Abstract
To evaluate the incidence of apoptosis within the testes of patients who died from severe acute respiratory syndrome coronavirus 2 (COVID-19) complications, testis tissue was collected from autopsies of COVID-19 positive (n = 6) and negative men (n = 6). They were then taken for histopathological experiments, and RNA extraction, to examine the expression of angiotensin-converting enzyme 2 (ACE2), transmembrane protease, serine 2 (TMPRSS2), BAX, BCL2 and Caspase3 genes. Reactive oxygen species (ROS) production and glutathione disulfide (GSH) activity were also thoroughly examined. Autopsied testicular specimens of COVID-19 showed that COVID-19 infection significantly decreased the seminiferous tubule length, interstitial tissue and seminiferous tubule volume, as well as the number of testicular cells. An analysis of the results showed that the Johnsen expressed a reduction in the COVID-19 group when compared to the control group. Our data showed that the expression of ACE2, BAX and Caspase3 were remarkably increased as well as a decrease in the expression of BCL2 in COVID-19 cases. Although, no significant difference was found for TMPRSS2. Furthermore, the results signified an increase in the formation of ROS and suppression of the GSH activity as oxidative stress biomarkers. The results of immunohistochemistry and TUNEL assay showed that the expression of ACE2 and the number of apoptotic cells significantly increased in the COVID-19 group. Overall, this study suggests that COVID-19 infection causes spermatogenesis disruption, probably through the oxidative stress pathway and subsequently induces apoptosis.
Keywords: Apoptosis, Testicular tissue, Spermatogenesis disrupt, COVID-19
Introduction
In December 2019, Wuhan, the capital of Hubei province in China, became the epicenter of an outbreak of pneumonia. Coronavirus 2019 (COVID-19) is an infectious disease caused by acute respiratory infection due to coronavirus 2 (SARS-CoV-2). The world health organization (WHO) announced the outbreak of COVID-19 on March 11, 2020 as a global pandemic. As of November 25, 2020, more than 59.8 million cases, as well as more than 1.41 million deaths, have been reported worldwide [1]. COVID-19, can lead to multiple organ failure in a short period such as the cardiovascular system [2], liver [3], kidney [4], spleen, lymph nodes, brain, testis and skin [5]. All damaged organs and tissues have been reported to have high levels of ACE2 and TMPRSS2 [6]. It has been shown that the pattern of ACE2 expression in adult testes is highly expressed in spermatogonia, spermatids, Leydig and Sertoli cells [7]. Co-expression of ACE2 and TMPRSS2 in testicular cells suggests that the testis may be a high-risk organ that is vulnerable to SARS-CoV-2 infection, which may lead to testicular cell destruction and impaired spermatogenesis, eventually leading to infertility in men [7]. Data from autopsies of 12 patients with COVID-19 was conducted by Neto et al. which, showed a dramatic reduction in Leydig cells in the interstitial tissue [8]. Recently, researchers confirmed impaired spermatogenesis in COVID-19 patients resulting from significant injury to Sertoli cells, seminiferous tubules, reduction of Leydig cells, and mild inflammatory infiltrates in the testis interstitium [9, 10]. It has been reported in SARS-CoV2 infections that the excessive production of ROS can lead to induction of germ cell apoptosis, spermatogenic disruptions and reduced semen quality [11]. In men with COVID-19, it has been shown to reduce testosterone levels as well as alter the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) by stimulating the hypothalamic-pituitary–gonadal (HPG) axis [12]. Therefore, this study was conducted to find answers to the following questions: Does over expression of ACE2 and TMPRSS2 following COVID-19 disease, observed? Is apoptosis associated with cell death mediated by COVID-19? Is deduction observed in testicular cells following COVID-19disease? Is decrease in GSH as the main marker for of antioxidant activity following COVID-19, signified? Is there an increase in ROS as the main source of oxidative stress following COVID-19? Finally, whether the change of BAX, BCL2 and Caspase3 expression a suitable marker for apoptosis following COVID-19?
Methods
Patient selection and patient criteria
Six patient samples and six healthy samples were included in the study (Table 1). For the control group, people in the age range 45 to 58 years with healthy reproductive status who died due to accident, electric shock or carbon monoxide poisoning, with no disease whatsoever were utilized.
Table 1.
Clinical characteristics of COVID-19 patients
| Case no | Age (yr) | Disease duration (d) | Reproductive system disorders | Fertile and have children | Corticosteroid therapy | Comorbidity | Cause of death |
|---|---|---|---|---|---|---|---|
| C1 | 58 | 23 | Non | Yes | Yes | Hypertension, type 2 diabetes | COVID-19, acute respiratory distress syndrome, septic shock |
| C2 | 48 | 28 | Non | Yes | Yes | Non | COVID-19, pneumonia, acute respiratory distress syndrome, myocardial infarction |
| C3 | 49 | 30 | Non | Yes | Yes | Non | COVID-19, respiratory failure, myocardial infarction |
| C4 | 54 | 32 | Non | Yes | Yes | Hypertension | COVID-19, respiratory failure, septic shock |
| C5 | 57 | 27 | Non | Yes | Yes | Hypertension, type 2 diabetes | COVID-19, respiratory failure, septic shock |
| C6 | 55 | 20 | Non | Yes | Yes | Hypertension | COVID-19, respiratory failure, septic shock |
COVID-19 coronavirus disease 2019, yr year, d day
Study design
This study is a method that involves the development of the interpretations of the results based on a blind review of the data analysis in group A versus group B. After agreeing that there would be no further change, the researchers recorded their decisions and signed the resulting document. The random code was then broken, the correct interpretation selected, and the final version finalized.
Postmortem examination and sampling of the lung and testes
Postmortem human lung and testes were collected from Iranian Legal Medicine Organization. This study was conducted in accordance with the principles of the Declaration of Helsinki and the guidelines of the Chinese National Health Commission, and was approved by the Ethics Committee at Shahid Beheshti University of Medical Sciences, (IR.SBMU.MSP.REC 1399.779). All cases were checked for their medical history of death, death certificate, and autopsy report. Cases with a history of reproductive system disorders were excluded from the present study. According to standard protocol, COVID-19 diagnosis was confirmed by positive nucleic acid testing of nasopharyngeal swabs, radiography (chest X-ray), computer tomography (CT) scan features of viral pneumonia, and clinical symptomatology. The deceased COVID-19 and control cases were transported by refrigerator from the hospital to forensic medicine. Postmortem examinations were carried out after consent from family members and were performed approximately 8 to 10 h after death. For six patients and six controls, a tissue sample of 3 × 3cm2 was obtained via incisional autopsy for histological, cellular and molecular study.
