Abstract
The blacklegged tick, Ixodes scapularis, is the primary vector of multiple human pathogens, including the causative agents of Lyme disease, anaplasmosis, and babesiosis. Both I. scapularis and its associated pathogens have expanded their geographic range throughout the northeastern Unites States and into northern New England. Through this study, we present an updated distribution of I. scapularis in Maine and report the first statewide passive surveillance infection and coinfection prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti within the state's I. scapularis population. In 2019, we collected 2016 ticks through a passive surveillance program, in which Maine residents submitted tick samples for identification and/or pathogen testing. We used a single multiplex quantitative PCR assay to detect tickborne pathogens in 1901 tick samples. At the state level, we found that Bo. burgdorferi and A. phagocytophilum infection rates of adults (42.4%, 11.1%) were nearly double that of nymphs (26.9%, 6.7%), whereas B. microti prevalence was similar for both adults (6.5%) and nymphs (5.2%). Spatially, we found an uneven distribution of both tick activity and pathogen prevalence, with both increasing on a north to south gradient. We also noted a potential association between the ratio of adult to nymphal ticks and the incidence of tickborne disease in human populations, with counties that exhibit high rates of human disease also maintaining low adult to nymph ratios.
Keywords: Ixodes scapularis, passive surveillance, qPCR, Borrelia burgdorferi, Anaplasma phagocytophilum, Babesia microti
Introduction
Tickborne illnesses constitute an increasing number of emerging infectious diseases, with ∼50,000 annual reported cases in the contiguous United States alone (Rosenberg et al. 2018, CDC 2019). While some tickborne diseases are still considered regional, the geographic expansion into new localities has been notable; for example, the number of United States counties with a high incidence of Lyme disease has increased by ∼300% since the mid-1990s (Kugeler et al. 2015). The expansion of tickborne disease cases directly corresponds to expanding geographic ranges of the tick vectors, themselves (Eisen and Eisen 2018). Several tick species of medical importance, particularly Ixodes scapularis and Amblyomma americanum, are expanding their geographic distribution, with contributing factors, including climate change, host availability, habitat suitability, and human landscape modification (Sonenshine 2018).
Of the nearly 90 tick species found in the United States, specimens from 15 different species have been identified in Maine, all belonging to the Ixodidae family (Smith et al. 1996, Rand et al. 2007). Some of the tick species identified in Maine have become widespread, while others do not have established permanent populations (Rand et al. 2007). The tick species of greatest medical and veterinary concern in Maine is the blacklegged tick (I. scapularis). In Maine, I. scapularis is known to transmit Borrelia burgdorferi, Anaplasma phagocytophilum, Babesia microti, Bo. miyamotoi, and Powassan virus, the causative agents of Lyme disease, anaplasmosis, babesiosis, Bo. miyamotoi disease, and Powassan encephalitis, respectively (Maine CDC 2020a). Reported human cases in Maine continue to increase and expand geographically.
I. scapularis was first reported in Maine in the late 1980s and has since been found in all of the state's 16 counties (Anderson et al. 1987, Ginsberg and Ewing 1988, Rand et al. 2007). The current distribution may represent the recolonization of a historic range that could have extended across much of the eastern United States (Eisen and Eisen 2018). Changes in habitat availability associated with evolving human land use and fluctuations in host abundance have facilitated this reemergence of I. scapularis that has rapidly continued over the past three decades (Sonenshine 2018). In addition, the effects of climate change have the potential to significantly impact the range of I. scapularis since its development and survival is heavily influenced by climatic factors when off host (Schulze et al. 2009). Increasing temperature and humidity, as well as variation in rainfall events, can enhance I. scapularis survival at higher latitudes, while also resulting in an expansion of suitable habitat (Simon et al. 2014).
In the 35 years since I. scapularis was first detected in Maine (Anderson et al. 1987, Ginsberg and Ewing 1988), its distribution has expanded throughout the state, however, its population density and pathogen infection prevalence remains geographically variable. Using a statewide passive surveillance program, we examined the distribution of the blacklegged tick in Maine and investigated the infection and coinfection prevalence of Bo. burgdorferi, A. phagocytophilum, and B. microti in I. scapularis. We also assessed differences in infection rates based upon tick life stage (adults vs. nymphs) and made comparisons to human cases of tickborne disease.
Materials and Methods
Collection of tick specimens
Ticks were collected as part of a passive surveillance program offered by the University of Maine Cooperative Extension Tick Laboratory in Orono, Maine. Starting in April of 2019, Maine residents submitted ticks for species identification and testing for the presence of Bo. burgdorferi, A. phagocytophilum, and B. microti. All clients submitting a tick sample for testing completed a survey, which asked the date, physical location, and activity they were doing when they encountered the tick; the species of the host; and the gender, age, and attachment site on the host (if the host was human). Samples submitted for identification only, or those that originated outside of Maine, were not included in this analysis.
