and performed literature search. LS, SG, AAM, GAR, IP, GFS, FC collected clinical data, wrote the paper and revised the manuscript. All authors read and approved the final manuscript.
Funding
No specific fundings were used for the current manuscript.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Parent’s informed written consent was provided.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Manti S, Licari A, Montagna L, Votto M, Leonardi S, Brambilla I, Castagnoli R, Foiadelli T, Marseglia GL, Cardinale F, Caffarelli C, Tosca MA, Cravidi C, Duse M, Chiappini E. SARS-CoV-2 infection in pediatric population. Acta Bio-Medica. 2020;91(11-S):e2020003. doi: 10.23750/abm.v91i11-S.10298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castagnoli R, Votto M, Licari A, Brambilla I, Bruno R, Perlini S, Rovida F, Baldanti F, Marseglia GL. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review. JAMA Pediatr. 2020;174(9):882–889. doi: 10.1001/jamapediatrics.2020.1467. [DOI] [PubMed] [Google Scholar]
- 3.Chiappini E, Licari A, Motisi MA, Manti S, Marseglia GL, Galli L, Lionetti P. Gastrointestinal involvement in children with SARS-COV-2 infection: an overview for the pediatrician. Pediatr Allergy Immunol. 2020;31(Suppl 26):92–95. doi: 10.1111/pai.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet (London, England) 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet (London, England) 2020;395(10239):1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, Behrens EM, Ferris A, Kernan KF, Schulert GS, Seo P, Son F, Tremoulet AH, Yeung R, Mudano AS, Turner AS, Karp DR, Mehta JJ. American College of Rheumatology Clinical Guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and Hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol (Hoboken, N.J.) 2020;72(11):1791–1805. doi: 10.1002/art.41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020;77(8):1018–1027. doi: 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JE, Asfour A, Sewell TB, Hooe B, Pryce P, Earley C, Shen MY, Kerner-Rossi M, Thakur KT, Vargas WS, Silver WG, Geneslaw AS. Neurological issues in children with COVID-19. Neurosci Lett. 2021;743:135567. doi: 10.1016/j.neulet.2020.135567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, Debray A, Basmaci R, Salvador E, Biscardi S, Frange P, Chalumeau M, Casanova JL, Cohen JF, Allali S. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ (Clinical research ed) 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical research ed.) 2009;339(1):b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdel-Mannan O, Eyre M, Löbel U, Bamford A, Eltze C, Hameed B, Hemingway C, Hacohen Y. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77(11):1–6. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abel D, Shen MY, Abid Z, Hennigan C, Boneparth A, Miller EH, Uhlemann AC, McBrian DK, Thakur K, Silver W, Bain JM. Encephalopathy and bilateral thalamic lesions in a child with MIS-C associated with COVID-19. Neurology. 2020;95(16):745–748. doi: 10.1212/WNL.0000000000010652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asif R, O' Mahony, M. S. Rare complication of COVID-19 presenting as isolated headache. BMJ Case Rep. 2020;13(10):e239275. doi: 10.1136/bcr-2020-239275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baccarella A, Linder A, Spencer R, Jonokuchi AJ, King PB, Maldonado-Soto A, Boneparth A, Hooe BS, Schweickert AJ, Carlin RF, Kingery F, Vargas WS, Sewell TB, Silver WG. Increased intracranial pressure in the setting of multisystem inflammatory syndrome in children, associated with COVID-19. Pediatr Neurol. 2021;115:48–49. doi: 10.1016/j.pediatrneurol.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basirjafari S, Rafiee M, Shahhosseini B, Mohammadi M, Aghayari Sheikh Neshin S, Zarei M. Association of pediatric COVID-19 and subarachnoid hemorrhage. J Med Virol. 