Tissue preparation
Lung specimens were fixed in 10% formalin for 72 h and submitted for standard tissue processing. Sectioning and hematoxylin and eosin (H&E) staining for histopathology evaluations was subsequently followed. Evaluation of tissue sections was done by an expert histologist. The testes samples were kept in Bouin's liquid for 48 h and then transferred to formalin 10% before placing them in paraffin blocks. We then made serial sections (which were 5 µm thick to estimate volume, or 25 µm to estimate number) using a microtome (Leica RM2125 RTS, Germany) consistent with the stereological techniques. The Systematic Uniform Random Sampling (SURS) was used to choose 10 sections in each sample by picking a random figure in the 1 to 10 range. They were then finally stained using H&E staining technique (Sigma, USA). It is worth noting that the testis cells were distinct in terms of morphology.
The length of the seminiferous tubules
To estimate the length of seminiferous tubules, six random fields for each tissue section were selected using an objectivity of × 10. The number of the selected seminiferous tubule profiles was counted using an unbiased counting frame. The length density of seminiferous tubules was also estimated using the following formula [13, 14]:
where ΣQ is the total number of the seminiferous tubules, a/f is the area per frame and ΣP is the total points superimposed on the testis tissue. The total length of the seminiferous tubules was estimated by multiplying the length density (Lv) by the total volume of the testis.
Total volumes of the testis
Using a projecting microscope, the live image of 8–10 testis sections was evaluated. The volumes were calculated using the Cavalieri method [13, 14]. Using stereological software, a point grid was superimposed on the testis images. The volume of the testis was estimated by the following formula:
where ΣP is the total points hitting the testis sections, a/p is the area associated with each point and t being the distance between the sampled sections.
Testicular cells number
The total number of testicular cells was determined using the optical dissector method [15]. In this study the microscopic field position of each tissue section was selected by systematic uniform random sampling. An unbiased counting frame was superimposed on the images. To measure the z-axis direction a microcrater was attached to the stage of the microscope being used. The height of the dissector was defined as a guard zone at the top and bottom of each section. Any nucleus of testicular cells coming into maximal focus was selected if it layed inside the counting frame and did not touch the exclusion line. The numerical density (NV) was estimated using the following equation:
where (ΣQ) is the number of cells, (ΣP), the number of counting frame grid in all fields; (a/f) is the area of frame; (h), the dissector height; (BA), the thickness of microtome section and (t) being the section’s real thickness.
Johnsen score
A Johnsen score was determined for each testicular biopsy. In total, 100 tubules were examined per slide and each slide was scored on a scale of 1–10 based on the level of spermatogenesis (Table 2).
Table 2.
Johnsen Score
| Score | Level of spermatogenesis |
|---|---|
| 10 | Full spermatogenesis |
| 9 | Slightly impaired spermatogenesis |
| 8 | Less than five spermatozoa per tubule |
| 7 | No late spermatids; many early spermatids |
| 6 | Few early spermatids; many of spermatogenesis at the spermatid stage |
| 5 | Many spermatocytes |
| 4 | Few spermatocytes, arrest of spermatogenesis at the primary spermatocyte stage |
| 3 | Spermatogonia only |
| 2 | No germ cells, Sertoli cells only |
| 1 | No seminiferous epithelial cell, tubular sclerosis |
Immunocytochemistry
The testes samples were transferred to 4% paraformaldehyde, after which, the testes were embedded in paraffin blocks and sections of 5 µm thickness were cut out with a microtome)Leica RM2125 RTS, Germany). The tissue sections were then placed on poly-l-lysine-coated slides. Immunohistochemistry was used to determine the expression of the receptor for SARS-CoV-2, ACE2 in testicular tissue. The primary antibodies used included monoclonal mouse anti-human ACE2 (1:100; MM0073- 11A31, Abcam, Cambridge, UK). Antigen retrieval was performed in EDTA antigen retrieval solution (G1206, Servicer Bio.) by microwave oven heating. Sections were incubated with a blocking solution And after blocking, sections were incubated overnight with the primary antibody at 4 °C. Alexa Flour 594 donkey anti-mouse IgG (Ref21203, Life) secondary antibody was used for the detection of ACE2. In order to count the percentage of ACE2 positive cells, at least 200 cells in the testis were counted for each specimen. Cells were counted under a light microscope at high magnification.
Reactive oxygen species in testicular tissue
After cell isolation from testis tissue, using trypsin Ethylene-diamine-tetra-acetic acid (EDTA), the suspension was centrifuged in PBS for 5 min at 4 °C at 1200RPM. Then, 2,7-dichlorofluorescein diacetate (DCFDA) was added to the sample at a concentration of 20 µM in a 100 µl aliquot and was stored in a 37 °C incubator for 45 min in the dark. Finally, the sample was examined by a flow cytometer with a wavelength of 495 nm [16].
Glutathione disulfide content assessments
Glutathione peroxidase (GPX) assay kit (Zelbio GmbH) was used for determining GPX in testis tissue samples with 5 U/ml sensitivity (5KU/L). In this assay GPX activity unit was considered as the amount of the sample that will catalyze decomposition of 1 µmol of GSH in one minute. Aliquots of the testicular cells suspension (0.5 ml) that were previously stained with O-Phthalaldehyde (OPA) and N-Ethylmaleimide (NEM) probe (5 μM) were separated from the incubation medium by 1 min centrifugation at 1000 rpm. The cell pellet was then suspended in 2 ml of fresh incubation medium. This washing process was carried out twice to remove the fluorescent dye from the media. Each sample was measured in quarts cuvettes using a Shimadzu RF5000U fluorescence spectrophotometer set for at 495 nm excitation and 530 nm emission wavelengths [16].
