Abstract
BACKGROUND:
To determine the effect of urologist and radiologist learning curves and changes in MRI-TRUS fusion platform during 9 years of NCI’s experience with multiparametric magnetic resonance imaging (mpMRI)/TRUS fusion biopsy.
METHODS:
A prospectively maintained database of patients undergoing mpMRI followed by fusion biopsy (Fbx) and systematic biopsy (Sbx) from 2007 to 2016 was reviewed. The patients were stratified based on the timing of first biopsy. Cohort 1 (7/2007 − 12/2010) accounted for learning curve. Cohort 2 (1/2011–5/2013) and cohort 3 (5/2013–4/2016) included patients biopsied prior to and after debut of a new software platform, respectively. Clinically significant (CS) disease was defined as Gleason 7 (3+4) or higher. McNemar’s test compared cancer detection rates (CDRs) of Sbx and Fbx between time periods.
RESULTS:
1528 patients were included in the study with 230, 537 and 761 patients included in three respective cohorts. Median age (interquartile range) was 61.0 (±9.0), 62.0 (±7.3), and 64.0 (±11.0) years in three cohorts, respectively (P < 0.001). Fbx and Sbx had comparable CS CDR in cohort 1 (24.8 vs 22.2%, P = 0.377). Fbx detected significantly more CS disease compared to Sbx in the following two periods (cohort 2: 31.5 vs 25.0%, P = 0.001; cohort 3: 36.4 vs 30.3%, P < 0.001) and detected significantly less low risk disease in the same period (cohort 2: 14.5 vs 19.6%, P < 0.001; cohort 3: 12.6 vs 16.7%, P < 0.001). Even after multivariate adjustment with age, PSA, race, clinical stage and MRI suspicion score, Fbx CS cancer detection increased in successive cohorts (cohort 2: OR 2.23, P = 0.043; cohort 3: OR 2.92, P = 0.007).
CONCLUSIONS:
In the past 9 years, there has been significant improvement in the accuracy of Fbx. Our results show that after an early learning period, Fbx detected higher rates of CS cancer and lower rates of clinically insignificant cancer than Sbx. Software advances allowed for even greater detection of CS disease.
INTRODUCTION
Prostate cancer (PCa) is the most common non-cutaneous cancer in men in the United States, and is the second most common cause of cancer-related deaths. The current gold standard of diagnosis involves a systematic 12-core TRUS guided biopsy (Sbx) of the prostate. However, this may miss up to 47% of tumors, and underdiagnose 38% of tumors when compared to whole mount prostatectomy specimens, making search of alternative biopsy methods desirable.1,2 In recent years, the addition of multiparametric MRI (mpMRI) as a diagnostic tool has allowed visualization of PCa where it may otherwise have been missed by Sbx.
MpMRI-TRUS fusion-guided biopsy (Fbx) has emerged as a possible alternative to Sbx, with early reports showing that Fbx may detect up to 30% more high risk cancers, and 17% fewer low risk cancer when compared to Sbx.3 However, outcomes of Fbx are not consistent across institutions.3–6 Reasons for this may include a steep learning curve associated with the reporting and interpretation of prostate MRI, operator experience in performing Fbx, and the type of software platform used for fusion guidance.7–9
At our institution, mpMRI was introduced as a supplement to Sbx in 2007. Over the following 10 years, Fbx evolved, incorporating multiple sequential software platforms meanwhile accumulating more collective imaging and surgical experience. The aim of the current study is to explore the effects of Fbx and a new software platform on the CDR at one institution over a 10-year experience.
METHODS
Patient selection
Data were collected prospectively on 1528 consecutive patients who underwent Fbx in addition to Sbx between June 2007 and April 2016. All patients were Fbx naive before their biopsies received as part of the current study. Only initial Fbx sessions on patients receiving multiple biopsies due to enrollment in active surveillance were included. We divided patient biopsies into three cohorts based on the year of biopsy. The first cohort consisted of patients who underwent their first biopsy between June 2007 and December 2010 (n = 230), presumably corresponding the learning curve for imaging and urology. The second cohort consisted of patients biopsied between January 2011 and May 2013 (n = 537), after more collective experience had been accumulated. The third consisted of patients between May 2013 and April 2016 (n = 761). In this cohort, patients benefitted from the introduction of a new software platform used in the application of Fbx (Uronav, Invivo, Gainesville, FL, USA), which improved the image calibration process, ultrasound tracker design, and MR-to-ultrasound registration software. In this cohort patients also benefitted from additional accumulated experience among the urologists and radiologists.
