Abstract
Background
The COVID-19 pandemic has drastically affected everyone in a hit or miss manner. Since it began, evidence of the neuro-invasive potential of the virus has been intensifying significantly. Several pathways have been hypothesized to elucidate the neurotropic nature of SARS-CoV2. It is the need of the hour to collect vital information.
Objective
To evaluate and correlate the neuro-radiological and neurological manifestations in patients diagnosed with SARS-CoV2.
To identify neuro-invasive pathways of COVID infection.
Methods
Relevant studies were identified through four databases—the Cochrane Library, PubMed, Science Direct, and Web of Science. These were searched using relevant keywords—“COVID-19,” “SARS-CoV2,” “neurological manifestations,” “neuroimaging,” “CT,” and “MRI.” Relevant articles were screened according to a pre-defined inclusion and exclusion criteria from December 2019 to August 2020.
Results
Our review included a total of 63 full text publications with 584 patients, composed mainly of observational studies, case reports, and case series. The most common neurological manifestations associated with COVID-19 were altered mental status, stroke, and paralysis. About 17.85% patients who underwent neuroimaging were found to be having ischemic changes suggestive of a stroke. This was followed by hemorrhagic changes as the second most common finding. The most commonly involved vessel was the Middle Cerebral Artery. Besides stroke, we found that SARS-CoV2 could be the cause for new-onset seizures, Guillain-Barre Syndrome, encephalitis, and many other severe neurological diseases.
Conclusion
The information that we have obtained so far will prove dynamic to healthcare providers working against the COVID-19 pandemic. It is necessary to be aware of these atypical neurological findings for the early diagnosis and treatment of COVID-19 infected patients. However, to completely understand the connection between SARS-CoV2 and the nervous system, further research is necessary.
Keywords: SARS-CoV 2, Stroke, Neuro-invasive, COVID-19, Neuroimaging
Introduction
The infamous COVID-19 pandemic has drastically involved everyone in a hit or miss manner. The world is currently fighting against a highly infectious novel coronavirus, known as SARS-CoV2. What began as an outbreak of pneumonia in Wuhan, China, has rapidly engulfed the entire world [1]. As of August 31, 2020, this virus has infected approximately 25 million people and caused 844 thousand deaths globally [2]. The pandemic has posed severe challenges to public health, and the medical community continues to struggle in hitherto mysterious zones, especially in terms of reliable therapeutic interventions. In one study, health care providers utilized extracorporeal membrane oxygenation (ECMO) for patients with acute respiratory distress syndrome secondary to COVID-19, although early reports seem to have a high mortality rate due to devastating neurological insult [3].
Though the respiratory symptoms are the most common, there have been studies which highlight the potential neurotropism of the virus. The incubation period of COVID-19 infected patients, whether asymptomatic or possessing wide spread signs and symptoms, varies from 2 to 11 days with an approximate mortality rate of 2-4% [4]. In an observational study in Wuhan, 36.4% of the patients had neurological involvement such as impaired consciousness, acute cerebrovascular events, headache, seizure, hyposmia, and hypogeusia [5]. There have also been several reports on patients presenting with neurological involvement as the initial symptoms [6, 7].
This initial data reflects that the brain seems to be a target organ for various infections and critical diseases, either due to direct insult or through secondary involvement. The peripheral nervous system (PNS) is also particularly susceptible during infection-related immune-mediated diseases [8].
Even though there is extensive data on the respiratory involvement of SARS-CoV2, documentation of its neurological aspect has been limited to observational studies and case reports. There is a further lack of information on the neuroimaging findings of COVID-19. In this rapidly evolving situation, it has become essential for healthcare providers to stay updated on the various atypical presentations of SARS-CoV2 and keep in mind COVID-19 as a potential diagnosis when encountering such cases. Therefore, we performed a comprehensive literature search in this systematic review to ascertain the different neurological manifestations and neuroimaging findings linked with COVID-19 infection.
Objective
To evaluate and correlate the neuro-radiological and neurological manifestations in patients diagnosed with SARS-CoV2.
To identify neuro-invasive pathways of COVID infection.
Methods
A comprehensive search of the literature was performed from the following databases: PubMed, Web of Science, Cochrane Library, and Science Direct. The following search terms were used in combination with the Boolean operators AND and OR; “COVID-19,” “SARS-CoV2,” “neurological manifestations,” “neuroimaging,” “MRI,” and “CT.” We selected for analysis only articles in which the title and abstract contained the aforementioned search terms. In an initial screen, we excluded articles which were duplicates, and those in which title and abstract were not relevant to our search terminology. Of the remaining studies, screening was done based on the full text of the article under the following inclusion criteria: (1) Studies reporting patients with laboratory confirmation of SARS-CoV2, (2) case reports, case series, cohort studies, and case-control studies, (3) studies in which subjects were above the age of 18, (4) studies containing neuroimaging (CT or MRI) of the brain, (5) studies performed between December 2019 and August 2020. The exclusion criteria were as follows: (1) reviews, editorials, or commentaries. (2) Studies in which subjects were in the pediatric age group, were pregnant, or had prior neurological conditions. (3) Studies with no neurological evaluation, (4) studies published in any language other than English, without available English translations. The articles were screened in their entirety, by two independent readers, in each of the aforementioned scientific databases, to determine eligibility for inclusion. Discrepancies were discussed among all authors, and a collective effort was undertaken to resolve them.
The search strategy and article selection process are depicted in the flowchart in Fig. 1 as per the PRISMA statement.
Fig. 1.
PRSIMA flow chart summarizing search strategy for the articles included in the study
Results
Through the search strategy, we identified 63 articles with neurological and neuroimaging manifestations in patients infected with COVID-19. We included 584 patients who presented with neurological manifestations and underwent different neuroimaging modalities. The age of patients ranged from 24-88 years.
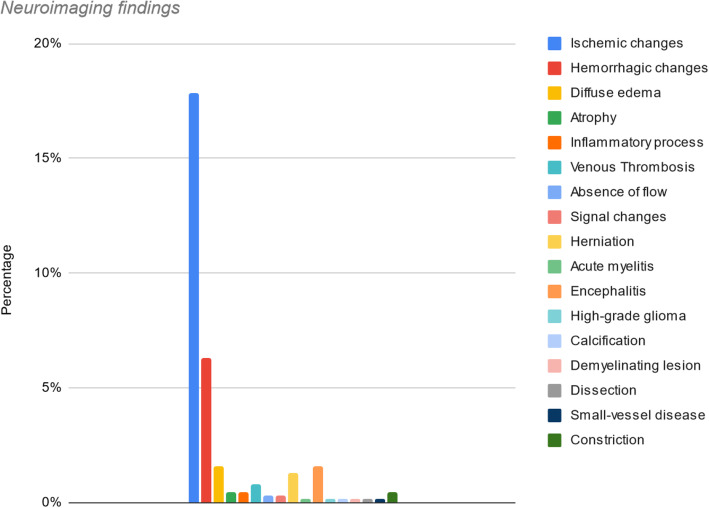
In terms of neuroimaging findings (Table 1), among these 63 articles, 584 patients underwent neuroimaging. Four hundred and twenty eight (67.61%) patients that underwent neuroimaging did not have any abnormality on CT or MRI. For the remaining 156 patients, neuroimaging findings were in descending order as follows: ischemic changes (17.85%), with the middle cerebral artery (MCA) being the most frequent anatomical location; hemorrhagic changes (6.31%), diffuse edema (1.57%), encephalitis (1.57%), herniation (with uncal and subfalcine as the most common) (1.26%), venous thrombosis (0.7%), atrophy (0.4%), inflammatory process (0.4%), and constriction (0.4%). The absence of flow and signal changes was 0.3% each. The least common findings were acute myelitis, high-grade glioma, calcification of the proximal left internal carotid artery (ICA), a demyelinating lesion in left temporal and right occipital lobes, dissection of the left vertebral artery, and small-vessel disease comprised the remaining 0.6% (0.1% each) (Fig. 2).
Table 1.
