Abstract
Nanomedicine has demonstrated substantial potential to improve the quality and efficacy of healthcare systems. Although the promise of nanomedicine to transform conventional medicine is evident, significant numbers of therapeutic nanomedicine products have failed in clinical trials. Most studies in nanomedicine have overlooked several important factors, including the significance of sex differences at various physiological levels. This report attempts to highlight the importance of sex in nanomedicine at cellular and molecular level. A more thorough consideration of sex physiology, among other critical variations (e.g., health status of individuals), would enable researchers to design and develop safer and more-efficient sex-specific diagnostic and therapeutic nanomedicine products.
Subject terms: Drug delivery, Nanomedicine
Given the early failing of therapeutic nanomedicine in the clinic the importance of understanding bio-nano interactions and biosystem parameters has gained much attention. Here, the authors review the work to date that looks at the impact of sex on the applications and outcomes of nanomedicine.
Introduction
According to the British Standards Institute, nanotechnology refers to “intentional design, characterization, production, and applications of materials, structures, devices, and systems by controlling their size and shape in the nanoscale range (1–100 nm)”1. Nanomedicine employs nanotechnology for medical applications including diagnosis and treatment of diseases2. As an example, for drug delivery, nanomedicine aims to design and develop biocompatible nanoparticles (NPs) that can protect payloads from degradation and unwanted interactions with biosystems (e.g., immune cells and biological barriers) and deliver them to the desired biosystems (e.g., cells)3. The purpose of this review is to explore the relevance of sex at cellular and molecular level for the laboratory and clinical research in the general field of nanomedicine.
Although the emergence and development of nanomedicine have demonstrated promising (and sometimes even tremendous) positive results in in vitro environmental and animal studies, therapeutic nanomedicines such as those for cancer faced significant translational challenges. More specifically, a 2019 clinical trial analysis of 75 cancer nanomedicines, that were registered through clinicaltrials.gov database, revealed that cancer nanomedicine products have had a success rate of 14% in phase 3 trials, which largely reflects the efficacy of nanomedicine products4. Remarkably, the trials of some nanomedicines have failed to show any improvement in pharmacokinetics or drug accumulation within tumor tissues, as compared to the parent drugs4.
These disheartening clinical translational results are driven at least in part by neglect and/or a failure to fully understand several crucial/influential factors (e.g., sex) at play in both nano- and bio-systems and in nano-bio interactions3,5,6. Therefore, identifying and gaining a much deeper understanding of these factors is an essential step in improving successful clinical translation of nanomedicine products.
Challenges to the identification of critical factors in safe/effective nanomedicines
Monoclonal antibodies failed their first decade of clinical trials owing to their murine-derived origin; only when humanized/human monoclonals were used did clinical trials succeed. Based on current evidence7,8, nanomedicine may be following a similar trajectory, but perhaps for different reasons. It is essential, therefore, that all factors critical to therapeutic efficacy–including differences in biological cellular responses between males and females—be robustly considered and incorporated into both laboratory and clinical research. This will enable development of more effective drug delivery systems and more reliable diagnostics and therapeutics.
The importance of sex as a biological variable in laboratory and clinical experiments has been recognized by the biomedical research community since the 1990s9. However, most researchers are still using only one sex of biosystems (e.g., cells, tissues, or animals) and applying the results to both males and females. With the emergence of nanomedicine, the importance of sex in biomedical research has become even more obvious. In a recent review article, Liyod-Parry et al.10 provide a comprehensive overview of the role of nanotechnology and nanomedicine in women’s health research. The authors present both the benefits and shortcomings of the application of nanomedicine to both laboratory-based research and clinical trials. Most importantly, in recent years, the importance of sex at the cellular level has also been recognized in biomedical research. Nevertheless, it is still the case that only a small number of published articles report the sex of biosystems used in their study11.
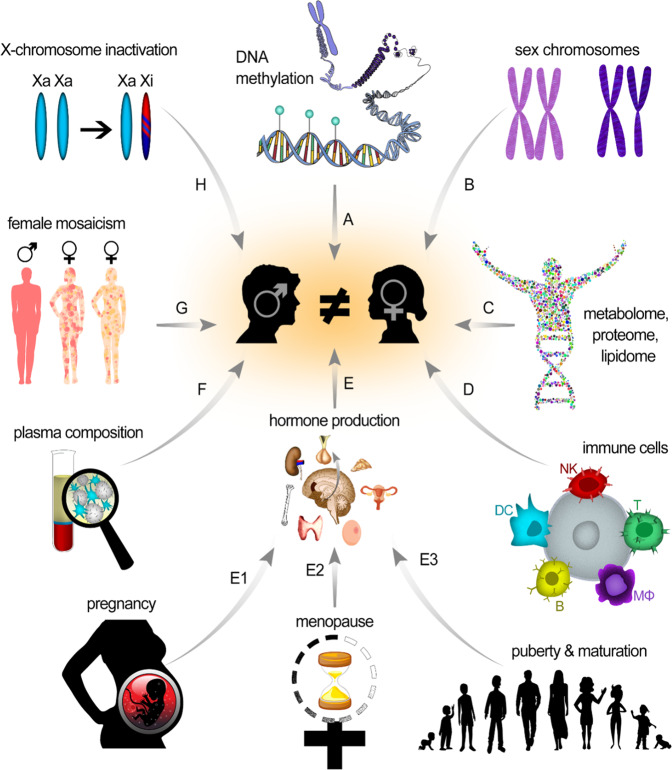
This review focuses on how sex-dependent physiological differences (Fig. 1) can affect the interaction of NPs with biosystems. Throughout this report, the term “sex” refers to the biological trait and not gender, the social identity of individuals. The terms sex and gender might be used exchangeably, but they have different meanings. According to Canadian Institutes of Health Research (CIHR), sex refers to biological attributes of humans and animals, including physical features, chromosomes, gene expression, hormones, and anatomy, while gender refers to socio-cultural factors including socially constructed roles, behaviors, expressions, and identities.
Fig. 1. Sex-dependent physiological differences.
Schematic representation of male/female differences in (A) DNA methylation, (B) sex chromosomes, (C) metabolome, lipidome, and proteome, (D) immune cells, (E) hormone production (during E1) pregnancy, E2 menopause, E3 puberty, (F) plasma composition, (G) mosaicism, and (H) X-chromosome inactivation.
Although only a handful of nanomedicine studies have focused on the sex-dependent effects of NPs, results indicate that the same NPs have different therapeutic and toxic impacts in male and female animals. For example, PEG-coated gold NPs show different toxic effects in male and female mice, producing more severe kidney damage in females, but higher liver toxicity in males12. In another study, amorphous silica NPs triggered more-severe inflammation and lung tissue damage in female rats compared to male rats13.
Relevance of sex differences in cellular NP interactions and nanomedicine
With the emergence of nanomedicine, a wide range of NPs has been developed for diagnostics/therapeutics application14,15. However, despite the extensive deployment of therapeutic nanomedicines in both the laboratory and clinical trials, they have had very limited success in reaching clinical practice. The safe and effective use of therapeutic nanomedicine remains a growing concern among biomedical and clinical researchers7,8,16. Among the many reasons for the frequent failure of clinical trials involving nanomedicine is our lack of a deep understanding of the mechanisms of interaction between NPs and body fluids, extracellular matrix, and cellular components. Another important stumbling block is the fact that most laboratory and clinical studies involving cellular interactions with nanomaterials do not take the significance of sex (or, indeed, many other relevant factors6) into account. This is more obvious in studies involving diseased cells and tissues.
Recent studies provide convincing evidence that sex differences can alter NP efficacy at the cellular level17. For example, the level and pathway of NP uptake and intracellular trafficking in human amniotic mesenchymal stem cells and cancer cells are strongly sex dependent; in addition, the composition of the biomolecular/protein corona (i.e., a layer of various biomolecules that forms on the surface of NPs upon contact with a biological fluid18) is affected by sex-specific paracrine factors17. Furthermore, it has been found that toxic silver NPs significantly alter the bacterial species harbored in male zebrafish, but not in females19. It is clear that challenges to the progress of NP-based nanomedicines will not be overcome until biological variable aspects are taken into consideration, including alteration of the biomolecular/protein corona on the surface of NPs and different structural and molecular responses to functionalized NPs by male and female cells.
