Abstract
Introduction:
It is estimated that 5%–10% of patients with newly diagnosed glioblastoma (GBM) fail to complete standard chemoradiation (CRT). We sought to determine the impact of failure to complete CRT on survival and to identify risk factors.
Methods:
We queried the National Cancer Database and identified a cohort of 17,451 adults with GBM diagnosed from 2005 to 2012. The cohort was restricted to patients that started conventionally fractionated adjuvant chemoradiation of 1.8 to 2.0 Gy per fraction to a dose of ≤66Gy. Patients were stratified by RT dose: a) completed RT ≥ 58Gy, b) nearly completed RT ≥ 50Gy - b58Gy, and c) did not complete RT ≤ 50Gy.
Results:
The CRT completion rate correlated with survival, 87% of patients completed CRT and had a median OS of 13.5 months, 4% were near completers (median OS 5.7 months), and 9% did not complete RT (median OS 1.9 months). Older age was associated with a higher risk of non-completion. Twenty-eight percent of patients ≥80 years old did not complete standard CRT (OR 2.99) and 19% of 70–79-year olds did not complete CRT (OR 1.99). The adjusted mortality hazard ratio was greater for patients that did not complete CRT across all age categories and for nearly complete CRT patients older than 40 (non-significant for age < 40).
Conclusions:
Failure to complete standard chemoradiation was associated with decreased survival in our cohort. Patients with risk factors for failure (like advanced age) should be considered for alternative treatments such as hypofractionated radiotherapy.
Keywords: Glioblastoma, Treatment failure, Radiation failure, Chemoradiation
1. Introduction
Standard therapy for newly diagnosed glioblastoma (GBM) patients is maximal safe resection followed by adjuvant chemoradiation (CRT) [1]. However, in some instances the intended course of radiation therapy (RT) cannot be completed, which may impact patient survival.
Failure to complete radiation occurs when treatment is discontinued before patients reach the intended total radiation dose. For conventional regimens this has been defined as 59.4 to 60 Gy given in 30 or 33 fractions of 2.0 or 1.8 Gy delivered daily, five days per week, over a six to seven-week period [2]. Based on prospective studies that detail the number of patients that start radiation but do not finish for any reasons (which could include patient choice, disease progression, intercurrent illness or other adverse events), failure to complete standard chemoradiation has been estimated to occur in approximately five to 10% of patients with newly diagnosed GBM1. The impact on survival of failure to complete standard radiation has not been well described. If survival for these patients is significantly decreased, identification of factors predictive for failure to complete standard RT would be of high clinical value and would facilitate consideration of alternative therapeutic management for patients at risk for failure.
In this study, we used the National Cancer Database (NCDB) to identify patients with newly diagnosed GBM who failed to complete conventional chemoradiation in order to determine the impact on their survival, and to determine factors associated with failure.
2. Materials and Methods
2.1. Database
We performed an analysis of the NCDB, a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. As a large, prospectively maintained database, the NCDB collects hospital-level data from over 1500 CoC accredited centers, encompassing an estimated 70% of all malignancies diagnosed in the United States [3]. The data used in this study are derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators. This study was approved by our institutional review board and meets the requirements for protection of human subjects.
2.2. Cohort Selection
We queried the NCDB for all adult patients with newly diagnosed GBM from 2005 to 2012. Patients with GBM were identified using International Classification of Diseases for Oncology (ICD—O—3) site codes (C700, C710-C719). Patients who did not undergo surgical resection or biopsy and patients who did not receive adjuvant RT and chemotherapy (CT) were also excluded. Additional exclusion criteria included receiving multiagent CT or initiating CT >14 days from first RT fraction. We restricted our cohort to patients receiving conventionally fractionated adjuvant RT of 1.8 to 2.0 Gy and a planned total dose of <66 Gy.
The cohort was categorized into treatment groups defined by total RT dose received, with patients deemed to have received a complete course of RT defined as ≥58 and ≤66 Gy, a near complete course of RT defined as total dose ≥50 and <58 Gy and incomplete courses of RT defined as <50 Gy. These dose categories were selected a priori with consideration of dose-response data [4].
2.3. Statistical Analysis
Patient demographic, tumor and treatment related factors including age, sex, race, insurance status, treatment facility type, facility case load, diagnosis year, and surgical procedure (resection vs. biopsy alone) were abstracted from the dataset and adjusted for in the performed multivariable analyses. Descriptive statistics using chi-squared tests were performed to characterize the cohort stratified by treatment groups categorized by RT completion status. Multivariable logistic regression models were considered to determine predictors associated with failure to receive a complete course of RT (either receiving incomplete or near complete courses of RT). Overall survival was defined as the interval from first RT fraction to death from any cause or censoring. Kaplan-Meier methods were used to estimate OS for the cohort with log-rank tests performed to compare OS by treatment group, as well as by age for those receiving an incomplete course of RT. Multivariable Cox proportional hazards regression models were considered to validate the relationship between RT completion and hazard of death while adjusting for known prognostic factors including age, sex, race, Charlson/Deyo comorbidity score, surgical procedure type and treatment facility type and case load. Schoenfeld residuals were studied to assess the proportional hazards assumption. Stratifying the baseline hazard by surgery vs biopsy and an interaction effect between RT completion and age were included to better meet the proportional hazard assumption; after these additions the correlation between Schoenfeld residuals and all predictors was <0.05 in absolute value. All statistical analysis was performed using the R statistical software, v.3.5.1 [5]. Statistical significance was defined as α <0.05.