Analysis of ACE2, TMPRSS2, BAX, BCL2 and caspase3 expression using real-time PCR
Following the extraction of all RNA samples, we treated them with DNase I (Roche, Basel, Switzerland) to remove contamination induced by genomic DNA. We used a commercial kit (Fermentas, Lithuania) to synthesize cDNA at 42 °C for 60 min in compliance with the protocols described in the manufacturer’s instructions. We used real-time PCR (TaqMan) based on QuantiTect SYBR Green RT-PCR kit (Takara Bio Inc, Japan) for the quantification of the relative expression of genes. We designed all pairs of reverse and forward primers by Primer 3 Plus software using an exon-exon junction method for the separation of cDNA from genomic DNA. Prior to that, we tested PCR primers using Primer-Blast tool available at the website, www.ncbi.nlm.nih.gov/tools/primer-blast. The sequences of the primer probe sets used are as follows: GAPDH, CAT#: P7732, Forward: 5′‐CTCAAGATTGTCAGCAATGC‐3′, Reverse: 5′‐CAGGATGCCCTTTAGTGGGC‐3′. ACE 2, CAT#: HP214223, Forward: 5′‐CTCAAGATTGTCAGCAATGC‐3′, Reverse: 5′‐CAGGATGCCCTTTAGTGGGC‐3′. TMPRSS, CAT#: HP208758, Forward: 5′‐CCTCTAACTGGTGTGATGGCGT‐3′, Reverse: 5′‐TGCCAGGACTTCCTCTGAGATG‐3′. BAX, CAT#: B8304, F: 5′‐AGGATAGAGCAGGGAGGATGG ‐3′, Reverse: 5′‐TGGTAGCAAAGTAGAAGAGGG‐3′. BCL2, CAT#: B9179, Forward: 5′‐AGGAATGTGTGGAATGTGGAGA‐3′, Reverse: 5′‐AGATGAATGGTAGAGGGTGTGA‐3′. Caspase3, HP100140, Forward: 5′‐AGCTTCTTCAGAGGCGA CTA, Reverse: 5′‐GGACACAATACACGGGATCT‐3′.
TUNEL assay
To evaluate the ratio of apoptotic cells using TUNEL assay (In-Situ Cell Death Detection Kit, POD; Roche), the tissue sections (5 μm) were mounted on poly-l-lysine-coated slides and were deparaffinized and rehydrated. Tissue sections were incubated with proteinase K solution (10–20 μg/ml) for 30 min. Tissue sections were then rinsed twice in PBS and were reacted with 50 μl of the TUNEL reaction mixture at room temperature for 60 min in a dark, humidified chamber. Tissue sections were once again rinsed in PBS and incubated for 30 min with 50 μl of the Converter-POD and followed by 3-amino-9-ethylcarbazole (AEC). Sections were then counterstained with hematoxylin. Finally, we quantified the percentage of TUNEL-positive cells in three sections per testis. The brown nuclei of the cells were considered as apoptotic testicular cells.
Statistical analysis
The quantitative data was extracted from six independent samples and presented as Mean ± SD. All statistical analyses were performed using the SPSS software, version 20. All quantitative data analyzed via Kolmogorov–Smirnov had normal distribution; therefore, the comparisons between groups were performed using t-test. Statistical significance was set at P < 0.05.
Results
Patients confirmation
Chest X-ray image of COVID-19 patients
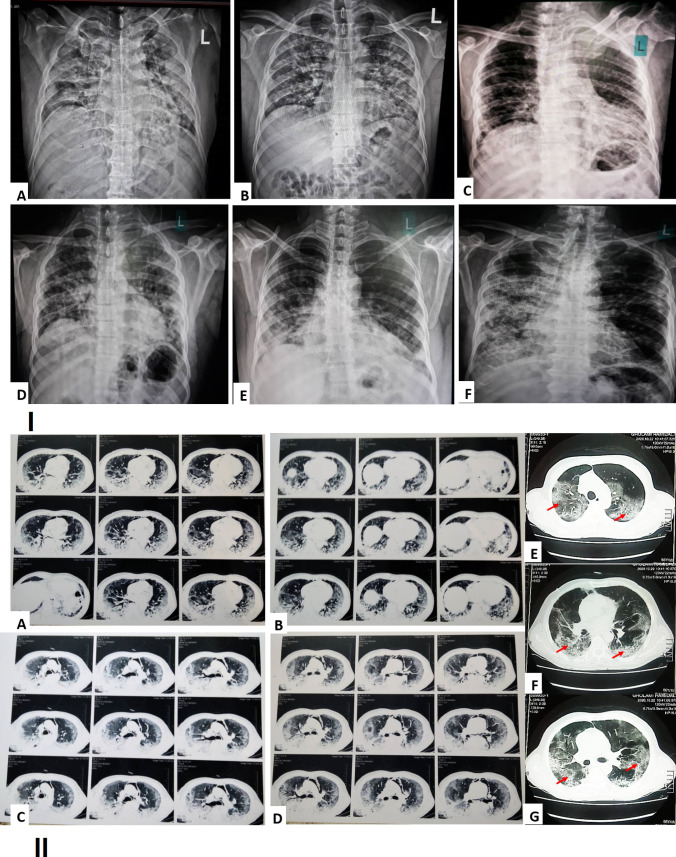
Chest X-ray images of COVID -19 positive adult males, showing bilateral mid and lower zones homogenous consolidation in peripheral distribution on both lungs with obscuration of both CP angles- Findings, fell in the category of indeterminate for COVID-19 (Fig. 1).
Fig. 1.
(I) Chest X-ray image of a COVID-19 positive old male, showing bilateral mid and lower zones homogenous consolidation in peripheral distribution along with Obscuration of both CP angles (red arrow), findings fall in the category of indeterminate for COVID-19 (A–F). (II) Axial thin-section chest computed tomography (CT) in patients with COVID-19 pneumonia from our institution. A–G Non-contrast chest CT scan exam showed bilateral ground glass opacities and interlobular septal thickening giving appearance of crazy paving and areas of surrounding consolidation giving appearance of reverse halo sign and air bronchogram (red arrow) (Color figure online)
Chest CT scan image of COVID-19 patients
Axial thin-section unenhanced CT scan of COVID-19 showed multifocal ground glass opacities and consolidation in the periphery and center of both lungs. Axial unenhanced imaging showed that the pleural effusion was loculated in areas and pleural thickening had occurred in the posterior aspect of the lungs. Other occurrences such as Smooth interlobular septal thickening, fibrotic bands in both lungs and collapse consolidation in both lungs were observed as well. (Fig. 1).
