Abstract
Kidney stones are painful, common, and increasing in incidence. Obesity and bariatric surgery rates are also on the rise in the United States. Although bariatric surgery is associated with improvements in metabolic outcomes, malabsorptive bariatric surgery procedures are also associated with increased risk of kidney stones. Restrictive bariatric surgeries have not been associated with kidney-stone risk. Higher risk of kidney stones after malabsorptive procedures is associated with postsurgical changes in urine composition, including high urine oxalate, low urine citrate, and low urine volume. Certain dietary recommendations after surgery may help mitigate these urine changes and reduce risk of kidney stones. Understanding risk of kidney stones after surgery is essential to improving patient outcomes after bariatric surgery.
Keywords: nephrolithiasis, bariatric surgery, kidney calculi, risk factors
Introduction
Kidney stones are a major cause of morbidity in the United States (1–4). The lifetime prevalence is estimated at 9% and expected to rise (5). Kidney stones are associated with acute pain and chronic conditions, such as bone disease (6,7), CKD (8,9), hypertension (10,11), and coronary heart disease (12,13). Prevention of kidney stones is critical to improving patient outcomes. Clinical prevention of kidney stones focuses on modifying risk factors for kidney stone formation. Obesity and certain bariatric surgeries are important clinical risk factors for kidney stones, particularly given that rates of both are on the rise in the United States (14–16). This review will discuss the risk of kidney stones after bariatric surgery and recommendations for mitigating that risk.
Obesity and Bariatric Surgery
Obesity and metabolic syndrome are well-established risk factors for kidney stones. Higher body mass index (BMI), larger body size, and weight gain are each strongly associated with higher risk of kidney stones in men and women (17,18). For example, compared with a BMI of 21–22.9 kg/m2, the multivariate relative risk for developing kidney stones with a BMI of ≥30 kg/m2 is 2.09 in young women, 1.90 in older women, and 1.33 in men (18). Furthermore, diabetes mellitus (19) and hypertension (20) are both independently associated with higher risk of developing kidney stones.
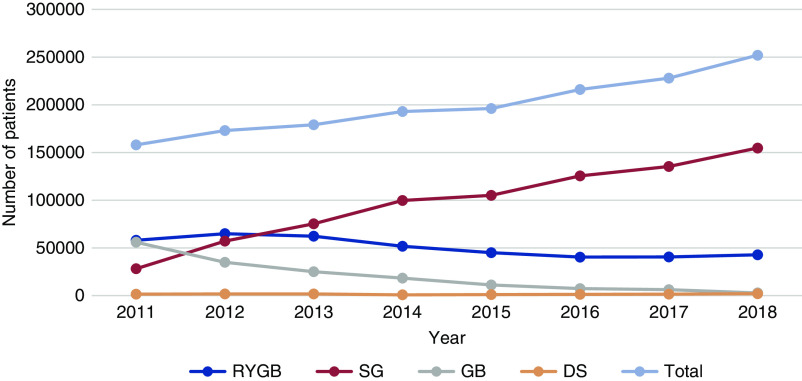
Bariatric surgery is a surgical option for the management of morbid obesity. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are currently the most common bariatric surgical procedures in the United States, making up nearly 80% of the 252,000 bariatric surgeries in 2018 (Figure 1) (21). Other procedures that were previously popular, such as adjustable gastric band and biliopancreatic diversion with duodenal switch, are now less common and account for <2% of procedures (Figure 1) (21). RYGB and SG are also the two most common procedures performed outside of the United States, but there is some variability between rates of RYGB versus SG (22). For example, RYGB makes up 84% of bariatric surgeries in Canada, whereas SG makes up 100% of bariatric surgeries in Australia (22). In general, bariatric surgery is much more common in women, with female patients accounting for nearly 80% of all bariatric surgery procedures (15,22,23). RYGB is both a restrictive and malabsorptive procedure involving the creation of a small, proximal, stomach pouch that connects to a more distal part of the small intestine (24). SG is a purely restrictive procedure with the creation of a small stomach pouch (24). RYGB and SG are highly effective in management of morbid obesity, with significant weight loss and improvement in metabolic syndrome and cardiovascular outcomes (25–28).
Figure 1.