Upon a tick sample's arrival at the laboratory, we made a species-level identification based on published identification keys (Keirans and Litwak 1989, Keirans et al. 1996) and recorded life stage (larva, nymph, or adult) and level of engorgement (unengorged, partially engorged, fully engorged). If samples arrived at the laboratory too damaged to be visually identified, or had ambiguous characteristics, we identified the species of tick by sequencing a portion of the mitochondrial genome (Table 1) and comparing the generated sequence to that of known specimens.
Table 1.
Quantitative PCR Primers and Probes Used to Detect Selected Pathogens and Tick DNA in Ixodes scapularis Ticks
| Target | Gene | Type | Sequence (5′-3′) | Con. (nM) | References |
|---|---|---|---|---|---|
| Borrelia burgdorferi sensu lato* | 23S | FWD | CGAGTCTTAAAAGGGCGATTT | 500 | Xu et al. (2016) |
| AGT | |||||
| REV | GCTTCAGCCTGGCCATAAATAG | 500 | |||
| Probe | FAM—AGATGTGGTAG | 250 | |||
| ACCCGAAGCCGAGTG—BHQ 1 | |||||
| Tick DNA control* | 16S | FWD | AATACTCTAGGGATAACAGCGT | 500 | Xu et al. (2016) |
| AATAATTTT | |||||
| REV | CGGTCTGAACTCAGATCAAGTA | 500 | |||
| GGA | |||||
| Probe | Cy5—AAATAGTTTGCGACCTC | 250 | |||
| GATGTTGGATTAGGA—BHQ 1 | |||||
| Anaplasma phagocytophilum* | MSP2 | FWD | ATGGAAGGTAGTGTTGGTTATG | 500 | Hojgaard et al. (2014) |
| GTATT | |||||
| REV | TTGGTCTTGAAGCGCTCGTA | 500 | |||
| Probe | HEX—TGGTGCCAGGGT | 250 | |||
| TGAGCTTGAGATTG—BHQ1 | |||||
| Babesia microti* | 18S | FWD | CGACTACGTCCCTGCCCTTTG | 500 | Hojgaard et al. (2014) |
| REV | ACGAAGGACGAATCCACGTTTC | 500 | |||
| Probe | Tex615—ACACCGCCCGTCGCTC | 250 | |||
| CTACCG—BHQ2 | |||||
| Babesia microti | SA1 | FWD | ACAGAATGCAGTCGGTGAAG | 1000 | Hojgaard et al. (2014) |
| REV | ATCAAGGAGAGTGGATAGGTTTG | 1000 | |||
| Tick species ID | COI | FWD | GGTCAACAAATCATAAAGATA | Folmer et al. (1994) | |
| TTGG | |||||
| REV | TAAACTTCAGGGTGACCAAAAA | ||||
| ATCA |
Targets denoted with an asterisk (*) were amplified together as a single multiplex qPCR. We completed each qPCR run on a Bio-Rad CFX 96 thermal cycler using the following: 5 μL Bio-Rad iQ Multiplex Powermix, 3 μL of primer and probe mixture, and 2 μL of tick DNA extract. We used an initial 95°C burn-in for 3 min followed by 40 cycles of 95°C denaturation (15 s) and 60°C annealing-extension (45 s). We ran samples testing positive for B. microti in multiplex for a confirmatory qPCR assay that used the same cycling conditions, but different reagents: 5 μL Applied Biosystems PowerUp SYBR Green Mastermix, 1 μL nuclease-free dH2O, 2 μL primer solution and 2 μL of tick DNA extract. The Tick Species ID fragment was sequenced to determine the species of origin of samples that could not be identified visually.
qPCR, quantitative PCR.
Tick DNA extraction
We extracted DNA from whole ticks using a Qiagen (Germantown, MD) the DNeasy Blood and Tissue (Cat no. 69506) Extraction Kit and a modified protocol. First, we placed each tick in an individually labeled 2.0 mL screw-top vial containing 400 mg of 2–2.5 mm yttria-stabilized zirconium (YSZ) beads (MSE Supplies, Tucson, AZ) and 150 mg of 0.2 mm YSZ beads. Next, we disrupted the bead tubes on a Qiagen TissueLyser II (Cat no. 85300) for 30 s at 30 Hz, rotated the tubes 180° horizontally, and disrupted again. Then, we added 230 μL of Buffer ATL and 20 μL of Proteinase K, mixed and centrifuged the samples, and placed them horizontally in a 250-mL beaker on an IBI Scientific (Dubuque, IA) Belly Button (model no. BBUAAUV1S) mixing platform inside of an incubating oven.
We incubated the samples with mixing overnight at 56°C. Next, each sample was disrupted again on the TissueLyser II for 30 s at 30 Hz. We added 200 μL of Buffer AL and 200 μL of 100% ethanol or 300 μL of Buffer AL and 300 μL of 100% ethanol to unengorged, and engorged ticks, respectively. We then followed the remainder of manufacturer's extraction protocol, except the final elution with Buffer AE was done in two steps—35 and 30 μL for unengorged ticks or 65 and 60 μL for engorged ticks.