Pathology assessment from each patient was used for analysis. For this study, clinically significant (CS) PCa was defined as a tumor containing Gleason 3+4 or higher PCa, and clinically insignificant was defined as Gleason 3+3. Biopsy and whole-mount specimens were assigned risk categories of low, intermediate or high according to maximum Gleason score detected in the sample (6, 7, or 8–10, respectively). Risk category upgrade was defined as a risk category found on whole-mount pathology greater than the risk category detected from the patient’s biopsy. Gleason score upgrade was defined as a higher maximum Gleason score detected from whole-mount pathology than the maximum Gleason score detected from biopsy. Imaging interpretation data were recorded for the purposes of monitoring changes in the number of biopsy targets assigned by radiologists over time.
Data collection and inclusion criteria
A retrospective review of prospectively acquired data was performed. Patient demographic information included age, prostate specific antigen (PSA), race, and clinical stage for each patient. Indications for mpMRI and biopsy were an elevated PSA level or abnormal digital rectal exam. The proportion of patients with clinical stage >cT1c was not significantly different between the three cohorts. All patients underwent mpMRI interpreted by two highly experienced genitourinary radiologists prior to Fbx. Criteria for Fbx was presence of visible lesions on prostate mpMRI suspicions for PCa and stayed the same throughout the study time period. Lesions on mpMRI were then segmented and recorded (DynaCAD, Invivo). Patients with suspicious lesions on mpMRI then underwent Fbx performed by a single urologist under previously described Fbx protocols.3
Multiparametric magnetic resonance imaging
All patients underwent prostate MRI on a 3.0 T (Achieva, Philips Healthcare, Andover, MA, USA) scanner using an endorectal coil (BPX-30, Medrad®) and a 16-channel surface coil (SENSE, Philips Healthcare) in accordance with previously described protocols.10,11 Sequences obtained included triplanar T2-weighted, axial dynamic contrast-enhanced, and axial diffusion weighted imaging with apparent diffusion coefficient mapping and high B-value images. The MRI protocol used was the same throughout the duration of the study period for T2-weighted, apparent diffusion coefficient mapping and dynamic contrast-enhanced images. High B-value imaging parameters were added to the protocol in December 2011. All suspicious areas on mpMRI were given suspicion scores of low, moderate or high on a Likert scale; PI-RADS criteria were not employed as the study began before these criteria were created.2,12,13
Statistical evaluation
Statistical analysis was performed using SPSS version 21 (Chicago, IL, USA). Overall and CS CDRs along with 95% confidence intervals for CS CDR were calculated separately for each cohort. To control for age and PSA differences between different cohorts, age and PSA adjusted rates were calculated. χ2-test was used to compare biopsy results between different cohorts, and McNemar test was used to compare Fbx results to Sbx results in patients from the same cohort. Continuous parameters between cohorts were compared with Kruskal–Wallis Test. Age and PSA adjustments were performed using a previously described technique of indirect standardization.14 Multivariate logistic regression was used to assess associations of clinical and imaging criteria on detection of CS cancer on biopsy.
RESULTS
Demographics
In total, 1528 patients met our inclusion criteria. Patient demographic, clinical and biopsy data are shown in Table 1. In the entire cohort, the median age with IQR, and PSA were 61.0 years (11.3) and 6.1 ng ml − 1 (5.7), respectively. The median age (IQR) increased in each successive cohort (61.0 years (9.0) vs 62.0 years (7.3) vs 64.0 years (11.0) in cohorts 1, 2 and 3, respectively; P < 0.001). The median PSA differed between cohorts (6.1 ng ml − 1 (5.7), 6.9 ng ml − 1 (6.57), 6.5 ng ml − 1 (5.6) in cohorts 1, 2 and 3, respectively; P = 0.03). There was no difference in race or clinical stage between cohorts. The median (IQR) number of Fbx targets assigned by radiologists decreased over each successive cohort (6.0 (4.0) vs 5.0 (2.0) vs 4.0 (4.0) lesions in cohorts 1, 2 and 3, respectively; P < 0.001).
Table 1.