It is noteworthy that the extent of sex-related differences in biosystem responses to NPs/drugs depends strongly on the type of disease and tissue involved. Sex difference of the whole organism at the physiological level is one of the most important biological variables determining the incidence, prevalence, and severity of many diseases, including cancer, neurodegenerative, cardiovascular, autoimmune, and psychological diseases20–26. For example, in cancers, sex significantly influences tumor incidence, growth, cellular and molecular phenotypes, and therapeutic efficacy27–29. This is at least partially due to sex-related genetic differences: abnormal reactivation of X-linked genes30, differences in duplication/deletion of segments on X/Y chromosomes28, differences in mutated genes in tumors31, transcription factor-binding patterns29, and sex-specific roles of hormones32. Drugs also have different pharmacodynamic and pharmacokinetic patterns in males and females and, therefore, exert sex-dependent therapeutic and toxic effects33–35. Male and female cells of different origin may respond differently to the same NPs. For example, quantum dots have higher uptake in female than in male human amniotic stem cells; however, the same particles have shown lower uptake in female salivary gland primary cells compared to their male counterparts17.
Biological identity of NPs determined by the biomolecular/protein corona
Once exposed to biological fluids, the surface of NPs becomes rapidly covered with lipids, metabolomes, proteins, and other biomolecules; this biomolecular/protein corona alters the original surface of the NPs and gives them a new biological identity18. Most studies in nanomedicine have not properly reported/considered biological variable factors in the in vitro and in vivo microenvironment that determine the fate, safety, and efficacy of NPs in both animal and human clinical trials6,8. Corrective efforts have mainly involved: (1) identifying important biological variable factors in the NP microenvironment; (2) improving NP toxicity assays; (3) optimizing in vitro protocols mimicking in vivo conditions; and (4) more-accurate computational modeling approaches to identify NP mechanisms of action8,36,37.
Human plasma is the most suitable biological fluid to study biomolecular/protein corona. One of the major sources of misprediction of the biological safety and efficacy of NPs is conflicting biomolecular/protein corona data, originating from the use of various biological fluids, including (1) aqueous non-biological fluids, (2) semi-biological fluids such as cell culture media, and (3) animal serum/plasma. Even when using human plasma, characteristics of the donor (e.g., sex, age, and health status) can significantly affect the composition of the biomolecular/protein corona. Unfortunately, much of the current literature on the corona does not report the details of the plasma used; in fact, in most cases, pooled plasmas from multiple individuals are employed6. Exposure of NPs to proteins in the plasma of healthy individuals vs. patients with cancerous conditions significantly affects the composition of biomolecular/protein coronas, pointing to NPs’ therapeutic and diagnostic properties38–40. It has been shown that changes in plasma composition during the disease development process substantially alter corona composition and thus NP biological function38,41–43. Other recent findings have revealed the role of sex-dependent secretion of paracrine factors in NPs’ interactions with cells17.
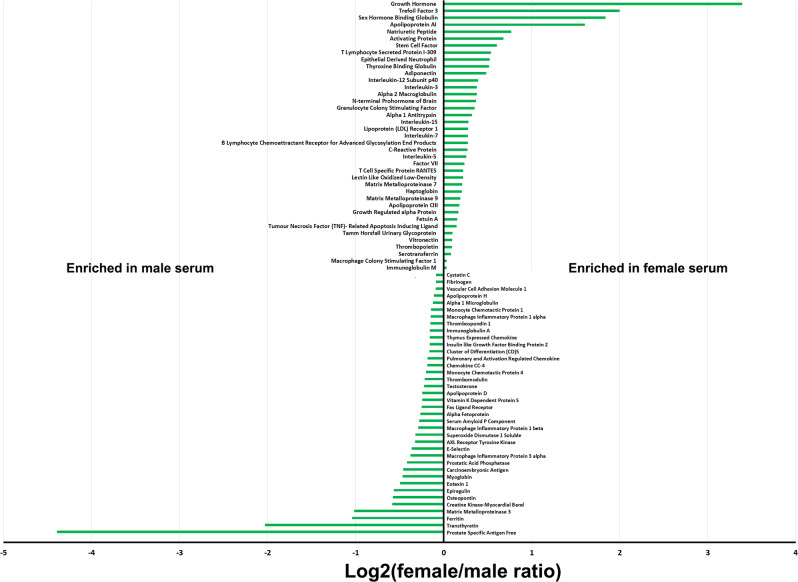
Based on both proteomics and metabolomics data, the composition of female and male plasma differs significantly, with significant variations in the abundance of 231 proteins. Another study analyzed 174 serum biomolecules in 196 male and 196 female human samples, from nine independent cohorts, and probed sex-specific differences44. The outcomes of this meta-analysis have revealed robust and reproducible sex differences in 77 of the biomolecules. Among these, 40 have higher concentrations in females (Fig. 2). Sex-specific variations in biomolecules (e.g., hormones) strongly depend on individual physiological condition (Table 1). Several studies have qualitatively probed the role of physiological conditions in plasma protein variations and revealed individual differences in plasma proteome patterns45–49. For example, serum concentrations of alpha 1-antitrypsin, alpha 2-HS-glycoprotein, beta 2-glycoprotein III, Gc-globulin, alpha 1-lipoprotein, and alpha 2-AP-glycoprotein decrease in females after menopause, while the concentrations of alpha 1-acid glycoprotein, haptoglobin, serum amyloid P-component, Zn-alpha 2-glycoprotein, beta-lipoprotein, and C1-components increase45. As another example, levels of some plasma proteins may change during pregnancy: plasglypican-3 (increases from 10 to 40 weeks), sialic acid-binding immunoglobulin-type lectin-6 (decreases from 10 to 22 weeks and increases from 23 to 40 weeks), placental growth factor (increases from 10 to 31 weeks), prolactin (increases from 10 to 35 weeks), and interleukin-1 receptor 4 (increases from 25 to 40 weeks)47.
Fig. 2. Molecular sex differences in human serum.
Log2-scale of female to male serum concentration ratio of 77 analytes (out of 174 identified serum molecules across nine independent cohorts of individuals consisting 196 males and 196 females) that had significant differences in male and female serum concentrations (data for the graph was extracted from reference44).
Table 1.
Summary of normal hormonal changes in males and females’ serum under different physiological conditions (data extracted from references126–130).
