Abstract
Repeat-associated non-ATG (RAN) proteins have been reported in 11 microsatellite expansion disorders but the factors that allow RAN translation to occur and the effects of different repeat motifs and alternative AUG-like initiation codons are unclear. We studied the mechanisms of RAN translation across myotonic dystrophy type 2 (DM2) expansion transcripts with (CCUG) or without (CAGG) efficient alternative AUG-like codons. To better understand how DM2 LPAC and QAGR RAN proteins are expressed, we generated a series of CRISPR/Cas9-edited HEK293T cell lines. We show that LPAC and QAGR RAN protein levels are reduced in protein kinase R (PKR)−/− and PKR-like endoplasmic reticulum kinase (PERK)−/− cells, with more substantial reductions of CAGG-encoded QAGR in PKR−/− cells. Experiments using mutant eIF2α-S51A HEK293T cells show that p-eIF2α is required for QAGR production. In contrast, LPAC levels were only partially reduced in these cells, suggesting that both non-AUG and close-cognate initiation occur across CCUG RNAs. Overexpression of the alternative initiation factor eIF2A increases LPAC and QAGR protein levels but, notably, has a much larger effect on QAGR expressed from CAGG-expansion RNAs that lack efficient close-cognate codons. The effects of eIF2A on increasing LPAC are consistent with previous reports that eIF2A affects CUG-initiation translation. The observation that eIF2A also increases QAGR proteins is novel because CAGG expansion transcripts do not contain CUG or similarly efficient close-cognate AUG-like codons. For QAGR but not LPAC, the eIF2A-dependent increases are not seen when p-eIF2α is blocked. These data highlight the differential regulation of DM2 RAN proteins and eIF2A as a potential therapeutic target for DM2 and other RAN diseases.
Introduction
Repeat-associated non-ATG (RAN) translation was first reported by Zu et al. (1), as a process in which repeat expansion mutations can produce proteins in all three reading frames in the absence of an AUG-initiation codon. RAN proteins have now been reported in 11 different microsatellite expansion diseases including both myotonic dystrophy type 1 (DM1) and type 2 (DM2) (1–11). DM2 is caused by an intronic CCTG expansion mutation in the cellular nucleic acid binding protein (CNPB) gene (12). Both sense CCUG and antisense CAGG expansion transcripts and their corresponding tetrapeptide LPAC and QAGR RAN proteins accumulate in patient autopsy brains (7). While RAN translation can occur without AUG- or AUG-like initiation codons in SCA8, DM1 and several other disease-sequence contexts, AUG and/or AUG-like sequences can be present in some of the reading frames (1,13). For example, in Huntington’s disease, the mutant polyglutamine-containing Huntingtin protein expressed from a large AUG-initiated ORF, plus polySer, polyLeu, polyCys and polyAla RAN proteins accumulate in patient brains (6). These results demonstrate that the presence of an AUG-initiation codon and a large ORF in one frame does not preclude the expression of RAN proteins in other reading frames from both sense and antisense transcripts (6).
Although RAN proteins have been reported in a growing number of diseases (1–11,13–16), little is known about how RAN translation is regulated, what specialized factors may be required for RAN translation or how different repeat motifs affect the production of RAN proteins. For example, it is not clear how AUG- and AUG-like close-cognate codons, which can use canonical translation initiation mechanisms, affect the production of proteins in these and other reading frames. Additionally, AUG and CUG close-cognate codons can also be expressed throughout the repeat tract as occurs for the SCA31 TGGAA●TTCCA (9) and DM2 (CCTG●CAGG) (7) repeat expansions, respectively. Thus, a combination of AUG, close-cognate AUG-like and non-cognate translation initiation mechanisms may occur in different reading frames of repeat expansion transcripts. Understanding how these various mechanisms, including RAN translation, are regulated and how sequence variations affect protein production across expansion transcripts will be important for understanding the mechanisms of microsatellite expansion diseases and for the development of therapeutic strategies.
Hairpin-forming expansion RNAs have been shown to activate the protein kinase R (PKR) pathway (17–19). Additional studies show endoplasmic reticulum (ER) and oxidative stress can upregulate RAN translation through the integrated stress response (ISR), which leads to the phosphorylation of the α-subunit of the eukaryotic initiation factor-2 (eIF2α) (20–23). These studies suggest that RAN proteins activate the PKR-like endoplasmic reticulum kinase (PERK) pathway, which in turn leads to further upregulation of RAN translation.
To better understand how RAN translation is regulated in the presence or absence of efficient close-cognate alternative initiation codons, we examined RAN proteins expressed from DM2 CCUG and CAGG expansion transcripts. Our data show that the levels of DM2 LPAC (CCUG) and QAGR (CAGG) RAN proteins are increased by double-strand RNA (dsRNA) and ER stress and reduced in PKR−/− and PERK−/− HEK293T cells, with the most substantial decreases seen for QAGR expression in PKR−/− cells. Additional experiments show that p-eIF2α is required for QAGR but not LPAC accumulation. Finally, we show that the alternative initiation factor eIF2A (24) is required for QAGR expression. In contrast, eIF2A contributes to, but is not required for, LPAC expression. In summary, our work shows that the alternative initiation factor eIF2A plays an important role in DM2 RAN translation and that RAN translation is differentially regulated by stress pathways across CCUG and CAGG expansion transcripts.
Results
DM2 RAN proteins are increased by stress and decreased in PKR and PERK KO cells
To understand the effect of repeat motif and role of stress pathways in RAN translation in DM2, we generated stable cell lines expressing CAGG or CCUG DM2 expansion nanoluciferase RNAs (Supplementary Material, Figs S1 and S2). We selected HEK293T cells for these experiments because they have been previously shown to undergo RAN translation efficiently (1). Luciferase assays show that the QAGR and LPAC nanoluciferase RAN protein levels increase in response to dsRNA- and thapsigargin-induced stress (Fig. 1). Additionally, protein blots show that p-eIF2α levels are significantly increased by these stresses (Fig. 1).
Figure 1 .
