Abstract
Background
Chemotherapy is one of the most common treatment options for breast cancer (BC) patients. However, about half of the BC patients are chemotherapeutic resistant. Doxorubicin (DOX) is considered as one of the first line drugs in the treatment of BC patients whose function is negatively affected by multi drug resistance. Due to the severe side effects of DOX, it is very important to diagnose the DOX resistant BC patients. Therefore, assessment of molecular mechanisms involved in DOX resistance can improve the clinical outcomes in BC patients by introducing the novel therapeutic and diagnostic molecular markers. MicroRNAs (miRNAs) as members of the non-coding RNAs family have pivotal roles in various cellular processes including cell proliferation and apoptosis. Therefore, aberrant miRNAs functions and expressions can be associated with tumor progression, metastasis, and drug resistance. Moreover, due to miRNAs stability in body fluids, they can be considered as non-invasive diagnostic markers for the DOX response in BC patients.
Main body
In the present review, we have summarized all of the miRNAs that have been reported to be associated with DOX resistance in BC for the first time in the world.
Conclusions
Since, DOX has severe side effects; it is required to distinguish the non DOX-responders from responders to improve the clinical outcomes of BC patients. This review highlights the miRNAs as pivotal regulators of DOX resistance in breast tumor cells. Moreover, the present review paves the way of introducing a non-invasive panel of prediction markers for DOX response among BC patients.
Keywords: Breast cancer, Chemo-resistance, Doxorubicin, MicroRNA, Chemotherapy
Background
Breast cancer (BC) is one of the leading causes of cancer related mortalities among females [1]. It is the most frequently occurring female malignancy which is responsible for nearly 31% of all cancers diagnosed in women. An estimated 1,200,000 newly diagnosed BC patients and 465,000 mortality are reported annually in the world [2]. BC can be classified to the various distinct histological types such as lobular, ductal, papillary, and tubular carcinomas [3]. It can be also classified according to the immuno-pathological features such as progesterone receptor (PR), HER2, and estrogen receptor (ER) expressions [4]. Triple-negative breast cancer (TNBC) accounts for almost 15–20% of all BC cases which is referred to any breast tumor lacking the expressions of ER, PR, and HER2 [5, 6]. Chemotherapy is a routine treatment option for BC, while almost half of the initially responsive tumors develop resistance to various chemotherapeutic regimens [7].
Adriamycin (ADR) or Doxorubicin (DOX) is regarded as the most effective chemotherapeutic medication used for BC treatment; however, DOX effectiveness is negatively affected by multidrug resistance in BC cells during chemotherapy [8]. About 30–50% of metastatic BC patients are responsive to the DOX treatment [9]. DOX as a topoisomerase II inhibitor suppresses tumor growth through DNA replication interfering [10]. Multidrug resistance (MDR) affects the efficacy of cancer therapy and is responsible for treatment failure, tumor progression, and recurrence in a large number of BC patients. Deregulation of drug efflux transporters such as ABCB1 and multiple resistance protein-1 (MRP1) are important factors associated with MDR. Abnormal increased DNA repair processes, drug detoxification, and aberrant expression of oncogenes and tumor suppressors, are also other driving forces behind the MDR development [11, 12]. Mechanisms of DOX resistance can be classified into: (1) up regulation of drug-resistant proteins and membrane multidrug pumps in cancer cells, and (2) disruption in cellular signaling pathways which leads to the suppression of the apoptosis induced by DOX.
MicroRNAs (miRNAs) are small non-coding RNAs with 9–22 nucleotides length serving as post-transcriptional regulators of gene expression via binding to the 3′-untranslated region (UTR) of their target mRNAs that results in mRNA degradation or translational suppression [13]. Dysregulation of various miRNAs have been reported to be associated with the tumor progression and drug resistance [14–16]. Inhibition of miRNA activity by competitive inhibitors including miRNA sponge or target mimic has been used to study their functions. Sponge miRNAs can bind with a non-coding transcript or 3′ UTR of target gene which are expressed by U6 or CMV promoters. Lentiviral and retroviral vectors with sponge RNAs have continuous miRNA suppression in either dividing or non-dividing cells [17]. There is not any efficient method to distinguish the non-responders from those who will respond to chemotherapy. Therefore, a reliable approach for classifying patients in order to prevent unwanted side effects of chemotherapy and optimize the treatment outcome is imperative. Regarding severe DOX side effects, it is required to clarify the molecular mechanisms involved in DOX resistance to provide novel efficient therapeutic modalities to improve the clinical outcomes of BC patients. Since, microRNAs are non-invasive and more stable factors compared with mRNAs, they can be introduced as efficient and reliable markers of DOX response in BC patients.
In the present review, we have summarized all of the miRNAs that have been reported to be associated with DOX resistance in BC for the first time in the world (Table 1). We categorized the reported miRNAs based on their targets to clarify the molecular mechanisms of miRNAs mediated DOX resistance in breast tumor cells.
Table 1.
All of the miRNAs associated with Doxorubicin resistance in BC
| Study | Year | Gene | Country | Target | samples | Results |
|---|---|---|---|---|---|---|
| Developmental factors and signaling pathways | ||||||
| Wu [24] | 2019 | miR-140-5p | China | WNT1 | MCF-7, MDA-MB-231 cell lines | Increased Dox sensitivity |
| Cheng [31] | 2019 | miR-137 | China | FSTL1 |
87 patients HCC38, MDA-MB-231, and MDA-MB-468 cell lines |
Increased Dox sensitivity |
| Xiong [36] | 2018 | miR-613 | China | DAAM1 |
123 patients MDA-MB-231, MCF-7, HEK-293 T, and SUM1315 cell lines |
Increased Dox sensitivity |
| Li [42] | 2012 | miR-34a | China | NOTCH1 |
38 patients MCF-7 cell line |
Increased Dox sensitivity |
| Hu [47] | 2016 | miR-760 | China | NANOG and SNAIL | MCF-7, MDA-MB-231 cell lines | Increased Dox sensitivity |
| Kim [55] | 2016 | miR-34a | Korea | PRKD1 | MCF-7, MDA-MB-231 cell lines | Increased Dox sensitivity |
| PI3K/AKT and MAPK signaling pathways | ||||||
| Shen [57] | 2016 | miR-29a | China | PTEN | MCF-7 cell line | Increased Dox resistance |
| Hu [58] | 2016 | miR-205 | China | VEGFA and FGF2 |
30 patients MCF-7 cell line |
Increased Dox sensitivity |
| Liu [59] | 2019 | miR-202-5p | China | PI3K and AKT |
62 patients MCF-10A and MCF-7 cell lines |
Increased Dox resistance |
| Kopp [70] | 2012 | miR-200c | Germany | TRKB and BMI1 | MDA-MB-436 and BT474 cell lines | Increased Dox sensitivity |
| Xie [73] | 2018 | miR-132 and miR-212 | China | PTEN |
53 patients MCF-7 cell line |
Increased Dox resistance |
| Shen [74] | 2017 | miR-222 | China | PTEN | MCF-7 cell line | Increased Dox resistance |
| Wang [75] | 2011 | miR-21 | China | PTEN | MCF-7 cell line | Increased Dox resistance |
| Chu [76] | 2017 | miR-93 | China | PTEN |
16 patients MCF-7 cell line |
Increased Dox resistance |
| Chen [77] | 2013 | miR-200c | China | ZEB1 | MCF-7 cell line | Increased Dox sensitivity |
| Fang [84] | 2014 | miR-30c | China | YWHAZ | MCF-7, MDA-MB-231 cell lines | Increased Dox sensitivity |
| Du [86] | 2019 | miR-137 | China | DUSP4 | MCF-7, HCC1937, and MDA-MB-468 cell lines | Increased Dox sensitivity |
| Mi [88] | 2018 | miR-381 | China | FYN | MCF-7, MDA-MB-231 cell lines | Increased Dox sensitivity |
| Zhao [92] | 2016 | miR-302 | China | MEKK1 | MCF-7 cell line | Increased Dox sensitivity |
| Apoptosis, cell cycle, and DNA repair | ||||||
| Zheng [96] | 2016 | miR-181b | China | BIM |
30 patients MCF-10A, T-47D, MCF-7, MDA-MB-231, and MDA-MB-435 cell lines |
Increased Dox resistance |
| Dai [97] | 2019 | miR-222 | China | BIM |
25 patients MCF-7 cell line |
Increased Dox sensitivity |
| Long [101] | 2015 | miR-193b | China | MCL1 | MCF-7 cell line | Increased Dox sensitivity |
| Hu [103] | 2015 | miR-218 | China | BIRC5 | MCF-7 and CAL-51 cell lines | Increased Dox sensitivity |
| Li [108] | 2019 | miR-3609 | China | PDL1 |
47 patients HBL-100, MCF-7, MDA-MB-231, and MDA-MB-468 cell lines |
Increased Dox sensitivity |
| Zhang [112] | 2019 | miR-192-5p | China | PPIA and BCL2 | MCF-10A, MCF-7 cell lines | Increased Dox sensitivity |
| Zhang [114] | 2016 | miR-214 | China | RFWD2 |
31 patients MCF-7, MDA-MB-231, and MDA-MB-468 cell lines |
Increased Dox sensitivity |
| Tormo [115] | 2019 | miR-449 | Spain | CDK2, E2F1, and E2F3 |
30 patients MDA-MB-231, MDA-MB-468, and MCF-7 cell lines |
Increased Dox sensitivity |
| Lu [121] | 2020 | miR-140 | China | FEN1 | MCF-7 cell line | Increased Dox sensitivity |
| Lin [124] | 2019 | miR-30c | China | REV1 and FANCF | MCF-7, ZR-75–1, T-47D, MDA-MB-231, and MCF-10A cell lines | Increased Dox sensitivity |
| Transporters | ||||||
| Lu [129] | 2015 | miR-134 | China | ABCC1 |
40 patients MCF-7 cell line |
Increased Dox sensitivity |
| Chang [131] | 2018 | miR-199a | China | MRP1 | MCF-7 | Increased Dox sensitivity |
| Gao [132] | 2016 | miR-145 | China | MRP1 |
112 patients MCF-7, MDA-MB-231, MDA-MB-453, MDA-MB-468, and MCF-10A cell lines |
Increased Dox sensitivity |
| Chen [135] | 2012 | miR-200c | China | MDR1 |
39 patients MCF-7 and MDA-MB-231 cell lines |
Increased Dox sensitivity |
| Kovalchuk [136] | 2008 | miR-451 | Canada | MDR1 | MCF-7 cell line | Increased Dox sensitivity |
| Hu [137] | 2019 | miR-124-3p | China | ABCC4 |
40 patients MCF-7 and MCF-10A cell lines |
Increased Dox sensitivity |
| Yuan [143] | 2015 | miR-133a | China | UCP2 | MCF-7 cell line | Increased Dox sensitivity |
| TGF-β and JAK/STAT signaling pathways | ||||||
| Sun [146] | 2018 | miR-574 | China | SMAD4 |
30 patients MCF-7 cell line |
Increased Dox resistance |
| Jiang [148] | 2014 | miR-489 | China | SMAD3 | MCF-7 cell line | Increased Dox sensitivity |
| Liang [150] | 2019 | miR-548-p | China | PBLD | MCF-7 and MDA-MB-231 cell lines | Increased Dox resistance |
| Liu [155] | 2019 | miR-124 | China | STAT3 and HIF1 | MCF-7 cell line | Increased Dox sensitivity |
| Enzymes and structural proteins | ||||||
| Han [161] | 2019 | miR-181c | China | OPN |
29 patients MCF-7 cell line |
Increased Dox sensitivity |
| Zhang [165] | 2019 | miR-135b-5p | China | AGR2 |
28 patients MCF-7 and MDA-MB-231 cell lines |
Increased Dox sensitivity |
| Bolandghamat pour [171] | 2019 | miR-154 | Iran | NAMPT | MCF-7, MCF-10A, and MDA-MB-231 cell lines | Increased Dox sensitivity |
| Li [175] | 2018 | miR-770 | China | STMN1 | MDA-MB-231, MDA-MB-468, and THP-1 cell lines | Increased Dox sensitivity |
Main text
Developmental factors and signaling pathways
Developmental signaling pathways such as WNT and NOTCH have pivotal roles in DOX response of breast tumor cells which can be regulated by miRNAs (Fig. 1). WNT signaling is a developmental pathway triggered by interaction between WNT ligands and Frizzled (FZD) receptors that result in the activation of non-canonical and canonical cascades. WNT family of proteins consists of a variety of cysteine-rich secreted glycoproteins involved in cell proliferation, polarity, apoptosis, DNA repair, embryogenesis, and tumor progression [18–20]. ALDH1 + breast cancer stem cells (BCSCs) are a sub population of tumor cells with a high self-renewal and tumorigenic capacities. MiR-140-5p modulates the BCSCs through inhibiting the self-renewal factors including WNT, SOX2, and SOX9 [21]. OCT4 is also the principal transcription factor for the regulation of pluripotency and self-renewing capabilities in the embryonic stem cells [22]. WNT1 induces tumor cell cycle progression and migration via interaction with specific FZD receptors in the surface of target cells which leads to β-catenin nuclear transportation and activation [23]. It has been reported that miR-140-5p reduced BCSCs proliferation, self-renewal, and sphere-formation via WNT signaling targeting. MiR-140-5p also decreased the levels of OCT4 and ALDH1 expressions and reduced the sphere formation. Moreover, miR-140-5p sensitized BCSCs to DOX mainly through the suppression of WNT1/ABCB1 axis [24].