Molecular pathogen detection
We used a single multiplex quantitative PCR (qPCR) assay to simultaneously test for the presence of Bo. burgdorferi sensu lato, A. phagocytophilum, B. microti, as well as a tick 16S rRNA internal positive control. We completed each qPCR run on a Bio-Rad CFX 96 thermal cycler (Hercules, CA) with the following reaction components: 5 μL Bio-Rad iQ Multiplex Powermix, 3 μL of primer and probe mixture (Table 1), and 2 μL of tick DNA extract. The thermal cycling conditions we used were an initial 95°C burn-in for 3 min followed by 40 cycles of 95°C denaturation (15 s) and 60°C annealing extension (45 s).
We amplified each sample in at least duplicate and sample runs were only considered valid if the internal tick DNA control was successfully amplified with a critical threshold (Ct) ≤35.0. We ran samples testing positive for B. microti in multiplex for a second qPCR assay (Table 1) to reduce the possibility of a false-positive result. We used the same thermal cycling conditions, but different reagents: 5 μL Applied Biosystems (Foster City, CA) PowerUp SYBR Green Mastermix, 1 μL nuclease-free water, 2 μL primer solution (Table 1), and 2 μL of tick DNA extract. We only considered samples to be positive for B. microti if amplification was successful for both assays. We used negative extraction controls, negative PCR controls, and positive controls from extracts of the target pathogens to reduce the possibility of erroneous qPCR results.
Human tickborne disease data
For the purposes of an exploratory comparison to our tick pathogen dataset we aggregated human tickborne disease case data provided by the Maine CDC (2020b) at the county level for 2019. We completed this work under the University of Maine Institutional Review Board protocol #A2018-11-07.
Results
Spatial and temporal distribution of collected ticks
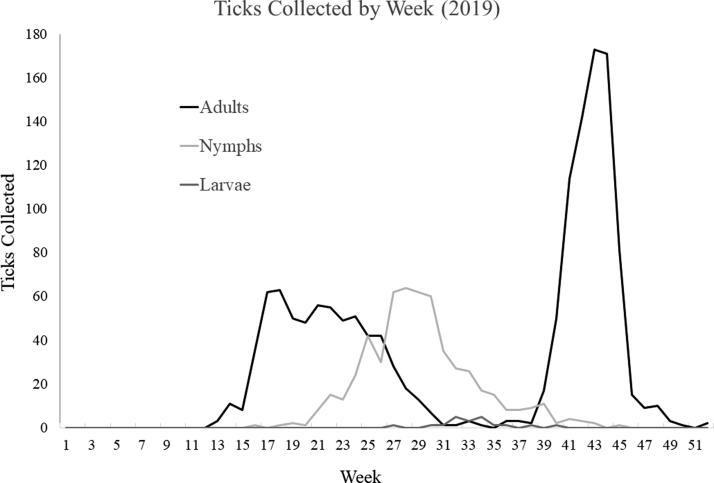
We received a total of 2016 I. scapularis ticks that were collected in Maine between March and December of 2019. Of the submitted I. scapularis samples, 70.1% (n = 1413) were adult females, whereas 27.4% (n = 553) were identified as nymphs. Adult males (n = 30) and larvae (n = 20) together comprised 2.5% of the total submitted samples. We observed two distinct peaks of adult activity, one in the spring and one in the fall, and a single peak of nymphal activity during mid-summer (Fig. 1). Weekly collections of adults peaked in the spring (n = 63) during week 17 (the week of April 22nd) and in the fall (n = 173) during week 43 (the week of October 21st). Nymphal activity peaked during week 28 (the week of July 15th) with 64 samples collected.
FIG. 1.
Weekly number of ticks, separated by life stage, collected by Maine residents in 2019. Samples were not accepted by the laboratory for testing until week 12.
When considering the human population size at the county level, we discovered that the number of ticks submitted for testing varied between counties (Table 2). Aroostook County had the lowest number of ticks submitted per 100,000 human residents at 7.0, in contrast to Lincoln County, the highest, at 634.8. Regionally, ticks received per human population were greatest from the counties of mid-coast Maine, with Lincoln (634.8), Hancock (607.5), Waldo (365.3), and Knox (354.5) being at least twice that of any other county.
Table 2.