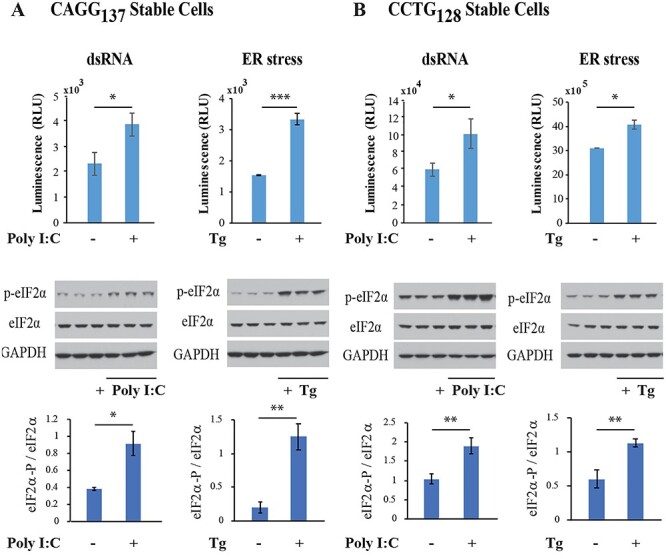
RAN translation is upregulated by dsRNA and ER stress. Stable cell lines expressing nanoluciferase-tagged QAGR (A) or LPAC (B) proteins from constructs containing CAGG or CCUG repeats, respectively, were transfected with double-stranded polyI:C RNA (5 μg/ml) or treated with thapsigargin (Tg) (1 μM) for 12 hrs. Upper graphs show relative luminescence units (RLU) of cells with and without polyIC or Tg treatment. Middle panels show protein blots of treated and untreated cells probed with antibodies against phospho-eIF2α (p-eIF2α), total eIF2α and GAPDH. Lower panels show quantification of p-eIF2α/eIF2α. Error bars show standard deviation (SD). n = 3. Statistical analyses were performed using the two-tailed t-test, *P < 0.05, **P < 0.01, ****P < 0.0001.
Microsatellite expansion RNAs, which form double-stranded RNA structures, can activate PKR (17,18) and misfolded RAN proteins can activate the PERK pathway (20–23), leading to elevated levels of p-PKR and p-PERK, respectively. To study the contributions of the PKR and PERK pathways in RAN translation in DM2, we generated PKR−/− and PERK−/− knockout (KO) HEK293T cells. CRISPR/Cas9 gene targeting experiments were performed using three different guide RNAs for each gene—PKR (EIF2AK2) and PERK (EIF2AK3) (Supplementary Material, Table S1) and PCR, sequencing and protein blotting data confirm successful KOs for at least two independently targeted cell lines for each of the kinases (Supplementary Material, Fig. S3A).
To test the effects of PKR and PERK on RAN translation across DM2 expansion transcripts, KO and control cells were transfected with constructs expressing CAGG or CCUG repeat expansion RNAs. For CAGG repeats, QAGR RAN protein levels were substantially decreased in PKR−/− cells (to <1%, P ≤ 0.001) and to a lesser extent in PERK−/− (to ~28%, P ≤ 0.01) cells compared to control cells (Fig. 2A). Additionally, LPAC levels are reduced in PKR−/− and PERK−/− cells (Fig. 2B). As expected, both PKR−/− and PERK−/− cells showed decreased p-eIF2-α levels compared to WT cells. No changes in RNA levels between KO and wild-type (WT) cells were detected (Supplementary Material, Fig. S4).
Figure 2 .
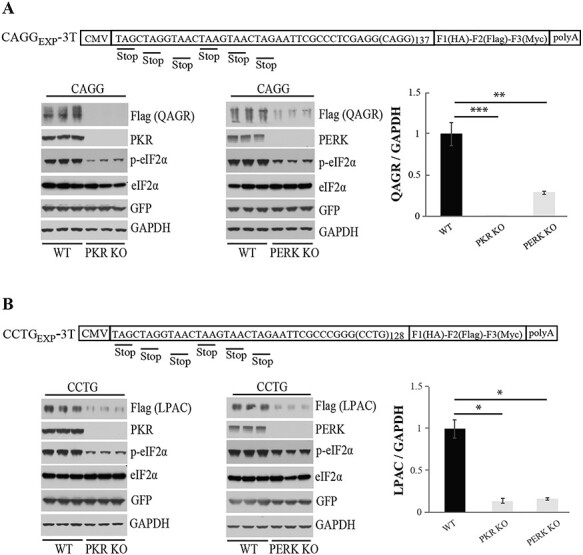
Knocking out PKR and PERK kinases decreases DM2 RAN protein levels. CAGG (A) and CCTG (B) repeat expansion constructs used to co-transfect WT and KO HEK293T cell lines with a GFP expressing control plasmid. These constructs have two upstream stop codons and a C-terminal epitope tag in each reading frame. Protein blots showing QAGR (A) or LPAC (B), PKR, p-eIF2α, total eIF2α, GFP and GAPDH levels in WT versus PKR KO (lower left) or WT versus PERK KO cells (lower middle). Quantification using ImageJ software shows normalized QAGR/GAPDH (A) or LPAC/GAPDH ratios (B) (lower right). Error bars show SD. n = 3. Statistical analyses were performed using the two-tailed t-test, *P < 0.05, **P < 0.01, ****P < 0.0001.
QAGR accumulation is highly affected by eIF2α phosphorylation while LPAC expression shows less dependence on p-eIF2α
To directly test if eIF2α phosphorylation is required for DM2 RAN translation in HEK293T, we used CRISPR/Cas9 to generate a cell line homozygous for the eIF2α-S51A mutation, which prevents phosphorylation at serine 51 (Supplementary Material, Fig. S5A). QAGR levels are dramatically reduced (to ~2%) in HEK293T-S51A compared to WT HEK293T cells, showing that the accumulation of QAGR is highly dependent on p-eIF2α (Fig. 3A). Complementation of eIF2α-S51A by overexpression of eIF2α restores eIF2α phosphorylation and rescues QAGR expression from CAGG repeats (Supplementary Material, Fig. S5B). In contrast, CCUG-encoded LPAC protein levels were only partially reduced in eIF2α-S51A cells, with 56% of the protein remaining compared to WT cells. These results suggest that for CCUG repeats, translation initiation occurs at both close-cognate AUG-like CUG codons, which do not require eIF2α-phosphorylation, and one or more non-cognate start codons in the other reading frames (e.g. CCU, UGC, GCC) (Fig. 3B).
