ABSTRACT
Introduction
: The ongoing SARS-CoV-2 pandemic is a serious threat for the health of immunocompromised patients. Among neutralizing antibody-based therapeutics, convalescent plasma containing polyclonal anti-SARS-CoV-2 immunoglobulins has promising results in both congenital and iatrogenic immunodeficiencies in oncohematological and transplant patients.
Areas covered
: This article discusses case reports, case series and controlled studies detailing the efficacy of convalescent plasma in immunocompromised patients.
Expert opinion
: Convalescent plasma, when administered at high neutralizing antibody titers, is a safe and effective treatment for frail immunocompromised patients. Genetic monitoring of refractory patients is recommended to intercept intra-host emergence of SARS-CoV-2 variants.
KEYWORDS: Convalescent plasma, hyperimmune serum, polyclonal immunoglobulins, neutralizing antibodies, covid-19, sars-cov-2
1. Introduction
The COVID19 pandemic has caused more than 125 million cases and 2.5 million deaths worldwide since January 2020. Few drugs have shown robust evidences of clinical efficacy, including a few neutralizing antibody (nAb)-based therapeutics. COVID19 convalescent plasma (CCP), despite poor definition [1] and many controversial trials (summarized in Figure 1), is one of the few SARS-CoV-2 therapeutics under massive investigation [2], having shown clinical benefit when used early (within 72 hours since onset of symptoms) and with high titers of neutralizing antibodies (nAb) [3–5]. CCP has also proven an extremely safe therapy with very few thromboembolic events [6]. Similarly, monoclonal antibodies (mAb) have been proven effective when administered early in the disease course and in seronegative recipients [7], but their cost and availability remain severe hurdles. Clinical trials with hyperimmune serum, an industrial derivative of CCP or derived from immunized animals, are still ongoing [8], but to date no experience has been reported in immunocompromised patients. The absolute requirement for early treatment in frail immunocompromised patients is still questioned, leaving hopes for benefit also in late usages [9]: accordingly, many case reports and series have documented success of CCP in late COVID19 stages in such patients. Long-term benefits still need to be assessed since immunocompromised patients are at risk for reinfection. Similarly, there is a lack of long-term follow-up studies assessing the outcome of CCP therapy on the underlying malignancy or immune deficiency.
Figure 1.
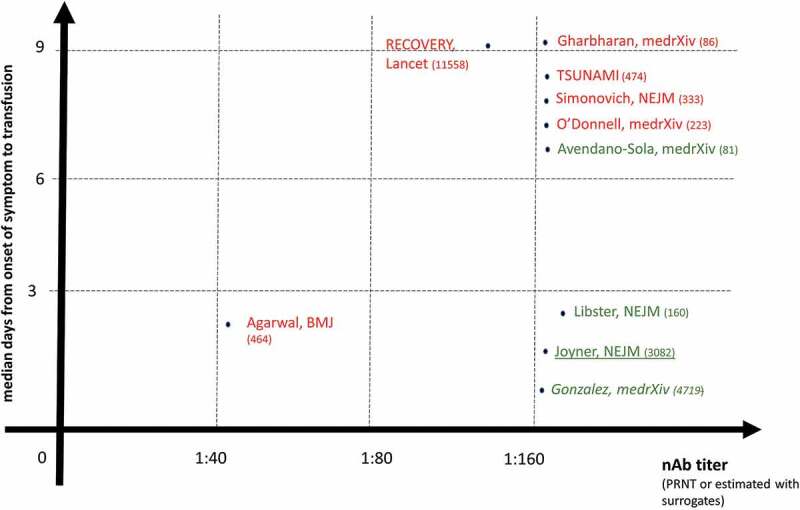
Graphical summary of COVID19 convalescent plasma (CCP) randomized controlled trials (RCT), propensity-score matched trials (italics), and matched controlled trials (underlined characters) for which nAb titer and days from onset of symptoms to transfusion were disclosed, and having placebo or best supportive care as a comparator. The green characters show trials reporting clinical benefits, while the red characters show trials which failed to evidence clinical benefit
Preliminary evidences suggest that commercial intravenous immunoglobulin (IVIg) formulations manufactured from plasma donations collected after the beginning of the pandemic could have high titers of anti-SARS-CoV-2 nAb [10], but no clinical trial has been initiated yet.
Frail immunosuppressed patients are prone to more severe COVID19, given the inability to control SARS-CoV-2 infection. The risk profile varies according to the type of immune deficiency (ranging from humoral to cellular to combined T- and B-) and depth (e.g. hypo- vs. agammaglobulinemia). Immunocompromised patients are likely to mount partial or no protective immune response after vaccination, making the availability of effective therapeutics mandatory for such cohort. Unfortunately, these patients have not been represented in large CCP randomized controlled trials (RCT) reported to date [11], and evidences of efficacy mostly stem from small-scale phase II trials (for oncohematological and transplant patients) or case reports (for rare congenital immune deficiencies).
In this narrative review we analyze evidences supporting the efficacy of CCP for post-exposure prophylaxis and early treatment in these frail cohorts.