Number of Ticks Collected, Adult to Nymph Ratios, Ticks Submitted Per 100 k Human Population, Pathogen Prevalence, and Coinfection Prevalence of Ixodes scapularis by Maine County
| County | Adults/nymphs | Adults:nymphs | Ticks/100 k pop | Bb (%) | Ap (%) | Bm (%) | Bb + Ap (%) | Bb + Bm (%) | Ap + Bm (%) | Bb + Ap + Bm (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Androscoggin | 53/13 | 4.1 | 65.0 | 43.4/23.1 | 7.5/7.7 | 11.3/7.7 | 1.9/0 | 7.5/0 | 0/0 | 0/0 |
| Aroostook | 5/0 | — | 7.5 | 0/— | 0/— | 0/— | 0/— | 0/— | 0/— | 0/— |
| Cumberland | 223/104 | 2.1 | 118.2 | 39.0/27.9 | 16.1/10.6 | 9.0/5.8 | 4.9/6.7 | 4.9/3.8 | 0.9/2.9 | 0.4/1.9 |
| Franklin | 26/5 | 5.2 | 117.1 | 50/40 | 3.8/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Hancock | 195/123 | 1.6 | 607.5 | 44.1/28.5 | 11.8/6.5 | 5.1/0.8 | 2.6/4.1 | 2.6/0.8 | 1.0/0 | 0.5/0 |
| Kennebec | 96/32 | 3.0 | 111.4 | 36.5/25.0 | 13.5/6.3 | 4.2/3.1 | 4.2/3.1 | 2.1/3.1 | 1.0/0 | 1.0/0 |
| Knox | 85/48 | 1.8 | 354.5 | 48.2/43.8 | 12.9/2.1 | 11.8/12.5 | 9.4/0 | 7.1/4.2 | 2.4/0 | 2.4/0 |
| Lincoln | 165/39 | 4.2 | 634.8 | 47.3/33.3 | 6.1/2.6 | 6.1/7.7 | 3.6/2.6 | 4.2/7.7 | 0/2.6 | 0/2.6 |
| Oxford | 37/12 | 3.1 | 90.2 | 45.9/8.3 | 21.6/0 | 5.4/8.3 | 13.5/0 | 2.7/0 | 2.7/0 | 2.7/0 |
| Penobscot | 186/42 | 4.4 | 164.8 | 39.8/38.1 | 5.9/4.8 | 2.2/0 | 2.2/2.4 | 1.6/0 | 0.5/0 | 0.5/0 |
| Piscataquis | 20/4 | 5.0 | 148.8 | 30/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Sagadahoc | 25/23 | 1.1 | 137.5 | 48.0/30.4 | 16.0/8.7 | 4.0/13.0 | 4.0/4.3 | 4.0/8.7 | 0/0 | 0/0 |
| Somerset | 30/6 | 5.0 | 77.1 | 46.7/0 | 6.7/16.7 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 |
| Waldo | 90/50 | 1.8 | 365.3 | 50/28.0 | 11.1/6.0 | 7.8/6.0 | 4.4/6.0 | 4.4/6.0 | 1.1/2.0 | 0/2.0 |
| Washington | 36/6 | 6.0 | 142.9 | 36.1/33.3 | 13.9/0 | 5.6/0 | 5.6/0 | 2.8/0 | 0/0 | 0/0 |
| York | 92/30 | 3.1 | 61.6 | 37.0/26.7 | 15.2/13.3 | 14.1/10 | 4.3/6.7 | 6.5/6.7 | 4.3/0 | 2.2/0 |
| Statewide | 1,364/537 | 2.5 | 150.6 | 42.4/26.9 | 11.1/6.7 | 6.5/5.2 | 4.0/3.9 | 3.7/3.4 | 1.0/0.9 | 0.7/0.7 |
For prevalence rates, the adult prevalence is listed before the slash and the nymphal rate is listed after. Ticks/100 k pop is the number of ticks submitted from that county per 100,000 human population.
Table fields marked with a dash (—) could not be calculated.
Ap, Anaplasma phagocytophilum; Bm, Babesia microti; Bb, Borrelia burgdorferi.