| Hormone | Female | Male |
|---|---|---|
| Hydroxyprogesterone |
Follicular <80 ng/dL Luteal <285 ng/dL Postmenopausal <51 ng/dL |
Adult <220 ng/dL |
| Androstenedione | 30–200 ng/dL | 40–150 ng/dL |
| Beta-human chorionic gonadotropin (beta-hCG) |
Premenopausal nonpregnant: <1.0 U/L Postmenopausal: <7.0 U/L |
<1.4 U/L |
| Calcitonin | ≤5 pg/mL | ≤10 pg/mL |
| Dehydroepiandrosterone sulfate (DHEA-S) | 44–332 μg/dL | 89–457 μg/dL |
| Estradiol |
Follicular: 10–180 pg/mL Mid-cycle peak: 100–300 pg/mL Luteal: 40–200 pg/mL Postmenopausal <10 pg/mL |
20–50 pg/mL |
| Follicle-stimulating hormone |
Follicular/luteal: 2–9 mIU/mL (2–9 U/L) Mid-cycle peak: 4–22 mIU/mL (4–22 U/L) Postmenopausal: >30 mIU/mL (>30 U/L) |
Adult: 1–7 mIU/mL (1–7 U/L) Children, Tanner stages: 1, 2 0.5–8.0 mIU/mL (0.5–8.0 U/L) Children, Tanner stages: 3, 4, 5 1–12 mIU/mL (1–12 U/L) |
| β-Human chorionic gonadotropin (β-hCG) |
Premenopausal nonpregnant: <1.0 U/L Postmenopausal: <7.0 U/L |
<1.4 U/L |
| Luteinizing hormone (LH) |
Follicular/luteal: 1–12 mIU/mL (1–12 U/L) Mid-cycle peak: 9–80 mIU/mL (9–80 U/L) Postmenopausal: >30 mIU/mL (>30 U/L) |
Adult: 2–9 mIU/mL (2–9 U/L) Children, Tanner stages: 1, 2, 3 <9.0 mIU/mL (<9.0 U/L) Children, Tanner stages: 4, 5 1–15 mIU/mL (1–15 U/L) |
| Osteocalcin | 7.2–27.9 ng/mL | 11.3–35.4 ng/mL |
| Progesterone |
Follicular: 0.02–0.9 ng/mL Luteal: 2–30 ng/mL |
Adult: 0.12–0.3 ng/mL |
| Testosterone | 18–54 ng/dL | 291–1100 ng/dL |
It has been increasingly recognized that plasma composition (e.g., due to individual variations and health status)42,43,50 significantly affects NP’s biomolecular/protein corona profiles. Therefore, one can expect that sex-based biomolecular differences in any given plasma sample to be reflected in NP biomolecular/protein corona composition. Although corona profiles have been widely investigated under a variety of conditions6, to the best of our knowledge, few reports have considered the effect of sex on the composition and decoration of biomolecular/protein corona. For example, it has been demonstrated that incubation of identical 70-nm SiO2 NPs with plasmas of male and female zebrafish leads to the formation of coronas with different compositions;51 the vitellogenin family (egg yolk precursor proteins with high plasma concentrations in female fish52,53) was found to represent one major difference in male and female corona compositions51. Blood cells therefore interact differently with NPs exposed to male and female plasma. The NPs with coronas containing proteins present in female plasma are preferentially recognized by leukocytes compared to their male counterparts. This finding suggests that blood circulation time and pharmacokinetics of identical NPs, together with the host immune system response to them, may differ significantly between males and females.
Sex-dependent toxicity and therapeutic efficacy of NPs
Sex-specific molecular and cellular structures
One of the main challenges to the vigorous study of NP interactions with biosystems is the selection of appropriate cells that originate at a very early stage of development, prior to the initiation of hormonal changes and genetically and structurally driven sexual dimorphisms. The use of embryonic cells is one strategy to address this issue. For example, male and female mouse embryonic stem cells (mESCs) have been used to determine the sex-specific effects of silver NPs on stem cell differentiation54. Low concentrations (0.2, 0.5, and 1.0 μg mL−1) of silver NPs delay the differentiation of female mESCs by interfering only with the X chromosome inactivation process. The same NPs, however, show no interference with the self-renewal process and exert no significant cytotoxic effect. It is well understood that even slight interference in X chromosome inactivation considerably impairs programmed ESC differentiation by regulating the expression of relevant genes55. The random inactivation of one X chromosome of the female cell is known to equilibrate gene content between the sexes56,57. Xist and Tsix are noncoding RNAs playing critical roles in silencing and activating the X chromosome, respectively. A low concentration of silver NPs interferes with the expression of Xist and Tsix and disrupts the optimal Xist/Tsix ratio for X chromosome inactivation. Silver NPs also prevent the expression of genes responsible for triggering programmed ESC differentiation by enhancing the trimethylation of histone (histone 3 lysine 27 trimethylation, H3K27me3). Therefore, even a low concentration of silver NPs can cause a series of transcriptome and epigenome changes interrupting X chromosome inactivation and subsequent ESC differentiation in females. The most striking observation has been that such epigenome and transcriptome changes are absent in male mESCs treated with low concentrations of silver NPs, revealing programmed differentiation even after long exposure to silver NPs. As X chromosome inactivation is a female cell-specific phenomenon, agents interfering with this process impair the differentiation of female cells only54.
As another example, human amniotic stem cells (hAMSCs), one of the earliest sources of somatic stem cells, have been employed to probe the effect of cell sex on NP uptake17. Exposure to quantum dots (QDs) reflects significant sex-dependent differences in cellular uptake efficacy in human amniotic stem cells (hAMSCs): female cells take up more QDs than male cells. Aside from sex-dependent biomolecular/protein coronas, sex-based differences in the structure and function of cells also play key roles in their response to NPs. It was shown that sex-dependent uptake of QDs is caused by differences in arrangement, shape, and distribution of actin filaments (cytoskeleton), which regulate the endocytosis and cellular trafficking of QDs. Remarkably, male and female cells show different capabilities in reprogramming hAMSCs into pluripotent stem cells when treated with nanosized Sendai virus commonly used for the preparation of pluripotent stem cells. The Sendai virus shows more effective transfection—and hence, better reprogramming efficacy—into female hAMSCs compared to male hAMSCs17.
Sex-specific biomolecules
For accurate evaluation of the interactions of NPs with non-embryonic cells, the possible roles of sex-specific hormonal effects on both cell behavior/characteristics and biomolecular/protein corona of NPs should be considered in detail. Hormones are sex-specific chemical messengers regulating the duration and level of crucial processes (e.g., growth, development, metabolism, and reproduction) in tissues and organs58,59. Therefore, small variations in hormone production/balance may produce significant changes in susceptibility/resistance to diseases and therapeutic drugs/NPs. For example, it is well understood that cell sex characteristics and sex hormones have significant impacts on both normal lung physiology and lung diseases60. New findings from the NELSON Trial provide evidence of sex-specific differences in lung cancer screening and survival61. Notably, the results of related research suggest that lung cancer progression may be more rapid and lethal in females. These important factors need to be considered in interpretation of the toxicity and therapeutic outcomes of NPs being used for tissues (e.g., lung) that are highly affected by sex-specific phenomena. In addition, sex-specific disease-dependent biomolecular changes in plasma can significantly affect the biomolecular/protein corona profiles of NPs and thus their interactions with biosystems. For example, different concentrations of vitellogenin in male and female zebrafish plasma significantly affected the composition of the corona formed on the surface of SiO2 NPs51. In addition, since the protein metabolome profiles of males and females reflect considerable differences in amino acids, fatty acids, and lipids62, their blood plasmas also have different metabolome and lipidome profiles. Depending on the sex and age of a person, different metabolic pathways are activated and different plasma metabolite association networks form63. As the metabolome can affect protein-NP interactions64, one could expect metabolomics variations to affect the biomolecular/protein corona composition of NPs in a sex-specific manner.
Sex-specific immunity
Immune system response to NPs is an important factor in their toxicity and therapeutic efficacy; and sex-related physiologic differences significantly affect host immune system responses to foreign materials including NP/biomolecular therapeutics. In rodents, for example, the phagocytic activity of macrophages is stronger in females than in males65. Indeed, female mice and rats have more CD45+ leukocytes and macrophages in both naive peritoneal and pleural cavities66. In the respiratory tract, sex hormones including estrogens, androgens, and progesterone regulate the number and function of innate immune cells in a sex-specific manner67. Sex can also affect patient responses to cancer immunotherapy23. Sex-specific differences have also been observed in the functions of other immune cells such as T-lymphocytes, B-lymphocytes, astrocytes (in the brain), and natural killer cells20,68. Therefore, males and females show different innate/adaptive immune responses to antigens/infections. The immune response to self-antigens, leading to autoimmune diseases, is more prevalent in females; ~80% of patients diagnosed with autoimmune disease are females69. As stated above, it is legitimate to hypothesize that immune system responses to identical NPs are sex-dependent. For example, female mice have stronger immune responses (e.g., through significant pathological changes in spleen and thymus index) to PEG-coated gold NPs compared to male mice12. In addition, male mice treated with gold NPs show more significant infection and inflammation compared to female mice12.