3. Results
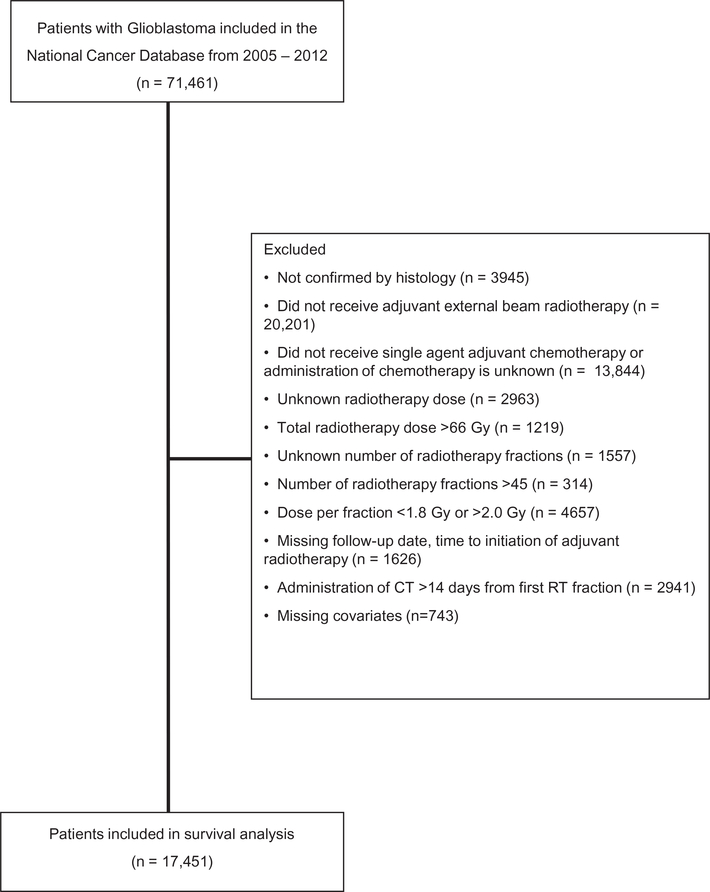
A total of 17,451 patients meeting all selection criteria were identified and included in the study cohort (Fig. 1). The median age for the entire cohort was 62 years (Interquartile Range (IQR): 53–69 years). Median survival for the entire cohort was 12.1 months (95% CI 11.9–12.3). Fifty-nine percent of the patients were male and 92% were Caucasian (Table 1).
Fig. 1.
Analytic diagram depicting inclusion and exclusion criterion for study cohort selection.
Table 1.
Baseline patient, tumor and treatment factors.
| Treatment regimen | N | Prop | N | Prop | N | Prop | N | Prop | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Complete | Near complete | Incomplete | |||||||
| 17,451 | 15,120 | 87% | 762 | 4% | 1569 | 9% | |||
| Age | <0.0001 | ||||||||
| [18–40) | 870 | 5% | 792 | 91% | 36 | 4% | 42 | 5% | |
| [40–50) | 2018 | 12% | 1851 | 92% | 70 | 3% | 97 | 5% | |
| [50–60) | 4634 | 27% | 4174 | 90% | 184 | 4% | 276 | 6% | |
| [60–70) | 5637 | 32% | 4896 | 87% | 229 | 4% | 512 | 9% | |
| [70–80) | 3459 | 20% | 2805 | 81% | 183 | 5% | 471 | 14% | |
| [80–+) | 833 | 5% | 602 | 72% | 60 | 7% | 171 | 21% | |
| Sex | 0.0002 | ||||||||
| Male | 10,382 | 59% | 9084 | 87% | 435 | 4% | 863 | 8% | |
| Female | 7069 | 41% | 6036 | 85% | 327 | 5% | 706 | 10% | |
| Race | 0.0125 | ||||||||
| White | XX | 92% | 13,960 | 87% | XX | 4% | 1434 | 9% | |
| Black | XX | 5% | 712 | 84% | XX | 6% | 92 | 11% | |
| Asian | XX | 1% | 226 | 92% | XX | 1% | 18 | 7% | |
| Other | XX | 1% | 222 | 86% | XX | 4% | 25 | 10% | |
| Hispanic Origin | 0.4428 | ||||||||
| Non-Hispanic | 16,773 | 96% | 14,532 | 87% | 727 | 4% | 1514 | 9% | |
| Hispanic | 678 | 4% | 588 | 87% | 35 | 5% | 55 | 8% | |
| Insurance Status | <0.0001 | ||||||||
| Private Insurance | 9067 | 52% | 8153 | 90% | 340 | 4% | 574 | 6% | |
| Not Insured | 598 | 3% | 527 | 88% | 27 | 5% | 44 | 7% | |
| Govt | 7786 | 45% | 6440 | 83% | 395 | 5% | 951 | 12% | |
| Facility Type | <0.0001 | ||||||||
| Academic | XX | 41% | XX | 89% | XX | 4% | XX | 8% | |
| Community | XX | 47% | XX | 85% | XX | 5% | XX | 11% | |
| Integrated | XX | 7% | XX | 86% | XX | 5% | XX | 9% | |
| Other | XX | 0% | XX | 82% | XX | 9% | XX | 9% | |
| Suppressed (age < 40) | XX | 5% | XX | 91% | XX | 4% | XX | 5% | |
| Facility Case Load | <0.0001 | ||||||||
| <1 | 1542 | 9% | 1255 | 81% | 93 | 6% | 194 | 13% | |