Histopathological features
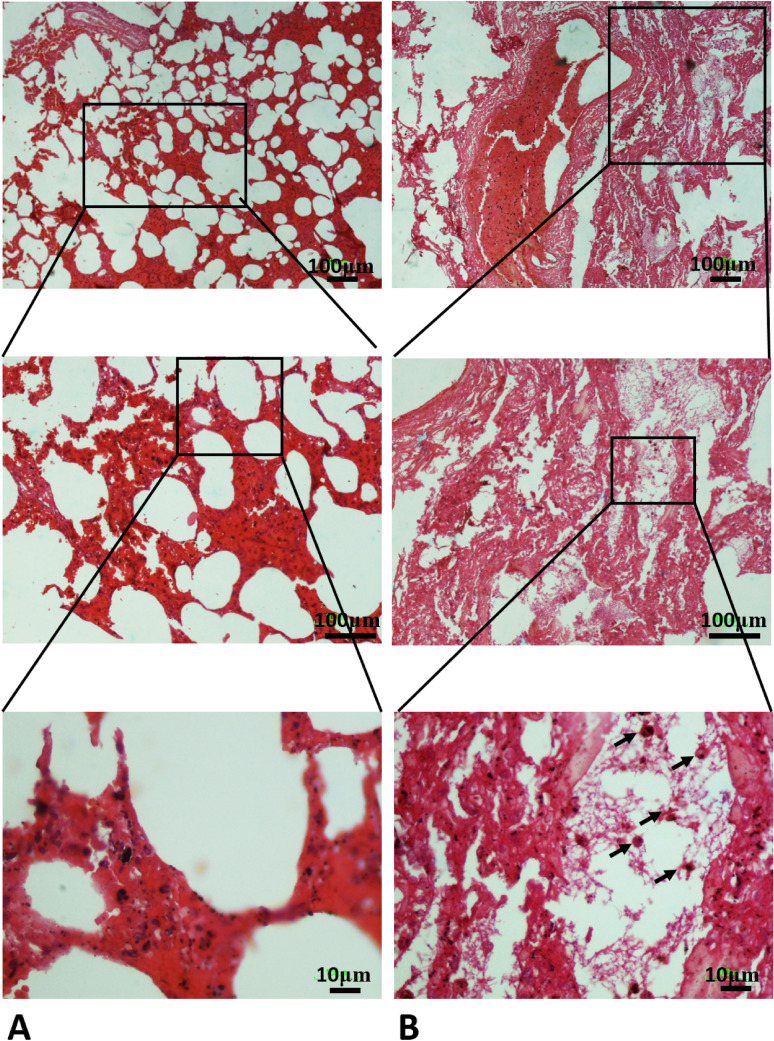
Histopathological examination of the pulmonary system showed a spectrum of diffuse alveolar damage in patients, which was evidenced by the presence of intra-alveolar fibrin and hyaline membranes in the alveolar septal walls. Airways and alveolar spaces contained large, reactive mononuclear inflammatory cells. Microscopic hemorrhage was identified with diffuse alveolar damage in patients. Most patients showed variable degrees of chronic interstitial inflammation, with some having more prominent perivascular lymphocytic inflammation (Fig. 2).
Fig. 2.
A In the control group, the lung parenchyma, including the alveolar sac and alveolar walls, has a normal appearance. B In Covid-19 group, infiltration of lung tissue by mononuclear inflammatory cells (arrow), along with desquamation of alveolar epithelium and formation of hyaline membrane together alveolar wall thickening was observed
The impact of COVID-19 in testis
Stereological parameters
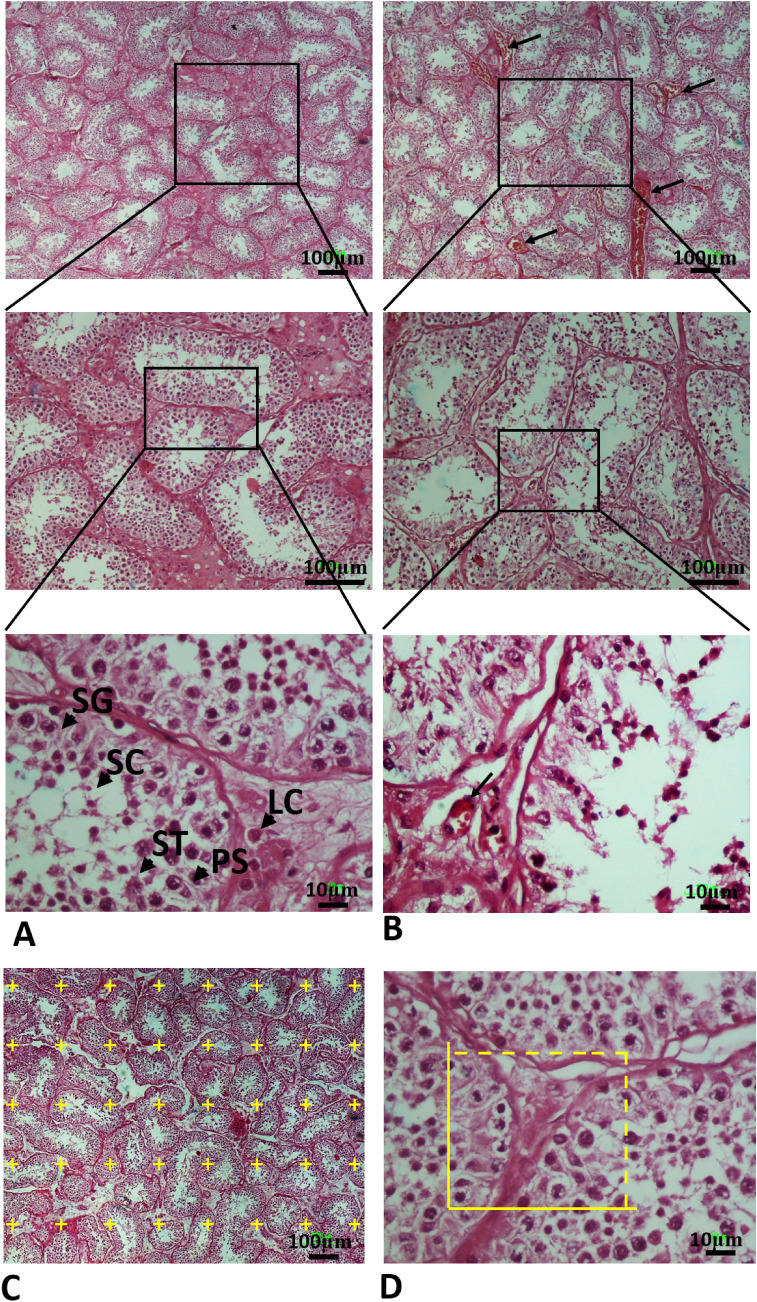
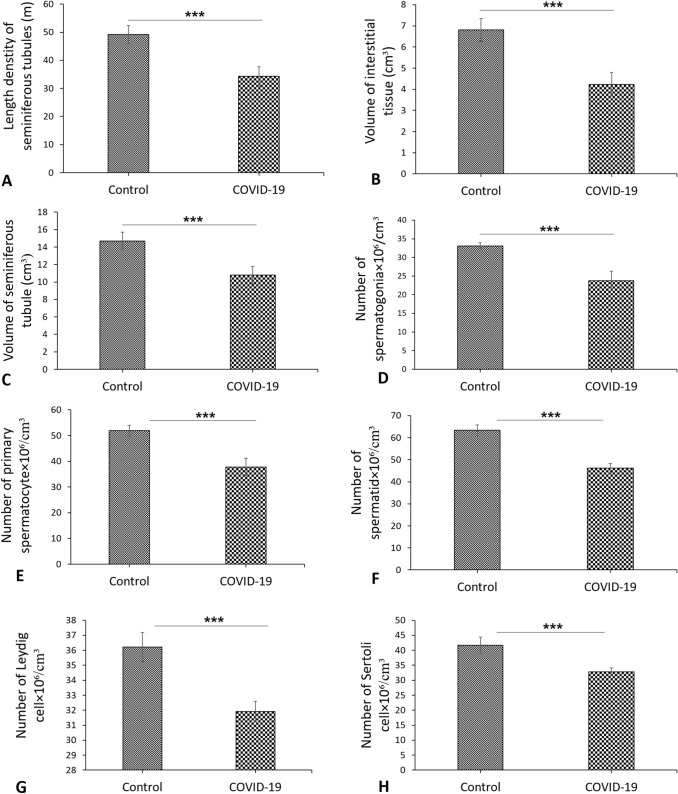
The advantage of using stereological methods was to obtain unbiased and accurate results. The control group showed normal seminiferous tubules with normal germinal epithelium and testis interstitial tissue (Fig. 3). However, exposure to COVID-19 caused degenerative changes in the seminiferous tubules associated with loss of spermatogenesis. Moreover, testis section in the COVID-19 group showed severe damage of seminiferous tubules and an absence of spermatogenic cells (Fig. 3). The graphs for evaluated stereological parameters are shown in Figs. 3 and 4. The control group showed normal histological parameters. Based on the obtained results, the length of seminiferous tubules witnessed reduction in the COVID-19 group when compared with the control group (P < 0.001) (Fig. 4A). In addition, the volume of interstitial tissue and seminiferous tubules significantly decreased in the COVID-19 group as compared to the control group (P < 0.001, P < 0.001, respectively) (Fig. 4B and C). At the cellular level, a significant reduction was observed in the total number of testicular cells such as spermatogonia, primary spermatocyte, spermatid, Leydig and Sertoli cells in the COVID-19 group as compared to the control group (P < 0.001) (Fig. 4D–H). The overall stereological findings showed normal appearance and normal spermatogenesis in the control group. Testicular tissue structure in the COVID-19 group showed changes in the seminiferous tubules including arrest of spermatogenesis and reduction in testicular cell numbers with in the germinal epithelium. It was also to be noted that Testicular tissue structure in the COVID-19 group showed reduction of testicular cells such as spermatogonia, primary spermatocyte, spermatid, Leydig and Sertoli cells (Fig. 3).