Tick pathogen testing
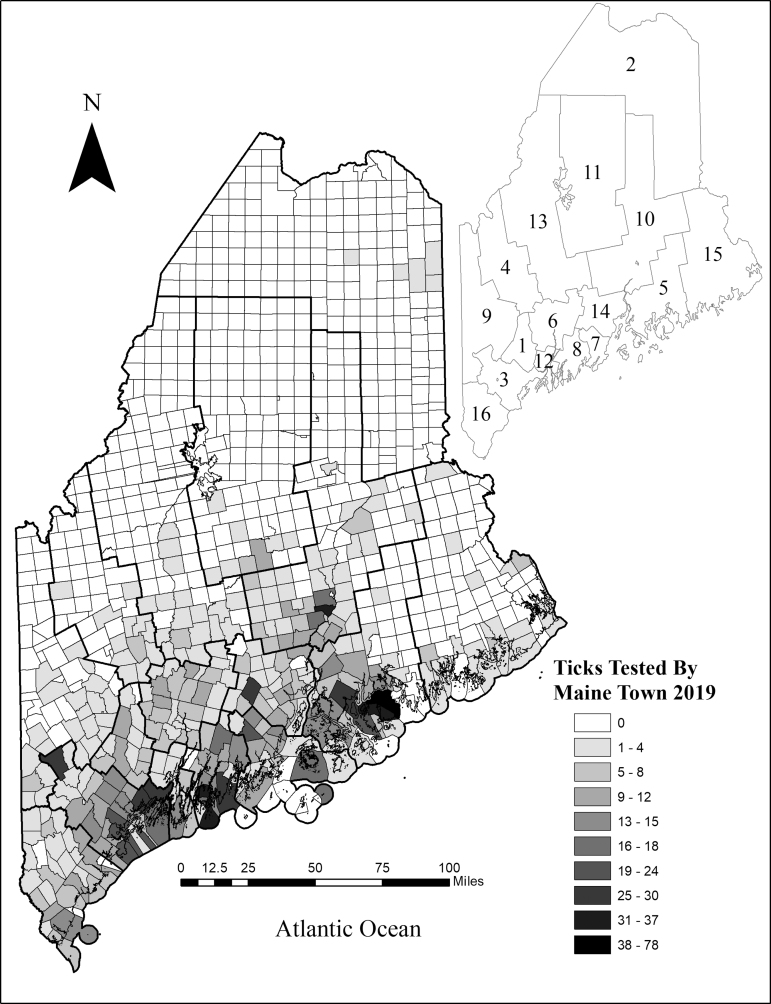
Of the 2016 I. scapularis ticks we received for identification, we tested 1960 for the presence of Bo. burgdorferi, A. phagocytophilum, and B. microti, but only 1901 adults and nymphs had accompanying Maine town and county geolocation information and were included in this study. We tested I. scapularis ticks from each of Maine's 16 counties and a total of 319 towns (Fig. 2).
FIG. 2.
Number of ticks tested from each Maine town during the 2019 season. The locations from which we received the greatest number of ticks are concentrated along the southern coast. Counties list: 1—Androscoggin, 2—Aroostook, 3—Cumberland, 4—Franklin, 5—Hancock, 6—Kennebec, 7—Knox, 8—Lincoln, 9—Oxford, 10—Penobscot, 11—Piscataquis, 12—Sagadahoc, 13—Somerset, 14—Waldo, 15—Washington, 16—York.
When comparing pathogen prevalence at the state level, we observed that the Bo. burgdorferi and A. phagocytophilum rates of adults (42.4%, 11.1%) were nearly double that of nymphs (26.9%, 6.7%), but we found the B. microti prevalence was similar for both adults (6.5%) and nymphs (5.2%) (Table 2). We also found that the statewide coinfection rates of both adults and nymphs were similar. Of the adults we tested, 4.0% were coinfected with Bo. burgdorferi and A. phagocytophilum, 3.7% with Bo. burgdorferi and B. microti, and 1.0% with A. phagocytophilum and B. microti, as compared with the 3.9%, 3.4%, and 0.9% we observed in nymphs, respectively (Table 2). We observed a 0.7% prevalence of triple coinfection in both adults and nymphal ticks.
We found that pathogen prevalence varied considerably at the county level. We detected Bo. burgdorferi in ticks from all counties, except Aroostook, although we only tested five samples from this county. Excluding Aroostook, the county-level Bo. burgdorferi prevalence ranged from 30.0% (Piscataquis) to 50.0% (Franklin and Waldo) in adults and 0% (Piscataquis and Somerset) to 43.8% (Knox) in nymphs. A. phagocytophilum was not detected in Aroostook or Piscataquis county adult ticks, but we found the prevalence in Oxford County adults (21.6%) to be nearly twice the statewide average (11.1%). A. phagocytophilum in nymphs was less prevalent than adults, and nymphs from Franklin, Oxford, Piscataquis, and Washington counties had 0% prevalence, but detection may have been limited by a small sample size.
B. microti was the least widely distributed pathogen with no detections in adults from Aroostook, Franklin, Piscataquis, and Somerset Counties, however, Androscoggin (11.3%), Knox (11.8%), and York (14.1%) all had adult prevalence rates considerably higher than the statewide average of 6.5%. We found a similar distribution of B. microti in nymphs and did not detect the pathogen in Franklin, Penobscot, Piscataquis, Somerset, or Washington counties, but found the prevalence was greatest in Knox (12.5%), Sagadahoc (13.0%), and York (10.0%) counties.
Discussion
While our data confirms that I. scapularis is currently active in each of Maine's 16 counties, we found an uneven distribution of both tick activity and pathogen prevalence. We found that both the number of ticks submitted per 100,000 human residents and the prevalence of Bo. burgdorferi, A. phagocytophilum, and B. microti increased along a gradient from northern Maine into southern Maine. These southern coastal areas with greater tick activity include the locations where I. scapularis was first detected in Maine (Ginsberg and Ewing 1988), and are thought to be continually reintroduced by avian migration (MacQueen et al. 2012).
