Abstract
The present study was designed to analyze the expression of pregnancy-associated plasma protein-A (PAPP-A) in the serum of patients with ectopic pregnancy (EP) and related factors inducing this condition. Seventy-five patients with EP admitted to the Affiliated Hospital of Jining Medical University from January 2018 to February 2019 were selected as the research group, and another 59 healthy pregnant women of the corresponding age, gravidity and gestational week were enrolled in the control group. ELISA was employed to detect the serum expression levels of PAPP-A and inflammatory factors such as interleukin-8 (IL-8) and tumor necrosis factor-α (TNF-α). ROC was adopted to evaluate the diagnostic value of serum PAPP-A in patients with EP, and Pearson correlation coefficient was applied to analyze the correlation of PAPP-A with inflammatory factors IL-8 and TNF-α. Serum PAPP-A expression was significantly lower in EP patients than those in the control group. The area under the curve (AUC) of serum PAPP-A in diagnosing EP patients was 0.812, and the PAPP-A value in the control group was significantly higher than that of the research group at 7-8 weeks and ≥9 weeks. With regard to the expression of inflammatory factors, the research group presented markedly higher IL-8 and TNF-α levels than the control group. PAPP-A was negatively related to inflammatory factors IL-8 and TNF-α in the research group. In addition, it was revealed that patients with a history of genital surgery, salpingotomy, pelvic infection, EP or low PAPP-A expression were at high risk of EP. In conclusion, PAPP-A was revealed to be lowly expressed in the serum of EP patients, and to negatively be correlated with inflammatory factors IL-8 and TNF-α, which may serve as a useful marker for the diagnosis and prognosis of EP.
Keywords: pregnancy-associated plasma protein-A, ectopic pregnancy, prognosis, diagnosis, risk factors
Introduction
Ectopic pregnancy (EP) is the main cause of maternal morbidity and mortality in the first trimester (1,2). With the occurrence of embryo transfer and in vitro fertilization, the incidence of EP has increased sharply, leading to mass maternal and sporadic deaths (3,4), which account for approximately 10% of all pregnancy-related deaths (5). Complications caused by EP remain a major cause of morbidity and mortality in early pregnancy (6). In addition, little is known about the treatment and predictive factors of this complication (7), and its early symptoms are not obvious, and easily confused with threatened abortion (8). Therefore, determining the development, occurrence, prognosis and potential mechanism of EP is conducive for clinicians to explore a more feasible treatment plan for this condition.
First found in the plasma of pregnant women, PAPP-A is a metalloproteinase that plays a key part in regulating the activity of insulin-like growth factors (9), and is subsequently acknowledged to be a multifunctional regulator in various pathological processes (10). In addition, it is a major physiological regulator of insulin-like growth factor binding protein-4 (IGFBP-4), which cleaves the IGFBP4/IGF1 complex to release insulin-like growth factor 1 (IGF-1), and then regulates its bioavailability (11). However, there are other studies indicating that the aberrant expression of PAPP-A disrupts the regulation of the availability of IGF-1 and thus affects the biology of tumors (12-14). Previous studies (15,16) have demonstrated that, the prevention and prognosis of an adverse pregnancy is of great clinical significance from a medical point of view, and is particularly challenging for the scientific community, family and society, and that ultrasounds combined with PAPP-A can better diagnose and predict an abnormal pregnancy. However, the relationship between serum PAPP-A and the diagnosis, prognosis, related factors, as well as the possible molecular mechanism of EP remains poorly understood.
Therefore, by examining the expression of PAPP-A in EP, its clinical value in EP, the related factors inducing the disease and the possible molecular mechanism were explored, with the aim to identify reliable diagnostic and prognostic markers and potential drug targets for EP.
Patients and methods
General information
From January 2018 to February 2019, 75 patients with EP admitted to the Affiliated Hospital of Jining Medical University were included in the research group, and another 59 healthy pregnant women of the corresponding age, gravidity and gestational week were enrolled in the control group (17). Patients in the research group were 21-35 years old, with an average age of 25.73±7.23 years, while those in the control group were 20-35 years old, with an average age of 26.64±7.35 years.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Aged 20-35 years and naturally conceived; patients diagnosed with EP laparoscopically (18); with complete clinical general data, amenorrhea between 27-88 days; and no history of fetus protection during pregnancy. Ultrasound examination confirmed that there was no pregnancy sac in the uterine cavity of the patient, and there were heterogeneous abnormal echogenic masses outside the uterine cavity revealing a trend of gradual increase, or there were fetal buds and fetal heart beats. All of the enrolled patients were informed of this study and signed the written informed consent. The experimental process was approved by the Medical Ethics Committee of the Affiliated Hospital of Jining Medical University and was in accordance with the 2013 version of the Declaration of Helsinki. The exclusion criteria were as follows: Patients with communication barriers or severe mental illness, those combined with malignant tumor or serious heart, lung, liver, kidney and other functional disorders, or those who were in urgent need of surgery for intraperitoneal hemorrhage with unstable vital signs were excluded.