| [1–5) | 8079 | 46% | 6871 | 85% | 377 | 5% | 831 | 10% | |
| [5–10) | 4351 | 25% | 3870 | 89% | 154 | 4% | 327 | 8% | |
| 10+ | 3479 | 20% | 3124 | 90% | 138 | 4% | 217 | 6% | |
| Distance to Treatment Facility | 0.0301 | ||||||||
| [0–50) | 15,360 | 88% | 13,269 | 86% | 676 | 4% | 1415 | 9% | |
| [50–100) | 1339 | 8% | 1174 | 88% | 60 | 4% | 105 | 8% | |
| [100+) | 752 | 4% | 677 | 90% | 26 | 3% | 49 | 7% | |
| Charlson/Deyo comorbidity score | <0.0001 | ||||||||
| 0 | 12,950 | 74% | 11,368 | 88% | 541 | 4% | 1041 | 8% | |
| 1 | 2824 | 16% | 2369 | 84% | 125 | 4% | 330 | 12% | |
| 2+ | 1677 | 10% | 1383 | 82% | 96 | 6% | 198 | 12% | |
| Diagnosis Year | 0.0087 | ||||||||
| 2005 | 1458 | 8% | 1221 | 84% | 82 | 6% | 155 | 11% | |
| 2006 | 1706 | 10% | 1464 | 86% | 80 | 5% | 162 | 9% | |
| 2007 | 1872 | 11% | 1633 | 87% | 78 | 4% | 161 | 9% | |
| 2008 | 1948 | 11% | 1712 | 88% | 91 | 5% | 145 | 7% | |
| 2009 | 2189 | 13% | 1871 | 85% | 102 | 5% | 216 | 10% | |
| 2010 | 2500 | 14% | 2166 | 87% | 113 | 5% | 221 | 9% | |
| 2011 | 2762 | 16% | 2430 | 88% | 105 | 4% | 227 | 8% | |
| 2012 | 3016 | 17% | 2623 | 87% | 111 | 4% | 282 | 9% | |
| Zip code income level | <0.0001 | ||||||||
| [$63K+) | 6013 | 34% | 5321 | 88% | 240 | 4% | 452 | 8% | |
| [$48K–63K) | 5098 | 29% | 4410 | 87% | 203 | 4% | 485 | 10% | |
| [$38K–$48K) | 3994 | 23% | 3399 | 85% | 197 | 5% | 398 | 10% | |
| [0–$38K) | 2346 | 13% | 1990 | 85% | 122 | 5% | 234 | 10% | |
| Surgery | <0.0001 | ||||||||
| Surgery | 14,215 | 81% | 12,666 | 89% | 542 | 4% | 1007 | 7% | |
| Biopsy only | 3236 | 19% | 2454 | 76% | 220 | 7% | 562 | 17% | |
Govt: Government, Surgery: includes both subtotal and gross total resection extent, Complete: total dose of RT ≥ 58 and ≤66 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy, Near Complete: total dose of RT ≥ 50 and <58 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy, Incomplete: total dose of RT <50 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy.
XX: To ensure anonymity of patients, NCDB requires that no cell counts <10 be reported. Cell with fewer than 10 individuals or cells with counts allowing back-calculation of small counts have been redacted.
Patients were stratified into three treatment groups defined as: a.) complete RT (those patients that reached a RT dose of at least ≥58 Gy), b.) near complete RT (RT between ≥50 and <58 Gy) and c.) the incomplete radiation therapy group (RT <50 Gy).
