Abstract
Recently, infections with emerging zoonotic bacteria of the genus Bartonella have been reported in association with a range of central nervous system (CNS) symptoms. Currently, it remains unknown if Bartonella spp. infection is associated with symptoms of schizophrenia/schizoaffective disorder (SCZ/SAD). The objective of this study was to determine if there is an association between Bartonella species infection and SCZ/SAD. A secondary objective was to determine if SCZ/SAD symptoms were more severe among participants with documented Bartonella spp. infection. Using a case–control study design, 17 cases and 13 controls were evaluated with a series of clinical and cognitive assessments. Blood samples were collected and tested for Bartonella spp. infection using serological, microbiological, and molecular techniques. People with SCZ/SAD were more likely than healthy volunteers to have Bartonella spp. DNA in their bloodstream, with 11 of 17 cases (65%) positive by Bartonella spp. droplet digital PCR (ddPCR). In comparison, only one healthy volunteer was Bartonella spp. ddPCR positive (8%, p = 0.0024). Based on serology, Bartonella spp. exposure was common among people with SCZ/SAD (12 of 17) as well as among healthy volunteers (12 of 13), with no significant difference between the groups (p = 0.196). Within the case group of people with SCZ/SAD, there was no significant difference in SCZ/SAD severity scores between people with and without ddPCR evidence of Bartonella spp. infection. This pilot study provides preliminary evidence in support of future investigations that should examine a potential contribution of Bartonella spp. infection to SCZ/SAD.
Keywords: vector-borne disease, infectious disease, cat, epidemiology, ddPCR, cat scratch disease
Introduction
Epidemiological and neuropathological studies of schizophrenia have suggested that some cases of schizophrenia are associated with environmental factors, including exposure to infectious agents (Yolken et al. 2001, Köhler-Forsberg et al. 2018). More recently there has been evidence that autoimmune encephalitis, which may have infectious triggers, can mimic predominantly psychiatric disorders such as schizophrenia (Endres et al. 2020a, 2020b). There is a well-established epidemiological association between Toxoplasma gondii infection and schizophrenia, but a causal link between toxoplasmosis and schizophrenia has remained elusive (Sutterland et al. 2015, Fuglewicz et al. 2017, Torrey and Yolken 2019). Given the potential association with infectious disease, and that cat ownership during childhood is robustly associated with an increased risk of developing schizophrenia, it is reasonable to consider a potential contribution of cat-transmitted infectious agents other than T. gondii—specifically Bartonella species—in the etiology of schizophrenia (Kolpakova and Bedwell 2013, Fuller Torrey et al. 2015, Divya and Rajajeyakumar 2019).
The domestic cat is the reservoir host for at least three zoonotic Bartonella spp.: Bartonella henselae, Bartonella clarridgeiae, and Bartonella koehlerae, and incidental infection with other Bartonella spp. has also been reported in cats. (Cheslock and Embers 2019). In humans. B. henselae remains the most well-characterized zoonotic Bartonella spp., with infection most often associated with the acute, self-limiting febrile lymphadenopathy called “cat scratch disease” (Relman et al. 1990, Regnery et al. 1992, Zangwill et al. 1993). However, with the advent of increasingly sensitive molecular and microbiological diagnostic assays, Bartonella spp. infections have now been implicated in a spectrum of central nervous system (CNS) diseases, including severe chronic neurological and neuropsychological manifestations and Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) (Breitschwerdt et al. 2008, 2010b, 2011, 2012, 2019a, Mascarelli et al. 2013, Canneti et al. 2018).
The objective of this study was to determine if an association exists between Bartonella spp. infection and schizophrenia/schizoaffective disorder (SCZ/SAD). A secondary objective was to determine if schizophrenia symptoms were more severe among participants with documented Bartonella spp. infection. Our hypothesis was that infection with Bartonella spp. would be more common in people with SCZ/SAD compared to healthy controls. Secondarily, we expected Bartonella-infected cases to exhibit more severe symptomatology.
Materials and Methods
Study design and setting
This was a prospective case–control study conducted at University of North Carolina-Chapel Hill and approved by the UNC-Chapel Hill Biomedical Institutional Review Board (No. 19-0114). Participants were enrolled between March 1 and October 31, 2019. Cases were recruited from the local community using fliers and newspaper advertisements. Controls were healthy volunteers recruited from the surrounding local community through targeted online and email advertising. To minimize selection bias, recruitment material did not specify the purpose of the study. Participants provided written informed consent before enrollment and were compensated $80 for their time. This article was prepared in accordance with strengthening the reporting of observational studies in epidemiology (STROBE) guidelines for case–control studies; the checklist is included as Supplementary Data S1 (Vandenbroucke et al. 2007).
Participants
Inclusion criteria for cases were a diagnosis of SCZ/SAD (confirmed by the Structured Clinical Interview for DSM-V); clinical stability as demonstrated by no psychiatric hospitalizations for the past 3 months; stable dosing of antipsychotic medications (no changes in medication or dose for 1 month before enrollment); ability to provide written informed consent; and at least one set of blood samples collected for microbiological and molecular testing.
Controls were considered healthy based on self-reported health status, and were excluded if reporting a previous diagnosis of SCZ/SAD. Controls were included from a local volunteer population, expected to have similar Bartonella spp. exposures.
Variables, data sources, and measurement
Whole blood and serum were collected from each participant. To increase the likelihood for detection of intermittent bacteremia, blood was collected twice within a 1-week period (Pultorak et al. 2013).
