Abstract
Novel strategies modulating the immune system yielded enhanced anticancer responses and improved cancer survival. Nevertheless, the success rate of immunotherapy in cancer treatment has been below expectation(s) due to unpredictable efficacy and off-target effects from systemic dosing of immunotherapeutic. As a result, there is an unmet clinical need for improving conventional immunotherapy. Nanotechnology offers several new strategies, multimodality, and multiplex biological targeting advantage to overcome many of these challenges. These efforts enable programming the pharmacodynamics, pharmacokinetics, delivery of immunomodulatory agents/co-delivery of compounds to prime at the tumor sites for improved therapeutic benefits. This review provides an overview of the design and clinical principles of biomaterials driven nanotechnology and their potential use in personalized nanomedicines, vaccines, localized tumor modulation, and delivery strategies for cancer immunotherapy. In this review, we also summarize the latest highlights and recent advances in combinatorial therapies avail in the treatment of cold and complicated tumors. It also presents key steps and parameters implemented for clinical success. Finally, we analyse, discuss, and provide clinical perspectives on the integrated opportunities of nanotechnology and immunology to achieve synergistic and durable responses in cancer treatment.
Keywords: nanoparticles, immunotherapy, adjuvants, biomaterials, vaccines, cancer treatment, tumor, imaging, theranostic
1. Introduction
The conventional therapies, such as surgery, radiation, and chemotherapies or their combination, have shown significant improvements in cancer treatment. Surgery is feasible only in removal of primary and solid tumors. [1] However, it is often difficult to detect tumors in the early stages.[2] While chemotherapy and radiation treatments are often recommended but encountered higher relapse.[3] Thus, research has been focused on immunotherapies in which the human body’s own defense mechanism acts against cancer cells. Cancer immunotherapy is considered a paradigm shift in cancer treatments.[4] The immune system is primarily responsible for protecting humans from foreign particles and microorganisms (bacteria, viruses, and organisms) in the human body. The effective function of immune system is coordinated by two cellular compartments, i.e., “innate” and “adaptive” immune system[5]. The innate immune system involves phagocytes (macrophages and dendritic cells) and granulocytes (neutrophils, eosinophils, basophils, and mast cells), while adaptive immune response is governed by T-lymphocytes and B-lymphocytes. The innate immune system provides the first line of defense by distinguishing widespread molecular recognitions on pathogens; thus, phagocytes and granulocytes are instantly stimulated and deployed to the sites of inflammation, infection, or tissue damage. In addition, lectin and other associated pathways are considered as complementary components of the innate immune system. Whereas, adaptive immune system recognizes pathogens via specific antigen presence. This immune component is also referred as “memory” to the immune response, i.e., it can rapidly recollect specific immune responsivity to molecule or antigens that are present on pathogen that had seen in the past. In general, immunotherapy is used to treat a disease either by inhibiting or enhancing the immune system. Cancer immunotherapy functions by producing the immune response and train the immune cells to search and destroy the cancer cells. The discovery of immune checkpoint inhibitors by James Allison and Tauku Honjo (the 2018 Noble laureates) reestablished the belief in immunotherapy for the treatment of cancer[6, 7]. Currently, immunotherapy is one of the clinically proven treatment options for many cancers to enhance the immune system by employing cytokines, immune checkpoint inhibitors, engineered T cells, monoclonal antibodies, and cancer vaccines.[8]
1.1. Cytokines
Cytokines are a group of proteins and major regulators of innate and adaptive immunity. Cytokines display significant role in boosting the immune system. Interferon (IFN), interleukins (IL), and Granulocyte-macrophage colony-stimulating factor (GM-CSF) are widely employed cytokines in cancer immunotherapy [9]. In response to microbial infection, immune cells produce interferons that are responsible for the maturation of other immune cells like natural killers (NK), macrophages, dendritic cells, and lymphocytes [10-12], The interferon also responsible for the inhibition of angiogenesis in the tumor, while interleukins help in stimulating CD4+ and CD8+ T-cells [12-14]. GM-CSF aids in the homeostasis of T-cells, which is critical for its survival, also supports the differentiation of dendritic cells to promote antigen presentation. GM-CSF, as well as granulocyte colony-stimulating factor (GCSF), are also used for maintaining the number of granulocytes after chemotherapy and radiotherapy [15]. Cytokines were first time introduced for immunotherapy with the approval of IFN. Currently, 3 recombinant cytokines have been approved for immunotherapy, and several more are in pipeline [16] (Table 1). However, high dosages of cytokines are required due to shorter half-life that leads to vascular leakage and cytokine release syndrome. Also, it might cause immune attack on the healthy tissue [9, 17]. Towards it, IL-15 and IL-21 have been found to be advantageous in comparison to IL-2 [18, 19]. Current treatment strategies include usage of cytokines in combination to other therapies like chemotherapy and checkpoint inhibitors for reducing the dosage-related adverse effects [11].
Table 1.
List of approved and under clinical trial cancer immunotherapies.
| Type | Name (trade name) |
Manufacturer/ CTid |
Target/ Platform |
Indication (Phase) |
|---|---|---|---|---|
| Cytokines | IFN-α2b (Intron A) | Merck & Co., Inc | Kaposi Sarcoma (II), leukemia (IV), lymphoma (IV), melanoma (III) | |
| IFN-α2a (Roferon-A) | Genentech, Inc. | Kaposi Sarcoma (II), leukemia (IV) | ||
| Recombinant IL-2 (Aldesleukin) | Novartis | RCC (III), melanoma (III) | ||
| 1-isobutyl-1H-imidazo (Imiquimod) | Perrigo | Superficial basal cell carcinoma (III) | ||
| IFN-α2b | NCT02634294 | Hematological malignancies (II/III) | ||
| IFN-α2a | NCT03253250 | Hepatocellular carcinoma (IV) | ||
| NCT02829775 | Advanced tumors (II/III) | |||
| GM-CSF | NCT03363373 | Neuroblastoma (II) | ||
| Checkpoint inhibitors | Ipilimumab (Yervoy) | Bristol-Myers Squibb | CTLA-4 | Melanoma (IV) |
| Pembrolizumab (Keytmda) | Merck & Co | PD-1 | HL (III), lung cancer (IV), melanoma (IV), head and neck cancer (IV), stomach cancer (III) | |
| Nivolumab (Opdivo) | Bristol-Myers Squibb | PD-1 | Melanoma (IV), RCC (IV), HL (III), liver cancer (IV), bladder cancer (III), NSCLC (IV) | |
| Atezolizumab (Tecentriq) | Roche, Genentech | PD-1 | Lung (IV), breast (III), bladder (III), urinary tract cancer (III) | |
| Avelumab (Bavencio) | Merck KGaA and Pfizer | PD-L1 | Merkel cell carcinoma (III) | |
| Durvalumab (Imfinzi) | Medimmune/AstraZe neca | PD-L1 | Bladder (III), urinary tract cancer (III), NSCLC (IV) | |
| Cemiplimab | Sanofi | PD-L1 | Squamous cell carcinoma (III) | |
| IMP321 | NCT00365937, NCT01308294, NCT02614833, NCT03625323 | LAG-3 | Advanced melanoma (I/II), Metastatic breast cancer (II), Advanced NSCLC and HNSCC (II), | |
| Relatlimab | NCT01968109, NCT02488759, NCT02061761, NCT03459222, NCT03623854, NCT03743766, NCT03642067, NCT03607890, NCT02996110, NCT02935634, NCT02750514, NCT02060188, NCT02519322 | LAG-3 | Virus associated cancer (I/II), hematologic malignancies (I/II), Advanced solid tumors (II), chordoma (II), melanoma (II), RCC (II), GC (II), NSCLC (II), CRC (II), | |
| LAG525 | NCT02460224, NCT03365791, NCT03499899, NCT03484923 | LAG-3 | Advanced Solid tumor (I/II), hematological malignancies (II), TNBC (II), melanoma (II) | |
| MK-4280 | NCT03598608, NCT03516981 | LAG-3 | Advanced NSCLC (II), hematological malignancies (I/II), | |
| BI754111 | NCT03697304 | LAG-3 | Advanced solid tumor (II) | |
| TSR-022 | NCT03680508 | TIM-3 | Liver Cancer (II) | |
| MBG453 | NCT02608268 | TIM-3 | Advanced malignancies (I/II) | |
| BMS-986258 | NCT03446040 | TIM-3 | Advanced solid tumor (I/II) | |
| Tiragolumab | NCT03563716 | TIGIT | Advanced NSCLC (II) | |
| BMS-986207 | NCT02913313 | TIGIT | Advanced solid tumor (I/II) | |
| Enoblituzumab | NCT02923180 | B7-H3 | Prostate cancer (II) | |
| 131I-8H9/omburtamab | NCT03275402 | B7-H3 | Neuroblastoma/leptomeningeal metastases (II/III) | |
| CAR-T cells | Tisagenlecleucel (Kymriah) | Novartis Pharma | CD19 | Leukemia (III), lymphoma (III) |
| Axicabtagene ciloleucel (Yescarta) | Kite Pharma | CD19 | NHL (II) | |
| NCT03631576, NCT02937103, NCT03398967 | CD123 | AML (II/III), myeloid malignancies (I/II) | ||
| NCT03398967 | CD19 and CD20 or CD22 | B cell leukemia and lymphoma (I/II) | ||
| NCT02958397 | CD33 | myeloid malignancies (I/II) | ||
| NCT03754764 | CD38 | B-ALL (I/II) | ||
| NCT03196414 | CART-138 | MM (I/II) | ||
| NCT03778346 | CD38/CD138 | MM (I) | ||
| NCT03767751 | Dual CD38/BCMA | MM (I/II) | ||
| NCT03222674 | Muc1/CLL1/CD33/CD38/CD56/CD123 | AML (I/II) | ||
| Vaccine | Spiuleucel-T (Provenge) | Dendreon Pharmaceuticals | Autologous immuno therapy | Hormone-refractory prostate cancer (III) |
| BCG | Merck & Co., Dianon Systems, Evans Vaccines, Statens Serum Institut, Japan BCG Laboratory NCT03300843 | Attenuated Mycobac terium bovis DC | Bladder cancer (IV) solid cancers (II) | |
| NCT03480152 | RNA | solid cancers (I/II) | ||
| NCT03598816 | DNA | RCC (II) | ||
| NCT03633110 | Synthetic peptide | Solid cancers (I/II) | ||
| NCT03639714 | RNA | Solid cancer (I/II) |
Abbreviation: Bacillus Calmette Guerin (BCG), acute myeloid leukemia (AML), multiple myeloma (MM), renal cell carcinoma (RCC), B-cell acute lymphoblastic leukemia (B-ALL), triple-negative breast cancer (TNBC), non-small cell lung cancer (NSCLC), gastric cancer (GC), colorectal cancer (CRC), head and neck squamous cell cancer (HNSCC), Hodgkin lymphoma (HL), non - Hodgkin lymphoma (NHL)
1.2. Checkpoint inhibitors
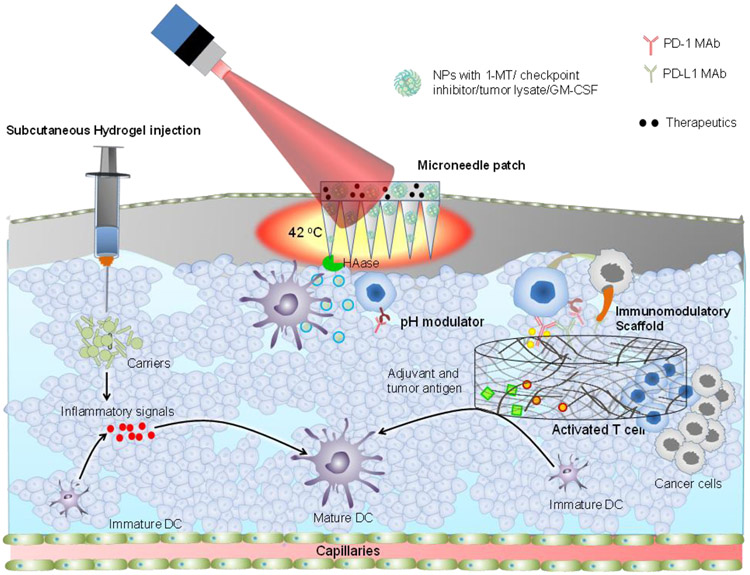
Checkpoint inhibition is a new approach in cancer immunotherapy and demonstrating clinical benefits. The immune checkpoint inhibitors are capable of maintaining healthy environment and protect the tissue from self-immune attack [20, 21]. These inhibitors release biological damper of the immune system; thus, immune cells can efficiently recognize and attack tumor cells. Therefore, such therapy is called immune checkpoint blockade (ICB). For example, during the inflammation, T cells get activated and start expressing the programmed cell death protein 1 (PD-1) to recognize the abnormal and cancerous cells. The cancerous cells evade the immune response by overexpressing the programmed death-ligand 1 (PD-L1) for binding to PD-1 and render T cells inactive [22, 23]. Thus, the blocking of this PD-1 and PD-L1 interaction with the help of checkpoint inhibitors (like monoclonal antibodies, mAbs) facilitates the death of cancer cells by preventing the inactivation of T cells (Fig. 1). Another checkpoint inhibitor is cytotoxic T lymphocyte antigen 4 (CTLA-4), a co-inhibitory molecule to regulate the extent of T cells activation. The ligands of CTLA-4 are CD80 and CD86, which inactivate the response of T cells [24]. It is also under investigation as some of the CTLA-4 blocking antibodies can also deplete the T cells population [25-27]. Currently, five checkpoint inhibitors for the PD-1/PD-L1 and one CTLA-4 are available for cancer treatment (Table 1). More than 700 clinical trials are underway to test the efficacy of several checkpoint inhibitors alone or in combining them with other cancer therapies [28].
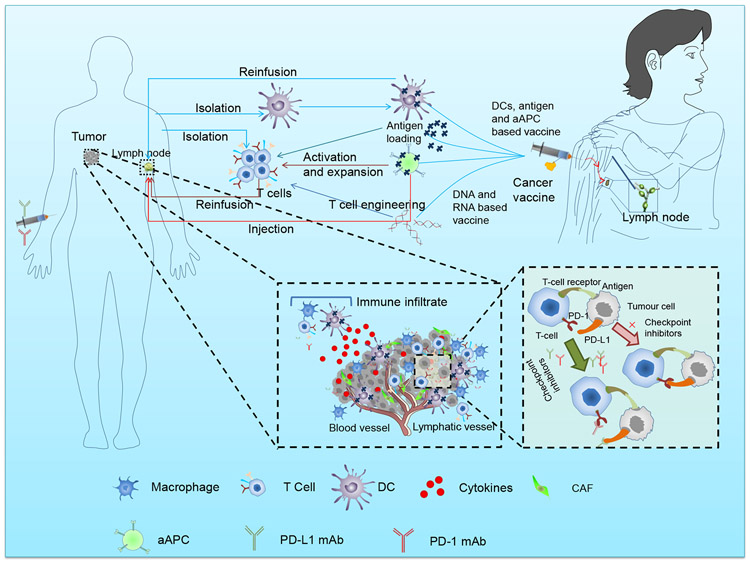
Fig. 1. Manipulation of T cells, infiltration of immune cells in tumor microenvironment, checkpoint inhibitor, and approaches followed in vaccine development.
Manipulation of T cells includes antigen representation by DC and artificial antigen presenting systems (aAPC), and nucleic acid transfer. The immune cells (macrophages, T cells, dendritic cells) infiltrate in the tumor microenvironment. The approaches for vaccine development include the injection of engineered dendritic cells, antigen, and aAPC.
Although checkpoint inhibitors have tremendous potential, it has been found to cause side-effects in different organs. Also, many patients have been found unresponsive to checkpoint inhibitors [29-32]. Thus, there is a need for the study of factors responsible for showing the responsiveness to the checkpoint inhibitors. It could be the low number of infiltrated T cells, adaptive resistance, low expression of checkpoints, deregulation of checkpoint inhibitors as well as tumor microenvironment capabilities of immune suppression [33, 34].
1.3. Engineered T cells
In this type of immunotherapy, the T cells are derived from the patient(s) and engineered to express the chimeric antigen receptors (CARs) that are specific to the particular type of antigen present on the cancer cells. These engineered T cells are re-administered to the same patients [35] (Fig. 1). Unlike other types of immunotherapy, it is a one-time treatment, and engineered T cells can retain their activity for more than a decade [36, 37]. Many patients have got complete remission from cancer. However, the long-term effect of this treatment is still under observation [38, 39]. The chimeric antigen receptor T (CAR T) cells initially target the CD19 that are present on the B cells' leukemia and lymphomas. At present, two CAR T-cell based therapy (tisagenlecleucel and axicabtagene ciloleucel) have been approved for acute lymphoblastic leukemia and large B cells lymphoma, respectively [40, 41] (Table 1). The success of CAR T-cell therapy has encouraged the development of different CAR T-cells to target multiple antigens towards developing generalized cancer therapy [35, 42].
Although CAR T-cell therapy is a most promising therapy, limitations, such as expensiveness, time-consuming, complexity of the process, neurotoxicity, and cytokine release syndrome, are commonly encountered [43-45], The penetration incompetence of engineered T cells into the core of the solid tumor is another drawback of the CAR T-cell therapy [29, 46].
CAR T-cell therapy is also successful in Epidermal growth factor receptor variant III (EGFRvIII) expressing glioblastoma [47]. T cell receptor-transduced T cells (TCR T) have come up instead of CART cells. Unlike the CART cells, TCR-T cells are major histocompatibility complexes (MHCs) specific and are highly matched to the patients’ immune profile [48]49]. Engineered T-cell therapy is seeking novel pathways for improving its therapeutic efficacy [50].
In addition, several agonist antibodies bind to the receptors of T cells, can stimulate and enhance the growth of T cells having higher specificity for cancer cells [51]. The most common costimulatory receptors are CD28 and tumor necrosis factor (TNF) family like glucocorticoidinduced TNFR related protein (GITR), OX40, 4-1BB, or CD137 [52, 53], The phosphoinositide-3-kinase–protein kinase B (PI3K-PKB), c-Jun amino-terminal kinases (JNK), and nuclear factor-kappa B (NF-κB) pathways are commonly used for the activation of T cells [54, 55]. The United States (US) Food and Drug Administration (FDA) has approved a recombinant fully human anti-OX40 monoclonal antibody (IBI101) for the clinical trials [56]. Also, utomilumab and urelumab, which target the 4-1BB (CD137), are in phase II clinical trials[57, 58]. Current studies are investigating to reduce dose-dependent toxicity by improving the administration strategy. For example, in lungs metastatic model, the agonist antibodies to 4-1BB show more efficacy with fewer side-effects if delivered in conjugation to liposomes [59].
1.4. Cancer vaccines
Dendritic cells (DCs) vaccine therapies are the most commonly used vaccines for the treatment of cancer [60]. DCs are taken out from the patient and engineered for the expression of specific antigen to activate the T cells against cancer cells [61] (Fig. 1). In 2010, PROVENGE® (sipuleucel-T, Dendreon Pharmaceuticals, LLC), a dendritic cell-based vaccine, has been approved for the treatment of asymptomatic or minimally symptomatic metastatic castrate-resistant (hormone-refractory) prostate cancer [62]. There are many cancer vaccines that have good safety profiles; however, got failed in the clinical trials due to poor efficacy [63] (Table 1). Thus, if a subset of dendritic cells presenting a high level of antigen could be identified and directly given to the lymph node, which may improve the efficacy of cancer vaccines.
DNA and RNA-based vaccines are an alternative to conventional vaccines, wherein exogenous nucleic acid is injected into the target cells. The antigen-presenting cells (APCs) uptake the nucleic acid and express the antigen. These antigens are presented to T cells to activate them against the given tumor type [64] (Fig. 1). The nucleic acid-based antigens may not require knowledge about the immunogenic epitopes and human leukocytes antigen (HLA) type. The nucleic acid-based antigens are also helpful in prolonging the antigen presentation in comparison to other types of vaccines. However, nucleic acid delivery barriers and immunogenicity have been the major hurdle in clinical implementation [65]. To address such clinical issues, mRNA-based vaccines have been developed to directly express the antigens. Even after having several advantages over the DNA based vaccines, mRNA is sensitive to nuclease driven degradation and has poor internalization into the cells [64, 66, 67]. Thus, there is a critical need for an efficient transfection or delivery carrier for improved intracellular delivery [68].
The neoantigens based cancer vaccines are also under development stage. It boosts the immune system(s) using the tumor-specific antigen called the neoantigens, which only present on the cancer cells arise due to somatic DNA alteration; thus, off-target effects are almost eliminated. These vaccines have encompassed number of neoantigens that are beneficial for the treatment of heterogeneous cancers[69, 70].
1.5. Cancer pathology/progress due to immune system:
There is a great debate from decades whether immune system checks the cancer progression or promotes it. It is established that although specific immune responses like immunosurveillance and immunoediting are required for checking the progression of cancer, the unresolved immune response like inflammation can promote the progression of cancer as the chronic inflammatory diseases are more associated with the risk of cancer and anti-inflammatory drugs reduce the risk of cancer [71]. There are many paths followed by the infiltrated innate immune cells that lead to the promotion of cancer like angiogenesis, free radical damage, adaptive immune suppression, tissue remodeling, and growth factors production. Although the innate immune response is critical for initiating the adaptive immune response, the innate inflammatory response might inhibit the activation of the adaptive immune response [72, 73]. For example, CCL22, chemokine produced by tumor-derived macrophage, enhance the infiltration of Tregs to the tumor. Also, MDSC inhibit the development of specific tumor immune responses. The effector cells like CD4+, CD8+ T cells, and cytokines like IFN-γ are anti-tumor while the MDSC, TAM, and cytokines produced like TNF, IL-6, IL-23, IL-β are pro-tumorigenic. Also, there are few other types of immune cells whose role still remains elusive, like Th17, CD25+, Foxp3+ regulatory T cells, and the cytokines like TGF-β.
In the early stages of tumors, TAM infiltrate in response to inflammation and releases pro-inflammatory chemokines and cytokines like CXCL10 and CXCL19 to attract as well as promote the development and differentiation of NK, Th1, and Th17 cells [72]. The GM-CSF and INF-γ direct towards the M1 phenotype of TAM, whereas IRF5 expression helps in maintaining the M1 markers, lymphocyte production, and response of Th1/Th17 by upregulating the TNF-α, IL-23, IL-12p40, and IL-12p35. In the advanced tumor condition, M1 phenotype changes to M2 type, which favors the Th2 differentiation and recruitment. The release of the different types of cytokines and chemokines like CCL24, CCL22, and CCL17 favoring the development of Tregs. Also, M2 supports the remodeling, tissue repair, and angiogenesis with the help of VEGF or EGF. Overall, these M2 types get proinflammatory with the release of cytokines and chemokines like IL-6, IL-12, IL-23, and TNF-α, which promote the anti-cancer immunity with the production of IL-10 and TGF-β. The molecular mechanism controlling the regulation of TAM is largely unknown. Its elucidation will help in finding out the more effective therapies and determining the inflammation associated progression of cancer.
The MDSC favor the inflammation and exerts suppressive effects to the T cells via the reactive oxygen species, nitric oxide, and secretion of TGF-β, which also lead the Treg induction [74], Also, it produces IL6 in high levels, and its expansion in the presence of VEGF factor is also reported. Usually, MDSC differentiates after migration, but in the tumor area in presence of cytokines, factors, and other chemokines, it remains undifferentiated to immature myeloid cells and causes the immunity suppression of tumor via different ways. IL6 is another factor that promotes the proliferation of cancer cells, along with inhibiting the apoptosis mechanism via the Stat3 [75]. It could also affect the differentiation of T cell subsets which further gets mighty in presence of other cytokines like TGF-β. Also, it has been found to play role in carcinogen driven liver cancer and act as angiogenic factor like TNF, which itself is the primary inflammatory mediator of NF-κB. IL-β polymorphism is linked to gastric cancer, and its role in the activation of NF-κB is also established [76]. The Stat3 and NF-κB appear to be working in positive feedback loop during the interaction of cancer cells and inflammatory cells, which helps in the cancer progression [72]. Like in the Src oncogene model, the induction of inflammation was helpful for the activation of Stat3 via NF-κB and IL-6 [77].
1.6. Drawbacks of conventional immunotherapy and implications of nanotechnology in immunotherapy
Although several checkpoint inhibitors have been approved by the FDA, still, several challenges need to be addressed before the widespread commercialization [78]. One of the hurdles is the limited response, i.e., only 10-30% of patients respond to immunotherapy, particularly to checkpoint inhibitors due to their cold tumors, which is characterized by low number of T cells in the tumor microenvironment, less number of PD-1, PD-L1, CTLA-4 expression, immunosuppressive microenvironment, and other factors. Towards this, there have been efforts on the administration of the combination of different checkpoint inhibitors like anti-PD-1, anti-CTLA-4, etc. However, adverse immune responses have been observed with the seriousness of grade 3 and 4, i.e., renal, hepatic, and gastrointestinal disorders. Additionally, there are prospects of generation of self-antigen reactive T cells, but damages to healthy tissue [79]. There has been research on neoantigen driven personalized immunotherapy [80]. The combination of different cellular therapies like tumor-specific T cells and APCs with the checkpoint inhibitors reflected the promising outcome. However, engineering of T cells and tumor antigen-specific dendritic cells has been very expensive, labor-intensive, and afflicted with lot of variations and quality issues [43-45,81].
