Abstract
Interest in monitoring long-term neurodevelopmental outcomes of children born moderate-to-late preterm (32–36 weeks gestation) is increasing. Moderate-to-late preterm birth has a negative impact on academic achievement, which may relate to differential development of executive function (EF). Prior studies reporting deficits in EF in preterm children have almost exclusively assessed EF in affectively neutral contexts in high-risk preterm children (<32 weeks gestation). Disrupted function in motivational or emotionally charged contexts (hot EF) following preterm birth remains uninvestigated, despite evidence that preterm children show differential development of neural circuitry subserving hot EF, including reduced orbitofrontal cortex volume. The present study is the first to examine whether low-risk, healthy children born moderate-to-late preterm exhibit impairments in the development of hot EF. Preterm children at age 4.5 years were less likely to choose larger, delayed rewards across all levels of reward magnitude on a delay discounting task using tangible rewards, but performed more similarly to their full-term peers on a delay aversion task involving abstract rewards and on measures of cool EF. The relationship between gestational age at birth and selection of delayed rewards extended across the entire gestational age range of the sample (32–42 weeks), and remained significant after controlling for intelligence and processing speed. Results imply there is not a finite cut-off point at which children are spared from potential long-term neurodevelopmental effects of PT birth. Further investigation of reward processing and hot EF in individuals with a history of PT birth is warranted given the susceptibility of prefrontal cortex development to early environmental variations.
Preterm birth is a significant public health problem in the United States. Children born moderate-to-late preterm (32–36 weeks gestation) represented >9% of total US births in 2011 (Martin et al., 2012). Since the risk of serious medical problems for moderate-to-late preterm (PT) infants is lower in comparison to very PT neonates (<32 weeks gestation), infants born within this gestational age range are not routinely followed in neurodevelopmental clinics. Furthermore, because moderate-to-late PT infants usually appear relatively healthy, parents, practitioners, and educational professionals do not perceive a heightened risk for developmental problems in moderate-to-late PT children, despite the increased risk of neonatal morbidity (Engle, Tomashek, & Wallman, 2007).
Critical developmental changes occur in the fetal brain late in the third trimester. At 34 weeks gestation, the brain has only reached two-thirds of its eventual term weight (Kinney, 2006), and dramatic changes in cortical tissue growth and connectivity occur over the last weeks of gestation (Collin & van den Heuvel, 2013). Cortical gray matter volume increases 45% between 34 and 40 weeks gestation, while white matter increases fivefold over a similar time period (Hüppi et al., 1998). For the moderate-to-late PT infant, neurodevelopment from 32–40 weeks postmenstrual age is occurring in an environment with increased visual, auditory, and tactile stimulation compared to the in-utero environment (Luciana, 2003).
The prefrontal cortex undergoes prolonged anatomical and functional development, beginning prenatally and extending into young adulthood (Giedd et al., 1999; Sowell, Thompson, Tessner, & Toga, 2001). In the adult, the prefrontal cortex is responsible for complex cognitive skills, including executive functions such as working memory, inhibition, and attention shifting. Although often perceived as late-developing, the prefrontal cortex undergoes rapid changes in gray matter volume (Gilmore et al., 2011; Matsuzawa et al., 2001; Nishida et al., 2006), white matter connectivity (Pandit et al., 2013), metabolism (Chugani & Phelps, 1986), and function (Franson et al., 2012; Gao et al., 2009; Grossman, 2013) beginning in the third trimester of gestation and extending into the early postnatal period. Early development of this brain region may be sensitive to variations in the environment, including moderate-to-late birth.
There is a growing literature reporting differences in prefrontal-dependent behavior such as executive function (EF) skills in individuals born very PT (e.g. Aarnoudse-Moens, Smidts, Oosterlaan, Duivenvoorden, & Weisglas-Kuperus, 2009; Anderson et al., 2004; Marlow et al., 2007). Group differences in EF in this population emerge early in development (Sun, Mohay, & O’Callaghan, 2009) and persist into adolescence and young adulthood (Narberhaus et al., 2008; Nosarti et al., 2007). However, neurodevelopmental outcomes of healthy, low-risk children born only moderate-to-late preterm are under studied. Recently, one study has demonstrated that EF deficits are also present in lower-risk PT children born specifically in the late preterm range (34–36 weeks gestation; Brumbaugh et al., 2013); these early differences in laboratory measures of EF in low-risk PT children may underlie functional impairment in real world environments, given poorer academic performance and increased risk of behavioral and emotional problems within this population (Chyi, Lee, Hintz, Gould, & Sutcliffe, 2008; Morse, Sheng, Tang, & Roth, 2009; van Baar, Vermaas, Knots, de Kleine, & Spons, 2009).
Almost all prior studies of the development of prefrontal-dependent functions in PT children have utilized abstract, decontextualized problems, which tap “cool” EF (Zelazo & Carlson, 2012). In contrast, EF can also be examined within an affective or motivational context (“hot” EF). Although there is significant controversy regarding the degree to which cool and hot EF tasks rely on dissociable cognitive and neural processes, some researchers have found evidence for dissociable (although somewhat interrelated) performance by children as young as preschool-aged (Hongwanishkul, Happaney, Lee, & Zelazo, 2005; Carlson, White, & Davis-Unger 2014). In adult samples, prefrontal lesion studies indicate that hot and cool EF have dissociable neural bases (Bechara, Damasio, Tranel, & Anderson, 1998; Bechara, 2004), with hot EF tasks relying predominantly on orbitofrontal cortex circuitry (Happaney, Zelazo, & Stuss, 2004). However, despite lesion studies suggesting a dissociable neural basis, it is also possible that both hot and cool EF tasks rely on similar prefrontal circuitry, with hot EF tasks involving interference from affective and reward processing regions such as the ventral striatum (Prencipe et al., 2011). Like cool EF, hot EF also undergoes rapid development during the early childhood years, as exhibited by children’s increasing likelihood to choose larger delayed rewards over smaller immediate ones (Hongwanishkul et al., 2005) and decreased selection of disadvantageous choices on gambling tasks (Kerr & Zelazo, 2004) with advancing age. Individual differences in hot EF during early childhood are predictive of concurrent and long-term measures of cognitive and socioemotional functioning, including academic achievement, social competence, stress-resilience, externalizing disorders, divorce rates, and adult body mass index (Ayduk et al., 2000; Casey et al., 2011; Eigsti et al., 2006; Mischel, Shoda, & Peake, 1988; Mischel, Shoda, & Rodriquez, 1989; Schlam, Wilson, Shoda, Mischel, & Ayduk, 2013; Shoda, Mischel, & Peake, 1990).
Converging evidence suggests that moderate-to-late PT children may exhibit altered hot EF development. Neuroimaging studies have reported orbitofrontal cortex grey matter volume reductions in children born very PT (Ball et al., 2012; Gimenez et al, 2006; Nagy et al., 2009; Thompson et al., 2007). PT children are also at a higher risk for development of attention problems (Talge et al., 2010), and hot EF skills are altered in children with ADHD (Barkley, Edwards, Laneri, Fletcher, & Metevia, 2001; Kuntsi, Oosterlaan, & Stevenson, 2001; Marco et al., 2009; Paloyelis, Asherson, & Kuntsi, 2009; Pauli-Pott & Becker, 2011; Solanto et al., 2001; Sonuga-Barke, Williams, Hall, & Saxton, 1996). Finally, a small literature with a temperament and individual differences framework has investigated the development of effortful control in PT children. Effortful control involves global self-regulatory abilities (Rothbart & Bates, 2007), including the ability to delay gratification, and thus overlaps somewhat with “hot” EF (see Zhou, Chen, & Main, 2012). During the toddler years, very PT children perform more poorly on laboratory effortful control tasks (Clark, Woodward, Horwood, & Moor, 2008; Voight, Pietz, Pauen, Kliegel, & Reuner, 2012), with effortful control deficits predicting increased ADHD symptom rates (Poehlman et al., 2010). Whether early differences in effortful control or related hot EF skills persist over later development and whether these differences are present in the lower risk PT population born moderate-to-late PT has not been investigated.