Trend in more overall bariatric surgery procedures and more sleeve gastrectomies over time in the United States. DS, duodenal switch; GB gastric band; RYGB, Roux-en-Y gastric bypass; SG, sleeve gastrectomy. Data from https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
Bariatric Surgery and Kidney-Stone Risk
Despite the many positive metabolic outcomes, RYGB is also associated with higher risk of kidney stones (29–33) and bone disease (34–38) after surgery. Three years after surgery, new kidney stone incidence is 8% (30), and this continues to rise to 14% (32) 10 years after surgery. In comparison, the rate for kidney stones in controls with obesity is 5% and 7% at 3 and 10 years, respectively (30,32). Similarly, the multivariate hazards ratio of developing a kidney stone is 2.3 for patients who have had RYGB compared with controls with obesity (32). In contrast, restrictive procedures, including SG, have not been associated with risk of kidney stones or fracture (32). Given that SG has increased in popularity only in the past few years, most studies of restrictive procedures and kidney stones have primarily included patients with adjustable gastric bands (31,39). One study that examined incidence rates in 85 patients with an SG found that only 1% of patients with an SG developed a stone after a mean of just over 2 years of follow-up (40). A recent, retrospective review found that 8% of patients who underwent RYGB surgery compared with 4% of patients with an SG (P<0.001) developed a kidney stone postoperatively, with a mean follow-up of nearly 3 years (41). However, additional studies of patients who have undergone an SG with longer follow-up time are needed to better characterize the overall stone risk associated with SG.
Kidney Stone Type and Urine Composition Changes after RYGB Surgery
Calcium oxalate stones are the most common stone type reported after RYGB (29,32,42). Prior studies have found that mean 24-hour urine calcium oxalate supersaturation (CaOx SS) increases post-RYGB (42–44). Similarly, one study found that individuals who form kidney stones after RYGB had higher 24-hour urine CaOx SS compared with those who did not form kidney stones after RYGB (32). These findings are consistent with data in patients without bariatric surgery that show higher urine CaOx SS is associated with higher risk of being a kidney-stone former (45). For this reason, understanding postsurgical changes in urine CaOx SS and its major contributors can provide insight into why some individuals form kidney stones after surgery. The primary individual urine components that influence urine CaOx SS level include urine calcium, oxalate, citrate, and volume (46). High levels of urine calcium or oxalate, or low levels of urine volume or citrate, each individually increase urine CaOx SS and kidney stone risk (46–48). After surgery, patients who have undergone RYGB surgery may experience changes in one or more of these urine components which leads to higher urine CaOx SS. Common alterations in urine composition seen after surgery include high urine oxalate, low urine volume, and low urine citrate.
Hyperoxaluria
High urine oxalate is one of the most prominent factors associated with higher CaOx SS and, thus, higher kidney-stone risk after malabsorptive bariatric surgery. This is unique to the malabsorptive procedures, such as RYGB, because the restrictive procedures have not been associated with high urine oxalate (31,39). Multiple studies have demonstrated that mean urine-oxalate levels rise significantly after RYGB when preoperative oxalate excretion was compared with postoperative oxalate excretion 6–12 months after surgery (Table 1) (39,42–44,49–57). For example, in a study of 151 patients who underwent RYGB surgery, Valezi et al. (53) found that urine oxalate increased from 24 mg/d pre-RYGB to 41 mg/d 12 months post-RYGB. In addition, some (56), but not all (58), studies in which patients with obesity were compared with patients who had undergone RYGB showed higher urine oxalate in the patients who had undergone surgery. In one cross-sectional study, 19 patients who had undergone RYGB surgery had a mean urine oxalate of 45 mg/d, compared with 30 mg/d in controls with obesity (56). Notably, both of these levels are well above the level of urine oxalate at which risk of kidney stones begins to rise (20–25 mg/d) (47). Frank hyperoxaluria, levels of oxalate above the normal range, occur in a significant number of patients after RYGB, which has been estimated at 42%–67% after at least 6 months of follow-up (50,55,56). In contrast, a recent study by Moreland et al. (57) found no difference in mean urine-oxalate levels in patients before versus after RYGB (61 versus 69 mg/d; P=0.92). However, given that urine-oxalate levels were very high both before and after surgery, high urine-oxalate levels were still an important finding in that study and a contributor to kidney-stone risk in those patients.
Table 1.