Using this definition 87% (n = 15,120) of patients completed radiation, 4% (n = 762) were near completers and 9% (n = 1569) did not complete RT. The median survival for patients that completed RT was 13.5 months (95% CI: 13.3–13.7) and 5.7 months (95% CI: 5.0–6.7) for those who nearly completed RT. The median survival for patients that did not complete therapy was 1.9 months (1.8–2.1; log-rank test: p < .0001) (Fig. 2A). The median age for patients in the 3 groups were: completers 61 years (IQR 53–69), near completers 64 years (IQR 59–75) and incomplete 67 years, (IQR 59–75; Kruskal Wallis test: p < .0001).
Fig. 2.
A. Subeffect interaction demonstrating the survival impact of RT completion differs by age group with adjusted mortality hazard rates estimated relative to patients aged 40 to 49 who completed standard RT. B. Subeffect interaction model demonstrating the survival impact of RT completion status within each age group with adjusted mortality hazrd rates estimated for each age category with respect to patients within the age grouping who completed standard radiotherapy. C. Kaplan-Meier figure comparing OS for patients unable to complete standard RT categorized by age decade.
Combining patients who were in the nearly complete or incomplete groups, older patients were more likely to fail to complete RT with 28% of patients 80 years or older and 21% of patients ages 70–79 in a failed to complete group. This is in comparison to the <40, 40–49, and 50–59 age groups where the rate was 10% or less (Table 1). Using logistic regression analysis for failure to complete RT controlling for all patient characteristics, age was significantly associated with a greater chance of failure for ages 60–69 (OR 1.48, 95% CI: 1.23–1.77, p < .0001), ages 70–79 (OR 1.99, 1.63–2.42, p < .0001), and ages 80 and greater (OR: 2.99, 2.36–3.80), all when compared to the 40–49 reference group (Table 2).
Table 2.
Multivariable logistic regression model for predictors of early radiotherapy stop.
| Odds ratio of early stop (either incomplete or near complete) |
P-values | ||||
|---|---|---|---|---|---|
| OR | 95% CI | ||||
| Age | <0.0001 | ||||
| (18–40) | 1.15 | 0.86 | 1.53 | 0.3586 | |
| [40–50) | Reference | ||||
| [50–60) | 1.17 | 0.97 | 1.42 | 0.0961 | |
| [60–70) | 1.48 | 1.23 | 1.77 | <0.0001 | |
| [70–80) | 1.99 | 1.63 | 2.42 | <0.0001 | |
| [80–+) | 2.99 | 2.36 | 3.80 | <0.0001 | |
| Sex | 0.0031 | ||||
| Male | Reference | ||||
| Female | 1.15 | 1.05 | 1.26 | 0.0031 | |
| Race | 0.0175 | ||||
| White | Reference | ||||
| Black | 1.29 | 1.06 | 1.57 | 0.0123 | |
| Asian | 0.66 | 0.41 | 1.05 | 0.0803 | |
| Other | 1.14 | 0.79 | 1.65 | 0.4804 | |
| Hispanic Origin | 0.9056 | ||||
| Non-Hispanic | Reference | ||||
| Hispanic | 1.01 | 0.80 | 1.28 | 0.9056 | |
| Insurance Status | 0.0010 | ||||
| Private Insurance | Reference | ||||
| Not Insured | 1.17 | 0.90 | 1.52 | 0.2462 | |
| Govt | 1.24 | 1.10 | 1.39 | 0.0002 | |
| Facility Type | 0.7782 | ||||
| Academic | Reference | ||||
| Community | 1.04 | 0.92 | 1.17 | 0.5367 | |
| Integrated | 1.09 | 0.90 | 1.31 | 0.3908 | |
| Other | 1.51 | 0.32 | 7.25 | 0.6056 | |
| Suppressed (age <40) | |||||
| Facility Case Load | <0.0001 | ||||
| <1 | Reference | ||||
| [1–5) | 0.82 | 0.71 | 0.95 | 0.0078 | |
| [5–10) | 0.62 | 0.52 | 0.75 | <0.0001 | |
| 10+ | 0.64 | 0.53 | 0.78 | <0.0001 | |
| Distance to Treatment Facility | 0.1820 | ||||
| [0–50) | Reference | ||||
| [50–100) | 0.93 | 0.78 | 1.11 | 0.4306 | |
| [100 – +) | 0.80 | 0.63 | 1.03 | 0.0903 | |
| Charlson/Deyo comorbidity score | <0.0001 | ||||
| 0 | Reference | ||||
| 1 | 1.25 | 1.11 | 1.40 | 0.0002 | |
| 2 + | 1.38 | 1.20 | 1.59 | <0.0001 | |
| Diagnosis Year | 0.0408 | ||||
| 2005 | Reference | ||||
| 2006 | 0.86 | 0.70 | 1.05 | 0.1282 | |
| 2007 | 0.77 | 0.63 | 0.94 | 0.0117 | |
| 2008 | 0.74 | 0.61 | 0.90 | 0.0032 | |
| 2009 | 0.88 | 0.73 | 1.06 | 0.1834 | |
| 2010 | 0.82 | 0.68 | 0.98 | 0.0327 | |
| 2011 | 0.74 | 0.61 | 0.89 | 0.0014 | |
| 2012 | 0.81 | 0.67 | 0.97 | 0.0205 | |
| Zip code income level | 0.0030 | ||||
| [$63 K – +) | Reference | ||||
| [$48 K–63 K) | 1.12 | 1.00 | 1.26 | 0.0538 | |
| [$38 K–$48 K) | 1.24 | 1.09 | 1.40 | 0.0007 | |
| [0–$38 K) | 1.24 | 1.07 | 1.44 | 0.0043 | |
| Surgery | |||||
| Surgery | Reference | ||||
| Biopsy only | 2.33 | 2.11 | 2.57 | <0.0001 | |
Govt: Government, Surgery: includes both subtotal and gross total resection extent, Complete: total dose of RT ≥ 58 and ≤66 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy, Near Complete: total dose of RT ≥ 50 and <58 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy, Incomplete: total dose of RT <50 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy.