Reported studies on COVID-19 patients with neurological manifestations with positive findings on major imaging modalities
| Article name | Imaging modality | Neuroimaging findings | ||
|---|---|---|---|---|
| 1 | A case of COVID-19 respiratory Illness with Subsequent seizure and hemiparesis [9] | CT—head | Subcortical hypoattenuation with sulcal effacement in the left occipital and posterior parietal lobes suggestive of ischemic changes | |
| 2 | A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19 [3] | CT—head |
Multicompartment intracranial hemorrhage with marked diffuse edema and secondary infarction of the left anterior and posterior cerebral artery territories due to vascular compression Multifocal intracerebral hemorrhage (ICH) with left hemispheric lobar hemorrhage and right cerebellar hemorrhage Small left frontal cortical subarachnoid hemorrhage (SAH) |
|
| 3 | A first case of meningitis/encephalitis associated with SARS-coronavirus-2 [10] | MRI—brain |
Diffusion weighted images (DWI) showed hyperintensity along the wall of inferior horn of right lateral ventricle. Fluid-attenuated inversion recovery (FLAIR) images showed hyperintense signal changes in the right mesial temporal lobe and hippocampus—suggestive of right lateral ventriculitis and encephalitis. |
|
| 4 | Acute abducens nerve palsy in a patient with the novel coronavirus disease (COVID-19) [11] | MRI—brain | Denervation of CN VI- as evident by hyperintensity on T2 weighting of atrophic left lateral rectus muscle | |
| 5 | Acute disseminated encephalomyelitis after SARS-CoV-2 infection [12] | MRI—brain and spine |
6 enhancing lesions, most with ring enhancement and some with nodular enhancement Hyperintense signal of the optic nerves bilaterally Hyperintense spindle-like T8 lesion |
|
| 6 | Acute myelitis as a neurological complication of COVID-19: a case report and MRI findings [13] | Gadolinium-enhanced MRI—spine |
Extensive diffuse hyperintense signal of the gray matter of cervical, dorsal, and lumbar regions of the spinal cord Mild enlargement and swelling of the cervical cord Areas of restricted diffusion on DWI and apparent diffusion coefficient (ADC) |
|
| 7 | Acute polyradiculoneuritis with locked-in syndrome in a patient with COVID-19 [14] | MRI—spine | Massive symmetrical contrast enhancement of the spinal nerve roots at all levels of the spine including the cauda equina | |
| 8 | Acute profound sensorineural hearing loss after COVID-19 pneumonia [15] | MRI—brain |
Pronounced contrast enhancement in the right cochlea and a partially decreased fluid signal in the basal turn of the right cochlea Adjacent to the temporal bone, meningeal contrast enhancement was seen at the base of the right temporal lobe Signs of an inflammatory process in the cochlea |
|
| 9 | Basal ganglia involvement and altered mental status: a unique neurological manifestation of coronavirus disease 2019 [16] |
CT—head MRI—brain |
B/L basal ganglia hyper-density suggestive of subacute hemorrhagic event Involvement of basal ganglia in subacute bleeding |
|
| 10 | Bilateral posterior cerebral artery territory infarction in a SARS-Cov-2 infected patient: discussion about an unusual case [17] | MRI—brain |
B/L and asymmetric acute occipito-temporal infarction of the posterior cerebral arteries (PCA) with occlusion of P3 segments Hemorrhagic transformation of the previous lesions |
|
| 11 | Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID-19 infection [18] | MRI—brain |
Signs of vasculitis of the vertebrobasilar system Inflammatory signs in the periaqueductal region, along the topography of the trochlear nuclei |
|
| 12 | Cerebral microhemorrhage and purpuric rash in COVID-19: the case for a secondary microangiopathy [19] | MRI—brain |
Multiple areas of micro-hemorrhage throughout the corpus callosum, B/L juxtacortical white matter, basal ganglia, cerebellum, and brain- stem, without clear asymmetry Discrete areas of FLAIR hyperintensity correlating with some of the larger areas of SWI changes suggesting larger macro-hemorrhage Areas of diffusion restriction |
|
| 13 | Cerebral nervous system vasculitis in a COVID-19 patient with pneumonia [20] | CT—headMRI—brain |
Cortical-subcortical blood-related hyperdensities in the right occipital lobes and B/L fronto-parietal Signal restriction of the cortex in a parietal and parieto-occipital region and at the pons level suggestive of subacute phase of cortical inflammation and ischemia |
|
| 14 | Cerebral venous thrombosis: a typical presentation of COVID-19 in the young [21] |
CT—head MRI—brain |
Left temporoparietal hemorrhagic venous infarct with edema and mass effect with 5 mm rightward shift Hyperintense DWI signal of the left temporoparietal hemorrhagic infarct with mass effect and effacement of the left lateral and third ventricle with 4 mm rightward shift Absence of flow in the sigmoid sinus, left transverse and internal jugular vein (IJV) secondary to venous thrombosis |
|
| 15 | Coexistence of COVID-19 and acute ischemic stroke report of four cases [22] | MRI—brain |
Total middle cerebral artery (MCA) infarction Left lenticulostriate artery infarction Right pontine infarction |
|
| 16 | Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019 [23] | CT—head | No abnormality | |
| 17 | Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the New Orleans experience [24] |
CT—head MRI—brain |
Focal encephalitides and vasculolitides Diffuse hypoattenuation, focal hypodensities in deep structures, subacute ischemic strokes, and subcortical parenchymal hemorrhages Viral encephalitis: restriction and FLAIR changes in corpus callosum as well as B/L deep structures |
|
| 18 | COVID-19 presenting as stroke [25] |
CT—head CTA MRI—brain |
Case 1—Loss of gray-white differentiation at the left occipital and parietal lobes, consistent with acute infarct. Evolution of a large acute infarct in the left MCA territory with hyperdense appearance of left MCA vessels—consistent with an acute thrombus Case 2—Moderate hypodensity in the right frontal lobe suggestive of an acute infarct Case 3—Occlusion of the right internal carotid artery (ICA) at origin with a core infarct in the right MCA distribution and a surrounding ischemic penumbra Case 4—acute infarct in the left medial temporal lobe Chronic microvascular ischemic changes Acute left MCA infarct Multiple small acute infarcts in B/L cerebral hemispheres Large acute hemorrhage in the brainstem and right cerebral hemisphere Ischemic and hemorrhagic stroke, hypoxic anoxic brain injury, encephalitis Severe cerebral edema with mass effect, diffuse cerebral sulcal effacement, brainstem compression with narrowing of the 4th ventricle due to downward cerebellar tonsillar herniation Severe diffuse cerebral arterial and dural venous sinus constriction |
|
| 19 | COVID-19 presenting with seizures [26] | CT—head | ||
| 20 | COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome [27] | CT—head | ||
| 21 | COVID-19-associated encephalopathy with fulminant cerebral vasoconstriction: CT and MRI findings [28] |
CT—Head MRI MRA MRV |
||
| 22 | COVID-19-associated encephalopathy: neurological manifestation of COVID-19 [29] |
CT—head MRI—brain |
Hypodensity of bilateral thalami Signal changes of brain parenchyma including insula, B/L dorsal frontal lobes, and thalamus with restricted diffusion of globus pallidus (features of encephalopathy) |
|
| 23 | COVID-19-associated ophthalmoparesis and hypothalamic involvement [30] | MRI—brain | T2/FLAIR Hyperintensity (HI) in the brainstem, including the medial temporal lobes, mammillary bodies, CN VI nuclei, thalami, and hypothalamus | |
| 24 | COVID-19-associated pulmonary and cerebral thromboembolic disease [31] |
CT—head MRI—brain |
Partial right Sylvian segment (M2), superior division occlusion and right opercular (M3), parietal segment occlusions Multiple, discrete, peripheral acute infarctions of the right MCA territory with some hemorrhagic conversion |
|
| 25 | COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia [32] |
CT—head MRI—brain |
Increased hypodensity and swelling of the brain stem, and a new area of cortical and subcortical hypodensity in the left occipital lobe suggestive of an acute posterior circulation infarct Extensive, symmetrical changes in the supratentorial and infratentorial compartments. Hemorrhage and diffuse swelling in the amygdalae and brain stem Microhemorrhage and extensive abnormal signal were found in a symmetrical distribution within the dorsolateral putamina, ventrolateral thalamic nuclei, sub-insular regions, splenium of the corpus callosum, cingulate gyri, and subcortical perirolandic regions |
|
| 26 | COVID-19-related strokes in adults below 55 years of age: a case series [33] | CT—head | Right MCA, Left MCA, and left basal ganglia infarction | |
| 27 | COVID-19-associated encephalitis mimicking glial tumor [34] | MRI—brain | Hyperintense signal in the left temporal lobe in T2 and T2 FLAIR imaging suggestive of high-grade glioma | |
| 28 | De novo status epilepticus in patients with COVID-19 [35] |
CT—head MRI—brain |
No abnormality | |
| 29 | Delirium as a presenting feature in COVID-19: neuroinvasive infection or autoimmune encephalopathy? [36] |
CT—head MRI—brain |
Case 1—3 hyperintense foci on diffusion suggesting cellular infiltration/inflammation or small infarcts Case 2—Changes in the limbic system with partial diffusion restriction, consistent with limbic encephalitis |
|
| 30 | Emergency room neurology in times of COVID-19: malignant ischaemic stroke and SARS-CoV-2 infection [7] |
CT—head CTA |
Established infarct in the territory of the left MCA with a mild deviation of the midline Occlusion of the left MCA, ACA and ICA with a free-floating thrombus in the ascending aorta |