Changes in 24-hour urine parameters in prospective studies of patients who have undergone bariatric surgery
| Study | Procedure | N | Time to FU (mo) | Sex (n M/F) | CaOx SS | Oxalate | Calcium | Citrate | Volume |
| Park et al. (50) | RYGB | 45 | 6–12 | 8/37 | ↑ | ↑ | ↓ | ↓ | ↓ |
| Duffey et al. (55) | RYGB | 21 | 24 | 5/16 | No change | ↑ | ↓ | ↓ | No change |
| Kumar et al. (52) | RYGB/BPD | 11 | 12 | 0/11 | ↑ | No change | No change | No change | No change |
| Wu et al. (54) | RYGB | 38 | 6 | 7/31 | ↑ | ↑ | ↑ | No change | ↓ |
| Valezi et al. (53) | RYGB | 151 | 12 | 42/109 | Not reported | ↑ | ↓ | ↓ | ↓ |
| Agrawal et al. (43) | RYGB | 13 | 6 | 2/11 | ↑ | ↑ | No change | ↓ | No change |
| Moreland et al. (57) | RYGB | 26 | 12 | 9/17 | No change | No change | Not reported | Not reported | Not reported |
Patients were studied before bariatric surgery and at the specified time point postoperatively. Most patients were nonstone formers. FU, follow-up; M, male; F, female; CaOx SS, calcium oxalate supersaturation; RYGB, Roux-en-y gastric bypass; BPD, biliopancreatic diversion with duodenal switch.
The high urinary oxalate pre-RYGB is likely related to the association between obesity and hyperoxaluria. Hyperoxaluria in obesity is thought to be secondary to inflammation causing increased oxalate absorption in the gut (59,60). High urine oxalate after a malabsorptive bariatric surgery is described as enteric hyperoxaluria. Enteric hyperoxaluria is the process of enhanced gut absorption of dietary oxalate that occurs in the setting of fat malabsorption (52,61,62). In the postsurgical gut, higher levels of free fatty acids bind to dietary calcium. This lowers the amount of calcium available in the gut to precipitate with dietary oxalate and leads to higher levels of unbound oxalate in the gut. This unbound oxalate is more likely to be absorbed by the gut. Additionally, higher levels of bile salts and fatty acids increase the permeability of oxalate in the colon (63). As a result, more oxalate is absorbed in the colon (64) and this oxalate is eventually excreted in the urine. Urine-oxalate levels begin to increase within months of surgery (42,43,51) and may remain high for years after surgery (39).
Urine Volume and Citrate
Low urine volume (43,53,54) and citrate (50,53,55,56) excretion are two other urine-composition changes that have been described after RYGB, although less consistently than increased oxalate, and both are important to understanding kidney -tone risk in this population (Table 1) (32,49,50). For example, Agrawal et al. (43) studied 13 patients from pre- to post-RYGB and found that, in addition to increased urine oxalate, urine volume decreased from 2.1 L to 1.4 L and urine citrate decreased from 540 mg/d to 305 mg/d. Other studies have found that the citrate is lower postoperatively in malabsorptive procedures compared with restrictive procedures. Penniston et al. (39) collected urine in patients with a gastric band and those with an RYGB for 3 years after surgery and found much lower urine citrate in patients with the RYGB compared with those with a gastric band. In the Penniston et al. study, both groups had low urine volume (39). Both Lieske et al. (32) and Valezi et al. (53) found that urine citrate was lower in those patients who formed stones after RYGB compared with those that did not. The etiology for low urine volume may be partially related to low fluid intake due to the restrictive nature of the procedure. Low urine volume may also be related to water losses in stool because diarrhea is common after RYGB (65), and may be more common in RYGB compared with SG (66). To our knowledge, this has not been more fully studied in the context of kidney-stone risk. Low urine citrate may be related to metabolic acidosis, as seen in other states of acid retention (67,68). Two studies that reported urine ammonia excretion found that it was higher after RYGB compared with either controls with obesity or normal subjects, suggesting increased acid excretion (51,56). Additional study is needed to better understand why urine volume and citrate levels are low in patients who have undergone bariatric surgery.
Diet and Supplements
Diet, including specific food choices and certain diet patterns, is a well-established risk factor for kidney stones in individuals who have not had bariatric surgery. For example, diets low in fruits and vegetables are associated with low urine citrate (69), and diets high in oxalate are associated with high urine oxalate (70). A low-calcium diet is associated with higher kidney stone recurrence compared with a normal-calcium, low-salt diet (71). The Dietary Approaches to Stop Hypertension diet pattern has been associated with lower kidney-stone risk (69). Low dietary fluid intake associates with low urine volume (72) and, thus, to higher risk of being a stone former (47).