The purpose of the present study was to determine whether low-risk, healthy children born moderate-to-late PT also exhibit impairments in the development of prefrontal-dependent hot EF skills in comparison to full-term (FT) children at preschool age. In addition to measuring performance on two hot EF tasks (a delay discounting task involving tangible rewards and a delay aversion task involving abstract rewards), we examined whether group differences in performance were accounted for by other factors impacted by PT birth, including putative differences in cool EF development (Brumbaugh et al., 2013), intelligence, and processing speed. Finally, we examined the association between laboratory measures of hot EF and parent report of EF. Characterization of hot EF in this population is critical both to understand the spectrum of neurodevelopmental sequelae following lower risk premature birth and to elucidate the mechanisms that underlie social and behavioral outcomes of PT children in everyday contexts.
Methods
Participants
Four–year-old children were recruited based on gestational age by telephone from a database of families who endorsed interest in participating in child development research. The sample consisted of 45 children born moderate-to-late PT (32–36 weeks gestation) and 46 children born FT (37–42 weeks gestation). Children were between 4.5–5.0 years of age at testing, and were predominantly Caucasian (92%), lived in college-educated (81%), two-parent families (95%), with median incomes between $51,000–$100,000. Hollingshead scores (Hollingshead, 1975), reflecting overall familial socioeconomic status, did not differ for the two groups. Exclusion criteria included uncorrected hearing or visual impairment, neurological insult, cyanotic congenital heart disease, and for FT children, admission to a special care or intensive care nursery for >24 hours. See Table 1 for demographic characteristics of the sample.
Table 1.
Sample Demographic Characteristics
| Preterm (n = 45) | Full-Term (n = 46) | ||
|---|---|---|---|
| n (%) | n (%) | p | |
| Child’s Sex - # male | 23 (51.1) | 25 (54.3) | .75 |
| Child’s Ethnicity - # Caucasian | 40 (88.9) | 44 (95.7) | .23 |
| Maternal Education | .24 | ||
| High school degree or GED | 6 (13.4) | 4 (8.7) | |
| Associate degree | 4 (8.9) | 3 (6.5) | |
| Bachelor’s degree | 13 (28.9) | 23 (50.0) | |
| Graduate or professional degree | 22 (48.9) | 16 (34.8) | |
| Maternal Work | .01* | ||
| Full-time work for pay | 27 (60.0) | 14 (30.4) | |
| Part-time work for pay | 4 (8.9) | 15 (32.6) | |
| Student | 1 (2.2) | 0 (0) | |
| Stay at home parent | 13 (28.9) | 17 (37.0) | |
| Annual Household Income | .10 | ||
| ≤ $50,000 | 6 (13.6) | 2 (4.4) | |
| $51,000 – $100,000 | 22 (50.0) | 20 (44.4) | |
| $101,000 – $150,000 | 6 (13.6) | 15 (33.3) | |
| ≥ $151,000 | 10 (22.7) | 8 (17.8) | |
| Marital Status - # married | 41 (91.1) | 45 (97.8) | .35 |
| Hollingshead Score | 55.13 | 54.66 | .81 |
Notes. Two families declined to provide household income.
p < .05.
Birth hospitalization records were obtained for 99% of the sample to confirm gestational age and to document perinatal history. See Table 2 for perinatal characteristics of the sample.
Table 2.
Sample Perinatal Characteristics
| Preterm (n = 45) | Full-Term (n = 45) | ||
|---|---|---|---|
| M (SD) | M (SD) | p | |
| Birth History | |||
| Gestational age (weeks) | 35.56 (1.16) | 39.59 (1.00) | < .01* |
| Birth weight (grams) | 2696.16 (468.80) | 3767.70 (478.35) | < .01* |
| Apgar at 1 minute | 7.82 (1.59) | 8.05 (1.09) | 41 |
| Apgar at 5 minutes | 8.57 (1.32) | 8.98 (.34) | .05* |
| Length of hospital stay (days) | 7.09 (6.90) | 2.02 (.75) | < .01* |
| Maternal age at delivery (years) | 31.18 (4.38) | 32.46 (4.27) | .16 |
| n (%) | n (%) | p | |
| Pregnancy Related Characteristics | |||
| Twin gestationa | 6 (13.3) | 0 (0) | < .01* |
| Cesarean delivery | 25 (55.6) | 9 (20.0) | < .01* |
| Preeclampsia or hypertension | 11 (24.4) | 1 (2.2) | < .01* |
| Diabetes mellitus | 7 (15.6) | 4 (8.9) | .52 |
| Neonatal Complications | |||
| Glucose treatment | 16 (35.6) | 1 (2.2) | < .01* |
| Phototherapy | 11 (24.4) | 1 (2.2) | < .01* |
| Respiratory distress | 10 (22.2) | 1 (2.2) | < .01* |
| Positive pressure ventilation | 5 (11.1) | 2 (4.4) | .43 |
| Apnea | 3 (6.7) | 0 (0) | .24 |
| Hypovolemia | 2 (4.4) | 0 (0) | .49 |
Notes. One full-term parent declined to provide access to medical records.
Only one twin per pair was tested.
p < .05.
Children completed two hot EF tasks (delay discounting, delay aversion) and three cool EF tasks (spatial working memory, verbal working memory, inhibitory control) as part of a study investigating neurobehavioral development following moderate-to-late PT birth. Children completed four subtests of the Wechsler Preschool and Primary Scale of Intelligence-III (WPPSI-III; Wechsler, 2002) to estimate intelligence quotient (vocabulary and matrix reasoning) and processing speed (symbol search and coding). Two FT children refused to complete the vocabulary measure and were not included in IQ analyses. Similarly, 21 children were not included in the processing speed analyses due to inability or refusal to complete the task (8 PT, 7 FT) or experimenter timing error (1 PT, 5 FT). Parents completed the Behavior Rating Inventory of Executive Function-Preschool Version (BRIEF-P, Gioia, Espy, & Isquith, 2002) to measure children’s executive function skills in everyday environments. Written informed consent was obtained from parents and verbal assent provided by the children. Parents received a gift card for travel, and children received prizes including a book, stickers, and candies. Study procedures were approved by the University of Minnesota’s Institutional Review Board.
Hot EF Measures
Delay discounting.
In the modified delay discounting paradigm, children made several choices between a smaller immediate reward or a delayed reward of higher value (modeled on Prencipe & Zelazo, 2005). Children were told that several special cards would be presented, with each card requiring them to decide whether treats should be received immediately or saved until later to take home. To ensure comprehension, the experimenter presented two demonstration trials where she selected a test card, verbally described the options, made a choice, and demonstrated the results. An immediate reward was chosen on the first demonstration trial and a delayed reward on the second trial.
Children completed six trials in random order, which used two reward types (candies, stickers) and three reward magnitudes (small: one now vs. two later; medium: one now vs. four later; large: one now vs. six later). The treats were in view of the child during the task. For the immediate reward, the child was allowed to eat the candy or to place the sticker on a piece of paper or on his/her clothing. Delayed treats were selected and set aside in an envelope. The experimenter provided no feedback about the children’s choices. The child’s response was recorded immediately by the experimenter and was also available for review from videotape. Based on Prencipe and Zelazo (2005), dependent measures included behavior on the first trial and the percentage of trials delayed overall.
One FT child did not complete the task due to parent refusal.
Delay aversion.
An adapted version of the Maudsley’s Index of Childhood Delay Aversion (MIDA) was used to assess children’s level of delay aversion (modeled on Kuntsi et al., 2001). The MIDA has high test-retest reliability within participants (Kuntsi, Stevenson, Oosterlaan, & Sonuga-Barke, 2001), discriminates between children diagnosed with ADHD and controls (Sonuga-Barke, Sergeant, Nigg, & Willcutt, 2008), and is predictive of both teacher ratings and behavioral observations of ADHD-related symptoms (Solanto et al., 2001). Children played a computerized game within a spaceship environment where they were asked to fire at asteroids to save a fictitious planet. For each trial the child could choose one of two options: 1) immediately fire at one asteroid, receiving one point with a two second pre-reward delay, or 2) wait to fire at two asteroids, receiving two points but requiring a thirty second pre-reward delay. Choosing small immediate rewards was associated with fewer potential points earned, but faster trials and less delay.