There are number of nanomaterial platforms focus on efficient delivery and addressing the challenges of immunotherapy [82]. (Fig. 2) Nanomaterials or nanoparticles (NPs) can help in the efficient delivery of the therapeutic agent(s) to the tumor(s) and accumulation in tumor(s) via enhanced permeability and retention (EPR) or active targeting mechanism. Such mechanisms not only reduce side-effects of therapeutic agents but also protect from degradation and quick systemic elimination. Further, release kinetics, as well as other physicochemical properties of nanomaterials, can be easily tuned as per the patient's profile [83]. However, the potential of nanotechnology in clinical trials is still underway for controlled delivery of cytokines, adjuvants, antigens, and other immunological moieties [59, 84-86]. Also, there have been efforts on combining conventional therapies like chemotherapy, radiotherapy, hyperthermia, and others with immunotherapy towards converting the cold tumors into hot tumors and observing the abscopal effect for the treatment of disseminated metastatic sites [87-90].
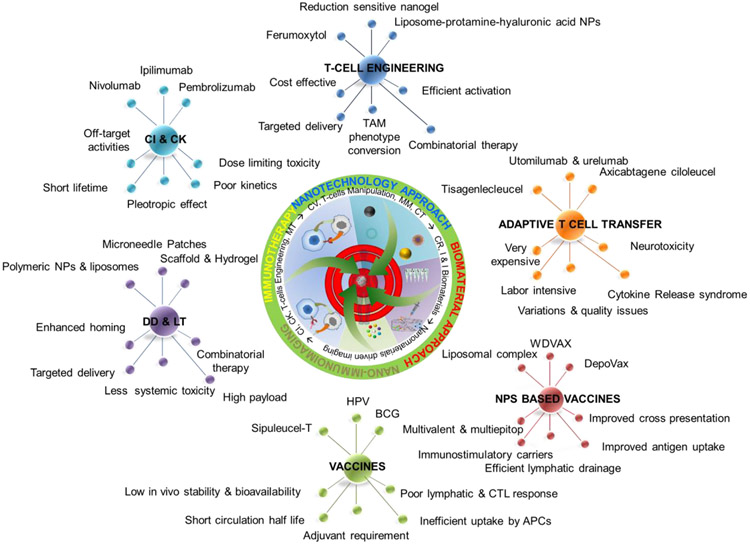
Fig. 2. Developmental Pathway and Interconnectivity of Immunotherapy and Nanotechnology:
The top 3 spikes show the current advancements, while the bottom spikes show the advantages and disadvantages of nano-immunotherapy and convention immunotherapy, respectively. Integration of nanotechnology, material science, and nano-immunoimaging towards reinforcing cancer immunotherapy. The area of pie sector represents the percentage of current development. Abbreviation: checkpoint inhibitor (CI), cytokines (CK), drug delivery (DD), localized therapy (LT), microenvironment tunning (MT), cancer vaccines (CV), microenivornment modulation (MM), combination therapy (CT), controlled release (CR), implantable and injectable biomaterials (I&I Biomaterials).
Herein, we summarize the current development of immunotherapy in reference to nanomaterials and combinatorial therapy approaches. It includes the immunological functionalization of NPs, advantages of combinatorial therapy, usage of scaffold in the localized release of adjuvant, antigen, intervention towards the maturation of dendritic cells, and activation of the T cells. The nanotechnology combination helps in accelerating the specificity, sensitivity, and improving safety profile of the immuno-therapeutic agents (Fig. 2). This review aimed to demonstrate NPs mediated immuno-theranostics for widening therapeutic and imaging window in cancer treatments.
2. Nanotechnology Approaches in Cancer Immunotherapy
Despite positive outcomes with conventional cancer immunotherapies, some inherent issues are noticed. These concerns include but not limited to convoluted tumor cell population, heterogeneity, desmoplasia, and their tumor microenvironment. All these limits the entry of therapeutic agents into tumors for their effective actions. To tackle these unsolved issues, nanotechnology-based cancer immunotherapy can be a viable option towards smooth translation to clinical applications. (Table 2) Currently, there are more than 80 FDA-approved therapeutic nanoparticle-based medicinal agents available for clinical use. Most of these NPs are found to be highly biocompatible for improved, targeted, and sustained delivery applications. They have potential to be tuned for different degrees of tissue penetration, distribution, pharmaco-kinetic, and pharmaco-dynamic profiles. Nanotechnology also helps in improving the stability, bioavailability, half-life of therapeutic molecule(s). Thus, this section is devoted to present novel principles, opportunities, and advances of nanotechnology in cancer immunotherapy.
Table 2:
Progress of nano-and micro-formulation in the improvement of immunotherapy.
| Micro and Nanoformulation |
Type | Surface functionalization |
Adjuvant | Average Size |
Publications, Patents, and Clinical trials |
|---|---|---|---|---|---|
| Polymeric NPs and MPs | PLGA, PLA, PGA, PCL, Chitosan, Polyester, Dextran, PLHMGA, polystyrene | PEG, polyhydroxy, PEI, Carboxyl, cancer cell membrane, sulfate, LHRH peptide, Cy5.5 | CpG, OVA, anisamide, listeriolysin, α-GalCer, HSPs, montanide | 20 mn-5μm | 82, 2, 0 |
| Lipid NPs (Liposomes, solid lipid NPs) | Phospholipid, cationic lipid, DOTAP, DSPE-PEG, phosphatidylcholine, DOPE, NBD-DOPE | Maleimide, PEG, protamine, hyaluronic acid, LHRH peptide, Cy5.5 | OVA, CpG, pIC, zymosan, R848, LPS, L-PAM, Pam3CSK4, MPLA, DiC14-amidine, BCG-CWS, HSP | 150 nm-1.5 μm | 500, 19, 23 |
| Inorganic NPs | Gold, iron oxide, zinc oxide, calcium phosphate, QDs, copper sulfide, CNT, Ce6, MOF, UCNPs, aluminum oxide, silica | Amino, mannose, dextran, avidin, polydopamine | CEA, CpG, GMCSF, 1MT, imiquimod, IDO inhibitors, HSPs, alum | 2-50 mn | 298, 4, 0 |
| Nanogels | Gelatin, chitosan | Quaternary amino | IL-2, IL-15SA | 130-250 mn | 15, 0, 0 |
| Dendrimers | Succinamic acid dendrimers G4), polyamidoamine dendrimers (G5) | DGBA, LHRH peptide, Cy5.5 | CpG, PADRE, | 58-68 nm | 28, 0, 0 21, 0, 0 |
| Cyclodextrins | α-CD, β-CD, γ-CD, Succinyl-β, Methacrylate β, HP-β-CD, M-β-CD, SBE-β-CD | PEG, Porphyrin, | |||
| Micelles | PPS, MPEG- OH, PLL-g-PEG, PEI, PCL, PLH–PEG, dextran-grafted-poly (histidine), PGA, LMWH-d- (TOS), PEG-CDM-PEI | Cancer cell hybrid membrane, CaCO3-crosslinked, heparin | CpG, Poly I:C, M-CSF, IMQ | 23 mn | 78, 0, 0 |
| Microneedles, Scaffold, Hydrogel, rods | Hyaluronic acid, Alginate, PLGA, Chitosan, PVA, silica, gelatin, aluminum hydroxide | Melanin, PEI | GMCSF, CpG, IL-2, IL-15SA, FLT3L, CCL20 | 258, 7, 1 |
Abbreviation: polystyrene nanoparticles (PSNPs), l-dioleoyl phosphatidylethanolamine (DOPE), N-(7-nitro-2,1,3-benzoxadiazol-4-yl) labeled dioleoyl phosphatidyl ethanolamine (NBD-DOPE), phenylalanine Mustard (L-PAM), monophosphoryl lipid A(MPLA), Bacille Calmette–Guerin cell wall skeleton (BCG-CWS), Carcinoenbryonic antigen (CEA), Poly(amidoamine)(PAMAM), Guanidinobenzoic acid (DGBA), Galactosylceramide (α-GalCer), Guanidinobenzoic acid (DGBA), cytosine-guanine dinucleotides (CpG), PAn DR epitope (PADRE),2-Hydroxypropyl-β-cyclodextrin (HP-β-CD), Methyl-β-cyclodextrin (MβCD), sulfobutylether-β-cyclodextrin (SBE-β-CD), Methoxy poly (ethylene glycol) (MPEG- OH), poly(l-histidine)–poly(ethylene glycol) (PLH–PEG), Poly (glutamic acid) (PGA20k), low molecular weight heparin (LMWH)-d-α-tocopheryl succinate (TOS), macrophage colony-stimulating factor (M-CSF), macrophage colony-stimulating factor (M-CSF), polypropylene sulfide (PPS), polycaprolactone (PCL), luteinizing hormone releasing hormone (LHRH), 1,2-dioleoyl-3-(trimethylammonium)propane (DOTAP), 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-Poly (ethylene glycol)(DSPE-PEG), TLR2 ligand Pam 3 CysSerLys 4 (Pam 3 CSK 4), Nt-butyl-N′-tetradecyl-3-tetradecylaminopropionamidine (diC 14-amidine), chlorine e6 (Ce6), Metal–organic frameworks (MOFs), Poly(L-lysine)-graft-poly(ethylene glycol) (PLL-g-PEG), 2-propionic-3-methylmaleic anhydride (CDM),
2.1. Nanoparticles-based cancer vaccines
The NPs can reach the lymphatic system (lymphatic vessels and lymphoid organs) and elicit the efficient activation of the immune response. Nanotechnology is extensively applied for developing targeted DC vaccines. The goal of NPs based vaccine is to enhance the delivery of antigen (Fig. 3) to DC for the activation of T cells, as well as sustained release of antigen(s) along with high biocompatibility. These vaccines are also being used for eliminating malignancies and prevent tumor relapse after post-surgery[91]. DepoVax, a liposome-based DC vaccine, is in Phase I clinical trial for prostate, breast, and ovarian cancer. The DepoVax is a blend of several tumor-specific epitopes like TNF-α converting enzyme (TACE), topoisomerase II α, epithelial discoidin domain receptor I (EDDR1), B cell receptor-specific protein 31 (CDM), and γ catenin. This mixture of epitopes and adjuvants can be manipulated as per the requirement of specific tumor and activation of immune cells[92] (Table 3). Similarly, iron and zinc-based nanoshells were applied to deliver the gene of interest to DC and for simultaneous imaging purposes[93]. An up-conversion NPs formulation conjugated with polymers [polyethylene glycol (PEG) and polyethylene imine (PEI)] has been used for delivering the ovalbumin (OVA, a model antigen) to DC and to track the DC migrations in in vivo conditions[94, 95]. This antigen nanocomplex system efficiently activated the CD8+ cytotoxic T cell population. Further, Cruz et al.,[96] showed the importance of antigen interactions with DC using the PEG-coated poly(lactic-co-glycolic acid) (PLGA) NPs[97]. This PLGA NPs formulation was loaded with OVA and Toll-like receptor (TLR) ligand and conjugated with CD40, CD11c, and DEC-205 monoclonal antibodies for effective DC targeting. This approach led to the production of IL-2 and results in robust immune response in comparison to non-targeted cancer vaccines. Further, the subcutaneously injected vaccine targeted to CD40 of DC helped in proliferating the growth and enhanced response of CD8+ T cells.
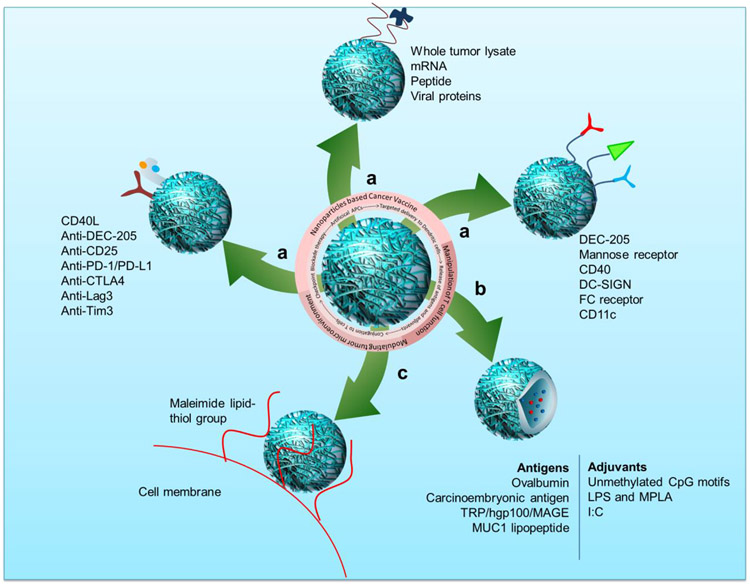
Fig. 3. Role of nanoparticles in the different areas of cancer immunotherapy.
Nanoparticles are helpful in a) targeted delivery of antigens to dendritic cells, b) controlled and triggered release of antigen and adjuvants to the tumor. c) Conjugation to the T cells for long-term activation and increasing the efficiency of checkpoint blockade therapy.
Table 3.
List of nano and macro-materials used for the cancer immunotherapy.
| Nanomaterial | Functionalization | Delivering agent | Target/Action |
|---|---|---|---|
| Vaccine development | |||
| Iron and Zinc NS | Gene of interest | DC | |
| Polymeric NPs | UPNP coated | OVA | DC |
| DepoVax | TACE, topoisomerase II α, EDDR1, B cell receptor specific protein 31 (CDM), γ catenin | DC | |
| PLGA NPs | PEG and mAbs | OVA and TLR ligand | DC |
| Liposomal complex | mRNA of neoantigen | DC | |
| WDVAX | Tumor lysate with GM-CSF and CpG | DC | |
| Immune cells manipulation | |||
| Poly (beta-amino ester) NPs | Anti-CD3e f(ab’)2 fragment-modified poly(glutamic) acid + peptides containing the microtubule-associated sequence + NLS | 194-1BBz CAR | T cells |
| Polymeric NPs | CAR transgene and plasmids encoding iPB7 transpose | T cells | |
| Liposomes | Maleimide, F(ab’)2 fragment | IL-15SA and IL-21 | T cells |
| Reduction sensitive nanogel | Anti-CD45 antibody and PEG-b-pol(l-lysine) | IL-15SA | T cells |
| PLGA NPs | Anti-CD3e f(ab’)2 fragment, Anti-PD-1 | SD208 | T cells |
| PLGA NPs | Anti-PD-1 | R848 | T cells |
| Iron oxide NPs (ferumoxytol) | TAM | ||
| L-lysine NPs | Crosslinking with Succinyl-β-CD | R848 inhibitor | TAM |
| Liposomes | Protamine and hyaluronic acid | siRNA against CD47 | CD47 immunoglobulin |
| IR700 | Anti-CD25 F(ab’)2 fragments | Tregs | |
| Methacrylated β-cyclodextrin + PEG-polylactide diacrylate NPs | SB505 + IL-2 | T cells, NK cells, Tregs | |
| Liposome-protaminehyaluronic acid NPs | siRNA against TGF-β + CpG | T cells, NK Cells, Tregs | |
| PEG-PLGA NPs. | Methyl-2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oate | Myeloid-suppressor Cells, Tregs | |
| PLHMGA microparticles | Anti-CD40 and anti-CTLA-4 antibodies | DC, T cells | |
Abbreviation: TNF-α converting enzyme (TACE), epithelial discoidin domain receptor I (EDDR1), nuclear localization signals (NLS), polyethylene glycol (PEG), poly lactic-co-glycolic acid (PLGA), toll-like receptor (TLR), poly (D, L-lactic-co-hydroxymethyl glycolic acid) (PLHMGA), FMSrelated tyrosine kinase 3 ligand (FLT3L), CC-chemokine ligand 20 (CCL20), 1-methyl-DL-tryptophan (1-MT), polyethyleneimine (PEI),
The combination of antigen and immunoadjuvant delivery using biodegradable nanocarriers elicit more potent and robust immune response.[98] In comparison to the liposomes, polymeric NPs have the advantage for sustained release of therapeutic antigen and adjuvants, which can induce the long-term memory of T-cell phenotype[99]. However, it was suggested that the short-lived vaccine depots overture the sequestration of T cells as well as dysfunction and deletion at the vaccination site [100, 101]. Such vaccine depots not only overcome the persistent antigen delivery limitations but also provide improved therapeutic benefits.
Nucleic acid vaccines have several advantages over epitope and adjuvant-based vaccines. However, the delivery of nucleic acids has been a major challenge [102, 103]. A set of recent studies demonstrated that DC has been engineered ex vivo with mRNA encapsulation and then supplemented back to the patient[103] for rational design of mRNA vaccines. Systemic delivery, targeting, and protection of nucleic acid from degradation were achieved using a complexation based liposomal formulation with neoantigen coding mRNA[104]. This mRNA-liposome complex can facilitate the cytosolic delivery of mRNA in the mouse tumor model to induce potent effector and memory T cell response. Such RNA based vaccine(s) can produce antigen and peptide, thus helping the development of broad-spectrum antitumor vaccines.
2.2. Manipulation of T cells
T cells act as executor in the immune response cascade. However, infiltration of T cells into tumor(s) is a major hurdle in immunotherapy. Also, activation and proliferation of T cells are often affected by several signaling pathways. A well-known example of such pathways is PD-1/PD-L1 interaction. Therefore, cutting off PD-1/PD-L1 interaction and engineering of T cells are successful approaches. However, harnessing the full potential of T cells driven therapy is still lacking. Thus, the usage of nano- and microbiomaterials has been adopted as follows[105] (Table 3).
2.2.1. Proliferation and reprogramming
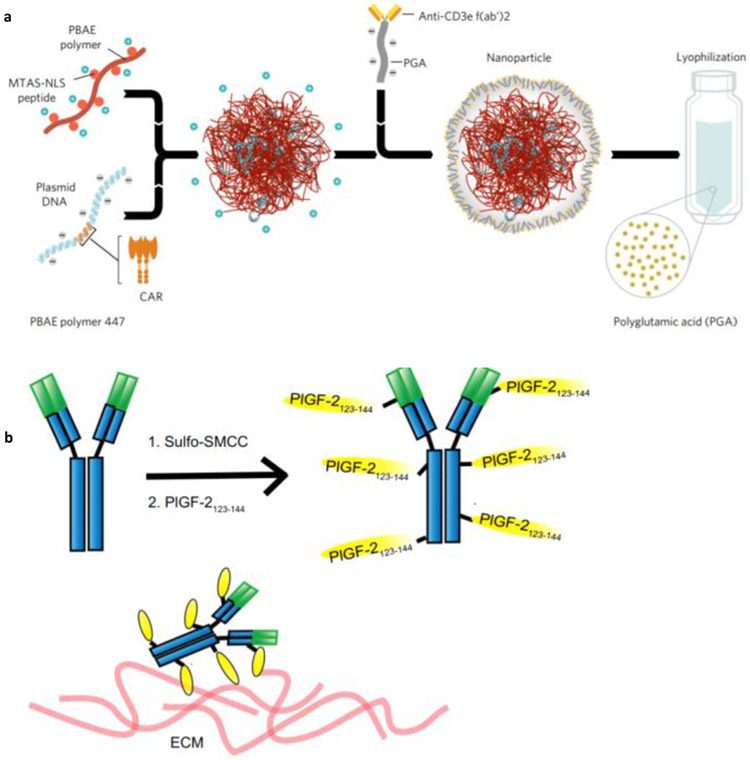
Although CAR T-cell therapy has shown promising results, there are only few centers worldwide that can implement such advanced therapy. Moreover, the financial hurdle (approx. 0.5 million USD for single infusion) restricts the widespread implementation [106]. Thus, in an attempt to simplify the adoptive T therapy, Smith et al. [107] have developed the in situ gene delivery technology. In this T cell programming approach, gene encoding the leukemia-specific 194-1BBz CAR were complexed with poly(β-amino ester)-based polymeric NPs (size 150 nm and zeta potential −7.8 mV). This NPs system was attached with anti-CD3e f(ab’)2 fragment-modified polyglutamic acid and peptides containing the microtubule-associated sequence and nuclear localization signals for targeting the T cells and nuclear homing (Fig. 4a). In in vitro studies, 4% of T cells were found to be CAR+ within 30 h of incubation, which is quite efficient. Further, intravenous administration of targeted polymeric nanocomplex was capable of entering the T lymphocytes and effectively reaching out to the spleen, bone marrow, and lymph nodes, while the non-targeted nanocomplex didn’t show the efficient accumulation in T lymphocytes and ended up in the liver. Similarly, polymeric NPs loaded with both CAR transgene and plasmids encoding iPB7 transpose showed better insertion of CAR gene in the genome of T cells [108]. The percentage of CAR+ T cells reached 5.8%, with an increase in efficacy of 70% and extended survival of mice to 58 more days in comparison to control.
Fig. 4.
a) Schematic showing the fabrication of the anti-CD3e f(ab′)2 fragments attached poly(β-amino ester) nanoparticles. b) Schematic showing the conjugation of PIGF-2 peptide to IgG antibody for improving the binding to ECM proteins. Adopted with permission from reference [107] (a) and [115] (b)
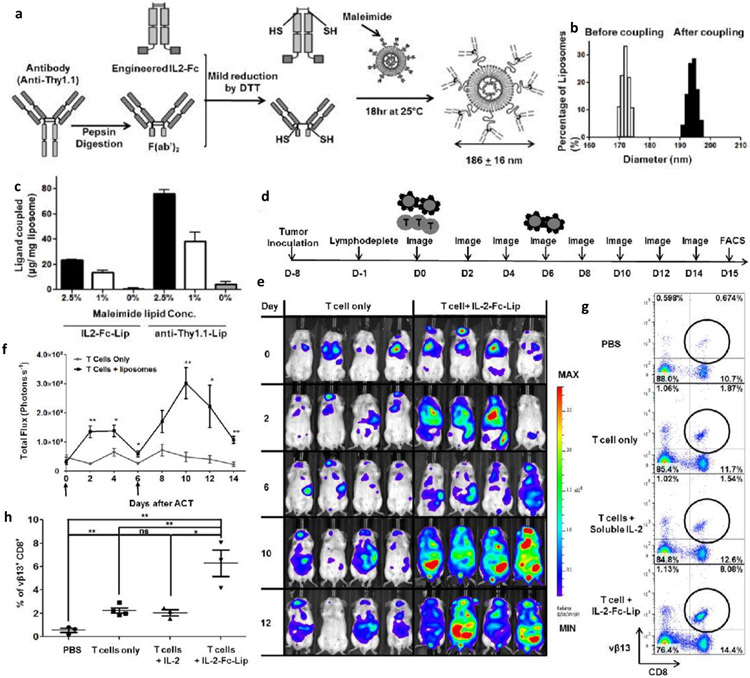
The effector T cells are sensitive to the immunosuppressive environment of the tumor. Although cytokines help in augmenting the efficacy of effector T cells, severe side effects may be encountered with bolus injections. To overcome such uninvited events, Stephan et al., [109] developed the multilamellar lipid NPs composed of superagonist IL-15SA and IL-21 and functionalized with maleimide group to react to the thiol of T cells. This engineering precisely allowed binding with T cells (100 liposomes on the surface of T cells) and did not affect the function, proliferation, transmission, and tumor homing capabilities of T cells. The liposome conjugated T cells showed the persistent immune response and amplified homing to lymph nodes and spleen, whereas unmodified T cells gradually cleared from systemic circulation. The disadvantage is the dilution of pharamcyte (i.e., attached liposome) with the division of T cells. Thus, the same group also developed the in vivo targeting of T cells with the engineered liposomes to avoid one-time injection limitation [110]. Herein, either the F(ab’)2 fragment attached liposomes were directed to the Thy1.1 antigen present on the T cell surface or IL-2 engineered on Fc fragment. This approach allows more than 90% T cells to bind with the liposome upon a single injection with no serious toxicities, indicating its clinical feasibility (Fig. 5a-h).
Fig. 5.
a) Schematic showing the preparation of immunoliposomes. b) Size distribution of immunoliposomes before and after coupling with T cells. c) Quantification of ligand (IL2 or anti-Thy1.1) to the liposomes containing different concentration of maleimide. d) Timeline representing the study regimen. e) Representative bioluminescent images of mice over different time periods. f) Quantitative analysis of whole-body imaging. g) Flow cytometric analysis after the adaptive transfer of the tumor-specific (vβ13 TCR+) T cells. h) Quantitative analysis of tumor-specific (vβ13 TCR+) T cells in the inguinal lymph nodes. Adopted with permission from reference [110].
A smart cytokine releasing nanocarrier can significantly activate higher number of T cells [111]. This reduction sensitive cross-linked nanogel comprised of cytokines (IL-15SA) and decorated with anti-CD45 antibody promotes effective release of payload to the T cells surface. Schmid et al. [112] have developed the SD208 [an effective inhibitor of transforming growth factor (TGF-β) kinase, which is responsible for the proliferation and activation of CD8+ T cells] loaded PLGA NPs. These NPs were tagged with anti-CD8 antibody using the fragments of F(ab’)2 via maleimide/thiol click chemistry. Up to 90% T cells showed efficient binding with targeted PLGA NPs, and less than 20% of these bound NPs were internalized. Flow cytometry analyses confirmed that 90-100% of immune cells were attached to the targeted NPs after 1 h of injection. Moreover, it has been found that the SD-208 loaded and PD-1 targeted NPs showed the efficient activation of CD8+ T cells and enhanced production of IFN-γ and granzyme-B. Further, the R848 (agonist of TLR7/8) loaded, and PD-1 targeted PLGA NPs were prepared for stimulating the infiltration of CD8+ T cells in the core of MC38 tumor. The mice treated with R848 loaded and PD-1 targeted NPs showed extended survival in comparison to control mice.