As described previously (Maggi et al. 2011, Lantos et al. 2014, Breitschwerdt et al. 2019b), each participant was tested using six indirect fluorescent antibody (IFA) assays, each representing a unique Bartonella species or subspecies. Bartonella vinsonii subsp. berkhoffii (genotypes I, II, and III), B. henselae, B. koehlerae, and Bartonella quintana IgG antibodies were determined using cell culture-grown bacteria as antigens and following standard IFA techniques. A sample was considered Bartonella spp. seroreactive at an IFA titer of ≥1:64 for any one or more antigen.
Bartonella alpha proteobacteria growth medium (BAPGM) enrichment blood culture and quantitative PCR (qPCR) were performed as previously described (Breitschwerdt et al. 2019b). Briefly, qPCR targeting the Bartonella intergenic 16S-23S rRNA (ITS) region was performed on DNA extracted from the following: each whole blood sample and whole blood culture enriched in BAPGM at 7, 14, and 21 days of culture. A sample was considered BAPGM/qPCR positive if any one or more of these four qPCR tests were positive on any one or more sample.
In addition to BAPGM/qPCR, all blood and BAPGM enrichment blood culture DNA extractions were tested for Bartonella spp. DNA by droplet digital PCR (ddPCR) using the QX200 Droplet Digital PCR (Bio-Rad, Hercules, CA) system. Digital PCR amplification of the Bartonella 16S-23S ITS region, and the human hydroxymethylbilane synthase (HMBS) as housekeeping human reference gene, was conducted as previously validated and described (Maggi et al. 2020). Bio-Rad QuantaSoft Analysis Pro software was utilized to analyze the fluorescent drop distribution and to define the positive DNA detection thresholds for each channel (FAM channel 1 for Bartonella and HEX channel 2 for housekeeping gene amplification). A sample was considered BAPGM/ddPCR positive if any one or more of these four ddPCR tests was positive on any one or more whole blood sample.
For the primary aim, study participants were considered to have Bartonella spp. exposure if they were Bartonella spp. seroreactive at an IFA titer of ≥1:64 for any one or more antigen. Participants were considered to have Bartonella spp. infection if they were positive on any one or more BAPGM/qPCR or BAPGM/ddPCR assay. All Bartonella testing was performed by researchers blinded to participant identity and group assignment.
SCZ/SAD symptom severity was measured with a series of clinical and cognitive assessments, including the Positive and Negative Syndrome Scale (PANSS) (Kay et al. 1988), Brief Assessment of Cognition in Schizophrenia (BACS) (Keefe et al. 2004), and Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES) (Ritsner et al. 2005). PANSS results were analyzed using the positive, negative, and general symptom subscores, and a total PANSS score. BACS results for each primary measure were first converted to Z scores, and then the Z scores from each primary measure were averaged to create a BACS composite score. For the Q-LES, the results were normalized to the possible total score and reported as a percentage.
Data on possible confounders or effect modifiers were collected with a questionnaire (health history questionnaire, Supplementary Data S2) assessing employment, geographic location, health history, and animal and insect vector contact.
Statistical methods
Summary statistics for demographics, Bartonella test results, schizophrenia symptom measures, and health history questionnaire results were calculated for cases and controls. For continuous variables, groups were compared using the Wilcoxon rank sum test for nonparametric data. For categorical variables, cases and controls were compared using chi-squared test (or Fisher exact test for small group sizes). Correction for multiple comparisons for the primary and secondary outcome was performed using Bonferroni correction. For the remaining comparisons, including demographics, animal and insect exposure, and self-reported symptoms from the health history questionnaire, statistical significance was set at p < 0.05. All statistical analysis was performed in R v. 4.0.2 (R Core Team 2019).
Results
During the 9-month recruitment period defined for this pilot study, 17 cases and 13 controls were enrolled. A total of 26 serum and whole blood samples were obtained from controls, and a total of 28 serum and 27 whole blood samples were obtained from cases. No samples were missing from controls, whereas six serum and seven whole blood samples were missing from cases: four cases did not return for the second blood draw, three cases returned, but only serum was able to be drawn due to difficulty in obtaining venous access, one case returned, but neither blood nor serum was able to be drawn due to difficulty obtaining venous access, and for one case, the serum was excessively hemolyzed and unable to be separated for IFA.
Demographic and geographic characteristics of the cases and controls are reported in Table 1. Of cases, 10 were diagnosed with schizophrenia and 7 with schizoaffective disorder. Cases were significantly older than controls. Race and employment status were also significantly different between cases and controls. Cases reported living in North Carolina for significantly longer than controls. Gender and urban/suburban/rural living environment did not differ significantly between cases and controls.
Table 1.