Methods
Elbow venous blood (5 ml) was extracted from an empty stomach in the morning into vacuum blood collection tubes without anticoagulant, centrifuged at 1,500 x g and 4˚C for 10 min, and then stored in a low-temperature refrigerator at -75˚C for later use. The serum was then removed from the freezer and dissolved in a 4˚C refrigerator before placing it at room temperature for complete dissolution. The serum expression levels of PAPP-A (cat. no. ab174314; Kemin Biotechnology Co., Ltd.), IL-8 [cat. no. Ant-111 (0.5 mg); Jingke Chemical Technology Co., Ltd.] and TNF-α (cat. no. BL-E1290h; Bdlisa Technology Co., Ltd.) were detected by enzyme-linked immunosorbent assay (ELISA) (19). The present study was carried out in strict accordance with the manufacturer's instructions. Firstly, sample, standard and blank wells were set up. Then, 50 µl of the sample to be tested was added to the sample wells, 50 µl of the standard was added to the standard wells, and blank wells contained no reagents. Subsequently, 100 µl of horseradish peroxidase-labeled detection antibody (cat. no. P39810-100 mg; Acmec Biochemical Co., Ltd.) was added to the sample wells and standard wells, and then the plates were sealed and incubated at 37˚C for 60 min. Next, the liquid was discarded, the plates were patted dry and washed repeatedly 5 times and the substrates A and B (1:1) (included in the kit) were thoroughly mixed before their addition (100 µl) to all the wells. The plates were then sealed and incubated at 37˚C for 15 min. Finally, 50 µl termination solution was added to each well, and the absorbance [optical density (OD)] of each well at 450 nm was read by a fully-automatic enzyme label analyzer (M15; Chenlian Biotechnology Development Co., Ltd.) to calculate the expression levels of PAPP-A, IL-8 and TNF-α.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 (IBM Corp.), and the data was visualized by GraphPad Prism 6 (GraphPad Software, Inc.). The counting data were expressed as case/percentage [n (%)] and the chi-square test was adopted for inter-group comparisons. The measurement data were expressed in the form of the mean ± SD, and the comparison of measurement data between the two groups was conducted by independent sample t-test. The area under the receiver operating characteristic (ROC) curve (AUC) was applied to evaluate the diagnostic value of peripheral blood PAPP-A in patients with EP. The correlation between PAPP-A and inflammatory factors IL-8 and TNF-α was assessed by Pearson correlation coefficient, and the independent risk factors affecting the incidence of EP were analyzed using Cox regression analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
General information
Seventy-five patients with EP admitted to our hospital were enrolled as the research group, and another 59 healthy pregnant women were enrolled as the control group. The participants in the research group were 21-35 years old, with an average age of 25.73±7.23 years, while those in the control group were 20-35 years old, with an average age of 26.64±7.35 years. No significant difference was observed in terms of age, gravidity, ethnicity, allergic reaction, smoking history, drinking history, diet, height, gestational age, abdominal circumference, systolic blood pressure, or diastolic blood pressure of patients in the two groups, while other baseline data represented by pre-pregnancy BMI, weight gain during pregnancy, and level of PAPP-A revealed statistically significant differences (P<0.05; Table I).
Table I.
Comparison of general data between two groups [n (%)] (mean ± SD).