Fig. 1.
miRNAs are involved in DOX response (resistance or sensitivity) of breast tumor cells via regulation of WNT and NOTCH signaling pathways
Integrin β3 belongs to the integral cell-surface receptors that mainly serves as a link between the cytoskeleton and extra cellular matrix (ECM) to regulate cell adhesion, proliferation, migration, angiogenesis, cytoskeletal organization, and tumorigenesis [25–27]. It also enhances the growth factor release, invasion, migration, and epithelial mesenchymal transition (EMT) process in breast tumor cells [28–30]. WNT/β-catenin pathway exerts its effect on intracellular signal transduction via cell surface receptors such as integrin β3. It has been shown that there was FSTL1 up regulation in TNBC samples and cell lines compared with non-TNBC samples and normal mammary epithelial cells, respectively. MiR-137 also inhibited WNT/β-catenin signaling and suppressed stemness and DOX resistance of BC cells through targeting FSTL1 [31]. Dishevelled-associated activator of morphogenesis 1 (Daam1) is involved in WNT/PCP signaling pathway through interaction with Dishevelled [32, 33]. It is associated with increased cell migration via stimulation of actin reorganization during gastrulation, filopodia formation, and female germ cells meiosis [34, 35]. It has been reported that there was a converse association between the levels of miR-613 expressions and lymph node involvement in BC patients. MiR-613 was involved in regulation of DOX sensitivity via inhibition of Daam1/RhoA pathway [36].
EMT is a biological process allows epithelial cells to lose their polarity and cell–cell adhesion to gain mesenchymal organization. It has pivotal roles in various physiological and pathological processes including embryogenesis, tissue homeostasis, and tumorigenesis [37, 38]. Tumor cells that undergo EMT, acquire stem cell-like properties correlated with malignant behavior and enhanced chemo resistance [39].
Notch signaling is one of the critical developmental pathways involved in cell differentiation, migration, and drug resistance via Notch receptors (Notch1-4) and ligands (DLL and Jagged). This signaling pathway also regulates the EMT especially in cancer stem cells (CSCs) that is a fundamental process in drug resistance and tumor relapse [18, 40, 41]. It has been reported that the miR-34a expression regulated the ADR response in BC cells through NOTCH1 targeting. There was also significant miR-34a down regulation in MCF-7/ADR cells compared with MCF-7 cells. MiR-34a significantly increased ADR sensitivity. Moreover, ADR responders had higher levels of miR-34a expressions compared with non-responders [42].
Nanog is a developmental transcription factor involved in self-renewal and differentiation of stem cells [43, 44]. It is also a critical factor for the regulation of EMT process and chemo resistance during tumor progression [45, 46]. It has been reported that there was significant miR-760 down regulation in MCF-7/DOX and DOX resistant BC tissues in comparison with MCF-7 cells and chemo sensitive tissues. MiR-760 increased DOX sensitivity in BC cells through NANOG inhibition and also reversed EMT by SNAIL down regulation and E-cadherin up regulation in MCF-7/DOX cells [47]. CSCs are a small subset of tumor cells with self-renewal, recurrence, and chemo resistance capabilities [48]. Various miRNA are implicated in the formation of BCSCs and self-renewal maintenance [49]. MiR-34c was shown to inhibit the EMT process and decrease the self-renewal capabilities of BCSCs [50]. Serine/threonine-protein kinase D1 (PRKD1) is a downstream effector of diacylglycerol and protein kinase C that mediates the function of growth factors, hormones, and neurotransmitters [51]. It is also involved in activation of NF-kB signaling, DNA synthesis, and cell cycle progression [52–54]. PRKD1 enhanced the self-renewal ability of BCSCs via the GSK3/β-catenin signaling pathway. MiR-34a targeted the PRKD1 and reduced breast cancer stemness through the GSK3/β-catenin signaling axis [55].
PI3K/AKT and MAPK signaling pathways
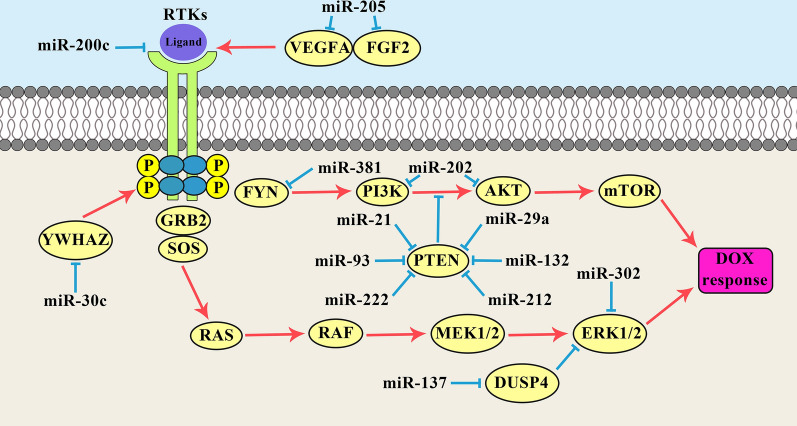
MiRNAs are involved in regulation of DOX response in BC through PI3K/AKT and MAPK signaling pathways (Fig. 2). The PI3K/AKT pathway has critical roles in regulation of cell proliferation and tumor progression. PI3K activates AKT that regulates various effectors such as CREB, p27, FOXO, and mTOR. Tyrosine kinase receptors and phosphatase and tensin homolog (PTEN) are known as the positive and negative regulators of the PI3K/AKT pathway, respectively. Glycogen Synthase Kinase 3β (GSK-3β) is a serine/threonine kinase involved in the PI3K/AKT signaling pathway [56]. It has been reported that miR-29a up regulation was associated with the p-AKT and p-GSK3β over expressions which promoted the DOX-resistance in breast tumor cells [57]. MiR-205 up regulation was significantly associated with sensitivity to TAC (docetaxol, doxorubicin plus cyclophosphamide). There were miR-205 down regulations in drug-resistant BC cell lines, however, ectopic expression of miR-205 resulted in DOX restoration and taxol sensitivity via inducing apoptosis in both of the aforementioned drug-resistant BC cells. Moreover, miR-205 suppressed the PI3K/AKT signaling by VEGFA and FGF2 down regulations which resulted in enhanced tumor cell apoptosis upon chemotherapy [58]. Another study showed that there were significant miR-202-5p up regulations in DOX resistant BC specimens and cell lines. MiR-2020-5p enhanced breast tumor cell proliferation and DOX-resistance through the PTEN/PI3K/AKT signaling pathway [59].
Fig. 2.
miRNAs have important roles in DOX response (resistance or sensitivity) of breast tumor cells via regulation of PI3K/AKT and MAPK signaling pathways
MiR-200c is an important regulator of EMT process through suppressing the E-cadherin transcriptional repressors (ZEB1 and ZEB2) [60–62]. Tropomyosin receptor kinase B (TrkB) is a tyrosine kinase receptor involved in cell differentiation, proliferation, and migration that functions through activation of the PI3K/AKT and MAP kinases [63]. The AKT phosphorylation plays an important role in promoting cell survival via phosphorylating and suppressing pro-apoptotic caspases and Bad [64, 65]. Bmi1 belongs to the polycomb-group protein family involved in self-renewal maintenance and inhibition of senescence [66–68]. It also down regulates the p19Arf that leads to p53 degradation by MDM2 [69]. It has been reported that the miR-200c increased DOX sensitivity via TrkB and Bmi1 inhibitions in breast tumor cells [70].