Demographics, Schizophrenia Severity, and Exposures
| Control, n = 13 | Case, n = 17 | p | |
|---|---|---|---|
| Demographics | |||
| Age | <0.0001* | ||
| Median (range) | 20.9 (18.1 to 45.4) | 43.3 (29.4 to 63.3) | |
| Sex | 0.0271* | ||
| Male | 3 | 12 | |
| Female | 10 | 5 | |
| Race | 0.0004* | ||
| Caucasian | 6 | 9 | |
| Asian | 7 | 0 | |
| African American | 0 | 7 | |
| Other | 0 | 1 | |
| Employment | <0.0001* | ||
| Student | 9 | 0 | |
| Medically disabled | 0 | 14 | |
| Part time | 3 | 2 | |
| Full time | 1 | 0 | |
| Retired | 0 | 1 | |
| Years in NC | 0.0003* | ||
| Median (range) | 14 (1 to 24) | 28.5 (3 to 49) | |
| Living area | |||
| Urban | 2 | 7 | 0.3105 |
| Suburban | 9 | 7 | |
| Rural | 2 | 3 | |
| SCZ severity scores | |||
| Q-LES | |||
| Percentage | 78.6 (57.1 to 96.4) | 69.6 (44.6 to 92.9) | 0.1799 |
| PANSS | |||
| Positive | 8.5 (7 to 18) | 18.5 (10 to 29) | <0.0001* |
| Negative | 9.5 (7 to 15) | 14 (10 to 23) | 0.0019* |
| General | 21 (16 to 29) | 33.5 (20 to 46) | 0.0003* |
| BACS | |||
| Composite | 0.739 (0.113 to 1.12) | −0.390 (−1.31 to 0.676) | <0.0001* |
| Reported exposures | |||
| Dog contact | |||
| No. of participants who currently own a dog | 6 | 4 | 0.3619 |
| Years participant has owned a dog (median, range) | 4 years (1 to 11) | 8 years (1 to 41) | 0.2887 |
| No. of participants bitten/scratched by dog | 7 | 6 | 0.5193 |
| Cat contact | |||
| No. of participants who currently own a cat | 4 | 3 | 0.6844 |
| Years participant has owned a cat (median, range) | 8.5 years (1 to 22) | 20 years (2.5 to 48) | 0.1325 |
| No. of participants bitten/scratched by cat | 9 | 7 | 0.2473 |
| Insect bites | |||
| Mosquitoes | 12 | 14 | 0.8003 |
| Bees | 8 | 11 | 1 |
| Ticks | 6 | 6 | 0.8215 |
| Fleas | 2 | 7 | 0.2603 |
| Spiders | 3 | 4 | 1 |
| Biting flies | 4 | 4 | 0.9778 |
| Bedbugs | 0 | 7 | 0.0273* |
| Lice | 3 | 1 | 0.406 |
Number of participants or median and range reported for each category. Median and range of severity scores based on three assessment tools for cases and controls. p Values for comparison between cases and controls, using chi-squared tests (or Fisher exact tests for small group numbers) for proportions and Wilcoxon rank sum test for continuous variables.
Statistical significant considered at p < 0.01 to correct for multiple comparisons for secondary outcome (severity scores), and at p < 0.05 for exploratory analysis (demographics and reported exposures).
BCAS, Brief Assessment of Cognition in Schizophrenia; PANSS, Positive and Negative Syndrome Scale; Q-LES, Quality of Life Enjoyment and Satisfaction Questionnaire; SCZ, schizophrenia.
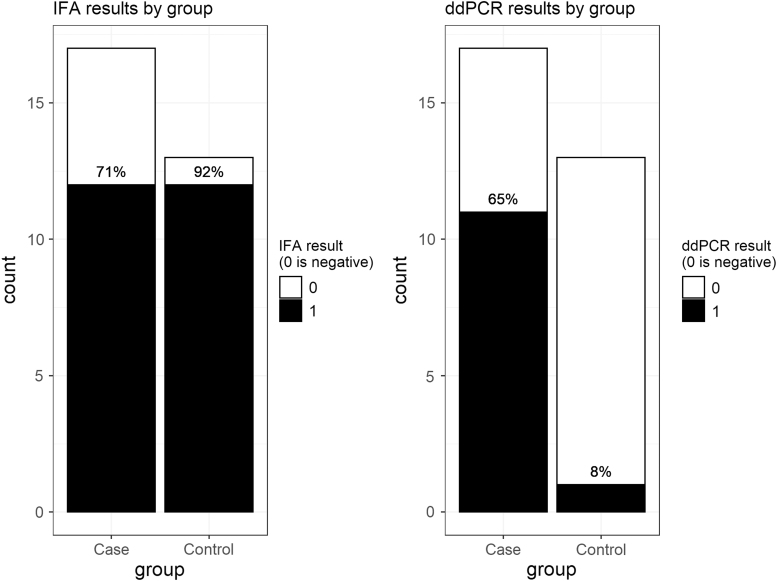
For the primary aim, Bartonella testing results are summarized in Fig. 1. Based on BAPGM/ddPCR testing, Bartonella spp. infection was significantly more common in cases (65%, 11/17) than controls (8%, 1/13; p = 0.0024). Of cases, 5 of 7 with schizoaffective disorder and 6 of 10 with schizophrenia were BAPGM/ddPCR positive. Based on IFA seroreactivity, Bartonella spp. exposure was not significantly different between cases and controls: 71% of cases and 92% of controls were seroreactive to one or more of the six Bartonella spp. antigens (p = 0.1961). Of cases, 4 of 7 with schizoaffective disorder and 8 of 10 with schizophrenia were seroreactive. BAPGM/qPCR failed to amplify Bartonella spp. DNA from any participant blood or enrichment culture sample.
FIG. 1.
Bartonella testing results. Left panel shows IFA results, right panel shows ddPCR results. No participant was positive on qPCR/BAPGM (not shown). Black, positive test result; white, negative test result. BAPGM, Bartonella alpha proteobacteria growth medium; ddPCR, droplet digital PCR; IFA, indirect fluorescent antibody; qPCR, quantitative PCR.
Of the 12 BAPGM/ddPCR-positive participants, 5 were positive only from whole blood and 7 were positive only after BAPGM enrichment blood culture. No participant was ddPCR positive both directly from whole blood and from BAPGM enrichment blood culture. Ten of the 12 BAPGM/ddPCR-positive participants only had one single positive test. One participant was ddPCR positive on BAPGM blood culture of their first blood sample at all three culture time points (7, 14, and 21 days of incubation); that participant's second blood sample was ddPCR negative. Another participant was ddPCR positive on BAPGM enrichment blood culture day 14 from both the first and second blood samples; blood and 7- and 21-day culture time points were ddPCR negative.
Based on IFA, B. henselae seroreactivity was the most common among both cases (65%) and controls (92%). Most seroreactive participants were reactive to more than one Bartonella species/strain. Bartonella spp. IFA results are reported in Table 2.