The immune system plays a critical role in many diseases, including cancers, in a sex-specific manner. Due to their genetic and epigenetic mosaicism due to random inactivation of one of the X chromosomes in XX female cells, females are more resistant and show stronger immune response to cancer compared to males70. In addition, in some cancers such as glioblastoma, current treatments show better outcomes in females than in male patients71. For example, the effectiveness of immune checkpoint inhibitors (e.g., antibodies against CTLA-4, PD-1, and PDLA-1), among the most efficient therapeutics, is sex-dependent72–74. One of the recent promising applications of NPs in cancer is immunotherapy75; here again, sex may have significant effects on the therapeutic efficacy of nanomedicine products, in an organ-dependent manner, for several reasons, including sex-related differences in tumor microenvironments76; sex-associated molecular differences in response to cancer immunotherapy;77 and the role of NPs in the efficacy of the immune system in tumor microenvironments78. For example, it was shown that ferumoxytol, a Food and Drug Administration (FDA)-approved iron supplement, could increase the number of pro-inflammatory M1 tumor-associated macrophages in female mice, demonstrating intrinsic inhibitory effects on the growth of early mammary cancers together with lung cancer metastases in liver and lungs78. Although the study was limited to female mice, one may expect to observe different therapeutic outcomes with ferumoxytol in male mice, as the production of cytokines and chemokines by macrophages differs between males and females79.
Sex-specific disease environments
NPs not only exert different toxic effects in males and females, but also show sex-dependent variations in therapeutic efficacy. The fetal hypoxia that often occurs during pregnancy may induce oxidative/nitrosative stress, which increases the risk of catastrophic diseases in mature offspring80. The placentas of male and female fetuses use different mechanisms to suppress oxidative stress and show different responses to identical stimuli. NPs encapsulating MtiQ, an antioxidant suppressing mitochondrial oxidative stress, have been used to treat fetal hypoxia and prevent subsequent placental dysfunction in animal models, showing sex-specific therapeutic effects81 in favor of female fetuses. Therefore, sex differences should be considered throughout the development of nano-based drugs for treatment of placenta- or embryo-related disorders.
Since sex differences affect the prevalence and progression of many diseases82–84, differences in therapeutic efficacies of drug/NPs between males and females should be considered in an organ-specific manner. This is because male and female cells have different sex chromosomes, display dissimilar gene expression patterns, and activate distinct sex-specific signaling pathways in response to the same treatment11,85,86. For example, the body’s natural healing process in response to cardiac diseases (e.g., cardiomyopathy) is sex specific87–89. Although nanotechnologies have been widely used to address cardiovascular diseases90,91, the role of sex in therapeutic efficacy has not been critically considered. It is noteworthy that even circulating biomarkers and/or biomolecules associated with cardiovascular diseases are sex dependent92, which may affect the biomolecular/protein corona of therapeutic NPs. Such variation in circulating biomolecules may also be related to the gut microbiota93, whose composition depends strongly on host sex94. Although NP-based oral treatments may affect the composition of the gut microbiome in a sex-dependent manner (e.g., the significant effects of silver NPs on the gut microbiome of male but not female zebrafish)19, we do not consider them in this review.
Some injuries may also have sex-specific physiological effects that may significantly affect NP delivery to the injured tissue. For example, it was shown that within the first couple of hours following traumatic brain injury (TBI), the blood–brain barrier (BBB) often undergoes less damage in females than in males95,96. This will significantly affect the delivery of identical NPs to the brain tissue in a sex-dependent manner. It has been demonstrated in transgenic mice models of TBI that PEGylated polystyrene NPs were more effectively delivered to female than to male brain tissue96,97. Although not evaluated in previous studies, the therapeutic efficacy of the NPs that accumulate in brain tissue is assumed to be sex-specific, at least in part due to the sex-specific response by brain immune cells. Recent reports have revealed significant differences in function, morphology, abundance, and gene expression profiles between male and female microglia, which are the main immune cells involved in central nervous system maturation, signal transduction, and clearance of protein/cell aggregates/debris in the brain98–101. As sex differences in microglia are involved in the cause of differing incidence and prevalence of neurodegenerative diseases such as Alzheimer’s, Parkinson’s disease, Amyotrophic lateral sclerosis, and frontotemporal dementia between male and female patients, the successful use of therapeutic nanomedicines for these diseases must also be sex-specific102–105. Microglia are the resident immune cells in the brain and maintain their homeostasis and functionality. The role of sex in microglial cell functions and its effect on physiological and pathological conditions in the brain has been discussed in a recent review article by Yanguas-Casás106.
Challenges and recommendations in considering sex in nanomedicine studies
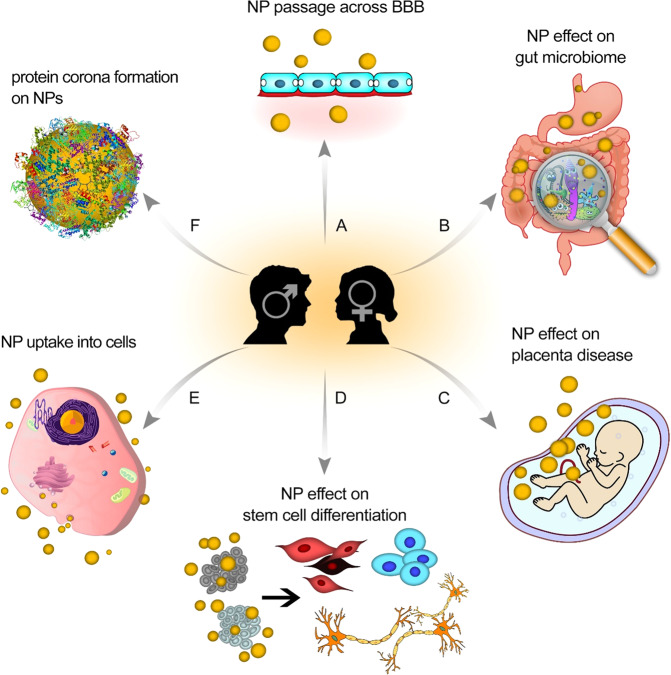
Taking sex into account will have a significant impact on the success of both laboratory and clinical research in nanomedicine, encompassing increased safety and efficacy, identifying sex differences in cellular characteristics, plasma composition, and secreted factors that affect NPs’ biomolecular/protein coronas and functions (e.g., in terms of their safety and therapeutic efficacies; Fig. 3). It is clear that many researchers in the field of (nano)medicine are aware of the importance of considering sex in their studies; there are, however, many challenges to overcome before the importance of sex can be systematically considered in studies and/or reports. In this section, we outline the central challenges and propose possible strategies to address them.
Fig. 3. Schematic representation of sex-dependent therapeutic/toxic effects of nanoparticles.
A Crossing the blood–brain barrier, (B) gut microbiome composition, (C) placenta-related diseases, (D) stem cell differentiation, (E) uptake of NPs into cells, (F) formation of biomolecular/protein corona.
Physiological complexity of considering sex
Description of the challenge
Compared to male biosystems, female biosystems (e.g., plasma composition) have more physiological complexity, due to conditions such as menstruation, pregnancy, lactation stage, and menopause, among others. These complexities may make the outcomes of studies involving females less reliable and/or reproducible than the findings of studies on males. Age also needs to be properly considered in study designs, as many sex-specific hormones are strongly dependent on age and individual health status. For example, during puberty, hormones differently induce the development of sex organs in males and females107. Menopause is characterized by reduced concentrations of progesterone and estrogens and increased levels of follicle stimulating hormone (FSH)108; premenopausal and postmenopausal females have different hormone profiles108; and blood concentrations of progesterone, estriol, and prolactin change considerably during pregnancy108. All these variables may have significant effects on the biomolecular/protein corona profile of NPs and their interactions with biological systems.
Mitigation strategies
To obtain useful biomolecular/protein corona data, researchers need to have full information on the age, sex, health, and physiological condition of plasma donors. Researchers should avoid using pooled plasma and/or plasma with no comprehensive information on the donor (except the personal information), as the outcomes may not be conclusive. When seeking to determine the role of sex in the biomolecular/protein corona profile of NPs, the characteristics/specification of plasma donors (e.g., age, health, and physiological conditions, and ethnicity) need to be the same for both sexes. In addition, other influential factors (e.g., blood collection and reservation tubes, duration, and temperature of plasma storage) need to be carefully considered in the experimental setup. The best approach to considering the effect of female-specific physiological conditions (e.g., menstruation, pregnancy, lactation stage, and menopause) on the biological efficacy of NPs is to use plasma samples from the same individuals in a prospective manner.