2.2.2. Delivery of immune checkpoint inhibitors
Checkpoint inhibitors are the crucial bases for the current developments in immunotherapy [113], Many immune checkpoint blockers (ICBs) have shown the complete regression of tumor(s) in small cohorts of patients[78]. However, along with the low response rate, the adverse effects are also associated with ICB therapy, which is life-threatening [79]. Thus, to minimize the off-targets and increase the efficacy, several different delivery methods have been adopted as following [114].
An ICB antibody conjugated to an extracellular matrix (ECM)–super-affinity peptide (derived from placenta growth factor–2, PIGF-2123–144) was engineered [115]. The unmodified antibodies rapidly leaked out from the tumor environment to the blood vessels, while peptide modified anti-CTLA-4 and anti-PD-L1 antibodies showed less off-target effects. The antitumor response was augmented with the enhanced infiltration of CD4+ and CD8+ T cells in the tumor environment of B16F10 melanoma. The local treatment of the primary tumor also inhibited the development of metastatic sites. The superior efficacy of the peptide modified ICB antibodies was further confirmed in both chemically induced and xenograft (MMTV-PyMT) tumors developed in genetically engineered Tyr:Cre-ER+/LSL-BrafV600E/Ptenfl/fl mice. Also, the memory effect against the MMTV-PyMT tumor was noticed in peptide modified ICB antibodies treated mice (Fig. 4b). Along with peptides, platelets have also been exploited for delivering immune checkpoint inhibitors. The anti-PD-L1 conjugated platelets using the maleimide based bifunctional linker was established without compromising the efficacy of antibody and function of platelets [116]. The anti-PD-L1 antibody functionalized platelets showed enhanced circulation (> 34 h) and accumulation at the surgical site in comparison to the bare anti-PD-L1 antibody (< 10 h). The modified antibody showed effective inhibition of tumor recurrence after the removal of the primary tumor. The treatment was associated with the high infiltration of CD4+ and CD8+ T cells in comparison to the controls (PBS, free anti-PD-L1 antibody, and platelets). Additionally, the platelets attached anti-PD-L1 antibody inhibited the metastasis of the lung and prolonged the survival of treated mice. The efficacy was also observed in the triple-negative breast cancer model, wherein the recurrence was delayed significantly. These data show that the platelets conjugated anti-PD-L1 antibody is effective in inhibiting the tumor recurrence after surgery as well as treating the metastatic sites.
The biodegradable polyester-based microparticles have been used for high loading capacity and long-term release profile. Seyednejad and Ghassemi teams efficiently fabricated anti-CTLA-4 antibody containing poly(lactic-co-hydroxymethyl glycolic acid) (PLHMGA) microparticles [117, 118]. The localized and sustained release profile of the anti-CTLA-4 antibody was evidenced for up to 20 days with minor systemic toxicity. In an in vivo MC-38 tumor-bearing mice study, it was confirmed that antibody was less in the blood, which illustrates the localized retention at the tumor site[119]. A DNA-based NPs system has also been applied for the successful delivery of anti-PD-1 antibodies to the resectioned sites [120]. This construction is quite efficient for subsequent release of anti-PD-1 due to the presence of guanine residues (CpG) and sites for the restriction endonuclease Cfo I (HhaI) enzyme. The localized applications of anti-PD-1 loaded DNA NPs were effective in inhibiting metastasis in the lungs after the removal of the primary tumor.
2.3. Modulation of the tumor microenvironment
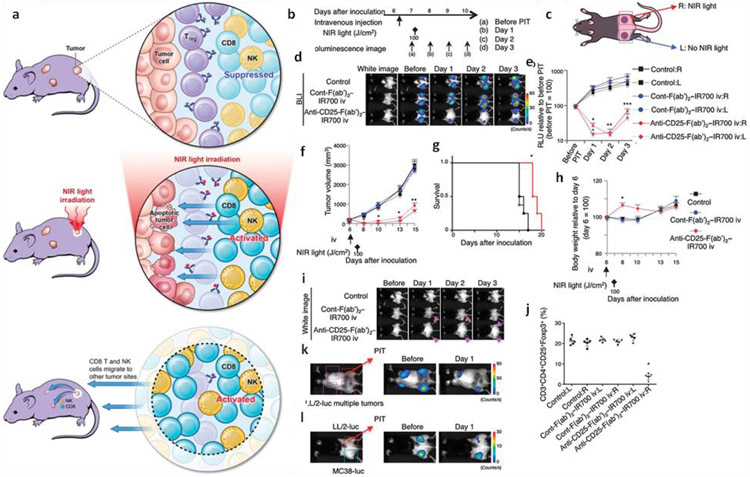
The immunosuppressive environment of the tumor is a major hurdle for immunotherapy. The immunosuppressive effects can be exerted both directly or indirectly with the help of regulatory T cells (Tregs), tumor cells, myeloid suppressor cells, tumor-associated macrophages, and several soluble immunosuppressive factors. Thus, to improve the efficacy of immunotherapy, the inhibition of these cells and factors is critically required. For example, PEI functionalization of siRNA helps in better uptake by the ovarian cancer cells and leads to more than 50% inhibition of PD-L1 [121]. Similarly, siRNA encapsulated in protamine and hyaluronic acid (HA)-functionalized liposome knocked down the overexpressed CD47 in a very effective manner [122], A nanolipo gel formulation comprised of liposomes, polymers, SB505124 (TGF-β inhibitor), and IL-2 (immunomodulant) [123] has proven to promote both innate and adaptive immune response by the activation of CD8+ T cells and NK cells. This gel formulation also extended the survival time of mice melanoma models. Tregs are also primarily responsible for the immunosuppressive environment. However, the inhibition of these cells often leads to autoimmune diseases. Thus, it is highly crucial to achieve selective targeting of the Tregs present in the tumor environment. Selective depletion of such Tregs in the tumor microenvironment was achieved by the near infraredphotoimmunotherapy (NIR-PIT) [124]. In this approach, IR700 molecules were conjugated to the F(ab’)2 fragments that target the CD25. After systemic administration in mice, these molecules reach the tumor(s), which were shined for 30 min. This process leads to the 85% selective depletion of CD4+CD25+Foxp3+ Tregs. This approach also leads to the activation and infiltration of CD8+ T cells, APCs, and NK cells for the complete regression of the tumor (Fig. 6).
Fig. 6.
a) Schematic representation of the induced immunotherapy after NIR-PIT. b) General regimen adopted for the NIR-PIT. c) Biflanked tumor model: right tumor was treated with NIR light and anti-CD25-F(ab′)2–IR700 or (ab′)2–IR700 driven phototherapy, while the left tumor was left untreated. d) Representative bioluminescence images showing the changes after the localized NIR-PIT. e) Reduction in the relative light units (RLU) of the right tumor after NIR-PIT as well as in the left non-irradiated tumor. f) Reduction in size of the tumor after the CD25 treated right tumor as well as non-irradiated one. g) Prolonged survival of mice after NIR-PIT. h) Change in weight after the NIR-PIT, i) Edema due to NIR-PIT at both tumors after CD25 targeted NIR-PIT, j) Depletion of CD4+CD25+Foxp3+ Tregs in the right tumor but not in the left untreated. k) Regression of multiple LL/2-luc tumors after the localized CD25 targeted NIR-PIT at the right tumor. l) Negligible effect on the MC38-luc tumor after NIR-PIT on the LL/2-luc tumors of the right side. Adopted with permission from reference [124].
2.3.1. Suppressive immune cells
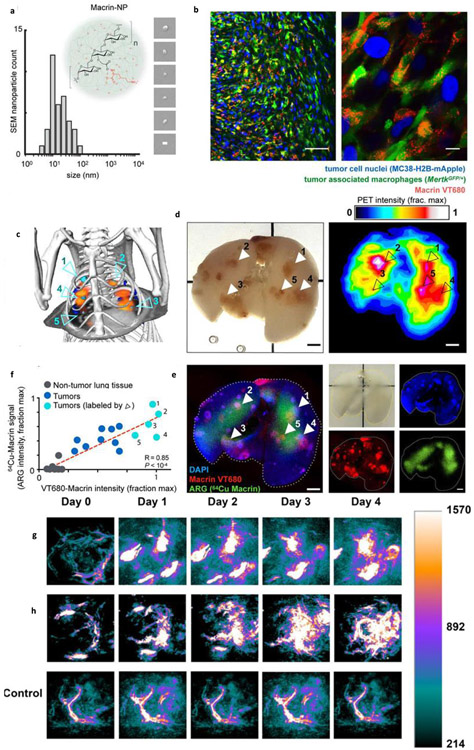
The tumor-associated macrophages (TAM) are a major population in the tumor, and its phenotype (usually M2 type) responsible for the proliferation, metastasis, and drug resistance of tumor cells. Thus, reversing the M2 phenotype to M1 type, i.e., antitumor type, has been accomplished using several strategies [125, 126]. Towards this, an FDA approved iron oxide NPs (ferumoxytol) formulation can be applied, which also has good safety profile [127]. Ferumoxytol treatment exhibited the upregulation of TNF-α and CD86 markers in the macrophages, which represent the M1 phenotype, while the downregulation of CD206 and IL-10 represents the M2 phenotype. The ferumoxytol treated tumor (bilateral tumor model) was found to have increased concentration of CD80+ cells and evidence of the depletion of TAM. This therapy was effective in the treatment of liver and lung metastatic models of mice. Although ferumoxytol is approved for iron-deficient anemia, its high concentration usage should be further evaluated for the treatment of cancer [128, 129].
NPs are preferably endocytosed by macrophages [130]. The R848 inhibitor of TLR7/8 is highly effective in changing the murine macrophage to M1 phenotype. A succinyl-β-cyclodextrin cross-linked with L-lysine nanoparticle formulation proved to be effective in delivering the R848 to the TAM of MC38 tumors with the help of EPR effect [131]. This R848 nanoparticle formulation can efficiently reach tumors and accumulates in TAM, which resulting in increased expression of IL-12 (represents the M1 phenotype). This treatment approach helped in prolonged survival and long-term inhibition of the tumor growth and enhanced the efficacy of the anti-PD-1 antibody.
Another major population of leukocytes relates to immune-oncology is neutrophils, which are responsible for the innate immune response. The programming of neutrophils is effective for eliciting antitumor immunity [132]. A nucleic acid devoid cowpea mosaic virus can enhance the production of IL-1β, IL-12p40, CCL3 (MIPl-α), IL-6, and TNF-α, which represent the stimulation of primary macrophage or bone marrow-derived dendritic cells (BMDCs). The intranasal delivery significantly increased the concentration of CD11b+Ly6G+ neutrophils in healthy mice and capable of inhibiting the metastasis of lung in the 4T1 tumor rechallenge model (immunogenic comparator: lipopolysaccharide, poly (I:C), and STING agonist).
2.3.2. Soluble suppressive factors
Soluble factors such as indoleamine 2,3-dioxygenase (IDO) and TGF-β also play an important role in immunosuppression. Transforming growth factor-beta (TGF-β) is a multifunctional cytokine and helps in inhibiting the activity of cytotoxic T lymphocytes (CTLs), NK cells, as well as the proliferation of Tregs [133]. The TGF-β inhibitor (SB525334) was successfully delivered using liposomes [134]. These liposomes were targeted to both internalization receptor (CD90) and noninternalization receptor (CD45). Anti-CD90 targeted liposomes were more effective than anti-CD45 targeted liposomes in in vivo studies. This may be due to the fact that anti-CD45 targeted liposomes undesirably up taken by the peripheral macrophages, B-cells, and dendritic cells. Another nanoparticle formulation (~ 120 nm) based on methacrylated β-cyclodextrin and PEGpolylactide diacrylate revealed efficient delivery of SB505 and IL-2 to inhibit TGF-β as well as the proliferation of T cells [123]. This dual (SB505 and IL-2) formulation showed greater efficacy in subcutaneous B16 melanoma mice model than free and single component loaded NPs. This was evidenced with the increase in CD8+ T cells and NK cells in the tumor microenvironment. siRNA delivery using 30 nm liposome-protamine-HA NP formulation is also promising to inhibit the effect of TGF-β [135]. The intravenous delivery of siRNA nanocomplex was found to inhibit ~50% expression of TGF-β in the late-stage tumors, thus increased the efficacy of the vaccine. Delivery of methyl-2-cyano-3,12- dioxooleana-1,9(11)-dien-28-oate through 120 nm PEG-PLGA NPs has also been reported to significantly decrease Tregs population [136].
3. Combination therapy
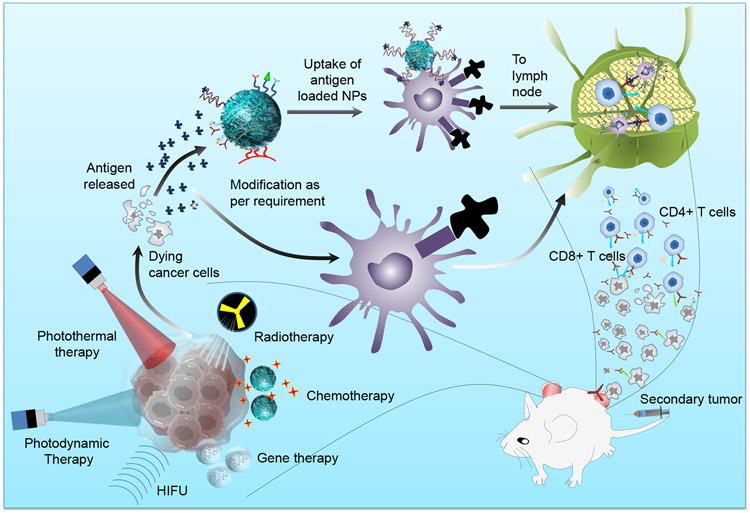
The conversion of the cold tumors to the hot tumors is difficult to achieve using conventional immunotherapy due to strong immunosuppressive environment [137]. The CAR T-cell therapy has been approved for the hematological related malignancies, but its application for the treatment of solid tumors is a challenge [138]. The treatment of primary tumor with the help of conventional treatments like chemotherapy, radiotherapy, photodynamic therapy, etc. lead to immunogenic cell death of cancer cells. The dying cancer cells express the specific antigen or damage-associated molecular patterns (DAMP) on the surface, thus help in activating the adaptive as well as innate immune response [139, 140]. It’s also used for developing the in situ vaccination for the broad repertoire of cancer antigens [141]. The current limitations of immunotherapy are being taken care of by the synergistic combination of it with conventional therapies like chemotherapy, photothermal, photodynamic, and radiotherapy using nanomaterials, as following (Fig. 7).
Fig. 7. Synergistic effect of conventional therapies and immunotherapy in reference to nanoparticles functionalization.
Chemotherapy, photothermal, photodynamic, radiotherapy, and gene therapy cause immunogenic cell death, and nanoparticle functionalization leads to robust activation of the immune response, which is helpful in observing the abscopal effect.
3.1. Chemo-immunotherapy
Chemotherapy has been the standard treatment for cancer management. There is a high interest in combining it with immunotherapy. In phase III clinical trial, albumin NPs assisted delivery of paclitaxel along with the PD-1/PD-L1 immune checkpoint inhibitor helped in progression-free long survival of non-small cell lung and triple-negative breast cancer patients [84-86, 142]. Similarly, the phase III clinical trial of chemoimmunotherapy is underway in urothelial cancer patients [143, 144]. It was noticed that the non-small cell lung cancer patients responded poorly to the alone PD-1/PD-L1 checkpoint inhibitors therapy by expressing low PD-L1. However, on the combination of chemotherapy, the response enhanced multifold[89]. Although a segment of patients also showed the adverse effect on the treatment of chemoimmunotherapy, which ranged from moderate to life-threatening. It leads to the discontinuation of treatment by 10-20% of patients [85, 86, 142, 145]. Thus, further interpretation of the off-target toxicity needs to be explored along with the focus on targeted and localized approaches. There are several chemotherapeutic drugs (mitoxantrone, doxorubicin, oxaliplatin, etc.) that can kill the cancer cells in immunogenic fashion and elicit the systemic immune response during cell death [139, 140]. During the immunogenic cell death, soluble factors like CX-chemokine ligand 10 (CXCL 10), high-mobility group box 1 (HMGB1), calreticulin (CRT), and ATP are released. These signals the dendritic cells to phagocytose the dying tumor cells and present antigen to facilitate the infiltration of T cells. In contrast to cancer vaccines which rely on tumor antigen, the chemotherapeutic driven immunogenic cell death leads to the immune response against multiple antigens. Therefore, targeted delivery of chemotherapeutics to the tumors needs to be promoted by choosing targeted nanocarriers (liposome, polymeric NPs, drug-polymer conjugates, etc.) to avoid the off-target effects and promoting the therapeutic index [146] (Table 3).
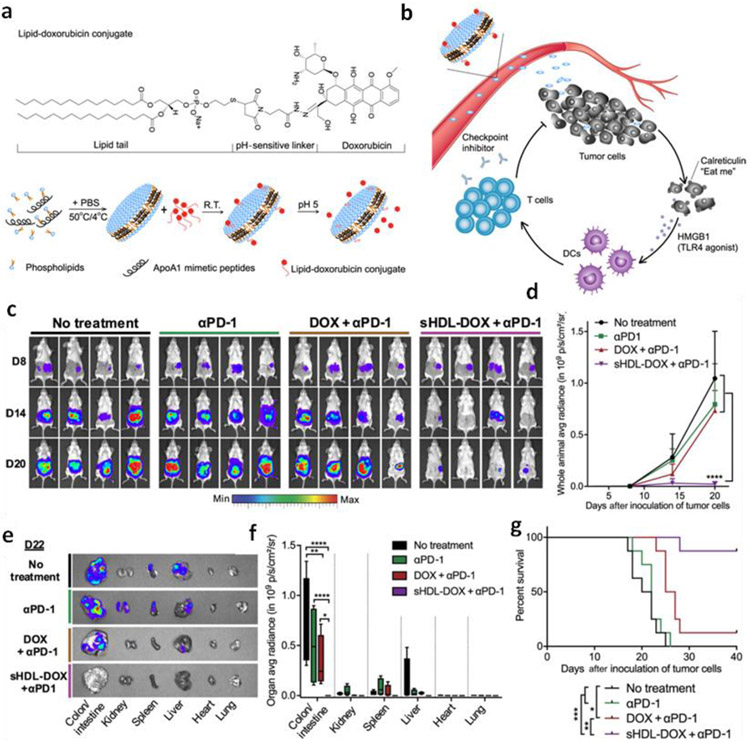
The combination of immune checkpoint inhibitors with the targeted chemotherapeutic NPs has shown promising results in preclinical studies. For example, doxorubicin (DOX) loaded nanodisc comprised of synthetic high-density lipoprotein exhibits higher blood circulation time and accumulation of DOX in tumor(s) [141]. It was experienced that the combination of anti-PD-1 immune checkpoint inhibitor with DOX loaded nanodisc lead to the seven-fold increase in the IFNγ+CD8+T cells and leads to complete regression of CT26 tumors (Fig. 8).
Fig. 8.
a) Schematic of sHDL-DOX synthesis for the chemo-immunotherapy. b) Schematic showing the intratumoral delivery of DOX due to ultra-small nature of sHDL, and also triggering ICD and danger signals like HMGB1 and calreticulin. c) Representative bioluminescence images of mice during the course of study. d) Quantification of the signals. e) Imaging of the major organs harvested. f) Quantification of signals from each organ harvested. g) Survival graph of mice. Adopted from reference [141] which is distributed under a Creative Commons Attribution Noncommercial License 4.0 (CC BY-NC).
The combination of chemotherapeutics with immune adjuvants also helps in increasing immunity. DOX loaded dendrimers entrapping CpG (TLR9 agonist) and targeted against the prostate-specific membrane antigen helped in potent immune response in 22RV1 tumor-bearing mice [147]. Paclitaxel (PTX), another potent chemotherapeutic drug, shows good combination with the immunotherapy by inducing the maturation of TLR4 mediated dendritic cells and infiltration of CD8+ T cells [148, 149]. The polyglutamic acid microparticles carrying the PTX and imiquimod (TLR7 agonist) lead to the robust localized and systemic immune response, which inhibited distant metastatic sites in B16F10 tumor-bearing mice [150]. The combinatorial delivery of paclitaxel and the detoxified bacterial lipopolysaccharide (LPS, a TLR4 agonist) exhibits to increase the number of T-helper cells [151]. Similarly, the sequential delivery of PTX, CpG, and siRNA against the IL-10 with the help of PLGA NPs can prolong the survival of B16F10-OVA melanoma tumor-bearing mice [152].
Furthermore, the combination of mitoxantrone, CpG, and the immune checkpoint inhibitor has been explored for the tumor vaccination to check the recurrence [153]. It was found that 78% of tumor-bearing mice showed complete remission. The co-delivery of DOX and CpG using PLGA NPs boosts the activation of dendritic cells and T cell response, which was further amplified when the CTLA-4 and or anti-OX40 (immune checkpoint inhibitors) was co-administered [154]. A nanogel formulation comprised of chitosan was loaded with PTX and IL-2, and also coated with the erythrocyte membrane for enhanced blood circulation [155]. This construct facilitated more exposure of CRT to the dendritic cells and thus increased response of CD8+ T cells in comparison to alone PTX or IL-2 in B16F10 tumor-bearing mice. Similarly, the combination of DOX and IFNy has been used for extending the survival of B16F10 melanoma mice [156]. Further, mesoporous silica NPs have been used for the co-delivery of DOX, IL-2, and all-trans-retinoic acid (ATRA, a potent immunomodulator and increases the infiltration of CD8+ T cells as well as NK cells) for chemoimmunotherapy of B16F10 tumor-bearing mice [157].
3.2. Photothermal-immunotherapy
Photothermal therapy (PTT) is a type of hyperthermia wherein the photon is converted to heat with the help of photothermal agents [158]. The release of tumor antigens and other immune stimulator molecules after photothermal ablation induces the cascading immune response. For example, the gold nanoshells based photothermal therapy has been reported to induce the expression of pro-inflammatory cytokines [IL6, IL-1, IL12p70, TNF-α, GCSF, GM-CSF, macrophage colony-stimulating factor (MCSF)], chemokines [CC-chemokine ligand (CCL2, CCL4), and chemokine (C-X-C motif) ligand 1 (CXCL1)] and maturation of dendritic cells in the lymph nodes [159]. However, the suboptimal immune activation, myeloid-derived suppressive cells, and other factors cause difficulty in the treatment of metastatic sites in photothermal monotherapy [159]. Further, it was noticed that if the temperature during the PTT raised above the 45 °C, then the immune response decreases due to adverse effects on the cytokines, chemokines, vasculature of the tumor, and immunogenic death of the cancer cells[160]. Thus, for the ablation of the big tumor and the suboptimal activation of immune response is a limitation, which results in relapse due to left-out cancer cells. Literature suggests that combining the PTT with the immune-modulating agents can ablate the big tumors and as well as metastatic sites [161, 162] (Table 4). For example, the chitosan-coated and CpG loaded copper sulfide NPs not only ablated the primary tumor with the help of PTT but also activate the plasmacytoid dendritic cells for the elimination of distant metastatic sites and untreated tumor [163]. Similarly, the combination of checkpoint inhibitors (anti-CTLA-4) and PTT with single-walled carbon nanotube demonstrate reversion of the immune suppression by the infiltration of Tregs to the tumor [88]. In another study, the combination of anti-PD-L1 with the gold nanostar helped in increasing the infiltration of T and B cells and decreasing the myeloid-derived suppressor cells (MDSC), thus helped in eliminating the distant tumor along with the primary one [164]. The synergistic effect of photothermal therapy with chemotherapy has been demonstrated in various xenograft and syngeneic tumor models[165]. The polydopamine coated spiky gold NPs with the subtherapeutic concentration of DOX and PTT can assist in the elimination of distant secondary tumor with the help of systemic immune response, i.e., abscopal effect [166].
Table 4.