Table 2.
Bartonella spp. Indirect Fluorescent Antibody Results
| Control, n = 13 | Case, n = 17 | |
|---|---|---|
| Bh | 12 (92%) | 11 (65%) |
| Bk | 7 (54%) | 7 (41%) |
| Bq | 6 (46%) | 7 (41%) |
| Bvb I | 2 (15%) | 3 (18%) |
| Bvb II | 7 (54%) | 4 (24%) |
| Bvb III | 9 (69%) | 7 (41%) |
Number (and percentage) of participants in each group who were seroreactive against each Bartonella species/genotype antigen tested.
Bh, Bartonella henselae; Bk, Bartonella koehlerae; Bq, Bartonella quintana; Bvb I, Bartonella vinsonii subsp. berkhoffii genotype I; Bvb II, Bartonella vinsonii subsp. berkhoffii genotype II; Bvb III, Bartonella vinsonii subsp. berkhoffii genotype III.
Clinical assessment test results for cases and controls are shown in Table 1. As expected, cases had significantly lower BACS composite scores, and significantly higher PANSS scores in each of the three primary categories (positive, negative, and general). There was no significant difference in quality of life between cases and controls as measured by the Q-LES. Among cases, there was no significant difference in any of the severity scores between BAPGM/ddPCR-positive and BAPGM/ddPCR-negative individuals. There were three participants with schizophrenia receiving medications previously reported to have activity against T. gondii (fluphenazine and loxapine) (Fond et al. 2014, Neville et al. 2015): all three participants were Bartonella spp. seroreactive, and two were also positive by Bartonella spp. BAPGM/ddPCR.
Animal and insect exposures are reported in Table 1. There were no significant differences in dog or cat ownership or reports of dog or cat bites between cases and controls. A significantly higher proportion of cases (41%) reported exposure to bedbugs compared to controls (0, p = 0.0273). Although a higher proportion of cases (41%) reported flea exposure compared to controls (15%), this difference was not statistically significant. There were no other significant differences in reported exposure to potential insect vectors between cases and controls. There were no significant differences in cat or dog ownership, reports of cat or dog bites, or exposure to potential insect vectors between seropositive or BAPGM/ddPCR-positive versus seronegative or BAPGM/ddPCR-negative participants.
Based on the health history questionnaire, the most commonly reported symptoms differed between cases and controls (Table 3). Cases reported a significantly higher number of symptoms (median 8, range 1–24) compared to controls (median 0, range 0–3; p < 0.0001).
Table 3.
Reported Health Symptoms
| Control, n = 13 | Case, n = 17 | ||
|---|---|---|---|
| Sleepiness | 3 | Hallucinations | 13 |
| Fatigue | 1 | Difficulty remembering | 10 |
| Headache | 1 | Mental confusion | 10 |
| Difficulty sleeping | 1 | Weight gain | 9 |
| N/A | Depression | 8 | |
Most common symptoms reported on the health history questionnaire, by controls and cases.
Paired Bartonella testing results were missing for six cases for IFA and seven cases for BAPGM/ddPCR. All controls had paired blood testing results available. Comparisons of Bartonella test results between paired samples are reported in Table 4. For IFA, paired (first and second time point for same participant) results showed substantial agreement (kappa = 0.71). For the three participants whose paired samples did not match, all three were positive solely to B. henselae on the first sample (with titers of 1:64, 1:64, and 1:128) and negative to all antigens on the second sample (with B. henselae titers all 1:32). In contrast to serology results, paired results for BAPGM/ddPCR showed agreement slightly less than chance alone (kappa = −0.01). When comparing IFA to BAPGM/ddPCR results for the same participant, agreement was also less than chance alone (kappa = −0.31).
Table 4.
Paired Bartonella Test Results
| All participants | Kappa | |
|---|---|---|
| IFA test/retest | n = 24 | 0.71 |
| Positive/positive | 15 | |
| Positive/negative | 3 | |
| Negative/positive | 0 | |
| Negative/negative | 6 | |
| ddPCR test/retest | n = 23 | −0.01 |
| Positive/positive | 1 | |
| Positive/negative | 5 | |
| Negative/positive | 3 | |
| Negative/negative | 14 | |
| ddPCR/IFA | n = 30 | −0.31 |
| Positive/positive | 7 | |
| Positive/negative | 5 | |
| Negative/positive | 17 | |
| Negative/negative | 1 |
Agreement between paired samples from all participants (drawn between 2 and 8 days apart) for IFA and ddPCR, and agreement between IFA and ddPCR for each participant. Cohen's kappa score indicates agreement between tests.
ddPCR, droplet digital PCR; IFA, indirect fluorescent antibody.
Discussion
People with SCZ/SAD were more likely than healthy volunteers to be infected with Bartonella spp. based on BAPGM/ddPCR evidence: 11 of 17 cases (65%) were Bartonella spp. BAPGM/ddPCR positive compared to only one healthy volunteer (8%). A surprisingly high proportion of both cases (12/17) and healthy volunteers (12/13) were seroreactive to one or more of the six Bartonella spp. IFA antigens.
Paired Bartonella spp. IFA testing, with samples drawn within 1 week, had substantial agreement for both cases and controls, indicating that most seropositive participants likely had previous exposures leading to relatively stable antibody titers. In contrast, paired BAPGM/ddPCR results had very poor agreement, potentially due to relapsing or intermittent bacteremia as documented in human B. quintana infection (Byam et al. 1867, Drancourt et al. 1995; Stein and Raoult 1995; Brouqui et al. 1999), and other Bartonella spp. in cats (Kordick and Breitschwerdt 1997) and rodents (Morick et al. 2013, Goodrich et al. 2020). IFA and BAPGM/ddPCR results often did not agree, potentially indicating the possibility that Bartonella antibodies suppress the number of circulating bacteria below the level of BAPGM/ddPCR detection (Rodriguez-Barradas et al. 1995, Koesling et al. 2001).