Biosystems
Description of the challenge
Results published from both in vitro and in vivo studies should include full details on the sex of the cells and/or animals. Unfortunately, in most studies, the sex of cell lines used in in vivo and in vitro (e.g., for tumor implantation) are not appropriately considered and reported in the current literature6. Even in the limited number of publications that do report cell sex, other crucial information (e.g., number of the passage)36 are often missing. The number of cell passages is critically important, as some male cell lines widely used in in vitro studies gradually lose their Y chromosome with increasing numbers of passages109.
Mitigation strategies
Researchers need to have full information about the cells they intend to use for in vitro and/or in vivo studies, including their sex, origin, passage numbers, and storage conditions. In addition, to completely investigate the effect of sex on cellular responses to NPs. The selected male and female cells should (i) have the same origin (e.g., organs and species), (ii) have been extracted from similar donors (e.g., in terms of age, health condition, and ethnicity), and (iii) have had minimal exposure to sex-specific hormones. For in vitro evaluations of the effects of sex on NPs, researchers must ensure that their culture media are free from sex-specific hormones that may differentially affect the behavior of male and female cells. Another major problem is the limited number of comparable male and female cells offered by vendors/distributors of cells [e.g., The Global Bioresource Center (ATCC)]. Therefore, efforts by bioresource companies, as important stakeholders, should be made to provide resources for studying sex as an important biological variation. In the absence of such resources, researchers may have to access suitable cells through their clinical collaborators.
Purity of biomolecular/protein corona
Description of the challenge
As discussed above, achieving rigorous and concise information on the composition of biomolecular/protein corona is essential to predict NPs’ interactions with biosystems and their biological impact. Recent findings obtained by combining cryo-electron microscopy, cryo-electron tomography, and image simulation have revealed that the biomolecular/protein corona formed on the surface of NPs contain some impurities in the form of non-specific clusters of biomolecules. This will influence the accuracy of the results of proteomics analysis of the biomolecular/protein corona110. Although the mechanism formation of these clusters and their function is unknown, the composition and concentration of these impurities associated with the corona formed by exposure of NPs to male and female plasma could be different.
Mitigation strategies
More in-depth attention needs to be paid for investigation of the purity of protein corona prior proteomics analysis. Another crucial aspect is experimental obstacles affecting the characterization of NPs and their corona profiles. Using diluted NPs to study biomolecular/protein corona is one practical approach that can minimize such impurity issues110,111.
Access to biological resources
Description of the challenge
Access to materials and data are easily available through extensive studies of differences between the sexes on the reproductive systems. Lack of availability of resources on sex differences in nonreproductive biology, where differences are much less recognized, creates critical challenges in the field. Although most of the differences in sex in nonreproductive areas of biology are difficult to detect, some of these differences can have significant implications for clinical and laboratory research within the discipline of nanomedicine. An additional problem is the fact that many researchers may not have access to comparable male and female biosystems (e.g., cells, plasmas, animals) for a variety of reasons, including lack of a clinical collaborator/center.
Mitigation strategies
Researchers should thoroughly report all available information, including sex, on the biosystems they study and be specific about their claims/conclusions. For example, if studies are performed on male plasma and/or cells, that should be clearly conveyed in an article’s conclusion to avoid apparently conflicting results arising from sex-specificity; that is, the results might not be reproducible if female plasma and/or cells are used by other researchers.
Conclusions and future perspectives
Beyond conventional medicine and commercially available parent drugs, therapeutic and diagnostic applications of nanomedicine have yet to be developed to the point of clinical use against devastating diseases such as cancer, heart disease, and neurological diseases. Among the reasons for slow progress in many areas of therapeutic nanomedicine are the lack of comprehensive, basic scientific research and an insufficient understanding of the mechanisms involved in cellular interactions with nanomaterials and functionalized NPs together with other complex biological variables, including sex, age, race, health status, and comorbidities6.
To accelerate successful clinical translation of diagnostic and therapeutic nanomedicine, future studies should consider the importance of overlooked factors described in this review and report critical information including the alteration of physicochemical and biological properties of NPs upon exposure to body fluid and most importantly, the significance of biological variables including sex. More specifically, to robustly consider and understand the role sex plays in the safety and therapeutic efficacy of nanomedicines. There is a need for advancement of our understanding of (i) the effects of ethnicity, disease stage, age, sex, and physiological conditions on the biomolecular/protein corona profiles of NPs, (ii) the interaction of NPs with sex-specific elements of the immune system (e.g., macrophages, T-cells, B-cells and natural killer cells), and (iii) the interactions of NPs with sex-specific disease environments (e.g., for cancer: tumor microenvironment, tissue, cellular components, and biochemical environment). As different laboratories may have significant variations in the characteristics readouts of identical NPs112, interlaboratory procedures and instrumentations should be standardized for assessment of the role of sex on diagnostic and therapeutic nanomedicine.
The importance of the topics outlined in this article is evident by the application of nanomedicine-based approach to develop and produce an effective vaccine against COVID-19. According to the initial reports113,114, the NP-based vaccines developed by Pfizer/BioNTech and Moderna performed slightly better in males [95.4% (Moderna) and 96.4% (Pfizer/BioNTech)] than in females [93.1% (Moderna) and 93.7% (Pfizer/BioNTech)]113,114. While monitoring of the sex-specific efficacy outcomes of these vaccines should be continuously monitored and reported, more fundamental studies are required to be conducted on the sex-specific efficacy of the NP-based vaccines at cellular and molecular level. The outcomes of such efforts will help the scientific community to better define potential sex-dependent therapeutic/toxic effect of COVID-19 NP-based vaccines which in turn pave a way for development of sex-specific vaccine design, development, and administration.
The other important factor that should be considered in the sex-specific meta-analysis of nanomedicine is to minimize publication bias (i.e., “the null results of studies may face a higher barrier to publication than those that yield statistically significant differences.”115)1,19. When only statistically significant results are published, it is obvious that the outcomes misrepresent the real findings116. According to Joober and co-workers117, “withholding negative results from publication—publication bias—could have major consequences for the health of millions”. The negative impact of publication bias has been studied and validated in most of the scientific fields including chemistry, medicine, and biomedical sciences118–123. Due to the multidisciplinary nature of nanomedicine, it is obvious that nanomedicine literature has the same issue which has been poorly considered so far124; despite extensive number of publication since its emergence, addressing the literature bias in nanomedicine has been ignord124.
Overall, this review suggests that the sex of biosystems (e.g., cells and plasmas) should be considered, as an essential parameter, in nanomedicine studies. Such consideration will be critical in addressing the reasons behind the alarming signals of failure in therapeutic cancer nanomedicine7.
A collective national and international effort among all involved stakeholders (e.g., basic scientists, clinician, pharmaceutical, and grant agencies) is required to introduce policies, guidelines and regulation to promote inclusion of sex-specific factors in nanomedicine research. A good example is the initiation of the Committee on Understanding the Biology of Sex and Gender Differences that was constructed by the National Academy of Science to address biology at the cellular, developmental, organ, organismal, and behavioral levels with emphasis on Sex and Gender Differences. The results of this comprehensive study have been published in a book by T.M. Wizemann and M-L. Pardue125. Unfortunately, most of the recommendations outlined in this book have been ignored so far. Such a framework of integrated responding of all involved stakeholders may increase responsibility and response-ability in understanding and solving problems in timely and efficient manner126. Considering the current challenges facing nanomedicine, it would be helpful to establish a similar undertaking to overcome some of the shortfalls in nanomedicine. Inclusion of sex-specific biological factors at cellular and molecular is essential for advancements of nanobiotechnology and nanomedicine for both sexes. Undoubtedly, nanomedicine holds the promise to improve the health care system in near future; therefore, it is important for the scientific community to pay more attention to the importance of sex differences in their studies. Last, but not least, if we are to move closer to the lofty goal of personalized nanomedicine, the role of gender must also become a central consideration in future studies.
Author contributions
M.J.H., H.A., V.S., H.V., S.S., and M.M. wrote the manuscript. M.J.H. and H.A. are co-first authors.