Fig. 3.
Photomicrograph of the testis stained with H&E; (× 4, × 10 and × 40). SG Spermatogonia (SG), primary spermatocyte (PS), round spermatid (ST), Sertoli cell (SC), Leydig cell (LC). A In the control group, the testis tissue, including the seminiferous tubules and germinal epithelium, has a normal appearance. B The testicular cells death, interstitial edema and thinning of the seminiferous epithelium was observed in all autopsy COVID-19 specimens of testes. C A point grid is superimposed over the photomicrograph for measuring the volume using Cavalieri method. D Counting frames are superimposed over the photomicrographs to measure the number of testicular cells using optical disector method. The yellow box is counting frame (Color figure online)
Fig. 4.
A–C Stereological results of testis tissue. Mean ± SD of the seminiferous tubules length, interstitial tissue volume and seminiferous tubules volume of testis in the study groups; (***P < 0.001). C Photomicrograph of the testis stained with H&E, × 4. D–H Stereological results of testis tissue. Mean ± SD of the total number of spermatogonia, total number of primary spermatocyte, total number of round spermatid, total number of Leydig cells and total number of Sertoli cells in the study groups; (***P < 0.001)
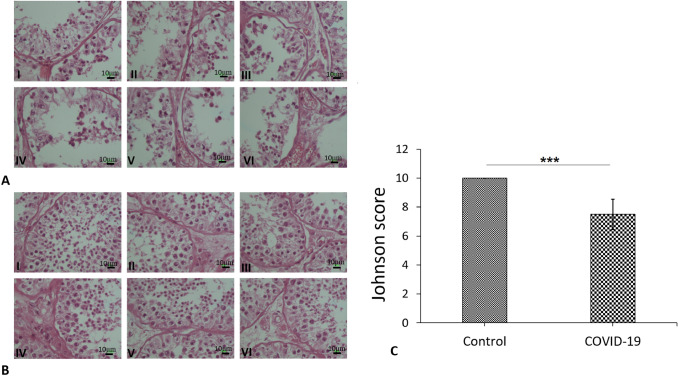
Histopathological analysis
The overall histopathological findings showed that the morphology of the tubes was completely normal. The epithelium manifested normal thickness in the control autopsy with a Johnsen score of 10. Testicular histopathology in the COVID-19 autopsy specimens showed changes in the seminiferous tubules of all samples including: one sample which had certain tubules with slightly impaired spermatogenesis whilst maintaining a Johnson score of 9. Two samples had tubules with less than five spermatozoa per tubule giving a Johnson score of 8. Two samples had tubules with no late spermatids and many early spermatids resulting in a 7 Johnson score. One sample had tubules with few early spermatids; most of the spermatogenesis was at the spermatid stage while the Johnson score was 6 (Fig. 5A and B). The Johnsen score in the COVID-19 group showed a significant reduction compared to the control group (P < 0.001) (Fig. 5C).
Fig. 5.