|
| 31 | Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient [37] |
CT—head MRI—brain |
No abnormality | |
| 32 | COVID-19-associated myositis with severe proximal and bulbar weakness [38] | MRI—brain |
Extensive edema and enhancement suggestive of inflammatory myopathy Central nonenhancement in the vastus medialis, consistent with myonecrosis |
|
| 33 | Evolution and resolution of brain involvement associated with SARS- CoV2 infection: a close clinical—paraclinical follow up study of a case [39] |
CT—head MRI—brain |
High signal abnormalities in B/L pons, thalami, and medial temporal lobes | |
| 34 | First case of focal epilepsy associated with SARS-coronavirus-2 [40] |
CTA MRI—brain |
Proximal left ICA plaques with focal calcification Dilated ventricular system with a prominent and patent aqueduct of Sylvius |
|
| 35 | First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease [41] | MRI—brain | No abnormality | |
| 36 | First motor seizure as presenting symptom of SARS-CoV-2 infection [42] | CT—head | No abnormality | |
| 37 | Focal EEG changes indicating critical illness associated cerebral microbleeds in a COVID-19 patient [43] | MRI—brain |
Focal injury without encephalopathy Diffuse microbleeds in B/L juxtacortical white matter, corpus callosum, and internal capsule |
|
| 38 | Fulminant cerebral edema as a lethal manifestation of COVID-19 [44] | CT—head |
Extensive vasogenic edema and herniation of temporal lobes toward the brain stem with obliteration of basal cerebral cisterns, multiple juxtacortical microbleeds, which may be compatible with venous hemorrhagic infarction, effacement of ventricles and peripheral sulci and gyri |
|
| 39 | Intracranial hemorrhage in a young COVID-19 patient [45] | CT—head | Large, multiloculated right ICH associated with vasogenic edema; uncal and sub-falcine herniation without an underlying ischemic stroke | |
| 40 | Ischemic stroke associated with novel coronavirus 2019: a report of three cases [46] | CT—head |
Case 1. Low-density lesion at right cerebellar suggestive of acute ischemic stroke Case 2. Attenuation and effacement at the right hemisphere around the Sylvian fissure Case 3. Hypo-density at left basal ganglion |
|
| 41 | Locked-in with COVID-19 [47] |
MRI—brain MRA |
Numerous foci of restricted diffusion within the pons, (correlating with FLAIR signal abnormality) consistent with acute pontine ischemic infarcts Decreased flow in distal right vertebral artery with a patent basilar artery |
|
| 42 | Macrothrombosis and stroke in patients with mild COVID-19 infection [48] |
CT—head MRI—brain |
Nonocclusive thrombus in the right common carotid artery, extending into the ICA Acute stroke in the territory of the right MCA |
|
| 43 | Malignant cerebral ischemia in a COVID-19 infected patient: case review and histopathological findings [49] | CT—head | Large right MCA infarct | |
| 44 | Multiple sclerosis following SARS-CoV-2 infection [50] | MRI—brain | Supratentorial periventricular demyelinating lesions in right occipital lobe and left temporal | |
| 45 | Necessity of brain imaging in COVID-19 infected patients presenting with acute neurological deficits [51] | CT—head |
Case 1—B/L subacute infarcts, basilar cistern effacement, a left-to-right midline shift, intraparenchymal hemorrhage, sub-falcine, and uncal herniation Case 2—Pre-op - large volume hemorrhage within the right temporal and parietal lobes, surrounding edema, midline shift, uncal herniation, and entrapment of the temporal horns. Post-op—right-sided craniectomy and anterior temporal lobectomy—improvement in overall mass effect |
|
| 46 | Neuralgic amyotrophy following infection with SARS-CoV-2 [52] | MRI—brain |
Edema and inflammatory contrast enhancement of the right distal median nerve Minor right C5-C6 disk protrusion without nerve root impingement, and mild T2-signal increase of the ipsilateral C7-C8 roots, suggestive of proximal edema |
|
| 47 | Neurological manifestations in critically ill patients with COVID-19: a retrospective study [53] | CT—head |
Low density lesions in the following: Case 1. B/L parietal and frontal lobes, right occipital lobe Case 2. Left hemisphere, B/L temporal, and occipital lobes Case 3. B/L parietal and frontal lobes Case 4. Right hemisphere Case 5. Left midbrain Case 6. Right side of the periventricular area |
|
| 48 | Novel coronavirus (COVID-19)-associated Guillain-Barré syndrome: case report [54] | MRI—spine | No evidence of myelopathy or radiculopathy | |
| 49 | Olfactory gyrus intracerebral hemorrhage in a patient with COVID-19 infection [55] |
CT—head MRI—brain |
Right olfactory gyrus ICH with surrounding edema, with no evidence of soft tissue injury or cerebral contusion | |
| 50 | Orbitofrontal involvement in a neuroCOVID-19 patient [56] | MRI—brain | Hyperintensity of the right orbital prefrontal cortex adjacent to the olfactory bulb, which seemed to spread toward the right caudate nucleus and mesial prefrontal cortex | |
| 51 | Posterior reversible encephalopathy syndrome (PRES): another imaging manifestation of COVID-19 [57] |
CT—head MRI—brain |
Symmetric hypoattenuation of the external capsules and posterior subcortical cerebral white matter Hyperintensity with increased diffusion in the internal and external capsules, subcortical, deep cerebral, and cerebellar white matter |
|
| 52 | Prolonged confusional state as first manifestation of COVID-19 [6] | CT—head | Mild chronic small vessel ischemic changes | |
| 53 | Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection [58] | CT—head |
B/L convexity SAH Left vertebral artery dissection |
|
| 54 | Reversible encephalopathy syndrome (PRES) in a COVID-19 patient [59] |
CT—head CTA MRI—brain |
Posterior frontal and temporo-parieto-occipital symmetrical B/L hypodensity of the subcortical white matter, and a small left occipital parenchymal hemorrhage Absence of vascular malformation and alterations of posterior circle vessel caliber- suggestive of vasoconstriction mechanism Onset of right temporal hypodensity, correlated to hemorrhagic process |
|
| 55 | SARS-CoV-2-associated Guillain-Barré syndrome with dysautonomia [60] | CT—head | No abnormalities | |
| 56 | Severe headache as the sole presenting symptom of COVID-19 pneumonia: a case report [61] |
MRI—brain MRA |
Nonspecific white matter hyperintensities Normal MRA |
|
| 57 | Steroid-responsive encephalitis in coronavirus disease 2019 [62] |
CT—head MRI—brain |
No abnormalities | |
| 58 | Stroke and COVID19: not only a large-vessel disease [63] |
CTA MRI—brain |
Small cortical acute ischemic lesions in the right pre- and post- central gyrus, without signs of previous ischemic lesions and hemorrhagic infarction | |
| 59 | Stroke in patients with SARS-CoV-2 infection: case series [64] |
CT—head MRI—brain |
Case 1—CT showed numerous hypodense lesions involving different cortical and subcortical regions of B/L cerebral hemispheres Case 2—Ischemic lesion involving the frontal lobe on the right side; Occlusion of the right pericallosal artery; multiple, B/L supratentorial and infra-tentorial ischemic lesions. Case 3—Small hypodense area in the right thalamus of presumed ischemic origin Case 4—Focal T2-FLAIR HI lesion in the left precentral gyrus with a bright signal on DWI sequence, and mild post-contrast enhancement of the head of right caudate nucleus Case 5—Large cerebellar hemorrhage compressing the brainstem and 4th ventricle causing a subsequent obstructive hydrocephalus Case 6—Diffuse cerebral edema with loss of normal gray—white matter differentiation and obliteration of CSF spaces; large right frontal hemorrhage with other smaller hemorrhages and a bright spot within the sagittal sinus suspected for dural sinus thrombosis |
|
| 60 | Subcortical myoclonus in COVID-19: comprehensive evaluation of a patient [65] | MRI—brain | Cerebral small-vessel disease of moderate severity | |
| 61 | Thalamic perforating artery stroke on computed tomography perfusion in a patient with coronavirus disease 2019 [66] |
CT—head MRI—brain |
Small focal hypoperfusion in the paramedian perforating vascular territory supplying the left medial thalamus 2 punctate acute ischemic lesions in each cerebellar hemisphere |
|
| 62 | Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection [67] | MRI—brain | Normal | |
| 63 | COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients [68] | MRI—brain | Microbleeds in unusual distribution, particularly involving the anterior/posterior limbs of internal capsule (five patients), middle cerebellar peduncles (5/9 patients), and the corpus callosum | |
Fig. 2.
Evaluation of positive neurological findings on CT scan and MRI of COVID-19 infected patients. To demonstrate positive neuroimaging findings, patients with normal findings on imaging or findings unrelated to COVID-19 were not included
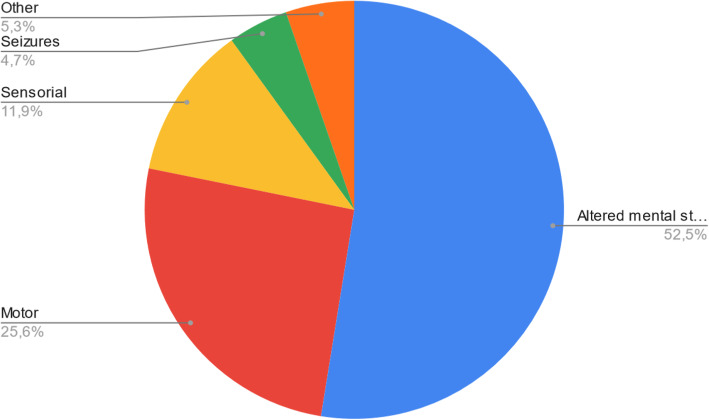
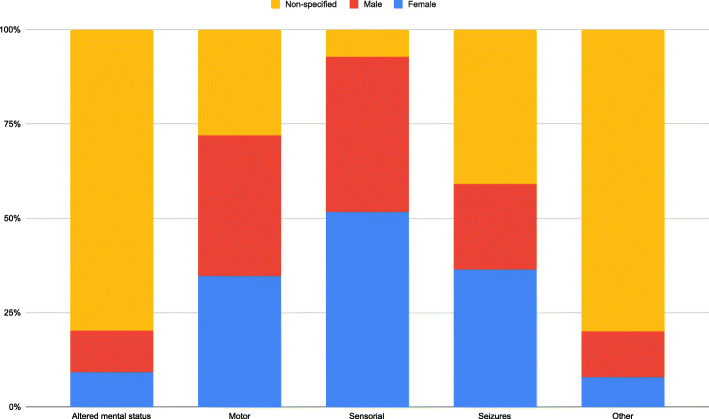
Out of the 157 distinct neurological manifestations presented in the 63 articles (Table 2), we were able to identify 5 possible groups. Patients were only included once per group. In order of prevalence: altered mental status (52.5%), sensory alterations (19.7%), motor alterations (17.7%), others (5.5%), and seizures (4.6%) (Fig. 3). Certain articles with a larger patient population did not specify its prevalence for the different neurological manifestations. The only group with a female predominance was sensory alterations (51.7%). No group had a defined male predominance. Altered mental status and others had a greater representation of un-specified sex (79.8% and 80% respectively) (Fig. 4).