Figure 3 .
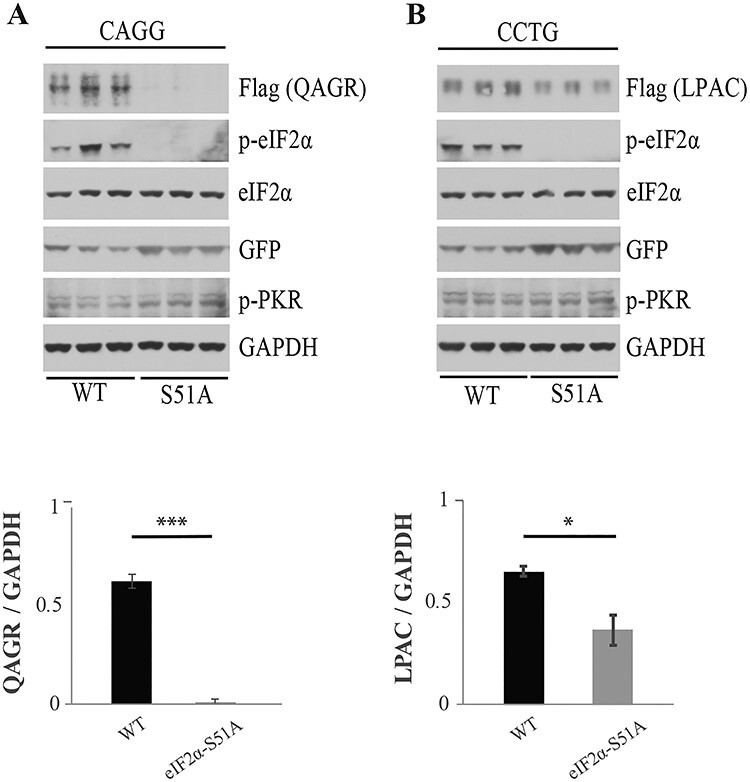
RAN proteins levels are decreased in eIF2α-S51A mutant cells. Protein blots of WT and S51A mutant HEK293T cells co-transfected with CAGG (A) or CCTG (B) expansion vectors and a GFP expressing control plasmid. Protein blots were probed with antibodies against Flag, p-eIF2α, total eIF2α, GFP, p-PKR or GAPDH. Quantification using ImageJ shows the ratio of QAGR or LPAC RAN proteins compared to GAPDH levels (lower panels). Error bars show SD. n = 3. Statistical analyses were performed using the two-tailed t-test, *P < 0.05, ****P < 0.0001.
The alternative translation initiation factor eIF2A increases QAGR and LPAC RAN protein levels
Because phosphorylation of eIF2α prevents the use of eIF2 and the formation of the ternary complex, we tested the effects of the alternative initiation factor eIF2A, which functions though a separate mechanism in eukaryotic translation (24). For these experiments, we generated HEK293T eIF2A−/− cell lines using CRISPR/Cas9 (Supplementary Material, Fig. S6). Protein blots show that LPAC RAN protein levels are decreased in eIF2A−/− cells (Fig. 4B) in the absence of changes in CCUG RNA levels (Supplementary Material, Fig. S7). The decreased levels of LPAC protein expressed from the AUG-like CUG containing transcripts (CCUG expansion) in the eIF2A−/− cells are consistent with previous reports that eIF2A can use CUGs as alternative initiation codons (14,20,25). Additionally, we show that eIF2A increases QAGR RAN protein levels expressed across CAGG expansion transcripts that do not contain efficient alternative close cognate codons (Fig. 4) (26). Overexpression of eIF2A, which increases the levels of QAGR and LPAC RAN proteins, provides further evidence that eIF2A can be used as an alternative initiation factor for RAN translation (Fig. 4C and D).
Figure 4 .
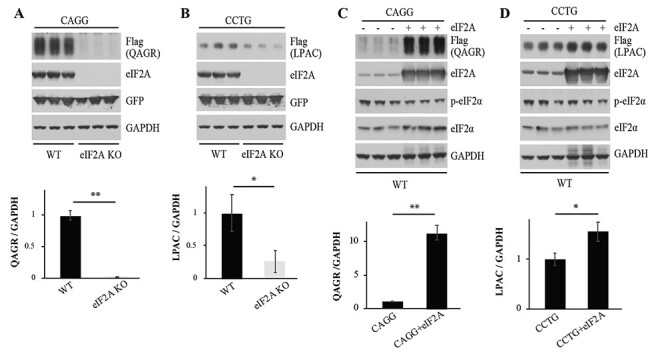
Alternative translation initiation factor 2A (eIF2A) contributes to RAN translation. Immunoblots of WT and eIF2A KO HEK293T cells after co-transfection with CAGG (A) or CCTG (B) expansion vectors and a GFP control plasmid. Protein blots were probed with antibodies against Flag, eIF2A, GFP and GAPDH (upper panels). Quantification using ImageJ presents the ratio of RAN proteins to GAPDH (lower panels). Protein blots of WT cells transfected with CAGG (C) or CCTG (D) expansion constructs with or without co-transfection of an eIF2A expressing construct. Protein blots were probed with antibodies against Flag, eIF2A, p-eIF2α, total eIF2α and GAPDH. Quantification was done using ImageJ with ratios of RAN proteins to GAPDH shown (lower panels). Error bars show SD. n = 3. Statistical analyses were performed using the two-tailed t-test, *P < 0.05, **P < 0.01.
eIF2A-dependent increases in QAGR but not LPAC require eIF2α phosphorylation
To further investigate the role of eIF2A in RAN translation, we overexpressed eIF2A in eIF2α-S51A mutant cells, in which eIF2α phosphorylation is prevented and the ISR is blocked. The increases in QAGR proteins seen with eIF2A overexpression in WT cells were not seen when eIF2A was overexpressed in S51A mutant cells (Fig. 5A). These data are consistent with the hypothesis that eIF2A effectively initiates QAGR expression under conditions in which canonical translation is inhibited by eIF2α phosphorylation. In contrast, LPAC proteins expressed from CCUG expansion transcripts are increased in eIF2α-S51A mutant cells overexpressing eIF2A (Fig. 5B). These data show that eIF2A differentially regulates RAN translation across CCUG and CAGG repeat expansions, and for CAGG, but not CCUG repeats, eIF2A increases in RAN translation require eIF2α phosphorylation.