Photomicrograph of the testis stained with H&E; (× 40). A Histopathological finding showed normal spermatogenesis in all control autopsy and the Johnsen score was 10. B Testicular histopathology in the six COVID-19 autopsy specimens such as: sample (I) had some tubules with slightly impaired spermatogenesis and the Johnson score was 9. Two samples (II and III) had some tubules with less than five spermatozoa per tubule and the Johnson score was 8. samples (IV and V) had some tubules with no late spermatids and many early spermatids and the Johnson score was 7. Sample (IV) had some tubules with few early spermatids; many of spermatogenesis at the spermatid stage and the Johnson score was 6. C Mean ± SD of the Johnsen score in the study groups; (*P < 0.001)
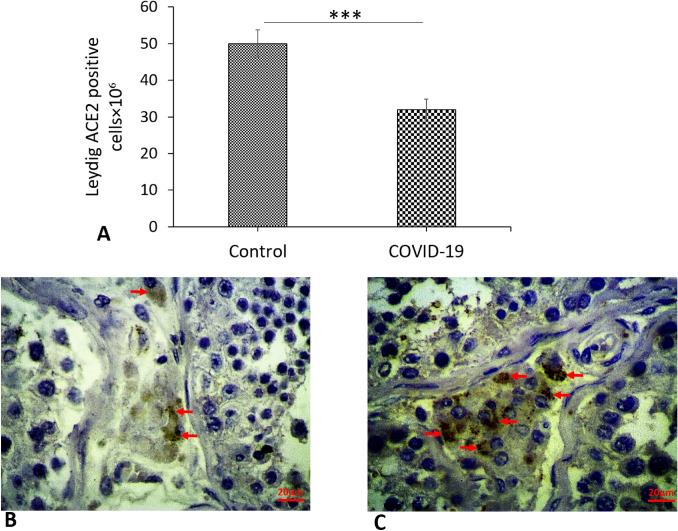
Expression of ACE2 in testis based on immunocytochemistry
Immunostaining showed that ACE2 was sporadically expressed in testicular cells and strongly expressed in Leydig cells in the COVID-19 group compared to the controls (P < 0.001) (Fig. 6A–C). Whilst in Sertoli cells, spermatogonia, primary spermatocytes and spermatids are less expressed, evaluation of such as was difficult because they are covered by Sertoli cell cytoplasm. However, no obvious differences in the ACE2 expression were observed between COVID-19 and control samples.
Fig. 6.
Photomicrograph of the testis immunohistochemistry staining for ACE2 (4X and 40X). A Control group and B COVID-19 groups. Expressed of ACE2 in Leydig cells (arrows) according to immunohistochemistry. C Mean ± SD of the genes expression in the different groups (***P < 0.001)
Reactive oxygen species (ROS) production
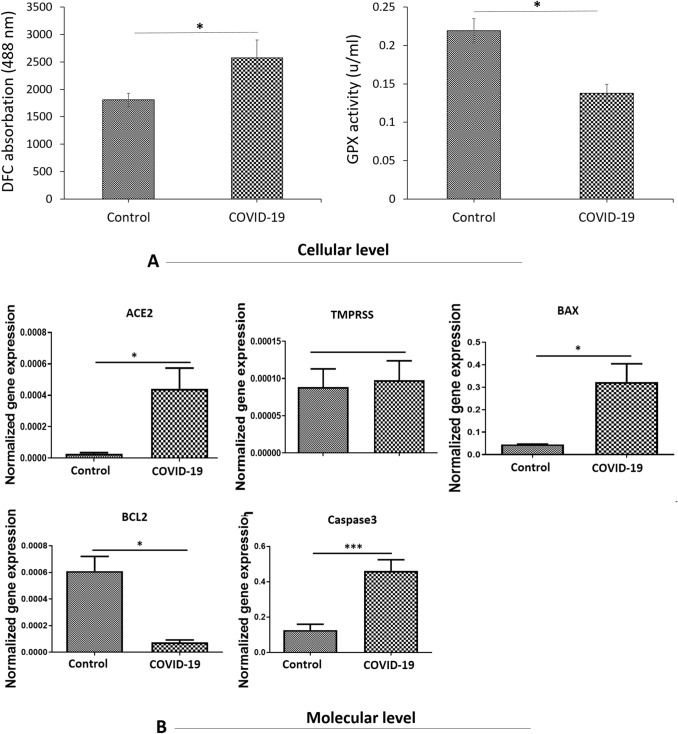
To understand probable oxidative stress mechanism at the COVID-19, the effects of COVID-19 on the formation of ROS was assayed (Fig. 7A). The ROS production in the COVID-19 group showed a significant increase compared to the control group (P < 0.001) (Fig. 7A).
Fig. 7.
A ROS production and level of GSH in testis tissue. Mean ± SD of the ROS production and GPX activity level in the study groups; (*P < 0.05). B Real-time-PCR analyses. mRNA expression levels of (ACE2, TMPRSS2, BAX, BCL2 and Caspase3) in the testis tissue. Mean ± SD of the genes expression in the different groups (*P < 0.05 and ***P < 0.001). PCR, polymerase chain reaction
Thiols metabolism
Since thiol metabolism has an important role in cellular defense against stress exposure, we assessed the concentration of glutathione (GSH). As Fig. 5 show, concentration of GSH in COVID-19 group had significantly reduced in comparison to the control group (P < 0.05) (Fig. 7A).
Real-time PCR analysis
To assess mRNA expression of target genes at molecular level, the amount of transcripts for two genes contributing to the cellular entry of the virus (ACE2 and TMPRSS) as well as three genes involved in cellular death (BAX, BCL2 and Caspase3) was analyzed in testis tissue. The transcript for ACE2 was significantly upregulated in the COVID-19 group as compared to the control group (P < 0.001) (Fig. 7B). Nonetheless, the relative levels of mRNA expression for TMPRSS did not signify any differences between the COVID-19 group, and control group (Fig. 7B). Based on the statistical results, expression levels of BAX and Caspase3 were notably upregulated in the COVID-19 group as compared to the control group (P < 0.01) (Fig. 7B). Finally, in comparison with the control group, the protein expression for BCL2 had seen reduction in the COVID-19 group (P < 0.01) (Fig. 7B).
Percentage of apoptotic cells
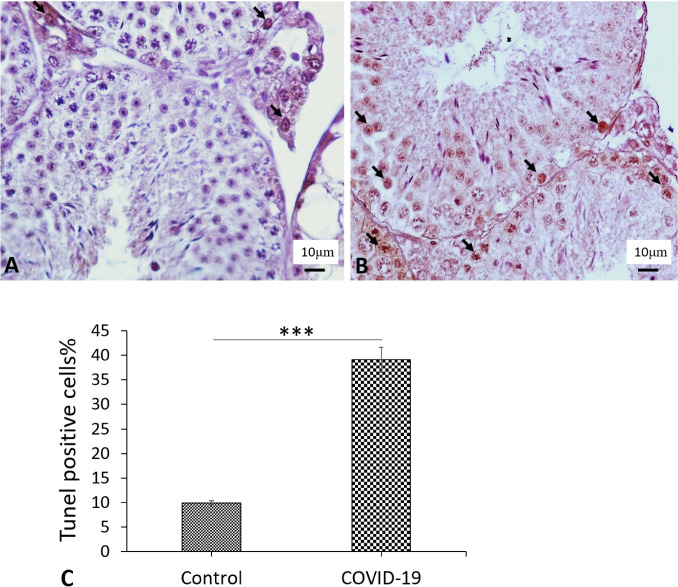
The percentage of apoptotic cells in testis was examined by TUNEL assay. TUNEL-positive cell counts illustrated a substantial increase in the apoptotic cells in the COVID-19 group (39.05%) compared to the control group (9.93%) (P < 0.001) (Fig. 8A–C).