List of nanomaterials driven combinatorial immunotherapies for the treatment of cancer.
| Combinatorial agent / Carrier | Immunotherapy agent | Cancer Model |
|---|---|---|
| Chemo-immunotherapy | ||
| DOX / Thermoresponsive NPs, Lipoprotein nanodisc, Poly(amidoamine) dendrimer, Mesoporous silica NPs, Poly(lactic-co-glycolic) acid NPs | IFNγ, anti-PD-1Ab, CpG, ATRA, IL-2 CpG, anti-CLA4 Ab, anti-OX40 Ab |
B16F10, CT26, 22RV1, B16 melanoma, EL4, A20 lymphomas |
| PTX / poly(glycolic acid) NPs, Poly(lactic-co-glycolic) acid NPs; IL-10 siRNA / Poly(lactic-co-glycolic) acid NPs, RBC coated chitosan nanogel | Imiquimod, LPS, CpG, IL-2 | B16F10 |
| Cisplatin / Liposomes | CpG | B16F10 |
| Photothermal-Immunotherapy | ||
| Copper sulfide, gold NPs, CNT, ICG, IR7, melanin | CpG, GC, GM-CSF, OVA | 4T1, EMT6, B16, CT26, EG-7OVA |
| Polydopamine coated spiky gold NPs | DOX | CT26 |
| SWCNT, ICG, Bremaclilorin, Ce6 | Anti-CTLA-4 Ab, R837, SLPs, OVA, CpG | 4T1, CT26, TCI RMAEG7-OVA |
| Gold nanostar, Pyrolipid, IR700, TBP | Anti-PD-L1 Ab | MB49, MC38, CT26, 4T1 |
| Gold nanoshell | Adoptive T cells | B16F10 |
| Temoporfin, Ce6 | Anti-GR1 Ab, Anti-CD25 Ab, LCL521 | SCCVII, B16F10, |
| PPa | PD-L1 gene silencing | B16F10 |
| Photodynamic-Immunotherapy | ||
| NCP-pyrolipid | Oxaliplatin + anti-PD-L1 Ab | CT26, MC38 |
| ZnP-pyrolipid | Anti-PD-L1 Ab | 4T1 and TUBO |
| MOF, PpIX, H4TBC | IDO inhibitor, 1MT | CT26, MC38 |
| UCNPs-Ce6 | Imiqimod + anti-CTLA-4 Ab | CT26 |
| Radio-immunotherapy | ||
| Gold NPs | Anti-CD40 Ab | |
| Hf NPs | IDO inhibitor | |
| Gene-Immunotherapy | ||
| STAT3, IDO, SOCS1 siRNA / Liposome | ||
| SOCS1 siRNA, STAT3 siRNA, IL-10 siRNA/ PLGA NPs | imiquimod, OVA, | EG7-OVA, B cell lymphoma model |
| TGFβ siRNA/Liposomes | CpG, (TRP2) peptide | B16F10 |
Abbreviation: Doxorubicin (DOX), paclitaxel (PTX), all-trans-retinoic acid (ATRA), lipopolysaccharide (LPS), carbon nanotubes (CNT), 5,10,15,20-tetra(p-benzoato)porphyrin (TBP), Pyropheophorbide-a (PPa), 5,10,15,20-tetra(p-benzoato)chlorin (H4TBC)
3.2.1. PTT-Immunoadjuvant therapy
The combination of PTT with immunoadjuvant is emerging as a promising field in cancer treatment. Hollow copper sulfide crystals (CuS, photothermal agent) with the immunoadjuvants oligodeoxynucleotides containing the cytosine-guanine (CpG) motifs efficiently release the IFN-α by the plasmacytoid dendritic cells (pDCs) in tumors [163]. The myeloid dendritic cells (mDCs) cross primed the T cells to the antigen-specific CD8+ T cells, hence triggered the adaptive immunity. Additionally, the level of IFN-γ was found higher, which not only inhibited the growth of primary tumor but also the secondary tumor in EMT6 tumor model. Besides inorganic crystals, gold NPs are also a potential photothermal agent due to its biocompatibility, ease of synthesis, and targeting capability [167-170]. The simple chemistry of functionalization with the protein, oligonucleotides, and other molecules gives several folds advantage over other types of photothermal agent [171, 172]. The CpG with thiol groups can be conjugated instantaneously to the surface of gold NPs for synergistic photothermal and immunotherapy [169]. The efficiency and absorbance wavelength of gold NPs can be controlled by varying the synthesis parameters [173]. The laser irradiation leads to the ablation of the primary tumor as well as generation of heatshock protein 70 (HSP70), which elicited the immune response and resulted in the reduction of distant tumor with 80% survival rate in C57BL/6 tumor model. Gold nanostars and iron hexacyanoferrate dye known as Prussian blue, have also been used for the synergistic application of photothermal and immunotherapy [174, 175]. Further, indocyanine green (ICG) is an FDA approved imaging and photothermal agent [176, 177]. It can be encapsulated inside the carrier due to small molecule size. The ICG and OVA can be formulated in ambient conditions [178]. The OVA-ICG nanovaccine enhanced the secretion of IL-6 and TNF-α, indicating the positive immune response. In the prophylactic model, the nanovaccine was injected before the transplantation of B16 melanoma cells for developing the immunogenic memory.
3.2.2. PTT-ICB Therapy
The alternative photothermal combination strategy is the usage of immune checkpoint inhibitors. PD-L1 receptors are upregulated in the cancer cells [164]. The gold NPs were injected in the C57BL/6 mice implanted with MB49 bladder cancer cells. It facilitated the photothermal induction for promoting anti-PD-L1 therapy. This synergistic therapeutic outcome is 40% more compared to anti-PD-L1 monotherapy. Similarly, hydrogel incorporating the tumor penetrating peptide with the ICG and JQ1 (inhibitor of PD-L1) promotes matured DC (~62%) and proinflammatory cytokines abundance in tumors [179]. The antibody against the CTLA-4 has also been exploited for photo-immunotherapy. It helps in the downregulation of Tregs in the draining lymph nodes [160, 180]. The PEGylated single-wall carbon nanotubes (SWCNT) can increase temperature up to 53 °C when irradiated with 808 nm laser [88]. While concurrent administration of anti-CTLA-4 antibody with these NPs helped in the downregulation of Tregs, thus increased the ratio of CD4+ and CD8+ T cells to the Tregs. In the case of photo-immunotherapy, 57% of the mice survived for 50 days, while in surgery, only 25% survived. An ICG, imiquimod (R837), and TLR-7 agonist combined encapsulation in PLGA nanoparticle formulation resulted in sustained release of R837 and TLR-7 increased the maturation of dendritic cells as well as the secretion of proinflammatory cytokines [181]. A pronounced inhibition of secondary tumors was achieved along with the increase in effector T memory cells (CD3+, CD62L+, and CD44+) in comparison to the control.
3.2.3. Considerations in hyperthermia
Hyperthermia generated in the temperature range 39-45 °C supports the DAMP, particularly HSPs, for the immune stimulation [182, 183]. The milder rise in temperature promoted the expression of HSPs as cell response to physiological changes; however, higher temperature hampered the HSPs expression and led to necrosis [170, 184]. Further, the modest temperature facilitated more infiltration of APCs and T cells due to enhancement in the interstitial and lymphatic blood flow [185]. The research in understanding the thermal dose for optimum immuno-stimulation is still in infancy[183], Li et al. [186] have significantly improved photo-immunotherapy of IR-7, and unmethylated CpG loaded liposomes in the CT26 colon cancer bi-flank model. Similarly, SWCNT and glucosamine polymer, N-dihydro-galacto-chitosan as immunoadjuvant based formulation induced a strong immune response with the enhanced infiltration of CD8+ T cells and increase in the secretion of IFN-γ and IL-2 in the spleen in EMT6 tumor model of BALB/c mice [163, 187]. Also, the OVA based nanovehicle delivered the ICG and antigen against the B16 melanoma cancer cells, and the hyperthermia temperature achieved was more than 60 °C [178].
3.3. Photodynamic therapy-immunotherapy
Besides PTT, photodynamic therapy (PDT) has also shown great promise in immunotherapy. The mechanism is similar to PTT (i.e., generation of DAMP). The tumor-specific antigen and DAMP are presented by APCs, which helps in the maturation of DC, cross-priming of T cells, and developing the immunologic memory. A long immunological memory with the help of bis (amino) silicon (IV) phthalocyanine (BAM-SiPc) mediated PDT in CT26 BALB/c mice model helped in the eradication of 70% tumor in the test group [188]. The 5-5-(4-N, N-diacetoxylphenyl-10, 15, 20-tetraphenylporphyrin) (DTPP) mediated therapy, which was evidenced with the increase in the ratio of CD4+/CD8+, percentage of NK cells, IL-1 and IFN-γ levels along with lymphocytes congregation at the site[189]. Burley et al. [190] have developed the affibody (ZEGFR:03115) to the phthalocyanine dye IR700DX for the treatment of brain tumor. Another report demonstrated higher stimulation of macrophages along with the increase in HSP70 on the PDT treatment of SCC VII mouse model [191].
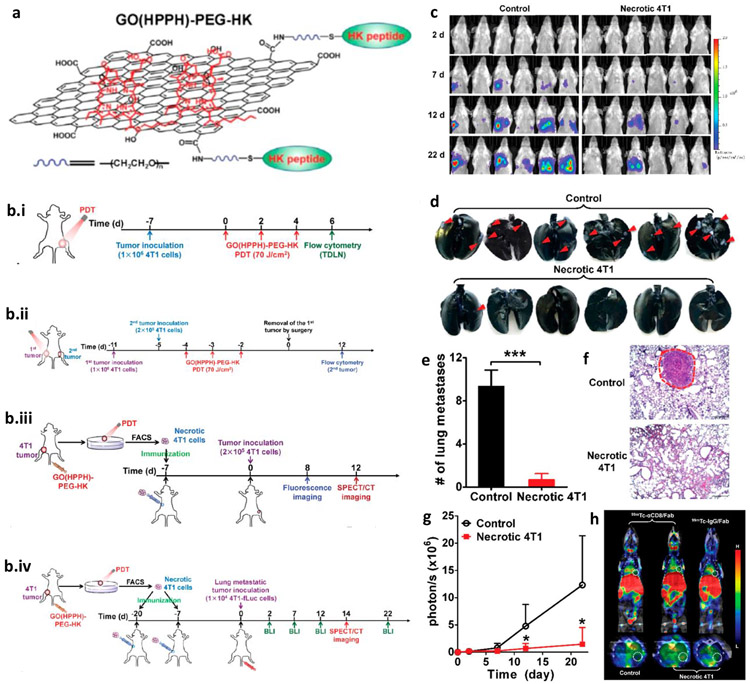
The development of 2nd and 3rd generation photosensitizers has given the global reorganization to PDT for the treatment of cancer [192]. Gollnick and Henderson used PDT as a prophylactic vaccine for the first time[193]. In contrast to conventional treatments, herein, tumor is taken out and treated with PDT, the lysate formed is injected into the same patient (autologous) or different patient (allogenic) [192, 194, 195]. Yu et al. [196] used it for developing vaccination using graphene-based PDT. 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-alpha coated with graphene, and HK peptide (high affinity for the αvβ6 integrin receptor which is highly expressed in the tumor) exhibited an enhanced generation of singlet oxygens and maturation of DCs (increase in the expression of CD40 and CD70 in the lymph nodes). Further, the ratio of CD8+ to CD4+ improved along with the higher level of IFN-γ showed the activation of antitumor immunity. In addition, this approach efficiently eliminated residual cells, inhibited the metastasis and relapse (Fig. 8). Though several groups have used PDT solely for the induction of antitumor immune response, there are other groups also wherein the combinatorial approach has been used for inducing the persistent and stronger immune response. (Table 4)
3.3.1. PDT-Immunoadjuvant therapy
Calreticulin (CRT, a well-known DAMP in apoptotic cells) is located in the endoplasmic reticulum. Under stress conditions, it translocate to the cell membrane and signals for the phagocytosis, i.e., ‘eat me.’ The immunogenic property of CRT replaces the immunoadjuvants like OVA and CpG [196]. CRT driven generation of antitumor immune response was observed upon PDT [197]. Synthetic long peptides (SLP) have shown therapeutic benefits and currently being researched for cancer vaccination [198]. For example, SLP with PDT offers enhanced CD8+ T cells infiltration and elimination of distant metastatic sites [87]. This combination vaccination aided in the treatment of 30 % more inhibition of tumor growth of mice (20 % for SLP and 0 % for the PDT and control. Another nanoscale coordination polymer (NCP) loaded with pyrolipid and oxaliplatin lead to CRT overexpressed on the surface of the cells and helped in the migration of DCs [199]. This treatment shows higher levels of IFN-γ, IL-6, and TNF-α, which helped in the inhibition of tumor growth in two animal models.
3.3.2. PDT-ICB therapy
PDT-ICB is another type of combinatorial therapy wherein immune checkpoints are blocked along with the PDT. A pH-sensitive micelleplex multifunctional nanoplatform was encapsulated with RNAi (for silencing the PD-L1 expression) and pheophorbide (PDT agent) [200]. This unique micelle formulation produces 5.6-fold higher reactive oxygen species generation and creates immune response due to the HSP70, NF-κB expression, and infiltrating CD8+ T cells. The monotherapy of PD-L1 and PDT leads to the inhibition of 65% and 73% tumor growth, respectively. In contrast, the combinatorial approach leads to the complete elimination of the tumor. The combination of PD-L1 with PDT and its pH triggered release significantly reduces the Tregs level and increase in CD8+ T cell population [201]. In both studies, effector memory T cells were noticed for rendering long-term immunization. Similarly, PDT in combination with anti-PD-L1 therapy inhibited the lung metastasis along with the complete elimination of primary tumor [202], Xu et al., [203] synthesized the upconversion NPs using 20% Yb and 2% Er-doped NaYF4 with PEG and loaded with immunoadjuvant R837 and chlorin e6 (photosensitizer). The antigen released due to PDT was amplified with the help of immunoadjuvant R837, which further activated the DC and release of cytokines like IL-12p40 and other molecules like TNF-α and IFN-γ.
The usage of IDO enzyme inhibitors has gained popularity along with the other checkpoint inhibitors [204]. A nanoconstruct of photosensitizer protoporphyrin IX (PpIX) to the caspase responsive peptide was linked with IDO enzyme inhibitor 1-methyltryptophan (1MT) via the PEG and palmitic acid [205]. The regression of CT26 tumor was noticed after the intravenous injection of PpIX-1MT NPs due to immunogenic cell death and expression of CRT on the cell surface. Further, the ratio of CD8+ against CD4+ increased in spleen and sera. Another report indicated synergistic actions of PDT and IDO enzyme inhibitors in primary and secondary tumor sites of CT26 and MC38 [206].
3.4. Radio-immunotherapy
Radiation therapy is used for the treatment of more than 50% of cancer patients. Cancer cells are vulnerable to radiotherapy because their DNA repair mechanism is impaired in comparison to healthy cells. Radiation also causes immunogenic cell death, which enhances the expression of radiation-responsive DNA damage repair proteins. This process can increase the infiltration of CD8+ T cells and activate the dendritic cells with the help of pro-inflammatory cytokines, type I interferon, cytosolic sensor, stimulator of interferon genes mediated DNA detection, IL-1β, CXCL16, and TNF-α [207, 208]. These complimentary events induce the abscopal effect and help in the inhibition of secondary distant metastatic sites [209]. It has been experienced that low dosage of radiotherapy preferably causes the expansion and recruitment of less radiosensitive immunosuppressive cells like Treg than other lymphocytes [210]. Also, it increases the expression of immune inhibitory proteins like PD-L1 and TGF-β [211]. Thus, there is a need for combining radiotherapy with immunotherapy for the optimum and desired action of immune cells showing significant abscopal effect.
The combination of radiotherapy with the cowpea mosaic virus has been noticed to convert the ID8 cold tumor to hot tumor [212]. In addition, the combination of radiotherapy with the checkpoint inhibitors has augmented the immune response even in poorly immunogenic tumors such as triple-negative 4T1 and TUBO spontaneous mammary tumors [213, 214]. Triple combinatorial therapy (radiotherapy with anti-CTLA-4 and anti-PD-L1 antibodies) further improved efficacy due to synergistic and non-redundant mechanisms of their action [211]. Radiotherapy has been combined with the CpG for the treatment of cutaneous T cell lymphoma and low-grade B cell lymphoma in phase I and II patients. This combination therapy has 36% response rate [215]. Inorganic NPs have been found very effective in reducing the dosage of radiotherapy, thus side effects to neighboring healthy cells. Nanomaterials are being researched out dynamically towards increasing the therapeutic index of radio-immunotherapy. For example, NPs having the ability to scavenge the free radicals and renders anti-oxidative properties are very effective in increasing the safety profile of radio-immunotherapy. The NPs, like gadolinium and hafnium, have already entered the clinical trial. Along with enhancing the radio-sensitization and radioprotection capability, they have also been noticed to improve the abscopal effect [216].
3.5. Gene-immunotherapy
Although the RNA based therapeutics are promising, it has been moderated due to instability, sensibility for degradation, poor delivery, and uptake problems. Thus, the NPs assisted delivery techniques had come to picture to address these limitations [217] (Table 4). For example, the efficient siRNA driven silencing of the PD-1/PD-L1 pathway has been achieved via the folic acid functionalized PEI NPs [121]. These NPs sensitized the ovarian epithelial cell and improved the antigen mediated cytotoxicity of CD4+ and CD8+ T cells. Other RNA nanotherapy was effective in silencing the other immunosuppressors like signal transducer and activator of transcription 3 (STAT3), IDO, suppressor of cytokine signaling 1 (SOCS1), etc., which are upregulated in the tumor microenvironment [218]. An ex-situ IDO silencing of dendritic cells was achieved using siRNA and injecting back into the patients, lead to more infiltration of CD8+ T cells, along with the decrease in the number of Tregs [219]. The liposomes have been loaded with IDO siRNA and conjugated with mannose for specifically targeting the dendritic cells in the lymph nodes [220, 221]. This regimen assists in decreasing the apoptosis of CD4+ and CD8+ T-cells and the maturation and function of dendritic cells. Similarly, another nanoconstruct comprised of the conjugation of STAT3 silencing siRNA with CpG was improved in TLR9 mediated uptake, thus the inhibition of myeloid-derived suppressor cells in murine prostate cancer model [222]. siRNA against SOCS1 delivered using PLGA NPs enhanced the antigen-specific CD8+ T cells, and expression of proinflammatory cytokines includes IL-2, IL-6, IL-12, and TNF in comparison to naked siRNA [82]. Another HA-functionalized liposomal formulation with TGFβ siRNA, CpG, as well as tyrosinase-related protein 2 (TRP2) peptide further increased the infiltration of CD8+ T cells in the B16F10 tumor model [135].
The application of photodynamic therapy after the PD-L1 siRNA delivery by cationic micelles improved the robust immune response [200]. Also, the combination of radiotherapy with PD-L1 siRNA micelles has helped in the elimination of the residual cells [223]. The complete regression of lung adenocarcinoma in xenograft mice tumor model was obtained when the gold NPs tagged with the M2 macrophage-specific targeting peptide (M2pep) and siRNA against vascular endothelial growth factor [224]. Alternatively, the lipid protamine NPs encoding the trap for the IL-10 and CXCL12 promotes the reversing of immunosuppression in the orthotopic 4T1 breast and KPC pancreatic tumor model of mice [225]. The combination of oxaliplatin enhanced the infiltration of CD4+ and CD8+ T cells, along with reducing the expression of PD-L1 in multiple tumor models like B16F10, CT26-FL3, and 4T1 tumor model. The plasmid encoding the IL-2 and IL-12 has been delivered successfully using liposome [226]. The combination of radiotherapy boosted the infiltration of CD8+ T cells, NK, and macrophages in head and neck squamous cell carcinoma. Moreover, the combination of IFNγ-inducible protein 10 gene and adoptive T cell therapy helped in the inhibition of the growth of tumors in melanoma and hepatocellular carcinoma model of mice [227].
In contrast to peptide and DNA, mRNA can be encoded for the stimulation of pattern recognition receptors and co-delivery of encoded antigens and danger signals. The combination of mRNA and chemotherapeutics against immune checkpoints has been found to act synergistically [228]. For example, the combination of protamine-mRNA and anti-CTLA-4 antibodies leads to the enhanced infiltration of cytotoxic T cells and NK cells [229]. The mannose tagged liposomes were proposed to target the dendritic cells for delivering the mRNA encoding the Mucin 1 (MUC1, tumor antigen) for eliciting the immune response in TNBC 4T1 tumor model [230]. The immune response was further enhanced when combined with the anti-CTLA-4 antibody. All the above literature suggests the versatility of gene therapy and its potential combination with the other cancer treatment modalities.
3.6. Combination Therapy: Sequential or Simultaneous
There are number of clinical trials focused on harnessing the advantage of combination therapies like experiencing high efficacy, fewer side-effects, and reducing the chances of resistance development. Although biological system is complex, and the interaction between several components decides whether the alone, sequential, or simultaneous intervention could lead to the best result. For example, the simultaneous application of chemotherapy and hormonal therapy didn’t have any significant improvement in the overall survival of breast cancer patient. However, in the case of prostate cancer, the combination of androgen ablation and taxane-based chemotherapy had more significant effect than the sequential administration in LNCaP and Shionogi tumor model [231]. It reflects the importance of dosing regimen and timing of combination therapy, as many times simultaneous delivery may often be difficult for the co-delivery as well as induce adverse side effects, like in chemotherapy, side effects are severe, and combination of drugs may further extend and aggravate it. Also, the combination of drugs may limit the maximum therapeutic effect of individual drugs. Herein, the importance of sequential delivery might be realized than the simultaneous one to reduce the drug-drug interaction and side-effects along with the easing of delivery and increasing the drug access to the targets [232].
The benefits of combined therapy like hyperthermia plus radiotherapy can be harnessed more if the changes in the delivery of treatment are rationalized. In the in vitro and animal studies, it has been found that if hyperthermia is given simultaneously instead of sequentially (conventional), the sensitization to radiotherapy is increased even at a lesser dosage. The mild hyperthermia (~41 °C) for 60 min produced more heat-induced radiosensitization during the simultaneous treatment than hyperthermia (> 42 °C) given during sequential treatment [232]. In contrast, the sequential combination of paclitaxel and carboplatin and later radiotherapy has been found to have more advantageous than the simultaneous combination of cisplatin and radiotherapy in high-risk cervical cancer patients [233]. In another study of breast cancer, the simultaneous application of radiotherapy and tamoxifen didn’t show any disadvantage in any of the in vitro studies; it also improved the survival of the patient. However, lung and subcutaneous breast fibrosis was found to increases in the later years [234].
4. Localized Immunotherapy
The systemic delivery of immunomodulators has been experienced to develop cytokine release syndrome and cause abnormal liver function. Thus, in many therapeutic decisions, the local and sustained release systems are required to reduce the off-target effects. Mineral oil has been used for the sustained and localized delivery of anti-CD40 antibodies in the immunotherapy clinical trial [235]. In comparison to systemic injection of antibodies, the localized injection of this formulation lowered the dosage of antibodies in generating the systemic immune response and activating the T cells [236]. However, this system also causes inflammation and swelling at the injection site[237]. This led to the usage of biodegradable polymeric based nano- and microparticles. The PLHMGA microparticles exhibited sustained delivery of anti-CD40 and anti-CTLA-4 antibodies [119]. PLHMGA protects antibodies from rapid biodegradation and provides a better-sustained release (over 30 days) compared to PLGA. The earlier systems include mineral oils and polymeric microspheres for sustained and controlled release. However, by the time, more advanced systems have emerged as follows.
4.1. Implantable scaffold
Most of the implanted immune cells can die upon the transplantation and involve a lot of modifications during ex-situ engineering. The polymeric scaffold used for drug delivery can be extended for the release of bioactive molecules, recruiting, and proliferation of the dendritic cells [238, 239] (Table 5). The antigens loaded in these scaffolds could activate and recruit the dendritic cells for efficient antigen presentation. PLGA is a widely used scaffold material that efficiently encapsulates GM-CSF for the recruitment and proliferation of the dendritic cells [240]. In contrast to bolus injection, the scaffold helped in the release of antigens and stimulation of dendritic cells over the 2 weeks study [241]. In general, intravenously injected T cells marginally reach tumor while the localized delivery suffers immunosuppression. The implantation scaffold-based approach can solve this issue [242]. Porous alginate-based scaffolds allow T cell entrapment and release in the cavity of post-operated tumors or nearby to the inoperable tumors [243] (Fig. 10). The collagen mimicking peptides were conjugated to alginate scaffold for the efficient migration (8-folds) and enhancing the viability of T cells (22-folds). This approach has also been applied for the sustained release of immunostimulatory molecules[244]. Further, the lysate of lung carcinoma, rat glioma, and others have been loaded on the scaffold towards improving the antigen presentation profile[245, 246].
Table 5.
List of biomaterials being used for the localized immunotherapy.
| Biomaterials for Localized tumor modulation |
Functionalization | Delivering agent | Target/Action |
|---|---|---|---|
| Hyaluronic acid-based microneedle patches | Melanin | Dextran NPs co-loaded with anti-PD-1 antibody/IDO inhibitor, glucose oxidase and catalase. | DC, T cells |
| PLHMGA microparticles loaded with anti-CTLA-4 antibody | T cells | ||
| Anti-PD-1 loaded DNA NPs | T cells | ||
| 1-MT + anti-PD-1 | T cells | ||
| Porous alginate scaffold | Adoptive T cells + IL-15SA | T cells | |
| PLGA scaffold | GM-CSF + tumor lysate | DC | |
| FLT3L, CCL20 | DC | ||
| Chitosan Hydrogel | T cells | T cells | |
| PVA hydrogel | Gemcitabine, anti-PD-L1 antibody | T cells | |
| Nanolipo gels: liposomes and polymers | SB505124 inhibitor + IL-2 | T cells and NK cells | |
| Self-assembled silica rods | PEI | GM-CSF, Oligodeoxynucleotides containing CpG motifs, and antigens | T cells |
Fig. 10. Approaches for the localized modulation of the tumor microenvironment.
The current approaches mainly include the scaffold, microneedle patches, and injectable hydrogels. The scaffold helps in the activation of T cells, immune checkpoint blockade, as well as the maturation of dendritic cells. The microneedle patches and injectable hydrogel are less invasive and used for the triggered and controlled release of different immune modulators.
4.2. Injectable scaffolds
The scaffold-based immunotherapy often requires surgical implantation. Therefore, injectable scaffolds comprised of gelatin, mesoporous silica rods, alginate hydrogel, etc., have shown alternative approach for immunotherapy application [247-249]. The high aspect ratio mesoporous silica rods have been administered in vivo to form the porous structure for the recruitment and activation of immune cells [250] (Fig. 10). Mesoporous silica is extensively used for the drug delivery application due to high surface area and biocompatibility[251]. On the administration of the silica rods, it self-assembled into porous structure to localize the immune cells and release the loaded GM-CSF, oligodeoxynucleotides containing CpG motifs (CpG-ODNs), and antigens [239]. Further, the nanomaterials can be modified with the PEI, which stimulates the secretion of pro-inflammatory cytokines, thus helps in increasing the immunogenicity of the neoantigens to target the heterogeneous tumor clones [251, 252].