When we made observational comparisons between our passive surveillance pathogen prevalence data and the actual human incidence rates of tickborne disease by Maine County from 2019 (Table 3; Maine CDC 2020b), we found some similarities. The counties with the greatest incidence rates of Lyme disease per 100,000 residents: Knox (588.4), Hancock (350.3), Waldo (357.7), and Lincoln (384.4) were the same counties from which we received the most ticks per 100,000 residents (Tables 2 and 3). We found that these same counties had comparatively high prevalence rates of Bo. burgdorferi. Our passive surveillance data also suggest that mid-coast Maine is currently the focal location of I. scapularis and Bo. burgdorferi activity in the state.
Table 3.
Average Human Tick-Borne Disease Incidence Rates for Each Maine County 2019
| County | Lyme disease | Anaplasmosis | Babesiosis |
|---|---|---|---|
| Androscoggin | 91.0 | 61.3 | 6.5 |
| Aroostook | 3.0 | 1.5 | 0.0 |
| Cumberland | 120.6 | 40.2 | 9.5 |
| Franklin | 130.4 | 13.4 | 6.7 |
| Hancock | 350.3 | 78.5 | 12.8 |
| Kennebec | 226.9 | 50.0 | 14.7 |
| Knox | 588.4 | 228.8 | 42.7 |
| Lincoln | 384.4 | 174.7 | 23.3 |
| Oxford | 152.7 | 62.5 | 13.9 |
| Penobscot | 73.5 | 9.9 | 2.6 |
| Piscataquis | 23.8 | 0.0 | 0.0 |
| Sagadahoc | 232.9 | 84.2 | 25.3 |
| Somerset | 134.4 | 17.8 | 4.0 |
| Waldo | 357.7 | 100.8 | 10.1 |
| Washington | 98.4 | 15.9 | 0.0 |
| York | 151.3 | 51.4 | 11.6 |
| Statewide | 161.9 | 51.2 | 10.3 |
The average number of confirmed and probable human cases of Lyme disease, anaplasmosis, and babesiosis by Maine County (2019) was provided by the Maine Center for Disease Control and Prevention.
When analyzing the geolocation data of our positive A. phagocytophilum samples, we found them not as widely or evenly distributed as Bo. burgdorferi. A. phagocytophilum prevalence in adult I. scapularis was greatest in the southwestern portion of the state and was generally lower than the state average for adults (11.1%) in inland and northern counties. Franklin County, which had one of the highest prevalence rates of Bo. burgdorferi in adult I. scapularis, had an A. phagocytophilum prevalence that was almost a third of the state average. However, Oxford County, which borders Franklin County, had the highest adult prevalence of A. phagocytophilum and A. phagocytophilum and Bo. burgdorferi coinfection at 21.6% and 13.5%, respectively. Differing levels of A. phagocytohilum prevalence among neighboring counties corroborates the uneven distribution of actual human cases of anaplasmosis in Maine (Maine CDC 2020b). When we observationally compared the prevalence of A. phagocytophilum in ticks with the geolocation data of human anaplasmosis cases, we found that high disease incidence counties did not necessarily have higher prevalence rates within submitted ticks.
The discrepancy between the prevalence of A. phagocytophilum in ticks and the human cases of anaplasmosis suggests that there may be a disconnect between these datasets. Elias et al. (2020) discovered a similar decoupling between I. scapularis abundance and Bo. burgdorferi prevalence in the southern counties of the state. It is also possible that there is a lag period between an increase in pathogen prevalence on the landscape, and when it influences human disease case numbers. Another possibility for this disconnect in the datasets is that knowledge of anaplasmosis is not as widespread as Lyme disease, which may lead to the underdiagnosis of this disease. In addition, individuals coinfected with A. phagocytophilum and Bo. burgdorferi may be treated with antibiotics for Lyme disease without ever being tested for anaplasmosis.
Of the three pathogens for which we tested, B. microti was the most limited in its distribution. We found the greatest prevalence of B. microti in adult I. scapularis in the counties of southeastern Maine, particularly Androscoggin (11.3%), Cumberland (9.0%), Knox (11.8%), and York (14.1%). We did not detect B. microti in Aroostook, Franklin, Piscataquis, and Somerset Counties, and the prevalence in other counties was relatively low. At the town level, we found that nearly half of the towns (42.9%), from which a B. microti-positive tick was submitted had multiple positive tests, suggesting a clustered distribution. The human incidence rate of babesiosis for 2019 (Maine CDC 2020b) was also unevenly distributed; with the highest case rates in mid-coast Maine and very low incidence rates for the same counties in which we failed to detect B. microti.
The sporadic distribution of B. microti is consistent with a pathogen that is colonizing a new location and has not yet reached an even spatial distribution (Diuk-Wasser et al. 2016). B. microti is also thought to spread more quickly in areas where Bo. burgdorferi is prevalent due to an immune interaction in reservoir hosts such as white-footed mice (Peromyscus leucopus) or deer mice (P. maniculatus) (Dunn et al. 2014). B. microti is likely to continue spreading throughout Maine. To document the range expansion of B. microti, we will be selectively collecting both active and passive surveillance data from locations where this pathogen is prevalent.