Other factors associated with failure to complete standard CRT (near complete or incomplete) were: female sex, African American race, having government insurance, a higher Charlson/Deyo comorbidity score, living in a lower income zip code and undergoing biopsy alone (all OR > 1 and p-values <.05). One factor associated with being more likely to complete CRT was receiving therapy at facility with higher case load of GBM patients per year. Hispanic origin, facility type (i.e. academic vs. community) and distance to treatment facility were not associated with likelihood of completion (Table 2).
A Cox proportional hazard model was used to assess the factors associated with overall survival (Table 3). Age and CRT completion were highly associated with survival, and the effect of failing to complete CRT differed by age (interaction effect: p < .0001; Fig. 2A,B). Increasing age was associated with greater hazard across all RT categories, and the detrimental effect of incomplete CRT increases in the older population. Across all ages, median survival for patients with incomplete CRT is found to be less than six months and less than two months for patients 60–69, 70–79, and 80 and older (Fig. 2C). Other factors associated with poor survival in the Cox model included government insurance, community and integrated facilities, higher comorbidity scores, and living in poorer neighborhoods (all HR > 1, p < .05). Female sex, African American and Asian race, Hispanic ethnicity, and high-volume facility were associated with improved survival (HR < 1, p < .05).
Table 3.
Multivariable cox proportional hazards regression model for overall survival.
| HR | LHR | UHR | P-values | ||
|---|---|---|---|---|---|
| Treatment Regimen | <0.0001 | ||||
| Complete | Reference | ||||
| Near Complete | 1.38 | 1.07 | 1.79 | 0.0136 | |
| Incomplete | 2.61 | 2.09 | 3.25 | <0.0001 | |
| Age | <0.0001 | ||||
| (18–40) | 0.72 | 0.65 | 0.80 | <0.0001 | |
| [40–50) | Reference | ||||
| [50–60) | 1.20 | 1.13 | 1.28 | <0.0001 | |
| [60–70) | 1.44 | 1.36 | 1.53 | <0.0001 | |
| [70–80) | 1.86 | 1.74 | 2.00 | <0.0001 | |
| [80 – +) | 2.30 | 2.08 | 2.55 | <0.0001 | |
| Treatment Regimen/Age Interaction | <0.0001 | ||||
| Near& < 40 | 0.84 | 0.52 | 1.38 | 0.5015 | |
| Incomplete& < 40 | 1.04 | 0.71 | 1.54 | 0.8273 | |
| Near&[50–60) | 1.12 | 0.83 | 1.52 | 0.4626 | |
| Incomplete&[50–60) | 1.08 | 0.84 | 1.40 | 0.5346 | |
| Near&[60–70) | 1.58 | 1.18 | 2.12 | 0.0021 | |
| Incomplete&[60–70) | 1.39 | 1.09 | 1.77 | 0.0071 | |
| Near&[70–79) | 1.32 | 0.98 | 1.78 | 0.0726 | |
| Incomplete&[70–79) | 1.41 | 1.11 | 1.80 | 0.0050 | |
| Near&[80 – +) | 1.58 | 1.09 | 2.30 | 0.0161 | |
| Incomplete&[80 – +) | 1.85 | 1.40 | 2.45 | <0.0001 | |
| Sex | <0.0001 | ||||
| Male | Reference | ||||
| Female | 0.89 | 0.87 | 0.92 | <0.0001 | |
| Race | <0.0001 | ||||
| White | Reference | ||||
| Black | 0.87 | 0.81 | 0.94 | 0.0007 | |
| Asian | 0.73 | 0.63 | 0.85 | <0.0001 | |
| Other | 0.89 | 0.78 | 1.02 | 0.0917 | |
| Hispanic Origin | <0.0001 | ||||
| Non-Hispanic | Reference | ||||
| Hispanic | 0.82 | 0.75 | 0.90 | <0.0001 | |
| Insurance Status | <0.0001 | ||||
| Private Insurance | Reference | ||||
| Not Insured | 1.09 | 0.99 | 1.20 | 0.0662 | |
| Govt | 1.13 | 1.08 | 1.17 | <0.0001 | |
| Facility Type | <0.0001 | ||||
| Academic | Reference | ||||
| Community | 1.11 | 1.07 | 1.16 | <0.0001 | |
| Integrated | 1.14 | 1.07 | 1.22 | <0.0001 | |
| Other | 0.91 | 0.47 | 1.75 | 0.7763 | |
| Suppressed (age < 40) | |||||
| Facility Case Load | 0.0210 | ||||
| <1 | Reference | ||||
| [1–5) | 1.00 | 0.94 | 1.06 | 0.9999 | |
| [5–10) | 0.98 | 0.92 | 1.05 | 0.5791 | |
| [10 – +) | 0.92 | 0.86 | 0.99 | 0.0333 | |
| Distance to Treatment Facility | |||||
| [0–50) | Reference | ||||
| [50–100) | 0.99 | 0.93 | 1.06 | 0.8717 | |
| [100 – +) | 1.01 | 0.93 | 1.10 | 0.7980 | |
| Charlson/Deyo comorbidity score | <0.0001 | ||||
| 0 | Reference | ||||