Patient demographics, clinical characteristics, and biopsy results for patients who underwent mpMRI followed by mpMRI-TRUS fusion-guided biopsy and systematic 12-core biopsy, separated into three cohorts
| Cohort 1 | Cohort 2 | Cohort 3 | Total | P-value | |
|---|---|---|---|---|---|
| Patients, n (%) | 230 (15.1) | 537 (35.1) | 761 (49.8) | 1528 | |
| Age, years, median (IQR) | 61.0 (11.3) | 62.0 (9.0) | 64.0 (11.0) | 63.0 (10.0) | < 0.001 |
| Race, n (%) | 0.33 | ||||
| White | 167 (72.6) | 430 (80.0) | 590 (77.5) | 1187 (79.1) | |
| Black | 34 (14.8) | 88 (16.4) | 116 (15.2) | 238 (15.9) | |
| Other | 29 (12.6) | 19 (3.5) | 55 (7.2) | 103 (6.7) | |
| PSA, ng ml−1, median (IQR) | 6.1 (5.7) | 6.9 (6.57) | 6.5 (5.6) | 6.5 (5.8) | 0.03 |
| Clinical Stage, n (%) | 0.57 | ||||
| cTIc | 46 (86.8) | 489 (91.2) | 685 (90.2) | 1220 (90.8) | |
| >T2a | 7 (13.2) | 47 (8.8) | 70 (9.3) | 124 (9.3) | |
| Fbx targets, median (IQR) | 6 (4) | 5 (2) | 4 (4) | 4(4) | < 0.001 |
| Highest MRI suspicion score, n (%) | |||||
| Low to moderate | 188 (81.7) | 434 (80.8) | 582 (76.5) | 1204 (78.8) | |
| Moderate-high to high | 42 (18.3) | 103 (19.2) | 179 (23.5) | 324 (21.2) | 0.122 |
| Systematic 12-core biopsy | |||||
| Gleason score, n (%) | 0.009 | ||||
| Benign | 126 (54.8) | 288 (53.6) | 382 (71.1) | 796 (52.1) | |
| 6 | 53 (23.0) | 115 (21.4) | 149 (19.7) | 317 (20.8) | |
| 7 | 32 (13.9) | 76 (14.2) | 161 (21.2) | 269 (17.6) | |
| >8 | 19 (8.3) | 58 (10.8) | 69 (9.1) | 144 (9.4) | |
| mpMRI-TRUS fusion-guided biopsy | |||||
| Gleason score, n (%) | < 0.001 | ||||
| Benign | 128 (55.7) | 290 (54.0) | 388 (51.0) | 806 (52.8) | |
| 6 | 45 (19.5) | 78 (14.5) | 96 (12.6) | 219 (14.3) | |
| 7 | 34 (14.8) | 83 (15.5) | 181 (23.8) | 298 (19.4) | |
| >8 | 23 (10) | 86 (16.0) | 96 (12.6) | 205 (13.4) | |
| CS PCa, n (%) | 69 (30.1) | 197 (36.8) | 332 (43.7) | 600 (39.3) | < 0.001 |
Abbreviations: IQR, interquartile range; MRI, magnetic resonance imaging; mpMRI, multiparametric MRI; PCa, prostate cancer.
Biopsy results
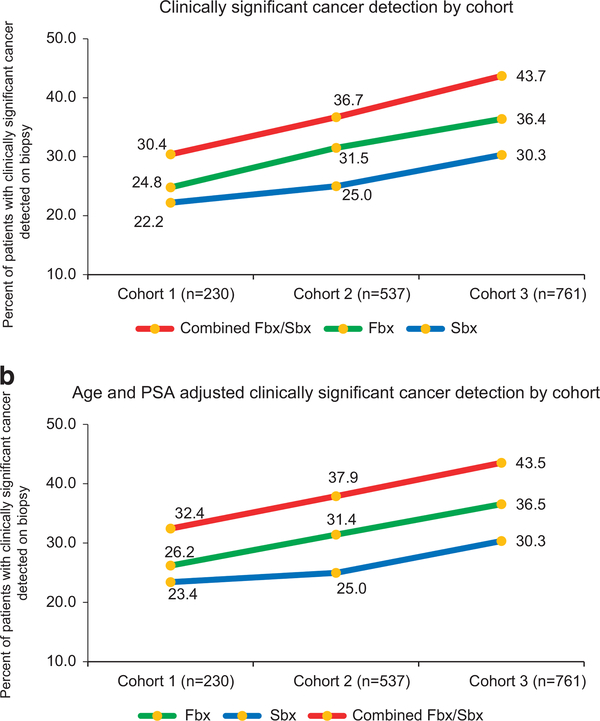
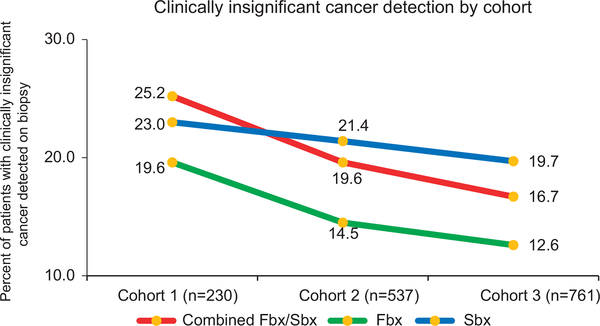
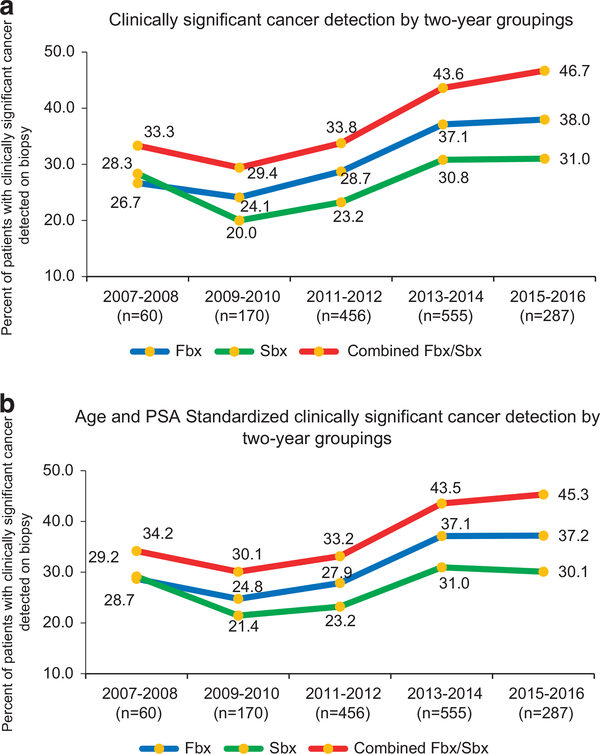
There was a significant increase (P < 0.001) in detection of CS PCa by Fbx with each successive cohort: 24.8% (57/230) in cohort 1, 31.5% (169/537) in cohort 2 and 36.4% (277/761) in cohort 3 (Figure 1a). Age and PSA adjusted CDR of CS disease by Fbx showed similar trends with significant increases in each successive cohort (cohort 2 vs 1: increase by 5.2%, 95% CI (2.1–8.5), P = 0.001), 3 vs 2 (increase by 5.2%, 95% CI (1.8–8.6), P = 0.003; Figure 1b). There was a decrease in the detection of clinically insignificant cancer by Fbx between each successive cohort (19.6% (45/230), 14.5% (78/537) and 12.6% (96/761); P < 0.001 in cohorts 1, 2 and 3, respectively; Figure 2). Fbx, on average, detected 2.6% more CS cancer, and 3.4% fewer clinically insignificant disease when