When comparing I. scapularis nymphs to adults, we found that while the pathogen prevalence rates of nymphs never exceeded those of adults within the same county, they did occasionally exceed the adult prevalence rates of other counties. We believe that this provides additional evidence of the uneven distribution of tickborne disease risk in Maine. The presence of questing nymphs in a location may be more indicative of I. scapularis reproduction in that area, as opposed to questing adults that may have been introduced to the area as fed nymphs by birds and other wildlife. The counties from which the fewest nymphal I. scapularis were submitted were generally northern locations, where establishment is thought to still be taking place.
We also examined the ratio of adult to nymph ticks submitted for testing from each Maine county and discovered that these ratios were variable based on geolocation. While not examined more closely due to the limited number of nymphal ticks received from some counties, we found that counties with the highest rates of tickborne disease generally had lower adult to nymph ratios. For instance, the counties with the highest average incidence rates of Lyme disease in 2019 (Maine CDC 2020b), in descending order, were Knox, Hancock, Waldo, Lincoln, Sagadahoc, and York Counties, which had adult to nymph ratios of 1.8, 1.6, 1.8, 4.2, 1.1, and 3.1, respectively. In contrast, the five counties with the lowest incidence rates of Lyme disease in 2019, in ascending order, were Aroostook, Piscataquis, Penobscot, Washington, and Franklin, which had the following adult to nymph ratios: no nymphs received, 5.0, 4.4, 6.0, and 5.2, respectively.
Due to the small size of I. scapularis nymphs, their bites may be less likely to be detected by a host, which can increase the probability of pathogen transmission (Yeh et al. 1995). Lower adult to nymph ratios in a location may be an indicator of greater risk of tickborne disease in the northeastern United States, where nymphal I. scapularis are believed to be the primary vector of Bo. burgdorferi and have been previously modeled as such (Mather et al. 1996, Diuk-Wasser et al. 2012). We will continue to investigate this apparent trend with additional, yearly passive surveillance data.
Conclusion
Our study represents the first statewide passive surveillance tick-testing program focused solely in Maine and provides important information on the geographic distribution of I. scapularis and its associated pathogens as they continue to expand their range within the state. Although passive surveillance for tickborne pathogens has some limitations, particularly as they relate to sampling bias, previous investigations have noted the important information that can be obtained using this surveillance method, including fine-scale environmental risk, pathogen detection, and assessment of tick colonization in new regions (Ripoche et al. 2018, Soucy et al. 2018).
In this study, we highlight the uneven distribution of I. scapularis and the tickborne pathogens Bo. burgdorferi, A. phagocytophilum, and B. microti in Maine. We also note a latitudinal gradient associated with both tick activity and pathogen prevalence, with both increasing from north to south. Through the continuation of this passive surveillance program and accumulation of additional data, we will continue to examine spatial relationships and begin to study temporal patterns associated with I. scapularis and tickborne pathogens in Maine.
Acknowledgments
The authors thank all of those who submitted ticks and thus contributed to Maine's passive tick surveillance program. They also thank the reviewers that helped us improve this article.
Authors' Contributions
T.F.R., Jr. led the writing of the article with the support of G.M.D. G.M.D. was the leader of tick collection and initial processing and T.F.R., Jr. led the DNA testing and subsequent data analyses. A.M.B. assisted in data collection and analysis and provided critical revision of the art. C.C.D. assisted with the data analysis. J.F.D. conceived this work and led the initial acquisition of tick samples. All authors participated in the article revision process and approved the publication of this final version.
Author Disclosure Statement
No conflicting financial interests exist.
Funding Information
This research was supported by the State of Maine, University of Maine Cooperative Extension, and the United States Department of Agriculture National Institute of Food and Agriculture Award No. 2017-70006-27160.