Competing interests
Morteza Mahmoudi discloses that (i) he is a co-founder and director of the Academic Parity Movement (www.paritymovement.org), a non-profit organization dedicated to addressing academic discrimination, violence, and incivility, and (ii) he receives royalties/honoraria for his published books, plenary lectures, and licensed patents.
Footnotes
Peer review information Nature Communications thanks Eun-Jung Park, Yuya Hayashi, and the other anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sara Sheibani, Email: sara.sheibani@mcgill.ca.
Morteza Mahmoudi, Email: mahmou22@msu.edu.
References
- 1.Specification PA. Terminology for nanomaterials. British Standards Institute, London, (2007).
- 2.Kim BYS, Rutka JT, Chan WCW. Nanomedicine. N. Eng. J. Med. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- 3.Nanomedicine and the COVID-19 vaccines. Nat. Nanotechnol. 15, 963-963 (2020). [DOI] [PMC free article] [PubMed]
- 4.He H, Liu L, Morin EE, Liu M, Schwendeman A. Survey of clinical translation of cancer nanomedicines - lessons learned from successes and failures. Acc. Chem. Res. 2019;52:2673–2683. doi: 10.1021/acs.accounts.9b00233. [DOI] [PubMed] [Google Scholar]
- 5.Dawson K. A., Yan Y. Current understanding of biological identity at the nanoscale and future prospects. Nat. Nanotechnol. 16, 229–242 (2021). [DOI] [PubMed]
- 6.Mahmoudi M. Debugging nano–bio interfaces: systematic strategies to accelerate clinical translation of nanotechnologies. Trends Biotechnol. 2018;36:755–769. doi: 10.1016/j.tibtech.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 7.Park K. The beginning of the end of the nanomedicine hype. J. Control. Release. 2019;305:221–222. doi: 10.1016/j.jconrel.2019.05.044. [DOI] [PubMed] [Google Scholar]
- 8.Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016;1:16014. doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- 9.Lee SK. Sex as an important biological variable in biomedical research. BMB Rep. 2018;51:167. doi: 10.5483/BMBRep.2018.51.4.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lloyd-Parry O, Downing C, Aleisaei E, Jones C, Coward K. Nanomedicine applications in women’s health: state of the art. Int. J. Nanomed. 2018;13:1963. doi: 10.2147/IJN.S97572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah K, McCormack CE, Bradbury NA. Do you know the sex of your cells? Am. J. Physiol. Cell Physiol. 2014;306:C3–C18. doi: 10.1152/ajpcell.00281.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J, et al. Sex differences in the toxicity of polyethylene glycol-coated gold nanoparticles in mice. Int. J. Nanomed. 2013;8:2409. doi: 10.2147/IJN.S46376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han H-Y, et al. Amorphous silica nanoparticle-induced pulmonary inflammatory response depends on particle size and is sex-specific in rats. Toxicol. Appl. Pharmacol. 2020;390:114890. doi: 10.1016/j.taap.2020.114890. [DOI] [PubMed] [Google Scholar]
- 14.Freitas RA., Jr. What is nanomedicine? Nanomed.: NNBM. 2005;1:2. doi: 10.1016/j.nano.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Wagner V, Dullaart A, Bock A-K, Zweck A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006;24:1211–1217. doi: 10.1038/nbt1006-1211. [DOI] [PubMed] [Google Scholar]
- 16.Saifi MA, Khan W, Godugu C. Cytotoxicity of nanomaterials: using nanotoxicology to address the safety concerns of nanoparticles. Pharm. Nanotechnol. 2018;6:3–16. doi: 10.2174/2211738505666171023152928. [DOI] [PubMed] [Google Scholar]
- 17.Serpooshan V, et al. Effect of cell sex on uptake of nanoparticles: the overlooked factor at the nanobio interface. ACS Nano. 2018;12:2253–2266. doi: 10.1021/acsnano.7b06212. [DOI] [PubMed] [Google Scholar]
- 18.Monopoli MP, Åberg C, Salvati A, Dawson KA. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012;7:779–786. doi: 10.1038/nnano.2012.207. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y, et al. Sex dependent effects of silver nanoparticles on the zebrafish gut microbiota. Environ. Sci. Nano. 2018;5:740–751. doi: 10.1039/C7EN00740J. [DOI] [Google Scholar]
- 20.Ober C, Loisel DA, Gilad Y. Sex-specific genetic architecture of human disease. Nat. Rev. Gen. 2008;9:911–922. doi: 10.1038/nrg2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jack CR, Jr., et al. Age-specific and sex-specific prevalence of cerebral β-amyloidosis, tauopathy, and neurodegeneration in cognitively unimpaired individuals aged 50–95 years: a cross-sectional study. Lancet Neurol. 2017;16:435–444. doi: 10.1016/S1474-4422(17)30077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orton S-M, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5:932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 23.Conforti F, et al. Cancer immunotherapy efficacy and patients’ sex: a systematic review and meta-analysis. Lancet Oncol. 2018;19:737–746. doi: 10.1016/S1470-2045(18)30261-4. [DOI] [PubMed] [Google Scholar]
- 24.Klein SL, Flanagan KL. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 25.Arnold AP, Cassis LA, Eghbali M, Reue K, Sandberg K. Sex hormones and sex chromosomes cause sex differences in the development of cardiovascular diseases. Arterioscler. Thromb. Vasc. Biol. 2017;37:746–756. doi: 10.1161/ATVBAHA.116.307301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norheim F, et al. Gene-by-Sex interactions in mitochondrial functions and cardio-metabolic traits. Cell Metabol. 2019;29:932–949. doi: 10.1016/j.cmet.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnold AP, Disteche CM. Sexual inequality in the cancer cell. Cancer Res. 2018;78:5504–5505. doi: 10.1158/0008-5472.CAN-18-2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li CH, Haider S, Shiah Y-J, Thai K, Boutros PC. Sex differences in cancer driver genes and biomarkers. Cancer Res. 2018;78:5527–5537. doi: 10.1158/0008-5472.CAN-18-0362. [DOI] [PubMed] [Google Scholar]
- 29.Lopes-Ramos CM, et al. Gene regulatory network analysis identifies sex-linked differences in colon cancer drug metabolism. Cancer Res. 2018;78:5538–5547. doi: 10.1158/0008-5472.CAN-18-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaligné R, Heard E. X-chromosome inactivation in development and cancer. FEBS Lett. 2014;588:2514–2522. doi: 10.1016/j.febslet.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 31.Dunford A, et al. Tumor-suppressor genes that escape from X-inactivation contribute to cancer sex bias. Nat. Gen. 2017;49:10. doi: 10.1038/ng.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laughlin G, Barrett-Connor E, May S. Sex-specific determinants of serum adiponectin in older adults: the role of endogenous sex hormones. Int. J. Obes. 2007;31:457–465. doi: 10.1038/sj.ijo.0803427. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu. Rev. Pharmacol. Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz JB. The influence of sex on pharmacokinetics. Clin. Pharm. 2003;42:107–121. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- 35.Ruddy RM, Adams KV, Morshead CM. Age-and sex-dependent effects of metformin on neural precursor cells and cognitive recovery in a model of neonatal stroke. Sci. Adv. 2019;5:eaax1912. doi: 10.1126/sciadv.aax1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faria M, et al. Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 2018;13:777–785. doi: 10.1038/s41565-018-0246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaddi CD, Phan JH, Wang MD. Computational nanomedicine: modeling of nanoparticle-mediated hyperthermal cancer therapy. Nanomedicine. 2013;8:1323–1333. doi: 10.2217/nnm.13.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajipour MJ, et al. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide. Nanoscale. 2015;7:8978–8994. doi: 10.1039/C5NR00520E. [DOI] [PubMed] [Google Scholar]
- 39.Schottler S, Klein K, Landfester K, Mailander V. Protein source and choice of anticoagulant decisively affect nanoparticle protein corona and cellular uptake. Nanoscale. 2016;8:5526–5536. doi: 10.1039/C5NR08196C. [DOI] [PubMed] [Google Scholar]
- 40.Bitounis D, et al. Detection and analysis of nanoparticles in patients: a critical review of the status quo of clinical nanotoxicology. Biomaterials. 2016;76:302–312. doi: 10.1016/j.biomaterials.2015.10.061. [DOI] [PubMed] [Google Scholar]
- 41.Strojan K, et al. Dispersion of nanoparticles in different media importantly determines the composition of their protein corona. PloS One. 2017;12:e0169552. doi: 10.1371/journal.pone.0169552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajipour MJ, Laurent S, Aghaie A, Rezaee F, Mahmoudi M. Personalized protein coronas: a “key” factor at the nanobiointerface. Biomater. Sci. 2014;2:1210–1221. doi: 10.1039/C4BM00131A. [DOI] [PubMed] [Google Scholar]
- 43.Caracciolo G, et al. Disease-specific protein corona sensor arrays may have disease detection capacity. Nanoscale Horiz. 2019;4:1063–1076. doi: 10.1039/C9NH00097F. [DOI] [Google Scholar]
- 44.Ramsey JM, et al. Molecular sex differences in human serum. PloS One. 2012;7:e51504. doi: 10.1371/journal.pone.0051504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hashimoto S, Miwa M, Akasofu K, Nishida E. Changes in 40 serum proteins of post-menopausal women. Maturitas. 1991;13:23–33. doi: 10.1016/0378-5122(91)90282-U. [DOI] [PubMed] [Google Scholar]