Figure 5 .
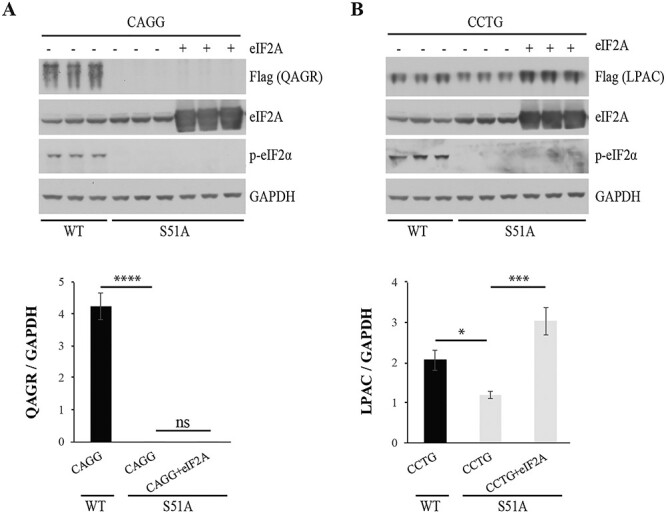
Contribution of eIF2A to RAN translation depends on phosphorylation of eIF2α. Protein blots of WT and S51A mutant HEK293T cells transfected with CAGG (A) or CCTG (B) expansion constructs with or without an eIF2A expressing construct. Protein blots were probed with antibodies against Flag, eIF2A, p-eIF2α and GAPDH. Quantification was done using ImageJ and ratios of RAN proteins to GAPDH are shown (lower panels). Error bars show SD. n = 3. Statistical analyses were performed using the two-tailed t-test, *P < 0.05, ***P < 0.001, ****P < 0.0001.
Discussion
RAN translation has been reported in a growing number of diseases with different types of repeat motifs, but little is known about the mechanisms of RAN translation or how these different motifs affect the production of RAN proteins. To address these questions, we studied how RAN translation across the DM2 tetranucleotide expansion with (CCUG) and without (CAGG) efficient close-cognate codons is affected by various translation initiation factors (7,12). Our data show that QAGR and LPAC RAN protein levels increase with dsRNA and ER stress and are reduced in PKR−/−, PERK−/− cells and eIF2α-S51A mutant HEK293T cells. These reductions were more substantial in PKR−/− and eIF2α-S51A cells for QAGR expressed from CAGG transcripts that lack efficient close-cognate codons. KO and overexpression experiments show that the alternative initiation factor eIF2A enhances the expression of CCUG-encoded LPAC RAN proteins. This result is consistent with previous studies, showing that eIF2A can initiate translation at CUG codons (14,20,25). Additionally, our data show that eIF2A is required for CAGG-encoded QAGR expression, which indicates for the first time that eIF2A is required for translation at additional non-CUG alternative codons. Finally, we show that the eIF2A-dependent increases in QAGR, but not LPAC levels, require p-eIF2α. Taken together, these data show that RAN translation in DM2 is mediated by the alternative initiation factor eIF2A and that LPAC and QAGR RAN protein expression is differentially regulated by stress.
It was previously suggested that RAN proteins expressed in C9orf72 ALS/FTD and FXTAS cause ER stress, which leads to PERK activation, eIF2α-phosphorylation and a feedforward cycle of increased RAN translation (20–23). Our data show that both PKR and PERK pathways are important drivers of RAN translation across CAGG and CCUG transcripts. Specifically, we show that RAN proteins expressed across CAGG and CCUG repeats are dramatically decreased in PKR KO cells. These data, combined with previous work showing that CUG repeats activate PKR (17–19), suggest that CAGG and CCUG expansion transcripts activate PKR and that PKR activation initiates a feedforward cycle in which both PKR and PERK activation lead to increased p-eIF2α and increased RAN translation.
In 2011, Zu et al. (1) showed that RAN translation is differentially affected by flanking sequence and the presence of close-cognate initiation codons. While several mechanistic studies have highlighted the use of upstream alternative CUG initiation codons (14,20,25), which use AUG-like initiation mechanisms, RAN translation also occurs in the absence of close-cognate codons (1). Direct comparisons of DM2 repeats with (CCUG) and without (CAGG) efficient close-cognate initiation codons (26) highlight several mechanistic differences. LPAC levels expressed from close-cognate containing CCUG repeats are less affected by PKR, p-eIF2α and eIF2A compared to QAGR levels expressed from CAGG repeats. These data are consistent with a model in which translation across CCUG repeats uses both non-canonical initiation at GCC, CCU and UGC codons and canonical mechanisms at AUG-like close-cognate CUG codons. In contrast, CAGG repeats do not contain efficient close-cognate codons in any frame (26), and therefore, non-canonical mechanisms are required for RAN translation across DM2 CAGG transcripts and possibly other repeats.
Starck et al. showed that translation initiation of antigenic precursor proteins can start at a CUG initiation codon and that CUG initiation can use a Leu tRNA and eIF2A as an alternative initiation factor (25). Upstream close-cognate initiation codons can also be used as translational start sites for polyGA RAN proteins, which are expressed in C9orf72 ALS/FTD, and other studies show decreased levels of polyGA expression in eIF2A−/− cells (14,20). Close-cognate codons have also been shown to be used for translation initiation of the polyGly repeat expansion protein that accumulates in FXTAS (16). Our data extend these studies by showing RAN translation across DM2 CCUG and CAGG repeats are both similar and distinct from C9orf72 and FXTAS. For example, LPAC levels are partially reduced in both eIF2A−/− and in eIF2α-S51A cells, suggesting that eIF2α and eIF2A both contribute to translation initiation across CCUG repeat transcripts. In contrast, QAGR expression was dramatically reduced in eIF2A−/− cells, indicating that eIF2A plays a prominent role in the initiation of RAN proteins across CAGG repeats, which does not contain efficient alternative close-cognate initiation codons.