Due to postsurgical anatomy, patients who have undergone bariatric surgery have unique dietary limitations and nutritional and supplemental requirements. Such patients receive dietician guidance before and after surgery. However, adherence to diet recommendations is poor both before and after surgery (73,74). There has been very limited study of diet and kidney-stone risk in patients who have undergone bariatric surgery. In one small study of diet in patients after RYGB surgery, Pang et al. (75) measured 24-hour urine in six patients after RYGB surgery who were on an individual home diet and after 4 days on a controlled diet. The controlled diet contained limited oxalate (70–80 mg/d), the recommended daily amount of calcium (1000 mg), and moderate sodium. The investigators found urinary oxalate did not statistically change from home diet to controlled diet. Interestingly, they did find that CaOx SS was lower on the controlled diet, despite the lack of statistical difference in urine oxalate, volume, citrate, or calcium on the home versus controlled diet (75). This may highlight the concept that a multimodal approach is key to managing kidney-stone risk in this patient population. Other studies have found that limiting dietary oxalate in patients after bariatric surgery is important for urine-oxalate levels. Froeder et al. (58) gave study participants an oral oxalate load and measured urine-oxalate levels for 6 hours. The urine-oxalate levels peaked 4 hours after the oral load and were higher in patients postsurgery compared with both patients who were presurgery and those with obesity (58). This demonstrates that, due to enteric hyperoxaluria, patients absorb more of their dietary oxalate after RYGB surgery.
There have not been larger studies in patients with an RYGB reviewing home versus controlled diets to further guide dietary recommendations for reducing kidney-stone risk. To our knowledge, there are no studies guiding a recommended daily intake for oxalate in the bariatric surgery patient population. Most experts on kidney stones agree that common dietary guidance should include recommendations to avoid high-oxalate foods. One good source for oxalate content of individual foods can be found at https://regepi.bwh.harvard.edu/health/Oxalate/files. This source provides lists of high- and low-oxalate-content foods and can help guide patients when making food choices with the goal to reduce hyperoxaluria. Patients should also consume at least the daily recommended amount of dietary calcium after bariatric surgery. An additional recommendation to reduce hyperoxaluria is to time calcium supplement intake with food. This has not been demonstrated to be effective in patients who have undergone bariatric surgery but, in nonbariatric patient populations, this strategy is often used to reduce urine-oxalate levels. Adequate dietary calcium and taking supplemental calcium with food may lower urine-oxalate levels because the calcium binds with dietary oxalate in the gut and the oxalate-calcium complex is unabsorbed and excreted by the gut (76–78). Some providers have also recommended reducing dietary fat intake to reduce fatty acids in the gut and the effect of enteric hyperoxaluria. To our knowledge, this has not been formally studied in modern RYGB procedures but has been shown to be effective in early malabsorptive procedures, such as jejunoileal bypass (79,80). Other dietary recommendations for reducing kidney stones in patients who have had bariatric surgery include increasing fluid intake to increase urinary volume. This recommendation should be attempted, while keeping in mind that a restricted stomach size may make this difficult to achieve. It is often recommended to consume fluids between meals instead of with the meal. In addition, a citrate-rich diet (fruits and vegetables) and consideration of use of citrate supplementation (often prescribed as potassium citrate) may increase urine citrate and reduce overall kidney-stone risk (33,81,82). Finally, study of potassium calcium citrate, an effervescent form of calcium and alkali supplementation, has been shown to increase urine citrate and decrease bone resorption after RYGB and, thus, may be a therapeutic option for these patients (83,84).
Conclusions
Bariatric surgery is effective at treating obesity and metabolic syndrome, but certain procedures, notably RYGB, are associated with increased calcium oxalate kidney-stone risk. Higher kidney-stone risk after RYGB is related to high CaOx SS from high urine oxalate, low urine citrate, and low urine volume. Restrictive bariatric surgery procedures have not been associated with the same alterations in urine composition or kidney-stone risk. Clinical management to reduce risk of kidney stones is focused on dietary modifications, although dietary studies in this population have been limited. Common recommendations include a low-oxalate diet, timing calcium supplementation with meals, increased fluid intake, and possible citrate supplementation. As obesity, metabolic syndrome, and bariatric surgery rates continue to rise, these strategies will be crucial for reducing kidney-stone risk and improving outcomes in patients who undergo bariatric surgery.
Disclosures
E. Worcester reports being a member of the Kidney Health Initiative Enteric Hyperoxaluria group and receiving personal fees from Allena, Alnylam, Bridgebio Pharmaceutical, Dicerna, and OxThera, outside the submitted work. The remaining author has nothing to disclose.
Funding
None.
Author Contributions
M. Prochaska conceptualized the study and wrote the original draft; E. Worcester provided supervision and reviewed and edited the manuscript.