References
- Anderson JF, Magnarelli LA, McAninch JB. Ixodes dammini and Borrelia burgdorferi in Northern New England and Upstate New York. J Parasitol 1987; 73:419–421 [PubMed] [Google Scholar]
- CDC (United States Centers for Disease Control and Prevention). Tickborne disease surveillance data summary. 2019. Available at https://www.cdc.gov/ticks/data-summary/index.html
- Diuk-Wasser MA, Hoen AG, Cislo P, Brinkerhoff R, et al. Human risk of infection with Borrelia burgdorferi, the Lyme disease agent, in eastern United States. Am J Trop Med Hyg 2012; 86:320–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: Ecological, epidemiological, and clinical consequences. Trends Parasitol 2016; 32:30–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JM, Krause PJ, Davis S, Vannier EG, et al. Borrelia burgdorferi promotes the establishment of Babesia microti in the Northeastern United States. PLoS One 2014; 9:e115494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L. The blacklegged tick, Ixodes scapularis: An increasing public health concern. Trends Parasitol 2018; 34:295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias SP, Maasch KA, Anderson NT, Rand PW, et al. Decoupling of blacklegged tick abundance and Lyme disease incidence in Southern Maine, USA. J Med Entomol 2020; 57:755–765 [DOI] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoeh, W, Lutz R, et al. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 1994; 3:294–299 [PubMed] [Google Scholar]
- Ginsberg HS, Ewing CP. Deer ticks, Ixodes dammini (Acari: Ixodidae), and Lyme disease spirochetes, Borrelia burgdorferi, in Maine. J Med Entomol 1988; 25:303–304 [DOI] [PubMed] [Google Scholar]
- Hojgaard A, Lukacik G, Piesman J. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum and Babesia microti, with two different multiplex PCR assays. Ticks Tick-Borne Dis 2014; 5:349–351 [DOI] [PubMed] [Google Scholar]
- Keirans JE, Hutcheson HJ, Durden LA, Klompen JSH. Ixodes (Ixodes) scapularis (Acari: Ixodidae): Redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol 1996; 33:297–318 [DOI] [PubMed] [Google Scholar]
- Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida, Ixodoidea), east of the Mississippi river. J Med Entomol 1989; 26:435–448 [DOI] [PubMed] [Google Scholar]
- Kugeler KJ, Farley GM, Forrester JD, Mead PS. Geographic distribution and expansion of human Lyme disease, United States. Emerg Infect Dis 2015; 21:1455–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen DD, Lubelczyk C, Elias SP, Cahill BK, et al. Genotypic diversity of an emergent population of Borrelia burgdorferi at a coastal maine island recently colonized by Ixodes scapularis. Vector-Borne Zoonotic Dis 2012; 12:456–461 [DOI] [PubMed] [Google Scholar]
- Maine CDC (Center for Disease Control and Prevention). Report to Maine legislature, Lyme and other tickborne illnesses. 2020a. Available at https://www.maine.gov/dhhs/mecdc/infectious-disease/epi/vector-borne/lyme/documents/Lyme-Legislative-Report-2020.pdf
- Maine CDC (Center for Disease Control and Prevention, Maine Tracking Network). Tickborne disease: 2001. –2019 data. Available at https://data.mainepublichealth.gov/tracking/
- Mather TN, Nicholson MC, Donnelly EF, Matyas BT. Entomologic index for human risk of Lyme disease. Am J Epidemiol 1996; 144:1066–1069 [DOI] [PubMed] [Google Scholar]
- Rand PW, Lacombe EH, Dearborn R, Cahill B, et al. Passive surveillance in Maine, an area emergent for tick-borne diseases. J Med Entomol 2007; 44, 12:1118–1129 [DOI] [PubMed] [Google Scholar]
- Ripoche M, Gasmi S, Adam-Poupart A, Koffi JK, et al. Passive tick surveillance provides an accurate early signal of emerging Lyme disease risk and human cases in Southern Canada. J Med Entomol 2018; 55:1016–1026 [DOI] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, et al. Vital signs: Trends in reported vectorborne disease cases—United States and territories, 2004–2016. MMWR Morb Mortal Wkly Rep 2018; 67:496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze TL, Jordan RA, Schulze CJ, Hung RW. Precipitation and temperature as predictors of the local abundance of Ixodes scapularis (Acari: Ixodidae) nymphs. J Med Entomol 2009; 46:1025–1029 [DOI] [PubMed] [Google Scholar]
- Simon JA, Marrotte RR, Desrosiers N, Fiset J, et al. Climate change and habitat fragmentation drive the occurrence of Borrelia burgdorferi, the agent of Lyme disease, at the northeastern limit of its distribution. Evol Appl 2014; 7:750–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RP, Rand PW, Lacombe EH, Morris SR, et al. Role of bird migration in the long-distance dispersal of Ixodes dammini, the vector of Lyme disease. J Infect Dis 1996; 174:221–224 [DOI] [PubMed] [Google Scholar]
- Sonenshine D. Range expansion of tick disease vectors in North America: Implications for spread of tick-borne disease. Int J Environ Res Public Health 2018; 15:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy, J-PR, Slatculescu AM, Nyiraneza C, Ogden NH, et al. High-resolution ecological niche modeling of Ixodes scapularis ticks based on passive surveillance data at the northern frontier of Lyme disease emergence in North America. Vector-Borne Zoonotic Dis 2018; 18:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Mather TN, Hollingsworth CS, Rich SM. Passive surveillance of Ixodes scapularis (Say), their biting activity, and associated pathogens in Massachusetts. Vector-Borne Zoonotic Dis 2016; 16:520–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh M-T, Bak JM. Hu R, Nicholson MC, et al. Determining the duration of Ixodes scapularis (Acari: Ixodidae) attachment to tick-bite victims. J Med Entomol 1995; 32:853–858 [DOI] [PubMed] [Google Scholar]