2. Convalescent plasma in primary immune deficiencies (PID)
A large survey has shown that more than 30% of patients with PID had mild coronavirus disease 2019 (COVID-19) and risk factors predisposing to severe disease/mortality in the general population also affect patients with PID, including more younger patients [12]. Mortality rate was 9% which was similar to the global data from the general population and it was mainly among patients with other comorbidities like heart and kidney diseases [12]. The severity of PID inversely correlate with the severity of COVID‐19 [13]. Stricter infection control measures (such as social isolation) compared to the general population likely lead to lower SARS-CoV-2 infection rates in PID patients [12–14]. Compared to the aggressive clinical course seen in patients with common variable immunodeficiency (CVID), patients with agammaglobulinemia (either Bruton’s X-linked (XLA) or autosomal recessive (ARA)) had very mild COVID19 courses [12,15,16]. This suggests that T-cell immunity (only moderately affected in XLA and ARA) could partly compensate the lack of neutralizing antibodies.
Overall, COVID19 has been reported to date in 13 Bruton’s XLA [12,15–17] (including one possible reinfection [18]), 1 ARA [16], and 11 CVID patients [15,19,20], and 1 with X-SCID [21]. 2 cases have been reported in Good syndrome (thymoma with immunodeficiency) [19,22], which is currently classified a phenocopy of PID. Table 1 reports the features of the ones of them who were treated with CCP: of interest, none of them died despite most of them being treated later during the course of the disease. Unfortunately, no data on nAb content in units of in patients were available. Underlying prophylaxis with intravenous immunoglobulins (likely derived from prepandemic donation batches) in patients with hypo/agammaglobulinemias was not sufficient to prevent COVID19, also excluding any beneficial impact from cross-reactive antibodies against seasonal, endemic related coronaviruses.
Table 1.
Summary of case reports detailing efficacy of CCP in congenital immune deficiencies
| Type of congenital immune deficiency | Notes | Concurrent drugs | COVID19 course | CCP regimen | Outcome | Ref |
| Bruton’s X-linked agammaglobulinemia (XLA) |
10-yo male with hereditary spherocytosis | Remdesivir + s.c.IVIg every other week | Pneumonia | 2 250-ml units at days 22 and 23 | nAb titer from 0 to 1:80 3 days after transfusion. Discharged on day 29 |
[17] |
| 24-yo male | IVIg every 3 weeks | Pneumonia | 2 200-ml units at day 16 | Discharged on day 19 | ||
| 40-yo male | IVIg every 3 weeks | Pneumonia | 2 200-ml units at day 44 | nAb titer from 0 to 1:16,012 hours after infusion. Discharged on day 45 | ||
| 39-yo male | IVIg every month + hydroxychloroquine |
Pneumonia | 1 200-ml unit at day 23 | Discharged at day 30 | [23] | |
| 28-yo male | 10 g s.c. IVIg weekly + remdesivir | Pneumonia | 500 ml on day 5 | Discharged on day 13 | [24] | |
| 26-yo male | 30 g IVIg + tocilizumab + remdesivir | Pneumonia | 2 300-ml doses on days 39 and 45. | Discharged on day 50 | [25] | |
| 34-yo male | 30 g IVIg for 5 days+weekly, tocilizumab 3 doses | Pneumonia | 2 0.5 ml/kg units 12 hours apart on day 10 | Discharged on day 56 | [26] | |
| 26-yo male | IVIg 1 g/kg | Diarrhea | 1 200-ml unit on day 11 | Discharged on day 14 | [27] | |
| Autosomal recessive agammaglobulinemia (ARA) | 40-yo male | IVIg, steroids | HLH, lung disease | n.a. | Recovered at day 50 | [12] |
| Good syndrome (thymoma with immunodeficiency) | 41-yo female | Hydroxychloroquine, prednisone | Pneumonia | 2 daily 200-ml infusions at days 71 and 72 (2 1:160 and 2 1:40) | Discharged at day 75 | [19] |
| Common variable immunodeficiency (CVID) | 25-yo female | Supportive care | Pneumonia under mechanical ventilation | 4 200-ml doses over 6 days | Rapid recovery | [28] |
| 40-yo female | Ig, steroids, | Kidney tx, lymphoma and cervical cancer in remission | n.a. | Deceased | [12] | |
| 15-yo male | Ig | Dyspnea, sepsis, HLH | n.a. | Discharged | ||
| 70-yo female | Ig, chloroquine | Dyspnea | n.a. | Discharged |
Chronic enterovirus infection is well known in patients with XLA [29], so it is not unexpected that SARS-CoV-2 can lead to chronic infections in congenital agammaglobulinemias.