Additional data show that QAGR but not LPAC protein expression requires both eIF2A and p-eIF2α. These data indicate that eIF2A can act as an effective alternative initiation factor for repeats lacking efficient close-cognate initiation codons under conditions of stress in which eIF2α is phosphorylated. These data also suggest that for CAGG repeats, eIF2A does not compete well with unphosphorylated eIF2α or other required protein initiation factors. In contrast, for CCUG repeats, the increase in LPAC levels seen with eIF2A overexpression does not require p-eIF2α, suggesting that eIF2α and eIF2A independently contribute to LPAC production and that eIF2A can compete with eIF2α for translation initiation across CCUG repeats. These data highlight the importance of examining translational requirements and factors for both sense and antisense repeat expansion transcripts and for expansions with different repeat motifs.
In conclusion, our data show that PKR and PERK pathways play an important role in DM2 RAN translation and that RAN translation is differentially regulated across CAGG and CCUG repeat motifs. Previous studies showing that QAGR and LPAC accumulate in different brain regions (7) provide additional support that distinct mechanisms are involved in QAGR and LPAC expression. Further studies of LPAC and QAGR in cell culture and animal models will provide insight into their respective contributions to disease and if targeting one or both of these proteins will improve disease. These findings will also impact the development of therapeutic strategies that may need to target multiple brain regions to reduce one or both of these proteins. Additionally, our data suggest a feedforward loop initiated by structured repeat expansion RNAs, which activate PKR, leading to p-eIF2α and RAN translation. Aggregated RAN proteins, in turn, activate PERK, which further increases p-eIF2α and RAN proteins levels. Moreover, we show that the alternative initiation factor eIF2A plays an important role in RAN translation of DM2 repeat expansion transcripts with (CCUG) and without (CAGG) efficient close-cognate codons. Taken together, these data show that RAN proteins in DM2 are differentially affected by stress and identify eIF2A as a novel therapeutic target for DM2, and possibly other RAN protein disorders (Fig. 6).
Figure 6 .
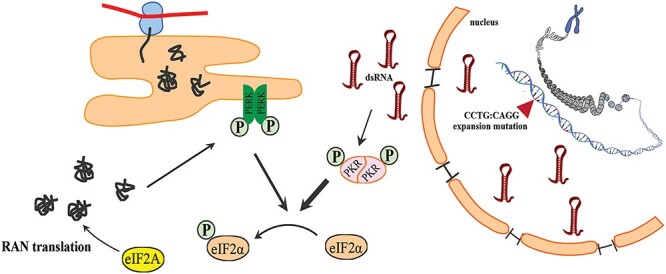
Feedforward loop and role of eIF2A in DM2 RAN translation. Schematic model of RAN translation showing PKR is activated by dsRNAs, which in turn leads to phosphorylation of eIF2α and conditions that allow that alternative initiation factor eIF2A to compete with eIF2α and initiate RAN translation. Activation of the PERK pathway by RAN proteins may lead to additional eIF2α phosphorylation and further increase RAN protein production.
Materials and Methods
DNA constructs
The CCTG-3T and CAGG-3T repeat expansion constructs used for transfecting WT, KO and S51A mutant HEK293T cells were previously described (7). All of these constructs contain six stop codons (two in each reading frame) upstream of the repeat expansion and different C-terminal epitope tags in each reading frame.
FRT/TO constructs with CAGG or CCTG expansions were generated by subcloning CAGG or CCTG expansions of 137 or 128 repeats, respectively, into the FRT/TO vector. FRT/TO, pFRT/lacZeo and pOG44 vectors were obtained from Flp-In™ T-REx™ Core Kit (Invitrogen, Cat# K6500-01).
Generation of stable cell lines
Stable HEK293T cell lines expressing QAGR or LPAC proteins were generated using the Flp-In T-Rex cell system according to the manufacturer’s instructions (Invitrogen, Cat # K6500-01). First, pFRT/lacZeo vector containing Flp Recombination Target (FRT) was transfected into HEK293T cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. After 48 h post-transfection, cells were split to 25% confluency, and 3 h later when cells had attached to the culture dish, zeocin was added to the culture for selection. Zeocin-resistant clones were selected with 200 μg/ml zeocin and then screened by Southern blotting to identify a clone containing a single integrated FRT site, which was selected as the host cell line for further recombination. Next, pcDNA5/FRT/TO vectors encoding QAGR or LPAC RAN proteins and pOG44 vector, which expresses Flp recombinase, were co-transfected at a ratio of 1:9 (w/w) into host cells using Lipofectamine 3000. After 48 h post-transfection, hygromycin B (85 μg/ml) was added to the culture for selection. After 2 weeks, hygromycin-resistant repeat expansion containing clones were harvested and used for further analysis.
Southern blotting
Genomic DNA was extracted using FlexiGene DNA Kit (QIAGEN, Cat No. 51206), following the manufacturer’s instructions. Eight μg of genomic DNA was digested using EcoRI at 37°C overnight and separated by electrophoresis, transferred to a nylon membrane (GE Healthcare). The membrane was UV cross-linked and pre-hybridized at 65°C for 2 h in Amersham™ Rapid-hyb buffer (GE Healthcare) and then was hybridized with a 32P-dCTP-labeled probe complementary to a region of the Zeocin or nanoluciferase genes at 65°C for 3 h. The membrane was then washed with 2 × SSC, 0.1% SDS solution (300 mM sodium chloride, 30 mM sodium citrate, pH 7.0) at room temperature (RT) for 20 min and three more times with 0.2 × SSC, 0.1% SDS solution at 65°C for 15 min per wash. The radioactive signal from the probe was detected using X-ray film after 24 h of exposure at −80°C.