Table 2.
General signs and symptoms, and associated neurological manifestations reported in the studies on COVID-19 infected patients
| Article name | Article type | N = no. of patients | Age/sex | General signs and symptoms | Neurological manifestations | |
|---|---|---|---|---|---|---|
| 1 | A case of COVID-19 respiratory illness with subsequent seizure and hemiparesis [9] | Case report | 1 | 38-year-old male |
Progressive cough Fever Dyspnea |
Generalized tonic—clonic seizure (GTCS) Left-sided hemiplegia Decreased right side spontaneous movements |
| 2 | A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19 [3] | Case series | 4 | Mean age—50.7 years |
Anisocoria Gaze defect Altered mental status (AMS) Agitation |
|
| 3 | A first case of meningitis/encephalitis associated with SARS-coronavirus-2 [10] | Case report | 1 | 24-year-old male |
Headache Generalized fatigue Fever and sore throat |
Neck stiffness Transient generalized seizures Glasgow coma scale (GCS)—6/15 |
| 4 | Acute abducens nerve palsy in a patient with the novel coronavirus disease (COVID-19) [11] | Case report | 1 | 32-year-old male |
Fever and cough Diarrhea Fatigue |
Diplopia (acute, binocular, horizontal) |
| 5 | Acute disseminated encephalomyelitis after SARS-CoV-2 infection [12] | Case report | 1 | 64-year-old female | Influenza-like syndrome |
Anosmia, ageusia B/L vision impairment Right leg sensory deficit |
| 6 | Acute myelitis as a neurological complication of COVID-19: a case report and MRI findings [13] | Case report | 1 | 32-year-old male | Flu-like symptoms |
Urinary retention B/L lower limb weakness |
| 7 | Acute polyradiculoneuritis with locked-in syndrome in a patient with COVID-19 [14] | Letter to the editor | 1 | 51-year-old male | Flu-like symptoms |
Progressive upper and lower limb weakness Acral paresthesia |
| 8 | Acute profound sensorineural hearing loss after COVID-19 pneumonia [15] | Correspondence (case report) | 1 | 60-year-old male | Fever with cough | Sensorineural hearing loss |
| 9 | Basal ganglia involvement and altered mental status: a unique neurological manifestation of coronavirus disease 2019 [16] | Case report | 1 | 54-year-old female |
Low-grade fever Cough |
AMS GCS- 10/15 |
| 10 | Bilateral posterior cerebral artery territory infarction in a SARS-Cov-2 infected patient: discussion about an unusual case [17] | Case report | 1 | 51-year-old male |
Cough Diarrhea |
Headache Dysgeusia Abrupt cortical blindness Disorientation |
| 11 | Bilateral trochlear nerve palsy due to cerebral vasculitis related to COVID-19 infection [18] | Case report | 1 | 69-year-old male |
Fever Abdominal pain Left posterior chest pain |
Binocular diplopia Severe stabbing occipital headache Bilateral paresis of CN IV |
| 12 | Cerebral microhemorrhage and purpuric rash in COVID-19: The case for a secondary microangiopathy [19] | Case report | 1 | 69-year-old male |
Dyspnea, cough Diarrhea Fever Diffuse rash |
Deterioration of mental status |
| 13 | Cerebral nervous system vasculitis in a COVID-19 patient with pneumonia [20] | Case report | 1 | 64-year-old male |
Fever Cough |
Tetraplegia and B/L mute plantar response GCS- 6/15 |
| 14 | Cerebral venous thrombosis: a typical presentation of COVID-19 in the young [21] | Case report | 1 | 25-year-old female |
Cough Low-grade fever Mild shortness of breath |
GTCS with post-ictal confusion Decreased level of arousal Global aphasia Right facial nerve palsy B/L CN VI palsy |
| 15 | Coexistence of COVID-19 and acute ischemic stroke report of four cases [22] | Case report | 4 |
45-year-old female 67-year-old female 72-year-old male 77-year-old male |
Fever Cough Shortness of breath |
Left facial paresis Dysarthria Hemiparesis Loss of consciousness Mild ataxia Left hemi-hypoesthesia |
| 16 | Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019 [23] | Case report | 1 | 64-year-old male |
Fever with mild cough Insomnia Muscle soreness |
Poor mental state B/L ankle clonus, Left Babinski sign + Neck Stiffness with Brudzinski sign + |
| 17 | Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the New Orleans experience [24] | Retrospective cohort study | 27 | Mean age—59.8 years |
Altered mental status Headache Dysgeusia Gaze deviation Focal deficits Hemiparesis/hemiplegia |
|
| 18 | COVID-19 presenting as stroke [25] | Case series | 4 |
73-year-old male 83-year-old male 80-year-old female 88-year-old female |
Fever Respiratory distress Nausea/vomiting Reduced oral intake |
Altered mental status Facial drop Slurred speech Left-sided hemiparesis Right-arm weakness Word-finding difficulty |
| 19 | COVID-19 presenting with seizures [26] | Case report | 1 | 72-year-old male |
Weakness, lightheadedness after a hypoglycemic episode Shortness of breath |
AMS Multiple episodes of tonic—clonic movements of upper and lower limbs |
| 20 | COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome [27] | Retrospective cohort study | 454 | Median age—64 years |
AMS/delirium (37.6%) Stroke (17.3%) Mechanical fall/ trauma (25.5%) Syncope (4%) Headache (3.8%) Dizziness (2.8%) Seizure (2.1%) Ataxia (1.4%) |
|
| 21 | COVID-19-associated encephalopathy with fulminant cerebral vasoconstriction: CT and MRI findings [28] | Case report | 1 | 50-year-old male |
Fatigue Nausea Vomiting |
Severe headache Worsening lethargy Fixed mydriasis with deviation toward the left |
| 22 | COVID-19-associated encephalopathy: neurological manifestation of COVID-19 [29] | Case report | 1 | 43-year-old male |
Fever, dry cough Generalized weakness |
Decreased level of consciousness GCS- 3/15 |
| 23 | COVID-19-associated ophthalmoparesis and hypothalamic involvement [30] | Case report | 2 |
60-year-old female 35-year-old female |
Patient 1. Fever Nausea Cough Patient 2. History of vomiting |
Patient 1. Right CN VI palsy Hyposmia Right hemi-cranial headache Diplopia Patient 2. Diplopia Paresthesia Decreased arousal Disorientation Episodic memory deficits B/L CN VI palsy Mild paraparesis |
| 24 | COVID-19-associated pulmonary and cerebral thromboembolic disease [31] | Case report | 1 | 79-year-old female |
Aphasia Left hemiparesis |
|
| 25 | COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia [32] | Case report | 1 | 59-year-old female |
Sore throat Shortness of breath Myalgia Vomiting |
Episodes of vacant staring Speech arrest Flexion of both shoulders GTCS with post-ictal reduced consciousness |
| 26 | COVID-19-related strokes in adults below 55 years of age: a case series [33] | Case series | 6 |
33-year-old female 39-year-old male 40-year-old male 47-year-old female 49-year-old female 53-year-old male |
Cough Dyspnea Myalgia Lethargy Headache |
Altered consciousness Global aphasia Hemiplegia Left side weakness Homonymous hemianopia Sensory deficit Dysarthria |
| 27 | COVID-19-associated encephalitis mimicking glial tumor [34] | Case report | 1 | 35-year-old female |
Headache Nausea |
Drug-refractory seizures Dizziness |
| 28 | De novo status epilepticus in patients with COVID-19 [35] | Case series | 2 |
49-year-old female 73-year-old female |
Patient 1. None Patient 2. Shortness of breath Lower limb edema |
Patient 1. B/L tonic clonic seizures Altered mental status Patient 2. Face and arm myoclonus Altered mental status |
| 29 | Delirium as a presenting feature in COVID-19: neuroinvasive infection or autoimmune encephalopathy? [36] | Case report (letter to the editor) | 2 |
46-year-old male 79-year-old female |
Patient 1. Status epilepticus Acute hypoactive delirium Disinhibition Headache Patient 2. Generalized seizure Dysphasia Impaired orientation, attention and memory |
|
| 30 | Emergency room neurology in times of COVID-19: malignant ischaemic stroke and SARS-CoV-2 infection [7] | Case report | 1 | 36-year-old female | Unconsciousness |
Global aphasia Right hemiplegia |
| 31 | Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient [37] | Case report | 1 | 41-year-old male |
Abdominal pain Intractable vomiting Dry cough Intermittent fever |
Confusion and agitation GTCS Left-sided ptosis |
| 32 | COVID-19-associated myositis with severe proximal and bulbar weakness [38] | Case report (letter to the editor) | 1 | 58-year-old female |
Cough Dyspnea Myalgia with severe generalized weakness Dysphagia Odynophagia |
Proximal bulbar weakness Bilateral ptosis Facial weakness Hypernasal dysarthria Profound symmetric proximal limb weakness |
| 33 | Evolution and resolution of brain involvement associated with SARS-CoV2 infection: a close clinical—paraclinical follow up study of a case [39] | Case report | 1 | 39-year-old female |
Fever with dry cough Myalgias and anorexia |
Decline in consciousness Multiple episodes of GTCS |
| 34 | First case of focal epilepsy associated with SARS-coronavirus-2 [40] | Case report | 1 | 73-year-old female |
Fatigue Dry cough Back pain |
Painful muscle stiffening and twitching in the left leg and arm (focal seizure) |
| 35 | First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease [41] | Case report | 1 | 42-year-old female | Mild respiratory symptoms | Paresthesia and hypoesthesia in left upper limb, left hemithorax, and hemiface |
| 36 | First motor seizure as presenting symptom of SARS-CoV-2 infection [42] | Case report | 1 | 54-year-old male |