4.3. Injectable hydrogels
The in-situ injectable hydrogels are being used for delivering the combined immunotherapy and chemotherapy agents. It’s beneficial for patients having poor immunogenic tumors or show side effects to the immune therapies [253]. Hydrogel based technology shows delivering the immunotherapy in a minimally invasive way [242]. A poly(vinyl alcohol) based nanogel enables sequential release of gemcitabine and anti-PD-L1 antibody to elicit antitumor immunity [254]. An injectable thermohydrogel composed of chitosan, phosphate buffer, and sodium hydrogen carbonate has been used to release T cells [255]. The 50 to 500 μm pore size hydrogel showed the optimum T cell proliferation and migration. This system promotes the production of Th1 cytokine (INFN-γ).
4.4. Transdermal delivery
The transdermal delivery has been proposed for sustained release and reducing the required dose of mAbs [256]. Microneedle technology is an advanced form of transdermal delivery which can reach the immune cell-rich epidermis and release the content [256, 257] (Fig. 10). Microneedles produced from HA, pH-sensitive polymers, and immune checkpoint inhibitors enhanced survival of the melanoma mouse model (40 days vs. 30 days alone anti-PD-1 antibodies) [256]. The loading of 1-methyl-DL-tryptophan (1-MT, an inhibitor of IDO) and anti-PD-1 in NPs embedded microneedles efficiently increased the survival rate of melanoma mice to 70% in comparison to controls [256].
5. Nano-Immunoimaging
The nano-immunoimaging is a representation of imaging, immunology, and nanotechnology. This novel tool enables to probe the unanswered questions of immunology, including visualizing the immunodynamics in a spatially and temporally controlled manner, insights of the immune cell biology, cell trafficking, and monitoring the therapeutic index (Fig. 11). For nano-immunoimaging, the cognate ability of imaging at optimal in vivo concentration without disturbing the immunological signaling is desirable (Table 6).
Fig. 11. Application of nanomaterials in immunoimaging.
The nano-immunoimaging involves the integration of diagnostic nanomaterials for the imaging of immune cells like dendritic cells, macrophages, T cells, and NK cells.
Table 6.
List of nano-immunoimaging agent used for the cancer immunotherapy.
| Modality | Imaging agent | Functionalization | Immune cells | Findings |
|---|---|---|---|---|
| MRI | SPIONs | Poly(aspartic acid)-b-poly(ecaprolactone), protamine, lipofectin, TAT peptide, dextran, HA, Annexin V, sLeX | Macrophages, DC, NK, CD8+ T cells, MSCs, exosomes | Non-invasive imaging of macrophages in inflammation, atherosclerotic plaques, allograft; Tracking and accurate delivery of DC and T cells; Early prediction of rejection of transplant, myocarditis; Tracking of exosomes; Understanding activation of T cells. |
| Optical Imaging | Luminescent imaging: Quantum dots, UCNPs; OCT: gold nanorods | PEI, Oligo-arginine, OVA, GFP/Luc | DC, TAM | Luminescent imaging of UCNPs to lymph node, |
| Nuclear Imaging | Polyglucose NPs, gold, silver, tantalum, and bismuth NPs | 89Zr, 124I, 64Cu | TAM | Quantification of TAM in the tumor; Tracking of CAR-T cells |
| Multimodal | Photoacoustic: gold nanorods; Magnetic and Optical: SPIONs | Silica, peptides | Cytokine-induced killer cells | Imaging of gastric cancer, endothelium |
5.1. Magnetic resonance imaging
Iron oxide NPs, primarily super paramagnetic iron oxide NPs (SPIONs) are the first example of immunoimaging agents that are used to label the immune cells and can be imaged under magnetic resonance imaging (MRI) [258]. The macrophages actively uptake the SPIONs due to high endocytic capabilities [259]. It was reported that SPIONs containing macrophages (~500 cells) could be tracked in vivo. Such non-invasive imaging of macrophages recruited to the lesion site and understanding the role of macrophages in the stroke associated neuroinflammation [260]. Besides macrophages, DC and NK can also be labeled with the SPIONs for immunoimaging. Effective labeling with biocompatible SPIONs was achieved by the coating of iron oxide NPs with the poly(aspartic acid)-b-poly(ε-caprolactone) for DC imaging [261]. These SPIONs were found to have no effect on the viability, proliferation, and differentiation capacity of DC. SPIONs provide excellent contrast in MRI and whole-body DC cell tracking; for example, after injection of labeled DC cells in the footpad, it was observed draining to the lymph nodes. The labeling of autologous dendritic cells with iron oxide and imaging with MRI helps not only tracking the inter and intranodal movement of dendritic cells but ensure its accurate delivery to the lymph node of melanoma patients. NK cells usually have low phagocytic capabilities; thus, transfecting agents like Protamine® or Lipofectin® are used for the labeling of SPIONs [262]. The SPIONs labeled NK cells have been used for the liver cancer model, wherein the increase of NK cells in the liver determined by MRI was also found to be correlated with the histology studies. The loading of SPIONs is further facilitated with the usage of the external magnetic field [263]. The NK-92MI cells were loaded with the Cy5.5 labeled SPIONs can be applied to track real-time infiltration of NK cells to the tumor with the help of the external magnet. Besides innate immune cells, adaptive immune cells like CD8+ cytotoxic T lymphocytes can also efficiently labeled with SPIONs using cell penetrating TAT peptide [264]. It increased the detection efficiency to 2 cells/voxel in in vitro studies and 3 cells/voxel in vivo studies.
Magnetic nano-immunoimaging has been explored for in vivo labeling. For this purpose, the dextran-coated SPIONs were injected intravenously (~ 56 mg per kg), which were preferentially uptaken by the macrophages [265]. The SPIONs were found in higher numbers in the atherosclerotic plaques having higher inflammation. A positive contrast image of the plaque can be achieved after 24 h injection of NPs in MRI [266]. Such imaging techniques not only provide qualitative images but the degree of inflammation. HA (target CD44 receptors on the macrophages) functionalized SPIONs were 4 times more uptaken than the non-functionalized SPIONs and can be imaged atherosclerotic plaques more efficiently [267]. This targeted SPION formulation can decrease the dosage of the imaging agent required for imaging. Another SPION formulation that was functionalized with Annexin V facilitates superior interaction with the phosphatidylserine expresses on the surface of dying macrophages and other cells in atherosclerotic plaques [268]. Such labeling also helped in reducing the dosage of SPIONs to more than 200-folds. Similarly, nano-immunoimaging is also helpful in the early determination of the rejection of the allograft and rejection induced apoptosis of mesenchymal stem cells by attaching the peptides showing the Tight up’ feature in the presence of caspase-3 (apoptosis marker) to SPIONs [269].
During the inflammation and metastasis, the E-selectin gets upregulated and recognizes the tetrasaccharide sialyl LewisX (sLeX) expressed on the leukocytes [270]. The sLeX-SPIONs reflected less attenuated MRI signal in hepatic mice than the uncoated SPIONs[271]. The ultrasmall SPIONs has been used for the labeling of exosomes for understanding the activation of T cell with MHCII bearing exosomes released from B-lymphocytes [272].
Magneto-motive ultrasound imaging (MM-US) is another imaging modality for immunoimaging[273]. Wherein a strong, focused magnetic field is applied to generate motion in tissue with the help of SPIONs, and then signals are measured using ultrasound transducers. After the injection of SPIONs, the motion of macrophages was readily noticed in the liver. These techniques have strong selectivity potential in tracking applications without the usage of radioactive materials. The limitations of the SPIONs are inherent inflammation, oxidative stress, and toxicity to the cells. The high chance of false-positive results if the labeled cells die and release the SPIONs, which could be taken up by the other cells[274].
5.2. Optical imaging
The benefits of optical imaging are the ability to even detect single cells with higher sensitivity, specificity, and higher temporal and spatial resolution [275]. There are instances wherein it has been used complementary to MRI and CT imaging for whole-body scanning and understanding the interaction of nanomaterials with the cell’s biology [276]. However, the limitations of optical imaging are the depth of imaging, which is usually trade-off with spatial resolution and background autofluorescence. Thus, the usage of NPs has come up to address these limitations. The high-resolution optical imaging includes fluorescence, bioluminescence, scattering, and absorbance based imaging[277]. Quantum dots are fluorescent nanomaterials, which are extensively used for optical immune imaging due to their size-tunable emission and robust fluorescence stability[278, 279]. To overcome the problem of shallow penetration of UV light, lanthanide-based upconversion nanoparticles (UCNPs) have been explored for the imaging of immune cells with low background [280]. They can fluoresce on the irradiation of near-infrared light (high penetration depth, 600-900 nm) due to the reverse stokes process. There are also reports of coating UCNPs with polyethyleneimine and oligo-arginine to facilitate its uptake by DCs on the incubation [281]. On the hypodermic injection of UCNPs to the footpad of the mice, it reached to the draining lymph nodes, which was determined with the help of luminescence imaging.
Optical coherence tomography is widely used for studying the movement and function of immune cells inside the tumor [282]. Towards this, TAM has been labeled with the gold nanorods as an optical scattering contrast agent. It helped in better and deeper imaging in the complex, hypoxic, and necrotic environment of the tumor. Optical imagining is also being used for tracking the multifunctional NPs in the course of immunotherapy. For example, multifunctional NPs were incorporated with GFP/Luc for tracking the activity and location inside the body [107].
5.3. Nuclear imaging
The usage of radioactive materials has given insights from the decades on finding the distribution and interaction of immune cells [283]. Recent advancement shows that NPs being surface decorated with radioactive isotopes like 89Zr, 124I, 64Cu via chelation chemistry or encapsulation for the positron emission tomography (PET) and single-photon emission computed tomography (SPECT) [284]. There are reports of labeling the TAM with the radioactive modified NPs for assessing the therapeutic response and stratifying the patients in the clinical trials. For example, polyglucose NPs were modified with the 64Cu, which helped in the quantification of TAM inside the tumor. It was observed that more than 700% strong radioactive signals were obtained in the TAM rich tumor than from the TAM deficient tumors [285] (Fig. 12a-f).
Fig. 12.
a) Size distribution of polygluocse nanoparticles (macrin) along with representative images of SEM, scale bar is 20 nm; inset: L-lysine (red) functionalized macrin (black) to attach the chelators for 64Cu and other labels. b) Confocal images of MC38 tumor at low and high magnification in MertkGFP/+ mice. c) Reconstructed PET/CT image showing the tumors in lungs (cyan and blue) and accumulation of macrin (orange). d-e) Ex vivo PET image corresponding to in vivo highlighted tumors(d), confocal images of optically cleared lung tissue(e). f) The relation of optical (VT680) and nuclear (64Cu) imaging. g, h) Phototoacoustic imaging with Au NR@SiO2 nanoparticles (g), NP-CIK cells (h) containing 6.7 μg of gold after peritumor injection. Part (a-f) adapted from reference [284], part (g-h) adapted from ref. adapted from reference [288].
The high contrast of nuclear imaging has also helped in monitoring the CAR-T cell therapy. For example, gold NPs modified with 64Cu2+ were used to track the CAR-T cells. The PET/CT imaging also ensured that CAR-T cells reach and remain at the intended site to avoid the side effects[286]. In comparison to other imaging modalities, radioactive material is also required in very less amount, thus an intangible chance of affecting the native functions of the cells or interactions, which has also been one of the reasons that nuclear imaging is rapidly being explored in the clinical trials.
5.4. Multimodal imaging
Multimodal imaging represents the combination of two or more imaging modalities, such as optical and acoustic in photoacoustic, and other combinations of different conventional imaging modalities. In photoacoustic imaging, ultrasound imaging is combined with optical imaging, wherein the multi-centimeter depth with micrometer resolution is achievable [287]. It gives the advantage of spatial contrast of optical imaging while understanding the anatomical and penetration depth of ultrasound imaging [288]. For example, human cytokine-induced killer (CIK) cells were loaded with the silica modified gold nanorods, which along with the photothermal therapy to gastric cancer, also helped in photoacoustic imaging of the subcutaneous gastric cancer after 4 h of injection [289] (Fig. 12g-h). It killed the MGC803 cancer cells, along with modulation of immune response due to the upregulation of cytokines and interferons.
To further harness the potential of magnetic and optical imaging modalities, peptides have also been used for decorating the SPIONs. For example, peptide (CDSDSDITWDQLWDLMK) has been attached to fluorescent SPIONs via the N-terminus cysteine to binds with the E selectin which facilitates the enhanced optical imaging of activated endothelium in mouse tumor model [290].
7. Challenges and opportunities
7.1. Challenges
The delivery of the required concentration of NPs to the specific site is one of the major limitations in cancer immunotherapy. The fabrication of NPs with due consideration on the shape, size, and pharmacokinetics can make the delivery of NPs more efficient with fewer side effects. However, challenges like a homogenous synthesis of NPs on a large scale and its interaction with immune cells inside the body need further investigation[291, 292]. It has been experienced that only less than 5% of the administered NPs reach the tumor due to the heterogeneous nature of the tumor, ECM, pharmacokinetics, and many other factors[291, 293]. In most of the studies, mouse tumor models have been used as pre-clinical models, which has been noted to differ significantly from the human in terms of heterogeneity, tumor growth, blood vessel diameter, and many more parameters. The safety of nanomaterials is another complication in immunotherapy. There are several biomaterials which are commonly used and have their own advantage and disadvantages (Table 7). Thus, the interaction between the nanomaterials and immune cells should be thoroughly evaluated. For example, it has been noticed that different sizes of NPs induced different types of immune response, e.g., ~40 nm particles induced the IFN-γ response while ~100 nm particles activated the CD4+ T cells and IL-4 secretion [294].The intrinsic immune regulating activities of nanomaterials is important to understand, which can significantly affect the physicochemical properties. For example, PEI (cationic polymer) known for gene delivery, has been reported to have self-adjuvant properties and affect the immunological response via TLR5 pathway [295]. Similarly, cationic liposomes are known to stimulate the APCs for expressing the costimulatory molecules like CD80 and CD86, while zwitterionic or anionic lipid shows no response towards DC.
Table 7.
Advantages and disadvantages of commonly used biomaterials for cancer immunotherapy.
| Biomaterials | Advantage | Disadvantage |
|---|---|---|
| Zinc | Easily functionalized, capability for multimodal imaging, optical and piezoelectric properties, antibacterial and antifungal nature. | Low stability, non-uniform size distribution, requirement of advanced equipment, risk of cytotoxicity and genotoxicity, skin and environment hazard |
| PLGA | High reproducibility and purity over natural polymer, no requirement of highly toxic solvents. | High size variability with change in concentration, low drug entrapment efficiency, high-cost, lack of surface functionalization |
| PLHMGA | Tailorable release rate, better compatibility with protein, not susceptible to acids during degradation | Difficult to prepare |
| Chitosan | High biocompatibility and biodegradability, wound healing and antimicrobial properties. | Low solubility in physiological pH, low porosity, stability & mechanical resistance. |
The load of mutations differs from cancer to cancer as well as between patients. It demands the approach of bioinformatics for finding the top neoantigen in a cancer type. However, the physicochemical and biological property of the neoantigen also needs to be considered as it affects the delivery to the draining lymph nodes, thus efficacy and immunogenicity of the vaccines. Additionally, the expeditious screening of patients reactive to neoantigen needs to be researched out. Towards achieving the broad T cell response, the competitive binding of neoantigen with MHC needs to be considered. Competitiveness and combination with the other antigens in the codelivery system need to be explored [296, 297].
The manufacturing process needs to be streamlined and cost-effective for the successful adoption of immunotherapy. It is another critical parameter to produce good manufacturing practice (GMP) grade vaccines which majorly critical in clinical trials or evaluation of particles. For example, the neoantigen-encoding mRNA vaccines take around 160 days to include the determination of neoantigen, production of mRNA, vaccine manufacturing, and product release [298]. Similarly, the neoantigen peptide vaccine takes around 18 weeks from the determination of neoantigen to vaccine production [299]. The cost of immunotherapy comprised of individual costs of biopsy of the tumor, bioinformatics, mRNA and DNA sequencing, current GMP manufactured biologics, carrier for the cells and drugs, and skilled personnel for delivering the tailored dosage. Thus, the requirement of a simplified approach and clear-cut manufacturing are highly sought.
The varying stability and physicochemical property of peptides, plasmids, biomaterials, and other components of immunotherapy demand the standardized formulation, analytical method, and sterilization protocol for quick product release [81]. The dosage and safety profiles of nanotherapeutics need to be established, as faster kinetic and potent therapeutics might require a lower dosage and vice versa.
7.2. Opportunities
The nature-derived NPs, like albumin NPs, protein nanocages, virus-like particles, exosomes, etc., have proven their place in clinical applications. In comparison to synthetic NPs, nature-derived NPs have advantages such as good biocompatibility, facile production, and immunotherapy boosting characteristics[300]. In contrast to conventional vaccines, albumin, and lipid conjugates having multiple sites, found to be more effective in eliciting the T cell response [301]. The coating of NPs with the cell membrane is another exciting field toward the NPs driven natural vaccines. These advancements help in maintaining the complex antigenic information, thus increase in the therapeutic efficacy in a very specific manner [302, 303]. High-density lipoprotein NPs have low immunogenicity and high stability in the blood and can deliver both the antigens and immunoadjuvant together[304]. Similarly, there are many reports which indicate that natural NPs themselves can elicit the augmented immune response and can work as cancer vaccines. For example, virus-like particles (VLPs) have been experienced to induce both cellular and humoral immune response. The VLPs based prophylactic vaccination (Gardasil or Cervarix) has been used for the treatment of HPV caused cervical cancer [305]. The cowpea mosaic virus in the form of NPs has been found to manipulate the tumor microenvironment and reduce immunosuppression[132].
Exosomes are 40-100 nm endogenous NPs with the properties of cell membrane. They often express the MHC-1 and 2, along with heat shock proteins, help in eliciting the immune response of T helper cells [306]. The key exosome markers like Alix, CD63, CD9, and TSG101 have been noticed to induce cellular immunity and activate the NK cells [306]. Exosomes are known to contain several antigens, which help in inducing specific immunity [307]. The role of exosomes has been determined in the immunosuppression and pro-metastatic preparation of the tumor.385Tumor-derived exosomes (TEX) can also induce antitumor immunity due to the antigen present on their surface [308]. It has been found that TEX causes the apoptosis of pancreatic cancer cells via PI3K/Akt/GSK-3β pathways and Notch signaling [309]. The exosomes driven activation of innate immunity with the help of IgG and NK activity has been reported along with adaptive immunity [310].
The usage of exosomes for presenting the antigen or delivering the chemotherapeutic or RNAi to the specific site has been very hopeful [311, 312]. It has been noticed that the dendritic cells derived exosomes (DEX) can work on boosting humoral and cellular immunity. The MHC complex, CD86, and adhesion molecules include MFG-E8, and ICAM-1 activate the CD8+, CD4+ T cell response [313]. DEX has been used in the cell-free vaccination of mice [312]. DEX activates innate immunity via NKG2D-dependent NK cell and IL-15Rα assisted cell proliferation [314]. Thus, it has been used for the inhibition of melanoma, and the results of early preclinical trials have been promising [315]. However, the challenges like suppression of host immune response and number of activated T cells on the usage of DEX as tumor vaccines need to be further investigated.
The nanomedicines are usually evaluated on the basis of parameters like the ability to target tumor cells and vasculature, active and passive drug delivery, high stability, slower body clearance, high tumor uptake, high drug loading efficiency, and release profile. Out of the several next-generation nanomedicines (e.g., liposomes, micelles, polymeric, and metal NPs), liposomes appear to be suitable for tuning to all the above parameters.
Since 1995 or earlier, FDA has approved several nanomedicines based on micelle, metallic, protein, nanocrystal, liposome, and polymeric materials. During 2001-2005, the approval of nanomedicine was at the peak with nanocrystals holding the highest percentage. However, after 2005, there was a decline in the FDA approval rate and an increase in the nanomedicines clinical trials. Currently, ~ 77 products are in clinical trials, and most of them are in phase 1/2 clinical trials. Further, it was noticed that 40% of the clinical trials listed in clinicaltrials.gov started in 2014 and 2015.
8. Conclusion and Future Direction
Although nanotechnology driven immunotherapy is still in the nascent stage, it holds great potential towards increasing the efficacy and safety profile of cancer treatment. The approaches discussed in this review article may help in facilitating opportunities that can be applied to increase the efficacy of combined therapies, designing biomaterials, and fabrication of multifunctional NPs towards the treatment of aggressive and complicated cold tumors. The outcome of clinical trials is also reflecting the potential of nanotechnology synergized immunotherapy; however, reproducibility, manufacturing feasibility, and undefined toxicity of the nanomaterials should be carefully considered before commercialization.
Fig. 9.
a) Schematic showing the fabrication of GO(HPPH)-PEG-HK. b.i, ii, iii, iv) Different regimens of GO(HPPH)-PEG-HK driven PDT in the subcutaneous 4T1 tumor model (i), GO(HPPH)-PEG-HK driven PDT at one tumor model to stimulate immunity at the second tumor (ii), GO(HPPH)-PEG-HK driven PDT to induce the necrotic 4T1 cells to use them for tumor vaccination (iii, iv). c,g) Representative bioluminescence images (c), and quantitative analysis (g). d) Representative images of the lung filled with Indian ink. e, f) Quantitative analysis (e) and H&E staining (f) of metastatic lesions in lungs. h) Representative SPECT/CT images of mice after the 99mTc-αCD8/Fab and 99mTc-IgG/Fab administration and vaccination using PBS and necrotic 4T1 cells, respectively. Adopted with permission from reference [196].
Acknowledgments
The study was partially supported by the Faculty Start up fund from UTRGV (to M.M.Y., M.J., and S.C.C.), Herb Kosten Foundation, and National Institute of Health of United States of America (R01 CA210192, R01 CA206069, R01 CA204552). This work is also supported by IIT Bombay Healthcare Initiative.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflict of interest.