Although Bartonella spp. DNA was not amplified by BAPGM/qPCR, the use of ddPCR increased sensitivity for low-concentration pathogen DNA detection, as expected. By instrumentation design, DNA amplification sensitivity of ddPCR is increased by massively partitioning the sample (15,000–20,000 1 nL droplets per sample) (Dong et al. 2015, Gutiérrez-Aguirre et al. 2015) and by sequestering target DNA among individual droplets to reduce the impact of inhibitory substances that compete with low-concentration pathogen DNA during amplification (Dingle et al. 2013, Maggi et al. 2020). While ddPCR allows increased sensitivity for detection of pathogen DNA compared to qPCR, unlike qPCR, it does not allow for subsequent confirmation of pathogen species identity using Sanger sequencing. Despite improved sensitivity, enrichment blood culture (BAPGM) was necessary to confirm infection in over half (7/12) of infected participants even with ddPCR.
There were no significant differences in animal exposures between cases and controls. While cat ownership has been previously implicated as a risk factor for schizophrenia (Kolpakova and Bedwell 2013, Fuller Torrey et al. 2015, Divya and Rajajeyakumar 2019), cat ownership was not associated with schizophrenia in this study. Of relevance to Bartonella transmission, bites or scratches from a cat, as well as exposure to fleas, were commonly reported among both cases and controls. These and other unknown factors may have contributed to the high Bartonella spp. seroprevalence in this study.
Previous studies of healthy control populations have found wide ranges of seroreactivity, depending on the number of Bartonella spp. antigens tested, the cutoff titer utilized to define a seroreactive result, and the population under study. At the high end, Bartonella spp. seroreactivity was documented in 83% of 100 participants, using the same six-antigen panel used in this study, when enrolling healthy volunteers from among workers at the Center of Biomedical Research (including the Center of Rickettsiosis and Arthropod-borne Diseases) and San Pedro's University Hospital at La Rioja, in northern Spain (Portillo et al. 2020). In contrast, early studies (now over 20 years old) using one or two antigens to test blood donors documented seroreactivity as low as 3–7% (Regnery et al. 1992, Dalton et al. 1995). More recently, sampling from diverse populations have reported seroreactivity anywhere from 3%, in a convenience sample at Duke University Medical Center (Durham, NC) (Lantos et al. 2014), to 32% of 500 blood donors in Campinas, a large city in southeastern Brazil, using IFA serology performed at the Centers for Disease Control and Prevention (Atlanta, GA) (Pitassi et al. 2015). The serological results reported in this pilot study emphasize the importance of sampling from an appropriate control population with similar exposures to the case population in a case–control study.
While drawing from the same geographic region, the cases and controls in this study had multiple significant differences, including age, race, employment status, and years living in North Carolina. The cases were significantly older than the controls, reflecting the fact that participants who enroll in research studies at medical centers are typically stable and often have been diagnosed years before study enrollment. This and other demographic and lifestyle factors were possible confounders, and could not be adjusted for in analysis due to the small sample size for this pilot study. Future epidemiological studies investigating a possible association between Bartonella spp. infection and schizophrenia should either use a matched design or have a sample size large enough to control for confounders in the statistical analysis.
We cannot exclude the possibility that the difference in Bartonella infection between cases and controls was due to secondary processes unrelated to the cause of their illness. There are known differences in immune system function in patients with schizophrenia, which may lead to a higher risk of chronic infection, (Müller and Schwarz 2010), and infection with Bartonella spp. itself may have long-term effects on the immune system (Pons et al. 2017). While no conclusions regarding a causal role for Bartonella in schizophrenia spectrum disorders can be inferred from the association found in this small study, considering whether Bartonella has the potential to contribute to the pathogenesis of schizophrenia is worthwhile. Bartonella infections can persist in cats and dogs for years (Kordick and Breitschwerdt 1998), and there is evidence for long-standing infection with Bartonella spp. (including B. henselae) in humans as well (Breitschwerdt et al. 2010a, 2019b). It is possible that pathology could be directly caused by the organism itself during chronic infection, as it has been shown to infect feline microglial cells in vitro (Muñana et al. 2001). Bartonella spp. can also induce encephalitis and microvascular injury, or could precipitate an autoimmune process, any of which could contribute to pathogenesis of neuropsychiatric symptoms (Scheidegger et al. 2011, Fan and Ali 2020, Tsukamoto et al. 2020).
Among participants with SCZ/SAD, Bartonella spp. infection was not associated with more severe symptoms. However, this study included a small sample size (17 people with schizophrenia), so it was likely underpowered to detect such differences. Based on previous studies of healthy volunteers, we estimated that 15% or less of the control group would have Bartonella spp. exposure (Lantos et al. 2014, Pitassi et al. 2015). This sample size therefore gave us 80% power at alpha <0.05 to detect a statistically significant difference in Bartonella infection between the cases and controls if <15% of controls and more than 70% of the cases were infected, but was not powered for the secondary aim. Funding to investigate a larger sample size will be needed to better understand whether Bartonella infection is associated with positive or negative symptoms, or differences in more specific domains of cognition and overall functional capacity.