3. Convalescent plasma in oncohematological patients
Immunocompromised patients with hematological cancers have a COVID19 mortality rate as high as 60% [30–32]. Patients with hematologic malignancies may have immune deficiencies from patient-related (i.e. age), disease-related, and treatment-related (i.e. chemo-immunotherapies) factors. Table 2 summarized the main evidences to date. In a single center cohort of patients with chronic lymphocytic leukemia and symptomatic COVID-19, 7 of 21 (33%) did not develop detectable anti-SARS-CoV-2 antibodies, markedly lower than the 100% seroconversion rate observed in a non-cancer population [33,34]. Immunocompromised patients suffering from oncohematological cancers, due to their inability to mount an appropriate humoral immune response to SARS-CoV-2, represent the ideal candidate for passive immunotherapy by means of CCP transfusion [35]. There is increasing interest toward the CCP use in patients with hematologic malignancies and several investigators have explored this therapeutic possibility [36–55]. Details about disease severity, timing of CCP administration, number of doses, pre- and post-treatment nAb titers were unfortunately not available for the vast majority of patients. In a recent case series published by Tremblay and colleagues [54], the authors identified 24 patients with cancer, 14 of whom with a hematological malignancy (5 non-Hodgkin lymphoma, 1 Hodgkin lymphoma, 4 multiple myeloma, 2 acute lymphoblastic leukemia, 1 myelofibrosis, 1 chronic lymphocytic leukemia), treated with high-titer (≥1:320) CCP within an expanded access protocol. Most patients (62.5%) were on anti-cancer therapy at the time of COVID-19 infection. The overall mortality rate was 41.7% (10/24). Non-intubated patients had favorable outcomes (death rate: 28.5%, 6/21), suggesting a potential clinical benefit of CCP in less advanced stages of COVID-19. In addition, a significant decrease of inflammatory markers (i.e. C-reactive protein, CRP) was observed after 3 days of CCP treatment. Transfusion reactions were uncommon and mild, occurring only in three patients. Of the 14 patients with hematologic malignancies, 8 patients (57.1%) were discharged, 1 (7.1%) was still hospitalized and mechanically ventilated and 5 (35.7%) expired. Hueso and colleagues [37] reported a series of 17 consecutive patients, of whom 15 had hematological malignancies (13 non-Hodgkin lymphoma, 1 chronic lymphocytic leukemia and 1 Waldenstrom macroglobulinemia) with profound B-cell lymphopenia due to anti-CD20 monoclonal antibody therapy and prolonged COVID-19 symptoms, negative SARS-CoV-2 IgG/IgM serology, and positive viral RNA-emia who were treated with 4 units of CCP. No serious adverse effects were observed during or after CCP therapy. Within 48 hours of transfusion, all but 1 patient experienced an improvement of clinical symptoms, including reduced oxygen requirements, which correlated strongly with the viral clearance documented in all the 9 patients evaluated. The hyper-inflammatory status faded within a week. Only 1 patient who needed mechanical ventilation for severe COVID-19 disease died of bacterial pneumonia. The authors concluded that CCP appeared to be a promising therapy for COVID-19 treatment in B-cell depleted patients unable to mount a specific humoral response against SARS-CoV-2. Interestingly, Betrains and colleagues [48] analyzed 5 patients with COVID-19 and B-cell lymphoma treated with anti-CD20 therapy and demonstrated that B-cell depletion was associated with decreased neutralized antibody formation, reduced viral clearance and protracted clinical manifestations of SARS-CoV-2 infection. Treatment with CCP was accompanied by an increase in neutralizing antibody titers in all patients and by a clinical response in all but one patient. The authors concluded that patients with B-cell-depleted lymphomas with protracted SARS-CoV-2 infection are the ideal candidates for passive immunotherapy by CCP. Other case reports confirmed this initial finding in similar groups of non-Hodgkin lymphoma patients [45,47,49]. In a retrospective analysis, Jeyaraman et al [53] identified 33 patients with hematologic malignancies (18 non-Hodgkin lymphoma, 4 acute leukemia, 7 multiple myeloma, 2 myelodysplastic syndrome, 2 chronic myeloid leukemia) treated for severe COVID-19 with CCP, in the majority of cases within 7 days of COVID-19 diagnosis. The majority of patients were on active chemotherapy (72.7%, 24/33) at the time of CCP infusion. The overall mortality rate in the cohort was 45.5% (15/33) and did not differ between early versus late CCP therapy. The largest experience on this issue is that pre-published by Thompson and colleagues on behalf of the COVID-19 and Cancer Consortium [52]. In this retrospective study, 143 patients with various hematologic malignancies (lymphoid neoplasms 123 and myeloid neoplasms 21) and COVID-19, in most cases moderate to severe, were treated with CCP and were compared to 823 untreated controls. After adjustment for potential confounding factors, CCP treatment was associated with a significantly improved 30-day mortality (hazard ratio [HR], 0.60; 95% CI 0.37–0.97), suggesting a potential survival benefit in the CCP treatment arm. The most recent study is that published by Ferrari and colleagues [50] where the authors reported their own experience on CCP therapy in 7 patients with COVID-19 previously treated with chemo-immunotherapy due to oncohematological disorders (4 non-Hodgkin lymphoma, 1 primary myelofibrosis, 1 chronic lymphocytic leukemia, 1 acute myeloid leukemia). CCP treatment was well tolerated and, in all cases, resulted in a clinical benefit in term respiratory symptoms with less intensive oxygen requirements. Viral clearance was detected by nasopharyngeal swabs in 5 out of 7 patients treated. The authors concluded that CCP can be a safe and effective therapeutic option for oncohematological patients with COVID-19 and immunodeficiency due to previous chemo-immunotherapy.
Table 2.