Nanoluciferase assay
Stable cells were washed with PBS and passive lysis buffer (Promega, Madison, WI) was added to each well of culture plate and incubated for 15 min on ice. Then, samples were centrifuged at 14 000×g for 10 min at 4°C, and subsequently, supernatants from each sample were collected. The protein concentration of each sample was measured using BCA protein assay kit (ThermoFisher, Waltham, MA). To measure luminescence signal, equal amounts of each sample (in terms of both volume and concentration) were added to individual wells on white luminescence plates (Thermo Fisher Scientific). Next, the same volume of Nano-Glo® Luciferase Assay Reagent (Promega) was added to each well, and after incubation for 3 min at RT, relative luminescence units were measured using a CLARIOstar Plus Microplate Reader (BMG LABTECH). All samples were tested in duplicate wells.
Cell culture and transfections
HEK293T cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and incubated at 37°C in a humid atmosphere containing 5% CO2. Plasmid transfection was done in 12-well tissue culture plate using Lipofectamine 2000 or 3000 (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions. Cells were collected 48 h post-transfection for subsequent analyses.
Generation of KO cell lines
CRISPR/Cas9 editing was used to develop a series of HEK293T KO cell lines. Guide RNAs targeting the EIF2AK2 (PKR), EIF2AK3 (PERK) and EIF2A genes (Supplementary Material, Table S1) were selected using www.chopchop.cbu.uib.no website and cloned into gRNA cloning vector (Addgene plasmid # 41824). The gRNA and cas9 (Addgene plasmid # 42230) plasmids were co-transfected (1:1) into HEK293T cells using Lipofectamine 2000, and 72 h post-transfection, single cell was seeded into the 96-well plates. Confirmation of target knock out was done using western blotting and sequencing.
Generation of S51A mutant cell line
Three gRNAs (Supplementary Material, Table S1) targeting exon 2 of the EIF2S1 gene, close to the desired mutation point: 67364921, were selected using www.chopchop.cbu.uib.no website and cloned into gRNA cloning vector (Addgene # 41824).
A 189 bp single-stranded donor oligonucleotide (ssODN) containing 5′ and 3′ homology arms to the target region was designed as a repair template for endogenous DNA repair mechanisms through homology-directed repair (HDR). The HDR donor has PAM-blocking mutation to block re-editing by CRISPR/Cas9 system. The gRNA and cas9 (Addgene # 42230) plasmids and ssODN (1:1:2) were co-transfected into HEK293T cells using Lipofectamine 2000, and single cells were seeded into 96-well plates. For confirmation of homozygous mutation, western blotting was performed and membranes were probed with antibodies that detect p-eIF2α protein and genomic DNA samples were sequenced to confirm correct gene targeting.
Western blotting
Transfected cells were washed with PBS and lysed with RIPA buffer (150 mM NaC1, 1% sodium deoxycholate, 1% Triton X-100, 50 mM Tris-HCl pH = 7.5, DNase I) containing protease (Roche) and phosphatase inhibitor cocktails (Sigma-Aldrich) for 15 min on ice. Then samples were centrifuged at 14 000×g for 10 min at 4°C, and the supernatant was collected. The protein concentration of each cell lysate was measured using BCA protein assay kit (ThermoFisher). Twenty-five micrograms of protein were run onto 4–12% Criterion Bis-Tris gels (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes (GE Healthcare, Lafayette, CO). Membranes are blocked with 5% dry milk in PBST (PBS containing 0.05% Tween-20) and probed with the primary antibody (1:1000) at 4°C overnight with shaking. Membrane are washed in PBST for 5 min three times and then probed with the HRP-conjugated secondary antibody (1:2500), (GE Healthcare, Lafayette, CO) for 1 h at RT. Membrane was washed again in PBST and then incubated with enhanced ElectroChemiLuminescence reagents (PerkinElmer, Waltham, MA) and exposed to X-ray film. Quantification of the results was performed by densitometric scan of films. Data analysis was done using ImageJ and measuring integrated density of bands after background subtraction.
Antibodies
For western blotting, the following primary antibodies were used: rabbit anti-Myc tag (Abcam, Cat. # ab9106, 1:1000 dilution), mouse anti-HA tag (BioLegent, Cat. # 901513, 1:2000 dilution), mouse anti-Flag tag (Sigma-Aldrich, Cat. # A8592, 1:1000 dilution), rabbit anti-PKR (Abcam, Cat. # ab32506, 1:2000 dilution), rabbit anti-PERK (Cell Signaling Technology, Cat. # C33E10, 1:2000 dilution), mouse anti-GAPDH (Millipore, Cat. # MAB374, 1:5000 dilution), rabbit anti-EIF2S1 (phospho S51) (Abcam, Cat. # ab32157, 1:1000 dilution), rabbit anti-EIF2A (Proteintech, Cat. # 11233–1-AP, 1:10000 dilution) and rabbit anti-GFP (Santa Cruz, Cat. # sc-8334, 1:3000 dilution). For western blotting, the following secondary antibodies were used: sheep anti-mouse IgG HRP (GE Healthcare, Cat. # NA931, 1:2000 dilution) and donkey anti-rabbit IgG HRP (GE Healthcare, Cat. # NA934, 1:2000 dilution).
For immunofluorescence, the following antibodies were used: rabbit anti-QAGR (Custom antibody, clone 3677, 1:500 dilution) (7), rabbit anti-LPAC (Custom antibody, clone 3674, 1:500 dilution) (7), goat anti-mouse IgG conjugated to Alexa Fluor 488 (Invitrogen, Cat. # A-11001, 1:500 dilution) and donkey anti-rabbit IgG conjugated to Alexa Fluor 488 (Invitrogen, Cat. # A-21206, 1:500 dilution).