compared to Sbx in cohort 1 (CS: 24.8% of Fbx vs 22.2% of Sbx P = 0.377; clinically insignificant: 19.6% of Fbx similar to in 23.0% of Sbx). After cohort 1; however, Fbx detected CS cancer at rates significantly higher than Sbx (31.5 vs 25.0% in cohort 2 (P < 0.001) and 36.4 vs 30.3% in cohort 3 (P < 0.001)), and clinically insignificant cancer at rates significantly lower than Sbx (14.5 vs 21.4% in cohort 2 (P < 0.001) and 12.6 vs 19.7% in cohort 3 (P < 0.001)). Figure 3 demonstrates crude as well as age and PSA adjusted CDRs in subcohorts each spanning 2 years.
Figure 1.
(a) Rates of clinically significant prostate cancer detection over the course of 10-year experience, comparing trends from mpMRI-TRUS fusion-guided biopsy, systematic 12-core biopsy, and combined mpMRI-TRUS fusion-guided biopsy/systematic 12-core biopsy. Patients were stratified by date of biopsy into corresponding time cohort. There was a significant increase (P < 0.001) in detection of clinically significant prostate cancer by mpMRI-TRUS fusion-guided biopsy with each successive cohort: 24.8% (57/230) in cohort 1, 31.5% (169/537) in cohort 2 and 36.4% (277/761) in cohort 3 (χ2-test). (b) Age and PSA adjusted rates of clinically significant prostate cancer detection over the course of 10-year experience, comparing trends from mpMRI-TRUS fusion-guided biopsy, systematic 12-core biopsy and combined mpMRI-TRUS fusion-guided biopsy/systematic 12-core biopsy. Cancer detection rate of mpMRI-TRUS fusion-guided biopsy demonstrated significant increases in each successive cohort (cohort 2 vs 1: increase by 5.2%, 95% CI (2.1–8.5), P = 0.001), 3 vs 2 (increase by 5.2%, 95% CI (1.8–8.6), P = 0.003).
Figure 2.
Rates of clinically insignificant cancer detection over the course of 10-year experience, comparing trends from mpMRI-TRUS fusion-guided biopsy, systematic 12-core biopsy and combined mpMRI-TRUS fusion-guided biopsy/systematic 12-core biopsy. Patients were stratified by date of biopsy into corresponding time cohort. There was a decrease in the detection of clinically insignificant cancer by mpMRI-TRUS fusion-guided biopsy between each cohort (19.6% (45/230), 14.5% (78/537) and 12.6% (96/761); P < 0.001 in cohorts 1, 2 and 3, respectively (χ2-test).
Figure 3.
(a) Rates of clinically significant cancer detection over the course of 10-year experience, comparing trends from mpMRI-TRUS fusion-guided biopsy, systematic 12-core biopsy and combined mpMRI-TRUS fusion-guided biopsy/systematic 12-core biopsy. Patients were stratified by date of biopsy into corresponding 2 year time cohort. (b) Age and PSA adjusted rates of CS prostate cancer detection over the course of 10-year experience, comparing trends from mpMRI-TRUS fusion-guided biopsy, systematic 12-core biopsy and combined mpMRI-TRUS fusion-guided biopsy/systematic 12-core biopsy. Patients were stratified by date of biopsy into corresponding 2 year time cohort.