References
- 1.Pearle MS, Calhoun EA, Curhan GC; The Urologic Diseases of America Project: Urologic diseases in America project: Urolithiasis. J Urol 173: 848–857, 2005. 10.1097/01.ju.0000152082.14384.d7 [DOI] [PubMed] [Google Scholar]
- 2.Hyams ES, Matlaga BR: Economic impact of urinary stones. Transl Androl Urol 3: 278–283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litwin MS, Saigal CS: Table 14-47: Economic Impact of Urologic Disease. Urologic Diseases in America, Washington, D.C., National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Public Health Service, US Department of Health and Human Services, 2012 [Google Scholar]
- 4.Feldstein L, Matlaga B: Urologic Diseases in America. NIH Publication No. 12-7865, Washington, D.C., US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018 [Google Scholar]
- 5.Scales CD Jr, Smith AC, Hanley JM, Saigal CS; Urologic Diseases in America Project: Prevalence of kidney stones in the United States. Eur Urol 62: 160–165, 2012. 10.1016/j.eururo.2012.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakhaee K, Maalouf NM, Kumar R, Pasch A, Moe OW: Nephrolithiasis-associated bone disease: Pathogenesis and treatment options. Kidney Int 79: 393–403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor EN, Feskanich D, Paik JM, Curhan GC: Nephrolithiasis and risk of incident bone fracture. J Urol 195: 1482–1486, 2016. 10.1016/j.juro.2015.12.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rule AD, Bergstralh EJ, Melton LJ 3rd, Li X, Weaver AL, Lieske JC: Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4: 804–811, 2009. 10.2215/CJN.05811108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhondup T, Kittanamongkolchai W, Vaughan LE, Mehta RA, Chhina JK, Enders FT, Hickson LJ, Lieske JC, Rule AD: Risk of ESRD and mortality in kidney and bladder stone formers. Am J Kidney Dis 72: 790–797, 2018. 10.1053/j.ajkd.2018.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madore F, Stampfer MJ, Willett WC, Speizer FE, Curhan GC: Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis 32: 802–807, 1998. 10.1016/S0272-6386(98)70136-2 [DOI] [PubMed] [Google Scholar]
- 11.Cappuccio FP, Strazzullo P, Mancini M: Kidney stones and hypertension: Population based study of an independent clinical association. BMJ 300: 1234–1236, 1990. 10.1136/bmj.300.6734.1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rule AD, Roger VL, Melton LJ 3rd, Bergstralh EJ, Li X, Peyser PA, Krambeck AE, Lieske JC: Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 21: 1641–1644, 2010. 10.1681/ASN.2010030253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferraro PM, Taylor EN, Eisner BH, Gambaro G, Rimm EB, Mukamal KJ, Curhan GC: History of kidney stones and the risk of coronary heart disease. JAMA 310: 408–415, 2013. 10.1001/jama.2013.8780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL: Trends in obesity among adults in the United States, 2005 to 2014. JAMA 315: 2284–2291, 2016. 10.1001/jama.2016.6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Angrisani L, Santonicola A, Iovino P, Vitiello A, Zundel N, Buchwald H, Scopinaro N: Bariatric surgery and endoluminal procedures: IFSO worldwide survey 2014 [published correction appears in Obes Surg 27: 2290–2292, 2017 10.1007/s11695-017-2773-8]. Obes Surg 27: 2279–2289, 2017. 10.1007/s11695-017-2666-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L: Changes in utilization of bariatric surgery in the United States from 1993 to 2016. Ann Surg 271: 201–209, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Curhan GC, Willett WC, Rimm EB, Speizer FE, Stampfer MJ: Body size and risk of kidney stones. J Am Soc Nephrol 9: 1645–1652, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Taylor EN, Stampfer MJ, Curhan GC: Obesity, weight gain, and the risk of kidney stones. JAMA 293: 455–462, 2005. 10.1001/jama.293.4.455 [DOI] [PubMed] [Google Scholar]
- 19.Taylor EN, Stampfer MJ, Curhan GC: Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 68: 1230–1235, 2005. 10.1111/j.1523-1755.2005.00516.x [DOI] [PubMed] [Google Scholar]