Children were told that they would have 20 chances to shoot the asteroids. The number of trials remaining was illustrated on a tally sheet and was updated after each trial. Children were informed that they could win a book of their choice at the end of the session if they earned enough points to save the planet. Points won after each trial were illustrated by stars and an accompanying cash register sound. The experimenter completed a demonstration of each choice, selecting an immediate reward on the first demonstration trial and a delayed reward on the second trial.
Children’s comprehension of the rules was assessed before beginning the task. The experimenter provided no feedback during the task and delay period. Responses during the task were recorded via a touch screen monitor press, with dependent measures of behavior on the first trial and percentage of trials delayed.
Data from six children were excluded (2 PT children refused to complete the task, 3 PT children could not pass the task comprehension questions, and 1 FT child was excluded due to experimenter error).
Cool EF Measures
Spatial working memory.
This measure of short-term spatial memory and spatial working memory was based on the Corsi block-tapping task (e.g. Kessels, van Zandvoort, Postma, Kappelle, & de Haan, 2000). For the forward task children were asked to touch a sequence of spatial locations (blocks on a wooden board) in the same order as demonstrated by the experimenter. Children progressed through sequences two to seven digits in length with two trials per length until they completed two trials incorrectly at the same span length. Subsequently, for the backward task children were asked to touch a sequence of spatial locations in the reverse order of the experimenter’s demonstration. Children progressed through sequences two to six digits in length, with two trials per length (although two sets of span length two trials were completed by each child, given this represents the limit of many 4-year-old’s abilities) until they completed two trials incorrectly at the same span length. To ensure comprehension, the experimenter presented two practice trials each before the forward and backward task that included corrective feedback. Forward and backward span length were defined as the longest accurate spatial span. A secondary measure was the number of correct trials completed.
Eleven PT and 8 FT children did not provide useable span data for the backward span task because they were unable to correctly complete any items from the shortest span length.
Verbal working memory.
This verbal working memory task was designed to be a direct analog of the spatial working memory task and was based on the digit span subtest of the WPPSI-III (Wechsler, 2002). Children verbally repeated a sequence of numbers spoken by the experimenter. Children progressed through sequences two to seven digits in length with two trials per length until they completed two trials incorrectly at the same span length. Subsequently, for the backward task children were asked to repeat the sequence in reverse order of the experimenter’s demonstration. Children progressed through sequences two to six digits in length, with two trials per length (although two sets of span length two trials were completed by each child, given this represents the limit of many 4-year-old’s abilities) until they completed two trials incorrectly at the same span length. To ensure comprehension, the experimenter presented two practice trials each before the forward and backward task that included corrective feedback. Forward and backward span lengths were defined as the longest accurate digit span. A secondary measure was the number of correct trials completed.
Two PT children were unable and/or refused to complete the task. Eleven PT and 8 FT children did not provide useable span data for the backward span task because they were unable to correctly complete any items from the shortest span length.
Inhibitory control.
A standard computerized Go/no-go paradigm was used to measure response inhibition. Animal stimuli appeared in a central location on the computer screen with one animal randomly assigned as the go stimulus and another as the no-go stimulus. Children were instructed to press the space bar for the frequent go stimulus (80% of trials) and to withhold pressing for the no-go stimulus (20% of trials). Following a practice round of 8 stimuli with corrective feedback, children completed two blocks consisting of 48 stimuli presented for 600 ms with an ITI of 800 ms. Accuracy on the infrequent no-go stimuli was recorded as a measure of inhibitory control. Accuracy on frequent go trials was also analyzed to assure task comprehension.
Two PT and 1 FT children failed to complete the task and were excluded from analyses.
Statistical Analyses
The effects of prematurity were analyzed first with gestational age broken down into a categorical variable: moderate-to-late PT vs. FT. Independent samples t-tests were used to compare group means for continuous variables, Chi-square tests (or Fisher’s exact test when necessary) were used to compare group means for categorical variables, and logistic regression was used to compare group performance on binary outcome variables.
For tasks that produced significant categorical group differences, the effects of prematurity were then also analyzed using gestational age as a continuous variable with linear regression for continuous variables and logistic regression for binary outcome measures. Effects of prematurity were first analyzed across the entire sample range (32 – 42 weeks gestation). If non-significant in the entire sample, we then examined effects of gestational age within the PT children only, given that we would not predict linear effects to necessarily extend into the FT range.
Since measures of EF and IQ encompass shared variability, analyses were also run separately adjusting for individual variation in IQ and processing speed using ANCOVA models. Similarly, since SES is an established predictor of EF (e.g. Evans & English, 2002), analyses were also run separately adjusting for familial Hollingshead scores using ANCOVA models; effects of SES are not reported as the inclusion of this covariate did not alter any reported results.
Last, given our specific interest in hot EF development, we assessed relationships between parental report of EF and performance on the hot EF laboratory tasks using Pearson’s correlations. These correlations were conducted using the entire sample of children. Follow-up analyses of significant correlations were then conducted separately within the FT and PT groups, given the possibility of unique relations between parent report and children’s laboratory performance within the PT sample.
All results are reported using group means and standard deviations. For regression and ANCOVA models, the unstandardized B value and parameter-level statistics are reported. Effect sizes are reported using Cohen’s d or partial eta squared. Effects of sex and potential interactions between sex and group status (FT vs. PT) are not reported throughout as they were not significant predictors of EF task performance.
Results
IQ and Processing Speed
IQ did not differ between groups, but was approximately one standard deviation above the population mean for both groups in this low-risk, highly resourced sample, t(87) = .39, p < 0.74, d = .08; MFT = 114.84 ± 10.58, MPT = 115.84 ± 13.63. Similarly, processing speed quotient also did not differ by group, t(68) = −.25, p < .81, d = .06; MFT = 111.50 ± 11.50, MPT = 110.78 ± 12.76.
Parent Report of EF
Parent reported global executive function composite scores were within the normal range for both PT and FT children and did not differ by group, t(89) = 1.61, p < .11, d = .34; MFT = 48.52 ± 9.98, MPT = 51.91 ± 10.17.
Hot EF Measures
Delay discounting.
We first conducted analyses using all trials as a composite measure of children’s delay discounting. As in previous reports (Prencipe & Zelazo, 2005), a paired-samples t-test indicated there was no difference in children’s responding for the two reward types (candies and stickers), t(89) = .79, p < .43, so these were collapsed. A repeated measures ANOVA with reward magnitude (small, medium, large) and group (PT vs. FT) as independent variables, and percent of trials delayed as the dependent variable, revealed a main effect of group, F(1, 88) = 16.85, p < .01, ηpartial2 = .16, and a main effect of reward magnitude F(2, 88) = 7.25, p < .01, ηpartial2 = .08, with no interaction between group and reward level, F(2, 88) = 1.34, p < .27, ηpartial2 = .02. PT children were less likely to choose the larger delayed reward across all levels of reward magnitude in comparison to FT children, see Figure 1. Regression analyses indicated there was a linear relationship between higher gestational age at birth and increased delayed choices across the entire gestational age at birth range, F(1, 87) = 8.00, p < .01, ηpartial2 = .08.
Figure 1.