- 46.Bellei E, et al. Proteomic serum profile in menstrual-related and post menopause migraine. J. Pharm. Biomed. Anal. 2020;184:113165. doi: 10.1016/j.jpba.2020.113165. [DOI] [PubMed] [Google Scholar]
- 47.Romero R, et al. The maternal plasma proteome changes as a function of gestational age in normal pregnancy: a longitudinal study. Am. J. Bbst. Gynecol. 2017;217:67. e61–67. e21. doi: 10.1016/j.ajog.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillips RJ, Heesom KJ, Trinder J, Bernal AL. Human maternal plasma proteomic changes with parturition. EuPA Open Proteom. 2014;5:10–20. doi: 10.1016/j.euprot.2014.09.001. [DOI] [Google Scholar]
- 49.Scholl P, et al. Maternal serum proteome changes between the first and third trimester of pregnancy in rural southern Nepal. Placenta. 2012;33:424–432. doi: 10.1016/j.placenta.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hadjidemetriou M, Al-Ahmady Z, Buggio M, Swift J, Kostarelos K. A novel scavenging tool for cancer biomarker discovery based on the blood-circulating nanoparticle protein corona. Biomaterials. 2019;188:118–129. doi: 10.1016/j.biomaterials.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 51.Hayashi Y, et al. Female versus male biological identities of nanoparticles determine the interaction with immune cells in fish. Environ. Sci. Nano. 2017;4:895–906. doi: 10.1039/C7EN00071E. [DOI] [Google Scholar]
- 52.Babaei F, et al. Novel blood collection method allows plasma proteome analysis from single zebrafish. J. Proteome Res. 2013;12:1580–1590. doi: 10.1021/pr3009226. [DOI] [PubMed] [Google Scholar]
- 53.Li C, Tan XF, Lim TK, Lin Q, Gong Z. Comprehensive and quantitative proteomic analyses of zebrafish plasma reveals conserved protein profiles between genders and between zebrafish and human. Sci. Rep. 2016;6:24329. doi: 10.1038/srep24329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, et al. Silver nanoparticles compromise female embryonic stem cell differentiation through disturbing X chromosome inactivation. ACS Nano. 2019;13:2050–2061. doi: 10.1021/acsnano.8b08604. [DOI] [PubMed] [Google Scholar]
- 55.Schulz EG. X-chromosome dosage as a modulator of pluripotency, signalling and differentiation? Philost. T. R. Soc. B. 2017;372:20160366. doi: 10.1098/rstb.2016.0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borensztein M, et al. Xist-dependent imprinted X inactivation and the early developmental consequences of its failure. Nat. Struct. Mol. Biol. 2017;24:226. doi: 10.1038/nsmb.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yue M, et al. Xist RNA repeat E is essential for ASH2L recruitment to the inactive X and regulates histone modifications and escape gene expression. PLoS Gen. 2017;13:e1006890. doi: 10.1371/journal.pgen.1006890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gubbels Bupp MR, Potluri T, Fink AL, Klein SL. The confluence of sex hormones and aging on immunity. Front. Immunol. 2018;9:1269. doi: 10.3389/fimmu.2018.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taneja V. Sex hormones determine immune response. Front. Immunol. 2018;9:1931. doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carey MA, et al. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;293:L272–L278. doi: 10.1152/ajplung.00174.2007. [DOI] [PubMed] [Google Scholar]
- 61.Walter J, et al. P2. 11-02 direct comparison of new solid nodules detected in women and men during incidence screening rounds of the NELSON trial. J. Thorac. Oncol. 2018;13:S779. doi: 10.1016/j.jtho.2018.08.1348. [DOI] [Google Scholar]
- 62.Krumsiek J, et al. Gender-specific pathway differences in the human serum metabolome. Metabolomics. 2015;11:1815–1833. doi: 10.1007/s11306-015-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vignoli A, Tenori L, Luchinat C, Saccenti E. Age and sex effects on plasma metabolite association networks in healthy subjects. J. Proteome Res. 2018;17:97–107. doi: 10.1021/acs.jproteome.7b00404. [DOI] [PubMed] [Google Scholar]
- 64.Tavakol M, et al. Disease-related metabolites affect protein–nanoparticle interactions. Nanoscale. 2018;10:7108–7115. doi: 10.1039/C7NR09502C. [DOI] [PubMed] [Google Scholar]
- 65.Spitzer JA. Gender differences in some host defense mechanisms. Lupus. 1999;8:380–383. doi: 10.1177/096120339900800510. [DOI] [PubMed] [Google Scholar]
- 66.Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kadel S, Kovats S. Sex hormones regulate innate immune cells and promote sex differences in respiratory virus infection. Front. Immunol. 2018;9:1653. doi: 10.3389/fimmu.2018.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fish EN. The X-files in immunity: sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nat. Rev. Immunol. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- 70.Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- 71.Yang W, et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019;11:eaao5253. doi: 10.1126/scitranslmed.aao5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Botticelli A, et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget. 2017;8:99336. doi: 10.18632/oncotarget.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev.Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bayry J. CTLA-4: a key protein in autoimmunity. Nat. Rev. Rheumatol. 2009;5:244–245. doi: 10.1038/nrrheum.2009.77. [DOI] [PubMed] [Google Scholar]
- 75.Goldberg MS. Improving cancer immunotherapy through nanotechnology. Nature Rev. Cancer. 2019;19:587–602. doi: 10.1038/s41568-019-0186-9. [DOI] [PubMed] [Google Scholar]
- 76.Caetano MS, et al. Sex specific function of epithelial STAT3 signaling in pathogenesis of K-ras mutant lung cancer. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-07042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ye Y, et al. Sex-associated molecular differences for cancer immunotherapy. Nat. Commun. 2020;11:1–8. doi: 10.1038/s41467-020-15679-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zanganeh S, et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torcia MG, et al. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PloS ONE. 2012;7:e39853. doi: 10.1371/journal.pone.0039853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schoots MH, Gordijn SJ, Scherjon SA, van Goor H, Hillebrands J-L. Oxidative stress in placental pathology. Placenta. 2018;69:153–161. doi: 10.1016/j.placenta.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 81.Ganguly E, et al. Sex-specific effects of nanoparticle-encapsulated MitoQ (nMitoQ) delivery to the placenta in a rat model of fetal hypoxia. Front. Physiol. 2019;10:562. doi: 10.3389/fphys.2019.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Regitz‐Zagrosek V. Sex and gender differences in health. EMBO Rep. 2012;13:596–603. doi: 10.1038/embor.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amur S, Parekh A, Mummaneni P. Sex differences and genomics in autoimmune diseases. J. Autoimmun. 2012;38:J254–J265. doi: 10.1016/j.jaut.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 84.Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front. Gen. 2012;3:268. doi: 10.3389/fgene.2012.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pollitzer E. Cell sex matters. Nature. 2013;500:23–24. doi: 10.1038/500023a. [DOI] [PubMed] [Google Scholar]
- 86.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nat. N. 2014;509:282. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.InanlooRahatloo K, et al. Sex-based differences in myocardial gene expression in recently deceased organ donors with no prior cardiovascular disease. PloS ONE. 2017;12:e0183874. doi: 10.1371/journal.pone.0183874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Babiker FA, et al. Estrogenic hormone action in the heart: regulatory network and function. Cardiovas. Res. 2002;53:709–719. doi: 10.1016/S0008-6363(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 89.Du X-J. Gender modulates cardiac phenotype development in genetically modified mice. Cardiovas. Res. 2004;63:510–519. doi: 10.1016/j.cardiores.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 90.Dormont F, Varna M, Couvreur P. Nanoplumbers: biomaterials to fight cardiovascular diseases. Mater. Today. 2018;21:122–143. doi: 10.1016/j.mattod.2017.07.008. [DOI] [Google Scholar]
- 91.Mahmoudi M, et al. Multiscale technologies for treatment of ischemic cardiomyopathy. Nat. Nanotechnol. 2017;12:845. doi: 10.1038/nnano.2017.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lau ES, et al. Sex differences in circulating biomarkers of cardiovascular disease. J. Am. Coll. Cardiol. 2019;74:1543–1553. doi: 10.1016/j.jacc.2019.06.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kazemian N, Mahmoudi M, Halperin F, Wu JC, Pakpour S. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome. 2020;8:36. doi: 10.1186/s40168-020-00821-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Markle JG, et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science. 2013;339:1084–1088. doi: 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 95.O’Connor CA, Cernak I, Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062:171–174. doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 96.Bharadwaj V. N., et al. Nanoparticle delivery precision based on sex-dependent opening of the blood–brain barrier after brain injury. In: 42nd Society for Biomaterials Annual Meeting and Exposition 2019: The Pinnacle of Biomaterials Innovation and Excellence). Society for Biomaterials (2019).