- 20.Borghi L, Meschi T, Guerra A, Briganti A, Schianchi T, Allegri F, Novarini A: Essential arterial hypertension and stone disease. Kidney Int 55: 2397–2406, 1999. 10.1046/j.1523-1755.1999.00483.x [DOI] [PubMed] [Google Scholar]
- 21.American Society for Metabolic and Bariatric Surgery: Estimate of bariatric surgery numbers, 2011–2018. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed October 13, 2020
- 22.International Federation for the Surgery of Obesity and Metabolic Disorders (IFSO): 5th IFSO global registry report, 2019. Available at: https://www.ifso.com/pdf/5th-ifso-global-registry-report-september-2019.pdf. Accessed October 13, 2020
- 23.Kochkodan J, Telem DA, Ghaferi AA: Physiologic and psychological gender differences in bariatric surgery. Surg Endosc 32: 1382–1388, 2018. 10.1007/s00464-017-5819-z [DOI] [PubMed] [Google Scholar]
- 24.Alverdy JC, Prachand V, Flanagan B, Thistlethwaite WA, Siegler M, Garfinkel M, Angelos P, Agarwal S, Santry H: Bariatric surgery: A history of empiricism, a future in science. J Gastrointest Surg 13: 465–477, 2009. 10.1007/s11605-008-0742-1 [DOI] [PubMed] [Google Scholar]
- 25.Adams TD, Davidson LE, Litwin SE, Kolotkin RL, LaMonte MJ, Pendleton RC, Strong MB, Vinik R, Wanner NA, Hopkins PN, Gress RE, Walker JM, Cloward TV, Nuttall RT, Hammoud A, Greenwood JL, Crosby RD, McKinlay R, Simper SC, Smith SC, Hunt SC: Health benefits of gastric bypass surgery after 6 years. JAMA 308: 1122–1131, 2012. 10.1001/2012.jama.11164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams TD, Davidson LE, Litwin SE, Kim J, Kolotkin RL, Nanjee MN, Gutierrez JM, Frogley SJ, Ibele AR, Brinton EA, Hopkins PN, McKinlay R, Simper SC, Hunt SC: Weight and metabolic outcomes 12 years after gastric bypass. N Engl J Med 377: 1143–1155, 2017. 10.1056/NEJMoa1700459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, Kattan MW, Nissen SE: Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 322: 1271–1282, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, Patterson EJ, Pender JR, Pomp A, Pories WJ, Thirlby RC, Yanovski SZ, Wolfe BM; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium: Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 310: 2416–2425, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson WK, Houghton SG, Milliner DS, Lieske JC, Sarr MG: Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: Potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis 1: 481–485, 2005. 10.1016/j.soard.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA: Effect of gastric bypass surgery on kidney stone disease. J Urol 181: 2573–2577, 2009. 10.1016/j.juro.2009.02.029 [DOI] [PubMed] [Google Scholar]
- 31.Semins MJ, Asplin JR, Steele K, Assimos DG, Lingeman JE, Donahue S, Magnuson T, Schweitzer M, Matlaga BR: The effect of restrictive bariatric surgery on urinary stone risk factors. Urology 76: 826–829, 2010. 10.1016/j.urology.2010.01.037 [DOI] [PubMed] [Google Scholar]
- 32.Lieske JC, Mehta RA, Milliner DS, Rule AD, Bergstralh EJ, Sarr MG: Kidney stones are common after bariatric surgery. Kidney Int 87: 839–845, 2015. 10.1038/ki.2014.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canales BK, Gonzalez RD: Kidney stone risk following Roux-en-Y gastric bypass surgery. Transl Androl Urol 3: 242–249, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu EW, Lee MP, Landon JE, Lindeman KG, Kim SC: Fracture risk after bariatric surgery: Roux-en-Y gastric bypass versus adjustable gastric banding. J Bone Miner Res 32: 1229–1236, 2017. 10.1002/jbmr.3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Wu D, Zhang J-F, Xu D, Xu WF, Chen Y, Liu BY, Li P, Li L: Changes in bone metabolism in morbidly obese patients after bariatric surgery: A meta-analysis. Obes Surg 26: 91–97, 2016. 10.1007/s11695-015-1724-5 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura KM, Haglind EGC, Clowes JA, Achenbach SJ, Atkinson EJ, Melton LJ 3rd, Kennel KA: Fracture risk following bariatric surgery: A population-based study. Osteoporos Int 25: 151–158, 2014. 