Summary of main studies reporting CCP usage in oncohematological patients
| Type of tumor | n | CCP regimen | Outcome | Notes | Ref |
| NHL MM ALL HL MF CLL |
5 4 2 1 1 1 |
High-titer (≥1:320) CCP | - Overall mortality rate 41.2% (10/24) - Overall mortality rate in hematological cancers: 35.7% (5/14) - Mortality in non-intubated patients: 6/21 (28.5%) |
Clinical benefit of CP when administered in less advanced COVID-19 |
[54] |
| NHL CLL WM |
11 3 1 |
4 CCP units (titer ≥ 1:40) |
- Rapid viral clearance following CCP - Overall mortality rate 6.7% (1/15) |
CCP is a promising therapy for COVID-19 B-cell depleted patients | [37] |
| NHL | 5 | 2 high-titer (≥1:160) CCP units | - Increase in neutralizing antibody titer following CCP - Overall mortality rate 20.0% (1/5) |
Patients with B-cell-depleted lymphomas are ideal candidate for CCP | [48] |
| NHL AL MM CML MDS |
18 4 7 2 2 |
1–2 CCP (>1:640)1 |
- Overall mortality rate 45.5% (15/33) - No mortality difference between early (< 7 days) versus late CCP transfusion |
Study with limitations (retrospective case series) | [53] |
| Lymphoid neoplasms Myeloid neoplasms |
12,321 | NA | - Adjusted 30-day mortality: HR 0.60; 95% CI 0.37–0.97 | CP is associated with improved survival in patients with hematologic malignancies | [52] |
| NHL CLL MF AML |
4 1 1 1 |
3 CCP units | - No deaths recorded - Viral clearance in 5/7 (71.4%) - No adverse effects |
CP is associated with clinical and radiological improvement in oncohematological patients | [50] |
Legend: CCP, COVID-19 convalescent plasma; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; MM, multiple myeloma; ALL, acute lymphoblastic leukemia; MF, myelofibrosis; CLL, chronic lymphocytic leukemia; WM, Waldenstrom macroglobulinemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome; NA, not available; HR, hazard ratio; AL, acute leukemia; AML, acute myeloid leukemia;
1Serologic assay.
In a recent pre-published review, Senefeld and colleagues [56] identified 54 patients with hematological malignancies, including lymphoma, leukemia, multiple myeloma and myelodysplastic syndrome, transfused with CCP in 18 peer-reviewed reports. A majority of patients recovered following CP transfusion, with many demonstrating rapid clinical improvements shortly after transfusion. Notably, a patient with persistent (> 100 days) COVID-19 and with lymphoma-associated B-cell immunodeficiency demonstrated rapid reductions in fever, oxygen requirements and lung infiltrates immediately after two CCP transfusions separated by approximately 90 days [46].
Kenig et al reported eight patients with iatrogenic B-cell depletion who received CCP as add-on therapy and showed prompt negativization of NPS PCR and clinical improvement [57]. Rodionov et al also reported 14 seronegative COVID-19 patients with acquired immunodeficiencies due to solid organ transplantation (8 patients), allogeneic stem cell transplantation (4 patients), or active hematological malignancy (2 patients) transfuses with CCP units having PRNT50 ≥ 40 at a median of 5 days after diagnosis: anti-SARS-CoV-2 IgG serum titers of more than 20 IU/mL are able to confer a more than 2-point improvement in the WHO Clinical progression Scale [58].
4. Convalescent plasma in solid organ transplant recipients
Recipients of solid organ transplants receiving immunosuppressive medications are at increased risk of severe COVID-19. Only a few case reports or case series have assessed the role of CCP in solid organ transplant patients with COVID-19 [59–62]. A clinical beneficial effect associated with passive immunotherapy was even seen in the extreme case of a liver transplant recipient with a life-threatening COVID-19: the patient rapidly recovered following a CCP transfusion received during a 17 day medically induced coma due to COVID-19 complications [63]. Fung and colleagues [39] reported 3 transplant patients (2 kidney transplant recipients and 1 lung transplant recipient) treated with CCP through an Expanded Access Program. All patients clinically improved after CCP administration without transfusion reactions. A recent systematic review and meta-analysis by Raja and colleagues [64] identified 215 studies including 2772 transplanted patients (1500 kidney, 505 liver, 141 heart, 97 lung, 1 face and 43 unidentified combined transplants). Although CCP was utilized only in a minority of patients (33 patients enrolled in 13 studies), the mortality rate in this subgroup seemed to be lower than that recorded in the entire population of patients (12.9% versus 18.6%, respectively). In their recent pre-published review, Senefeld and colleagues [56] identified 9 articles reporting 29 COVID-19 patients receiving immunosuppressive therapies for previous solid organ transplants and transfused with hyperimmune plasma. In most case CCP administration was accompanied by improved clinical symptomatology and oxygen requirements and reduction in hospital stay. Despite the scarcity of the literature data and the potential bias of reporting (cases with positive outcomes preferentially reported with respect to those with negative outcomes), the currently reported cases suggest a potential beneficial effect of CP also in this particularly complex category of fragile patients.