Immunofluorescence
Stable HEK293T cells were seeded in L-lysine-coated 8-well chamber, and 24 h later, cells were fixed in 4% paraformaldehyde in PBS at RT for 30 min and permeabilized in 0.5% Triton X-100 in PBS at RT for 30 min. After three times washing with PBS for 5 min per wash, the slide was blocked in 1% normal goat serum in PBS at RT for 30 min. Next, the cells were incubated in blocking solution containing the α-FLAG antibody (1:500) (A8592, Sigma, St. Louis, MO) or α-LPAC or α-QAGR primary antibodies (1:500) for 1 h at 37°C. The slide was washed three times in PBS for 5 min per wash and subsequently incubated in blocking solution containing secondary antibodies for 1 h at 37°C. After washing step, cells were mounted with DAPI (Invitrogen, Carlsbad, CA) containing diamond Prolong mounting solution and coverslipped. Images were taken using confocal microscopy (LSM880, METALaser scanning microscope, Zeiss).
RNA extraction and cDNA synthesis
To check the transcript expression levels from transfections, RNAs were extracted from the different cell lines including wild type, different knock out and S51A mutant using Trizol Reagent (Invitrogen). First, TURBO DNA-free kit (Invitrogen) was used for DNAse treatment following the manufacturer’s protocol. Next, 1 μg of RNA was used to synthesize cDNA using SuperScript III Reverse Transcriptase System (Invitrogen) following the manufacturer’s protocol.
Quantitative PCR
Quantification of transcript levels in transfected wild type, different knock out and S51A mutant cells was performed using the 33T forward (5′ GGA CGA CGA CGA CAA GTA GC 3′) and 33T reverse (5′ CAG CTT CTG CTC GCT ATG C 3′) primers. eGFP forward (5′ CGA GCT GGA CGG CGA CGT AAA C 3′) and eGFP reverse (5′ GCC GGT GGT GCA GAT GAA CTT C 3’) primers were used for control reaction (27). qPCR results were analyzed using the 2−ΔΔCT method (28).
Statistical analysis
All data are represented as the mean ± standard deviation and processed by the software GraphPad Prism 5 (GraphPad Software Inc., GraphPad Software, Inc., La Jolla, CA). Comparison between groups was made by a two-tailed Student’s t-test or a one-way analysis of variance for multiple comparisons.
Supplementary Material
Acknowledgements
We thank Tammy Reid and Ramadan Ajredini for technical support.
Conflict of Interest statement. Drs Ranum, Zu and Nguyen are inventors on University of Florida patents and patent applications related to RAN translation.
Contributor Information
Solaleh Khoramian Tusi, Center for NeuroGenetics, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Department of Molecular Genetics and Microbiology, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Genetics Institute, University of Florida, Gainesville, FL 32610, USA.
Lien Nguyen, Center for NeuroGenetics, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Department of Molecular Genetics and Microbiology, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Genetics Institute, University of Florida, Gainesville, FL 32610, USA.
Kiruphagaran Thangaraju, Center for NeuroGenetics, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Department of Molecular Genetics and Microbiology, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Genetics Institute, University of Florida, Gainesville, FL 32610, USA.
Jian Li, Center for NeuroGenetics, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Department of Molecular Genetics and Microbiology, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Genetics Institute, University of Florida, Gainesville, FL 32610, USA.
John D Cleary, Center for NeuroGenetics, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Department of Molecular Genetics and Microbiology, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Genetics Institute, University of Florida, Gainesville, FL 32610, USA.
Tao Zu, Center for NeuroGenetics, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Department of Molecular Genetics and Microbiology, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Genetics Institute, University of Florida, Gainesville, FL 32610, USA.
Laura P W Ranum, Center for NeuroGenetics, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Department of Molecular Genetics and Microbiology, College of Medicine, University of Florida, Gainesville, FL 32610, USA; Genetics Institute, University of Florida, Gainesville, FL 32610, USA; McKnight Brain Institute, University of Florida, Gainesville, FL 32610, USA; Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, FL 32610, USA.
Funding
National Institutes of Health (RO1 NS098819, R37NS040389, PO1-NS058901); Target ALS; ALS Association; Packard Center; Myotonic Dystrophy Foundation; Department of Defense (W81XWH1910654); Muscular Dystrophy Association for support.
References
- 1. Zu, T., Gibbens, B., Doty, N.S., Gomes-Pereira, M., Huguet, A., Stone, M.D., Margolis, J., Peterson, M., Markowski, T.W., Ingram, M.A. et al. (2011) Non-ATG-initiated translation directed by microsatellite expansions. Proc. Natl. Acad. Sci. U. S. A., 108, 260–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zu, T., Liu, Y., Banez-Coronel, M., Reid, T., Pletnikova, O., Lewis, J., Miller, T.M., Harms, M.B., Falchook, A.E., Subramony, S.H. et al. (2013) RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl. Acad. Sci. U. S. A., 110, E4968–E4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Todd, P.K., Oh, S.Y., Krans, A., He, F., Sellier, C., Frazer, M., Renoux, A.J., Chen, K.C., Scaglione, K.M., Basrur, V. et al. (2013) CGG repeat-associated translation mediates neurodegeneration in fragile X tremor ataxia syndrome. Neuron, 78, 440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mori, K., Weng, S.M., Arzberger, T., May, S., Rentzsch, K., Kremmer, E., Schmid, B., Kretzschmar, H.A., Cruts, M., Van Broeckhoven, C. et al. (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science, 339, 1335–1338. [DOI] [PubMed] [Google Scholar]