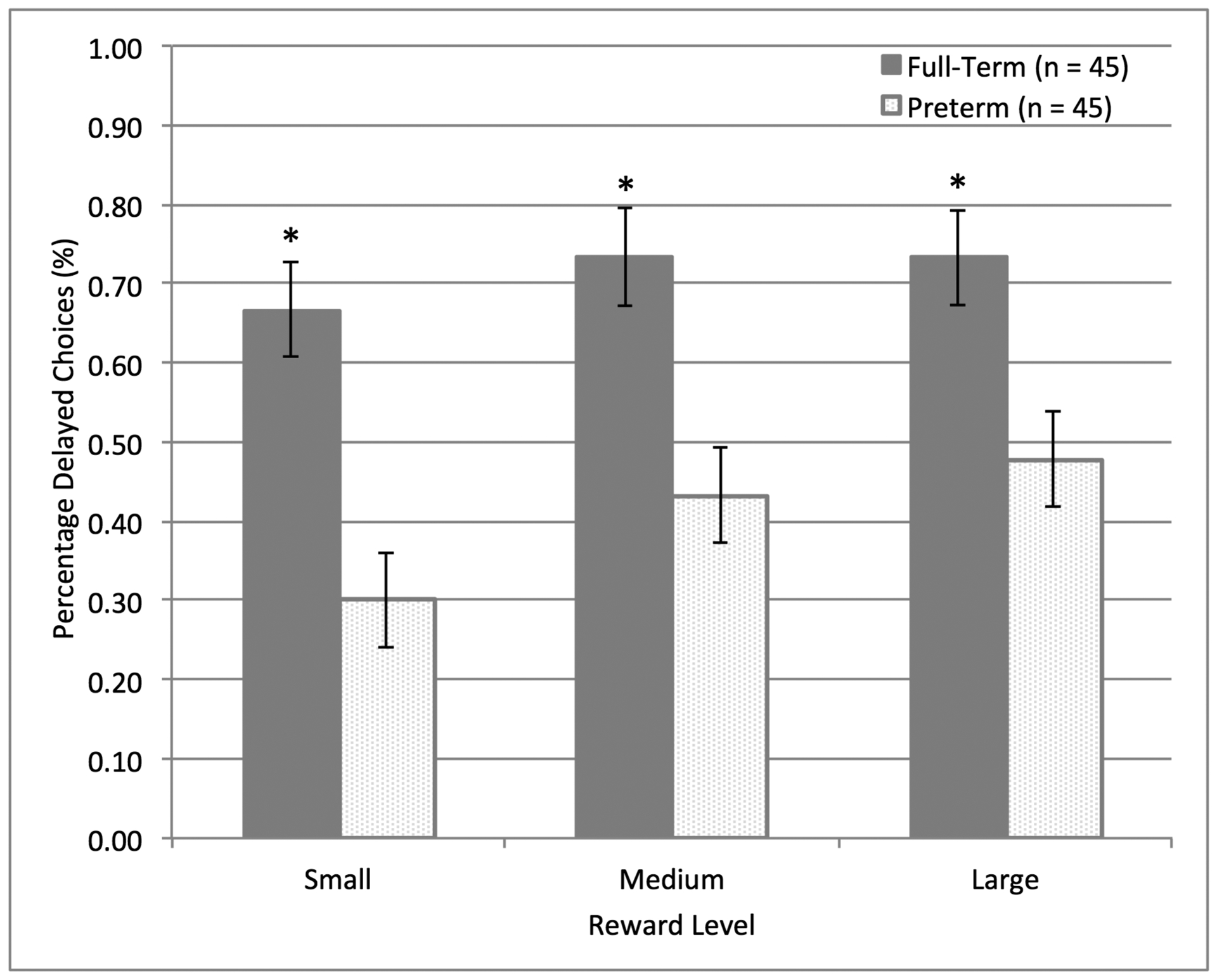
Group differences in delay discounting at age 4. PT children were less likely to choose the larger delayed reward across all levels of reward magnitude in comparison to FT children. Means ± standard errors are illustrated.
Analyses of children’s likelihood to delay on the initial trial (where differences in delayed vs. non-delayed choices may be reflective of inhibitory control failure) produced equivalent results. PT children were less likely to choose the larger, delayed reward on the first task trial, B = 1.49, se(B) = .45, p < .01, Exp(B) = 4.43; MFT = .67 ± 0.48, MPT = .31 ± .47, and gestational age at birth was a continuous predictor of likelihood to delay on the first trial across the entire sample of children, B = .28, se(B) =.10, p < .01, Exp(B) = 1.33, see Figure 2.
Figure 2.

Gestational age at birth and delay likelihood on the delay discounting task. Gestational age at birth was a predictor of likelihood to delay on the first task trial (as well as the overall percentage of delayed reward choices) across the entire sample of children. Figure illustrates the fitted logistic regression function with 95% confidence interval.
Group differences in delay percentage over all trials, F(1, 85) = 16.07, p < .01, ηpartial2 = .16, and likelihood to delay on the initial trial, B = 1.53, se(B) = .46, p < .01, Exp(B) = 4.63, remained significant after controlling for individual variability in IQ scores. Similarly, separate analyses indicated that group differences in delay percentage over all trials, F(1, 66) = 5.46, p < .02, ηpartial2 = .08, and likelihood to delay on the initial trial, , B = 1.25, se(B) = .51, p < .02, Exp(B) = 3.48, also remained significant after controlling for individual variability in processing speed scores.
Delay aversion.
PT and FT children were equally likely to choose the larger, delayed reward on the first task trial, B = −.44, se(B) = .45, p < .32, Exp(B) = .65; MFT = .47 ± 0.51, MPT = .58 ± 0.50.
We also conducted analyses using all 20 trials as a composite measure of children’s delay aversion. PT and FT children showed equivalent levels of overall delay aversion; an ANOVA with group (PT vs. FT) as the independent variable and percent of trials delayed as the dependent variable was non-significant, F(1, 83) = .31, p < .58, ηpartial2 = .00. Analyses that examined group differences in delay likelihood over time were also non-significant. Controlling for individual variability in intelligence or processing speed scores did not change these results.
Performance on the delay aversion task did not predict children’s likelihood to choose larger delayed rewards on the delay discounting task, r(84) = .02, p < .87, suggesting that the two tasks measured different aspects of hot EF within this preschool age sample.
Cool EF Measures
Spatial working memory.
Neither forward, t(86) = .12, p < 0.91, d = .02; MFT = 3.72 ± .81, MPT = 3.74 ± .83, nor backward, t(67) = −.25, p < 0.80, d = .06; MFT = 2.76 ± .85, MPT = 2.71 ± .90, spatial span scores differed between groups. Similarly, the number of correct trials on the forward, t(86) = −.11, p < 0.91, d = .03; MFT = 4.37 ± 1.51, MPT = 4.33 ± 1.49, and backward, t(67) = −.59, p < 0.59, d = .13; MFT = 4.26 ± 1.57, MPT = 4.03 ± 1.96, tasks did not differ by group. Controlling for individual variability in intelligence or processing speed scores did not alter these results.
Verbal working memory.
Neither forward, t(87) = .15, p < 0.88, d = .03; MFT = 4.00 ± .70, MPT = 4.02 ± .77, nor backward, t(68) = −.41, p < 0.68, d = .10; MFT = 2.39 ± .50, MPT = 2.34 ± .55, verbal working memory scores differed between groups. Similarly, the number of correct trials on the forward, t(87) = .45, p < 0.65, d = .10; MFT = 5.26 ± 1.36, MPT = 5.40 ± 1.42, and backward, t(68) = −1.27, p < 0.21, d = .31; MFT = 4.08 ± 1.17, MPT = 3.72 ± 1.20, tasks did not differ by group. However, following correction for individual variation in IQ, PT children received trend-level lower total scores on the backward digit span task in comparison to their FT peers, F(1, 69) = 2.91, p < .09, ηpartial2 = .04.
Inhibitory control.
PT and FT children had equivalent accuracy on both the frequent go trials, t(86) = .75, p < 0.46, d = .12; MFT = .89 ± .09, MPT = .90 ± .08, and the more difficult no-go trials, t(86) = −.49, p < 0.63, d = .10; MFT = .65 ± .18, MPT = .63 ± .21. Controlling for individual variability in intelligence or processing speed scores did not alter these results.
Relationship between Hot EF Tasks & Parent Report
Correlational analyses indicated that parent report of children’s daily life executive function-related behaviors predicted individual differences in performance on laboratory hot EF tasks.
On the delay discounting task, higher percentage of delayed reward choices was correlated with better global EF skills, r(90) = −.27, p < .01, driven primarily by significant relations between delayed reward choice and the inhibitory control, r(90) = −.26, p < .03, and planning/organization subscales of the BRIEF-P, r(90) = −.30, p < .01. The relationship between parent report of global EF skills and delay discounting performance was statistically significant only in the FT group, rFT(45) = −.35, p < .02, potentially due to the more restricted range of task performance in PT children, rPT(45) = −.11, p < .48. See Figure 3 for an illustration of the relation between parent report of EF and likelihood to delay on the first delay discounting trial.
Figure 3.

Parent ratings of EF and delay likelihood on delay discounting task. Lower parent reported EF difficulties in everyday contexts predicted a higher likelihood to delay on the first task trial (as well as the overall percentage of delayed reward choices) across the entire sample of children. Figure illustrates the fitted logistic regression function with 95% confidence interval.