| Categories | Research group (n=75) | Control group (n=59) | t/χ2 value | P-value |
|---|---|---|---|---|
| Age (years) | 25.73±7.23 | 26.64±7.35 | 0.718 | 0.474 |
| Gravidity (times) | 1.40±0.52 | 1.50±0.62 | 1.015 | 0.312 |
| Ethnicity | 0.273 | 0.602 | ||
| Han | 39 (52.00) | 28 (47.46) | ||
| Ethnic minorities | 36 (48.00) | 31 (52.54) | ||
| Allergic reaction | 0.970 | 0.325 | ||
| Yes | 42 (56.00) | 38 (64.41) | ||
| No | 33 (44.00) | 21 (35.59) | ||
| Smoking | 0.158 | 0.691 | ||
| Yes | 23 (30.67) | 20 (33.90) | ||
| No | 52 (69.33) | 39 (66.10) | ||
| Drinking | 0.001 | 0.980 | ||
| Yes | 24 (32.00) | 19 (32.20) | ||
| No | 51 (68.00) | 40 (67.80) | ||
| Diet | 0.970 | 0.325 | ||
| Dietary restriction | 33 (44.00) | 21 (35.59) | ||
| None dietary restriction | 42 (56.00) | 38 (64.41) | ||
| Height (cm) | 161.54±5.23 | 162.01±5.12 | 0.521 | 0.603 |
| Pre-pregnancy BMI (kg/m2) | 9.104 | 0.002 | ||
| ≥23 | 11 (14.67) | 22 (37.29) | ||
| <23 | 64 (85.33) | 37 (62.71) | ||
| Weight gain during pregnancy (kg) | 13.52±4.26 | 15.61±4.53 | 2.742 | 0.007 |
| Gestational age (weeks) | 23.95±1.85 | 24.45±1.55 | 1.666 | 0.098 |
| Abdominal circumference (cm) | 99.98±6.36 | 101.87±6.65 | 1.674 | 0.097 |
| Systolic blood pressure (mmHg) | 114.12±9.06 | 115.59±8.99 | 0.936 | 0.351 |
| Diastolic blood pressure (mmHg) | 72.98±7.16 | 75.04±6.88 | 1.682 | 0.095 |
| PAPP-A (pg/ml) | 4.94±1.36 | 5.68±1.59 | 2.902 | 0.004 |
PAPP-A, pregnancy-associated plasma protein-A.
Expression and diagnostic value of PAPP-A in the two groups
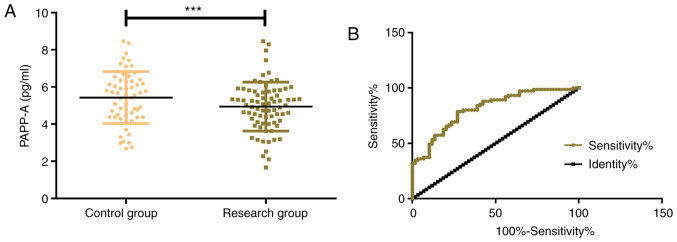
The expression levels of PAPP-A in the control group and the research group were 5.68±1.59 pg/ml and 4.94±1.36 pg/ml, respectively, which indicated that PAPP-A in the control group was significantly higher than that in the research group (P<0.001). By further drawing the ROC curve, it was determined that the serum PAPP-A in the diagnosis of EP was 0.812 (95% CI, 0.741-0.884), with an optimal cut-off value of 5.648, a sensitivity of 92.13, and a specificity of 78.33 (Fig. 1).
Figure 1.
Expression and diagnostic value of PAPP-A. (A) The expression of PAPP-A in the research group was significantly lower than that in the control group. (B) ROC curve of PAPP-A in the diagnosis of ectopic pregnancy. ***P<0.001 vs. the control group. PAPP-A, pregnancy-associated plasma protein A; ROC, receiver operating characteristic.
Changes of serum PAPP-A expression at different gestational weeks in the two groups
The corresponding serum PAPP-A expression levels at different gestational weeks in the control group and the research group were as follows: ≤6 weeks: 15.16±15.27 vs. 11.71±10.97 mU/ml; 6-7 weeks: 16.08±7.60 vs. 15.12±9.30 mU/ml; 7-8 weeks: 19.37±10.23 vs. 8.86±7.62 mU/ml; 8-9 weeks: 18.52±16.92 vs. 15.52±5.15 mU/ml; ≥9 weeks: 22.33±14.64 vs. 12.02±10.11 mU/ml. From the aforementioned data, no significant difference in serum PAPP-A expression was observed between the two groups at weeks ≤6, week 6-7, and week 8-9 (P>0.001), while the PAPP-A value of the control group at week 7-8 and ≥9 weeks was significantly higher than that of the research group (P<0.001; Table II and Fig. 2).
Table II.
Changes in the serum PAPP-A level at different gestational weeks in two groups [(mean ± sd), mU/ml].
| Groups | ≤6 weeks | 6-7 weeks | 7-8 weeks | 8-9 weeks | ≥9 weeks |
|---|---|---|---|---|---|
| Control group | 15.16±15.27 | 16.08±7.60 | 19.37±10.23 | 18.52±16.92 | 22.33±14.64 |
| Research group | 11.71±10.97 | 15.12±9.30 | 8.86±7.62 | 15.52±5.15 | 12.02±10.11 |
| t | 1.521 | 0.742 | 6.815 | 1.454 | 4.814 |
| P-value | 0.131 | 0.459 | <0.001 | 0.148 | <0.001 |
PAPP-A, pregnancy-associated plasma protein-A.