| 1 | 1.17 | 1.12 | 1.22 | <0.0001 | |
| 2+ | 1.30 | 1.23 | 1.37 | <0.0001 | |
| Diagnosis Year | <0.0001 | ||||
| 2005 | Reference | ||||
| 2006 | 0.94 | 0.88 | 1.01 | 0.0950 | |
| 2007 | 0.96 | 0.89 | 1.03 | 0.2178 | |
| 2008 | 0.87 | 0.81 | 0.93 | 0.0001 | |
| 2009 | 0.88 | 0.83 | 0.95 | 0.0005 | |
| 2010 | 0.90 | 0.84 | 0.96 | 0.0022 | |
| 2011 | 0.85 | 0.80 | 0.91 | <0.0001 | |
| 2012 | 0.86 | 0.81 | 0.92 | <0.0001 | |
| Zip code income level | <0.0001 | ||||
| [$63 K – +) | Reference | ||||
| [$48 K–63 K) | 1.07 | 1.03 | 1.12 | 0.0008 | |
| [$38 K–$48 K) | 1.14 | 1.09 | 1.19 | <0.0001 | |
| [0–$38K) | 1.10 | 1.04 | 1.16 | 0.0009 | |
| Surgery | |||||
| Surgery | Enter model through strata | ||||
| Biopsy | Enter model through strata | ||||
Govt: Government, Surgery: includes both subtotal and gross total resection extent, Complete: total dose of RT ≥ 58 and ≤66 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy, Near Complete: total dose of RT ≥ 50 and <58 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy, Incomplete: total dose of RT <50 Gy delivered in dose per fraction ≥1.8 Gy and ≤2.0 Gy.
4. Discussion
In this large-scale analysis of a national database, we show that approximately 9–13% of all newly diagnosed patients with GBM did not complete a full course of standard chemoradiation, and failure to complete standard CRT was associated with a poorer survival. Patients who completed RT had a median overall survival of 13.5 months (95%CI: 13.3–13.7); patients with nearly complete RT had a median survival of 5.7 months; and patients that did not reach 50Gy had a strikingly poor overall survival of 1.9 months, (p < .0001).
The strongest factor associated with not completing standard chemoradiation was advancing age. Focusing on the poorest outcome group, while 9% (1569/17,451) of patients in the total cohort failed to reach 50 Gy, of these patients 41% (642/1569) were 70 years or older. Comparatively, 9% (512/5637) of patients between 60 and 69 years old and 5% (42/870) of patients 18–39 did not reach 50 Gy.
In addition to advancing age we show that patients undergoing biopsy without further resection, with a high comorbidity score, or receiving treatment at low volume health centers were more likely not to finish standard radiotherapy. In support of this investigation, others have also found these same factors to be associated with a generally poorer survival in patients with GBM [6,7].
Advanced age has long been an issue in managing patients with GBM [8]. Previous studies have shown older patients with GBM do in fact benefit from radiotherapy [9]. But as seen in this and prior investigations, older patients also tend to have inherently shorter survivals, so management can be challenging given the poor prognosis and frequent comorbidities [10,11]. These limitations have led to older patients often being treated using hypofractionated radiotherapy (HFRT), which is radiation delivered in fewer fractions with a larger dose per fraction.
Hypofractionated radiotherapy was initially explored primarily as a method of patient convenience, to reduce overall treatment time in patients with a poor prognosis but was limited in use by an accompanying increased risk of toxicity. More recently, through advanced radiation techniques including intensity modulated radiotherapy and volumetric arc radiation therapy it has become possible to deliver high radiation doses to tumor while minimizing the volume to normal tissue [12]. Radiobiological models suggest HFRT can limit potential tumor repopulation and facilitate cell kill in a manner different than conventional fractionation, potentially minimizing the effects of a hypoxic tumor environment which may be more effective for certain tumor types. HFRT can also achieve intratumoral dose escalation, with the possibility of a superior therapeutic benefit compared to standard radiation, particularly in rapidly proliferating tumors [13–15].