Parent report of EF was less predictive of performance on the delay aversion task, with only the inhibitory control subscale showing a trend-level correlation with children’s likelihood to select larger, delayed rewards, r(85) = −.20, p < .07. This trend-level relationship was driven primarily by PT children, rPT(40) = −.30, p < .06, rFT(45) = −.10, p < .51.
Discussion
This is the first study to report hot EF function differences in children born preterm. Specifically, we found evidence that preschoolers who were born moderate-to-late PT were less likely to choose larger delayed rewards on a delay discounting task in comparison to their FT peers. This group difference was not accounted for by group or individual differences in processing speed or IQ. These results extend those of a previous study reporting subtle differences in cool EF (specifically verbal working memory, but not spatial working memory or inhibitory control) with a late-preterm, preschool-age cohort (Brumbaugh et al., 2013). As in this prior study, group differences in cool EF between low-risk PT children and their FT peers were absent or small in magnitude, and were not consistently present across all cool EF tasks. Given these relatively small differences in cool EF performance, the current results emphasize that EF in an affective context may be particularly taxing for PT children at preschool age. Last, we reported that individual variability in children’s delay discounting performance was also correlated with parent report of EF, suggesting that this task taps behaviors that are relevant for EF in everyday contexts. Interestingly, the relationship between gestational age at birth and the ability to choose larger delayed rewards extended across the entire gestational age range of the sample (32–42 weeks). This implies that there is not a specific gestational age beyond which children are spared from the potential long-term neurodevelopmental impacts of PT birth, consistent with recent studies describing subtle long-term risks associated with early-term (37–38 weeks gestation) birth (see Quigley et al., 2012; Ruth et al., 2012; Sengupta et al., 2013 for discussion).
In contrast, moderate-to-late PT children performed equivalently to their FT peers on a distinct, yet similar hot EF task measuring delay aversion, indicating that PT birth may not globally disrupt hot EF development. Instead it is possible that the impact of PT birth on hot EF depends on the context and task demands. For example, one prior study (Brumbaugh et al., 2013) failed to find differences between FT and late PT (34–36 weeks gestation) preschool-age children’s performance on a classic delay of gratification task involving one choice between an immediate smaller reward (candies) or a delayed larger reward. Instead, late PT and FT children were equivalently likely to wait 5 minutes to receive a larger tangible reward. More recent studies of delay of gratification with preschool-age children have utilized substantially longer delay periods (e.g. up to 15 minute delay; Kidd, Palmeri, & Aslin, 2013), suggesting a 5-minute delay may not be challenging enough to observe individual differences in this age group. The delay discounting task in the present experiment also involved repeated choices between rewards of various magnitude, which may compound EF demands across trials. Our current sample included a broader range of prematurity (32–36 weeks gestation) than the Brumbaugh et al., (2013) study, and we detected a linear relationship between gestational age and delay likelihood. Therefore, detection of hot EF effects in low-risk populations (e.g. moderate-to-late or exclusively late PT children) requires a task that adequately challenges hot EF (i.e. does not produce ceiling effects) and is thus sensitive to both individual and group differences in behavior.
Similar to previous studies that have reported only small or non-existent relationships between various developmental measures of hot EF (Bitsakou, Psychogiou, Thompson, & Sonuga-Barke, 2009; Hongwanishkul et al., 2005; Carlson & Wang, 2007), we did not find a relationship in performance between the two hot EF tasks in the current study. Furthermore, parent report of EF was only a weak (i.e. trend-level) predictor of children’s performance on the delay aversion task, but was sensitive to variability in children’s performance on the delay discounting task. Although both of these tasks ostensibly measure the ability to delay gratification, the delay aversion task is more sensitive to motivational style to escape or avoid delay (Bitsakou et al., 2009), whereas the delay discounting task measures the ability to delay within the context of tangible rewards, directly invoking the ability to resist temptation.
Although speculative, we hypothesize that one difference between the tasks that may have resulted in performance differences for PT children was the presence and receipt of tangible rewards in the delay discounting task. Reward motivation can improve performance on EF tasks in school-age children, adolescents, and adults, due to joint enhancements in executive attention and positive mood (Qu, Finestone, Quin, & Reena, 2013). Similarly, preschool-aged children can show improvements in EF following promise of a reward, although not universally across all EF tasks (Qu et al., 2013). However, if rewards become too attractive (Pessoa, 2009) or salient (Yates & Mischel, 1979), performance may be strongly impaired. For example, focusing on the treat of interest during the classic delay of gratification task is associated with shorter delay time (Mischel et al., 1989). In contrast, distraction of attention away from the attractive treat, actively “cooling” down reward stimuli by reappraisal (e.g. think of the marshmallow treat as a fluffy cloud; Mischel et al., 1989) or replacing rewarding stimuli with a symbolic representation (Carlson, Davis, & Leach, 2005), improves children’s performance. The repeated presence of the tangible rewards during the delay discounting task may have been especially taxing on PT children’s EF skills, given their equivalent performance to FT controls on the delay aversion task that instead involved the receipt of abstract points. Alternatively, it is also possible that the appeal of the rewards (i.e. candy and stickers for delayed discounting; abstract points to exchange for a book for delay aversion), performance demands associated with the testing method (i.e. in-person choices versus a computerized game), or the validity of the measures used (i.e. the delay aversion task is a downward extension of a task used with older children) differed systematically by group and/or task. Since these two hot EF tasks were not designed to be directly comparable, we are unable to determine the specific factors responsible for poorer performance by PT children on only the delay discounting task.
Although PT children were more likely to select the smaller, immediate reward than their FT peers on the delay discounting task, there was no interaction between the magnitude of the reward and group status, providing preliminary evidence that PT children show similar sensitivity to changes in reward magnitude as FT controls, but with a shifted baseline. Although several MRI studies have reported alterations in orbitofrontal cortex volume in children born PT (Ball et al., 2012; Gimenez et al., 2006; Nagy et al., 2009; Thompson et al., 2007), the literature on the long-term effects of PT birth has focused almost exclusively on broad population based outcomes (e.g. educational attainment), potential for IQ impairments, and on “cool” EF and dorso-lateral circuitry differences (e.g. Espy et al., 2002). Further investigation of reward processing and hot EF following PT birth is warranted, especially given additional literature suggesting that perinatal risk factors (e.g. Boksa & El-Khodor, 2003; Juarez, Gratton, & Flores, 2008; Müller et al., 2013) and variations in early life experience (e.g. Matthews & Robbins, 2003; Pechtel & Pizzagalli, 2011) impact reward processing at neural and behavioral levels.
Hot EF tasks inherently involve both cool and hot cognitive processes, raising the possibility that differences in cool EF, particularly in inhibitory control (Brumbaugh et al., 2013), may contribute to the poorer performance observed in the PT group. Effect size for the delay discounting task was in the moderate to large range, while previously reported statistical effects of late PT birth on inhibitory control were smaller (Brumbaugh et al., 2013). Similarly, our sample of low-risk moderate-to-late PT children showed only subtle differences in cool EF performance. Finally, both hot EF tasks utilized in the present study are similar on cool inhibitory demands (e.g. repeated, low time pressure choices between two options with similar response demands), yet PT children performed more poorly on only one task. In general, the development of hot EF is proposed to lag behind the maturation of cool EF skills (e.g. Prencipe et al., 2011), perhaps because hot EF tasks rely on much of the same basic neural circuitry as their cool counterparts but involve additional interference from bottom-up affective processes. Thus, differences in the development of hot EF function in moderate-to-late PT children at preschool-age could reflect cascading effects of developmental differences in more basic cool cognitive skills, as well as potential disruptions in reward processing and/or EF specifically within motivational and affective contexts.
In conclusion, we provide preliminary evidence for disrupted hot EF in children born moderate-to-late PT at preschool age as measured on a delay discounting task. The presence of such a large group difference in behavioral performance was surprising given that our sample of children was restricted to individuals of low medical risk and was over-representative of children with high socioeconomic status (SES). Across a wider demographic range, SES contributes to performance on delay of gratification tasks during childhood (e.g. Evans & English, 2002), and higher SES is also a protective factor for neurobehavioral outcomes following preterm birth (e.g. Vohr et al., 2000). Effects of PT birth on hot EF abilities are likely exacerbated in children born at earlier gestational ages and/or higher levels of medical risk (e.g. Patrianakos-Hoobler, Msall, Marks, Huo, & Schreiber, 2009) and lower SES status (e.g. Ford et al., 2011; Roberts, Bellinger, & McCormick, 2007; Parker, Greer, & Zuckerman, 1998; Potijk, Kerstjens, Bos, Reijneveld, & de Winter, 2013), suggesting that group differences may be more prevalent in the general PT population.