Figure 2.
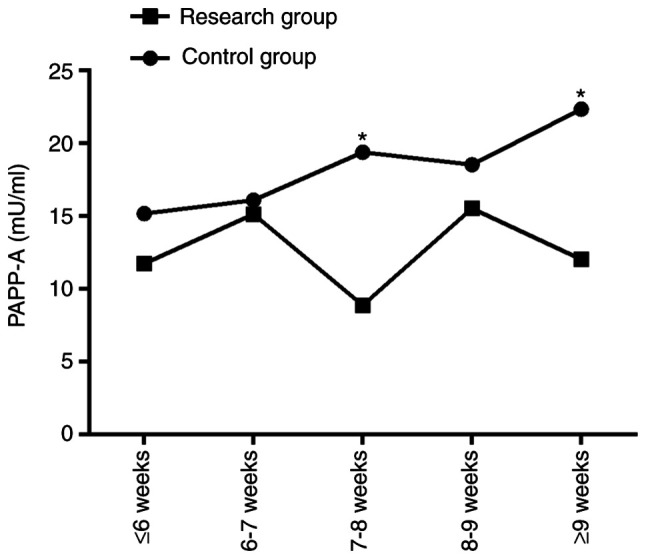
Changes of the serum PAPP-A expression at different gestational weeks in the two groups of patients (research and control groups). *P<0.05 vs. the control group. PAPP-A, pregnancy-associated plasma protein-A.
Expression of inflammatory factors IL-8 and TNF-α in the two groups
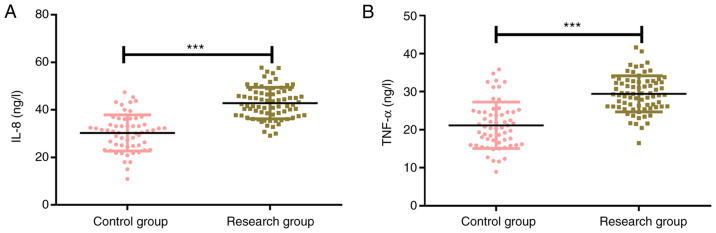
The expression levels of IL-8 in the control group and the research group were 27.73±4.79 ng/l and 42.93±6.28 ng/l, respectively, and the corresponding expression levels of TNF-α in the control group and the research group were 20.83±4.37 ng/l and 29.37±4.38 ng/l. The results revealed that the expression levels of inflammatory factors IL-8 and TNF-α in the control group were significantly lower than those in the research group (P<0.001; Fig. 3).
Figure 3.
Expression levels of markers IL-8 and TNF-α. (A) The expression of IL-8 in the research group was significantly higher than that in the control group. (B) The expression of TNF-α in the research group was significantly higher than that in the control group. ***P<0.001 vs. the control group. IL-8, interleukin-8; TNF-α, tumor necrosis factor-α.
Correlation analysis between inflammatory factors IL-8, TNF-α and PAPP-A
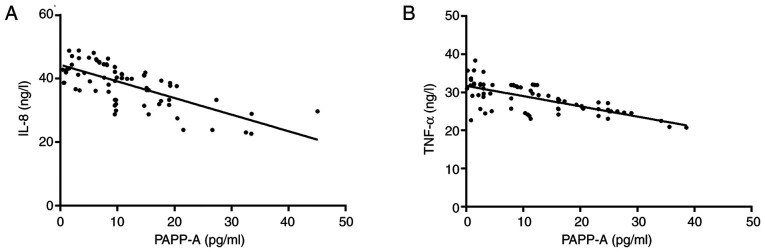
Pearson correlation coefficients were applied to analyze the correlation of PAPP-A with IL-8 and TNF-α. The results revealed that serum PAPP-A and IL-8 were negatively correlated (r=-0.691; P<0.001), and in addition, serum PAPP-A was negatively correlated with TNF-α (r=-0.692; P<0.001; Fig. 4).
Figure 4.
Correlation analysis between inflammatory factors IL-8, TNF-α and PAPP-A. (A) PAPP-A was negatively correlated with marker IL-8 (r=-0.691; P<0.001). (B) PAPP-A was negatively correlated with marker TNF-α (r=-0.692; P<0.001). IL-8, interleukin-8; TNF-α, tumor necrosis factor-α; PAPP-A, pregnancy-associated plasma protein-A.