Conjunctivitis Fever |
Clonic movements in the right arm Loss of consciousness |
| 37 | Focal EEG changes indicating critical illness associated cerebral microbleeds in a COVID-19 patient [43] | Case report | 1 | 56-year-old female |
Cough Fever |
Agitation Impaired cognition and vigilance Executive dysfunction |
| 38 | Fulminant cerebral edema as a lethal manifestation of COVID-19 [44] | Case report | 1 | 57-year-old male |
Fatigue and fever Dyspnea Nausea/vomiting Diarrhea |
Dilated and nonreactive pupils Absent brain stem reflexes |
| 39 | Intracranial hemorrhage in a young COVID-19 patient [45] | Case report | 1 | 42-year-old male |
Severe cough Fever (103°F) Dyspnea Pleuritic chest pain |
U/L pupillary changes- progressed to B/L fixed and dilated pupils Loss of all brain stem reflexes |
| 40 | Ischemic stroke associated with novel coronavirus 2019: a report of three cases [46] | Case reports | 3 |
88-year-old female 85-year-old female 55-year-old male |
Fever Dry cough Asthenia |
Ataxia Dysarthria Impaired orientation Drowsiness Peripheral/central facial paresis Limb weakness Impaired memory Acute hemiplegia Broca’s aphasia |
| 41 | Locked-in with COVID-19 [47] | Case report | 1 | 25-year-old female |
Cough Shortness of breath Fever Malaise |
Unable to exhibit motor functions Only able to follow commands through horizontal eye movement and eye blinking B/L Babinski sign + |
| 42 | Macrothrombosis and stroke in patients with mild COVID-19 infection [48] | Case report | 3 |
33-year-old female 77-year-old female 55-year-old male |
Cough |
Patient 1—Left sided hemiplagia with hemisensory loss Patient 2—Sudden onset aphasia with left side hemiparesis Patient 3—Left sided weakness |
| 43 | Malignant cerebral ischemia in a COVID-19 infected patient: case review and histopathological findings [49] | Case report | 1 | 48-year-old male |
Dyspnea Cough |
Left-sided hemiplegia and neglect Speech abnormalities |
| 44 | Multiple sclerosis following SARS-CoV-2 infection [50] | Case report | 1 | 29-year-old female |
Anosmia, dysgeusia Asthenia |
Reduced visual acuity in right eye Eye movements associated with increased retro-ocular pain and color desaturation Pyramidal tract dysfunction |
| 45 | Necessity of brain imaging in COVID-19 infected patients presenting with acute neurological deficits [51] | Case study | 2 |
37-year-old female 47-year-old female |
Patient 1. Fever, cough Shortness of breath Patient 2. Lethargy |
AMS |
| 46 | Neuralgic amyotrophy following infection with SARS-CoV-2 [52] | Case report | 1 | 52-year-old male |
Rhinorrhea Headache |
Persistent severe pain in the right shoulder aggravated by arm extension with gradual shift to forearm and hand Paresthesia of index and long fingers Progressive weakness of right hand |
| 47 | Neurological manifestations in critically ill patients with COVID-19: a retrospective study [53] | Retrospective case series | 7 | Mean age—66 ± 11.1 years |
Fever Cough Myalgia Fatigue Headache Dizziness |
Delirium Acute ischemic stroke Intracerebral hemorrhage Hypoxic-ischemic brain injury Flaccid paralysis |
| 48 | Novel coronavirus (COVID-19)-associated Guillain-Barré syndrome: case report [54] | Case report | 1 | 54-year-old male |
Rhinorrhea Odynophagia Fevers, chills, and night sweats |
Ascending limb weakness and numbness Quadriparesis Facial diplegia Mild ophthalmoparesis |
| 49 | Olfactory gyrus intracerebral hemorrhage in a patient with COVID-19 infection [55] | Case report | 1 | 72-year-old male |
Anosmia Loss of appetite |
Focal onset status epilepticus with Todd’s paralysis |
| 50 | Orbitofrontal involvement in a neuroCOVID-19 patient [56] | Case report | 1 | 69-year-old male |
Cough Fever |
Anosmia Status epilepticus |
| 51 | Posterior reversible encephalopathy syndrome (PRES): another imaging manifestation of COVID-19 [57] | Case report | 1 | 59-year-old male |
Fever Dyspnea |
Encephalopathy |
| 52 | Prolonged confusional state as first manifestation of COVID-19 [6] | Case report | 1 | 77-year-old male | Lethargy | Prolonged confusion |
| 53 | Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection [58] | Case report | 1 | 30s female | Severe cough | Severe thunderclap headache |
| 54 | Reversible encephalopathy syndrome (PRES) in a COVID-19 patient [59] | Case report | 1 | 64-year-old female |
Fever Dyspnea |
Drowsiness Blurred vision AMS Decreased left nasolabial fold Decreased strength and tone in B/L lower limbs DTRs decreased |
| 55 | SARS-CoV-2-associated Guillain-Barré syndrome with dysautonomia [60] | Letter to the editor | 1 | 72-year-old male |
Mild diarrhea Anorexia Chills |
Symmetric paresthesia Ascending appendicular weakness Tendon reflexes- absent Diminished sensation to light touch SIADH and Dysautonomia |
| 56 | Severe headache as the sole presenting symptom of COVID-19 pneumonia: a case report [61] | Case reports and case series | 1 | 76-year-old female |
Severe generalized headache Neck pain |
|
| 57 | Steroid-responsive encephalitis in coronavirus disease 2019 [62] | Case report | 1 | 60-year-old male |
Fever Cough Asthenia |
Cognitive fluctuations Severe akinetic syndrome associated with mutism Palmomental and glabella reflexes + Moderate nuchal rigidity |
| 58 | Stroke and COVID-19: not only a large-vessel disease [63] | Case report | 1 | 49-year-old female |
Dysarthria Left side hemiparesis, hemianesthesia, and facial weakness |
|
| 59 | Stroke in patients with SARS-CoV-2 infection: case series [64] | Retrospective observational case series | 6 | Median age—69 years |
Fever Cough Dyspnea |
Left-sided hemiparesis B/L fixed and dilated pupils Loss of consciousness Confusion Behavioral abnormalities |
| 60 | Subcortical myoclonus in COVID-19: comprehensive evaluation of a patient [65] | Case report | 1 | 58-year-old male | Fever Cough Dyspnea | Myoclonus elicited by action and tactile stimuli predominant in right proximal inferior limb muscles |
| 61 | Thalamic perforating artery stroke on computed tomography perfusion in a patient with coronavirus disease 2019 [66] | Case report | 1 | 50-year-old male | Bilateral pneumonia |
Sudden right facial palsy Mild Right limb weakness |
| 62 | Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection [67] | Case report | 2 |
64-year-old female 67-year-old female |
Flu-like symptoms |
Tonic clonic seizures Headache Psychotic symptoms Disorientation with motor perseverations with B/L grasping Aggressiveness Left hemianopia Sensory hemineglect |
| 63 | COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients [68] | Case series | 9 | Mean age—67.7 years |
Fever Cough Dyspnea |
Delayed recovery of consciousness Psychomotor agitation Confusion |
Fig. 3.
Neurological manifestations in patients infected with SARS-CoV2
Fig. 4.
Gender wise allocation of neurological manifestations in patients with COVID-19
Discussion
Since the outbreak of the SARS-CoV2 virus in December 2019, the majority of research has been centered around respiratory pathogenesis and manifestations of the virus. However, recent focus has shifted toward its invasive nature and complications in the nervous system. There has been a surge in the number of cases documenting the nervous system involvement in COVID-19 positive patients with minimal respiratory involvement. Some studies reported absence of SARS-CoV-2 RNA in the nasal and throat swabs even though it was found to be present in the cerebrospinal fluid upon further investigations [10]. However, our understanding of the pathophysiology behind such neurological manifestations and the data on neuroimaging still remains limited.
Pathogenesis
Currently, there are 4 mechanisms of neuro-invasion that have been hypothesized.
Receptor modulation
The body has a traditional angiotensin-converting enzyme (ACE) in lung capillaries which is a part of the renin-angiotensin-aldosterone system (RAAS) and is involved in regulating blood pressure. COVID-19 is known to use ACE2 receptors, present in the endothelium of the heart, kidneys, and alveolar cells, especially alveolar type 2 (AT2), for cell entry. Binding to these receptors, the virus hampers the body’s natural mechanism of decreasing blood pressure thus increasing the likelihood of intracranial hemorrhages and stroke [69–71]. The neurons and glial cells are known to have ACE2 receptors, possibly explaining the neurotropism of the virus [72]. The mechanism of entry hypothesized is that the spikes present on the virus might link with ACE2 on the capillary endothelium, damaging the blood-brain barrier (BBB) and thus gaining entry into CNS [71]. The two areas are involved in the central regulation of respiration—nucleus of the tractus solitarius and ventrolateral medulla also express ACE2 receptors.
Trans-cribrial transmission
The anosmia in many cases points toward viral entry via olfactory bulb and across the cribriform plate [71]. This mechanism has been linked with murine experiments which led to the detection of the virus in the midbrain, basal ganglia, infralimbic cortex, and the piriform via intranasal inoculation of COVID-19 [69, 73]. SARS-CoV-2 may use ACE2 or trans-membrane protease serine 2 (TMPRSS2) receptors to infect olfactory receptor neurons in the olfactory epithelium [74].