PTEN is a tumor suppressor that functions as a negative regulator of the AKT pathway, tumor cell migration, and apoptosis [71, 72]. It is a dual-specificity phosphatase that dephosphorylates lipid and protein substrates. It has been reported that there were miR-132 and miR-212 up-regulations in DOX resistant BC tumors and cell lines by PTEN inhibition. The miR-132 and miR-212 up regulations were also associated with NF-κB activation [73]. The FOXO is a family of transcription factors which are the downstream targets of AKT. It has been reported that the miR-222 was correlated with DOX resistance in BC cells through regulation of PTEN/AKT/FOXO1 axis [74]. MiR-21 also regulates the DOX-sensitivity in BC cells through targeting PTEN. There was a significant miR-21 up regulation in MCF-7/DOX cells compared with parental MCF-7 cells. PTEN was significantly suppressed in MCF-7/DOX cells compared to MCF-7 cells. Down regulation of miR-21 promoted the CASP3-mediated apoptosis in MCF-7/DOX cells which may be the possible explanation for increased sensitivity of MCF-7/DOX cells to DOX following transfection of miR-21 inhibitor [75]. It has been shown that there was significant miR-93 up regulation in ductal BC tissues compared with normal margins. MiR-93 markedly increased MCF-7 proliferation and survival after DOX treatment compared with control. Multidrug resistance-related genes (MDR, MRP, and BCRP) were also significantly up regulated in the MCF-7-miR-93 mimic cells. MiR-93 regulated DOX-resistance and EMT in BC cells through targeting PTEN [76]. It has been shown that miR-200c up regulated the E-cadherin through ZEB1 suppression. It also reduced AKT phosphorylation by PTEN up regulation that resulted in increased DOX sensitivity in breast tumor cells [77].
Mitogen-activated protein kinase (MAPK) is a signaling pathway that functions via sequential activation of a MAPK module including MAPKKK, MAPKK, and MAPK. There are various MAPKs such as ERK, JNK, and p38 involving in the cell growth, metabolism, and apoptosis [78]. The p38MAPK signaling has important role in apoptosis resistance in tumor cells [79]. YWHAZ encodes the 14–3-3f as an anti-apoptotic protein through the p38MAPK signaling pathway [80]. Moreover, YWHAZ has an important role in stabilization of EGFR, HER2, PKC, and b-catenin which are involved in signaling pathways, cell proliferation, and apoptosis [81–83]. It has been reported that the miR-30c increased DOX sensitivity in BC cells by targeting YWHAZ. There was significant miR-30c down regulation in DOX resistant breast cell lines [84]. DUSP4 belongs to the mitogen-activated protein kinase phosphatase (MKP) family that inhibits the MAPK signaling pathway [85]. It has been reported that miR-137 up regulation attenuated the DOX resistance in BC cells. MiR-137 also suppressed the EMT of breast tumor cells by DUSP4 targeting upon DOX treatment [86].
FYN is a non-receptor tyrosine kinase involved in cell growth, apoptosis, and motility [87]. It has been shown that there was miR-381 down regulation in DOX-resistant BC cells. MiR-381 re-sensitized DOX resistant BC cells via FYN inhibition and MAPK signaling inactivation [88]. During the chemo resistance process, tumor cells are able to develop resistance mechanisms by drug efflux, inactivation of detoxification enzymes, apoptosis regulation, tumor suppressor regulation, and DNA repair induction [89–91]. ABCB1 is a drug efflux transporter involved in multidrug resistance by increasing the intracellular levels of anticancer drugs. It has been shown that the miR-302 cluster reversed the BC cells drug resistance through ABCB1 down regulation. The miR-302 cluster also down regulated the MEKK1 as a member of the MAPK Kinase family. Therefore, miR-302 increased DOX sensitivity in BC cells by MEKK1 targeting and ABCB1 inhibition [92].
Apoptosis, cell cycle, and DNA repair
Bcl-2 interacting mediator of cell death (Bim) is a pro-apoptotic member of Bcl-2 protein family [93]. It is a key regulator of the intrinsic apoptosis pathways which directly initiates pro-apoptotic effect and induces cell apoptosis through interacting with all pro-apoptotic members of the Bcl-2 family [94, 95]. It has been reported that there were miR-181b and miR-222 up regulations in BC patients which were associated with DOX sensitivity through Bim targeting [96, 97]. The up regulation of myeloid cell leukemia 1 (MCL-1) as a pro-survival member of the Bcl-2 family, has been reported in various malignancies and shown to be correlated with a worse prognosis [98, 99]. MCL-1 enhances tumor cell survival while inhibiting their apoptosis through disrupting the normal activity of Noxa and other pro-apoptotic members of the BCL-2 family [100]. It has been shown that there was a significant miR-193b down regulation in the MCF-7/DOX resistant cells in comparison with its parental MCF-7 cells. MiR-193b increased the DOX sensitivity via MCL-1 targeting [101].
Survivin (BIRC5) belongs to the inhibitor of apoptosis (IAP) protein family. It was initially identified as a negative regulator of apoptosis which functions through inhibiting the caspase activation; however, it is now known that the survivin has a bi functional roles in survival and cell cycle [102]. Survivin exerts its anti-apoptotic activity through blocking the CASP9 function in a complex with hepatitis B X-interacting protein (HBXIP) thereby playing a crucial role in chemo resistance. It has been reported that there were significant miR-218 down regulation in drug-resistant breast cancer cell lines. MiR-218 restored the sensitivity of drug-resistant cell lines to doxorubicin and taxol through survivin targeting and apoptosis induction [103]. External antigens induce the proliferation of CD8+ and/or CD4+ helper cells that inhibit tumor progression [104].
The programmed death-ligand 1 (PD-L1) is an immune suppressor receptor expressed in T-cell membranes that reduces the proliferation of antigen-specific T-cell in the lymph nodes and increased regulatory T cells apoptosis during immune tolerance of cancer patients [105]. PD-L1 is also involved in increased chemo resistance in BC [106, 107]. It has been reported that there were miR-3609 down regulation and PD-L1 up regulation in DOX-resistant BC cell lines compared with the sensitive cells. Therefore, miR-3609 reversed DOX resistance by PD-L1 targeting and CD8+ T cells activation in BC cells. The miR-3609 down regulation was also correlated with poor prognosis in BC patients [108]. Peptidylprolyl isomerase A (PPIA) belongs to the peptidyl-prolyl cis/trans isomerase (PPIases) family and constitutes the cytosolic binding domain of cyclosporine A as an immunosuppressive agent. PPIA has key roles in various cellular processes such as cell proliferation, migration, apoptosis, immune regulation, and protein folding [109–111]. It has been reported that the miR-192-5p sensitized breast tumor cells to DOX and promotes apoptosis by the PPIA and BCL-2 targeting. MiR-192-5p also induced JNK-mediated apoptosis and up regulated the pro-apoptotic proteins such as CASP9 and BAD [112]. The RFWD2 is an E3 ubiquitin ligase that promotes tumor growth through p53 degradation [113]. It has been reported that there was miR-214 down regulation in BC tissues which was associated with longer disease free survival. MiR-214 increased apoptosis and DOX sensitivity in BC cells via RFWD2 targeting [114].
The findings indicated that the DOX treatment disrupted the normal cell cycle regulation by modulating the levels of miR-449 family and even its theoretically targeted genes (CDC25A, SIRT1, GMNN, E2F1, E2F3, BCL2, CDK2, and CCNE2). MiR-449 promoted DOX sensitivity by significant inhibition of cell cycle regulators including CDK2, E2F1, and E2F3 in BC cells [115]. Various mechanisms are involved in DNA repair in mammalian cells [116].
The flap endonuclease 1 (FEN1) is a critical factor during long-patch base excision repair process [117]. FEN1 has also a pivotal role during maturation of Okazaki fragments, telomere stability, and replication fork progression [118]. YY1 is a developmental transcription factor associated with cellular differentiation and proliferation [119, 120]. It has been reported that the miR-140 inhibited BC tumor progression and reduced DOX resistance through FEN1 down regulation and BER suppression. YY1 was also shown as a suppressor of FEN1 expression through miR-140 up regulation [121]. DOX-induced DNA damage activates DNA repair machinery in tumor cells. Therefore, aberrant DNA repair processes greatly influence cancer cells’ responsiveness to chemotherapy [122, 123]. It has been reported that the miR-30c was involved in DNA repair by regulation of REV1 and FANCF expressions. MiR-30c also promoted DOX-sensitivity in p53-mutant BC cells. DOX chemo resistance in p53-mutant BC cells was correlated with the miR-30c/FANCF/REV1-associated DNA damage response [124].
Transporters
ATP-binding cassette (ABC) family of transporters are drug efflux pumps involved in tumor cells MDR [125, 126]. Multidrug resistance protein 1 (MRP1) belongs to the superfamily of ABC transporters and is encoded by the ABCC1 gene. ABCC1 is correlated with the DOX resistance in MDR cancer cells [127, 128]. It has been observed that there was miR-134 down regulation in DOX-resistant breast tumor cells. MiR-134 significantly inhibited the cell proliferation and induced apoptosis in MCF-7/DOX cells via ABCC1 targeting [129]. Long non-coding RNAs (LncRNAs) are a class of non-coding RNAs (> 200 nucleotides length) with pivotal roles in tumor progression and chemo resistance [130]. It has been shown that there were linc00518 and ABCC1 up regulations in BC tissues and cell line. DOX-resistant MCF-7 cells (MCF-7/DOX) had also increased expression levels of linc00518 and ABCC1 compared to parental MCF-7 cell line. Linc00518 promoted MDR via regulating the miR-199a/ABCC1 axis in BC cells [131]. Another study has been reported that the miR-145 sensitized BC cells to DOX via ABCC1 targeting [132].
MiR-200 family is an essential regulator of EMT process which exerts its inhibitory function on tumor cell migration and invasion through down regulating E-cadherin transcriptional repressors such as ZEB1 and ZEB2 [133, 134]. It has been reported that there was a correlation between miR-200c down regulation and a poorer response of BC patients to neoadjuvant chemotherapy. Increased sensitivity of BC to epirubicin following transfection of miR-200c mimic was achieved at least in part via the inhibitory effect of miR-200c on ABCB1 expression. There were significant different levels of miR-200c expression between clinical responders and non-responders. DOX-resistant cells had significantly increased ABCB1 and decreased miR-200c levels compared with parental MCF-7 cells [135]. Another study also showed that the miR-451 increased DOX sensitivity of BC cells via ABCB1 targeting [136]. It has been reported that the miR-124-3p up regulation and ABCC4 inhibition increased DOX sensitivity in BC cells. There were significant correlations between tumor size, stage, and ABCC4 up regulation. ABCC4 down regulation inhibited the cell proliferation and migration, and induced DOX sensitivity. The miR-124-3p up regulation also significantly suppressed ABCB1 expression in MCF-7-DOX cells [137].