Cox regression analysis of factors affecting the incidence of EP
Multivariate logistic regression analysis was carried out for the factors with differences. The results indicated that a history of genital surgery (P=0.022), salpingotomy (P=0.005), pelvic infection (P=0.041), EP (P=0.013) and PAPP-A (P=0.003) were independent risk factors affecting the incidence of EP. Among the risk factors aforementioned above, history of salpingotomy, EP and low PAPP-A expression increased the risk of EP (Tables III and IV).
Table III.
Cox regression analysis assignment table.
| Factors | Variable | Assignment |
|---|---|---|
| Age | X1 | No=0, yes=1 |
| Allergic reaction | X2 | No=0, yes=1 |
| Smoking history | X3 | No=0, yes=1 |
| Drinking history | X4 | No=0, yes=1 |
| Diet | X5 | No=0, yes=1 |
| History of genital surgery | X6 | No=0, yes=1 |
| History of salpingotomy | X7 | No=0, yes=1 |
| History of pelvic infection | X8 | No=0, yes=1 |
| History of lower abdominal surgery | X9 | No=0, yes=1 |
| History of ectopic pregnancy | X10 | No=0, yes=1 |
| PAPP-A | X11 | No=0, yes=1 |
PAPP-A, pregnancy-associated plasma protein-A.
Table IV.
Univariate and multivariate Cox regression analysis of ectopic pregnancy.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Factors | HR (95 CI%) | P-value | HR (95 CI%) | P-value |
| Age | 1.549 (0.519-4.628) | 0.438 | ||
| Allergic reaction | 0.553 (0.157-2.042) | 0.372 | ||
| Smoking history | 1.008 (0.991-1.022) | 0.368 | ||
| Drinking history | 0.564 (0.201-1.580) | 0.276 | ||
| Diet | 2.748 (0.705-10.701) | 0.143 | ||
| History of genital surgery | 1.406 (1.092-1.812) | 0.043 | 1.138 (0.857-1.514) | 0.022 |
| History of salpingotomy | 6.459 (1.408-29.575) | 0.017 | 12.852 (2.297-71.238) | 0.005 |
| History of pelvic infection | 6.108 (1.385-24.954) | 0.018 | 2.601 (1.032-6.538) | 0.041 |
| History of lower abdominal surgery | 2.270 (0.901-1.792) | 0.175 | - | - |
| History of ectopic pregnancy | 0.292 (0.124-0.689) | 0.005 | 0.357 (0.158-0.735) | 0.013 |
| PAPP-A | 8.356 (2.128-3.846) | 0.002 | 5.817 (2.157-15.756) | 0.003 |
PAPP-A, pregnancy-associated plasma protein-A.
Discussion
Worldwide, gestational placenta related-diseases are one of the leading causes of maternal and neonatal morbidity and mortality (20). Of these, EP is a pregnancy implanted outside the endometrium, which hazards the health of patients and is a major cause of sudden death in women of childbearing age (21). In addition, these women often suffer from complications, such as organ rupture with massive bleeding, treatment-related risks, recurrent EP and infertility risk (22). Therefore, the diagnosis of EP is of vital significance for reducing the morbidity and mortality associated with this disease (23).
PAPP-A, a placental-derived glycoprotein produced by trophoblast cells that gradually increases during the first few weeks of a viable pregnancy, has been revealed to be diagnostic in a variety of abnormal obstetric conditions (24). The present study firstly detected the expression of PAPP-A in EP. It was observed that the serum PAPP-A expression was significantly lower in EP patients in the research group than that of patients in the control group, and the AUC of patients diagnosed with EP by PAPP-A was 0.812, with a sensitivity of 92.13 and a specificity of 78.33, indicating that PAPP-A may be a potential diagnostic and therapeutic target for EP. PAPP-A expression was further analyzed in the two groups at different gestational weeks, and different PAPP-A levels were observed, that is, the control group presented a stable increasing trend of PAPP-A at each gestational week, while the research group exhibited an irregular but insignificant increase. At 7-8 weeks and ≥9 weeks, the PAPP-A value in the control group was significantly higher than that in the research group, which was probably due to the fact that the PAPP-A value between the two groups gradually increased with the prolonging of amenorrhea. Therefore, according to the detection of low expression of PAPP-A in the serum of EP patients, it was theorized that the determination of PAPP-A in serum could be used as a routine measurement for pregnant patients. In a study of Kaijomaa et al on adverse pregnancy (25), PAPP-A was underexpressed in patients with adverse pregnancy, indicating that PAPP-A was of certain diagnostic value and was an important risk factor for EP, which was consistent with the results of the present study.