5. Convalescent plasma and SARS-CoV-2 variants of concern: efficacy and risks of accelerated viral evolution.
Spike mutations are rare after immunosuppressive treatment without anti-Spike treatment [65]. Nevertheless Bazykin et al reported emergence of Y453F and Δ69-70HV mutations (‘the ΔF combination’) (together with S50L, Δ141-144, T470N, and D737G) in a 47-year-old female with diffuse large B cell lymphoma treated with rituximab plus chemotherapy (R-ICE regimen) [66]. Borges et al reported another DLBCL patients with persistent infection for 6 months who developed four mutations (V3G, S50L, N87S and A222V) and two deletions (Δ18-30 and Δ141-144) in Spike [67]. Finally, Truong et al reported the emergence of escape mutations in 2 more patients with acute lymphoblastic leukemia who were persistently positive for SARS-CoV-2 for up to 162 days [68].
Several lines of evidence support the hypothesis that widespread deployment of antibody-based therapeutics could drive Spike immune escape.
In vitro evidences include the emergence of mutations during SARS-CoV-2 culture with convalescent plasma. Continuous passaging of SARS-CoV-2 in the presence of a CCP unit with nAb titer >1:104 led to ΔF140 at day 45, followed by E484K at days 73 and an insertion in the NTD: these accumulating mutations led to complete lack of neutralization [69]. Accordingly, K417N, E484K, and N501Y mutations. were selected when pseudotyped SARS-CoV-2 was cultured in the presence of the vaccine elicited mAbs [70].
Although within host SARS-CoV-2 mutation accumulation is typically very low [71], faster rates have been found in longitudinal studies of immunodeficient patients with persistent SARS-CoV-2 infections for up to several months. In particular, this has happened in case reports after treatment with CCP: the phenomenon does not seem very common or very fast, since none out of eight onco-hematological patients (recipients of hematopoietic stem-cell transplants or chimeric antigen receptor T lymphocytes) treated with CCP who remained SARS-CoV-2 positive for 2 months showed significant mutations compared to wild-type strain [65]. Nevertheless, Avanzato et al reported within-host genomic evolution in a patient affected by chronic lymphocytic leukemia and iatrogenic hypogammaglobulinemia treated with CCP and shedding infectious SARS-CoV-2 for 70 days, and subgenomic RNA for 105 days [36]. Similarly, Kemp et al reported an immune suppressed individual who showed little evolutionary change in the first 65 days while on remdesivir, but who developed D796H and ΔH69/ΔV70 mutations twice after 2 unsuccessful courses of CCP. In vitro, such mutant showed similar infectivity to wild type strain but resistance to many CCP donors [72]. Finally, Truong et al reported the emergence of 7 major and 3 minor allele variants (including ∆141-143, ∆145, ∆141-144, ∆211-212, N440K, V483A, and E484Q) in a patient with acute lymphoblastic leukemia who was treated with weekly CCP and tested persistently positive for SARS-CoV-2 until day 144 [68].
Such serial monitoring of immunocompromised patients receiving CCP with next-generation sequencing is extremely expensive and time-consuming. Despite lack of details from these sporadic case reports, we cannot exclude that treatment with subneutralizing antibody levels could have facilitated accelerated viral evolution. In this regard, the introduction of a weight- and titer-adjusted loading dose of CCP seems a prudent approach. Patients showing delayed refractoriness to CCP are encouraged to undergo at least SARS-CoV-2 Spike gene sequencing to exclude variants.
6. Expert opinion
COVID19 convalescent plasma (CCP) is a promising drug for treatment and post-exposure prophylaxis of COVID-19. Besides the safety of CCP therapy, which has been recognized by almost all previous trials [2], the most important issue regards its effectiveness. The great majority of the published literature data strengthen the importance of CCP transfusion in COVID-19 patients as close to symptom onset as possible, in order to promptly block SARS-CoV-2 replication and the consequent progression, often irreversible, of COVID-19 pathology [11]. Another key issue of CCP therapy pertains to the quality of CCP produced by transfusion services. Assuming that the antiviral activity of the CCP is mostly linked to the amount of antibodies present, it follows that the more the neutralizing antibodies, the more effective the plasma will be in blocking viral replication [11]. Although the plaque reduction neutralization test (PRNT), which measures the ability of neutralizing antibodies to prevent infection in vitro calculated as a reduction in the formation of plaques, is the current gold standard to assess viral neutralization by CCP, a number of commercial serological high throughput SARS-CoV-2 assay are being tested to replace PRNT for the determination of CCP neutralizing potency [11]. Although some national and international guidelines [73] recommend the use in immunocompromised patients of CCP at titer ≥ 1:320, the exact dose and the timing of CCP administration are yet to be established and need to be assessed by further trials. Finally, another important factor contributing to the CCP clinical effectiveness regards the COVID-19 patients’ characteristics. Controlled studies have shown clinical benefit in iatrogenic immunosuppression (oncohematological patients and solid organ transplant recipients). Among patients with hematological malignancies, those suffering from lymphoproliferative B-cell disorders or receiving B-cell depleting therapies are the ones who are likely to benefit most from CCP therapy. Such patients, indeed, are unable to mount an adequate immune response against SARS-CoV-2 and thus may benefit from the passive transfer of anti-SARS-CoV-2 antibodies through CCP transfusion administered during the early phase of viral infection [35]. Many case reports and cases series have also shown clinical benefit in congenital immune deficiencies, particularly those with genetically determined impaired humoral response. For such reasons, the US Food and Drug Administration (FDA) recently revised the Emergency Use Authorization (EUA) of COVID-19 CP authorizing its use at high titer for the treatment of hospitalized COVID-19 patients early in the course of disease and those hospitalized with impaired humoral immunity [9]. Studies testing CCP as post-exposure prophylaxis are still ongoing, and have the potential to lead to better outcomes than when used as treatment.