In addition to small sample size, there are several limitations to the interpretation of the results of this study. The specificity of Bartonella spp. IFA has been questioned due to the potential for cross-reactivity with other bacterial organisms—mainly Coxiella spp., and other Rickettsia-like pathogens, including Ehrlichia and Chlamydia species (La Scola and Raoult 1996, Maurin et al. 1997, Graham et al. 2000, Vermeulen et al. 2010). However epidemiologic studies do not consistently support the existence of cross-reactivity, but rather suggest that at least in some cases, presumed “cross-reactivity” is due to prior exposure to multiple bacterial pathogens and that Bartonella IFA specificity is high (Lantos et al. 2014, Noden et al. 2014, Oteo et al. 2017). In contrast, Bartonella IFA sensitivity is often poor (Breitschwerdt et al. 2007, Maggi et al. 2012, Yanagihara et al. 2018). When positive, serology can only provide evidence for previous exposure; when negative, serology does not rule out prior or current infection. Low sensitivity was also a concern for BAPGM/qPCR, as evidenced by no participant being BAPGM/qPCR positive despite multiple participants being positive by BAPGM/ddPCR (Oteo et al. 2017). Unfortunately, the droplets generated during ddPCR cannot be concentrated for DNA sequencing, so it is impossible to determine the Bartonella species detected by ddPCR in these participants. To increase the likelihood of Bartonella DNA detection, two blood samples were to be collected from each participant. However, paired samples were unable to be obtained from 7 of the 17 cases, either because they did not return for their second visit or because of difficult blood draws; in contrast, all controls had both blood samples available. This difference could lead to bias, since fewer samples for cases were tested than controls; however, this would bias the results toward the null hypothesis and lead to an underestimate of the difference between cases and controls.
Conclusion
While no conclusion regarding a causal role for Bartonella in SCZ/SAD can be drawn from this study, the results do support the need for a more in-depth examination of the epidemiology of Bartonella infection in people with schizophrenia. Large, well-funded follow-up studies that control for confounding variables could determine if any potentially observed effect is replicable across sample populations. If the epidemiological association between Bartonella infection and SCZ/SAD is replicated, studies could be designed to test whether Bartonella-targeted antimicrobial therapy can ameliorate one or more domains of schizophrenia symptoms and determine if Bartonella infection contributes to the pathogenesis of schizophrenia in a subset of affected individuals.
Data Availability Statement
All relevant data are available from Dryad at https://doi.org/10.5061/dryad.95x69p8jz
Supplementary Material
Acknowledgments
We thank Alana (Katie) Atkins, Kaelin Kennedy, Tonya Elliott, and Toni Richardson for their help with recruitment, data collection, and sample processing for this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authors' Contributions
E.L.: study conception and design, analysis and interpretation of data, and writing and revision of article. R.M.: study conception, acquisition and interpretation of data, and revision of article. L.F.J.: study design, acquisition and interpretation of data, and revision of article. J.B.: acquisition and interpretation of data, and revision of article. E.B.: study conception and design, interpretation of data, and revision of article. F.F.: study conception and design, acquisition, analysis and interpretation of data, and revision of article.
Author Disclosure Statement
In conjunction with Dr. Sushama Sontakke and North Carolina State University, E.B. holds U.S. Patent number 7,115,385: “Media and Methods for Cultivation of Microorganisms,” which was issued October 3, 2006. He is a co-founder, shareholder, and Chief Scientific Officer for Galaxy Diagnostics, a company that provides advanced diagnostic testing for the detection of Bartonella species infections. F.F. is founder, shareholder, and Chief Scientific Officer of Pulvinar Neuro, a neurotechnology company unrelated to the work presented in this study. In the last 12 months, F.F. has received honoraria from Sage Therapeutics, Strategic Innovation, Insel Spital, and Elsevier. All other authors declare no potential conflicts of interest.
Funding Information
The project described was partially supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through grant award number UL1TR002489. E.L.'s research was supported in part by the Comparative Medicine and Translational Research Program of the National Institutes of Health under award number T32OD011130. Microbiological testing was supported by the North Carolina State University College of Veterinary Medicine Bartonella/Vector Borne Disease Research Foundation Fund. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Supplementary Material
References
- Breitschwerdt EB, Greenberg R, Maggi RG, Mozayeni BR, et al. . Bartonella henselae bloodstream infection in a boy with Pediatric Acute-Onset Neuropsychiatric Syndrome. J Cent Nerv Syst Dis 2019a; 11:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Duncan AW, Nicholson WL, et al. . Bartonella species in blood of immunocompetent persons with animal and arthropod contact. Emerg Infect Dis 2007; 13:938–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Farmer P, Mascarelli PE. Molecular evidence of perinatal transmission of Bartonella vinsonii subsp. berkhoffii and Bartonella henselae to a child. J Clin Microbiol 2010a; 48:2289–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Lantos PM, Woods CW, et al. . Bartonella vinsonii subsp. berkhoffii and Bartonella henselae bacteremia in a father and daughter with neurological disease. Parasit Vectors 2010b; 3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Nicholson WL, Cherry NA, et al. . Bartonella sp. bacteremia in patients with neurological and neurocognitive dysfunction. J Clin Microbiol 2008; 46:2856–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Maggi RG, Quach C, Bradley JM. Bartonella spp. bloodstream infection in a Canadian family. Vector Borne Zoonotic Dis 2019b; 19:234–241 [DOI] [PubMed] [Google Scholar]