IL-8 is a chemokine that has been clinically demonstrated to play a major role in tumor immune escape by promoting an immunosuppressive tumor microenvironment, and it has been revealed that high levels of IL-8 are associated with poor prognosis in a variety of tumors (26). According to previous studies, serum IL-8 was revealed to be significantly higher in EP women than in women with normal pregnancy in utero, and the diagnosis and identification of EP women with chlamydia trachomatis infection was related to the level of IL-8 (27,28). TNF-α is an inflammatory cytokine secreted by macrophages and monocytes, which exerts a marked effect on inflammatory response, cell apoptosis and proliferation (29). There is also research showing that TNF-α has attracted the attention of clinicians and scholars due to its involvement in the development of inflammatory response, autoimmunity, neoplastic disease and the endocrine system (30). For example, TNF-α induces the apoptosis of cytotrophoblast cells, which also suggests that abnormal expression of TNF-α may adversely affect the development and function of the placenta (31). Growing evidence reveals that TNF-α mediates pregnancy complications and increases the sensitivity of infertility, while increased TNF-α in the placenta increases the abortion rate (32). Soriano et al (33) revealed that the expression of serum inflammatory factors IL-6, IL-8 and TNF-α in EP women was significantly higher than that in normal pregnant women, suggesting that the overexpression of these three inflammatory mediators stimulated the inflammatory cascade in patients and aggravated the progression of EP. Furthermore, other researchers have reported that the pro-inflammatory factors TNF-α, IL-1β and IL-6 stimulate the expression of PAPP-A in cultured cells (34). In the present study, the detection of inflammatory factors demonstrated that the expression levels of inflammatory factors IL-8 and TNF-α in the research group were significantly higher than those in the control group, while PAPP-A was negatively correlated with the two, suggesting that the low expression of PAPP-A in an inflammatory environment may be related to the occurrence and development of EP. Concerning the risk factors of EP, Zhang et al (35) revealed that a history of EP, infertility, and salpingotomy were all risk factors. In the present study, Cox regression analysis revealed that a history of genital surgery, salpingotomy, pelvic infection, EP and low expression of PAPP-A were the risk factors of EP, among which a history of salpingotomy, EP and low expression of PAPP-A increased the risks. Therefore, PAPP-A is anticipated to be a biomarker for the diagnosis and prognosis of EP.
The present study strictly screened the participants according to the inclusion and exclusion criteria and ensured the rigor and reliability of the research. Although in the present study it was confirmed that PAPP-A is lowly expressed in EP patients and is negatively correlated with inflammatory factors IL-8 and TNF-α, there are still some certain limitations in this study. We will perform more basic experiments in the future, investigating the specific regulatory mechanism of PAPP-A on EP, thus further verifying that PAPP-A can be a potential therapeutic target for EP.
In conclusion, PAPP-A was downregulated in patients with EP and may be a useful marker for the diagnosis and prognosis assessment of this condition.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XZ and CW conceived and designed the study, collected, analyzed and interpreted the experimental data, drafted this paper, and revised the manuscript critically for important intellectual content. Both authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Affiliated Hospital of Jining Medical University (Jining, China). Signed written informed consents were obtained from the patients and/or guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Perkins KM, Boulet SL, Kissin DM, Jamieson DJ. National ART Surveillance (NASS) Group. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001-2011. Obstet Gynecol. 2015;125:70–78. doi: 10.1097/AOG.0000000000000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bronson R. Ectopic pregnancy-still a challenge. Fertil Steril. 2018;110:1265–1266. doi: 10.1016/j.fertnstert.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Refaat B, Dalton E, Ledger WL. Ectopic pregnancy secondary to in vitro fertilisation-embryo transfer: Pathogenic mechanisms and management strategies. Reprod Biol Endocrinol. 