Given the low chances of mounting a protective immune response after vaccination, nAb-based therapeutics remain a potential drug for early treatment of COVID19 in frail immunocompromised patients. High-titer CCP bulks should hence be maintained, even after reaching herd immunity, for the coming years. The level of protection from commercial aspecific IVIg batches manufactured from plasma donations collected after January 2020 remains to be established. In addition, a number of trials are currently exploring the beneficial effect of specific polyclonal anti-SARS-CoV-2 IVIg [8]. It is important to underline, however, that the production of specific immunoglobulins requires significant investments by the manufacturing companies which take years to recover. It is therefore conceivable that, in the face of herd immunity in the Western world by the end of 2021, the interest of pharmaceutical companies in this type of products will be reduced.
Funding Statement
This paper was not funded.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors should have (1) substantially contributed to the conception and design of the review article and interpreting the relevant literature, and (2) been involved in writing the review article or revised it for intellectual content.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Focosi D, Farrugia A.. Urgent need to regulate convalescent plasma differently from thawed plasma, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Focosi D, Anderson AO, Tang JW, et al. Convalescent Plasma Therapy for COVID-19: state of the Art. Clin Microbiol Rev. 2020;33(4):e00072–00020. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This review provides historical background
- 3.Gonzalez SE, Regairaz L, Salazar M, et al. Timing of Convalescent plasma administration and 28-day mortality for COVID-19 pneumonia. medrXiv 2021;2002(2002):21250758 (2021. [DOI] [PubMed] [Google Scholar]
- 4.Joyner MJ, Carter RE, Senefeld JW, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med. 2021(11). doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This paper reports the results of the US Expanded Access Program
- 5.Libster R, Pérez Marc G, Wappner D, et al. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N Engl J Med. 2021;7. Epub ahead of print. DOI: 10.1056/NEJMoa2033700 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This trial provides evidence of benefit in nonhospitalized patients having mild symptoms since less than 3 days
- 6.Joyner MJ, Bruno KA, Klassen SA, et al. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin Proc. 2020;95(9):1888–1895. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N Engl J Med. 2020;384(3):238–251. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Focosi D, Franchini M, Tuccori M. The road towards polyclonal anti-SARS-CoV-2 immunoglobulins (hyperimmune serum) for passive immunization in COVID19. Life (Basel). 2021 Feb 15;11(2):144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FDA . Recommendations for Investigational COVID-19 Convalescent Plasma. (Ed.^(Eds)
- 10.Farcet MR, Karbiener M, Schwaiger J, et al. Rapidly Increasing SARS-CoV-2 Neutralization by Intravenous Immunoglobulins Produced from Plasma Collected During the 2020 Pandemic. J Infect Dis. 2021. DOI: 10.1093/infdis/jiab142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Focosi D, Franchini MCOVID. 19 convalescent plasma therapy: hit fast, hit hard! Vox Sang. 2021. DOI: 10.1111/vox.13091 [DOI] [PMC free article] [PubMed] [Google Scholar]; • This commentary summarizes evidences from randomized clinical trials and propensity score matched trials
- 12.Meyts I, Bucciol G, Quinti I, et al. Coronavirus disease 2019 in patients with inborn errors of immunity: an international study. J Allergy Clin Immunol. 2021;147(2):520–531. . [DOI] [PMC free article] [PubMed] [Google Scholar]; •• The largest study on CCP in inherited immunedeficiencies
- 13.Babaha F, Rezaei N. Primary Immunodeficiency Diseases in COVID-19 Pandemic: a Predisposing or Protective Factor? Am J Med Sci. 2020;360(6):740–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delavari S, Abolhassani H, Abolnezhadian F, et al. Impact of SARS-CoV-2 Pandemic on Patients with Primary Immunodeficiency. J Clin Immunol. 2021;41(2):345–355. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID-19? Lesson from patients with agammaglobulinemia. J Allergy Clin Immunol. 2020;146(1):211–213.e214. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soresina A, Moratto D, Chiarini M, et al. Two X-linked agammaglobulinemia patients develop pneumonia as COVID-19 manifestation but recover. Pediatr Allergy Immunol. 2020;31(5):565–569. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin H, Reed JC, Liu STH, et al. Three patients with X-linked agammaglobulinemia hospitalized for COVID-19 improved with convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(10):3594–3596.e3593. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh SY, Bassett J, Hoodless EJ, et al. COVID-19 reinfection in a patient with X-linked agammaglobulinaemia. BMJ Case Rep. 2021;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.London J, Boutboul D, Lacombe K, et al. Severe COVID-19 in Patients with B Cell Alymphocytosis and Response to Convalescent Plasma Therapy. J Clin Immunol. 