Uncoupling proteins (UCPs) are three structurally similar mitochondrial inner membrane transporters (UCP1/2/3) belong to the mitochondrial anion transporters family [138]. UCP-2 has a ubiquitous tissue expression and is implicated in cellular energy expenditures, mitochondrial ROS regulation, and ATP synthesis [139–142]. It has been reported that the miR-133a reduced DOX resistance in BC cells via UCP-2 targeting [143].
TGF-β and JAK/STAT signaling pathways
Transforming growth factor beta (TGFβ) signaling is a pivotal pathway involved in cell growth, cell differentiation, and apoptosis. This pathway is triggered by TGFβ ligands and receptors, which activates and translocates the SMAD proteins into the nucleus where they functions as transcription factors. SMAD4 is a mediator of TGF-β signaling pathway involved in the MDR of different tumors [144, 145]. It has been reported that there was significant miR-574 up regulation in Dox-resistant MCF-7 cells in comparison with parental cells. There were also increased levels of miR-574 in blood samples of advanced BC patients following chemotherapy. MiR-574 induced DOX resistance in BC cells through SMAD4 targeting [146]. SMAD3 is essential for the TGF-β-induced EMT and mediates the mammary epithelial cells invasion [147]. It has been reported that there was a significant miR-489 down regulation in DOX-resistant BC cells. MiR-489/SMAD3 axis regulated the DOX-resistance of breast tumor cells via EMT process [148]. PBLD is a negative regulator of TGF-β1-induced EMT during tumor progression [149]. It has been reported that there was significant reduced levels of circKDM4C expressions in BC samples which was inversely correlated with chemo resistance through the miR-548p regulation. There was also a significant direct association between circKDM4C expression and overall survival. CircKDM4C reduced the BC progression and DOX-resistance via miR-548p sponging and PBLD activating [150].
BCSCs are a sub population of tumor cancers mainly associated with tumor relapse, chemo resistance, and poor prognosis [151]. Therefore, elimination of BCSCs seems to be effective for the solving of clinical issues like drug resistance and tumor recurrence [152]. STAT family of transcription factors regulates the multiple cellular processes. Hypoxia-inducible factor-1 (HIF-1) is the main transcription factor implicated in cellular response to hypoxia. It also regulates the different genes associated with tumor aggressiveness [153]. HIF-1 signaling is critical for the activation of NOTCH pathway that affects the EMT process [154]. It has been reported that the miR-124 was involved in DOX-resistance of BCSCs via STAT3/HIF-1 signaling pathway. DOX-resistant BCSCs showed increased levels of STAT3. STAT3 up regulated the ALDH1, OCT4, and SOX2. MiR-124 reduced the DOX-resistance in BCSCs through modulation of STAT3/HIF-1 signaling pathway [155]. Role of miRNAs in regulation of DOX response in BC through TGFb and JAK/STAT signaling pathways is illustrated in Fig. 3.
Fig. 3.
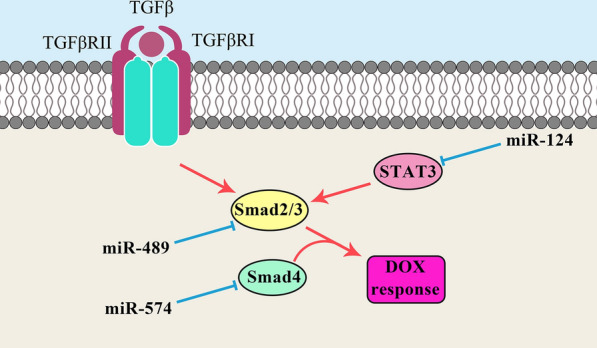
Roles of miRNAs in regulation of DOX response (resistance or sensitivity) in breast tumor cells through TGFb and JAK/STAT signaling pathways
Enzymes and structural proteins
Osteopontin (OPN) is a hydrophilic non-collagenous phosphorylated glycoprotein which is present in ECM and mediates the multiple biological functions. OPN is recognized as secreted (sOPN) or intracellular (iOPN) proteins [156]. Secreted OPN functions via interaction with the cell surface receptors including the integrin and CD44 families [157]. OPN has key roles in the diverse pathophysiological processes such as immune-mediated and inflammatory diseases as well as tissue and bone remodeling [158, 159] and is also implicated in tumor progression, metastasis, and angiogenesis [160]. It has been reported that there was a significant miR-181c down regulation in BC cells. MiR-181c suppressed the breast tumor cell proliferation and invasion while promoted DOX sensitivity. There was an inverse correlation between the miR-181c and OPN expression levels which was associated with the DOX response, metastasis, and BC patients’ overall and disease-free survival. Moreover, miR-181c inhibited the EMT of BC cells via vimentin and N-cadherin down regulations and E-cadherin up regulation [161].
Anterior gradient 2 (AGR2) belongs to the protein disulfide isomerases (PDIs) family which plays an important role in mammary epithelial proliferation, lobuloalveolar development, and protein folding [162, 163]. AGR2 up regulation early in tumorigenesis or in response to anti-hormone treatment is associated with intrinsic or acquired resistance to therapies in ER-positive breast cancers, respectively [164]. DOX-resistant BC cells were observed to have AGR2 over expression. Up regulated and down regulated AGR2 were correlated with increased and reduced DOX-sensitivity, respectively. It was also found that miR-135b-5p enhanced the DOX-sensitivity of BC cells through AGR2 targeting. MiR-135b-5p/AGR2 axis was suggested as an important pathway responsible for DOX-sensitivity in BC cells [165].
Nicotinamide phosphoribosyl transferase (NAMPT) as an important factor involved in NAD synthesis has pivotal roles in the immune response and metabolism [166, 167]. NAD is a substrate for the sirtuin deacetylase in transcriptional regulation of other genes [168]. NAMPT also promotes BC cell proliferation through stimulation of ER activity [169]. Moreover, NAMPT up regulation can be associated with DOX resistance in BC patients [170]. It has been reported that there was significant miR-154 down regulation in BC cell lines compared with normal mammary cells. There was an inverse association between the NAMPT and miR-154 expressions in BC cells. MiR-154 sensitized the BC cells to DOX through NAMPT targeting [171]. Stathmin1 (STMN1) is a microtubule-destabilizing factor involved in the regulation of cytoskeleton and microtubule dynamics [172]. STMN1 enhances the microtubule depolymerization via sequestering free tubulins [173, 174]. MiR-770 was significantly down regulated in chemo-resistant BC tissues. It also increased the DOX-sensitivity through STMN1 targeting [175].
Conclusions
DOX is one of the common first line chemotherapeutic drugs used for BC treatment; however there is a high ratio of DOX resistance among the BC patients. Since, DOX has severe side effects; it is required to distinguish the non DOX-responders from responders and also clarify the molecular mechanisms involved in DOX resistance to provide novel efficient therapeutic modalities to improve the clinical outcomes of BC patients. MiRNAs are important factors involved in drug resistance through regulation of drug efflux, DNA repair, cell cycle, and signaling pathways. They are also non-invasive and more stable factors compared with mRNAs. This review highlights the miRNAs as pivotal regulators of DOX resistance in breast tumor cells. Moreover, present review paves the way of introducing a non-invasive panel of prediction markers for DOX response among BC patients.
Acknowledgements
Not applicable.
Abbreviations
- BC
Breast cancer
- DOX
Doxorubicin
- miRNAs
MicroRNAs
- PR
Progesterone receptor
- ER
Estrogen receptor
- TNBC
Triple-negative breast cancer
- ADR
Adriamycin
- MDR
Multidrug resistance
- MRP1
Multiple resistance protein-1
- UTR
Untranslated region
- BCSCs
Breast cancer stem cells
- FZD
Frizzled
- ECM
Extra cellular matrix
- EMT
Epithelial mesenchymal transition
- Daam1
Dishevelled-associated activator of morphogenesis 1
- CSCs
Cancer stem cells
- PRKD1
Protein kinase D1
- PTEN
Phosphatase and tensin homolog
- GSK-3β
Glycogen Synthase Kinase 3β
- TrkB
Tropomyosin receptor kinase B
- MAPK
Mitogen-activated protein kinase
- MKP
Mitogen-activated protein kinase phosphatase
- Bim
Bcl-2 interacting mediator of cell death
- MCL-1
Myeloid cell leukemia 1
- IAP
Inhibitor of apoptosis
- HBXIP
Hepatitis B X-interacting protein
- PD-L1
Programmed death-ligand 1
- PPIA
Peptidylprolyl isomerase A
- PPIases
Peptidyl-prolyl cis/trans isomerase
- ABC
ATP-binding cassette
- MRP1
Multidrug resistance protein 1
- LncRNAs
Long non-coding RNAs
- UCPs
Uncoupling proteins
- TGFβ
Transforming growth factor beta
- HIF-1
Hypoxia-inducible factor-1
- OPN
Osteopontin
- sOPN
Secreted Osteopontin
- iOPN
Intracellular Osteopontin
- AGR2
Anterior gradient 2
- PDIs
Protein disulfide isomerases
- NAMPT
Nicotinamide phosphoribosyl transferase
- STMN1
Stathmin1
Authors' contributions
ASZ and MA were involved in search strategy and drafting. MM supervised the project and revised and edited the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Amir Sadra Zangouei, Email: zangoueias961@mums.ac.ir.
Maliheh Alimardani, Email: Alimardanim981@mums.ac.ir.
Meysam Moghbeli, Email: moghbelim@mums.ac.ir, Email: Meysam_moghbeli@yahoo.com.