2015;13(30) doi: 10.1186/s12958-015-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashshi AM, Batwa SA, Kutbi SY, Malibary FA, Batwa M, Refaat B. Prevalence of 7 sexually transmitted organisms by multiplex real-time PCR in Fallopian tube specimens collected from Saudi women with and without ectopic pregnancy. BMC Infect Dis. 2015;15(569) doi: 10.1186/s12879-015-1313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Zhao WH, Zhu Q, Cao SJ, Ping H, Xi X, Qin GJ, Yan MX, Zhang D, Qiu J, Zhang J. Risk factors for ectopic pregnancy: A multi-center case-control study. BMC Pregnancy Childbirth. 2015;15(187) doi: 10.1186/s12884-015-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagana AS, Vitale SG, De Dominici R, Padula F, Rapisarda AM, Biondi A, Cianci S, Valenti G, Capriglione S, Frangez HB, Sturlese E. Fertility outcome after laparoscopic salpingostomy or salpingectomy for tubal ectopic pregnancy A 12-years retrospective cohort study. Ann Ital Chir. 2016;87:461–465. [PubMed] [Google Scholar]
- 7.Hsu JY, Chen L, Gumer AR, Tergas AI, Hou JY, Burke WM, Ananth CV, Hershman DL, Wright JD. Disparities in the management of ectopic pregnancy. Am J Obstet Gynecol. 2017;217:49.e1–49.e10. doi: 10.1016/j.ajog.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanana C, Gupta N, Bansal I, Hooda K, Sharma P, Gupta M, Gandhi D, Kumar Y. Different sonographic faces of ectopic pregnancy. J Clin Imaging Sci. 2017;7(6) doi: 10.4103/jcis.JCIS_105_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bøtkjær JA, Noer PR, Oxvig C, Yding Andersen C. A common variant of the pregnancy-associated plasma protein-A (PAPPA) gene encodes a protein with reduced proteolytic activity towards IGF-binding proteins. Sci Rep. 2019;9(13231) doi: 10.1038/s41598-019-53957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bale LK, Chakraborty S, Conover CA. Inducible reduction in pregnancy-associated plasma protein-A gene expression inhibits established atherosclerotic plaque progression in mice. Endocrinology. 2014;155:1184–1187. doi: 10.1210/en.2013-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith YE, Toomey S, Napoletano S, Kirwan G, Schadow C, Chubb AJ, Mikkelsen JH, Oxvig C, Harmey JH. Recombinant PAPP-A resistant insulin-like growth factor binding protein 4 (dBP4) inhibits angiogenesis and metastasis in a murine model of breast cancer. BMC Cancer. 2018;18(1016) doi: 10.1186/s12885-018-4950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prithviraj P, Anaka M, McKeown SJ, Permezel M, Walkiewicz M, Cebon J, Behren A, Jayachandran A. Pregnancy associated plasma protein-A links pregnancy and melanoma progression by promoting cellular migration and invasion. Oncotarget. 2015;6:15953–15965. doi: 10.18632/oncotarget.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sert A, Leung K, Waring ME, Rojas-Rodriguez R, Corvera S, Moore Simas TA. Association between first trimester pregnancy associated plasma protein-A (PAPP-A) and gestational diabetes mellitus development. 2016. [Google Scholar]
- 14.Peeva G, Oakley L, Von Rège I, Nicolaides K, Oteng-Ntim E. Does first trimester serum pregnancy-associated plasma protein A differ in pregnant women with sickle cell disease? Prenat Diagn. 2019;39:921–924. doi: 10.1002/pd.5507. [DOI] [PubMed] [Google Scholar]
- 15.Antsaklis P, Fasoulakis Z, Theodora M, Diakosavvas M, Kontomanolis EN. Association of low maternal pregnancy-associated plasma protein A with adverse perinatal outcome. Cureus. 2019;11(e4912) doi: 10.7759/cureus.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papastefanou I, Wright D, Syngelaki A, Lolos M, Anampousi K, Nicolaides KH. Competing-risks model for prediction of small-for-gestational-age neonate from maternal characteristics and serum pregnancy-associated plasma protein-A at 11-13 weeks' gestation. Ultrasound Obstet Gynecol. 2020;56:541–548. doi: 10.1002/uog.23118. [DOI] [PubMed] [Google Scholar]
- 17.Xin H, Liu W, Li P. Diagnostic value of detection of serum β-HCG and CT-IgG combined with transvaginal ultrasonography in early tubal pregnancy. Exp Ther Med. 2018;16:277–281. doi: 10.3892/etm.2018.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann S, Abele H, Bachmann C. Spontaneous bilateral tubal ectopic pregnancy: Incidental finding during laparoscopy-brief report and review of literature. Geburtshilfe Frauenheilkd. 2016;76:413–416. doi: 10.1055/s-0041-110394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hornbeck PV. Enzyme-linked immunosorbent assays. Curr Protoc Immunol. 2015;110:2.1.1–2.1.23. doi: 10.1002/0471142735.im0201s110. [DOI] [PubMed] [Google Scholar]