- Breitschwerdt EB, Mascarelli PE, Schweickert LA, Maggi RG, et al. . Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae. J Clin Microbiol 2011; 49:3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitschwerdt EB, Sontakke S, Hopkins S. Neurological manifestations of bartonellosis in immunocompetent patients: a composite of reports from 2005-2012. J Neuroparasitology 2012; 3:1–15 [Google Scholar]
- Brouqui P, Lascola B, Roux V, Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med 1999; 340:184–189 [DOI] [PubMed] [Google Scholar]
- Byam W, Carroll JH, Churchill JH, et al. . Trench Fever, a Louse-Borne Disease, 1st edition. Preston: Halewood:ABA:ILAB: Booksellers, 1867. [Google Scholar]
- Canneti B, Cabo-López I, Puy-Núñez A, García García JC, et al. . Neurological presentations of Bartonella henselae infection. Neurol Sci 2019; 40:261–268 [DOI] [PubMed] [Google Scholar]
- Cheslock MA, Embers ME. Human bartonellosis: an underappreciated public health problem? Trop Med Infect Dis 2019; 4:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton MJ, Robinson LE, Cooper J, Regnery RL, et al. . Use of bartonella antigens for serologic diagnosis of cat-scratch disease at a National Referral Center. Arch Intern Med 1995; 155:1670–1676 [PubMed] [Google Scholar]
- Dingle TC, Sedlak RH, Cook L, Jerome KR. Tolerance of droplet-digital PCR vs real-time quantitative PCR to inhibitory substances. Clin Chem 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divya R, Rajajeyakumar M. Can cats cause schizophrenia? An insight into the role of Bartonella henselae in neuropsychiatric disorders. Acta Sci Pharm Sci 2019; 3:110–110 [Google Scholar]
- Dong L, Meng Y, Sui Z, Wang J, et al. . Comparison of four digital PCR platforms for accurate quantification of DNA copy number of a certified plasmid DNA reference material. Sci Rep 2015; 5:13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drancourt M, Carta A, Raoult D, Mainardi JL, et al. . Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med 1995; 332:419–423 [DOI] [PubMed] [Google Scholar]
- Endres D, Leypoldt F, Bechter K, Hasan A, et al. . Autoimmune encephalitis as a differential diagnosis of schizophreniform psychosis: clinical symptomatology, pathophysiology, diagnostic approach, and therapeutic considerations. Eur Arch Psychiatry Clin Neurosci 2020a; 270:803–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres D, Maier V, Leypoldt F, Wandinger K-P, et al. . Autoantibody-associated psychiatric syndromes: a systematic literature review resulting in 145 cases. Psychol Med 2020b; 7:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Ali H. Cat scratch disease causing encephalitis. Baylor Univ Med Cent Proc 2020; 33:440–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fond G, Macgregor A, Tamouza R, Hamdani N, et al. . Comparative analysis of anti-toxoplasmic activity of antipsychotic drugs and valproate. Eur Arch Psychiatry Clin Neurosci 2014; 264:179–183 [DOI] [PubMed] [Google Scholar]
- Fuglewicz AJ, Piotrowski P, Stodolak A. Relationship between toxoplasmosis and schizophrenia: a review. Adv Clin Exp Med 2017; 26:1031–1036 [DOI] [PubMed] [Google Scholar]
- Fuller Torrey E, Simmons W, Yolken RH. Is childhood cat ownership a risk factor for schizophrenia later in life? Schizophr Res 2015; 165:1–2 [DOI] [PubMed] [Google Scholar]
- Goodrich I, McKee C, Kosoy M. Longitudinal study of bacterial infectious agents in a community of small mammals in New Mexico. Vector Borne Zoonotic Dis 2020; 20:496–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JV, Baden L, Tsiodras S, Karchmer AW. Q fever endocarditis associated with extensive serological cross-reactivity. Clin Infect Dis 2000; 30:609–610 [DOI] [PubMed] [Google Scholar]
- Gutiérrez-Aguirre I, Rački N, Dreo T, Ravnikar M. Droplet digital PCR for absolute quantification of pathogens. Methods Mol Biol 2015; 1302:331–347 [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res 1998; 23:99–110 [DOI] [PubMed] [Google Scholar]
- Keefe RSE, Goldberg TE, Harvey PD, Gold JM, et al. . The Brief Assessment of Cognition in Schizophrenia: reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophr Res 2004; 68:283–297 [DOI] [PubMed] [Google Scholar]
- Koesling J, Aebischer T, Falch C, Schülein R, et al. . Cutting edge: antibody-mediated cessation of hemotropic infection by the intraerythrocytic mouse pathogen Bartonella grahamii. J Immunol 2001; 167:11–14 [DOI] [PubMed] [Google Scholar]
- Köhler-Forsberg O, Petersen L, Gasse C, Mortensen PB, et al. . A nationwide study in Denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents. JAMA Psychiatry 2019; 76:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpakova J, Bedwell JS. Childhood cat bites and disorganized symptoms of schizotypy in adulthood. Schizophr Res 2013; 146:370–371 [DOI] [PubMed] [Google Scholar]
- Kordick DL, Breitschwerdt EB. Relapsing bacteremia after blood transmission of Bartonella henselae to cats. Am J Vet Res 1997; 58:492–497 [PubMed] [Google Scholar]
- Kordick DL, Breitschwerdt EB. Persistent infection of pets within a household with three Bartonella species. Emerg Infect Dis 1998; 4:325–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scola B, Raoult D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol 1996; 34:2270–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantos PM, Maggi RG, Ferguson B, Varkey J, et al. . Detection of Bartonella species in the blood of veterinarians and veterinary technicians: a newly recognized occupational hazard? Vector Borne Zoonotic Dis 2014; 14:563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi RG, Mascarelli PE, Pultorak EL, Hegarty BC, et al. . Bartonella spp. bacteremia in high-risk immunocompetent patients. Diagn Microbiol Infect Dis 2011; 71:430–437 [DOI] [PubMed] [Google Scholar]