There is now level 1 evidence for use of HFRT in older patients with GBM provided by clinical trials showing similar survival outcomes for older patients with GBM receiving HFRT (25–40 Gy in 5–15 daily fractions) compared to patients receiving standard RT [16–21]. The Nordic study showed a higher treatment adherence for patients with GBM over 70 receiving HFRT, (34 Gy in 10 fractions, 98% completion rate), than those receiving standard RT (77% completion rate) with an accompanying improvement in overall patient survival (7 months vs. 5 months HR: 0.59, p = .02) [22].
Other reports demonstrate better than 90% of older patients are able to complete an effective hypofractionated radiotherapy course. Whereas a treatment adherence of only approximately 70% – 80% is seen in patients over 70 receiving standard RT in this and other analysis [17,18,22–24] (supplemental Table 4.). In these studies, radiotherapy was discontinued primarily due to clinical deterioration and/or disease progression.
Therefore, current trends where hypofractionated radiotherapy schemes are used to treat older patients with GBM are strongly supported by this retrospective analysis of the NCDB, since in this cohort patients over 70 years were almost two to three times more likely not to finish a standard CRT course, which was associated with an exceptionally poor survival. However, the majority of older patients do not undergo HFRT [25,26]. A recently published NCDB study showed HFRT utilization rates for the years 2005–2012 in patients over 65 years old, stratified by age, ranged from only 7%–18%, despite prospective evidence supporting its use [26]. Currently it is unclear what barriers limit the broader use of HFRT in the older GBM population. And one must also consider the findings of low HFRT utilization in older GBM patients from 2005 to 2012 may not accurately reflect current clinical practice. A more contemporary analysis will need to be done in light of two additional practice changing prospective studies published in 2012 and 2017, that lend further support for the use of HFRT in older GBM patients [21,22].
The limitations of this study include those associated with any retrospective analysis, such as selection bias. The failure to complete radiotherapy data may actually be more profound as previous population-based studies have shown approximately 30% of older patients do not attempt any treatment at all after surgery [25,27]. If this additional 30% of patients were alternatively treated with standard CRT it is likely a majority would also be unable to complete the longer therapy. As such, these results may underestimate incompletion rates and possibly overestimate survival outcomes. Although with the large number of patients we hope to mitigate this bias. Other limitations include a lack of detailed patient information such as performance status, tumor molecular characterization, extent of resection, treatment-related toxicities, specific chemotherapies or comorbidities that could allow us to further detail exactly why patients in different age groups discontinued radiation. For instance, it would be of interest to determine if chemotherapy plays a role in a patient’s ability to complete chemoradiation vis-à-vis tumor MGMT status [28]. Additionally, there is also no information on salvage therapy after resection, which could impact overall survival and we are unable to track cancer specific endpoints such as cancer specific death or progression free survival. Because of these limitations, one possible interpretation of the data is that incomplete radiation, instead of being a cause of poor survival is in fact a proxy for other poor prognostic factors that could result in patients not completing RT. An analysis of large prospective studies with more granular information available could potentially allow for a more detailed stratification system to determine marginal patients at risk for not completing RT regardless of age.
5. Conclusions
We performed an analysis of a large national database and demonstrated that patients were at increased risk of failing to complete standard chemoradiation if they were over 60 years old, received a biopsy alone, or had an increased Charlson/Deyo comorbidity score. Our results in aggregate with other data strongly supports the use of hypofractionated radiotherapy to treat older patients with GBM, which evidence suggest may be underutilized in this population. Established benefits of HFRT are less cost and shorter treatment time. Additionally, there is clinical and radiobiological evidence to suggest this approach may in fact be superior to standard RT for older patients with GBM. Barriers to its broader use should be identified but increased national support may be needed in the form of an additional prospective study that test not only whether hypofractionated chemoradiation increases RT completion rates, but also results in improved survival compared to standard chemoradiation.
Supplementary Material
Acknowledgements
The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
This research was supported in part by the intramural research program of the National Institutes of Health, National Cancer Institute, Neuro-Oncology Branch.
Footnotes
Declaration of Competing Interest
None.
Financial support
None.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jgo.2019.08.014.