10.1007/s00198-013-2463-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu EW, Bouxsein ML, Putman MS, Monis EL, Roy AE, Pratt JS, Butsch WS, Finkelstein JS: Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab 100: 1452–1459, 2015. 10.1210/jc.2014-4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagnon C, Schafer AL: Bone health after bariatric surgery. JBMR Plus 2: 121–133, 2018. 10.1002/jbm4.10048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penniston KL, Kaplon DM, Gould JC, Nakada SY: Gastric band placement for obesity is not associated with increased urinary risk of urolithiasis compared to bypass. J Urol 182: 2340–2346, 2009. 10.1016/j.juro.2009.07.041 [DOI] [PubMed] [Google Scholar]
- 40.Chen T, Godebu E, Horgan S, Mirheydar HS, Sur RL: The effect of restrictive bariatric surgery on urolithiasis. J Endourol 27: 242–244, 2013. 10.1089/end.2012.0408 [DOI] [PubMed] [Google Scholar]
- 41.Mishra T, Shapiro JB, Ramirez L, Kallies KJ, Kothari SN, Londergan TA: Nephrolithiasis after bariatric surgery: A comparison of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy. Am J Surg 219: 952–957, 2020. 10.1016/j.amjsurg.2019.09.010 [DOI] [PubMed] [Google Scholar]
- 42.Sinha MK, Collazo-Clavell ML, Rule A, Milliner DS, Nelson W, Sarr MG, Kumar R, Lieske JC: Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int 72: 100–107, 2007. 10.1038/sj.ki.5002194 [DOI] [PubMed] [Google Scholar]
- 43.Agrawal V, Liu XJ, Campfield T, Romanelli J, Enrique Silva J, Braden GL: Calcium oxalate supersaturation increases early after Roux-en-Y gastric bypass. Surg Obes Relat Dis 10: 88–94, 2014. 10.1016/j.soard.2013.03.014 [DOI] [PubMed] [Google Scholar]
- 44.Upala S, Jaruvongvanich V, Sanguankeo A: Risk of nephrolithiasis, hyperoxaluria, and calcium oxalate supersaturation increased after Roux-en-Y gastric bypass surgery: A systematic review and meta-analysis. Surg Obes Relat Dis 12: 1513–1521, 2016. 10.1016/j.soard.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 45.Prochaska M, Taylor E, Ferraro PM, Curhan G: Relative supersaturation of 24-hour urine and likelihood of kidney stones. J Urol 199: 1262–1266, 2018. 10.1016/j.juro.2017.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werness PG, Brown CM, Smith LH, Finlayson B: EQUIL2: A BASIC computer program for the calculation of urinary saturation. J Urol 134: 1242–1244, 1985. 10.1016/S0022-5347(17)47703-2 [DOI] [PubMed] [Google Scholar]
- 47.Curhan GC, Taylor EN: 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008. 10.1038/sj.ki.5002708 [DOI] [PubMed] [Google Scholar]
- 48.Curhan GC, Willett WC, Speizer FE, Stampfer MJ: Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney Int 59: 2290–2298, 2001. 10.1046/j.1523-1755.2001.00746.x [DOI] [PubMed] [Google Scholar]
- 49.Asplin JR, Coe FL: Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol 177: 565–569, 2007. 10.1016/j.juro.2006.09.033 [DOI] [PubMed] [Google Scholar]
- 50.Park AM, Storm DW, Fulmer BR, Still CD, Wood GC, Hartle JE 2nd: A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol 182: 2334–2339, 2009. 10.1016/j.juro.2009.07.044 [DOI] [PubMed] [Google Scholar]
- 51.Patel BN, Passman CM, Fernandez A, Asplin JR, Coe FL, Kim SC, Lingeman JE, Assimos DG: Prevalence of hyperoxaluria after bariatric surgery. J Urol 181: 161–166, 2009. 10.1016/j.juro.2008.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar R, Lieske JC, Collazo-Clavell ML, Sarr MG, Olson ER, Vrtiska TJ, Bergstralh EJ, Li X: Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 149: 654–661, 2011. 10.1016/j.surg.2010.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valezi AC, Fuganti PE, Junior JM, Delfino VD: Urinary evaluation after RYGBP: A lithogenic profile with early postoperative increase in the incidence of urolithiasis. Obes Surg 23: 1575–1580, 2013. 10.1007/s11695-013-0916-0 [DOI] [PubMed] [Google Scholar]
- 54.Wu JN, Craig J, Chamie K, Asplin J, Ali MR, Low RK: Urolithiasis risk factors in the bariatric population undergoing gastric bypass surgery. Surg Obes Relat Dis 9: 83–87, 2013. 10.1016/j.soard.2011.08.024 [DOI] [PubMed] [Google Scholar]