2021 Feb;41(2):356-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fill L, Hadney L, Graven K, et al. The clinical observation of a patient with common variable immunodeficiency diagnosed as having coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125(1):112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nsc VO, Hanners NW, Sue PK, et al. SARS-CoV-2 infection associated with hepatitis in an infant with X-linked severe combined immunodeficiency. Clin Immunol. 2021;224:108662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzi MR, Baronio M, Janetti MB, et al. Fatal SARS-CoV-2 infection in a male patient with Good’s syndrome. Clin Immunol. 2021;223:108644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mira E, Yarce OA, Ortega C, et al. Rapid recovery of a SARS-CoV-2-infected X-linked agammaglobulinemia patient after infusion of COVID-19 convalescent plasma. J Allergy Clin Immunol Pract. 2020;8(8):2793–2795. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iaboni A, Wong N, Betschel SDA. Patient with X-Linked Agammaglobulinemia and COVID-19 Infection Treated with Remdesivir and Convalescent Plasma. J Clin Immunol. 2021 Feb 6;1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delgado-Fernández M, García-Gemar GM, Fuentes-López A, et al. Treatment of COVID-19 with convalescent plasma in patients with humoral immunodeficiency - Three consecutive cases and review of the literature. Enferm Infecc Microbiol Clin. 2021. DOI: 10.1016/j.eimc.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milošević I, Jovanović J, Stevanovic O. Atypical course of COVID-19 in patient with Bruton agammaglobulinemia. J Infect Developing Countries. 2020;14(11):1248–1251. [DOI] [PubMed] [Google Scholar]
- 27.Hovey JG, Tolbert D, Howell D. Bruton’s Agammaglobulinemia and COVID-19. Cureus. 2020;12(11):e11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ribeiro LC, Benites BD, Ulaf RG, et al. Rapid clinical recovery of a SARS-CoV-2 infected common variable immunodeficiency patient following the infusion of COVID-19 convalescent plasma. Allergy Asthma Clin Immunol. 2021;17(1):14. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bearden D, Collett M, Quan PL, et al. Enteroviruses in X-Linked Agammaglobulinemia: update on Epidemiology and Therapy. J Allergy Clin Immunol Pract. 2016;4(6):1059–1065. [DOI] [PubMed] [Google Scholar]
- 30.Passamonti F, Cattaneo C, Arcaini L, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Haematol. 2020;7(10):e737–e745. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34(6):1637–1645. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aries JA, Davies JK, Auer RL, et al. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol. 2020;190(2):e64–e67. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roeker LE, Knorr DA, Pessin MS, et al. Anti-SARS-CoV-2 antibody response in patients with chronic lymphocytic leukemia. Leukemia. 2020;34(11):3047–3049. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. . [DOI] [PubMed] [Google Scholar]
- 35.Focosi D, Franchini M. COVID. 19 neutralizing antibody-based therapies in humoral immune deficiencies: a narrative review. Transfus Apher Sci. 2021 Jan 27;103071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avanzato VA, Matson MJ, Seifert SN, et al. Case Study: prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer. Cell. 2020(7). doi: 10.1016/j.cell.2020.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136(20):2290–2295. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khan AM, Ajmal Z, Raval M, et al. Concurrent Diagnosis of Acute Myeloid Leukemia and COVID-19: a Management Challenge. Cureus. 2020;12(8):e9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fung M, Nambiar A, Pandey S, et al. Treatment of immunocompromised COVID-19 patients with convalescent plasma. Transplant Infect Dis. 2020; (2):e13477. DOI: 10.1111/tid.13477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang LL, Liu Y, Guo YG, et al. Convalescent Plasma Rescued a Severe COVID-19 Patient with Chronic Myeloid Leukemia Blast Crisis and Myelofibrosis. Turk J Haematol. 2020(1). doi: 10.4274/tjh.galenos.2020.2020.0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lancman G, Mascarenhas J, Severe B-NM. COVID-19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luetkens T, Metcalf R, Planelles V, et al. Successful transfer of anti-SARS-CoV-2 immunity using convalescent plasma in an MM patient with hypogammaglobulinemia and COVID-19. Blood Adv. 2020;4(19):4864–4868. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karataş A, İnkaya A, Demiroğlu H, et al. Prolonged viral shedding in a lymphoma patient with COVID-19 infection receiving convalescent plasma. Transfus Apher Sci. 2020;59(5):102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright Z, Bersabe A, Eden R, et al. Successful Use of COVID-19 Convalescent Plasma in a Patient Recently Treated for Follicular Lymphoma. Clin Lymphoma Myeloma Leuk. 2021 Jan;21(1):66-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clark E, Guilpain P, Filip IL, et al. Convalescent plasma for persisting COVID-19 following therapeutic lymphocyte depletion: a report of rapid recovery. Br J Haematol. 2020;190(3):e154–e156. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baang JH, Smith C, Mirabelli C, et al. Prolonged SARS-CoV-2 replication in an immunocompromised patient. J Infect Dis. 2021 Jan 4;223(1):23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Malsy J, Veletzky L, Heide J, et al. Sustained response after remdesivir and convalescent plasma therapy in a B-cell depleted patient with protracted COVID-19. Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Betrains A, Godinas L, Woei AJF, et al. Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies. Br J Haematol. 2020(6). doi: 10.1111/bjh.17266. [DOI] [PubMed] [Google Scholar]