2021;93(2):658–660. doi: 10.1002/jmv.26434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bektaş G, Akçay N, Boydağ K, Şevketoğlu E. Reversible splenial lesion syndrome associated with SARS-CoV-2 infection in two children. Brain Dev. 2021;43(2):230–233. doi: 10.1016/j.braindev.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatta S, Sayed A, Ranabhat B, Bhatta RK, Acharya Y. New-onset seizure as the only presentation in a child with COVID-19. Cureus. 2020;12(6):e8820. doi: 10.7759/cureus.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burr T, Barton C, Doll E, Lakhotia A, Sweeney M. N-methyl-d-aspartate receptor encephalitis associated with COVID-19 infection in a toddler. Pediatr Neurol. 2021;114:75–76. doi: 10.1016/j.pediatrneurol.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, Fitzgerald JC, Topjian A, John A. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: a case series. J Pediatric Infect Dis Soc. 2020;9(3):393–398. doi: 10.1093/jpids/piaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curtis M, Bhumbra S, Felker MV, Jordan BL, Kim J, Weber M et al. Guillain-Barré syndrome in a child with COVID-19 infection. Pediatrics. 2021;147(4):e2020015115. 10.1542/peds.2020-015115. [DOI] [PubMed]
- 22.de Miranda Henriques-Souza AM, de Melo A, de Aguiar Coelho Silva Madeiro B, Freitas LF, Sampaio Rocha-Filho PA, Gonçalves FG. Acute disseminated encephalomyelitis in a COVID-19 pediatric patient. Neuroradiology. 2021;63(1):141–145. doi: 10.1007/s00234-020-02571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Paulis M, Oliveira D, Vieira RP, Pinto IC, Machado R, Cavalcanti MP, Soares CP, de Araujo A, Araujo DB, Bachi A, Leal FB, Dorlass EG, Gilio AE, Durigon EL, Barreira ER. Multisystem inflammatory syndrome associated with COVID-19 with neurologic manifestations in a child: a brief report. Pediatr Infect Dis J. 2020;39(10):e321–e324. doi: 10.1097/INF.0000000000002834. [DOI] [PubMed] [Google Scholar]
- 24.Emami A, Fadakar N, Akbari A, Lotfi M, Farazdaghi M, Javanmardi F, Rezaei T, Asadi-Pooya AA. Seizure in patients with COVID-19. Neurol Sci. 2020;41(11):3057–3061. doi: 10.1007/s10072-020-04731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enner S, Hormozdyaran S, Varughese R, Milillo J, Pavkovic I, Laureta E, Schneider J, Kothare S. Central apnea in an adolescent with COVID-19. Pediatr Neurol. 2020;110:87–88. doi: 10.1016/j.pediatrneurol.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frank C, Almeida T, Marques EA, de Sousa Monteiro Q, Feitoza P, Borba M, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection in a pediatric patient. J Trop Pediatr. 2020;0:1–6. 10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed]
- 27.Gaur P, Dixon L, Jones B, Lyall H, Jan W. COVID-19-associated cytotoxic lesions of the Corpus callosum. AJNR Am J Neuroradiol. 2020;41(10):1905–1907. doi: 10.3174/ajnr.A6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulko E, Overby P, Ali S, Mehta H, Al-Mufti F, Gomes W. Vessel Wall enhancement and focal cerebral Arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. AJNR Am J Neuroradiol. 2020;41(12):2348–2350. doi: 10.3174/ajnr.A6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaur H, Mason JA, Bajracharya M, McGee J, Gunderson MD, Hart BL, Dehority W, Link N, Moore B, Phillips JP, Rogers D. Transverse myelitis in a child with COVID-19. Pediatr Neurol. 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalifa M, Zakaria F, Ragab Y, Saad A, Bamaga A, Emad Y, Rasker JJ. Guillain-Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J Pediatr Infect Dis Soc. 2020;9(4):510–513. doi: 10.1093/jpids/piaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim MG, Stein AA, Overby P, Kleinman G, Nuoman R, Gulko E, Al-Mufti F, Pisapia JM, Muh CR. Fatal cerebral edema in a child with COVID-19. Pediatr Neurol. 2021;114:77–78. doi: 10.1016/j.pediatrneurol.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin J, Lawson EC, Verma S, Peterson RB, Sidhu R. Cytotoxic lesion of the Corpus callosum in an adolescent with multisystem inflammatory syndrome and SARS-CoV-2 infection. AJNR Am J Neuroradiol. 