References
- [1].Tannock IF, Conventional cancer therapy: promise broken or promise delayed?, Lancet, 351 Suppl 2 (1998) SII9–16. [DOI] [PubMed] [Google Scholar]
- [2].Pollock BH, Krischer JP, Vietti TJ, Interval between Symptom Onset and Diagnosis of Pediatric Solid Tumors, J Pediatr-Us, 119 (1991) 725–732. [DOI] [PubMed] [Google Scholar]
- [3].Sieniawski M, Franklin J, Nogova L, Glossmann JP, Schober T, Nisters-Backes H, Diehl V, Josting A, Outcome of patients experiencing progression or relapse after primary treatment with two cycles of chemotherapy and radiotherapy for early-stage favorable Hodgkin's lymphoma, J Clin Oncol, 25 (2007) 2000–2005. [DOI] [PubMed] [Google Scholar]
- [4].Tan SZ, Li DP, Zhu X, Cancer immunotherapy: Pros, cons and beyond, Biomed Pharmacother, 124 (2020). [DOI] [PubMed] [Google Scholar]
- [5].Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ, Natural innate and adaptive immunity to cancer, Annual review of immunology, 29 (2011) 235–271. [DOI] [PubMed] [Google Scholar]
- [6].Sharma P, Allison JP, Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential, Cell, 161 (2015) 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Huang PW, Chang JWC, Immune checkpoint inhibitors win the 2018 Nobel Prize, Biomed J, 42 (2019) 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shahid K, Khalife M, Dabney R, Phan AT, Immunotherapy and targeted therapy-the new roadmap in cancer treatment, Ann Transl Med, 7 (2019) 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee S, Margolin K, Cytokines in cancer immunotherapy, Cancers, 3 (2011) 3856–3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Katze MG, He Y, Gale M Jr., Viruses and interferon: a fight for supremacy, Nat Rev Immunol, 2 (2002) 675–687. [DOI] [PubMed] [Google Scholar]
- [11].Sun T, Yang Y, Luo X, Cheng Y, Zhang M, Wang K, Ge C, Inhibition of tumor angiogenesis by interferon-γ by suppression of tumor-associated macrophage differentiation, Oncology research, 21 (2014) 227–235. [DOI] [PubMed] [Google Scholar]
- [12].Müller L, Aigner P, Stoiber D, Type I Interferons and Natural Killer Cell Regulation in Cancer, Frontiers in immunology, 8 (2017) 304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cox MA, Harrington LE, Zajac AJ, Cytokines and the inception of CD8 T cell responses, Trends in immunology, 32 (2011) 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wu SW, Duan CX, Kong LH, Tu X, Wang LF, Guo Z, Ye JM, Interleukin-10 (IL-10) participates in host defense against bacterial pathogens and promotes IgM antibody production in Nile tilapia (Oreochromis niloticus), Aquaculture, 531 (2021). [Google Scholar]
- [15].Yan WL, Shen KY, Tien CY, Chen YA, Liu SJ, Recent progress in GM-CSF-based cancer immunotherapy, Immunotherapy, 9 (2017) 347–360. [DOI] [PubMed] [Google Scholar]
- [16].Ahmed S, Rai KR, Interferon in the treatment of hairy-cell leukemia, Best practice & research. Clinical haematology, 16 (2003) 69–81. [DOI] [PubMed] [Google Scholar]
- [17].Milling L, Zhang Y, Irvine DJ, Delivering safer immunotherapies for cancer, Adv Drug Deliv Rev, 114 (2017) 79–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hasan AN, Selvakumar A, Shabrova E, Liu XR, Afridi F, Heller G, Riviere I, Sadelain M, Dupont B, O'Reilly RJ, Soluble and membrane-bound interleukin (IL)-15 Rα/IL-15 complexes mediate proliferation of high-avidity central memory CD8(+) T cells for adoptive immunotherapy of cancer and infections, Clinical and experimental immunology, 186 (2016) 249–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chapuis AG, Lee SM, Thompson JA, Roberts IM, Margolin KA, Bhatia S, Sloan HL, Lai I, Wagener F, Shibuya K, Cao J, Wolchok JD, Greenberg PD, Yee C, Combined IL-21-primed polyclonal CTL plus CTLA4 blockade controls refractory metastatic melanoma in a patient, The Journal of experimental medicine, 213 (2016) 1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Postow MA, Callahan MK, Wolchok JD, Immune Checkpoint Blockade in Cancer Therapy, J Clin Oncol, 33 (2015) 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pardoll DM, The blockade of immune checkpoints in cancer immunotherapy, Nat Rev Cancer, 12 (2012) 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, Iyer AK, PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome, Front Pharmacol, 8 (2017) 561–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Munn DH, Bronte V, Immune suppressive mechanisms in the tumor microenvironment, Current opinion in immunology, 39 (2016) 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Darvin P, Toor SM, Sasidharan Nair V, Elkord E, Immune checkpoint inhibitors: recent progress and potential biomarkers, Experimental & molecular medicine, 50 (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, Solomon I, Lesko MH, Ruef N, Roddie C, Henry JY, Spain L, Ben Aissa A, Georgiou A, Wong YNS, Smith M, Strauss D, Hayes A, Nicol D, O'Brien T, Mårtensson L, Ljungars A, Teige I, Frendéus B, Pule M, Marafioti T, Gore M, Larkin J, Turajlic S, Swanton C, Peggs KS, Quezada SA, Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies, Cancer cell, 33 (2018) 649–663.e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Du X, Tang F, Liu M, Su J, Zhang Y, Wu W, Devenport M, Lazarski CA, Zhang P, Wang X, Ye P, Wang C, Hwang E, Zhu T, Xu T, Zheng P, Liu Y, A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy, Cell research, 28 (2018) 416–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA, Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma, The Journal of experimental medicine, 210 (2013) 1695–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ellis PM, Vella ET, Ung YC, Immune Checkpoint Inhibitors for Patients With Advanced Non-Small-Cell Lung Cancer: A Systematic Review, Clinical lung cancer, 18 (2017) 444–459.e441. [DOI] [PubMed] [Google Scholar]
- [29].Hege KM, Bergsland EK, Fisher GA, Nemunaitis JJ, Warren RS, McArthur JG, Lin AA, Schlom J, June CH, Sherwin SA, Safety, tumor trafficking and immunogenicity of chimeric antigen receptor (CAR)-T cells specific for TAG-72 in colorectal cancer, Journal for immunotherapy of cancer, 5 (2017) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Postow MA, Sidlow R, Hellmann MD, Immune-Related Adverse Events Associated with Immune Checkpoint Blockade, The New England journal of medicine, 378 (2018) 158–168. [DOI] [PubMed] [Google Scholar]
- [31].Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin AM, Rosenberg J, Voss MH, Rudin CM, Rizvi H, Hou X, Rodriguez K, Albano M, Gordon RA, Leduc C, Rekhtman N, Harris B, Menzies AM, Guminski AD, Carlino MS, Kong BY, Wolchok JD, Postow MA, Long GV, Hellmann MD, Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy, J Clin Oncol, 35 (2017) 709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Byun DJ, Wolchok JD, Rosenberg LM, Girotra M, Cancer immunotherapy - immune checkpoint blockade and associated endocrinopathies, Nature reviews. Endocrinology, 13 (2017) 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Maleki Vareki S, Garrigós C, Duran I, Biomarkers of response to PD-1/PD-L1 inhibition, Critical reviews in oncology/hematology, 116 (2017) 116–124. [DOI] [PubMed] [Google Scholar]
- [34].Restifo NP, Smyth MJ, Snyder A, Acquired resistance to immunotherapy and future challenges, Nat Rev Cancer, 16 (2016) 121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lim WA, June CH, The Principles of Engineering Immune Cells to Treat Cancer, Cell, 168 (2017) 724–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fesnak AD, June CH, Levine BL, Engineered T cells: the promise and challenges of cancer immunotherapy, Nat Rev Cancer, 16 (2016) 566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Scholler J, Brady TL, Binder-Scholl G, Hwang WT, Plesa G, Hege KM, Vogel AN, Kalos M, Riley JL, Deeks SG, Mitsuyasu RT, Bernstein WB, Aronson NE, Levine BL, Bushman FD, June CH, Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells, Science translational medicine, 4 (2012) 132ra153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Porter DL, Hwang WT, Frey NV, Lacey SF, Shaw PA, Loren AW, Bagg A, Marcucci KT, Shen A, Gonzalez V, Ambrose D, Grupp SA, Chew A, Zheng Z, Milone MC, Levine BL, Melenhorst JJ, June CH, Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia, Science translational medicine, 7 (2015) 303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak Ö, Brogdon JL, Pruteanu-Malinici I, Bhoj V, Landsburg D, Wasik M, Levine BL, Lacey SF, Melenhorst JJ, Porter DL, June CH, Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas, The New England journal of medicine, 377 (2017) 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, Reagan P, Bot A, Rossi J, Navale L, Jiang Y, Aycock J, Elias M, Chang D, Wiezorek J, Go WY, Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma, The New England journal of medicine, 377 (2017) 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, Zhang Y, Sen K, Lebwohl D, Pulsipher MA, Grupp SA, Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia, The New England journal of medicine, 378 (2018) 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Frigault MJ, Lee J, Basil MC, Carpenito C, Motohashi S, Scholler J, Kawalekar OU, Guedan S, McGettigan SE, Posey AD Jr., Ang S, Cooper LJ, Platt JM, Johnson FB, Paulos CM, Zhao Y, Kalos M, Milone MC, June CH, Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells, Cancer immunology research, 3 (2015) 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Fitzgerald JC, Weiss SL, Maude SL, Barrett DM, Lacey SF, Melenhorst JJ, Shaw P, Berg RA, June CH, Porter DL, Frey NV, Grupp SA, Teachey DT, Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy for Acute Lymphoblastic Leukemia, Critical care medicine, 45 (2017) e124–e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Levine BL, Miskin J, Wonnacott K, Keir C, Global Manufacturing of CAR T Cell Therapy, Molecular therapy. Methods & clinical development, 4 (2017) 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].van den Berg JH, Gomez-Eerland R, van de Wiel B, Hulshoff L, van den Broek D, Bins A, Tan HL, Harper JV, Hassan NJ, Jakobsen BK, Jorritsma A, Blank CU, Schumacher TN, Haanen JB, Case Report of a Fatal Serious Adverse Event Upon Administration of T Cells Transduced With a MART-1-specific T-cell Receptor, Molecular therapy : the journal of the American Society of Gene Therapy, 23 (2015) 1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Migliorini D, Dietrich PY, Stupp R, Linette GP, Posey AD Jr., June CH, CAR T-Cell Therapies in Glioblastoma: A First Look, Clin Cancer Res, 24 (2018) 535–540. [DOI] [PubMed] [Google Scholar]
- [47].O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, Martinez-Lage M, Brem S, Maloney E, Shen A, Isaacs R, Mohan S, Plesa G, Lacey SF, Navenot JM, Zheng Z, Levine BL, Okada H, June CH, Brogdon JL, Maus MV, A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma, Science translational medicine, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cameron BJ, Gerry AB, Dukes J, Harper JV, Kannan V, Bianchi FC, Grand F, Brewer JE, Gupta M, Plesa G, Bossi G, Vuidepot A, Powlesland AS, Legg A, Adams KJ, Bennett AD, Pumphrey NJ, Williams DD, Binder-Scholl G, Kulikovskaya I, Levine BL, Riley JL, Varela-Rohena A, Stadtmauer EA, Rapoport AP, Linette GP, June CH, Hassan NJ, Kalos M, Jakobsen BK, Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells, Science translational medicine, 5 (2013) 197ra103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang J, Wang L, The Emerging World of TCR-T Cell Trials Against Cancer: A Systematic Review, Technol Cancer Res Treat, 18 (2019) 1533033819831068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH, Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma, Blood, 122 (2013) 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Peggs KS, Quezada SA, Allison JP, Cancer immunotherapy: co-stimulatory agonists and co-inhibitory antagonists, Clinical and experimental immunology, 157 (2009) 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shinde P, Bharat V, Rodriguez-Oquendo A, Zhou BY, Vella AT, Understanding how combinatorial targeting of TLRs and TNFR family costimulatory members promote enhanced T cell responses, Expert Opin Biol Th, 18 (2018) 1073–1083. [DOI] [PubMed] [Google Scholar]
- [53].Buchan SL, Rogel A, Al-Shamkhani A, The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy, Blood, 131 (2018) 39–48. [DOI] [PubMed] [Google Scholar]
- [54].Chester C, Sanmamed MF, Wang J, Melero I, Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies, Blood, 131 (2018) 49–57. [DOI] [PubMed] [Google Scholar]
- [55].Croft M, Co-stimulatory members of the TNFR family: keys to effective T-cell immunity?, Nat Rev Immunol, 3 (2003) 609–620. [DOI] [PubMed] [Google Scholar]
- [56].Innovent Receives an Approval from the US FDA to Initiate Clinical Trials for its Anti-OX40 Monoclonal Antibody IBI101, in.
- [57].Tolcher AW, Sznol M, Hu-Lieskovan S, Papadopoulos KP, Patnaik A, Rasco DW, Di Gravio D, Huang B, Gambhire D, Chen Y, Thall AD, Pathan N, Schmidt EV, Chow LQM, Phase Ib Study of Utomilumab (PF-05082566), a 4-1BB/CD137 Agonist, in Combination with Pembrolizumab (MK-3475) in Patients with Advanced Solid Tumors, Clin Cancer Res, 23 (2017) 5349–5357. [DOI] [PubMed] [Google Scholar]
- [58].Segal NH, Logan TF, Hodi FS, McDermott D, Melero I, Hamid O, Schmidt H, Robert C, Chiarion-Sileni V, Ascierto PA, Maio M, Urba WJ, Gangadhar TC, Suryawanshi S, Neely J, Jure-Kunkel M, Krishnan S, Kohrt H, Sznol M, Levy R, Results from an Integrated Safety Analysis of Urelumab, an Agonist Anti-CD137 Monoclonal Antibody, Clin Cancer Res, 23 (2017) 1929–1936. [DOI] [PubMed] [Google Scholar]
- [59].Zhang Y, Li N, Suh H, Irvine DJ, Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity, Nature communications, 9 (2018) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mullard A, The cancer vaccine resurgence, Nature reviews. Drug discovery, 15 (2016) 663–665. [DOI] [PubMed] [Google Scholar]
- [61].Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P, Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape, Trends in immunology, 38 (2017) 577–593. [DOI] [PubMed] [Google Scholar]
- [62].Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Sipuleucel-T immunotherapy for castration-resistant prostate cancer, The New England journal of medicine, 363 (2010) 411–422. [DOI] [PubMed] [Google Scholar]
- [63].Bhatt D, Daemen T, Therapeutic Vaccines and Cancer Immunotherapy, Vaccines (Basel), 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Pardi N, Hogan MJ, Porter FW, Weissman D, mRNA vaccines - a new era in vaccinology, Nature reviews. Drug discovery, 17 (2018) 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang B, Jeang J, Yang A, Wu TC, Hung CF, DNA vaccine for cancer immunotherapy, Human vaccines & immunotherapeutics, 10 (2014) 3153–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].McNamara MA, Nair SK, Holl EK, RNA-Based Vaccines in Cancer Immunotherapy, J Immunol Res, 2015 (2015) 794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ, Developing mRNA-vaccine technologies, RNA biology, 9 (2012) 1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kauffman KJ, Webber MJ, Anderson DG, Materials for non-viral intracellular delivery of messenger RNA therapeutics, Journal of controlled release : official journal of the Controlled Release Society, 240 (2016) 227–234. [DOI] [PubMed] [Google Scholar]
- [69].Li L, Goedegebuure SP, Gillanders WE, Preclinical and clinical development of neoantigen vaccines, Ann Oncol, 28 (2017) xii11–xii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lauss M, Donia M, Harbst K, Andersen R, Mitra S, Rosengren F, Salim M, Vallon-Christersson J, Törngren T, Kvist A, Ringnér M, Svane IM, Jönsson G, Mutational and putative neoantigen load predict clinical benefit of adoptive T cell therapy in melanoma, Nature communications, 8 (2017) 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Franks AL, Slansky JE, Multiple Associations Between a Broad Spectrum of Autoimmune Diseases, Chronic Inflammatory Diseases and Cancer, Anticancer Res, 32 (2012) 1119–1136. [PMC free article] [PubMed] [Google Scholar]
- [72].Zamarron BF, Chen WJ, Dual Roles of Immune Cells and Their Factors in Cancer Development and Progression, Int J Biol Sci, 7 (2011) 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA, Chronic Inflammation and Cytokines in the Tumor Microenvironment, J Immunol Res, 2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gabrilovich DI, Nagaraj S, Myeloid-derived suppressor cells as regulators of the immune system, Nat Rev Immunol, 9 (2009) 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Yu H, Pardoll D, Jove R, STATs in cancer inflammation and immunity: a leading role for STAT3, Nat Rev Cancer, 9 (2009) 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Lin WW, Karin M, A cytokine-mediated link between innate immunity, inflammation, and cancer, J Clin Invest, 117 (2007) 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Iliopoulos D, Hirsch HA, Struhl K, An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation, Cell, 139 (2009) 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sambi M, Bagheri L, Szewczuk MR, Current Challenges in Cancer Immunotherapy: Multimodal Approaches to Improve Efficacy and Patient Response Rates, Journal of oncology, 2019 (2019)4508794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Champiat S, Lambotte O, Barreau E, Belkhir R, Berdelou A, Carbonnel F, Cauquil C, Chanson P, Collins M, Durrbach A, Ederhy S, Feuillet S, François H, Lazarovici J, Le Pavec J, De Martin E, Mateus C, Michot JM, Samuel D, Soria JC, Robert C, Eggermont A, Marabelle A, Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper, Ann Oncol, 27 (2016) 559–574. [DOI] [PubMed] [Google Scholar]
- [80].Schumacher TN, Schreiber RD, Neoantigens in cancer immunotherapy, Science (New York, N.Y.), 348 (2015) 69–74. [DOI] [PubMed] [Google Scholar]
- [81].Scheetz L, Park KS, Li Q, Lowenstein PR, Castro MG, Schwendeman A, Moon JJ, Engineering patient-specific cancer immunotherapies, Nature biomedical engineering, 3 (2019) 768–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Heo MB, Lim YT, Programmed nanoparticles for combined immunomodulation, antigen presentation and tracking of immunotherapeutic cells, Biomaterials, 35 (2014) 590–600. [DOI] [PubMed] [Google Scholar]
- [83].Barenholz Y, Doxil®--the first FDA-approved nano-drug: lessons learned, Journal of controlled release : official journal of the Controlled Release Society, 160 (2012) 117–134. [DOI] [PubMed] [Google Scholar]
- [84].Peters S, Ramalingam SS, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Reck M, Borghaei H, Brahmer JR, O’Byme KJ, Geese WJ, Bhagavatheeswaran P, Nathan FE, Hellmann MD, LBA4_PR - Nivolumab (NIVO) + low-dose ipilimumab (IPI) vs platinumdoublet chemotherapy (chemo) as first-line (1L) treatment (tx) for advanced non-small cell lung cancer (NSCLC): CheckMate 227 part 1 final analysis, Annals of Oncology, 30 (2019) v913–v914. [Google Scholar]
- [85].Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, Hermes B, ÇayŞenler F, Csőszi T, Fülöp A, Rodríguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM, Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer, The New England journal of medicine, 379 (2018) 2040–2051. [DOI] [PubMed] [Google Scholar]
- [86].Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer, The New England journal of medicine, 378 (2018) 2078–2092. [DOI] [PubMed] [Google Scholar]
- [87].Kleinovink JW, van Driel PB, Snoeks TJ, Prokopi N, Fransen MF, Cruz LJ, Mezzanotte L, Chan A, Löwik CW, Ossendorp F, Combination of Photodynamic Therapy and Specific Immunotherapy Efficiently Eradicates Established Tumors, Clin Cancer Res, 22 (2016) 1459–1468. [DOI] [PubMed] [Google Scholar]
- [88].Wang C, Xu L, Liang C, Xiang J, Peng R, Liu Z, Immunological responses triggered by photothermal therapy with carbon nanotubes in combination with anti-CTLA-4 therapy to inhibit cancer metastasis, Advanced materials (Deerfield Beach, Fla.), 26 (2014) 8154–8162. [DOI] [PubMed] [Google Scholar]
- [89].Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr., Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB, Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial, Lancet, 387 (2016) 1540–1550. [DOI] [PubMed] [Google Scholar]
- [90].Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr., Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G, Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial, Lancet, 393 (2019) 1819–1830. [DOI] [PubMed] [Google Scholar]
- [91].Bhargava A, Bunkar N, Khare NK, Mishra D, Mishra PK, Nanoengineered strategies to optimize dendritic cells for gastrointestinal tumor immunotherapy: from biology to translational medicine, Nanomedicine (London, England), 9 (2014) 2187–2202. [DOI] [PubMed] [Google Scholar]
- [92].Karkada M, Berinstein NL, Mansour M, Therapeutic vaccines and cancer: focus on DPX-0907, Biologics : targets & therapy, 8 (2014) 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D, Yang JS, Kim S, Kim YK, Seong SY, A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy, Nature nanotechnology, 6 (2011) 675–682. [DOI] [PubMed] [Google Scholar]
- [94].Basto AP, Badenes M, Almeida SC, Martins C, Duarte A, Santos DM, Leitao A, Immune response profile elicited by the model antigen ovalbumin expressed in fusion with the bacterial OprI lipoprotein, Molecular immunology, 64 (2015) 36–45. [DOI] [PubMed] [Google Scholar]
- [95].Xiang J, Xu L, Gong H, Zhu W, Wang C, Xu J, Feng L, Cheng L, Peng R, Liu Z, Antigen-Loaded Upconversion Nanoparticles for Dendritic Cell Stimulation, Tracking, and Vaccination in Dendritic Cell-Based Immunotherapy, ACS nano, 9 (2015) 6401–6411. [DOI] [PubMed] [Google Scholar]
- [96].Cruz LJ, Rosalia RA, Kleinovink JW, Rueda F, Löwik CW, Ossendorp F, Targeting nanoparticles to CD40, DEC-205 or CD11c molecules on dendritic cells for efficient CD8(+) T cell response: a comparative study, Journal of controlled release : official journal of the Controlled Release Society, 192 (2014) 209–218. [DOI] [PubMed] [Google Scholar]
- [97].Rosalia RA, Cruz LJ, van Duikeren S, Tromp AT, Silva AL, Jiskoot W, de Gruijl T, Löwik C, Oostendorp J, van der Burg SH, Ossendorp F, CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses, Biomaterials, 40 (2015) 88–97. [DOI] [PubMed] [Google Scholar]
- [98].Gorbet MJ, Ranjan A, Cancer immunotherapy with immunoadjuvants, nanoparticles, and checkpoint inhibitors: Recent progress and challenges in treatment and tracking response to immunotherapy, Pharmacol Therapeut, 207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, Fahmy TM, Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype, Biomaterials, 33 (2012) 4957–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, Moore JP, Crotty S, Langer R, Anderson DG, Chakraborty AK, Irvine DJ, Sustained antigen availability during germinal center initiation enhances antibody responses to vaccination, Proceedings of the National Academy of Sciences of the United States of America, 113 (2016) E6639–e6648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Hailemichael Y, Dai Z, Jaffarzad N, Ye Y, Medina MA, Huang XF, Dorta-Estremera SM, Greeley NR, Nitti G, Peng W, Liu C, Lou Y, Wang Z, Ma W, Rabinovich B, Sowell RT, Schluns KS, Davis RE, Hwu P, Overwijk WW, Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion, Nature medicine, 19 (2013) 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Liao X, Li Y, Bonini C, Nair S, Gilboa E, Greenberg PD, Yee C, Transfection of RNA encoding tumor antigens following maturation of dendritic cells leads to prolonged presentation of antigen and the generation of high-affinity tumor-reactive cytotoxic T lymphocytes, Molecular therapy : the journal of the American Society of Gene Therapy, 9 (2004) 757–764. [DOI] [PubMed] [Google Scholar]
- [103].Pollard C, De Koker S, Saelens X, Vanham G, Grooten J, Challenges and advances towards the rational design of mRNA vaccines, Trends in molecular medicine, 19 (2013) 705–713. [DOI] [PubMed] [Google Scholar]
- [104].Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, Vormehr M, Hüsemann Y, Selmi A, Kuhn AN, Buck J, Derhovanessian E, Rae R, Attig S, Diekmann J, Jabulowsky RA, Heesch S, Hassel J, Langguth P, Grabbe S, Huber Ö, Türeci O, Sahin U, Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy, Nature, 534 (2016) 396–401. [DOI] [PubMed] [Google Scholar]
- [105].Sun Q, Barz M, De Geest BG, Diken M, Hennink WE, Kiessling F, Lammers T, Shi Y, Nanomedicine and macroscale materials in immuno-oncology, Chemical Society reviews, 48 (2019)351–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Rosenbaum L, Tragedy, Perseverance, and Chance - The Story of CAR-T Therapy, The New England journal of medicine, 377 (2017) 1313–1315. [DOI] [PubMed] [Google Scholar]
- [107].Smith TT, Stephan SB, Moffett HF, McKnight LE, Ji W, Reiman D, Bonagofski E, Wohlfahrt ME, Pillai SPS, Stephan MT, In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers, Nature nanotechnology, 12 (2017) 813–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ruella M, Xu J, Barrett DM, Fraietta JA, Reich TJ, Ambrose DE, Klichinsky M, Shestova O, Patel PR, Kulikovskaya I, Nazimuddin F, Bhoj VG, Orlando EJ, Fry TJ, Bitter H, Maude SL, Levine BL, Nobles CL, Bushman FD, Young RM, Scholler J, Gill SI, June CH, Grupp SA, Lacey SF, Melenhorst JJ, Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell, Nature medicine, 24 (2018) 1499–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ, Therapeutic cell engineering with surface-conjugated synthetic nanoparticles, Nature medicine, 16 (2010) 1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zheng Y, Stephan MT, Gai SA, Abraham W, Shearer A, Irvine DJ, In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes, Journal of controlled release : official journal of the Controlled Release Society, 172 (2013) 426–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Tang T, Weng T, Jia H, Luo S, Xu Y, Li L, Zhang P, Harnessing the layer-by-layer assembly technique to design biomaterials vaccines for immune modulation in translational applications, Biomaterials science, 7 (2019) 715–732. [DOI] [PubMed] [Google Scholar]
- [112].Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, Goldberg MS, T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity, Nature communications, 8 (2017) 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Couzin-Frankel J, Breakthrough of the year 2013. Cancer immunotherapy, Science (New York, N.Y.), 342 (2013) 1432–1433. [DOI] [PubMed] [Google Scholar]
- [114].Riley RS, June CH, Langer R, Mitchell MJ, Delivery technologies for cancer immunotherapy, Nature reviews. Drug discovery, 18 (2019) 175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ishihara J, Fukunaga K, Ishihara A, Larsson HM, Potin L, Hosseinchi P, Galliverti G, Swartz MA, Hubbell JA, Matrix-binding checkpoint immunotherapies enhance antitumor efficacy and reduce adverse events, Science translational medicine, 9 (2017). [DOI] [PubMed] [Google Scholar]
- [116].Yang J, Wang S, Liu P, Dai L, Chen B, Luan J, Zhou J, Platelet-inspired medicine for tumor therapy, Oncotarget, 8 (2017) 115748–115753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Seyednejad H, Ghassemi AH, van Nostrum CF, Vermonden T, Hennink WE, Functional aliphatic polyesters for biomedical and pharmaceutical applications, Journal of controlled release : official journal of the Controlled Release Society, 152 (2011) 168–176. [DOI] [PubMed] [Google Scholar]
- [118].Ghassemi AH, van Steenbergen MJ, Talsma H, van Nostrum CF, Crommelin DJ, Hennink WE, Hydrophilic polyester microspheres: effect of molecular weight and copolymer composition on release of BSA, Pharmaceutical research, 27 (2010) 2008–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Rahimian S, Fransen MF, Kleinovink JW, Amidi M, Ossendorp F, Hennink WE, Polymeric microparticles for sustained and local delivery of antiCD40 and antiCTLA-4 in immunotherapy of cancer, Biomaterials, 61 (2015) 33–40. [DOI] [PubMed] [Google Scholar]
- [120].Wang C, Sun W, Wright G, Wang AZ, Gu Z, Inflammation-Triggered Cancer Immunotherapy by Programmed Delivery of CpG and Anti-PD1 Antibody, Advanced materials (Deerfield Beach, Fla.), 28 (2016) 8912–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Teo PY, Yang C, Whilding LM, Parente-Pereira AC, Maher J, George AJ, Hedrick JL, Yang YY, Ghaem-Maghami S, Ovarian cancer immunotherapy using PD-L1 siRNA targeted delivery from folic acid-functionalized polyethylenimine: strategies to enhance T cell killing, Advanced healthcare materials, 4 (2015) 1180–1189. [DOI] [PubMed] [Google Scholar]
- [122].Wang Y, Xu Z, Guo S, Zhang L, Sharma A, Robertson GP, Huang L, Intravenous delivery of siRNA targeting CD47 effectively inhibits melanoma tumor growth and lung metastasis, Molecular therapy : the journal of the American Society of Gene Therapy, 21 (2013) 1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Park J, Wrzesinski SH, Stem E, Look M, Criscione J, Ragheb R, Jay SM, Demento SL, Agawu A, Licona Limon P, Ferrandino AF, Gonzalez D, Habermann A, Flavell RA, Fahmy TM, Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy, Nature materials, 11 (2012) 895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Sato K, Sato N, Xu B, Nakamura Y, Nagaya T, Choyke PL, Hasegawa Y, Kobayashi H, Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy, Science translational medicine, 8 (2016) 352ra110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Peranzoni E, Lemoine J, Vimeux L, Feuillet V, Barrin S, Kantari-Mimoun C, Bercovici N, Guérin M, Biton J, Ouakrim H, Régnier F, Lupo A, Alifano M, Damotte D, Donnadieu E, Macrophages impede CD8 T cells from reaching tumor cells and limit the efficacy of anti-PD-1 treatment, Proceedings of the National Academy of Sciences of the United States of America, 115 (2018) E4041–e4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, Miller MA, Carlson JC, Freeman GJ, Anthony RM, Weissleder R, Pittet MJ, In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy, Science translational medicine, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, Daldrup-Link HE, Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues, Nature nanotechnology, 11 (2016) 986–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Crielaard BJ, Lammers T, Rivella S, Targeting iron metabolism in drug discovery and delivery, Nature reviews. Drug discovery, 16 (2017) 400–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Choi JY, Neuhouser ML, Barnett MJ, Hong CC, Kristal AR, Thomquist MD, King IB, Goodman GE, Ambrosone CB, Iron intake, oxidative stress-related genes (MnSOD and MPO) and prostate cancer risk in CARET cohort, Carcinogenesis, 29 (2008) 964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Weissleder R, Nahrendorf M, Pittet MJ, Imaging macrophages with nanoparticles, Nature materials, 13 (2014) 125–138. [DOI] [PubMed] [Google Scholar]
- [131].Rodell CB, Arlauckas SP, Cuccarese MF, Garris CS, Li R, Ahmed MS, Kohler RH, Pittet MJ, Weissleder R, TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy, Nature biomedical engineering, 2 (2018) 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, Fiering S, In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer, Nature nanotechnology, 11 (2016) 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yang L, Pang Y, Moses HL, TGF-beta and immune cells: an important regulatory axis in the tumor microenvironment and progression, Trends in immunology, 31 (2010) 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zheng Y, Tang L, Mabardi L, Kumari S, Irvine DJ, Enhancing Adoptive Cell Therapy of Cancer through Targeted Delivery of Small-Molecule Immunomodulators to Internalizing or Noninternalizing Receptors, ACS nano, 11 (2017) 3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Xu Z, Wang Y, Zhang L, Huang L, Nanoparticle-delivered transforming growth factor-β siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment, ACS nano, 8 (2014) 3636–3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Zhao Y, Huo M, Xu Y, Wang L, Huang L, Nanoparticle delivery of CDDO-Me remodels the tumor microenvironment and enhances vaccine therapy for melanoma, Biomaterials, 68 (2015) 54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A, Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy, Cell, 168 (2017) 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Restifo NP, Dudley ME, Rosenberg SA, Adoptive immunotherapy for cancer: harnessing the T cell response, Nat Rev Immunol, 12 (2012) 269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G, Immunogenic cell death in cancer and infectious disease, Nat Rev Immunol, 17 (2017) 97–111. [DOI] [PubMed] [Google Scholar]
- [140].Kroemer G, Galluzzi L, Kepp O, Zitvogel L, Immunogenic cell death in cancer therapy, Annual review of immunology, 31 (2013) 51–72. [DOI] [PubMed] [Google Scholar]
- [141].Kuai R, Yuan W, Son S, Nam J, Xu Y, Fan Y, Schwendeman A, Moon JJ, Elimination of established tumors with nanodisc-based combination chemoimmunotherapy, Science advances, 4 (2018) eaao1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA, Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer, The New England journal of medicine, 379 (2018) 2108–2121. [DOI] [PubMed] [Google Scholar]
- [143].Study of Atezolizumab as Monotherapy and in Combination With Platinum-Based Chemotherapy in Participants With Untreated Locally Advanced or Metastatic Urothelial Carcinoma (IMvigor130), in.