Fig. 8.
The percent of apoptotic cells. Tunel detection of apoptotic cells in the testes of the A Cont group and B COVID-19 group. TUNEL positive cells with brown neuleous (Arrows). C Mean ± SD of the percent of apoptotic cells of testis in control and COVID-19 groups. (***P < 0.001) (Color figure online)
Discussion
Many studies have been carried out to clarify the disease’s course and therapeutic approach during the pandemic outbreak of COVID-19 [17]. Findings of the negative effects of COVID -19 on the male reproductive system remains of significant concern for clinicians. Infection caused by the virus have been associated with deterioration in sperm quality, but yet the exact mechanisms of SARS-CoV-2 infection remain poorly understood upon male fertility [18]. This study provides important results on the effect of SARS-CoV-2 virus on induction of oxidative stress pathway followed by the onset of apoptosis in testicular cells. Our first finding based on stereological examination of testicular tissue autopsy samples of severely ill deceased COVID-19 showed a significant decrease in the volume of interstitial tissue and seminiferous tubules. Reduction was also noted in seminiferous tubule length in deceased COVID-19 patients caused by the significant depletion of testicular cells such as spermatogonia, primary spermatocyte, spermatid, Leydig and Sertoli cells in the testis. This indicated injury of seminiferous tubules, interstitial tissue and germinal epithelium. Recently, a research also reported oligospermia in recovered male patients with a moderate COVID-19 infection who were fertile and have had offspring. Li et al. (2020) reported that decreased cell layers of seminiferous epithelium and a significant increase in apoptotic cells within seminiferous tubules in deceased COVID-19 patients due to the extensive germ cell destruction, which is an indication of impaired spermatogenesis [9].
The seminiferous tubule injury, testicular cell depletion, and mild lymphocytic inflammation were also considered to be another key factor in the histopathological findings of testicular tissue in COVID-19 patients (10). A recent report by Nora et al. [19] also showed decreased sperm concentration in ameliorated men with a moderate COVID-19 infection [19]. Apoptosis trigger by human CoV (HCoV) infection was described in previous in vitro studies and animal models in SARS-CoV-infected lung, spleen, and thyroid tissues [20]. Examination of BAX, BCL2 and Caspase3 expression and TUNEL assay in the testicular tissues of deceased COVID-19 autopsy showed a significant increase in testicular cell apoptosis which is an indication of impaired spermatogenesis. Furthermore, the results of this study also showed a significant increase in ROS production, as well as, a decrease in GSH content in deceased COVID-19 autopsy in compared to controls. One of the possible effective factors in causing damage to testicular tissue is oxidative stress. Oxidative stress is a mechanisms of cell and tissue damage caused by viruses of the Corona family, such as influenza. Thus COVID-19 infection can lead to oxidative damage to the testis [11]. Oxidative stress by ROS is related to all the main changes observed in inflammatory and infectious diseases by viruses of the Corona family, such as influenza. This, could be the connecting point that unites all these events. It has also been suggested that apoptosis can be triggered by oxidative stress in testicular cell apoptosis. These results suggest that SARS-CoV-2 infection can induce apoptosis in testicular cells due to the high levels of oxidative stress which, was consistent with previous studies [21]. It is assumed that oxidative stress plays a role in the pathogenesis of COVID-19 infection through the cytokine storm cycle, blood clotting mechanism, and exacerbation of hypoxia [22]. There is crosstalk between the cytokine storm and oxidative stress which may have deleterious effects on the testis tissue in the patients with COVID-19 infection. In addition, evidence suggests that SARS-CoV-2 infection in men increases levels of pro-inflammatory cytokines, mainly IL-1β, IL-6, and TNF-α, and ultimately acute stage hypogonadism which has been linked with increased levels of oxidative stress [23].
Furthermore, we also investigated the relative mRNA expression levels of ACE2 and TMPRSS genes in testicular tissue. The ACE2 and TMPRSS2 receptors has been shown to be a target for SARS-CoV-2 infection in testicular cells, suggesting that the testis is potentially a target organ for the SARS-CoV-2 virus [7, 24–26]. RNA-Seq revealed the presence of ACE2 transcripts in human spermatogonia, spermatocyte, spermatids, Leydig cells and Sertoli cells in the testis tissue [27, 28]. Interestingly, high levels of ACE2 receptor expression in testicular cells were observed in infertile men compared to fertile men, suggesting that increased ACE2 gene expression may have adverse effects on spermatogenesis [29]. In the present study, the expression of ACE-2 genes was increased in the testis of the COVID-19 group, which could be due to the fact that most of the samples in the COVID-19 group had high blood pressure, mainly angiotensin-converting enzyme inhibitor and angiotensin-receptor blocker therapy can modulate the expression of ACE2 protein and increase the expression of ACE2 and patient susceptibility to viral host cell entry and proliferation [30]. Previous studies thought that SARS-CoV-2 virus had a destructive effect on the testes by gaining entry to testicular cells via ACE2 receptors [29]. Recent findings, indicated that the expression of both ACE2 and TMPRSS2 receptors was required for SARS-CoV-2 virus to enter host testicular cells [25, 30]. However, our results showed that the expression of ACE2 gene and protein was increased in testicular cells, but the expression of TMPRSS2 in testicular cells was not significantly different in the COVID-19 samples compared to the control group. Therefore, the possibility of SARS-CoV-2 virus entry to the testicular cells are unlikely.
The present study has limitations that include a relatively smaller sample size, and the study could not investigate these COVID-19 patients prospectively. However, with these limitations, this study can be considered as a comprehensive study to assess the severity of reproductive health in COVID-19 patients. Our results can be considered preliminary and further studies are invited by researchers to confirm the findings of our study.