- Maggi RG, Mozayeni BR, Pultorak EL, Hegarty BC, et al. . Bartonella spp. bacteremia and rheumatic symptoms in patients from Lyme disease–endemic region. Emerg Infect Dis 2012; 18:783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi RG, Richardson T, Breitschwerdt EB, Miller JC. Development and validation of a droplet digital PCR assay for the detection and quantification of Bartonella species within human clinical samples. J Microbiol Methods 2020; 176:106022. [DOI] [PubMed] [Google Scholar]
- Mascarelli PE, Maggi RG, Hopkins S, Mozayeni BR, et al. . Bartonella henselae infection in a family experiencing neurological and neurocognitive abnormalities after woodlouse hunter spider bites. Parasit Vectors 2013; 6:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin M, Eb F, Etienne J, Raoult D. Serological cross-reactions between Bartonella and Chlamydia species: implications for diagnosis. J Clin Microbiol 1997; 35:2283–2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morick D, Krasnov BR, Khokhlova IS, Gottlieb Y, et al. . Transmission dynamics of Bartonella sp. strain OE 1-1 in Sundevall's jirds (Meriones crassus). Appl Environ Microbiol 2013; 79:1258–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller N, Schwarz MJ. Immune system and schizophrenia. Curr Immunol Rev 2010; 6:213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñana KR, Vitek SM, Hegarty BC, Kordick DL, et al. . Infection of fetal feline brain cells in culture with Bartonella henselae. Infect Immun 2001; 69:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden BH, Tshavuka FI, van der Colf BE, Chipare I, et al. . Exposure and risk factors to Coxiella burnetii, spotted fever group and typhus group rickettsiae, and Bartonella henselae among volunteer blood donors in Namibia. PLoS One 2014; 9:e108674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville AJ, Zach SJ, Wang X, Larson JJ, Judge AK, Davis LA, et al. . Clinically available medicines demonstrating anti-toxoplasma activity. Antimicrobial Agents and Chemotherapy. 2015; 59:7161–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteo JA, Maggi R, Portillo A, Bradley J, et al. . Prevalence of Bartonella spp. by culture, PCR and serology, in veterinary personnel from Spain. Parasit Vectors 2017; 10:553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitassi LHU, de Paiva Diniz PPV, Scorpio DG, Drummond MR, et al. . Bartonella spp. bacteremia in blood donors from Campinas, Brazil. PLoS Negl Trop Dis 2015; 9:e000; 3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons MJ, Gomes C, Aguilar R, Barrios D, et al. . Immunosuppressive and angiogenic cytokine profile associated with Bartonella bacilliformis infection in post-outbreak and endemic areas of Carrion's disease in Peru. PLoS Negl Trop Dis 2017; 11:e0005684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo A, Maggi R, Oteo JA, Bradley J, et al. . Bartonella spp. prevalence (serology, culture, and PCR) in sanitary workers in La Rioja Spain. Pathogens 2020; 9:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pultorak EL, Maggi RG, Mascarelli PE, Breitschwerdt EB. Serial testing from a 3-day collection period by use of the Bartonella Alphaproteobacteria growth medium platform may enhance the sensitivity of Bartonella species detection in bacteremic human patients. J Clin Microbiol 2013; 51:1673–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2019 [Google Scholar]
- Regnery RL, Olson JG, Perkins BA, Bibb W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet 1992; 339:1443–1445 [DOI] [PubMed] [Google Scholar]
- Relman DA, Loutit JS, Schmidt TM, Falkow S, et al. . The agent of bacillary angiomatosis. N Engl J Med 1990; 323:1573–1580 [DOI] [PubMed] [Google Scholar]
- Ritsner M, Kurs R, Gibel A, Ratner Y, et al. . Validity of an abbreviated Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q-18) for schizophrenia, schizoaffective, and mood disorder patients. Qual Life Res 2005; 14:1693–1703 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Barradas MC, Bandres JC, Hamill RJ, Trial J, et al. . In vitro evaluation of the role of humoral immunity against Bartonella henselae. Infect Immun 1995; 63:2367–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidegger F, Quebatte M, Mistl C, Dehio C. The Bartonella henselae VirB/Bep system interferes with vascular endothelial growth factor (VEGF) signalling in human vascular endothelial cells. Cell Microbiol 2011; 13:419–431 [DOI] [PubMed] [Google Scholar]
- Stein A, Raoult D. Return of trench fever. Lancet 1995; 345:P450–P451 [DOI] [PubMed] [Google Scholar]
- Sutterland AL, Fond G, Kuin A, Koeter MWJ, et al. . Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. Acta Psychiatr Scand 2015; 132:161–179 [DOI] [PubMed] [Google Scholar]
- Torrey EF, Yolken RH. Schizophrenia as a pseudogenetic disease: a call for more gene-environmental studies. Psychiatry Res 2019; 278:146–150 [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Shinzawa N, Kawai A, Suzuki M, et al. . The Bartonella autotransporter BafA activates the host VEGF pathway to drive angiogenesis. Nat Commun 2020; 11:3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbroucke JP, Von Elm E, Altman DG, Gøtzsche PC, et al. . Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med 2007; 4:1628–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen MJ, Verbakel H, Notermans DW, Reimerink JHJ, et al. . Evaluation of sensitivity, specificity and cross-reactivity in Bartonella henselae serology. J Med Microbiol 2010; 59:743–745 [DOI] [PubMed] [Google Scholar]
- Yanagihara M, Tsuneoka H, Tanimoto A, Otsuyama KI, et al. . Bartonella henselae DNA in seronegative patients with cat-scratch disease. Emerg Infect Dis 2018; 24:924–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken RH, Bachmann S, Rouslanova I, Lillehoj E, et al. . Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clin Infect Dis 2001; 32:842–844 [DOI] [PubMed] [Google Scholar]
- Zangwill KM, Hamilton DH, Perkins BA, Regnery RL, et al. . Cat scratch disease in Connecticut—epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med 1993; 329:8–13 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available from Dryad at https://doi.org/10.5061/dryad.95x69p8jz