- [144].Study of Nivolumab in Combination With Ipilimumab or Standard of Care Chemotherapy Compared to the Standard of Care Chemotherapy Alone in Treatment of Participants With Untreated Inoperable or Metastatic Urothelial Cancer (CheckMate901), in.
- [145].Rizvi NA, Hellmann MD, Brahmer JR, Juergens RA, Borghaei H, Gettinger S, Chow LQ, Gerber DE, Laurie SA, Goldman JW, Shepherd FA, Chen AC, Shen Y, Nathan FE, Harbison CT, Antonia S, Nivolumab in Combination With Platinum-Based Doublet Chemotherapy for First-Line Treatment of Advanced Non-Small-Cell Lung Cancer, J Clin Oncol, 34 (2016) 2969–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R, Nanocarriers as an emerging platform for cancer therapy, Nature nanotechnology, 2 (2007) 751–760. [DOI] [PubMed] [Google Scholar]
- [147].Lee IH, An S, Yu MK, Kwon HK, Im SH, Jon S, Targeted chemoimmunotherapy using drug-loaded aptamer-dendrimer bioconjugates, Journal of controlled release : official journal of the Controlled Release Society, 155 (2011) 435–441. [DOI] [PubMed] [Google Scholar]
- [148].Pfannenstiel LW, Lam SS, Emens LA, Jaffee EM, Armstrong TD, Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice, Cellular immunology, 263 (2010) 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM, Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice, Cancer research, 61 (2001) 3689–3697. [PubMed] [Google Scholar]
- [150].Seth A, Heo MB, Lim YT, Poly (γ-glutamic acid) based combination of water-insoluble paclitaxel and TLR7 agonist for chemo-immunotherapy, Biomaterials, 35 (2014) 7992–8001. [DOI] [PubMed] [Google Scholar]
- [151].Roy A, Singh MS, Upadhyay P, Bhaskar S, Nanoparticle mediated co-delivery of paclitaxel and a TLR-4 agonist results in tumor regression and enhanced immune response in the tumor microenvironment of a mouse model, International journal of pharmaceutics, 445 (2013)171–180. [DOI] [PubMed] [Google Scholar]
- [152].Heo MB, Kim SY, Yun WS, Lim YT, Sequential delivery of an anticancer drug and combined immunomodulatory nanoparticles for efficient chemoimmunotherapy, International journal of nanomedicine, 10 (2015) 5981–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Fan Y, Kuai R, Xu Y, Ochyl LJ, Irvine DJ, Moon JJ, Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy, Nano letters, 17 (2017) 7387–7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Makkouk A, Joshi VB, Wongrakpanich A, Lemke CD, Gross BP, Salem AK, Weiner GJ, Biodegradable microparticles loaded with doxorubicin and CpG ODN for in situ immunization against cancer, The AAPS journal, 17 (2015) 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Song Q, Yin Y, Shang L, Wu T, Zhang D, Kong M, Zhao Y, He Y, Tan S, Guo Y, Zhang Z, Tumor Microenvironment Responsive Nanogel for the Combinatorial Antitumor Effect of Chemotherapy and Immunotherapy, Nano letters, 17 (2017) 6366–6375. [DOI] [PubMed] [Google Scholar]
- [156].Yin Y, Hu Q, Xu C, Qiao Q, Qin X, Song Q, Peng Y, Zhao Y, Zhang Z, Co-delivery of Doxorubicin and Interferon-γ by Thermosensitive Nanoparticles for Cancer Immunochemotherapy, Molecular pharmaceutics, 15 (2018) 4161–4172. [DOI] [PubMed] [Google Scholar]
- [157].Kong M, Tang J, Qiao Q, Wu T, Qi Y, Tan S, Gao X, Zhang Z, Biodegradable Hollow Mesoporous Silica Nanoparticles for Regulating Tumor Microenvironment and Enhancing Antitumor Efficiency, Theranostics, 7 (2017) 3276–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Riley RS, Day ES, Gold nanoparticle-mediated photothermal therapy: applications and opportunities for multimodal cancer treatment, Wiley interdisciplinary reviews. Nanomedicine and nanobiotechnology, 9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Bear AS, Kennedy LC, Young JK, Perna SK, Mattos Almeida JP, Lin AY, Eckels PC, Drezek RA, Foster AE, Elimination of metastatic melanoma using gold nanoshellenabled photothermal therapy and adoptive T cell transfer, PloS one, 8 (2013) e69073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E, Turk MJ, Hoopes PJ, Conejo-Garcia JR, Fiering S, Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors, Nanomedicine : nanotechnology, biology, and medicine, 10 (2014) 1273–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Chen WR, Singhal AK, Liu H, Nordquist RE, Antitumor immunity induced by laser immunotherapy and its adoptive transfer, Cancer research, 61 (2001) 459–461. [PubMed] [Google Scholar]
- [162].Zhou F, Li X, Naylor MF, Hode T, Nordquist RE, Alleruzzo L, Raker J, Lam SS, Du N, Shi L, Wang X, Chen WR, InCVAX--a novel strategy for treatment of late-stage, metastatic cancers through photoimmunotherapy induced tumor-specific immunity, Cancer letters, 359 (2015) 169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Guo L, Yan DD, Yang D, Li Y, Wang X, Zalewski O, Yan B, Lu W, Combinatorial photothermal and immuno cancer therapy using chitosan-coated hollow copper sulfide nanoparticles, ACS nano, 8 (2014) 5670–5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Liu Y, Maccarini P, Palmer GM, Etienne W, Zhao Y, Lee CT, Ma X, Inman BA, Vo-Dinh T, Synergistic Immuno Photothermal Nanotherapy (SYMPHONY) for the Treatment of Unresectable and Metastatic Cancers, Scientific reports, 7 (2017) 8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Abadeer NS, Murphy CJ, Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles, The Journal of Physical Chemistry C, 120 (2016) 4691–4716. [Google Scholar]
- [166].Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ, Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer, Nature communications, 9 (2018) 1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Yata T, Takahashi Y, Tan M, Nakatsuji H, Ohtsuki S, Murakami T, Imahori H, Umeki Y, Shiomi T, Takakura Y, Nishikawa M, DNA nanotechnology-based composite-type gold nanoparti cl e-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy, Biomaterials, 146 (2017) 136–145. [DOI] [PubMed] [Google Scholar]
- [168].Nakatsuji H, Kawabata Galbraith K, Kurisu J, Imahori H, Murakami T, Kengaku M, Surface chemistry for cytosolic gene delivery and photothermal transgene expression by gold nanorods, Scientific reports, 7 (2017) 4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Lee IH, Kwon HK, An S, Kim D, Kim S, Yu MK, Lee JH, Lee TS, Im SH, Jon S, Imageable antigen-presenting gold nanoparticle vaccines for effective cancer immunotherapy in vivo, Angewandte Chemie (International ed. in English), 51 (2012) 8800–8805. [DOI] [PubMed] [Google Scholar]
- [170].Chauhan DS, Reddy BPK, Mishra SK, Prasad R, Dhanka M, Vats M, Ravichandran G, Poojari D, Mhatre O, De A, Srivastava R, Comprehensive Evaluation of Degradable and Cost-Effective Plasmonic Nanoshells for Localized Photothermolysis of Cancer Cells, Langmuir : the ACS journal of surfaces and colloids, 35 (2019) 7805–7815. [DOI] [PubMed] [Google Scholar]
- [171].Ghaznavi H, Hosseini-Nami S, Kamrava SK, Irajirad R, Maleki S, Shakeri-Zadeh A, Montazerabadi A, Folic acid conjugated PEG coated gold-iron oxide core-shell nanocomplex as a potential agent for targeted photothermal therapy of cancer, Artificial cells, nanomedicine, and biotechnology, 46 (2018) 1594–1604. [DOI] [PubMed] [Google Scholar]
- [172].Tao Y, Ju E, Liu Z, Dong K, Ren J, Qu X, Engineered, self-assembled near-infrared photothermal agents for combined tumor immunotherapy and chemo-photothermal therapy, Biomaterials, 35 (2014) 6646–6656. [DOI] [PubMed] [Google Scholar]
- [173].Link S, Mohamed MB, El-Sayed MA, Simulation of the Optical Absorption Spectra of Gold Nanorods as a Function of Their Aspect Ratio and the Effect of the Medium Dielectric Constant, The Journal of Physical Chemistry B, 103 (1999) 3073–3077. [Google Scholar]
- [174].Yuan H, Fales AM, Vo-Dinh T, TAT peptide-functionalized gold nanostars: enhanced intracellular delivery and efficient NIR photothermal therapy using ultralow irradiance, Journal of the American Chemical Society, 134 (2012) 11358–11361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Hoffman HA, Chakrabarti L, Dumont MF, Sandler AD, Fernandes R, Prussian blue nanoparticles for laser-induced photothermal therapy of tumors, RSC Advances, 4 (2014) 29729–29734. [Google Scholar]
- [176].Fox IJ, Brooker LG, Heseltine DW, Essex HE, Wood EH, A tricarbocyanine dye for continuous recording of dilution curves in whole blood independent of variations in blood oxygen saturation, Proceedings of the staff meetings. Mayo Clinic, 32 (1957) 478–484. [PubMed] [Google Scholar]
- [177].Zhou F, Nordquist RE, Chen WR, Photonics immunotherapy — A novel strategy for cancer treatment, Journal of Innovative Optical Health Sciences, 09 (2015) 1630001. [Google Scholar]
- [178].Pan J, Wang Y, Zhang C, Wang X, Wang H, Wang J, Yuan Y, Wang X, Zhang X, Yu C, Sun SK, Yan XP, Antigen-Directed Fabrication of a Multifunctional Nanovaccine with Ultrahigh Antigen Loading Efficiency for Tumor Photothermal-Immunotherapy, Advanced materials (Deerfield Beach, Fla.), 30 (2018). [DOI] [PubMed] [Google Scholar]
- [179].Wang T, Wang D, Yu H, Feng B, Zhou F, Zhang H, Zhou L, Jiao S, Li Y, A cancer vaccine-mediated postoperative immunotherapy for recurrent and metastatic tumors, Nature communications, 9 (2018) 1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Nishikawa H, Sakaguchi S, Regulatory T cells in cancer immunotherapy, Current opinion in immunology, 27 (2014) 1–7. [DOI] [PubMed] [Google Scholar]
- [181].Chen Q, Xu L, Liang C, Wang C, Peng R, Liu Z, Photothermal therapy with immuneadjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy, Nature communications, 7 (2016) 13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Ostberg JR, Dayanc BE, Yuan M, Oflazoglu E, Repasky EA, Enhancement of natural killer (NK) cell cytotoxicity by fever-range thermal stress is dependent on NKG2D function and is associated with plasma membrane NKG2D clustering and increased expression of MICA on target cells, Journal of leukocyte biology, 82 (2007) 1322–1331. [DOI] [PubMed] [Google Scholar]
- [183].Moy AJ, Tunnell JW, Combinatorial immunotherapy and nanoparticle mediated hyperthermia, Adv Drug Deliv Rev, 114 (2017) 175–183. [DOI] [PubMed] [Google Scholar]
- [184].Toraya-Brown S, Fiering S, Local tumour hyperthermia as immunotherapy for metastatic cancer, International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group, 30 (2014) 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Evans SS, Repasky EA, Fisher DT, Fever and the thermal regulation of immunity: the immune system feels the heat, Nat Rev Immunol, 15 (2015) 335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Li L, Yang S, Song L, Zeng Y, He T, Wang N, Yu C, Yin T, Liu L, Wei X, Wu Q, Wei Y, Yang L, Gong C, An Endogenous Vaccine Based on Fluorophores and Multivalent Immunoadjuvants Regulates Tumor Micro-Environment for Synergistic Photothermal and Immunotherapy, Theranostics, 8 (2018) 860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Zhou F, Wu S, Song S, Chen WR, Resasco DE, Xing D, Antitumor immunologically modified carbon nanotubes for photothermal therapy, Biomaterials, 33 (2012) 3235–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Yeung HY, Lo PC, Ng DK, Fong WP, Anti-tumor immunity of BAM-SiPc-mediated vascular photodynamic therapy in a BALB/c mouse model, Cellular & molecular immunology, 14 (2017) 223–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Zheng L, Li Y, Cui Y, Yin H, Liu T, Yu G, Lv F, Yang J, Generation of an effective anti-lung cancer vaccine by DTPP-mediated photodynamic therapy and mechanistic studies, Lasers in medical science, 28 (2013) 1383–1392. [DOI] [PubMed] [Google Scholar]
- [190].Burley TA, Mączyhńska J, Shah A, Szopa W, Harrington KJ, Boult JKR, Mrozek-Wilczkiewicz A, Vinci M, Bamber JC, Kaspera W, Kramer-Marek G, Near-infrared photoimmunotherapy targeting EGFR-Shedding new light on glioblastoma treatment, International journal of cancer, 142 (2018) 2363–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Korbelik M, Merchant S, Photodynamic therapy-generated cancer vaccine elicits acute phase and hormonal response in treated mice, Cancer immunology, immunotherapy : CII, 61 (2012) 1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Korbelik M, Stott B, Sun J, Photodynamic therapy-generated vaccines: relevance of tumour cell death expression, British journal of cancer, 97 (2007) 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Gollnick SO, Vaughan L, Henderson BW, Generation of effective antitumor vaccines using photodynamic therapy, Cancer research, 62 (2002) 1604–1608. [PubMed] [Google Scholar]
- [194].Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, Wei Z, Wang L, Kong L, Sun H, Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence, Nature nanotechnology, 12 (2017) 692. [DOI] [PubMed] [Google Scholar]
- [195].Korbelik M, Cancer vaccines generated by photodynamic therapy, Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 10 (2011) 664–669. [DOI] [PubMed] [Google Scholar]
- [196].Yu X, Gao D, Gao L, Lai J, Zhang C, Zhao Y, Zhong L, Jia B, Wang F, Chen X, Liu Z, Inhibiting Metastasis and Preventing Tumor Relapse by Triggering Host Immunity with Tumor-Targeted Photodynamic Therapy Using Photosensitizer-Loaded Functional Nanographenes, ACS nano, 11 (2017) 10147–10158. [DOI] [PubMed] [Google Scholar]
- [197].Korbelik M, Banáth J, Saw KM, Zhang W, Čiplys E, Calreticulin as cancer treatment adjuvant: combination with photodynamic therapy and photodynamic therapy-generated vaccines, Frontiers in oncology, 5 (2015) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Renter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van derMeer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, Offringa R, van der Burg SH, Melief CJ, Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity, Clin Cancer Res, 14 (2008) 169–177. [DOI] [PubMed] [Google Scholar]
- [199].He C, Duan X, Guo N, Chan C, Poon C, Weichselbaum RR, Lin W, Core-shell nanoscale coordination polymers combine chemotherapy and photodynamic therapy to potentiate checkpoint blockade cancer immunotherapy, Nature communications, 7 (2016) 12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Wang D, Wang T, Liu J, Yu H, Jiao S, Feng B, Zhou F, Fu Y, Yin Q, Zhang P, Zhang Z, Zhou Z, Li Y, Acid-Activatable Versatile Micelleplexes for PD-L1 Blockade-Enhanced Cancer Photodynamic Immunotherapy, Nano letters, 16 (2016) 5503–5513. [DOI] [PubMed] [Google Scholar]
- [201].Dai L, Li K, Li M, Zhao X, Luo Z, Lu L, Luo Y, Cai K, Size/Charge Changeable Acidity-Responsive Micelleplex for Photodynamic-Improved PD-L1 Immunotherapy with Enhanced Tumor Penetration, Advanced Functional Materials, 28 (2018) 1707249. [Google Scholar]
- [202].Duan X, Chan C, Guo N, Han W, Weichselbaum RR, Lin W, Photodynamic Therapy Mediated by Nontoxic Core-Shell Nanoparticles Synergizes with Immune Checkpoint Blockade To Elicit Antitumor Immunity and Antimetastatic Effect on Breast Cancer, Journal of the American Chemical Society, 138 (2016) 16686–16695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Xu J, Xu L, Wang C, Yang R, Zhuang Q, Han X, Dong Z, Zhu W, Peng R, Liu Z, Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer, ACS nano, 11 (2017) 4463–4474. [DOI] [PubMed] [Google Scholar]
- [204].Munn DH, Blocking IDO activity to enhance anti-tumor immunity, Frontiers in bioscience (Elite edition), 4 (2012) 734–745. [DOI] [PubMed] [Google Scholar]
- [205].Song W, Kuang J, Li CX, Zhang M, Zheng D, Zeng X, Liu C, Zhang XZ, Enhanced Immunotherapy Based on Photodynamic Therapy for Both Primary and Lung Metastasis Tumor Eradication, ACS nano, 12 (2018) 1978–1989. [DOI] [PubMed] [Google Scholar]
- [206].Lu K, He C, Lin W, A Chlorin-Based Nanoscale Metal-Organic Framework for Photodynamic Therapy of Colon Cancers, Journal of the American Chemical Society, 137 (2015) 7600–7603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, Schwendener R, Pruschy M, Knuth A, van den Broek M, Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation, Journal of immunology (Baltimore, Md. : 1950), 189 (2012) 558–566. [DOI] [PubMed] [Google Scholar]
- [208].Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR, STINGDependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors, Immunity, 41 (2014) 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC, Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated, International journal of radiation oncology, biology, physics, 58 (2004) 862–870. [DOI] [PubMed] [Google Scholar]
- [210].Kachikwu EL, Iwamoto KS, Liao YP, DeMarco JJ, Agazaryan N, Economou JS, McBride WH, Schaue D, Radiation enhances regulatory T cell representation, International journal of radiation oncology, biology, physics, 81 (2011) 1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Ngiow SF, McArthur GA, Smyth MJ, Radiotherapy complements immune checkpoint blockade, Cancer cell, 27 (2015) 437–438. [DOI] [PubMed] [Google Scholar]
- [212].Patel R, Czapar AE, Fiering S, Oleinick NL, Steinmetz NF, Radiation Therapy Combined with Cowpea Mosaic Virus Nanoparticle in Situ Vaccination Initiates Immune-Mediated Tumor Regression, ACS omega, 3 (2018) 3702–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC, Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer, Clin Cancer Res, 11 (2005) 728–734. [PubMed] [Google Scholar]
- [214].Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX, Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice, J Clin Invest, 124 (2014) 687–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Brody JD, Ai WZ, Czerwinski DK, Torchia JA, Levy M, Advani RH, Kim YH, Hoppe RT, Knox SJ, Shin LK, Wapnir I, Tibshirani RJ, Levy R, In situ vaccination with a TLR9 agonist induces systemic lymphoma regression: a phase I/II study, J Clin Oncol, 28 (2010) 4324–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Xie J, Gong L, Zhu S, Yong Y, Gu Z, Zhao Y, Emerging Strategies of Nanomaterial-Mediated Tumor Radiosensitization, Advanced materials (Deerfield Beach, Fla.), 31 (2019) el802244. [DOI] [PubMed] [Google Scholar]
- [217].Huang L, Liu Y, In vivo delivery of RNAi with lipid-based nanoparticles, Annual review of biomedical engineering, 13 (2011) 507–530. [DOI] [PubMed] [Google Scholar]
- [218].Sheng WY, Huang L, Cancer immunotherapy and nanomedicine, Pharmaceutical research, 28 (2011)200–214. [DOI] [PubMed] [Google Scholar]
- [219].Zheng X, Koropatnick J, Chen D, Velenosi T, Ling H, Zhang X, Jiang N, Navarro B, Ichim TE, Urquhart B, Min W, Silencing IDO in dendritic cells: a novel approach to enhance cancer immunotherapy in a murine breast cancer model, International journal of cancer, 132 (2013) 967–977. [DOI] [PubMed] [Google Scholar]
- [220].Luo Z, Wang C, Yi H, Li P, Pan H, Liu L, Cai L, Ma Y, Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo, Biomaterials, 38 (2015) 50–60. [DOI] [PubMed] [Google Scholar]
- [221].Alshamsan A, Haddadi A, Hamdy S, Samuel J, El-Kadi AO, Uludağ H, Lavasanifar A, STAT3 silencing in dendritic cells by siRNA polyplexes encapsulated in PLGA nanoparticles for the modulation of anticancer immune response, Molecular pharmaceutics, 7 (2010) 1643–1654. [DOI] [PubMed] [Google Scholar]
- [222].Hossain DM, Pal SK, Moreira D, Duttagupta P, Zhang Q, Won H, Jones J, D'Apuzzo M, Forman S, Kortylewski M, TLR9-Targeted STAT3 Silencing Abrogates Immunosuppressive Activity of Myeloid-Derived Suppressor Cells from Prostate Cancer Patients, Clin Cancer Res, 21 (2015) 3771–3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Ahmed M, Kumar G, Navarro G, Wang Y, Gourevitch S, Moussa MH, Rozenblum N, Levchenko T, Galun E, Torchilin VP, Goldberg SN, Systemic siRNA Nanoparticle-Based Drugs Combined with Radiofrequency Ablation for Cancer Therapy, PloS one, 10 (2015) e0128910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Conde J, Bao C, Tan Y, Cui D, Edelman ER, Azevedo HS, Byrne HJ, Artzi N, Tian F, Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumour-associated macrophages and cancer cells, Adv Funct Mater, 25 (2015) 4183–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Shen L, Li J, Liu Q, Song W, Zhang X, Tiruthani K, Hu H, Das M, Goodwin TJ, Liu R, Huang L, Local Blockade of Interleukin 10 and C-X-C Motif Chemokine Ligand 12 with Nano-Delivery Promotes Antitumor Response in Murine Cancers, ACS nano, 12 (2018) 9830–9841. [DOI] [PubMed] [Google Scholar]
- [226].Xian J, Yang H, Lin Y, Liu S, Combination nonviral murine interleukin 2 and interleukin 12 gene therapy and radiotherapy for head and neck squamous cell carcinoma, Archives of otolaryngology--head & neck surgery, 131 (2005) 1079–1085. [DOI] [PubMed] [Google Scholar]
- [227].Duan S, Song M, He J, Zhou N, Zhou S, Zhao J, Fang Y, Yi P, Huang X, Luo G, Lai C, Yu X, Zhang Z, Xie Y, Zhao Y, Lu X, Folate-Modified Chitosan Nanoparticles Coated Interferon-Inducible Protein-10 Gene Enhance Cytotoxic T Lymphocytes' Responses to Hepatocellular Carcinoma, Journal of biomedical nanotechnology, 12 (2016) 700–709. [DOI] [PubMed] [Google Scholar]
- [228].Fotin-Mleczek M, Zanzinger K, Heidenreich R, Lorenz C, Thess A, Duchardt KM, Kallen KJ, Highly potent mRNA based cancer vaccines represent an attractive platform for combination therapies supporting an improved therapeutic effect, The journal of gene medicine, 14 (2012) 428–439. [DOI] [PubMed] [Google Scholar]
- [229].Fotin-Mleczek M, Zanzinger K, Heidenreich R, Lorenz C, Kowalczyk A, Kallen KJ, Huber SM, mRNA-based vaccines synergize with radiation therapy to eradicate established tumors, Radiation oncology (London, England), 9 (2014) 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J, Huang L, Combination Immunotherapy of MUC1 mRNA Nano-vaccine and CTLA-4 Blockade Effectively Inhibits Growth of Triple Negative Breast Cancer, Molecular therapy : the journal of the American Society of Gene Therapy, 26 (2018) 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Eigl BJ, Eggener SE, Baybik J, Ettinger S, Chi KN, Nelson C, Wang Z, Gleave ME, Timing is everything: preclinical evidence supporting simultaneous rather than sequential chemohormonal therapy for prostate cancer, Clin Cancer Res, 11 (2005) 4905–4911. [DOI] [PubMed] [Google Scholar]