Conclusion
It can be concluded from this study that COVID-19 infection can cause spermatogenesis disruption. This disruption is likely thought to be through the oxidative stress pathway and subsequently induces apoptosis. It is not yet fully determined whether SARS-CoV-2 virus particles can transmission to the testicular blood barrier and enter testicular tissue, therefore, further experiments are needed to validate this association. Due to the lack of information on the SARS-CoV-2 virus further studies are need to be undertaken to better understand the effects of this virus on reproductive organs.
Acknowledgements
This work has been performed at the School of Medicine, Shahid Beheshti University of Medical Science (Registration No: 1399. 26274). The present article is supported by “Research Department of the School of Medicine Shahid Beheshti University of Medical Sciences” (Registration No. 26274). We would like to express our special thanks to the forensic center of Tehran, Iran.
Author contributions
MAA designed the present research, implemented a stereological investigation, and drafted the manuscript. MG, GRM and MF provided the clinical information and samples and involved in the draft of the manuscript. MAA, AHH, and ARK, AP, AA and HAA conducted statistical analyses and involved in the draft of the manuscript. In addition, SA accomplished the histological study and involved in the draft of the manuscript. Molecular and cellular examination was also performed by NM and BEF and involved in immunohistochemistry. Each of the authors read and confirmed the resulting paper.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Negin Moghimi and Bahram Eslami Farsani are co-first authors.
Contributor Information
Mohammad-Amin Abdollahifar, Email: abdollahima@sbmu.ac.ir, Email: m_amin58@yahoo.com.
Mehdi Forozesh, Email: forozeshiran@gmail.com.
References
- 1.Organization WH. World Health Organization coronavirus disease 2019 (COVID-19) situation report. Geneva: World Health Organization; 2020. [Google Scholar]
- 2.Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durvasula R, Wellington T, McNamara E, Watnick S. COVID-19 and kidney failure in the acute care setting: our experience from Seattle. Am J Kidney Dis. 2020;76:4–6. doi: 10.1053/j.ajkd.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nunes Duarte-Neto A, de Almeida Monteiro RA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro Filho J, et al. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uhlén M, Fagerberg L, Hallström BM, Lindskog C, Oksvold P, Mardinoglu A, et al. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, Xu X. scRNA-seq profiling of human testes reveals the presence of the ACE2 receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neto FTL, Bach PV, Najari BB, Li PS, Goldstein M. Spermatogenesis in humans and its affecting factors, Seminars in cell & developmental biology. Semin Cell Dev Biol. 2016;59:10–26. doi: 10.1016/j.semcdb.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;9:1–8. doi: 10.1016/j.eclinm.2020.100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Chen S, Huang B, Zhong J-M, Su H, Chen Y-J, et al. Pathological findings in the testes of COVID-19 patients: clinical implications. Eur Urol Focus. 2020;6:1124–1129. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li R, Yin T, Fang F, et al. Potential risks of SARS-Cov-2 infection on reproductive health. Reprod BioMed Online. 2020;41(1):89–95. doi: 10.1016/j.rbmo.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sexrelated hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol. 2021;93(1):456–462. doi: 10.1002/jmv.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panahi S, Karamian A, Sajadi E, Aliaghaei A, Nazarian H, Abdi S, et al. Sertoli cell–conditioned medium restores spermatogenesis in azoospermic mouse testis. Cell Tissue Res. 2020;379:577–587. doi: 10.1007/s00441-019-03092-w. [DOI] [PubMed] [Google Scholar]
- 14.Ziaeipour S, Ahrabi B, Naserzadeh P, Aliaghaei A, Sajadi E, Abbaszadeh H-A, et al. Effects of Sertoli cell transplantation on spermatogenesis in azoospermic mice. Cell Physiol Biochem. 2019;52:421–434. doi: 10.33594/000000030. [DOI] [PubMed] [Google Scholar]
- 15.Reed M, Howard C, de Yanés GS. One-stop stereology: the estimation of 3D parameters using Isotropic Rulers. J Microsc. 2010;239:54–65. doi: 10.1111/j.1365-2818.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 16.Kanter M, Aktas C, Erboga M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: an immunohistochemical and ultrastructural study. Toxicol Ind Health. 2013;29:99–113. doi: 10.1177/0748233711425082. [DOI] [PubMed] [Google Scholar]
- 17.Machhi J, Herskovitz J, Senan AM, Dutta D, Nath B, Oleynikov MD, et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J Neuroimmun Pharmacol. 2020;1:28. doi: 10.1007/s11481-020-09944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalili MA, Leisegang K, Majzoub A, Finelli R, Selvam MKP, Henkel R, et al. Male fertility and the COVID-19 pandemic: systematic review of the literature. World J Men's Health. 2020;38:506. doi: 10.5534/wjmh.200134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nora H, Philippos E, Marcel A, Cornelius D, Dunja B-B, Ortwin A, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114:233–238. doi: 10.1016/j.fertnstert.2020.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J, Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am J Pathol. 2007;170(4):1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ighodaro O, Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria J Med. 2018;54:287–293. doi: 10.1016/j.ajme.2017.09.001. [DOI] [Google Scholar]
- 22.Cecchini R, Cecchini AL. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma L, Xie W, Li D, et al. Effect of SARS-CoV-2 infection upon male gonadal function: a single center-based study. medRxiv. 2020;4:1–14. [Google Scholar]
- 24.Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of SARS-CoV-2 in semen of males recovering from COVID-19. Fertil Steril. 2020;113:1135–1139. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenberg ML. Coronavirus disease 2019 and men’s reproductive health. Fertil Steril. 2020;113:1154. doi: 10.1016/j.fertnstert.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vishvkarma R, Rajender S. Could SARS-CoV-2 affect male fertility? Andrologia. 2020;52:13712. doi: 10.1111/and.13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Douglas GC, O’Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 28.Jan SZ, Vormer TL, Jongejan A, Röling MD, Silber SJ, de Rooij DG, et al. Unraveling transcriptome dynamics in human spermatogenesis. Development. 2017;144:3659–3673. doi: 10.1242/dev.152413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Q, Xiao X, Aierken A, Yue W, Wu X, Liao M, et al. The ACE2 expression in Sertoli cells and germ cells may cause male reproductive disorder after SARS-CoV-2 infection. J Cell Mol Med. 2020;24:9472–9477. doi: 10.1111/jcmm.15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Wang X, Chen J, Zhang H, Deng A (2019) Association of Renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease, (COVID-19) infection in Wuhan, China. JAMA Cardiol 2020:1624 [DOI] [PMC free article] [PubMed]