Blood-brain barrier spread
Prior research of SARS-CoV and MERS has shown that cytokines like tumor necrosis factor (TNF-α) and interleukins (IL-6 and IL-1) led to direct death of neurons in the respiratory center in the medulla [73, 75]. The prolific response of the immune system leads to an enormous release of these cytokines and chemokines. They lead to increased permeability and breakdown of the BBB resulting in increased entry of leukocytes. They can also precipitate glutamate receptor-induced neuronal hyperexcitability which may be the reason behind acute seizures linked with the virus. Furthermore, hyperinflammatory and immune responses can result in cytokine storm syndrome which is a severe manifestation of COVID-19 [72].
Trans-synaptic transmission
The entry of the virus into CNS through the peripheral nerves is another hypothesized secondary pathway. The alveoli in the lungs have sensory innervations that detect changes in O2 and CO2. These pathways run-up to the respiratory centers in the brainstem and send signals to the pre-synapses there. Porcine hepatitis E virus studies depict a similar pathway of transmission and since HEV is almost homologous to hCoV-OC432, a close relative of SARS-CoV-2, it might be the same case here [76].
The neuropathological mechanisms reported to play a role in the development of neurological disorders in COVID-19 are—hypoxic brain injury and immune-mediated damage. The hypoxic brain injury is believed to be due to the alveolar gas exchange disorders caused by proliferation of virus in the alveolar cells [71]. As mentioned above, severe immune response resulting in a cytokine storm can also lead to the development of neurological manifestations [72].
Neuro-radiological manifestations
About 17.85% patients who underwent neuroimaging were found to be having ischemic changes suggestive of a stroke. Rajan Jain [27] and colleagues found that the inpatient COVID-19 positive population with stroke had a poor outcome. Similarly, in a systematic review by Sebastian Fredman [77] and colleagues, mortality rate of 45% was reported in the admitted COVID-19 positive patients affected with ischemic stroke. Large vessel involvement was found to be the most common, particularly the MCA. The association of COVID-19 and cerebrovascular disease has been well established but it is still unclear whether this is a de novo occurrence or a complication of already existing atheromatous plaques [78]. The role of stenotic lesions resulting in ischemic changes is also unclear. Hemorrhagic changes were found to be the second most common positive imaging finding particularly involving the corpus callosum and subcortical parenchyma. Aikaterini Fitsiori [68] and colleagues reported that COVID-19 or its treatment may cause unusual microbleeds, predominantly affecting the corpus callosum. All these patients were suffering from severe or moderate acute respiratory distress. This could be due to microangiopathic changes resulting from the cytokine-induced pathogenesis discussed above. Simon Pao [79] and colleagues concluded that ischemic changes were seen in both mild and severe infections whereas hemorrhagic changes were more prevalent in severely affected patients.
Neurological findings
In this study, we observe that COVID-19 patients presented with a variety of neurological complications. In our review, the most prevalent finding has been altered mental status (52.5%). Among the earliest articles about COVID-19 by Mao [5] and colleagues was a retrospective study that showed that 36.4% of patients presented with nervous system abnormalities, and among them, patients who had severe disease were more vulnerable to acute cerebrovascular disease and altered consciousness. The neurotropism of the virus leading to inflammation in the CNS may be a cause of altered mental status. Macrophages and microglia which proliferate to the areas concentrated by viral antigen have shown to cause demyelination leading to memory and cognitive deficits. This was observed in a murine study conducted with several strains of the virus [80, 81]. Nepal G [80]. and colleagues mention the importance of early identification of altered mental status in SARS-CoV-2 patients to check for a possible reversible cause leading to its early management. Confusion, agitation, drowsiness, lethargy, and psychotic symptoms were some of the most commonly observed subsets of symptoms included in altered mental status (Table 2).
Stroke has been observed to be the most frequent finding in neuroimaging of patients affected by COVID-19. A peculiar thing about COVID-19 related strokes is that they can be found in younger patients as observed in a case series by Ashrafi [33] which explores this association in patients younger than the age of 55, where the youngest patient, a 33-year-old, was without any previous comorbidities. Several studies have mentioned the prothrombotic and inflammatory nature of COVID-19, and some reports mention stroke symptoms being the first presentation in many cases. Lee SG [82] and Spence JD [83] mention that about 20-55% of SARS-CoV-2 patients exhibited laboratory values indicating coagulopathies. The prevalence of ischemic strokes is slightly higher than that of hemorrhagic strokes as seen in a 6-patient case series by Morassi [64] where 4 were affected by ischemic stroke and 2 by hemorrhagic. Other frequently seen manifestations include paralysis, headaches, and altered speech.
As far as we know, this is the only study with documentation of reports published until August 2020 which is based on the nervous system involvement and neuroradiological findings of COVID-19 patients. The limitations of our study were that a subset of reported neurological or neuroimaging findings in severely ill and elderly patients may be incidental. The radiological findings might have been susceptible to clinical bias hence it is difficult to standardize them. Radiological imaging presumably is performed selectively on those presenting with notable neurological involvement, leaving out the probable findings in those diseases which are milder in nature, as routine imaging may increase the risk of transmission of the virus. Our study only included articles published in the English language.
Conclusion
In the past few months of the global pandemic, the connection between COVID-19 and neurological manifestations has been growing substantially. Having strong knowledge about such associations will prove to be instrumental in early detection, isolation, and care of patients who present with unusual neurologic symptoms, especially during the ongoing pandemic. Focus on long-term neurologic sequelae and neuroimaging findings is necessary to further the research on the neurotropic involvement of SARS-CoV-2.
Acknowledgements
Not applicable
Abbreviations
- ACE
Angiotensin-converting enzyme
- ACE2
Angiotensin-converting enzyme 2
- AT2
Alveolar type 2 cells
- BBB
Blood-brain barrier
- CNS
Central nervous system
- CT
Computed tomography
- HEV
Hepatitis E virus
- ICA
Internal carotid artery
- IL-1
Interleukin 1
- IL-6
Interleukin 6
- MCA
Middle cerebral artery
- MRA
Magnetic resonance angiography
- MRI
Magnetic resonance imaging
- RAAS
Renin angiotensin aldosterone system
- SARS-CoV2
Severe acute respiratory syndrome-coronavirus 2
- TMPRSS2
Transmembrane protease serine 2
- TNF
Tumor necrosis factor
Authors’ contributions
NM contributed to the conception, design, acquisition, analysis of data, drafted the work and approved the submitted version, and has agreed to be personally accountable for their contributions. MAF contributed to the conception, design, acquisition, analysis of data, drafted the work and approved the submitted version, and has agreed to be personally accountable for their contributions. CR contributed to the conception, design, acquisition, analysis of data, drafted the work and approved the submitted version, and has agreed to be personally accountable for their contributions. NK contributed to the conception, design, acquisition, analysis of data, drafted the work and approved the submitted version, and has agreed to be personally accountable for their contributions. SV contributed to the conception, design, acquisition, analysis of data, drafted the work and approved the submitted version, and has agreed to be personally accountable for their contributions. ES contributed to the conception, design, acquisition, analysis of data, drafted the work and approved the submitted version, and has agreed to be personally accountable for their contributions. JJ contributed to the conception, design, acquisition, analysis of data, drafted the work and approved the submitted version, and has agreed to be personally accountable for their contributions. AA contributed to the conception, design, acquisition, analysis of data, drafted the work and approved the submitted version, and has agreed to be personally accountable for their contributions. The authors read and approved the final manuscript.
Funding
The authors declare that no funding was received for this research.
Availability of data and materials
The authors declare that the data supporting the findings of this study are available within the article [and its supplementary information files].
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nikita Mohan, Email: mohan.nikita@gmail.com.
Muhammad Ali Fayyaz, Email: mafayyazbonamana1@gmail.com.
Christopher del Rio, Email: christodelrio@gmail.com.
Navpreet Kaur Rajinder Singh Khurana, Email: rhythmkhurana@gmail.com.
Sampada Sandip Vaidya, Email: drsampadavaidya@gmail.com.
Esteban Salazar, Email: salazar.c.esteban@gmail.com.
John Joyce, Email: johnjoyce1224@gmail.com.