- 97.Bharadwaj VN, et al. Sex-dependent macromolecule and nanoparticle delivery in experimental brain injury. Tissue Eng. A. 2020;26:688–701. doi: 10.1089/ten.tea.2020.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Asai H, et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015;18:1584. doi: 10.1038/nn.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kettenmann H, Kirchhoff F, Verkhratsky A. Microglia: new roles for the synaptic stripper. Neuron. 2013;77:10–18. doi: 10.1016/j.neuron.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 100.Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J. Neurochem. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kodama L, Gan L. Do microglial sex differences contribute to sex differences in neurodegenerative diseases? Trends Mol. Med. 2019;25:741–749. doi: 10.1016/j.molmed.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oveisgharan S, et al. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta Neuropathol. 2018;136:887–900. doi: 10.1007/s00401-018-1920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Curtis AF, et al. Sex differences in the prevalence of genetic mutations in FTD and ALS: A meta-analysis. Neurology. 2017;89:1633–1642. doi: 10.1212/WNL.0000000000004494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Augustine EF, et al. Sex differences in clinical features of early, treated Parkinson’s disease. PloS ONE. 2015;10:e0133002. doi: 10.1371/journal.pone.0133002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McCombe PA, Henderson RD. Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 2010;7:557–570. doi: 10.1016/j.genm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 106.Yanguas-Casás N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol. Neuroinflam. 2020;7:13–22. [Google Scholar]
- 107.Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol. Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 108.Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Rec. Prog. Horm. Res. 2002;57:257–276. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- 109.Park S-J, Jeong S-Y, Kim HJ. Y chromosome loss and other genomic alterations in hepatocellular carcinoma cell lines analyzed by CGH and CGH array. Cancer Gen. Cytogen. 2006;166:56–64. doi: 10.1016/j.cancergencyto.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 110.Sheibani S, et al. Nanoscale characterization of the biomolecular corona on the surface of polystyrene nanoparticles by cryo-electron microscopy, cryo-electron tomography, and image simulation. Nat. Commun. 2020;12:573. doi: 10.1038/s41467-020-20884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lundqvist M, Cedervall T. Three decades of research about the corona around nanoparticles: lessons learned and where to go now. Small. 2020;16:2000892. doi: 10.1002/smll.202000892. [DOI] [PubMed] [Google Scholar]
- 112.Langevin D, et al. Inter-laboratory comparison of nanoparticle size measurements using dynamic light scattering and differential centrifugal sedimentation. NanoImpact. 2018;10:97–107. doi: 10.1016/j.impact.2017.12.004. [DOI] [Google Scholar]
- 113.Polack, F. P. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed]
- 114.Baden, L. R. et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine 384, 403–416 (2020). [DOI] [PMC free article] [PubMed]
- 115.Franco A, Malhotra N, Simonovits G. Publication bias in the social sciences: unlocking the file drawer. Science. 2014;345:1502–1505. doi: 10.1126/science.1255484. [DOI] [PubMed] [Google Scholar]
- 116.Rosenberg MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution. 2005;59:464–468. doi: 10.1111/j.0014-3820.2005.tb01004.x. [DOI] [PubMed] [Google Scholar]
- 117.Joober R, Schmitz N, Annable L, Boksa P. Publication bias: what are the challenges and can they be overcome? J. Psych. Neurosci. JPN. 2012;37:149. doi: 10.1503/jpn.120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Young SS, Bang H, Kennedy D. The file-drawer problem, revisited [3] (multiple letters) Science. 2004;306:1133–1134. doi: 10.1126/science.306.5699.1133d. [DOI] [PubMed] [Google Scholar]
- 119.Khushf G. Upstream ethics in nanomedicine: A call for research. Nanomedicine. 2007;2:511–521. doi: 10.2217/17435889.2.4.511. [DOI] [PubMed] [Google Scholar]
- 120.Easterbrook PJ, Gopalan R, Berlin JA, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-Y. [DOI] [PubMed] [Google Scholar]
- 121.Begg CB, Berlin JA. Publication bias: a problem in interpreting medical data. J. R. Stat. Soc. A. 1988;151:419–445. doi: 10.2307/2982993. [DOI] [Google Scholar]
- 122.Berlin JA, Begg CB, Louis TA. An assessment of publication bias using a sample of published clinical trials. J. Am. Stat. Assoc. 1989;84:381–392. doi: 10.1080/01621459.1989.10478782. [DOI] [Google Scholar]
- 123.McAuley L, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet. 2000;356:1228–1231. doi: 10.1016/S0140-6736(00)02786-0. [DOI] [PubMed] [Google Scholar]
- 124.Ashkarran A. A., Swann J., Hollis L., Mahmoudi M. The file drawer problem in nanomedicine. Trends Biotechnol. (2021). [DOI] [PubMed]
- 125.Pardue M.-L., Wizemann T. M. Exploring the biological contributions to human health: does sex matter?. Committee on understanding the biology of sex and gender differences. 39, 425–427 (2021).
- 126.Mahmoudi M, Keashly L. Filling the space: a framework for coordinated global actions to diminish academic bullying. Angew. Chem. Int. Ed. 2021;60:3338–3344. doi: 10.1002/anie.202009270. [DOI] [PubMed] [Google Scholar]
- 127.Lobo R. Menopause: endocrinology, consequences of estrogen deficiency, effects of hormone replacement therapy, treatment regimens. Comprehensive Gyncology 5th edn (Mosby Elsevier, 1039–1071 2007).
- 128.Lobo R. Primary and secondary amenorrhea and precocious puberty: etiology, diagnostic evaluation, management. Comprehensive Gynecology 5th edn (Mosby, 2007).
- 129.Melmed S., Polonsky K. S., Larsen P. R., Kronenberg H. M. Williams Textbook of Endocrinology E-Book. (Elsevier Health Sciences, 2015).
- 130.https://www.abim.org/~/media/ABIM%20Public/Files/pdf/exam/laboratory-reference-ranges.pdf.