On multivariate analysis, after adjustment for age, PSA, clinical stage, race and MRI suspicion score; inclusion in cohort 2 (OR 2.232, 95% CI 1.026–4.859, P = 0.043) and cohort 3 (OR 2.918, 95% CI 1.348–6.317, P = 0.007) remained independent predictors of CS cancer detection on Fbx (Table 2). On the other hand, similar multivariate analysis for CS cancer detection by Sbx demonstrate that inclusion in cohorts 2 and 3 were not significant predictors, implying that cancer detection by Fbx alone significantly increased in successive cohorts while the increase in Sbx CS cancer detection was not statistically significant (cohort 2: OR 1.65, 95% CI 0.765–3.548, P = 0.11 and cohort 3: OR 2.015, 95% CI 0.942–4.306, P = 0.071).
Table 2.
Multivariate analysis used to assess the relationship between clinical and imaging variables on CDRs of clinically significant cancer on biopsy
| Factors |
Systematic biopsy |
Fusion biopsy |
||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Age | 1.049 | 1.028–1.070 | < 0.001 | 1.032 | 1.012–1.053 | 0.002 |
| PSA | 1.002 | 0.993–1.012 | 0.654 | 1.036 | 1.019–1.054 | < 0.001 |
| Clinical stage | 0.01 | |||||
| T1c | Ref | Ref | ||||
| >T1c | 2.997 | 1.721–5.221 | < 0.001 | 2.242 | 1.213–4.144 | 0.01 |
| Race | ||||||
| White | Ref | Ref | ||||
| Black | 1.692 | 1.183–2.420 | 0.004 | 1.31 | 0.900–1.906 | 0.158 |
| Other | 0.739 | 0.385–1.420 | 0.364 | 0.898 | 0.477–1.694 | 0.741 |
| Highest MRI suspicion score | ||||||
| Low to moderate | Ref | Ref | ||||
| Moderate-high to high | 4.525 | 3.350–6.111 | < 0.001 | 9.451 | 6.840–13.060 | < 0.001 |
| Cohorts | ||||||
| 1 (6/2007–12/2010) | Ref | Ref | ||||
| 2 (1/2011–5/2013) | 1.65 | 0.765–3.548 | 0.202 | 2.232 | 1.026–4.859 | 0.043 |
| 3 (6/2013–4/2016) | 2.015 | 0.942–4.306 | 0.071 | 2.918 | 1.348–6.317 | 0.007 |
Abbreviations: CI, confidence interval; MRI, magnetic resonance imaging.
Of the entire cohort, 1280 (84%) patients had history of prior prostate biopsy, of which 692 (54.0%) were found to be negative for cancer. In patients with a prior negative biopsy, Fbx detected significantly more CS disease than did Sbx in cohorts 2 (24.2%, 15.2%, respectively; P < 0.001) and 3 (23.9%, 19.7%, respectively; P = 0.036).
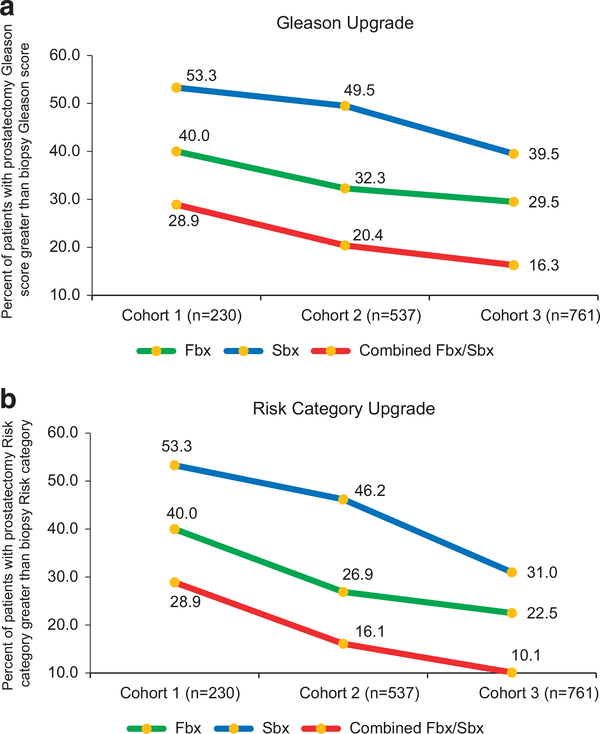
There was a nonsignificant decrease in the rate of Gleason score upgrade and risk category upgrade from Fbx to whole-mount prostatectomy pathology between cohorts 1, 2 and 3 (40.0%, 32.3%, 29.5%; P = 0.428 and 40.0%, 26.9%, 22.5%; P = 0.074, respectively; Figures 4a and b). However, there was a significant decrease in risk category upgrade on prostatectomy pathology from combined Fbx/Sbx between each cohort (28.9%, 16.1%, 10.1%; P = 0.010, respectively).