Amrat Ayaz Ali, Email: amrat.ayaz@outlook.com.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO . Coronavirus Disease (COVID-19) Dashboard. 2020. [Google Scholar]
- 3.Usman AA, Han J, Acker A, Olia SE, Bermudez C, Cucchiara B, Mikkelsen ME, Wald J, Mackay E, Szeto W, Vernick WJ, Gutsche JT. A case series of devastating intracranial hemorrhage during venovenous extracorporeal membrane oxygenation for COVID-19. J Cardiothorac Vasc Anesth. 2020;34(11):3006–3012. doi: 10.1053/j.jvca.2020.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. Neurol Sci. 2020;41(7):1667–1671. doi: 10.1007/s10072-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butt I, Sawlani V, Geberhiwot T. Prolonged confusional state as first manifestation of COVID-19. Annals of Clinical and Translational Neurology. 2020;7(8):1450–1452. doi: 10.1002/acn3.51067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonzalez-Pinto T, Luna-Rodriguez A, Moreno-Estebanez A, Agirre-Beitia G, Rodriguez-Antiguedad A, Ruiz-Lopez M. Emergency room neurology in times of COVID-19: malignant ischaemic stroke and SARS-CoV-2 infection. Eur J Neurol. 2020;27(9):e35–e36. doi: 10.1111/ene.14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsivgoulis G, Palaiodimou L, Katsanos AH, Caso V, Kohrmann M, Molina C, et al. Neurological manifestations and implications of COVID-19 pandemic. Ther Adv Neurol Disord. 2020;13:1756286420932036. doi: 10.1177/1756286420932036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamidi A, Sabayan B, Sorond F, Nemeth AJ, Borhani-haghighi A. A case of Covid-19 respiratory illness with subsequent seizure and hemiparesis. Galen Medical Journal. 2020;9:1915. doi: 10.31661/gmj.v9i0.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falcone MM, Rong AJ, Salazar H, Redick DW, Falcone S, Cavuoto KM. Acute abducens nerve palsy in a patient with the novel coronavirus disease (COVID-19) J AAPOS. 2020;24(4):216–217. doi: 10.1016/j.jaapos.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e797. 10.1212/NXI.0000000000000797. [DOI] [PMC free article] [PubMed]
- 13.AlKetbi R, AlNuaimi D, AlMulla M, AlTalai N, Samir M, Kumar N, et al. Acute myelitis as a neurological complication of Covid-19: a case report and MRI findings. Radiology Case Reports. 2020;15(9):1591–1595. doi: 10.1016/j.radcr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfefferkorn T, Dabitz R, von Wernitz-Keibel T, Aufenanger J, Nowak-Machen M, Janssen H. Acute polyradiculoneuritis with locked-in syndrome in a patient with Covid-19. J Neurol. 2020;267(7):1883–1884. doi: 10.1007/s00415-020-09897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degen C, Lenarz T, Willenborg K. Acute profound sensorineural hearing loss after COVID-19 pneumonia. Mayo Clin Proc. 2020;95(8):1801–1803. doi: 10.1016/j.mayocp.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddadi K, Ghasemian R, Shafizad M. Basal ganglia involvement and altered mental status: a unique neurological manifestation of coronavirus disease 2019. Cureus. 2020;12(4):e7869. doi: 10.7759/cureus.7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonardel C, Bonnerot M, Ludwig M, Vadot W, Beaune G, Chanzy B, Cornut L, Baysson H, Farines M, Combes I, Macheda G, Bing F. Bilateral posterior cerebral artery territory infarction in a SARS-Cov-2 infected patient: discussion about an unusual case. Journal of Stroke and Cerebrovascular Diseases. 2020;29(9):105095. doi: 10.1016/j.jstrokecerebrovasdis.2020.105095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira RdMC d, Santos DH, Olivetti BC, Takahashi JT. Bilateral trochlear nerve palsy due to cerebral vasculitis related to covid-19 infection. Arquivos de Neuro-Psiquiatria. 2020;78(7):385–386. doi: 10.1590/0004-282x20200052. [DOI] [PubMed] [Google Scholar]
- 19.Shoskes A, Migdady I, Fernandez A, Ruggieri P, Rae-Grant A. Cerebral microhemorrhage and purpuric rash in COVID-19: the case for a secondary microangiopathy. J Stroke Cerebrovasc Dis. 2020;29(10):105111. doi: 10.1016/j.jstrokecerebrovasdis.2020.105111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaschetto R, Cena T, Sainaghi PP, Meneghetti G, Bazzano S, Vecchio D, Pirisi M, Brustia D, Barini M, Cammarota G, Castello L, Della Corte F. Cerebral nervous system vasculitis in a Covid-19 patient with pneumonia. J Clin Neurosci. 2020;79:71–73. doi: 10.1016/j.jocn.2020.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein DE, Libman R, Kirsch C, Arora R. Cerebral venous thrombosis: a typical presentation of COVID-19 in the young. J Stroke Cerebrovasc Dis. 2020;29(8):104989. doi: 10.1016/j.jstrokecerebrovasdis.2020.104989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tun CA, UnlUba SY, Alemdar M, AkyUz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. 2020;77:227–229. doi: 10.1016/j.jocn.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin R, Feng W, Wang T, Chen G, Wu T, Chen D, et al. Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J Med Virol. 2020;92(10):1782–4. 10.1002/jmv.25888. [DOI] [PMC free article] [PubMed]
- 24.Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L. Coronavirus 2019 (COVID-19)-associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg. 2020;141:e437–ee46. doi: 10.1016/j.wneu.2020.05.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avula A, Nalleballe K, Narula N, Sapozhnikov S, Dandu V, Toom S, Glaser A, Elsayegh D. COVID-19 presenting as stroke. Brain, Behavior, and Immunity. 2020;87:115–119. doi: 10.1016/j.bbi.2020.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sohal S, Mansur M. COVID-19 presenting with seizures. IDCases. 2020;20:e00782. doi: 10.1016/j.idcr.2020.e00782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, Bilaloglu S, Hochman K, Raz E, Galetta S, Horwtiz L. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sirous R, Taghvaei R, Hellinger JC, Krauthamer AV, Mirfendereski S. COVID-19-associated encephalopathy with fulminant cerebral vasoconstriction: CT and MRI findings. Radiol Case Rep. 2020;15(11):2208–2212. doi: 10.1016/j.radcr.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al Mazrouei SS, Saeed GA, Al Helali AA, Ahmed M. COVID-19-associated encephalopathy: neurological manifestation of COVID-19. Radiol Case Rep. 2020;15(9):1646–1649. doi: 10.1016/j.radcr.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascual-Goni E, Fortea J, Martinez-Domeno A, Rabella N, Tecame M, Gomez-Oliva C, et al. COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e823. 10.1212/NXI.0000000000000823. [DOI] [PMC free article] [PubMed]
- 31.Gill I, Chan S, Fitzpatrick D. COVID-19-associated pulmonary and cerebral thromboembolic disease. Radiol Case Rep. 2020;15(8):1242–1249. doi: 10.1016/j.radcr.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon L, Varley J, Gontsarova A, Mallon D, Tona F, Muir D, et al. COVID-19-related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e789. 10.1212/NXI.0000000000000789. [DOI] [PMC free article] [PubMed]
- 33.Ashrafi F, Zali A, Ommi D, Salari M, Fatemi A, Arab-Ahmadi M, Behnam B, Azhideh A, Vahidi M, Yousefi-Asl M, Jalili khoshnood R, Advani S. COVID-19-related strokes in adults below 55 years of age: a case series. Neurol Sci. 2020;41(8):1985–1989. doi: 10.1007/s10072-020-04521-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Efe IE, Aydin OU, Alabulut A, Celik O, Aydin K. COVID-19-associated encephalitis mimicking glial tumor. World Neurosurg. 2020;140:46–48. doi: 10.1016/j.wneu.2020.05.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Somani S, Pati S, Gaston T, Chitlangia A, Agnihotri S. De novo status epilepticus in patients with COVID-19. Ann Clin Transl Neurol. 2020;7(7):1240–1244. doi: 10.1002/acn3.51071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosseini AA, Shetty AK, Sprigg N, Auer DP, Constantinescu CS. Delirium as a presenting feature in COVID-19: neuroinvasive infection or autoimmune encephalopathy? Brain Behav Immun. 2020;88:68–70. doi: 10.1016/j.bbi.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haddad S, Tayyar R, Risch L, Churchill G, Fares E, Choe M, Montemuro P. Encephalopathy and seizure activity in a COVID-19 well controlled HIV patient. IDCases. 2020;21:e00814. doi: 10.1016/j.idcr.2020.e00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID-19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020;62(3):E57–E60. doi: 10.1002/mus.27003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Afshar H, Yassin Z, Kalantari S, Aloosh O, Lotfi T, Moghaddasi M, Sadeghipour A, Emamikhah M. Evolution and resolution of brain involvement associated with SARS- CoV2 infection: a close clinical - paraclinical follow up study of a case. Mult Scler Relat Disord. 2020;43:102216. doi: 10.1016/j.msard.2020.102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elgamasy S, Kamel MG, Ghozy S, Khalil A, Morra ME, Islam SMS. First case of focal epilepsy associated with SARS-coronavirus-2. J Med Virol. 2020;92(10):2238–2242. doi: 10.1002/jmv.26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Domingues RB, Mendes-Correa MC, de Moura Leite FBV, Sabino EC, Salarini DZ, Claro I, Santos DW, de Jesus JG, Ferreira NE, Romano CM, Soares CAS. First case of SARS-COV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. 2020;267(11):3154–3156. doi: 10.1007/s00415-020-09996-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fasano A, Cavallieri F, Canali E, Valzania F. First motor seizure as presenting symptom of SARS-CoV-2 infection. Neurol Sci. 2020;41(7):1651–1653. doi: 10.1007/s10072-020-04460-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Stefano P, Nencha U, De Stefano L, Megevand P, Seeck M. Focal EEG changes indicating critical illness associated cerebral microbleeds in a Covid-19 patient. Clin Neurophysiol Pract. 2020;5:125–129. doi: 10.1016/j.cnp.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Enden AJM, van Gils L, Labout JAM, van der Jagt M, Moudrous W. Fulminant cerebral edema as a lethal manifestation of COVID-19. Radiol Case Rep. 2020;15(9):1705–1708. doi: 10.1016/j.radcr.2020.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khattar NK, Sharma M, McCallum AP, Oxford BG, Zeb H, Suliman SA, et al. Intracranial hemorrhage in a young COVID-19 patient. Interdiscip Neurosurg. 2020;22:100878. doi: 10.1016/j.inat.2020.100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharifi-Razavi A, Karimi N, Zarvani A, Cheraghmakani H, Baghbanian SM. Ischemic stroke associated with novel coronavirus 2019: a report of three cases. Int J Neurosci. 2020:1–5. 10.1080/00207454.2020.1782902. [DOI] [PMC free article] [PubMed]