- 5. Ash, P.E., Bieniek, K.F., Gendron, T.F., Caulfield, T., Lin, W.L., Dejesus-Hernandez, M., van Blitterswijk, M.M., Jansen-West, K., Paul, J.W., 3rd, Rademakers, R. et al. (2013) Unconventional translation of C9ORF72 GGGGCC expansion generates insoluble polypeptides specific to c9FTD/ALS. Neuron, 77, 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Banez-Coronel, M., Ayhan, F., Tarabochia, A.D., Zu, T., Perez, B.A., Tusi, S.K., Pletnikova, O., Borchelt, D.R., Ross, C.A., Margolis, R.L. et al. (2015) RAN translation in Huntington disease. Neuron, 88, 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zu, T., Cleary, J.D., Liu, Y., Banez-Coronel, M., Bubenik, J.L., Ayhan, F., Ashizawa, T., Xia, G., Clark, H.B., Yachnis, A.T. et al. (2017) RAN translation regulated by Muscleblind proteins in myotonic dystrophy type 2. Neuron, 95, 1292–1305 e1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soragni, E., Petrosyan, L., Rinkoski, T.A., Wieben, E.D., Baratz, K.H., Fautsch, M.P. and Gottesfeld, J.M. (2018) Repeat-associated non-ATG (RAN) translation in Fuchs’ endothelial corneal dystrophy. Invest. Ophthalmol. Vis. Sci., 59, 1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishiguro, T., Sato, N., Ueyama, M., Fujikake, N., Sellier, C., Kanegami, A., Tokuda, E., Zamiri, B., Gall-Duncan, T., Mirceta, M. et al. (2017) Regulatory role of RNA chaperone TDP-43 for RNA misfolding and repeat-associated translation in SCA31. Neuron, 94, 108–124 e107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buijsen, R.A., Visser, J.A., Kramer, P., Severijnen, E.A., Gearing, M., Charlet-Berguerand, N., Sherman, S.L., Berman, R.F., Willemsen, R. and Hukema, R.K. (2016) Presence of inclusions positive for polyglycine containing protein, FMRpolyG, indicates that repeat-associated non-AUG translation plays a role in fragile X-associated primary ovarian insufficiency. Hum. Reprod., 31, 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McEachin, Z.T., Gendron, T.F., Raj, N., Garcia-Murias, M., Banerjee, A., Purcell, R.H., Ward, P.J., Todd, T.W., Merritt-Garza, M.E., Jansen-West, K. et al. (2020) Chimeric peptide species contribute to divergent dipeptide repeat pathology in c9ALS/FTD and SCA36. Neuron, 107, 292–305 e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liquori, C.L., Ricker, K., Moseley, M.L., Jacobsen, J.F., Kress, W., Naylor, S.L., Day, J.W. and Ranum, L.P. (2001) Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science, 293, 864–867. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen, L., Cleary, J.D. and Ranum, L.P.W. (2019) Repeat-associated non-ATG translation: molecular mechanisms and contribution to neurological disease. Annu. Rev. Neurosci., 42, 227–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabet, R., Schaeffer, L., Freyermuth, F., Jambeau, M., Workman, M., Lee, C.Z., Lin, C.C., Jiang, J., Jansen-West, K., Abou-Hamdan, H. et al. (2018) CUG initiation and frameshifting enable production of dipeptide repeat proteins from ALS/FTD C9ORF72 transcripts. Nat. Commun., 9, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glineburg, M.R., Todd, P.K., Charlet-Berguerand, N. and Sellier, C. (2018) Repeat-associated non-AUG (RAN) translation and other molecular mechanisms in fragile X tremor ataxia syndrome. Brain Res., 1693, 43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kearse, M.G., Green, K.M., Krans, A., Rodriguez, C.M., Linsalata, A.E., Goldstrohm, A.C. and Todd, P.K. (2016) CGG repeat-associated non-AUG translation utilizes a cap-dependent scanning mechanism of initiation to produce toxic proteins. Mol. Cell, 62, 314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tian, B., White, R.J., Xia, T., Welle, S., Turner, D.H., Mathews, M.B. and Thornton, C.A. (2000) Expanded CUG repeat RNAs form hairpins that activate the double-stranded RNA-dependent protein kinase PKR. RNA, 6, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zu, T., Guo, S., Bardhi, O., Ryskamp, D.A., Li, J., Khoramian Tusi, S., Engelbrecht, A., Klippel, K., Chakrabarty, P., Nguyen, L. et al. (2020) Metformin inhibits RAN translation through PKR pathway and mitigates disease in C9orf72 ALS/FTD mice. Proc. Natl. Acad. Sci. U. S. A., 117, 18591–18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edery, I., Petryshyn, R. and Sonenberg, N. (1989) Activation of double-stranded RNA-dependent kinase (dsl) by the TAR region of HIV-1 mRNA: a novel translational control mechanism. Cell, 56, 303–312. [DOI] [PubMed] [Google Scholar]
- 20. Sonobe, Y., Ghadge, G., Masaki, K., Sendoel, A., Fuchs, E. and Roos, R.P. (2018) Translation of dipeptide repeat proteins from the C9ORF72 expanded repeat is associated with cellular stress. Neurobiol. Dis., 116, 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Green, K.M., Glineburg, M.R., Kearse, M.G., Flores, B.N., Linsalata, A.E., Fedak, S.J., Goldstrohm, A.C., Barmada, S.J. and Todd, P.K. (2017) RAN translation at C9orf72-associated repeat expansions is selectively enhanced by the integrated stress response. Nat. Commun., 8, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng, W., Wang, S., Mestre, A.A., Fu, C., Makarem, A., Xian, F., Hayes, L.R., Lopez-Gonzalez, R., Drenner, K., Jiang, J. et al. (2018) C9ORF72 GGGGCC repeat-associated non-AUG translation is upregulated by stress through eIF2alpha phosphorylation. Nat. Commun., 9, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Westergard, T., McAvoy, K., Russell, K., Wen, X., Pang, Y., Morris, B., Pasinelli, P., Trotti, D. and Haeusler, A. (2019) Repeat-associated non-AUG translation in C9orf72-ALS/FTD is driven by neuronal excitation and stress. EMBO Mol. Med., 11, e9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Komar, A.A. and Merrick, W.C. (2020) A retrospective on eIF2A-and not the alpha subunit of eIF2. Int. J. Mol. Sci., 21, 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Starck, S.R., Jiang, V., Pavon-Eternod, M., Prasad, S., McCarthy, B., Pan, T. and Shastri, N. (2012) Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science, 336, 1719–1723. [DOI] [PubMed] [Google Scholar]
- 26. Peabody, D.S. (1989) Translation initiation at non-AUG triplets in mammalian cells. J. Biol. Chem., 264, 5031–5035. [PubMed] [Google Scholar]
- 27. Jiwaji, M., Daly, R., Pansare, K., McLean, P., Yang, J., Kolch, W. and Pitt, A.R. (2010) The Renilla luciferase gene as a reference gene for normalization of gene expression in transiently transfected cells. BMC Mol. Biol., 11, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Livak, K.J. and Schmittgen, T.D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