Figure 4.
(a) Rates of Gleason score upgrade on whole-mount prostatectomy specimen from mpMRI-TRUS fusion-guided biopsy, systematic 12-core biopsy and combined mpMRI-TRUS fusion-guided biopsy/systematic 12-core biopsy. There was a decrease in Gleason score upgrade from mpMRI-TRUS fusion-guided biopsy/ systematic biopsy to whole-mount prostatectomy pathology between each cohort (28.9%, 20.4%, 16.3%; P = 0.186). (b) Rates of risk category upgrade on whole-mount prostatectomy specimen from mpMRI-TRUS fusion-guided biopsy, systematic 12-core biopsy and combined mpMRI-TRUS fusion-guided biopsy/systematic 12-core biopsy. There was a significant decrease in risk category upgrade on prostatectomy pathology from combined mpMRI-TRUS fusion-guided biopsy/systematic 12-core biopsy between each cohort (28.9%, 16.1%, 10.1%; P = 0.010, respectively).
DISCUSSION
Despite several reports suggesting improved CDR of Fbx compared to Sbx, there is still some resistance to its widespread adoption in the urology community. This is largely due to inconsistent reproducibility across centers, the cause of which may be influenced by user experience and type of software platform used for biopsy.
Ten years ago, when Fbx was introduced at our institution, initial results were less encouraging and showed that Sbx outperformed Fbx. By comparing Fbx vs Sbx CDRs longitudinally at a single center, we hoped to delineate the effects of user experience and type of software platform on CDR.
In other fields, variability of user experience as it relates to performance has been extensively studied.15,16 A reflection on the history of mammography may give historical precedent for user experience and upgraded technology improving CDR. From general purpose X-rays used in the 1960s, to ‘screen film’ scans in the following decades, to the recent advances of digital mammography at the turn of the century, technology has been at the forefront of the evolution of mammography and is thought to be an important contributor to improving CDR throughout the years.17 Further, a recent study concluded that increased radiologist experience with mammography has been shown to improve cancer detection rates (CDR) while decreasing the number of false positive reports.18 From these reports it is fair to say that increased user experience and technology improvement have had considerable effect on the evolution of mammography as a screening tool for cancer. Our study aimed to measure the effect of these two variables on the evolution of Fbx.
Our results demonstrated that across a nearly 10-year study period, Fbx yielded progressively improved CDR compared to Sbx. The difference between Fbx and Sbx CDRs was the smallest in the first cohort, a period of learning at our institution, when urologists were gaining experience with performing Fbx and radiologists were simultaneously gaining experience interpreting prostate MRIs. An important aspect of this was the increasing recognition of anterior tumors of the prostate that hitherto had not been detected.19,20 In cohort 2, Fbx CDRs were distinctly improved compared to Sbx, and the difference remained significant in cohort 3 with the introduction of a new software platform. Furthermore, our attempt to eliminate potential bias from significantly different age and PSA levels between cohorts yielded similar rates between crude and adjusted CDRs.
It is likely that the increased accuracy of Fbx in cohort 2 is a product of increased urologist, radiologist and pathologist experience. With increased exposure to a variety of lesions on mpMRI with validation by pathology comes improved confidence about what constitutes a CS vs non CS lesion. While previously Gaziev et al.21 demonstrated a learning curve for the interpretation of mpMRI and use of targeted biopsy in 340 patients over the course of 2 years, there is a lack of long-term data on the duration of the learning curve for targeted biopsy of the prostate. Learning curves have been well documented for many other urologic procedures including the application of brachytherapy, performance of robotic prostatectomy, and accuracy of interpreting and reporting prostate MRI.7,22–30
Cohort 3’s increased CDR from Fbx reflected the addition of an improved software platform. Before 2013, our institution used a research platform that allowed for user preferences to influence settings for imaging, processing and registration of the prostate. With the adoption of a commercialized platform in May of 2013, the system utilized a more standard operating protocol that ensured consistency with each use.
It is interesting that the CDR for CS cancer increased for both Fbx and Sbx modalities over time. The rates of upgrade between combined biopsy and whole-mount pathology decreased throughout each cohort, which may suggest technique improvement was responsible for the overall increase in CDR. Alternatively, this finding may be related to the USPSTF recommendation of 2012 which recommended against widespread PSA screenings. Biopsy patterns have emerged in the years following the recommendation, and recent data confirms that fewer biopsies have been performed than in the years preceding.31–33 There has been a higher rate of positive biopsy from biopsies performed after the USPSTF recommendation, and among these there is a higher rate of detection of CS cancers.34,35 Our results showing overall increase in CS cancers from all biopsy modalities is consistent with these observed trends which would explain higher age and PSA score of patients in cohort 3.