Individual differences in EF during early childhood have been proposed as a pathway through which differences emerge in life outcome metrics, including educational attainment and achievement (Fitzpatrick, McKinnon, Blair, & Willoughby, 2014), with hot EF skills purportedly better predicting real-world functioning than cool EF (Brock, Rimm-Kaufman, Nathanson, & Grimm 2009; Hongwanishkul et al., 2005; Willoughby, Kupersmidt, Voegler-Lee, & Bryant, 2011). Because the current project was not longitudinal in design, future studies must examine whether the observed hot EF differences in moderate-to-late PT children reflect a temporary developmental delay during the preschool period or a persistent deviation. Recently, behavioral therapies that encourage children to make “if-then” contingency plans have been found to improve self-control and delay of gratification in children with ADHD (Gawrilow, Gollwitzer, & Oettingen, 2011), but these interventions have not been extended to other populations such as PT children. Ultimately, the present findings highlight the broad vulnerability of the prefrontal cortex to early variations in the environment, including deviations from the biologically expected environment following PT birth, and emphasize the need for future, developmental characterization of hot EF and reward processing in children that experience diverse forms of early risk.
Research Highlights.
Children born moderate-to-late preterm show discrepant executive function development at preschool-age.
Deficits are particularly salient in the domain of hot executive function.
Potential alterations in reward processing and prefrontal cortex development are suggested as mechanisms.
Acknowledgments
This research was supported by the NIH under the Ruth L. Kirschstein National Research Service Award (#5HT-32HD007151), a training grant at the University of Minnesota (#T32-DA022616, PI Walter C. Low, PhD), a University of Minnesota Graduate Fellowship Award (Amanda S. Hodel), the Benjamin Walker Hanson Neonatology Fund, and the University of Minnesota Center for Neurobehavioral Development.
The authors thank members of the Cognitive Developmental Neuroimaging lab for their help and support, especially Shelby Rentmeester and Sara E. Van Den Heuvel for their assistance with participant testing and recruitment. We also thank Dr. Jason M. Cowell for discussions during experimental task development. Finally, we thank all the children and families who participated in this research.
References
- Aarnoudse-Moens CS, Smidts DP, Oosterlaan J, Duivenvoorden HJ, & Weisglas-Kuperus N (2009). Executive function in very preterm children at early school age. Journal of Abnormal Child Psychology, 37(7), 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson PJ, & Doyle LW (2004). Executive functioning in school-aged children who were born very preterm or with extremely low birth weight in the 1990s. Pediatrics, 114(1), 50–57. [DOI] [PubMed] [Google Scholar]
- Ayduk Ol, Mendoza-Denton R, Mischel W, Downey G, Peake PK, & Rodriguez M (2000). Regulating the interpersonal self: Strategic self-regulation for coping with rejection sensitivity. Journal of Personality and Social Psychology, 79, 776–792. [DOI] [PubMed] [Google Scholar]
- Ball G, Boardman JP, Rueckert D, Aljabar P, Arichi T, Merchant N, … Counsell SJ (2012). The effect of preterm birth on thalamic and cortical development. Cerebral Cortex, 22(5),1016–1024. doi: 10.1093/cercor/bhr176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA, Edwards G, Laneri M, Fletecher K, & Metevia L (2001). Executive functioning, temporal discounting, and sense of time in adolescents with Attention Deficit Hyperactivity Disorder (ADHD) and Oppositional Defiant Disorder (ODD). Journal of Abnormal Child Psychology, 29(6), 541–556. doi: 10.1023/A:1012233310098 [DOI] [PubMed] [Google Scholar]
- Bechara A (2004). The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain and Cognition, 55, 30–40. doi: 10.1016/j.bandc.2003.04.001 [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, & Anderson SW (1998). Dissociation of working memory from decision making within the human prefrontal cortex. Journal of Neuroscience, 18(1), 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitsakou P, Psychogiou L, Thompson M, & Sonuga-Barke EJS (2009). Delay Aversion in Attention Deficit/Hyperactivity Disorder: An empirical investigation of the broader phenotype, Neuropsychologia, 47(2), 446–456. doi: 10.1016/j.neuropsychologia.2008.09.015 [DOI] [PubMed] [Google Scholar]
- Boksa P, & El-Khodor BF (2003). Birth insult interacts with stress at adulthood to alter dopaminergic function in animal models: possible implications for schizophrenia and other disorders. Neuroscience & Biobehavioral Reviews, 27(1), 91–101. [DOI] [PubMed] [Google Scholar]
- Brock LL, Rimm-Kaufman SE, Nathanson L, & Grimm KJ (2009). The contributions of ‘hot’ and ‘cool’executive function to children’s academic achievement, learning-related behaviors, and engagement in kindergarten. Early Childhood Research Quarterly, 24(3), 337–349. [Google Scholar]
- Brumbaugh JE, Hodel AS, & Thomas KM (2013). The Impact of Late Preterm Birth on Executive Function at Preschool Age. American Journal of Perinatology, (EFirst). [DOI] [PubMed] [Google Scholar]
- Carlson SM, Davis AC, & Leach JG (2005). Less is more: Executive function and symbolic representation in preschool children. Psychological Science, 16(8), 609–615. doi: 10.1111/j.1467-9280.2005.01583.x [DOI] [PubMed] [Google Scholar]
- Carlson SM, & Wang TS (2007). Inhibitory control and emotion regulation in preschool children. Cognitive Development, 22(4), 489–510. doi: 10.1016/j.cogdev.2007.08.002 [DOI] [Google Scholar]
- Carlson SM, White RE, Davis-Unger AC (2014). Evidence for a relation between executive function and pretense representation in preschool children. Cognitive Development, 29, 1–16. doi: 10.1016/j.cogdev.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, … Shoda Y (2011). Behavioral and neural correlates of delay of gratification 40 years later. PNAS, 108(36), 14998–15003. doi: 10.1073/pnas.1108561108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, & Phelps ME (1986). Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science, 231, 840–843. [DOI] [PubMed] [Google Scholar]
- Chyi LJ, Lee HC, Hintz SR, Gould JB, & Sutcliffe TL (2008). School outcomes of late preterm infants: special needs and challenges for infants born at 32 to 36 weeks gestation. The Journal of Pediatrics, 153(1), 25–31. [DOI] [PubMed] [Google Scholar]
- Clark CAC, Woodward LJ, Horwood LJ, & Moor S (2008). Development of emotional and behavioral regulation in children born extremely preterm and very preterm: Biological and social influences. Child Development, 79(5), 1444–1462. doi: 10.1111/j.1467-8624.2008.01198.x [DOI] [PubMed] [Google Scholar]
- Collin C, & van den Heuvel MP (2013). The ontogeny of the human connectome: Development and dynamic changes of brain connectivity across the life span. The Neuroscientist, 19(6), 616–628. doi: 10.1177/1073858413503712 [DOI] [PubMed] [Google Scholar]
- Eigsti IM, Zayas V, Mischel W, Shoda Y, Ayduk O, Dadlani, … Casey BJ, (2006). Predicting cognitive control from preschool to late adolescence and young adulthood. Psychological Science, 17(6), 478–484. [DOI] [PubMed] [Google Scholar]
- Engle WA, Tomashek KM, & Wallman C (2007). “Late-preterm” infants: a population at risk. Pediatrics, 120(6), 1290–1401. doi: 10.1542/peds.2007-2952 [DOI] [PubMed] [Google Scholar]
- Espy KA, Stalets MM, McDiarmid MM, Senn TE, Cwik MF, & Hamby A (2002). Executive functions in preschool children born preterm: Application of cognitive neuroscience paradigms. Child Neuropsychology, 8(2), 83–92. [DOI] [PubMed] [Google Scholar]
- Evans GW, & English K (2002). The environment of poverty: Multiple stressor exposure, psychophysiological stress, and socioemotional adjustment. Child Development, 73(4), 1238–1248. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick C, McKinnon RD, Blair CB, & Willoughby MT (2014). Do preschool executive function skills explain the school readiness gap between advantaged and disadvantaged children?. Learning and Instruction, 30, 25–31. [Google Scholar]