2020;41(11):2017–2019. doi: 10.3174/ajnr.A6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz N, Treptow A, Schmidt S, Hofmann R, Raumer-Engler M, Heubner G, Gröber K. Neonatal early-onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr Infect Dis J. 2020;39(8):e212. doi: 10.1097/INF.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 34.Manji HK, George U, Mkopi NP, Manji KP. Guillain-Barré syndrome associated with COVID-19 infection. Pan Afr Med J. 2020;35(Suppl 2):118. doi: 10.11604/pamj.supp.2020.35.2.25003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McAbee GN, Brosgol Y, Pavlakis S, Agha R, Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirzaee S, Gonçalves FG, Mohammadifard M, Tavakoli SM, Vossough A. Focal cerebral Arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297(2):E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreno-Galarraga L, Urretavizcaya-Martínez M, Alegría Echauri J, García Howard M, Ruperez García E, Aguilera-Albesa S, Alzina de Aguilar V, Herranz Aguirre M. SARS-CoV-2 infection in children requiring hospitalization: the experience of Navarra, Spain. World J Pediatr. 2020;16(6):614–622. doi: 10.1007/s12519-020-00393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natarajan S, Ganesh R, Palaniappan N, Kannan L. SARS-CoV- 2 encephalitis in an adolescent girl. Indian Pediatr. 2020;57(12):1186–1187. doi: 10.1007/s13312-020-2080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paybast S, Gorji R, Mavandadi S. Guillain-Barré syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurologist. 2020;25(4):101–103. doi: 10.1097/NRL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raj SL, Vasanthi T, Baineni R, Sivabalan S. Neurological manifestations of COVID-19 in children. Indian Pediatr. 2020;57(12):1185–1186. doi: 10.1007/s13312-020-2079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Regev T, Antebi M, Eytan D, Shachor-Meyouhas Y, Ilivitzki A, Aviel YB, Ben-Ari J. Pediatric inflammatory multisystem syndrome with central nervous system involvement and Hypocomplementemia following SARS-COV-2 infection. Pediatr Infect Dis J. 2020;39(8):e206–e207. doi: 10.1097/INF.0000000000002804. [DOI] [PubMed] [Google Scholar]
- 42.Roussel A, Germanaud D, Bouchoucha Y, Ouldali N, Vedrenne-Cloquet M, Castelle M, Baruchel A. Cranial polyneuropathy as the first manifestation of a severe COVID-19 in a child. Pediatr Blood Cancer. 2021;68(3):e28707. doi: 10.1002/pbc.28707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saeed A, Shorafa E. Status epilepticus as a first presentation of COVID-19 infection in a 3 years old boy; case report and review the literature. IDCases. 2020;22:e00942. doi: 10.1016/j.idcr.2020.e00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Savić D, Alsheikh TM, Alhaj AK, Lazovic L, Alsarraf L, Bosnjakovic P, Yousef W. Ruptured cerebral pseudoaneurysm in an adolescent as an early onset of COVID-19 infection: case report. Acta Neurochir. 2020;162(11):2725–2729. doi: 10.1007/s00701-020-04510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schupper AJ, Yaeger KA, Morgenstern PF. Neurological manifestations of pediatric multi-system inflammatory syndrome potentially associated with COVID-19. Child Nervous Syst. 2020;36(8):1579–1580. doi: 10.1007/s00381-020-04755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seth V, Kushwaha S. Headache due to COVID-19: a disabling combination. Headache. 2020;60(10):2618–2621. doi: 10.1111/head.14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenker J, Trogen B, Schroeder L, Ratner AJ, Kahn P. Multisystem inflammatory syndrome in children associated with status epilepticus. J Pediatr. 2020;227:300–301. doi: 10.1016/j.jpeds.2020.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swarz JA, Daily S, Niemi E, Hilbert SG, Ibrahim HA, Gaitanis JN. COVID-19 infection presenting as acute-onset focal status epilepticus. Pediatr Neurol. 2020;112:7. doi: 10.1016/j.pediatrneurol.2020.