- 47.Avula A, Gill A, Nassar R, Nalleballe K, Siddamreddy S, Chalhoub M. Locked-in with COVID-19. J Clin Neurosci. 2020;79:80–83. doi: 10.1016/j.jocn.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fara MG, Stein LK, Skliut M, Morgello S, Fifi JT, Dhamoon MS. Macrothrombosis and stroke in patients with mild Covid-19 infection. J Thromb Haemost. 2020;18(8):2031–2033. doi: 10.1111/jth.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patel SD, Kollar R, Troy P, Song X, Khaled M, Parra A, Pervez M. Malignant cerebral ischemia in a COVID-19 infected patient: case review and histopathological findings. J Stroke Cerebrovasc Dis. 2020;29(11):105231. doi: 10.1016/j.jstrokecerebrovasdis.2020.105231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palao M, Fernandez-Diaz E, Gracia-Gil J, Romero-Sanchez CM, Diaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. 2020;45:102377. doi: 10.1016/j.msard.2020.102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohammad LM, Botros JA, Chohan MO. Necessity of brain imaging in COVID-19 infected patients presenting with acute neurological deficits. Interdiscip Neurosurg. 2020;22:100883. doi: 10.1016/j.inat.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Siepmann T, Kitzler HH, Lueck C, Platzek I, Reichmann H, Barlinn K. Neuralgic amyotrophy following infection with SARS-CoV-2. Muscle Nerve. 2020;62(4):E68–E70. doi: 10.1002/mus.27035. [DOI] [PubMed] [Google Scholar]
- 53.Fan S, Xiao M, Han F, Xia P, Bai X, Chen H, Zhang H, Ding X, Zhao H, Zhao J, Sun X, Jiang W, Wang C, Cao W, Guo F, Tian R, Gao P, Wu W, Ma J, Wu D, Liu Z, Zhou X, Wang J, Guan T, Qin Y, Li T, Xu Y, Zhang D, Chen Y, Xie J, Li Y, Yan X, Zhu Y, Peng B, Cui L, Zhang S, Guan H. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front Neurol. 2020;11:806. doi: 10.3389/fneur.2020.00806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rana S, Lima AA, Chandra R, Valeriano J, Desai T, Freiberg W, Small G. Novel coronavirus (COVID-19)-associated Guillain-Barre syndrome: case report. J Clin Neuromuscul Dis. 2020;21(4):240–242. doi: 10.1097/CND.0000000000000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thu SS, Matin N, Levine SR. Olfactory gyrus intracerebral hemorrhage in a patient with COVID-19 infection. J Clin Neurosci. 2020;79:275–276. doi: 10.1016/j.jocn.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Guennec L, Devianne J, Jalin L, Cao A, Galanaud D, Navarro V, et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020;61(8):e90–4. 10.1111/epi.16612. [DOI] [PMC free article] [PubMed]
- 57.Rogg J, Baker A, Tung G. Posterior reversible encephalopathy syndrome (PRES): another imaging manifestation of COVID-19. Interdiscip Neurosurg. 2020;22:100808. doi: 10.1016/j.inat.2020.100808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dakay K, Kaur G, Gulko E, Santarelli J, Bowers C, Mayer SA, Gandhi CD, al-Mufti F. Reversible cerebral vasoconstriction syndrome and dissection in the setting of COVID-19 infection. J Stroke Cerebrovasc Dis. 2020;29(9):105011. doi: 10.1016/j.jstrokecerebrovasdis.2020.105011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Princiotta Cariddi L, Tabaee Damavandi P, Carimati F, Banfi P, Clemenzi A, Marelli M, Giorgianni A, Vinacci G, Mauri M, Versino M. Reversible encephalopathy syndrome (PRES) in a COVID-19 patient. J Neurol. 2020;267(11):3157–3160. doi: 10.1007/s00415-020-10001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su XW, Palka SV, Rao RR, Chen FS, Brackney CR, Cambi F. SARS-CoV-2-associated Guillain-Barre syndrome with dysautonomia. Muscle Nerve. 2020;62(2):E48–EE9. doi: 10.1002/mus.26988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kimambo H, Chin JH, Mnacho M, Punatar P, Msilanga D, Chagula AC. Severe headache as the sole presenting symptom of COVID-19 pneumonia: a case report. Interdiscip Neurosurg. 2020;22:100882. doi: 10.1016/j.inat.2020.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, et al. Steroid-responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88(2):423–7. 10.1002/ana.25783. [DOI] [PMC free article] [PubMed]
- 63.Frisullo G, Bellavia S, Scala I, Piano C, Morosetti R, Brunetti V, Calabresi P, Della Marca G. Stroke and COVID19: not only a large-vessel disease. J Stroke Cerebrovasc Dis. 2020;29(10):105074. doi: 10.1016/j.jstrokecerebrovasdis.2020.105074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morassi M, Bagatto D, Cobelli M, D’Agostini S, Gigli GL, Bna C, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. 2020;267(8):2185–2192. doi: 10.1007/s00415-020-09885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muccioli L, Rondelli F, Ferri L, Rossini G, Cortelli P, Guarino M. Subcortical myoclonus in COVID-19: comprehensive evaluation of a patient. Mov Disord Clin Pract. 2020;7(8):971–3. 10.1002/mdc3.13046. [DOI] [PMC free article] [PubMed]
- 66.Rudilosso S, Esteller D, Urra X, Chamorro A. Thalamic perforating artery stroke on computed tomography perfusion in a patient with coronavirus disease 2019. J Stroke Cerebrovasc Dis. 2020;29(8):104974. doi: 10.1016/j.jstrokecerebrovasdis.2020.104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernard-Valnet R, Pizzarotti B, Anichini A, Demars Y, Russo E, Schmidhauser M, Cerutti-Sola J, Rossetti AO, du Pasquier R. Two patients with acute meningoencephalitis concomitant with SARS-CoV-2 infection. Eur J Neurol. 2020;27(9):e43–e44. doi: 10.1111/ene.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fitsiori A, Pugin D, Thieffry C, Lalive P, Vargas MI. COVID-19 is associated with an unusual pattern of brain microbleeds in critically ill patients. J Neuroimaging. 2020;30(5):593–597. doi: 10.1111/jon.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R373–R381. doi: 10.1152/ajpregu.00292.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abboud H, Abboud FZ, Kharbouch H, Arkha Y, El Abbadi N, El Ouahabi A. COVID-19 and SARS-Cov-2 infection: pathophysiology and clinical effects on the nervous system. World Neurosurg. 2020;140:49–53. doi: 10.1016/j.wneu.2020.05.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katal S, Balakrishnan S, Gholamrezanezhad A. Neuroimaging and neurologic findings in COVID-19 and other coronavirus infections: a systematic review in 116 patients. J Neuroradiol. 2021;48(1):43–50. 10.1016/j.neurad.2020.06.007. [DOI] [PMC free article] [PubMed]
- 73.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264–7275. doi: 10.1128/JVI.00737-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yavarpour-Bali H, Ghasemi-Kasman M. Update on neurological manifestations of COVID-19. Life Sci. 2020;257:118063. doi: 10.1016/j.lfs.2020.118063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, Gibson-Corley KN, Meyerholz DK, McCray PB., Jr Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712–722. doi: 10.1093/infdis/jiv499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Das M, Penn C, Martinez T, Mayilsamy K, McGill A, Wiling A, et al. COVID-19 neurotropism and implications for therapy. Neuroimmunology and Neuroinflammation. 2020;2020(2):141–149. [Google Scholar]
- 77.Fridman S, Bullrich MB, Jimenez-Ruiz A, Costantini P, Shah P, Just C, et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology. 2020;95(24):e3373–e3385. doi: 10.1212/WNL.0000000000010851. [DOI] [PubMed] [Google Scholar]
- 78.Gulko E, Oleksk ML, Gomes W, Ali S, Mehta H, Overby P, al-Mufti F, Rozenshtein A. MRI brain findings in 126 patients with COVID-19: initial observations from a descriptive literature review. AJNR Am J Neuroradiol. 2020;41(12):2199–2203. doi: 10.3174/ajnr.A6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pan S, Chen WC, Baal JD, Sugrue LP. Neuroradiological features of mild and severe SARS-CoV-2 infection. Acad Radiol. 2020;27(11):1507–1514. doi: 10.1016/j.acra.2020.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nepal G, Rehrig JH, Shrestha GS, Shing YK, Yadav JK, Ojha R, Pokhrel G, Tu ZL, Huang DY. Neurological manifestations of COVID-19: a systematic review. Crit Care. 2020;24(1):421. doi: 10.1186/s13054-020-03121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Templeton SP, Kim TS, O’Malley K, Perlman S. Maturation and localization of macrophages and microglia during infection with a neurotropic murine coronavirus. Brain Pathol. 2008;18(1):40–51. doi: 10.1111/j.1750-3639.2007.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee SG, Fralick M, Sholzberg M. Coagulopathy associated with COVID-19. CMAJ. 2020;192(21):E583. doi: 10.1503/cmaj.200685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spence JD, de Freitas GR, Pettigrew LC, Ay H, Liebeskind DS, Kase CS, del Brutto OH, Hankey GJ, Venketasubramanian N. Mechanisms of stroke in COVID-19. Cerebrovasc Dis. 2020;49(4):451–458. doi: 10.1159/000509581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article [and its supplementary information files].