- Ford RM, Neulinger K, O’Callaghan M, Mohay H, Gray P, & Shum D (2011). Executive function in 7–9-year-old children born extremely preterm or with extremely low birth weight: effects of biomedical history, age at assessment, and socioeconomic status. Archives of Clinical Neuropsychology, 26(7), 632–644. [DOI] [PubMed] [Google Scholar]
- Franson P, Metsaranta M, Blennow M, Aden U, Lagercrantz H, & Vanhatalo S (2012). Early development of spatial patterns of power-law frequency scaling in fMRI resting-state and EEG data in the newborn brain. Cerebral Cortex, 23(30), 638–646. doi: 10.1093/cercor/bhs047 [DOI] [PubMed] [Google Scholar]
- Gao W, Zhu H, Giovanello KS, Smith JK, Shen D, Gilmore JH, & Lin W (2009). Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. PNAS, 106(16), 6790–6795. doi: 10.1073/pnas.0811221106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawrilow C, Gollwitzer PM, & Oettingen G (2011). If-then plans benefit delay of gratification performance in children with and without ADHD. Cognitive Therapy and Research, 35(5), 442–455. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, … Rapoport JL (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2, 861–863. doi: 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin, … Shen D (2011). Longitudinal development of cortical and subcortical gray matter from birth to 2 years. Cerebral Cortex, 22(11), 2478–2485. doi: 10.1093/cercor/bhr327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez M, Junqué C, Narberhaus A, Bargalló N, Botet F, Mercader JM (2006). White matter volume and concentration reductions in adolescents with history of very preterm birth: A voxel-based morphometry study. NeuroImage, 32(4), 1485–1498. doi: 10.1016/j.neuroimage.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Espy KA, & Isquith PK (2002). Behavior Rating Inventory of Executive Function, Preschool Version (BRIEF-P). Odessa, FL: Psychological Assessment Resources [Google Scholar]
- Grossman T (2013). Mapping prefrontal cortex functions in human infancy. Infancy, 18(3), 303–324. doi: 10.1111/infa.12016 [DOI] [Google Scholar]
- Happaney K, Zelazo PD, & Stuss DT (2004). Development of orbitofrontal function: Current themes and future directions. Brain and Cognition, 55(1), 1–10. doi: 10.1016/j.bandc.2004.01.001 [DOI] [PubMed] [Google Scholar]
- Hollingshead AA (1975). Four-factor index of social status. Unpublished manuscript, Yale University, New Haven, CT. [Google Scholar]
- Hongwanishkul D, Happaney KR, Lee WSC, & Zelazo PD (2005). Assessment of hot and cool executive function in young children: Age-related changes and individual differences. Developmental Neuropsychology, 28(2), 617–644. doi: 10.1207/s15326942dn2802_4 [DOI] [PubMed] [Google Scholar]
- Hüppi PS, Warfield S, Kikinis R, Barnes PD, Zientara GP, Jolesz FA, Tsuji MK & Volpe JJ (1998). Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Annals of Neurology, 43(2), 224–235. doi: 10.1002/ana.410430213 [DOI] [PubMed] [Google Scholar]
- Juárez I, Gratton A, & Flores G (2008). Ontogeny of altered dendritic morphology in the rat prefrontal cortex, hippocampus, and nucleus accumbens following Cesarean delivery and birth anoxia. Journal of Comparative Neurology, 507(5), 1734–1747. [DOI] [PubMed] [Google Scholar]
- Kerr A, & Zelazo PD (2004). Development of “hot” executive function: The children’s gambling task. Brain and Cognition, 55(1), 148–157. doi: 10.1016/S0278-2626(03)00275-6 [DOI] [PubMed] [Google Scholar]
- Kessels RPC, van Zandvoort MJE, Postma A, Kappelle LJ, & de Haan EHF (2000). The corsi block-tapping task: Standardization and normative data. Applied Neuropsychology, 7(4), 252–258. doi: 10.1207/S15324826AN0704_8 [DOI] [PubMed] [Google Scholar]
- Kidd C, Palmeri H, & Aslin RN (2013). Rational snacking: Young children’s decision-making on the marshmallow task is moderated by beliefs about environmental reliability. Cognition, 126, 109–114. doi: doi: 10.1016/j.cognition.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC (2006). The near-term (late preterm) human brain and risk for periventricular leukomalacia: A review. Seminars in Perinatology, 30(2), 81–88. doi: 10.1053/j.semperi.2006.02.006 [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Oosterlaan J, & Stevenson J (2001). Psychological mechanisms in hyperactivity I: Response inhibition deficit, working memory impairment, delay aversion, or something else? Journal of Child Psychology and Psychiatry, 42(2), 199–210. doi: 10.1017/S0021963001006709 [DOI] [PubMed] [Google Scholar]
- Kuntsi J, Stevenson J, Oosterlaan J & Sonuga-Barke EJS (2001), Test-retest reliability of a new delay aversion task and executive function measures. British Journal of Developmental Psychology, 19, 339–348. doi: 10.1348/026151001166137 [DOI] [Google Scholar]
- Luciana M (2003). Cognitive development in children born preterm: implications for theories of brain plasticity following early injury. Developmental Psychopathology, 15(4), 1017–1047. doi: 10.1017/S095457940300049X [DOI] [PubMed] [Google Scholar]
- Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Müller U, … Sonuga-Barke EJS (2009). Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology, 23(3), 367–380. doi: 10.1037/a0014914 [DOI] [PubMed] [Google Scholar]
- Marlow N, Hennessy EM, Bracewell MA, & Wolke D (2007). Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics, 120(4), 793–804. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, & Mathews TJ (2013). Births: Final data for 2011. National Vital Statistics Report, 62(1). Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Matsuzawa J, Matsui M, Konishi T, Noguchi K, Gur RC, Bilker W, & Miyawaki T (2001). Age-related volumetric changes of brain gray and white matter in healthy infants and children. Cerebral Cortex, 11(4), 335–342. doi: 10.1093/cercor/11.4.335 [DOI] [PubMed] [Google Scholar]
- Matthews K, & Robbins TW (2003). Early experience as a determinant of adult behavioral responses to reward: The effects of repeated maternal separation in the rat. Neuroscience & Biobehavioral Reviews, 27(1–2), 45–55. doi: 10.1016/S0149-7634(03)00008-3 [DOI] [PubMed] [Google Scholar]
- Morse SB, Zheng H, Tang Y, & Roth J (2009). Early school-age outcomes of late preterm infants. Pediatrics, 123(4), e622–e629. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, Peake PK (1988). The nature of adolescent competencies predicted by preschool delay of gratification. Journal of Personality and Social Psychology, 54, 687–696. [DOI] [PubMed] [Google Scholar]
- Mischel W, Shoda Y, & Rodriguez ML (1989). Delay of gratification in children. Science, 244, 933–938 [DOI] [PubMed] [Google Scholar]
- Müller KU, Mennigen E, Ripke S, Banaschewski T, Barker GJ, Büchel C, … & Smolka MN (2013). Altered reward processing in adolescents with prenatal exposure to maternal cigarette smoking. JAMA Psychiatry, 1–10. [DOI] [PubMed] [Google Scholar]
- Nagy Z, Asbhurner J, Andersson J, Jbabdi S, Draganski B, Skare S, Bohm B, Smedler A, Forssberg H, & Lagercrantz H (2009). Structural correlates of preterm birth in the adolescent brain. Pediatrics, 124(5), e964–e972. doi: 10.1542/peds.2008-3801 [DOI] [PubMed] [Google Scholar]
- Narberhaus A, Segarra D, Caldú X, Giménez M, Pueyo R, Botet F, & Junqué C (2008). Corpus callosum and prefrontal functions in adolescents with history of very preterm birth. Neuropsychologia, 46(1), 111–116. [DOI] [PubMed] [Google Scholar]
- Nishida M, Makris N, Kennedy DN, Vangel M, Fischl B, Krishnamoorthy KS, … Grant PE (2006). Detailed semiautomated MRI based morphometry of the neonatal brain: Preliminary results. NeuroImage, 32(3), 1041–1049. doi: 10.1016/j.neuroimage.2006.05.020 [DOI] [PubMed] [Google Scholar]