References
- [1].Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352(10):987–96. [DOI] [PubMed] [Google Scholar]
- [2].Kirkpatrick JP, Laack NN, Shih HA, Gondi V. Management of GBM: a problem of local recurrence. J Neurooncol 2017;134(3):487–93. [DOI] [PubMed] [Google Scholar]
- [3].Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15(3):683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys 1979;5(10):1725–31. [DOI] [PubMed] [Google Scholar]
- [5].Development Core Team R. R: A language and environment for statistical computing. vol. 12011. [Google Scholar]
- [6].Zhu P, Du XL, Zhu JJ, Esquenazi Y. Improved survival of glioblastoma patients treated at academic and high-volume facilities: a hospital-based study from the National Cancer Database. J Neurosurg 2019:1–12. [DOI] [PubMed] [Google Scholar]
- [7].Dressler EV, Liu M, Garcia CR, et al. Patterns and disparities of care in glioblastoma. Neurooncol Pract 2019;6(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Halperin EC. Malignant gliomas in older adults with poor prognostic signs. Getting nowhere, and taking a long time to do it. Oncology (Williston Park) 1995;9(3): 229–34 [discussion 237–228, 243]. [PubMed] [Google Scholar]
- [9].Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med 2007;356(15):1527–35. [DOI] [PubMed] [Google Scholar]
- [10].Asmaa A, Dixit S, Rowland-Hill C, et al. Management of elderly patients with glioblastoma-multiforme-a systematic review. Br J Radiol 2018;91 (1088):20170271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Curran WJ Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three radiation therapy oncology group malignant glioma trials. J Natl Cancer Inst 1993;85(9):704–10. [DOI] [PubMed] [Google Scholar]
- [12].Sheu T, Briere TM, Olanrewaju AM, McAleer MF. Intensity modulated radiation therapy versus volumetric arc radiation therapy in the treatment of glioblastoma-does clinical benefit follow Dosimetric advantage? Adv Radiat Oncol 2019;4(1):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hingorani M, Colley WP, Dixit S, Beavis AM. Hypofractionated radiotherapy for glioblastoma: strategy for poor-risk patients or hope for the future? Br J Radiol 2012;85 (1017):e770–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Budach W, Gioioso D, Taghian A, Stuschke M, Suit HD. Repopulation capacity during fractionated irradiation of squamous cell carcinomas and glioblastomas in vitro. Int J Radiat Oncol Biol Phys 1997;39(3):743–50. [DOI] [PubMed] [Google Scholar]
- [15].Nahum AE. The radiobiology of hypofractionation. Clin Oncol (R Coll Radiol) 2015; 27(5):260–9. [DOI] [PubMed] [Google Scholar]
- [16].McAleese JJ, Stenning SP, Ashley S, et al. Hypofractionated radiotherapy for poor prognosis malignant glioma: matched pair survival analysis with MRC controls. Radiother Oncol 2003;67(2):177–82. [DOI] [PubMed] [Google Scholar]
- [17].Roa W, Kepka L, Kumar N, et al. International Atomic Energy Agency randomized phase III study of radiation therapy in elderly and/or frail patients with newly diagnosed glioblastoma Multiforme. J Clin Oncol 2015;33(35):4145–50. [DOI] [PubMed] [Google Scholar]
- [18].Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol 2004;22(9):1583–8. [DOI] [PubMed] [Google Scholar]
- [19].Minniti G, De Sanctis V, Muni R, et al. Hypofractionated radiotherapy followed by adjuvant chemotherapy with temozolomide in elderly patients with glioblastoma. J Neurooncol 2009;91(1):95–100. [DOI] [PubMed] [Google Scholar]
- [20].Minniti G, Lanzetta G, Scaringi C, et al. Phase II study of short-course radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys 2012;83(1):93–9. [DOI] [PubMed] [Google Scholar]
- [21].Perry JR, Laperriere N, O’Callaghan CJ, et al. Short-course radiation plus Temozolomide in elderly patients with glioblastoma. N Engl J Med 2017;376(11):1027–37. [DOI] [PubMed] [Google Scholar]
- [22].Malmstrom A, Gronberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol 2012;13(9): 916–26. [DOI] [PubMed] [Google Scholar]
- [23].Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 2012;13(7):707–15. [DOI] [PubMed] [Google Scholar]
- [24].Yusuf M, Ugiliweneza B, Amsbaugh M, et al. Interim results of a phase II study of Hypofractionated radiotherapy with concurrent Temozolomide followed by adjuvant Temozolomide in patients over 70 years old with newly diagnosed glioblastoma. Oncology 2018;95(1):39–42. [DOI] [PubMed] [Google Scholar]
- [25].Burton E, Ugiliweneza B, Woo S, Skirboll S, Boaky M. A surveillance, epidemiology and end results-Medicare data analysis of elderly patients with glioblastoma multiforme: treatment patterns, outcomes and cost. Mol Clin Oncol 2015;3(5):971–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nead KT, Swisher-McClure S. Utilization of hypofractionated radiation therapy in older glioblastoma patients. J Geriatr Oncol 2019;10(1):155–8. [DOI] [PubMed] [Google Scholar]
- [27].Scott J, Tsai YY, Chinnaiyan P, Yu HH. Effectiveness of radiotherapy for elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys 2011;81(1): 206–10. [DOI] [PubMed] [Google Scholar]
- [28].Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.