- 20.Browne JL, Klipstein-Grobusch K, Koster MP, Ramamoorthy D, Antwi E, Belmouden I, Franx A, Grobbee DE, Schielen PC. Pregnancy associated plasma protein-a and placental growth factor in a sub-Saharan African population: A nested cross-sectional study. PLoS One. 2016;11(e0159592) doi: 10.1371/journal.pone.0159592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sowter M. Finding an ectopic pregnancy. Imaging. 2015;17 [Google Scholar]
- 22.Perlman BE, Guerrero K, Karsalia R, Heller DS. Reproductive outcomes following a ruptured ectopic pregnancy. Eur J Contracept Reprod Health Care. 2020;25:206–208. doi: 10.1080/13625187.2020.1755032. [DOI] [PubMed] [Google Scholar]
- 23.Murano T, Shaker L, Marco CA. Evaluation and management of ectopic pregnancy in the emergency department. Emergency Med Rep. 2019;40 [Google Scholar]
- 24.Batson RJ, Mills BB, Nagy Z, Roudebush W. Pregnancy-associated plasma protein A (PAPP-A): A biomarker for the aid in risk stratification of nonviable pregnancy. Fertil Steril. 2015;104(e351) [Google Scholar]
- 25.Kaijomaa M, Rahkonen L, Ulander VM, Hämäläinen E, Alfthan H, Markkanen H, Heinonen S, Stefanovic V. Low maternal pregnancy-associated plasma protein A during the first trimester of pregnancy and pregnancy outcomes. Int J Gynaecol Obstet. 2017;136:76–82. doi: 10.1002/ijgo.12002. [DOI] [PubMed] [Google Scholar]
- 26.Melero Bermejo I, Jaffee EM, Davar D, Cardarelli J, Williams D, Phillips P, Phillips P, Carleton M, Zhou M, De Henau O, et al. Phase 1b/2 study of nivolumab in combination with an anti-IL-8 monoclonal antibody, BMS-986253, in a biomarker-enriched population of patients with advanced cancer. J Clin Oncol. 2018;36 (Suppl 15)(TPS3109) [Google Scholar]
- 27.Shao R, Feng Y, Zou S, Li X, Cui P, Billig H. Quantitative analysis of hormones and inflammatory cytokines in Chlamydia trachomatis-infected women with tubal ectopic pregnancy and early intrauterine pregnancy. Data Brief. 2015;6:135–142. doi: 10.1016/j.dib.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma L, Li Z, Xi S, Guo Q, Zhao P, Li W, Ai J, Chen X. Tubal ectopic pregnancy occurrence is associated with high expressions of prokineticin receptors and aberrant secretion of inflammatory cytokines. Am J Transl Res. 2020;12:5741–5751. [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang PR, Cao Z, Qiu ZL, Pan JW, Zhang N, Wu YF. Plasma levels of TNF-α and MMP-9 in patients with silicosis. Eur Rev Med Pharmacol Sci. 2015;19:1716–1720. [PubMed] [Google Scholar]
- 30.Zaka M, Abbasi BH, Durdagi S. Novel tumor necrosis factor-α (TNF-α) inhibitors from small molecule library screening for their therapeutic activity profiles against rheumatoid arthritis using target-driven approaches and binary QSAR models. J Biomol Struct Dyn. 2019;37:2464–2476. doi: 10.1080/07391102.2018.1491423. [DOI] [PubMed] [Google Scholar]
- 31.Cha HH, Hwang JR, Kim HY, Choi SJ, Oh SY, Roh CR. Autophagy induced by tumor necrosis factor α mediates intrinsic apoptosis in trophoblastic cells. Reprod Sci. 2014;21:612–622. doi: 10.1177/1933719113508816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li HH, Xu XH, Tong J, Zhang KY, Zhang C, Chen ZJ. Association of TNF-α genetic polymorphisms with recurrent pregnancy loss risk: A systematic review and meta-analysis. Reprod Biol Endocrinol. 2016;14(6) doi: 10.1186/s12958-016-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano D, Hugol D, Quang NT, Darai E. Serum concentrations of interleukin-2R (IL-2R), IL-6, IL-8, and tumor necrosis factor alpha in patients with ectopic pregnancy. Fertil Steril. 2003;79:975–980. doi: 10.1016/s0015-0282(02)04853-7. [DOI] [PubMed] [Google Scholar]
- 34.Tang SL, Zhao ZW, Liu SM, Wang G, Yu XH, Zou J, Wang SQ, Dai XY, Fu MG, Zheng XL, et al. Pregnancy-associated plasma protein-A accelerates atherosclerosis by regulating reverse cholesterol transport and inflammation. Circ J. 2019;83:515–523. doi: 10.1253/circj.CJ-18-0700. [DOI] [PubMed] [Google Scholar]
- 35.Zhang D, Shi W, Li C, Yuan JJ, Xia W, Xue RH, Sun J, Zhang J. Risk factors for recurrent ectopic pregnancy: A case-control study. BJOG. 2016;123 (Suppl 3):S82–S89. doi: 10.1111/1471-0528.14011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.