Our study has several limitations. The observed trend of increased Fbx CDR with simultaneous decreased median number of targets assigned to each successive cohort suggests a radiologist learning curve may be contributory; however, the presence of concomitant urologist, radiologist and pathologist learning curves makes ascertaining the true source of improved CDR difficult. It is likely that the radiologists and urologists learned together and from each other via weekly multidisciplinary conferences, in which biopsy data were reviewed with the imaging.
In cohort 3 it is particularly difficult to determine the source for improved CDR from Fbx. In addition to the introduction of the commercialized software platform for Fbx users were even more experienced, and therefore the effects of either of these factors on CDR cannot be isolated. Also, due to the longitudinal nature of this study it is difficult to compare our results to an appropriate control group.
CONCLUSION
This study shows that the performance of Fbx improves over time. It is likely that accumulating experience both on the part of the radiologist and the urologist contributes to this improvement, however, other factors such as software improvements may also improve CDR of CS cancers. Studies comparing Fbx and Sbx should reflect this development by incorporating user experience, as a variable that may influence biopsy results, into the design of the study. This study supports the adoption of Fbx into urological practice in addition to Sbx and provides evidence that user experience and software improvements influence its successful application in the field.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute, Center for Cancer Research, and the Center for Interventional Oncology. NIH and Philips Healthcare have a cooperative research and development agreement. NIH and Philips share intellectual property in the field. This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH by the Doris Duke Charitable Foundation (Grant #2014194), the American Association for Dental Research, the Colgate-Palmolive Company, Genentech, and other private donors. For a complete list, visit the foundation website at http://www.fnih.org.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Lecornet E, Ahmed HU, Hu Y, Moore CM, Nevoux P, Barratt D et al. The accuracy of different biopsy strategies for the detection of clinically important prostate cancer: a computer simulation. J Urol 2012; 188: 974–980. [DOI] [PubMed] [Google Scholar]
- 2.Kvale R, Moller B, Wahlqvist R, Fossa SD, Berner A, Busch C et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU Int 2009; 103: 1647–1654. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015; 313: 390–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore CM, Robertson NL, Arsanious N, Middleton T, Villers A, Klotz L et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol 2013; 63: 125–140. [DOI] [PubMed] [Google Scholar]
- 5.Kuru TH, Roethke MC, Seidenader J, Simpfendorfer T, Boxler S, Alammar K et al. Critical evaluation of magnetic resonance imaging targeted, transrectal ultrasound guided transperineal fusion biopsy for detection of prostate cancer. J Urol 2013; 190: 1380–1386. [DOI] [PubMed] [Google Scholar]
- 6.Bjurlin MA, Mendhiratta N, Wysock JS, Taneja SS. Multiparametric MRI and targeted prostate biopsy: improvements in cancer detection, localization, and risk assessment. Central Eur J Urol 2016; 69: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris RD, Schned AR, Heaney JA. Staging of prostate cancer with endorectal MR imaging: lessons from a learning curve. Radiographics 1995; 15: 813–829. discussion 829–832. [DOI] [PubMed] [Google Scholar]
- 8.Rosenkrantz AB, Ayoola A, Hoffman D, Khasgiwala A, Prabhu V, Smereka P et al. The learning curve in prostate MRI interpretation: self-directed learning versus continual reader feedback. AJR Am J Roentgenol 2017; 208: W92–w100. [DOI] [PubMed] [Google Scholar]
- 9.Rosenkrantz AB, Pujara AC, Taneja SS. Use of a quality improvement initiative to achieve consistent reporting of level of suspicion for tumor on multiparametric prostate MRI. AJR Am J Roentgenol 2016; 206: 1040–1044. [DOI] [PubMed] [Google Scholar]
- 10.Emberton M Has magnetic resonance-guided biopsy of the prostate become the standard of care? Eur Urol 2013; 64: 720–721. [DOI] [PubMed] [Google Scholar]
- 11.Greer MD, Choyke PL, Turkbey B. PI-RADSv2: How we do it. J Magn Reson Imaging 2017; 46: 11–23. [DOI] [PubMed] [Google Scholar]
- 12.Yerram NK, Volkin D, Turkbey B, Nix J, Hoang AN, Vourganti S et al. Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer. BJU Int 2012; 110: E783–E788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012; 22: 746–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naing NN. Easy way to learn standardization: direct and indirect methods. Malays J Med Sci 2000; 7: 10–15. [PMC free article] [PubMed] [Google Scholar]