References
- [1].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- [3].Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6(12):718. doi: 10.1038/nrclinonc.2009.166. [DOI] [PubMed] [Google Scholar]
- [4].Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni LJ. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol. 2016;13(11):674. doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Anders C, Carey LAJO. Understanding and treating triple-negative breast cancer. 2008;22(11):1233. [PMC free article] [PubMed] [Google Scholar]
- [7].O’Driscoll L, Clynes MJC. Biomarkers and multiple drug resistance in breast cancer. Current Cancer Drug Targets. 2006;6(5):365–84. doi: 10.2174/156800906777723958. [DOI] [PubMed] [Google Scholar]
- [8].Austreid E, Lonning PE, Eikesdal HPJE. The emergence of targeted drugs in breast cancer to prevent resistance to endocrine treatment and chemotherapy. Exp Opin Pharmacother. 2014;15(5):681–700. doi: 10.1517/14656566.2014.885952. [DOI] [PubMed] [Google Scholar]
- [9].Ali SM, Harvey H, Lipton AJCO, Research R Metastatic breast cancer: overview of treatment. Clin Orthopaedics Relat Res. 2003;415:S132–S137. doi: 10.1097/01.blo.0000092981.12414.7b. [DOI] [PubMed] [Google Scholar]
- [10].Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31(7):860–7. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- [11].Naci D, El Azreq M-A, Chetoui N, Lauden L, Sigaux F, Charron D, et al. α2β1 integrin promotes chemoresistance against doxorubicin in cancer cells through extracellular signal-regulated kinase (ERK) J Biol Chem. 2012;287(21):17065–76. doi: 10.1074/jbc.M112.349365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lee AJ, Endesfelder D, Rowan AJ, Walther A, Birkbak NJ, Futreal PA, et al. Chromosomal instability confers intrinsic multidrug resistance. Can Res. 2011;71(5):1858–70. doi: 10.1158/0008-5472.CAN-10-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- [14].Liang Y, Ridzon D, Wong L, Chen CJ. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics. 2007;8(1):166. doi: 10.1186/1471-2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lowery AJ, Miller N, Devaney A, McNeill RE, Davoren PA, Lemetre C, et al. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neureceptor status in breast cancer. Breast Cancer Res. 2009;11(3):R27. doi: 10.1186/bcr2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dvinge H, Git A, Gräf S, Salmon-Divon M, Curtis C, Sottoriva A, et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature. 2013;497(7449):378–82. doi: 10.1038/nature12108. [DOI] [PubMed] [Google Scholar]
- [17].Ebert MS, Sharp PA. Emerging roles for natural microRNA sponges. Curr Biol. 2010;20(19):R858–61. doi: 10.1016/j.cub.2010.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Abbaszadegan MR, Riahi A, Forghanifard MM, Moghbeli M. WNT and NOTCH signaling pathways as activators for epidermal growth factor receptor in esophageal squamous cell carcinoma. Cell Mol Biol Lett. 2018;23:42. doi: 10.1186/s11658-018-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Moghbeli M, Abbaszadegan MR, Golmakani E, Forghanifard MM. Correlation of Wnt and NOTCH pathways in esophageal squamous cell carcinoma. J Cell Commun Signal. 2016;10(2):129–35. doi: 10.1007/s12079-016-0320-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Moghbeli M, Sadrizadeh A, Forghanifard MM, Mozaffari HM, Golmakani E, Abbaszadegan MR. Role of Msi1 and PYGO2 in esophageal squamous cell carcinoma depth of invasion. J Cell Commun Signal. 2016;10(1):49–53. doi: 10.1007/s12079-015-0314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wolfson B, Eades G, Zhou QJWJ. Roles of microRNA-140 in stem cell-associated early stage breast cancer. World J Stem Cells. 2014;6(5):591. doi: 10.4252/wjsc.v6.i5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee MY, Lim HW, Lee SH, Han HJJ. Smad, PI3K/Akt, and Wnt-dependent signaling pathways are involved in BMP-4-induced ESC self-renewal. Stem Cells. 2009;27(8):1858–68. doi: 10.1002/stem.124. [DOI] [PubMed] [Google Scholar]
- [23].Prakash N, Wurst WJND. A Wnt signal regulates stem cell fate and differentiation in vivo. Neurodegener Dis. 2007;4(4):333–8. doi: 10.1159/000101891. [DOI] [PubMed] [Google Scholar]
- [24].Wu D, Zhang J, Lu Y, Bo S, Li L, Wang L, et al. miR-140–5p inhibits the proliferation and enhances the efficacy of doxorubicin to breast cancer stem cells by targeting Wnt1. Cancer Gene Ther. 2019;26(3):74–82. doi: 10.1038/s41417-018-0035-0. [DOI] [PubMed] [Google Scholar]
- [25].Barczyk M, Carracedo S, Gullberg DJC, research t Integrins. Cell Tissue Res. 2010;339(1):269. doi: 10.1007/s00441-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hynes ROJ. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- [27].Sheldrake HM, Patterson LHJC. Function and antagonism of β. Curr Cancer Drug Targets. 2009;9(4):519–40. doi: 10.2174/156800909788486713. [DOI] [PubMed] [Google Scholar]
- [28].Galliher AJ, Schiemann WPJB. β 3 integrin and Src facilitate transforming growth factor-β mediated induction of epithelial-mesenchymal transition in mammary epithelialcells. Breast Cancer Res. 2006;8(4):R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima MJ. The relationship between bone metastasis from human breast cancer and integrin αvβ3 expression. Anticancer Res. 2005;25(1A):79–83. [PubMed] [Google Scholar]
- [30].Wang R, Li ZQ, Han X, Li BL, Mi XY, Sun LM, et al. Integrin β3 and its ligand regulate the expression of uPA through p38 MAPK in breast cancer. APMIS. 2010;118(12):909–17. doi: 10.1111/j.1600-0463.2010.02687.x. [DOI] [PubMed] [Google Scholar]
- [31].Cheng S, Huang Y, Lou C, He Y, Zhang Y, Zhang QJC, et al. FSTL1 enhances chemoresistance and maintains stemness in breast cancer cells via integrin β3/Wnt signaling under miR-137 regulation. Cancer Biol Ther. 2019;20(3):328–37. doi: 10.1080/15384047.2018.1529101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107(7):843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- [33].Zhu Y, Tian Y, Du J, Hu Z, Yang L, Liu J, et al. Dvl2-dependent activation of Daam1 and RhoA regulates Wnt5a-induced breast cancer cell migration. PLoS ONE. 2012;7(5):e37823. doi: 10.1371/journal.pone.0037823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jaiswal R, Breitsprecher D, Collins A, Correa IR, Jr, Xu MQ, Goode BL. The formin Daam1 and fascin directly collaborate to promote filopodia formation. Curr Biol. 2013;23(14):1373–9. doi: 10.1016/j.cub.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lu Y, Zhang Y, Pan MH, Kim NH, Sun SC, Cui XS. Daam1 regulates fascin for actin assembly in mouse oocyte meiosis. Cell Cycle. 2017;16(14):1350–6. doi: 10.1080/15384101.2017.1325045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xiong H, Yan T, Zhang W, Shi F, Jiang X, Wang X, et al. miR-613 inhibits cell migration and invasion by downregulating Daam1 in triple-negative breast cancer. Cell Signal. 2018;44:33–42. doi: 10.1016/j.cellsig.2018.01.013. [DOI] [PubMed] [Google Scholar]
- [37].Kotiyal S, Bhattacharya SJB, communications br Breast cancer stem cells, EMT and therapeutic targets. Biochem Biophys Res Commun. 2014;453(1):112–6. doi: 10.1016/j.bbrc.2014.09.069. [DOI] [PubMed] [Google Scholar]
- [38].Shibue T, Weinberg RAJN. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mitra A, Mishra L, Li SJO. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget. 2015;6(13):10697. doi: 10.18632/oncotarget.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Moghbeli M, Forghanifard MM, Sadrizadeh A, Mozaffari HM, Golmakani E, Abbaszadegan MR. Role of Msi1 and MAML1 in regulation of notch signaling pathway in patients with esophageal squamous cell carcinoma. J Gastrointest Cancer. 2015;46(4):365–9. doi: 10.1007/s12029-015-9753-9. [DOI] [PubMed] [Google Scholar]
- [41].Moghbeli M, Mosannen Mozaffari H, Memar B, Forghanifard MM, Gholamin M, Abbaszadegan MR. Role of MAML1 in targeted therapy against the esophageal cancer stem cells. J Transl Med. 2019;17(1):126. doi: 10.1186/s12967-019-1876-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li XJ, Ji MH, Zhong SL, Zha QB, Xu JJ, Zhao JH, et al. MicroRNA-34a modulates chemosensitivity of breast cancer cells to adriamycin by targeting Notch1. Arch Med Res. 2012;43(7):514–21. doi: 10.1016/j.arcmed.2012.09.007. [DOI] [PubMed] [Google Scholar]
- [43].Kohler EE, Cowan CE, Chatterjee I, Malik AB, Wary KK. NANOG induction of fetal liver kinase-1 (FLK1) transcription regulates endothelial cell proliferation and angiogenesis. Blood. 2011;117(5):1761–9. doi: 10.1182/blood-2010-07-295261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Lee MT, Bonneau AR, Takacs CM, Bazzini AA, DiVito KR, Fleming ES, et al. Nanog, Pou5f1 and SoxB1 activate zygotic gene expression during the maternal-to-zygotic transition. Nature. 2013;503(7476):360–4. doi: 10.1038/nature12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Ding Y, Yu AQ, Li CL, Fang J, Zeng Y, Li DS. TALEN-mediated Nanog disruption results in less invasiveness, more chemosensitivity and reversal of EMT in Hela cells. Oncotarget. 2014;5(18):8393–401. doi: 10.18632/oncotarget.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Iv Santaliz-Ruiz LE, Xie X, Old M, Teknos TN, Pan Q. Emerging role of nanog in tumorigenesis and cancer stem cells. Int J Cancer. 2014;135(12):2741–8. doi: 10.1002/ijc.28690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hu SH, Wang CH, Huang ZJ, Liu F, Xu CW, Li XL, et al. miR-760 mediates chemoresistance through inhibition of epithelial mesenchymal transition in breast cancer cells. Eur Rev Med Pharmacol Sci. 2016;20(23):5002–8. [PubMed] [Google Scholar]
- [48].Park EY, Chang E, Lee EJ, Lee H-W, Kang H-G, Chun K-H, et al. Targeting of miR34a–NOTCH1 axis reduced breast cancer stemness and chemoresistance. Can Res. 2014;74(24):7573–82. doi: 10.1158/0008-5472.CAN-14-1140. [DOI] [PubMed] [Google Scholar]