- 49.Moore JL, Ganapathiraju PV, Kurtz CP, et al. A 63-Year-Old Woman with a History of Non-Hodgkin Lymphoma with Persistent SARS-CoV-2 Infection Who Was Seronegative and Treated with Convalescent Plasma. Am J Case Rep. 2020;21:e927812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ferrari S, Caprioli C, Weber A, et al. Convalescent hyperimmune plasma for chemo-immunotherapy induced immunodeficiency in COVID-19 patients with hematological malignancies. Leuk Lymphoma. 2021;1–9. DOI: 10.1080/10428194.2021.1872070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balashov D, Trakhtman P, Livshits A, et al. SARS-CoV-2 convalescent plasma therapy in pediatric patient after hematopoietic stem cell transplantation. Transfus Apher Sci. 2021 Feb;60(1):102983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson MA, Henderson JP, Shah PK, et al. Convalescent Plasma and Improved Survival in Patients with Hematologic Malignancies and COVID-19. medrXiv 2021;2002(2005):21250953 (2021. [Google Scholar]
- 53.Jeyaraman P, Agrawal N, Bhargava R, et al. Convalescent plasma therapy for severe Covid-19 in patients with hematological malignancies. Transfus Apher Sci. 2021 Feb 3:103075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tremblay D, Seah C, Schneider T, et al. Convalescent Plasma for the Treatment of Severe COVID-19 Infection in Cancer Patients. Cancer Med. 2020;9(22):8571–8578. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Çınar OE, Sayınalp B, Aladağ Karakulak E, et al. Convalescent (immune) plasma treatment in a myelodysplastic COVID-19 patient with disseminated tuberculosis. Transfus Apher Sci. 2020;59(5):102821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Senefeld JW, Klassen SA, Ford SK, et al. Therapeutic use of convalescent plasma in COVID-19 patients with immunodeficiency. medrXiv 2020;2011(2008):20224790 (2020. [Google Scholar]
- 57.Kenig A, Ishay Y, Kharouf F, et al. Treatment of B-cell depleted COVID-19 patients with convalescent plasma and plasma-based products. Clin Immunol. 2021 Apr 7;227:108723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodionov RN, Biener A, Spieth P, et al. Potential benefit of convalescent plasma transfusions in immunocompromised patients with COVID-19. Lancet. 2021;2(4):e138. . Microbe [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamir I, Lohia P, Pande RK, et al. Convalescent plasma therapy and remdesivir duo successfully salvaged an early liver transplant recipient with severe COVID-19 pneumonia. Ann Hepatobiliary Pancreat Surg. 2020;24(4):526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lima B, Gibson GT, Vullaganti S, et al. COVID-19 in recent heart transplant recipients: clinicopathologic features and early outcomes. Transplant Infect Dis. 2020;22(5):e13382. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naeem S, Gohh R, Bayliss G, et al. Successful recovery from COVID-19 in three kidney transplant recipients who received convalescent plasma therapy. Transplant Infect Dis. 2020; (1):e13451. DOI: 10.1111/tid.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang J, Miao Y, Zhao Y, et al. Convalescent plasma therapy: helpful treatment of COVID-19 in a kidney transplant recipient presenting with severe clinical manifestations and complex complications. Clin Transplant. 2020;34(9):e14025. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.NHS Blood and Transplant N. One of the first COVID-19 convalescent plasma recipients supports donor appeal. [Google Scholar]
- 64.Raja MA, Mendoza MA, Villavicencio A, et al. COVID-19 in solid organ transplant recipients: a systematic review and meta-analysis of current literature. Transplant Rev (Orlando). 2020;35(1):100588.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of Viable SARS-CoV-2 after Immunosuppressive Therapy for Cancer. N Engl J Med. 2020 Dec 24;383(26):2586-2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bazykin G, Stanevich O, Danilenko D. et al. Emergence of Y453F and Δ69-70HV mutations in a lymphoma patient with long-term COVID-19. (Ed.^(Eds) (2021)
- 67.Borges V, Isidro J, Cunha M. et al. Long-term evolution of SARS-CoV-2 in an immunocompromised patient with non-Hodgkin lymphoma. (Ed.^(Eds) [DOI] [PMC free article] [PubMed]
- 68.Truong TT, Ryutov A, Pandey U, et al. Persistent SARS-CoV-2 infection and increasing viral variants in children and young adults with impaired humoral immunity. bioRxiv. 2020 Dec 28:2020.12.28.424451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andreano E, Piccini G, Licastro D, et al. SARS-CoV-2 escape in vitro from a highly neutralizing COVID-19 convalescent plasma. bioRxiv. 2020 Dec 28:2020.12.28.424451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. biorXiv [Preprint]. (Ed.^(Eds) (2021) 2021.2001.2015… 426911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Valesano AL, Rumfelt KE, Dimcheff DE, et al. Temporal dynamics of SARS-CoV-2 mutation accumulation within and across infected hosts. 2021;2001(2019):427330 (2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kemp SA, Collier DA, Datir R, et al. Neutralising antibodies in Spike mediated SARS-CoV-2 adaptation. Nature. 2020;2020(2012):2005.20241927. [Google Scholar]
- 73.Franchini M, Marano G, Velati C, et al. Operational protocol for donation of anti-COVID-19 convalescent plasma in Italy. Vox Sang. 2020;116(1):136–137. [DOI] [PMC free article] [PubMed] [Google Scholar]