- 15.Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT. Assessment of the learning curve in health technologies. A systematic review. Int J Technol Assess Health Care 2000; 16: 1095–1108. [DOI] [PubMed] [Google Scholar]
- 16.Subramonian K, Muir G. The ‘learning curve’ in surgery: what is it, how do we measure it and can we influence it? BJU Int 2004; 93: 1173–1174. [DOI] [PubMed] [Google Scholar]
- 17.Gold RH, Bassett LW, Widoff BE. Highlights from the history of mammography. Radiographics 1990; 10: 1111–1131. [DOI] [PubMed] [Google Scholar]
- 18.Miglioretti DL, Gard CC, Carney PA, Onega TL, Buist DS, Sickles EA et al. When radiologists perform best: the learning curve in screening mammogram interpretation. Radiology 2009; 253: 632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkin D, Turkbey B, Hoang AN, Rais-Bahrami S, Yerram N, Walton-Diaz A et al. Multiparametric magnetic resonance imaging (MRI) and subsequent MRI/ultrasonography fusion-guided biopsy increase the detection of anteriorly located prostate cancers. BJU Int 2014; 114: E43–E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kongnyuy M, Sidana A, George AK, Muthigi A, Iyer A, Fascelli M et al. The significance of anterior prostate lesions on multiparametric magnetic resonance imaging in African-American men. Urol Oncol 2016; 34: e215–e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaziev G, Wadhwa K, Barrett T, Koo BC, Gallagher FA, Serrao E et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int 2016; 117: 80–86. [DOI] [PubMed] [Google Scholar]
- 22.Buus S, Rylander S, Hokland S, Sondergaard CS, Pedersen EM, Tanderup K et al. Learning curve of MRI-based planning for high-dose-rate brachytherapy for prostate cancer. Brachytherapy 2016; 15: 426–434. [DOI] [PubMed] [Google Scholar]
- 23.Ou YC, Yang CK, Chang KS, Wang J, Hung SW, Tung MC et al. The surgical learning curve for robotic-assisted laparoscopic radical prostatectomy: experience of a single surgeon with 500 cases in Taiwan, China. Asian J Androl 2014; 16: 728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atug F, Castle EP, Srivastav SK, Burgess SV, Thomas R, Davis R. Positive surgical margins in robotic-assisted radical prostatectomy: impact of learning curve on oncologic outcomes. Eur Urol 2006; 49: 866–871 discussion 871–862. [DOI] [PubMed] [Google Scholar]
- 25.Ahlering TE, Eichel L, Edwards RA, Lee DI, Skarecky DW. Robotic radical prostatectomy: a technique to reduce pT2 positive margins. Urology 2004; 64: 1224–1228. [DOI] [PubMed] [Google Scholar]
- 26.Zorn KC, Wille MA, Thong AE, Katz MH, Shikanov SA, Razmaria A et al. Continued improvement of perioperative, pathological and continence outcomes during 700 robot-assisted radical prostatectomies. Can J Urol 2009; 16: 4742–4749. [PubMed] [Google Scholar]
- 27.Patel VR, Palmer KJ, Coughlin G, Samavedi S. Robot-assisted laparoscopic radical prostatectomy: perioperative outcomes of 1500 cases. J Endourol 2008; 22: 2299–2305. [DOI] [PubMed] [Google Scholar]
- 28.Vickers AJ, Bianco FJ, Serio AM, Eastham JA, Schrag D, Klein EA et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst 2007; 99: 1171–1177. [DOI] [PubMed] [Google Scholar]
- 29.Latchamsetty KC, Borden LS Jr, Porter CR, Lacrampe M, Vaughan M, Lin E et al. Experience improves staging accuracy of endorectal magnetic resonance imaging in prostate cancer: what is the learning curve? Can J Urol 2007; 14: 3429–3434. [PubMed] [Google Scholar]
- 30.Akin O, Riedl CC, Ishill NM, Moskowitz CS, Zhang J, Hricak H. Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur Radiol 2010; 20: 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleshner K, Carlsson SV, Roobol MJ. The effect of the USPSTF PSA screening recommendation on prostate cancer incidence patterns in the USA. Nat Rev Urol 2017; 14: 26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGinley KF, McMahon GC, Brown GA. Impact of the US Preventive Services Task Force Grade D Recommendation: Assessment of Evaluations for elevated prostate-specific antigen and prostate biopsies in a large urology group practice following statement revision. Rev Urol 2015; 17: 171–177. [PMC free article] [PubMed] [Google Scholar]
- 33.Banerji JS, Wolff EM, Massman JD 3rd, Odem-Davis K, Porter CR, Corman JM. Prostate needle biopsy outcomes in the era of the U.S. Preventive Services Task Force Recommendation against prostate specific antigen based screening. J Urol 2016; 195: 66–73. [DOI] [PubMed] [Google Scholar]
- 34.Olsson C, Anderson A, Kapoor D. MP39–04 initial prostate cancer detection before and after United States Preventive Services Task Force Recommendation on prostate cancer screening. J Urol 195: e542. [Google Scholar]
- 35.Rosenberg M, Crawford D, Newmark J, Steiner M. MP39–01 use of PSA screening guidelines among primary care physicians. J Urol 195: e541. [Google Scholar]