- [49].Schwarzenbacher D, Balic M, Pichler M. The role of microRNAs in breast cancer stem cells. Int J Mol Sci. 2013;14(7):14712–14723. doi: 10.3390/ijms140714712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Yu F, Jiao Y, Zhu Y, Wang Y, Zhu J, Cui X, et al. MicroRNA 34c gene down-regulation via DNA methylation promotes self-renewal and epithelial-mesenchymal transition in breast tumor-initiating cells. J Biol Chem. 2012;287(1):465–73. doi: 10.1074/jbc.M111.280768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fu Y, Rubin CSJE. Protein kinase D: coupling extracellular stimuli to the regulation of cell physiology. EMBO Rep. 2011;12(8):785–796. doi: 10.1038/embor.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Storz P, Toker AJTE. Protein kinase D mediates a stress-induced NF-κB activation and survival pathway. EMBO J. 2003;22(1):109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sinnett-Smith J, Zhukova E, Hsieh N, Jiang X, Rozengurt EJJ. Protein kinase D potentiates DNA synthesis induced by Gq-coupled receptors by increasing the duration of ERK signaling in swiss 3T3 cells. J Biol Chem. 2004;279(16):16883–93. doi: 10.1074/jbc.M313225200. [DOI] [PubMed] [Google Scholar]
- [54].Harikumar KB, Kunnumakkara AB, Ochi N, Tong Z, Deorukhkar A, Sung B, et al. A novel small-molecule inhibitor of protein kinase D blocks pancreatic cancer growth in vitro and in vivo. Mol Cancer Ther. 2010;9(5):1136–1146. doi: 10.1158/1535-7163.MCT-09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Kim DY, Park EY, Chang E, Kang H-G, Koo Y, Lee EJ, et al. A novel miR-34a target, protein kinase D1, stimulates cancer stemness and drug resistance through GSK3/β-catenin signaling in breast cancer. Oncotarget. 2016;7(12):14791. doi: 10.18632/oncotarget.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Zhang X, Zhong S, Xu Y, Yu D, Ma T, Chen L, et al. MicroRNA-3646 contributes to docetaxel resistance in human breast cancer cells by GSK-3β/β-catenin signaling pathway. PLoS ONE. 2016;11(4):e0153194. doi: 10.1371/journal.pone.0153194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Shen H, Li L, Yang S, Wang D, Zhong S, Zhao J, et al. MicroRNA-29a contributes to drug-resistance of breast cancer cells to adriamycin through PTEN/AKT/GSK3β signaling pathway. Gene. 2016;593(1):84–90. doi: 10.1016/j.gene.2016.08.016. [DOI] [PubMed] [Google Scholar]
- [58].Hu Y, Qiu Y, Yagüe E, Ji W, Liu J, Zhang JJC, et al. miRNA-205 targets VEGFA and FGF2 and regulates resistance to chemotherapeutics in breast cancer. Cell Death Dis. 2016;7(6):e2291. doi: 10.1038/cddis.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Liu T, Guo J, Zhang XJC. MiR-202–5p/PTEN mediates doxorubicin-resistance of breast cancer cells via PI3K/Akt signaling pathway. Cancer Biol Ther. 2019;20(7):989–998. doi: 10.1080/15384047.2019.1591674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- [61].Hurteau GJ, Carlson JA, Spivack SD, Brock GJJC. Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Can Re. 2007;67(17):7972–6. doi: 10.1158/0008-5472.CAN-07-1058. [DOI] [PubMed] [Google Scholar]
- [62].Park S-M, Gaur AB, Lengyel E, Peter MEJG. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Segal RA. Selectivity in neurotrophin signaling: theme and variations. Annu Rev Neurosci. 2003;26(1):299–330. doi: 10.1146/annurev.neuro.26.041002.131421. [DOI] [PubMed] [Google Scholar]
- [64].Altomare DA, Testa JRJO. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- [65].Kim D, Chung JJJ, Biology M. Akt: versatile mediator of cell survival and beyond. BMB Rep. 2002;35(1):106–115. doi: 10.5483/bmbrep.2002.35.1.106. [DOI] [PubMed] [Google Scholar]
- [66].Gil J, Bernard D, Peters GJD. Role of polycomb group proteins in stem cell self-renewal and cancer. DNA Cell Biol. 2005;24(2):117–25. doi: 10.1089/dna.2005.24.117. [DOI] [PubMed] [Google Scholar]
- [67].Rajasekhar VK, Begemann MJSC. Concise review: roles of polycomb group proteins in development and disease: a stem cell perspective. Stem Cells. 2007;25(10):2498–2510. doi: 10.1634/stemcells.2006-0608. [DOI] [PubMed] [Google Scholar]
- [68].Sparmann A, van Lohuizen M. Polycomb silencers control cell fate, development and cancer. Nat Rev Cancer. 2006;6(11):846–856. doi: 10.1038/nrc1991. [DOI] [PubMed] [Google Scholar]
- [69].Park I-K, Morrison SJ, Clarke M. Bmi1, stem cells, and senescence regulation. J Clin Invest. 2004;113(2):175–179. doi: 10.1172/JCI20800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kopp F, Oak PS, Wagner E, Roidl AJP. miR-200c sensitizes breast cancer cellsto doxorubicin treatment by decreasing TrkB and Bmi1 expression. PLoS ONE. 2012;7(11):e50469. doi: 10.1371/journal.pone.0050469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Carnero A, Paramio JM. The PTEN/PI3K/AKT pathway in vivo cancer mouse models. Front Oncol. 2014;4:252. doi: 10.3389/fonc.2014.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Li Z, Wang L, Zhang W, Fu Y, Zhao H, Hu Y, et al. Restoring E-cadherin-mediated cell–cell adhesion increases PTEN protein level and stability in human breast carcinoma cells. Biochem Biophys Res Commun. 2007;363(1):165–70. doi: 10.1016/j.bbrc.2007.08.154. [DOI] [PubMed] [Google Scholar]
- [73].Xie M, Fu Z, Cao J, Liu Y, Wu J, Li Q, et al. MicroRNA-132 and microRNA-212 mediate doxorubicin resistance by down-regulating the PTEN-AKT/NF-kappaB signaling pathway in breast cancer. Biomed Pharmacother. 2018;102:286–94. doi: 10.1016/j.biopha.2018.03.088. [DOI] [PubMed] [Google Scholar]
- [74].Shen H, Wang D, Li L, Yang S, Chen X, Zhou S, et al. MiR-222 promotes drug-resistance of breast cancer cells to adriamycin via modulation of PTEN/Akt/FOXO1 pathway. Gene. 2017;596:110–8. doi: 10.1016/j.gene.2016.10.016. [DOI] [PubMed] [Google Scholar]
- [75].Wang Z-X, Lu B-B, Wang H, Cheng Z-X, Yin YMJA. MicroRNA-21 modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch Med Res. 2011;42(4):281–90. doi: 10.1016/j.arcmed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- [76].Chu S, Liu G, Xia P, Chen G, Shi F, Yi T, et al. miR-93 and PTEN: key regulators of doxorubicin-resistance and EMT in breast cancer. Oncol Rep. 2017;38(4):2401–7. doi: 10.3892/or.2017.5859. [DOI] [PubMed] [Google Scholar]
- [77].Chen Y, Sun Y, Chen L, Xu X, Zhang X, Wang B, et al. miRNA-200c increases the sensitivity of breast cancer cells to doxorubicin throughthe suppression of E-cadherin-mediated PTEN/Akt signaling. Mol Med Rep. 2013;7(5):1579–84. doi: 10.3892/mmr.2013.1403. [DOI] [PubMed] [Google Scholar]
- [78].Grossi V, Peserico A, Tezil T, Simone C. p38alpha MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol. 2014;20(29):9744–58. doi: 10.3748/wjg.v20.i29.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, et al. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther. 2007;6(2):178–84. doi: 10.4161/cbt.6.2.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Tzivion G, Gupta VS, Kaplun L, Balan V. 14-3-3 proteins as potential oncogenes. Semin Cancer Biol. 2006;16(3):203–13. doi: 10.1016/j.semcancer.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [81].Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, et al. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. J Biol Chem. 2004;279(24):25549–61. doi: 10.1074/jbc.M314182200. [DOI] [PubMed] [Google Scholar]
- [82].Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9(2):102–14. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- [83].McPherson RA, Harding A, Roy S, Lane A, Hancock JF. Interactions of c-Raf-1 with phosphatidylserine and 14-3-3. Oncogene. 1999;18(26):3862–9. doi: 10.1038/sj.onc.1202730. [DOI] [PubMed] [Google Scholar]
- [84].Fang Y, Shen H, Cao Y, Li H, Qin R, Chen Q, et al. Involvement of miR-30c in resistance to doxorubicin by regulating YWHAZ in breast cancer cells. Braz J Med Biol Res. 2014;47(1):60–9. doi: 10.1590/1414-431X20133324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Groschl B, Bettstetter M, Giedl C, Woenckhaus M, Edmonston T, Hofstadter F, et al. Expression of the MAP kinase phosphatase DUSP4 is associated with microsatellite instability in colorectal cancer (CRC) and causes increased cell proliferation. Int J Cancer. 2013;132(7):1537–46. doi: 10.1002/ijc.27834. [DOI] [PubMed] [Google Scholar]
- [86].Du F, Yu L, Wu Y, Wang S, Yao J, Zheng X, et al. miR-137 alleviates doxorubicin resistance in breast cancer through inhibition of epithelial-mesenchymal transition by targeting DUSP4. Cell Death Dis. 2019;10(12):1–10. doi: 10.1038/s41419-019-2164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Saito YD, Jensen AR, Salgia R, Posadas EM. Fyn: a novel molecular target in cancer. Cancer. 2010;116(7):1629–37. doi: 10.1002/cncr.24879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Mi H, Wang X, Wang F, Li L, Zhu M, Wang N, et al. miR-381 induces sensitivity of breast cancer cells to doxorubicin by inactivation of MAPK signaling via FYN. Eur J Pharmacol. 2018;839:66–75. doi: 10.1016/j.ejphar.2018.09.024. [DOI] [PubMed] [Google Scholar]
- [89].Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46(3):308–16. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- [90].Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- [91].Martin HL, Smith L, Tomlinson DC. Multidrug-resistant breast cancer: current perspectives. Breast Cancer (Dove Med Press). 2014;6:1–13. doi: 10.2147/BCTT.S37638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Zhao L, Wang Y, Jiang L, He M, Bai X, Yu L, et al. MiR-302a/b/c/d cooperatively sensitizes breast cancer cells to adriamycin via suppressing P-glycoprotein(P-gp) by targeting MAP/ERK kinase kinase 1 (MEKK1) J Exp Clin Cancer Res. 2016;35:25. doi: 10.1186/s13046-016-0300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kivits RA, Furneaux C. BIM: enabling sustainability and asset management through knowledge management. Sci World J. 2013 doi: 10.1155/2013/983721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Youle RJ, Strasser AJN. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- [95].O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17(2):384–95. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zheng Y, Lv X, Wang X, Wang B, Shao X, Huang Y, et al. MiR-181b promotes chemoresistance in breast cancer by regulating Bim expression. Oncol Rep. 2016;35(2):683–90. doi: 10.3892/or.2015.4417. [DOI] [PubMed] [Google Scholar]