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Theophanous C, Santoro JD, Itani R. Bell's palsy in a pediatric patient with hyper IgM syndrome and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Brain Dev. 2021;43(2):357–359. doi: 10.1016/j.braindev.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tiwari L, Shekhar S, Bansal A, Kumar S. COVID-19 associated arterial ischaemic stroke and multisystem inflammatory syndrome in children: a case report. The Lancet Child Adolesc Health. 2021;5(1):88–90. doi: 10.1016/S2352-4642(20)30314-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verkuil LD, Liu GT, Brahma VL, Avery RA. Pseudotumor cerebri syndrome associated with MIS-C: a case report. Lancet (London, England) 2020;396(10250):532. doi: 10.1016/S0140-6736(20)31725-6. [DOI] [PubMed] [Google Scholar]
- 52.Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11(1):3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yousefi K, Poorbarat S, Abasi Z, Rahimi S, Khakshour A. Viral meningitis associated with COVID-19 in a 9-year-old child: a case report. Pediatr Infect Dis J. 2021;40(2):e87–e98. doi: 10.1097/INF.0000000000002979. [DOI] [PubMed] [Google Scholar]
- 54.Zombori L, Bacon M, Wood H, Chatterjee F, Venkateswaran R, Lampariello S, Yoong M. Severe cortical damage associated with COVID-19 case report. Seizure. 2021;84:66–68. doi: 10.1016/j.seizure.2020.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E, International Pediatric Multiple Sclerosis Study Group International pediatric multiple sclerosis study group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Multiple sclerosis (Houndmills, Basingstoke, England) 2013;19(10):1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 56.Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1 Pt 1):e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 57.Cole J, Evans E, Mwangi M, Mar S. Acute disseminated encephalomyelitis in children: an updated review based on current diagnostic criteria. Pediatr Neurol. 2019;100:26–34. doi: 10.1016/j.pediatrneurol.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 58.Pezzini A, Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nature reviews. Neurology. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aghagoli G, Gallo Marin B, Katchur NJ, Chaves-Sell F, Asaad WF, Murphy SA. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocritical Care. 2020:1-10. 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed]
- 60.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet (London, England) 2016;388(10045):717–727. doi: 10.1016/S0140-6736(16)00339-1. [DOI] [PubMed] [Google Scholar]
- 61.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son M, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG, Overcoming COVID-19 Investigators., CDC COVID-19 Response Team. CDC COVID-19 Response Team Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H, New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383(4):347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M, PIMS-TS Study Group and EUCLIDS and PERFORM Consortia Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D. Acute heart failure in multisystem inflammatory syndrome in children in the context of global SARS-CoV-2 pandemic. Circulation. 2020;142(5):429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 65.Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, Bensaid P, Pichard S, Kouider H, Morelle G, Craiu I, Pondarre C, Deho A, Maroni A, Oualha M, Amoura Z, Haroche J, Chommeloux J, Bajolle F, Beyler C, Bonacorsi S, Carcelain G, Koné-Paut I, Bader-Meunier B, Faye A, Meinzer U, Galeotti C, Melki I. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79(8):999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Starkey J, Kobayashi N, Numaguchi Y, Moritani T. Cytotoxic lesions of the Corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics. 2017;37(2):562–576. doi: 10.1148/rg.2017160085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.