- [232].Shim G, Kim MG, Kim D, Park JY, Oh YK, Nanoformulation-based sequential combination cancer therapy, Adv Drug Deliv Rev, 115 (2017) 57–81. [DOI] [PubMed] [Google Scholar]
- [233].Sehouli J, Runnebaum IB, Fotopoulou C, Blohmer U, Belau A, Leber H, Hanker LC, Hartmann W, Richter R, Keyver-Paik MD, Oberhoff C, Heinrich G, du Bois A, Olbrich C, Simon E, Friese K, Kimmig R, Boehmer D, Lichtenegger W, Kuemmel S, A randomized phase III adjuvant study in high-risk cervical cancer: simultaneous radiochemotherapy with cisplatin (S-RC) versus systemic paclitaxel and carboplatin followed by percutaneous radiation (PC-R): a NOGGO-AGO Intergroup Study, Ann Oncol, 23 (2012) 2259–2264. [DOI] [PubMed] [Google Scholar]
- [234].Fodor J, [Interactions between radiation and hormonal therapy in breast cancer: simultaneous or sequential treatment], Orv Hetil, 147 (2006) 121–125. [PubMed] [Google Scholar]
- [235].Sanderson K, Scotland R, Lee P, Liu D, Groshen S, Snively J, Sian S, Nichol G, Davis T, Keler T, Yellin M, Weber J, Autoimmunity in a phase I trial of a fully human anticytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma, J Clin Oncol, 23 (2005) 741–750. [DOI] [PubMed] [Google Scholar]
- [236].Fransen MF, Sluijter M, Morreau H, Arens R, Melief CJ, Local activation of CD8 T cells and systemic tumor eradication without toxicity via slow release and local delivery of agonistic CD40 antibody, Clin Cancer Res, 17 (2011) 2270–2280. [DOI] [PubMed] [Google Scholar]
- [237].Graham BS, McElrath MJ, Keefer MC, Rybczyk K, Berger D, Weinhold KJ, Ottinger J, Ferarri G, Montefiori DC, Stablein D, Smith C, Ginsberg R, Eldridge J, Duerr A, Fast P, Haynes BF, Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity, PloS one, 5 (2010)e11995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ, Infection-mimicking materials to program dendritic cells in situ, Nature materials, 8 (2009) 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Kim J, Li WA, Choi Y, Lewin SA, Verbeke CS, Dranoff G, Mooney DJ, Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy, Nature biotechnology, 33 (2015) 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Gu L, Mooney DJ, Biomaterials and emerging anticancer therapeutics: engineering the microenvironment, Nat Rev Cancer, 16 (2016) 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Zhang C, Zhang J, Shi G, Song H, Shi S, Zhang X, Huang P, Wang Z, Wang W, Wang C, Kong D, Li C, A Light Responsive Nanoparticle-Based Delivery System Using Pheophorbide A Graft Polyethylenimine for Dendritic Cell-Based Cancer Immunotherapy, Molecular pharmaceutics, 14 (2017) 1760–1770. [DOI] [PubMed] [Google Scholar]
- [242].Weiden J, Tel J, Figdor CG, Synthetic immune niches for cancer immunotherapy, Nat Rev Immunol, 18 (2018) 212–219. [DOI] [PubMed] [Google Scholar]
- [243].Stephan SB, Taber AM, Jileaeva I, Pegues EP, Sentman CL, Stephan MT, Biopolymer implants enhance the efficacy of adoptive T-cell therapy, Nature biotechnology, 33 (2015) 97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Ali OA, Tayalia P, Shvartsman D, Lewin S, Mooney DJ, Inflammatory cytokines presented from polymer matrices differentially generate and activate DCs in situ, Adv Funct Mater, 23 (2013) 4621–4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Ali OA, Verbeke C, Johnson C, Sands RW, Lewin SA, White D, Doherty E, Dranoff G, Mooney DJ, Identification of immune factors regulating antitumor immunity using polymeric vaccines with multiple adjuvants, Cancer research, 74 (2014) 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Ali OA, Doherty E, Bell WJ, Fradet T, Hudak J, Laliberte MT, Mooney DJ, Emerich DF, The efficacy of intracranial PLG-based vaccines is dependent on direct implantation into brain tissue, Journal of controlled release : official journal of the Controlled Release Society, 154 (2011) 249–257. [DOI] [PubMed] [Google Scholar]
- [247].Hori Y, Winans AM, Huang CC, Horrigan EM, Irvine DJ, Injectable dendritic cellcarrying alginate gels for immunization and immunotherapy, Biomaterials, 29 (2008) 3671–3682. [DOI] [PubMed] [Google Scholar]
- [248].Koshy ST, Ferrante TC, Lewin SA, Mooney DJ, Injectable, porous, and cell-responsive gelatin cryogels, Biomaterials, 35 (2014) 2477–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Bencherif SA, Warren Sands R, Ali OA, Li WA, Lewin SA, Braschler TM, Shih TY, Verbeke CS, Bhatta D, Dranoff G, Mooney DJ, Injectable cryogel-based whole-cell cancer vaccines, Nature communications, 6 (2015) 7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Li AW, Sobral MC, Badrinath S, Choi Y, Graveline A, Stafford AG, Weaver JC, Dellacherie MO, Shih TY, Ali OA, Kim J, Wucherpfennig KW, Mooney DJ, A facile approach to enhance antigen response for personalized cancer vaccination, Nature materials, 17 (2018) 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI, Nel AE, Polyethyleneimine coating enhances the cellular uptake of mesoporous silica nanoparticles and allows safe delivery of siRNA and DNA constructs, ACS nano, 3 (2009) 3273–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Oh YK, Park JS, Kang MJ, Ko JJ, Kim JM, Kim CK, Enhanced adjuvanticity of interleukin-2 plasmid DNA administered in polyethylenimine complexes, Vaccine, 21 (2003) 2837–2843. [DOI] [PubMed] [Google Scholar]
- [253].Wang C, Wang J, Zhang X, Yu S, Wen D, Hu Q, Ye Y, Bomba H, Hu X, Liu Z, Dotti G, Gu Z, In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy, Science translational medicine, 10 (2018). [DOI] [PubMed] [Google Scholar]
- [254].Nathan C, Cunningham-Bussel A, Beyond oxidative stress: an immunologist's guide to reactive oxygen species, Nat Rev Immunol, 13 (2013) 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Monette A, Ceccaldi C, Assaad E, Lerouge S, Lapointe R, Chitosan thermogels for local expansion and delivery of tumor-specific T lymphocytes towards enhanced cancer immunotherapies, Biomaterials, 75 (2016) 237–249. [DOI] [PubMed] [Google Scholar]
- [256].Wang C, Ye Y, Hochu GM, Sadeghifar H, Gu Z, Enhanced Cancer Immunotherapy by Microneedle Patch-Assisted Delivery of Anti-PD1 Antibody, Nano letters, 16 (2016) 2334–2340. [DOI] [PubMed] [Google Scholar]
- [257].Yu J, Zhang Y, Ye Y, DiSanto R, Sun W, Ranson D, Ligler FS, Buse JB, Gu Z, Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucoseresponsive insulin delivery, Proceedings of the National Academy of Sciences of the United States of America, 112 (2015) 8260–8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Yeh TC, Zhang W, Ildstad ST, Ho C, Intracellular labeling of T-cells with superparamagnetic contrast agents, Magnetic resonance in medicine, 30 (1993) 617–625. [DOI] [PubMed] [Google Scholar]
- [259].Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C, Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10, Investigative radiology, 39 (2004) 56–63. [DOI] [PubMed] [Google Scholar]
- [260].Selt M, Tennstaedt A, Beyrau A, Nelles M, Schneider G, Löwik C, Hoehn M, In Vivo Non-Invasive Tracking of Macrophage Recruitment to Experimental Stroke, PloS one, 11 (2016)e0156626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Wu C, Xu Y, Yang L, Wu J, Zhu W, Li D, Cheng Z, Xia C, Guo Y, Gong Q, Song B, Ai H, Negatively Charged Magnetite Nanoparticle Clusters as Efficient MRI Probes for Dendritic Cell Labeling and In Vivo Tracking, Advanced Functional Materials, 25 (2015) 3581–3591. [Google Scholar]
- [262].Sheu AY, Zhang Z, Omary RA, Larson AC, MRI-monitored transcatheter intra-arterial delivery of SPIO-labeled natural killer cells to hepatocellular carcinoma: preclinical studies in a rodent model, Investigative radiology, 48 (2013) 492–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Jang ES, Shin JH, Ren G, Park MJ, Cheng K, Chen X, Wu JC, Sunwoo JB, Cheng Z, The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles, Biomaterials, 33 (2012) 5584–5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Kircher MF, Allport JR, Graves EE, Love V, Josephson L, Lichtman AH, Weissleder R, In vivo high resolution three-dimensional imaging of antigen-specific cytotoxic T-lymphocyte trafficking to tumors, Cancer research, 63 (2003) 6838–6846. [PubMed] [Google Scholar]
- [265].Sigovan M, Boussel L, Sulaiman A, Sappey-Marinier D, Alsaid H, Desbleds-Mansard C, Ibarrola D, Gamondès D, Corot C, Lancelot E, Raynaud JS, Vives V, Laclédère C, Violas X, Douek PC, Canet-Soulas E, Rapid-clearance iron nanoparticles for inflammation imaging of atherosclerotic plaque: initial experience in animal model, Radiology, 252 (2009) 401–409. [DOI] [PubMed] [Google Scholar]
- [266].Morishige K, Kacher DF, Libby P, Josephson L, Ganz P, Weissleder R, Aikawa M, High-resolution magnetic resonance imaging enhanced with superparamagnetic nanoparticles measures macrophage burden in atherosclerosis, Circulation, 122 (2010) 1707–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].El-Dakdouki MH, El-Boubbou K, Kamat M, Huang R, Abela GS, Kiupel M, Zhu DC, Huang X, CD44 targeting magnetic glyconanoparticles for atherosclerotic plaque imaging, Pharmaceutical research, 31 (2014) 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Smith BR, Heverhagen J, Knopp M, Schmalbrock P, Shapiro J, Shiomi M, Moldovan NI, Ferrari M, Lee SC, Localization to atherosclerotic plaque and biodistribution of biochemically derivatized superparamagnetic iron oxide nanoparticles (SPIONs) contrast particles for magnetic resonance imaging (MRI), Biomedical microdevices, 9 (2007) 719–727. [DOI] [PubMed] [Google Scholar]
- [269].Li K, Chan CT, Nejadnik H, Lenkov OD, Wolterman C, Paulmurugan R, Yang H, Gambhir SS, Daldrup-Link HE, Ferumoxytol-based Dual-modality Imaging Probe for Detection of Stem Cell Transplant Rejection, Nanotheranostics, 2 (2018) 306–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].McEver RP, Selectins: initiators of leucocyte adhesion and signalling at the vascular wall, Cardiovascular research, 107 (2015) 331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [271].Chantarasrivong C, Ueki A, Ohyama R, Unga J, Nakamura S, Nakanishi I, Higuchi Y, Kawakami S, Ando H, Imamura A, Ishida H, Yamashita F, Kiso M, Hashida M, Synthesis and Functional Characterization of Novel Sialyl LewisX Mimic-Decorated Liposomes for E-selectin-Mediated Targeting to Inflamed Endothelial Cells, Molecular pharmaceutics, 14 (2017) 1528–1537. [DOI] [PubMed] [Google Scholar]
- [272].Busato A, Bonafede R, Bontempi P, Scambi I, Schiaffino L, Benati D, Malatesta M, Sbarbati A, Marzola P, Mariotti R, Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes, International journal of nanomedicine, 11 (2016) 2481–2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Wang P, Kim T, Harada M, Contag C, Huang X, Smith BR, Nano-immunoimaging, Nanoscale Horiz, 5 (2020) 628–653. [DOI] [PubMed] [Google Scholar]
- [274].Singh N, Jenkins GJ, Asadi R, Doak SH, Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION), Nano reviews, 1 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].Sutton EJ, Henning TD, Pichler BJ, Bremer C, Daldrup-Link HE, Cell tracking with optical imaging, European radiology, 18 (2008) 2021–2032. [DOI] [PubMed] [Google Scholar]
- [276].Smith BR, Cheng Z, De A, Koh AL, Sinclair R, Gambhir SS, Real-time intravital imaging of RGD-quantum dot binding to luminal endothelium in mouse tumor neovasculature, Nano letters, 8 (2008) 2599–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Smith BR, Gambhir SS, Nanomaterials for In Vivo Imaging, Chemical reviews, 117 (2017) 901–986. [DOI] [PubMed] [Google Scholar]
- [278].Medintz IL, Uyeda HT, Goldman ER, Mattoussi H, Quantum dot bioconjugates for imaging, labelling and sensing, Nature materials, 4 (2005) 435–446. [DOI] [PubMed] [Google Scholar]
- [279].Voura EB, Jaiswal JK, Mattoussi H, Simon SM, Tracking metastatic tumor cell extravasation with quantum dot nanocrystals and fluorescence emission-scanning microscopy, Nature medicine, 10 (2004) 993–998. [DOI] [PubMed] [Google Scholar]
- [280].Park YI, Lee KT, Suh YD, Hyeon T, Upconverting nanoparticles: a versatile platform for wide-field two-photon microscopy and multi-modal in vivo imaging, Chemical Society reviews, 44 (2015) 1302–1317. [DOI] [PubMed] [Google Scholar]
- [281].Wang C, Cheng L, Xu H, Liu Z, Towards whole-body imaging at the single cell level using ultra-sensitive stem cell labeling with oligo-arginine modified upconversion nanoparticles, Biomaterials, 33 (2012) 4872–4881. [DOI] [PubMed] [Google Scholar]
- [282].SoRelle ED, Yecies D, Liba O, Bennett FC, Graef CM, Dutta R, Mitra SS, Joubert L-M, Cheshier SH, Grant GA, de la Zerda A, Wide-field dynamic monitoring of immune cell trafficking in murine models of glioblastoma, bioRxiv, (2017) 220954. [Google Scholar]
- [283].Tang J, van Panhuys N, Kastenmuller W, Germain RN, The future of immunoimaging--deeper, bigger, more precise, and definitively more colorful, European journal of immunology, 43 (2013) 1407–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Hong H, Zhang Y, Sun J, Cai W, Molecular imaging and therapy of cancer with radiolabeled nanoparticles, Nano today, 4 (2009) 399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Kim HY, Li R, Ng TSC, Courties G, Rodell CB, Prytyskach M, Kohler RH, Pittet MJ, Nahrendorf M, Weissleder R, Miller MA, Quantitative Imaging of Tumor-Associated Macrophages and Their Response to Therapy Using (64)Cu-Labeled Macrin, ACS nano, 12 (2018) 12015–12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Bhatnagar P, Li Z, Choi Y, Guo J, Li F, Lee DY, Figliola M, Huls H, Lee DA, Zal T, Li KC, Cooper LJ, Imaging of genetically engineered T cells by PET using gold nanoparticles complexed to Copper-64, Integrative biology : quantitative biosciences from nano to macro, 5 (2013) 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Kim C, Erpelding TN, Jankovic L, Pashley MD, Wang LV, Deeply penetrating in vivo photoacoustic imaging using a clinical ultrasound array system, Biomedical optics express, 1 (2010) 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Weber J, Beard PC, Bohndiek SE, Contrast agents for molecular photoacoustic imaging, Nature methods, 13 (2016) 639–650. [DOI] [PubMed] [Google Scholar]
- [289].Yang Y, Zhang J, Xia F, Zhang C, Qian Q, Zhi X, Yue C, Sun R, Cheng S, Fang S, Jin W, Yang Y, Cui D, Human CIK Cells Loaded with Au Nanorods as a Theranostic Platform for Targeted Photoacoustic Imaging and Enhanced Immunotherapy and Photothermal Therapy, Nanoscale research letters, 11 (2016) 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Funovics M, Montet X, Reynolds F, Weissleder R, Josephson L, Nanoparticles for the optical imaging of tumor E-selectin, Neoplasia (New York, N.Y.), 7 (2005) 904–911. [DOI] [PubMed] [Google Scholar]
- [291].Fang J, Nakamura H, Maeda H, The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect, Adv Drug Deliv Rev, 63 (2011) 136–151. [DOI] [PubMed] [Google Scholar]
- [292].Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA, Exploiting lymphatic transport and complement activation in nanoparticle vaccines, Nature biotechnology, 25 (2007) 1159–1164. [DOI] [PubMed] [Google Scholar]
- [293].Chauhan DS, Bukhari AB, Ravichandran G, Gupta R, George L, Poojari R, Ingle A, Rengan AK, Shanavas A, Srivastava R, De A, Enhanced EPR directed and Imaging guided Photothermal Therapy using Vitamin E Modified Toco-Photoxil, Scientific reports, 8 (2018) 16673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Mottram PL, Leong D, Crimeen-Irwin B, Gloster S, Xiang SD, Meanger J, Ghildyal R, Vardaxis N, Plebanski M, Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus, Molecular pharmaceutics, 4 (2007) 73–84. [DOI] [PubMed] [Google Scholar]
- [295].Cubillos-Ruiz JR, Engle X, Scarlett UK, Martinez D, Barber A, Elgueta R, Wang L, Nesbeth Y, Durant Y, Gewirtz AT, Sentman CL, Kedl R, Conejo-Garcia JR, Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity, J Clin Invest, 119 (2009) 2231–2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Adalsteinsson VA, Ha G, Freeman SS, Choudhury AD, Stover DG, Parsons HA, Gydush G, Reed SC, Rotem D, Rhoades J, Loginov D, Livitz D, Rosebrock D, Leshchiner I, Kim J, Stewart C, Rosenberg M, Francis JM, Zhang CZ, Cohen O, Oh C, Ding H, Polak P, Lloyd M, Mahmud S, Helvie K, Merrill MS, Santiago RA, O'Connor EP, Jeong SH, Leeson R, Barry RM, Kramkowski JF, Zhang Z, Polacek L, Lohr JG, Schleicher M, Lipscomb E, Saltzman A, Oliver NM, Marini L, Waks AG, Harshman LC, Tolaney SM, Van Allen EM, Winer EP, Lin NU, Nakabayashi M, Taplin ME, Johannessen CM, Garraway LA, Golub TR, Boehm JS, Wagle N, Getz G, Love JC, Meyerson M, Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors, Nature communications, 8 (2017) 1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, Trebska-McGowan K, Wunderlich JR, Yang JC, Rosenberg SA, Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients, Nature medicine, 22 (2016) 433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, Omokoko T, Vormehr M, Albrecht C, Paruzynski A, Kuhn AN, Buck J, Heesch S, Schreeb KH, Müller F, Ortseifer I, Vogler I, Godehardt E, Attig S, Rae R, Breitkreuz A, Tolliver C, Suchan M, Martic G, Hohberger A, Sorn P, Diekmann J, Ciesla J, Waksmann O, Brück AK, Witt M, Zillgen M, Rothermel A, Kasemann B, Langer D, Bolte S, Diken M, Kreiter S, Nemecek R, Gebhardt C, Grabbe S, Höller C, Utikal J, Huber C, Loquai C, Türeci Ö, Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer, Nature, 547 (2017) 222–226. [DOI] [PubMed] [Google Scholar]
- [299].Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, Zhang W, Luoma A, Giobbie-Hurder A, Peter L, Chen C, Olive O, Carter TA, Li S, Lieb DJ, Eisenhaure T, Gjini E, Stevens J, Lane WJ, Javeri I, Nellaiappan K, Salazar AM, Daley H, Seaman M, Buchbinder EI, Yoon CH, Harden M, Lennon N, Gabriel S, Rodig SJ, Barouch DH, Aster JC, Getz G, Wucherpfennig K, Neuberg D, Ritz J, Lander ES, Fritsch EF, Hacohen N, Wu CJ, An immunogenic personal neoantigen vaccine for patients with melanoma, Nature, 547 (2017) 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Lee EJ, Lee NK, Kim IS, Bioengineered protein-based nanocage for drug delivery, Adv Drug Deliv Rev, 106 (2016) 157–171. [DOI] [PubMed] [Google Scholar]
- [301].Liu H, Moynihan KD, Zheng Y, Szeto GL, Li AV, Huang B, Van Egeren DS, Park C, Irvine DJ, Structure-based programming of lymph-node targeting in molecular vaccines, Nature, 507 (2014) 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Guo Y, Wang D, Song Q, Wu T, Zhuang X, Bao Y, Kong M, Qi Y, Tan S, Zhang Z, Erythrocyte Membrane-Enveloped Polymeric Nanoparticles as Nanovaccine for Induction of Antitumor Immunity against Melanoma, ACS nano, 9 (2015) 6918–6933. [DOI] [PubMed] [Google Scholar]
- [303].Fang RH, Hu CM, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L, Cancer cell membrane-coated nanoparticles for anticancer vaccination and drug delivery, Nano letters, 14 (2014) 2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ, Designer vaccine nanodiscs for personalized cancer immunotherapy, Nature materials, 16 (2017) 489–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Draper E, Bissett SL, Howell-Jones R, Waight P, Soldan K, Jit M, Andrews N, Miller E, Beddows S, A randomized, observer-blinded immunogenicity trial of Cervarix(®) and Gardasil(®) Human Papillomavirus vaccines in 12-15 year old girls, PloS one, 8 (2013) e61825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Théry C, Ostrowski M, Segura E, Membrane vesicles as conveyors of immune responses, Nat Rev Immunol, 9 (2009) 581–593. [DOI] [PubMed] [Google Scholar]
- [307].Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L, Tumourreleased exosomes and their implications in cancer immunity, Cell death and differentiation, 15 (2008) 80–88. [DOI] [PubMed] [Google Scholar]
- [308].Cho JA, Yeo DJ, Son HY, Kim HW, Jung DS, Ko JK, Koh JS, Kim YN, Kim CW, Exosomes: a new delivery system for tumor antigens in cancer immunotherapy, International journal of cancer, 114 (2005) 613–622. [DOI] [PubMed] [Google Scholar]
- [309].Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A, Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles, International journal of cancer, 125 (2009) 1016–1026. [DOI] [PubMed] [Google Scholar]
- [310].Temchura VV, Tenbusch M, Nchinda G, Nabi G, Tippler B, Zelenyuk M, Wildner O, Uberla K, Kuate S, Enhancement of immunostimulatory properties of exosomal vaccines by incorporation of fusion-competent G protein of vesicular stomatitis virus, Vaccine, 26 (2008) 3662–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Beauvillain C, Ruiz S, Guiton R, Bout D, Dimier-Poisson I, A vaccine based on exosomes secreted by a dendritic cell line confers protection against T. gondii infection in syngeneic and allogeneic mice, Microbes and infection, 9 (2007) 1614–1622. [DOI] [PubMed] [Google Scholar]
- [312].André F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T, Amigorena S, Angevin E, Zitvogel L, Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells, Journal of immunology (Baltimore, Md. : 1950), 172 (2004) 2126–2136. [DOI] [PubMed] [Google Scholar]
- [313].Théry C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S, Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles, Journal of immunology (Baltimore, Md. : 1950), 166 (2001) 7309–7318. [DOI] [PubMed] [Google Scholar]
- [314].Viaud S, Terme M, Flament C, Taieb J, André F, Novault S, Escudier B, Robert C, Caillat-Zucman S, Tursz T, Zitvogel L, Chaput N, Dendritic cell-derived exosomes promote natural killer cell activation and proliferation: a role for NKG2D ligands and IL-15Ralpha, PloS one, 4 (2009) e4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK, A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer, Journal of translational medicine, 3 (2005) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]