- [97].Dai H, Xu L-y, Qian Q, Zhu Q-w, Chen W-x. MicroRNA-222 promotes drug resistance to doxorubicinin breast cancer via regulation of miR-222/bim pathway. Biosci Rep. 2019;39(7):BSR20190650. doi: 10.1042/BSR20190650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Luo L, Zhang T, Liu H, Lv T, Yuan D, Yao Y, et al. MiR-101 and Mcl-1 in non-small-cell lung cancer: expression profile and clinical significance. Med Oncol. 2012;29(3):1681–6. doi: 10.1007/s12032-011-0085-8. [DOI] [PubMed] [Google Scholar]
- [99].Zhang T, Zhao C, Luo L, Zhao H, Cheng J, Xu FJ. The expression of Mcl-1 in human cervical cancer and its clinical significance. Med Oncol. 2012;29(3):1985–91. doi: 10.1007/s12032-011-0005-y. [DOI] [PubMed] [Google Scholar]
- [100].Geserick P, Wang J, Feoktistova M, Leverkus MJ. The ratio of Mcl-1 and Noxa determines ABT737 resistance in squamous cell carcinoma of the skin. Cell Death Dis. 2014;5(9):e1412. doi: 10.1038/cddis.2014.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Long J, Ji Z, Jiang K, Wang Z, Meng GJB. miR-193b modulates resistance to doxorubicin in human breast cancer cells by downregulating MCL-1. BioMed Res Int. 2015 doi: 10.1155/2015/373574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Li F, Ambrosini G, Chu EY, Plescia J, Tognin S, Marchisio PC, et al. Control of apoptosis and mitotic spindle checkpoint by survivin. Nature. 1998;396(6711):580–584. doi: 10.1038/25141. [DOI] [PubMed] [Google Scholar]
- [103].Hu Y, Xu K, Yagüe EJB. miR-218targets survivin and regulates resistance to chemotherapeutics in breast cancer. Breast Cancer Res Treat. 2015;151(2):269–80. doi: 10.1007/s10549-015-3372-9. [DOI] [PubMed] [Google Scholar]
- [104].Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425(6957):516–21. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- [105].Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580–7. doi: 10.1158/1078-0432.CCR-12-1362. [DOI] [PubMed] [Google Scholar]
- [106].Carosella ED, Ploussard G, LeMaoult J, Desgrandchamps F. A systematic review of immunotherapy in urologic cancer: evolving roles for targeting of CTLA-4, PD-1/PD-L1, and HLA-G. Eur Urol. 2015;68(2):267–79. doi: 10.1016/j.eururo.2015.02.032. [DOI] [PubMed] [Google Scholar]
- [107].Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol. 2017;14(4):203–20. doi: 10.1038/nrclinonc.2016.168. [DOI] [PubMed] [Google Scholar]
- [108].Li D, Wang X, Yang M, Kan Q, Duan Z. miR3609 sensitizes breast cancer cells to adriamycin by blocking the programmed death-ligand 1 immune checkpoint. Exp Cell Res. 2019;380(1):20–8. doi: 10.1016/j.yexcr.2019.03.025. [DOI] [PubMed] [Google Scholar]
- [109].Yang H, Li M, Chai H, Yan S, Lin P, Lumsden AB, et al. Effects of cyclophilin A on cell proliferation and gene expressions in human vascular smooth muscle cells and endothelial cells1. J Surg Res. 2005;123(2):312–9. doi: 10.1016/j.jss.2004.08.026. [DOI] [PubMed] [Google Scholar]
- [110].Kim S-H, Lessner SM, Sakurai Y, Galis ZS. Cyclophilin A as a novel biphasic mediatorof endothelial activation and dysfunction. Am J Pathol. 2004;164(5):1567–74. doi: 10.1016/S0002-9440(10)63715-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Colgan J, Asmal M, Neagu M, Yu B, Schneidkraut J, Lee Y, et al. Cyclophilin A regulates TCR signal strength in CD4+ T cells via a proline-directed conformational switch in Itk. Immunity. 2004;21(2):189–201. doi: 10.1016/j.immuni.2004.07.005. [DOI] [PubMed] [Google Scholar]
- [112].Zhang Y, He Y, Lu LL, Zhou ZY, Wan NB, Li GP, et al. miRNA-192-5p impacts the sensitivity of breast cancer cells to doxorubicin via targeting peptidylprolyl isomerase A. Kaohsiung J Med Sci. 2019;35(1):17–23. doi: 10.1002/kjm2.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Dornan D, Wertz I, Shimizu H, Arnott D, Frantz GD, Dowd P, et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429(6987):86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- [114].Zhang J, Su B, Gong C, Xi Q, Chao T. miR-214 promotes apoptosis and sensitizes breast cancer cells to doxorubicin by targeting the RFWD2-p53 cascade. Biochem Biophys Res Commun. 2016;478(1):337–42. doi: 10.1016/j.bbrc.2016.07.054. [DOI] [PubMed] [Google Scholar]
- [115].Tormo E, Ballester S, Adam-Artigues A, Burgués O, Alonso E, Bermejo B, et al. The miRNA-449 family mediates doxorubicin resistance in triple-negative breast cancer by regulating cell cycle factors. Sci Rep. 2019;9(1):1–14. doi: 10.1038/s41598-019-41472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].O'Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60(4):547–60. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- [117].Zheng L, Jia J, Finger LD, Guo Z, Zer C, Shen B. Functional regulation of FEN1 nuclease and its link to cancer. Nucleic Acids Res. 2011;39(3):781–94. doi: 10.1093/nar/gkq884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Balakrishnan L, Bambara RA. Flap endonuclease 1. Annu Rev Biochem. 2013;82:119–38. doi: 10.1146/annurev-biochem-072511-122603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25(8):1125–42. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- [120].Wang CC, Tsai MF, Hong TM, Chang GC, Chen CY, Yang WM, et al. The transcriptional factor YY1 upregulates the novel invasion suppressor HLJ1 expression and inhibits cancer cell invasion. Oncogene. 2005;24(25):4081–93. doi: 10.1038/sj.onc.1208573. [DOI] [PubMed] [Google Scholar]
- [121].Lu X, Liu R, Wang M, Kumar AK, Pan F, He L, et al. MicroRNA-140 impedes DNA repair by targeting FEN1 and enhances chemotherapeutic response in breast cancer. Oncogene. 2020;39(1):234–47. doi: 10.1038/s41388-019-0986-0. [DOI] [PubMed] [Google Scholar]
- [122].Pitroda SP, Bao R, Andrade J, Weichselbaum RR, Connell PP. Low Recombination Proficiency Score (RPS) predicts heightened sensitivity to DNA-damaging chemotherapy in breast cancer. Clin Cancer Res. 2017;23(15):4493–4500. doi: 10.1158/1078-0432.CCR-16-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kuptsova-Clarkson N, Ambrosone CB, Weiss J, Baer MR, Sucheston LE, Zirpoli G, et al. XPD DNA nucleotide excision repair gene polymorphisms associated with DNA repair deficiency predict better treatment outcomes in secondary acute myeloid leukemia. Int J Mol Epidemiol Genet. 2010;1(4):278. [PMC free article] [PubMed] [Google Scholar]
- [124].Lin S, Yu L, Song X, Bi J, Jiang L, Wang Y, et al. Intrinsic adriamycin resistance in p53-mutated breast cancer is related to the miR-30c/FANCF/REV1-mediated DNA damage response. Cell Death Dis. 2019;10(9):1–15. doi: 10.1038/s41419-019-1871-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Deeley RG, Westlake C, Cole SPJP. Transmembrane transport of endo-and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86(3):849–899. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- [126].Fodale V, Pierobon M, Liotta L, Petricoin EJC. Mechanism of cell adaptation: when and how do cancer cells develop chemoresistance? Cancer J. 2011;17(2):89. doi: 10.1097/PPO.0b013e318212dd3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bonhoure E, Pchejetski D, Aouali N, Morjani H, Levade T, Kohama T, et al. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting shingosine kinase-1. Leukemia. 2006;20(1):95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- [128].Angelini A, Ciofani G, Baccante G, Di Febbo C, Di Ilio C, Cuccurullo F, et al. Modulatory effects of heparin on cellular accumulation and cytotoxicity of doxorubicin in MRP1-overexpressing HL60/doxo cells. Anticancer Res. 2007;27(1A):351–5. [PubMed] [Google Scholar]
- [129].Lu L, Ju F, Zhao H, Ma XJ. MicroRNA-134 modulates resistance to doxorubicin in human breast cancer cells by downregulating ABCC1. Biotech Lett. 2015;37(12):2387–94. doi: 10.1007/s10529-015-1941-y. [DOI] [PubMed] [Google Scholar]
- [130].Majidinia M, Yousefi BJD. Long non-coding RNAs in cancer drug resistance development. DNA Repair. 2016;45:25–33. doi: 10.1016/j.dnarep.2016.06.003. [DOI] [PubMed] [Google Scholar]
- [131].Chang L, Hu Z, Zhou Z, Zhang HJ. Linc00518 contributes to multidrug resistance through regulating the MiR-199a/MRP1 axisin breast cancer. Cell Physiol Biochem. 2018;48(1):16–28. doi: 10.1159/000491659. [DOI] [PubMed] [Google Scholar]
- [132].Gao M, Miao L, Liu M, Li C, Yu C, Yan H, et al. miR-145 sensitizes breast cancer to doxorubicin by targeting multidrug resistance-associated protein-1. Oncotarget. 2016;7(37):59714. doi: 10.18632/oncotarget.10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Korpal M, Lee ES, Hu G, Kang YJJ. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283(22):14910–4. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Xia H, Ng SS, Jiang S, Cheung WK, Sze J, Bian X-W, et al. miR-200a-mediated downregulation of ZEB2 and CTNNB1 differentially inhibits nasopharyngeal carcinoma cell growth, migration and invasion. Biochem Biophys Res Commun. 2010;391(1):535–41. doi: 10.1016/j.bbrc.2009.11.093. [DOI] [PubMed] [Google Scholar]
- [135].Chen J, Tian W, Cai H, He H, Deng YJ. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Med Oncol. 2012;29(4):2527–34. doi: 10.1007/s12032-011-0117-4. [DOI] [PubMed] [Google Scholar]