- Nosarti C, Giouroukou E, Micali N, Rifkin L, Morris RG, & Murray RM (2007). Impaired executive functioning in young adults born very preterm. Journal of the International Neuropsychological Society, 13(04), 571–581. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, & Kuntsi J (2009). Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. Journal of the American Academy of Child and Adolescent Psychiatry, 48(8), 837–846. doi: 10.1097/CHI.0b013e3181ab8c97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit AS, Robinson E, Aljabar P, Ball G, Gousias IS, Wang Z, … Edwards AD (2013). Whole-brain mapping of structural connectivity in infants reveals altered connection strength associated with growth and preterm birth. Cerebral Cortex, (EFirst). doi: 10.1093/cercor/bht086 [DOI] [PubMed] [Google Scholar]
- Parker S, Greer S, & Zuckerman B (1988). Double jeopardy: the impact of poverty on early child development. Pediatric Clinics of North America, 35(6), 1227. [DOI] [PubMed] [Google Scholar]
- Patrianakos-Hoobler AI, Msall ME, Marks JD, Huo D, & Schreiber MD (2009). Risk factors affecting school readiness in premature infants with respiratory distress syndrome. Pediatrics, 124(1), 258–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli-Pott U, & Becker K (2011). Neuropsychological basic deficits in preschoolers at risk for ADHD: A meta-analysis. Clinical Psychology Review, 31(4), 626–637. doi: 10.1016/j.cpr.2011.02.005 [DOI] [PubMed] [Google Scholar]
- Pechtel P, & Pizzagalli DA (2011). Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology, 214(1), 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L (2009). How do emotion and motivation direct executive control? Trends in Cognitive Science, 13, 160–166. doi: 10.1016/j.tics.2009.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlmann J, Miller Schwichtenberg AJ, Shah PE, Shlafer RJ, Hahn E, & Maleck S (2010). The development of effortful control in children born preterm. Journal of Clinical Child and Adolescent Psychology, 39(4), 522–536. doi: 10.1080/15374416.2010.486319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potijk MR, Kerstjens JM, Bos AF, Reijneveld SA, & de Winter AF (2013). Developmental Delay in Moderately Preterm-Born Children with Low Socioeconomic Status: Risks Multiply. The Journal of Pediatrics, 163(5), 1289–1295. [DOI] [PubMed] [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, & Zelazo PD (2011). Development of hot and cool executive function during the transition to adolescence. Journal of Experimental Child Psychology, 108(3), 621–637. [DOI] [PubMed] [Google Scholar]
- Prencipe A, & Zelazo PD (2005). Development of affective decision making for self and other evidence for the integration of first- and third-person perspectives. Psychological Science, 16(7), 501–505. doi: 10.1111/j.0956-7976.2005.01564.x [DOI] [PubMed] [Google Scholar]
- Qu L, Finestone DL, Qin LJ, & Reena LZ (2013). Focused but fixed: The impact of expectation of external rewards on inhibitory control and flexibility in preschoolers. Emotion, 13(3), 562–572. doi: 10.1037/a0027263 [DOI] [PubMed] [Google Scholar]
- Quigley MA, Poulsen G, Boyle E, Wolke D, Field D, Alfirevic Z, & Kurinczuik JJ (2012). Early term and late preterm birth are associated with poorer school performance at age 5 years: a cohort study. Arch Dis Child Fetal Neonatal Ed, 97, F167–F173. doi: 10.1136/archdischild-2011-300888 [DOI] [PubMed] [Google Scholar]
- Roberts G, Bellinger D, & McCormick MC (2007). A cumulative risk factor model for early identification of academic difficulties in premature and low birth weight infants. Maternal and Child Health Journal, 11(2), 161–172. [DOI] [PubMed] [Google Scholar]
- Rothbart MK & Bates JE (2007). Temperament. Handbook of Child Psychology. [Google Scholar]
- Ruth CA, Roos N, Hildes-Ripstein E, & Brownell M (2012). The influence of gestational age and socioeconomic status on neonatal outcomes in late preterm and early term gestation: a population based study. BMC Pregnancy and Childbirth, 12(62), 1–8. doi: 10.1186/1471-2393-12-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlam TR, Wilson NL, Shoda Y, Mischel W, & Ayduk O (2013). Preschoolers’ delay of gratification predicts their body mass 30 years later. Journal of Pediatrics, 162(1), 90–93. doi: 10.1016/j.jpeds.2012.06.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, Carrion V, Shelton J, Wynn RJ, Ryan RM, Singhal K, & Lakshminrusimha S (2013). Adverse neonatal outcomes associated with early-term birth. JAMA Pediatrics, 167(11), 1053–1059. doi: 10.1001/jamapediatrics.2013.2581. [DOI] [PubMed] [Google Scholar]
- Shoda Y, Mischel W, & Peake PK (1990). Predicting adolescent cognitive and self-regulatory competencies from preschool delay of gratification: Identifying diagnostic conditions. Developmental Psychology, 26, 978–986. [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, Schachar R, Logan GD, Wigal T, … Turkel E (2001). The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: A supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology, 29(3), 215–228. doi: 10.1023/A:1010329714819 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Sergeant JA, Nigg J, & Willcutt E (2008). Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: Nosologic and diagnostic implications. Child and Adolescent Psychiatric Clinics of North America, 17(2), 367–384. doi : 10.1016/j.chc.2007.11.008 [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Williams E, Hall M & Saxton T (1996), Hyperactivity and delay aversion III: The effect on cognitive style of imposing delay after errors. Journal of Child Psychology and Psychiatry, 37, 189–194. doi: 10.1111/j.1469-7610.1996.tb01390.x [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, & Toga AW (2001). Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. Journal of Neuroscience, 21(22), 8818–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Mohay H, & O’Callaghan M (2009). A comparison of executive function in very preterm and term infants at 8 months corrected age. Early Human Development, 85(4), 225–230. [DOI] [PubMed] [Google Scholar]
- Talge NM, Holzman C, Wang J, Lucia V, Gardiner J, & Breslau N (2010). Late preterm birth and its association with cognitive and socio-emotional outcomes at age 6. Pediatrics, 126, 1124–1131. [DOI] [PubMed] [Google Scholar]
- Thompson DK, Warfield SK, Carlin JB, Pavlovic M, Wang HX, Bear M, … Inder TE (2007). Perinatal risk factors altering regional brain structure in the preterm infant. Brain, 130(3), 667–677. doi: 10.1093/brain/awl277 [DOI] [PubMed] [Google Scholar]
- van Baar AL, Vermaas J, Knots E, de Kleine MJ, & Soons P (2009). Functioning at school age of moderately preterm children born at 32 to 36 weeks’ gestational age. Pediatrics, 124(1), 251–257. [DOI] [PubMed] [Google Scholar]
- Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ,… Kaplan MD (2000). Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics, 105(6), 1216–1226. [DOI] [PubMed] [Google Scholar]
- Voight B, Pietz J, Pauen S, Kliegel M, & Reuner G (2012). Cognitive development in very vs. moderately to late preterm and full-term children: Can effortful control account for group differences in toddlerhood? Early Human Development, 88, 307–313. doi: 10.1016/j.earlhumdev.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Wechsler D (2002). Wechsler Preschool and Primary Scale of Intelligence - III. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Willoughby M, Kupersmidt J, Voegler-Lee M, & Bryant D (2011). Contributions of hot and cool self-regulation to preschool disruptive behavior and academic achievement. Developmental Neuropsychology, 36(2), 162–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates BT, & Mischel W (1979). Young children’s preferred attentional strategies for delay gratification. Journal of Personality and Social Psychology, 37(2), 286–300. [Google Scholar]
- Zelazo PD & Carlson SM (2012). Hot and cool executive function in childhood and adolescence: Development and plasticity. Child Development Perspectives, 6(4), 354–360. doi: 10.1111/j.1750-8606.2012.00246.x [DOI] [Google Scholar]
- Zhou Q, Chen SH & Main A (2012), Commonalities and differences in the research on children’s effortful control and executive function: A call for an integrated model of self-regulation. Child Development Perspectives, 6, 112–121. doi: 10.1111/j.1750-8606.2011.00176.x [DOI] [Google Scholar]