- [136].Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7(7):2152–9. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- [137].Hu D, Li M, Su J, Miao K, Qiu X. Dual-targeting of miR-124-3p and ABCC4 promotes sensitivity to adriamycin in breast cancer cells. Genet Test Mol Biomarkers. 2019;23(3):156–65. doi: 10.1089/gtmb.2018.0259. [DOI] [PubMed] [Google Scholar]
- [138].Boss O, Muzzin P, Giacobino JP. The uncoupling proteins, a review. Eur J Endocrinol. 1998;139(1):1–9. doi: 10.1530/eje.0.1390001. [DOI] [PubMed] [Google Scholar]
- [139].Brand MD, Esteves TCJC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metabol. 2005;2(2):85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- [140].Nübel T, Emre Y, Rabier D, Chadefaux B, Ricquier D, Bouillaud FJ. Modified glutamine catabolism in macrophages of Ucp2 knock-out mice. Biochim Biophys Acta. 2008;1777(1):48–54. doi: 10.1016/j.bbabio.2007.11.002. [DOI] [PubMed] [Google Scholar]
- [141].Saleh MC, Wheeler MB, Chan CBJJ. Endogenous islet uncoupling protein-2 expression and loss of glucose homeostasis in ob/ob mice. J Endocrinol. 2006;190(3):659–67. doi: 10.1677/joe.1.06715. [DOI] [PubMed] [Google Scholar]
- [142].Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Yuan Y, Yao YF, Hu SN, Gao J, Zhang L. MiR-133a is functionally involved in doxorubicin-resistance in breast cancer cells MCF-7 via its regulation of the expression of uncoupling protein 2. PLoS ONE. 2015;10(6):e0129843. doi: 10.1371/journal.pone.0129843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Moon SU, Kang MH, Sung JH, Kim JW, Lee JO, Kim YJ, et al. Effect of Smad3/4 on chemotherapeutic drug sensitivity in colorectal cancer cells. Oncol Rep. 2015;33(1):185–92. doi: 10.3892/or.2014.3582. [DOI] [PubMed] [Google Scholar]
- [145].Roland CL, Starker LF, Kang Y, Chatterjee D, Estrella J, Rashid A, et al. Loss of DPC4/SMAD4 expression in primary gastrointestinal neuroendocrine tumors is associated with cancer-related death after resection. Surgery. 2017;161(3):753–9. doi: 10.1016/j.surg.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Sun FD, Wang PC, Luan RL, Zou SH, Du X. MicroRNA-574 enhances doxorubicin resistance through down-regulating SMAD4 in breast cancer cells. Eur Rev Med Pharmacol Sci. 2018;22(5):1342–50. doi: 10.26355/eurrev_201803_14476. [DOI] [PubMed] [Google Scholar]
- [147].Kohn EA, Du Z, Sato M, Van Schyndle CM, Welsh MA, Yang Y-A, et al. A novel approach for the generation of genetically modified mammary epithelial cell cultures yields new insights into TGFβ signaling in the mammary gland. Breast Cancer Res. 2010;12(5):1–16. doi: 10.1186/bcr2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Jiang L, He D, Yang D, Chen Z, Pan Q, Mao A, et al. MiR-489 regulates chemoresistance in breast cancer viaepithelial mesenchymal transition pathway. FEBS Lett. 2014;588(11):2009–15. doi: 10.1016/j.febslet.2014.04.024. [DOI] [PubMed] [Google Scholar]
- [149].Li DM, Zhang J, Li WM, Cui JT, Pan YM, Liu SQ, et al. MAWBP and MAWD inhibit proliferation and invasion in gastric cancer. World J Gastroenterol. 2013;19(18):2781–92. doi: 10.3748/wjg.v19.i18.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Liang Y, Song X, Li Y, Su P, Han D, Ma T, et al. circKDM4C suppresses tumor progression and attenuates doxorubicin resistance by regulating miR-548p/PBLD axis in breast cancer. Oncogene. 2019;38(42):6850–66. doi: 10.1038/s41388-019-0926-z. [DOI] [PubMed] [Google Scholar]
- [151].Hong D, Fritz AJ, Finstad KH, Fitzgerald MP, Weinheimer A, Viens AL, et al. Suppression of breast cancer stem cells and tumor growth by the RUNX1 transcription factor. Mol Cancer Res. 2018;16(12):1952–64. doi: 10.1158/1541-7786.MCR-18-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Bai X, Ni J, Beretov J, Graham P, Li YJ. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152–163. doi: 10.1016/j.ctrv.2018.07.004. [DOI] [PubMed] [Google Scholar]
- [153].De Francesco EM, Lappano R, Santolla MF, Marsico S, Caruso A, Maggiolini MJ. HIF-1α/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs) Breast Cancer Res. 2013;15(4):R64. doi: 10.1186/bcr3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].De Francesco EM, Maggiolini M, Musti AMJI. Crosstalk between Notch, HIF-1α and GPER in breast cancer EMT. Int J Mol Sci. 2018;19(7):2011. doi: 10.3390/ijms19072011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Liu C, Xing H, Guo C, Yang Z, Wang Y, Wang YJCC. MiR-124 reversed the doxorubicin resistance of breast cancer stem cells through STAT3/HIF-1 signaling pathways. Cell Cycle. 2019;18(18):2215–27. doi: 10.1080/15384101.2019.1638182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Inoue M, Shinohara MLJI. Intracellular osteopontin (iOPN) and immunity. Immunol Res. 2011;49(1–3):160–172. doi: 10.1007/s12026-010-8179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Piccirillo CA, Bjur E, Topisirovic I, Sonenberg N, Larsson OJN. Translational control of immune responses: from transcripts to translatomes. Nat Immunol. 2014;15(6):503–511. doi: 10.1038/ni.2891. [DOI] [PubMed] [Google Scholar]
- [158].Rittling S, Singh RJJ. Osteopontin in immune-mediated diseases. J Dental Res. 2015;94(12):1638–1645. doi: 10.1177/0022034515605270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Leavenworth JW, Verbinnen B, Wang Q, Shen E, Cantor HJP. Intracellular osteopontin regulates homeostasis and function of natural killer cells. Proc Natl Acad Sci. 2015;112(2):494–499. doi: 10.1073/pnas.1423011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Lin F, Li Y, Cao J, Fan S, Wen J, Zhu G, et al. Overexpression of osteopontin in hepatocellular carcinoma and its relationships with metastasis, invasion of tumor cells. Mol Biol Rep. 2011;38(8):5205–10. doi: 10.1007/s11033-010-0671-4. [DOI] [PubMed] [Google Scholar]
- [161].Han B, Huang J, Han Y, Hao J, Wu X, Song H, et al. The microRNA miR-181c enhances chemosensitivity and reduces chemoresistance in breast cancer cells via down-regulating osteopontin. Int J Biol Macromol. 2019;125:544–56. doi: 10.1016/j.ijbiomac.2018.12.075. [DOI] [PubMed] [Google Scholar]
- [162].Brychtova V, Mohtar A, Vojtesek B, Hupp TR, editors. Mechanisms of anterior gradient-2 regulation and function in cancer. Seminars in cancer biology; 2015: Elsevier. [DOI] [PubMed]
- [163].Verma S, Salmans ML, Geyfman M, Wang H, Yu Z, Lu Z, et al. The estrogen-responsive Agr2 gene regulates mammary epithelial proliferationand facilitates lobuloalveolar development. Dev Biol. 2012;369(2):249–60. doi: 10.1016/j.ydbio.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Salmans ML, Zhao F, Andersen BJ. The estrogen-regulated anterior gradient 2 (AGR2) protein in breast cancer: a potential drug target and biomarker. Breast Cancer Res. 2013;15(2):1–14. doi: 10.1186/bcr3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Zhang Y, Xia F, Zhang F, Cui Y, Wang Q, Liu H, et al. miR-135b-5p enhances doxorubicin-sensitivity of breast cancer cells through targeting anterior gradient 2. J Exp Clin Cancer Res. 2019;38(1):26. doi: 10.1186/s13046-019-1024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Grolla AA, Travelli C, Genazzani AA, Sethi JK. Extracellular nicotinamide phosphoribosyltransferase, a new cancer metabokine. Br J Pharmacol. 2016;173(14):2182–94. doi: 10.1111/bph.13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Shackelford RE, Mayhall K, Maxwell NM, Kandil E, Coppola D. Nicotinamide phosphoribosyltransferase in malignancy: a review. Genes Cancer. 2013;4(11–12):447–56. doi: 10.1177/1947601913507576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Menssen A, Hydbring P, Kapelle K, Vervoorts J, Diebold J, Luscher B, et al. The c-MYC oncoprotein, the NAMPT enzyme, the SIRT1-inhibitor DBC1, and the SIRT1 deacetylase form a positive feedback loop. Proc Natl Acad Sci USA. 2012;109(4):E187–96. doi: 10.1073/pnas.1105304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Zangooei M, Nourbakhsh M, Ghahremani MH, Meshkani R, Khedri A, Shadboorestan A, et al. Investigating the effect of visfatin on ERalpha phosphorylation (Ser118 and Ser167) and ERE-dependent transcriptional activity. EXCLI J. 2018;17:516–25. doi: 10.17179/excli2018-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Folgueira MA, Carraro DM, Brentani H, Patrao DF, Barbosa EM, Netto MM, et al. Gene expression profile associated with response to doxorubicin-based therapy in breast cancer. Clin Cancer Res. 2005;11(20):7434–43. doi: 10.1158/1078-0432.CCR-04-0548. [DOI] [PubMed] [Google Scholar]
- [171].Bolandghamat Pour Z, Nourbakhsh M, Mousavizadeh K, Madjd Z, Ghorbanhosseini SS, Abdolvahabi Z, et al. Suppression of nicotinamide phosphoribosyltransferase expression by miR-154 reduces the viability of breast cancer cells and increases their susceptibility to doxorubicin. BMC Cancer. 2019;19(1):1027. doi: 10.1186/s12885-019-6221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Bai T, Yokobori T, Altan B, Ide M, Mochiki E, Yanai M, et al. High STMN1 level is associated with chemo-resistance and poor prognosis in gastric cancer patients. Br J Cancer. 2017;116(9):1177–85. doi: 10.1038/bjc.2017.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Marklund U, Larsson N, Gradin HM, Brattsand G, Gullberg MJT. Oncoprotein 18 is a phosphorylation-responsive regulator of microtubule dynamics. EMBO J. 1996;15(19):5290–8. [PMC free article] [PubMed] [Google Scholar]
- [174].Rubin CI, Atweh GFJJ. The role of stathmin in the regulation of the cell cycle. J Cell Biochem. 2004;93(2):242–250. doi: 10.1002/jcb.20187. [DOI] [PubMed] [Google Scholar]
- [175].Li Y, Liang Y, Sang Y, Song X, Zhang H, Liu Y, et al. MiR-770 suppresses the chemo-resistance and metastasis of triple negative breast cancer via direct targeting of STMN1. Cell Death Dis. 2018;9(1):1–12. doi: 10.1038/s41419-017-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.