- 55.Duffey BG, Alanee S, Pedro RN, Hinck B, Kriedberg C, Ikramuddin S, Kellogg T, Stessman M, Moeding A, Monga M: Hyperoxaluria is a long-term consequence of Roux-en-Y gastric bypass: A 2-year prospective longitudinal study. J Am Coll Surg 211: 8–15, 2010. 10.1016/j.jamcollsurg.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 56.Maalouf NM, Tondapu P, Guth ES, Livingston EH, Sakhaee K: Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol 183: 1026–1030, 2010. 10.1016/j.juro.2009.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreland AM, Santa Ana CA, Asplin JR, Kuhn JA, Holmes RP, Cole JA, Odstrcil EA, Van Dinter TG Jr, Martinez JG, Fordtran JS: Steatorrhea and hyperoxaluria in severely obese patients before and after Roux-en-Y gastric bypass. Gastroenterology 152: 1055–1067.e3, 2017. 10.1053/j.gastro.2017.01.004 [DOI] [PubMed] [Google Scholar]
- 58.Froeder L, Arasaki CH, Malheiros CA, Baxmann AC, Heilberg IP: Response to dietary oxalate after bariatric surgery. Clin J Am Soc Nephrol 7: 2033–2040, 2012. 10.2215/CJN.02560312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amin R, Asplin J, Jung D, Bashir M, Alshaikh A, Ratakonda S, Sharma S, Jeon S, Granja I, Matern D, Hassan H: Reduced active transcellular intestinal oxalate secretion contributes to the pathogenesis of obesity-associated hyperoxaluria. Kidney Int 93: 1098–1107, 2018. 10.1016/j.kint.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakhaee K: Unraveling the mechanisms of obesity-induced hyperoxaluria. Kidney Int 93: 1038–1040, 2018. 10.1016/j.kint.2018.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Asplin JR: The management of patients with enteric hyperoxaluria. Urolithiasis 44: 33–43, 2016. 10.1007/s00240-015-0846-5 [DOI] [PubMed] [Google Scholar]
- 62.Nazzal L, Puri S, Goldfarb DS: Enteric hyperoxaluria: An important cause of end-stage kidney disease. Nephrol Dial Transplant 31: 375–382, 2016. 10.1093/ndt/gfv005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caspary WF, Tönissen J, Lankisch PG: ‘Enteral’ hyperoxaluria. Effect of cholestyramine, calcium, neomycin, and bile acids on intestinal oxalate absorption in man. Acta Hepatogastroenterol (Stuttg) 24: 193–200, 1977 [PubMed] [Google Scholar]
- 64.Dobbins JW, Binder HJ: Importance of the colon in enteric hyperoxaluria. N Engl J Med 296: 298–301, 1977. 10.1056/NEJM197702102960602 [DOI] [PubMed] [Google Scholar]
- 65.Borbély YM, Osterwalder A, Kröll D, Nett PC, Inglin RA: Diarrhea after bariatric procedures: Diagnosis and therapy. World J Gastroenterol 23: 4689–4700, 2017. 10.3748/wjg.v23.i26.4689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramadan M, Loureiro M, Laughlan K, Caiazzo R, Iannelli A, Brunaud L, Czernichow S, Nedelcu M, Nocca D: Risk of dumping syndrome after sleeve gastrectomy and Roux-en-Y gastric bypass: Early results of a multicentre prospective study. Gastroenterol Res Pract 2016: 2570237, 2016. 10.1155/2016/2570237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gianella FG, Prado VE, Poindexter JR, Adams-Huet B, Li X, Miller RT, Sakhaee K, Maalouf NM, Moe OW: Spot urinary citrate-to-creatinine ratio is a marker for acid-base status in chronic kidney disease [published online ahead of print July 25, 2020]. Kidney Int 10.1016/j.kint.2020.07.006 [DOI] [PubMed] [Google Scholar]
- 68.Madias NE. Metabolic acidosis and CKD progression [published online ahead of print August 7, 2020]. Clin J Am Soc Nephrol 10.2215/CJN.07990520 [DOI] [PMC free article] [PubMed]
- 69.Taylor EN, Stampfer MJ, Mount DB, Curhan GC: DASH-style diet and 24-hour urine composition. Clin J Am Soc Nephrol 5: 2315–2322, 2010. 10.2215/CJN.04420510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taylor EN, Curhan GC: Determinants of 24-hour urinary oxalate excretion. Clin J Am Soc Nephrol 3: 1453–1460, 2008. 10.2215/CJN.01410308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Borghi L, Schianchi T, Meschi T, Guerra A, Allegri F, Maggiore U, Novarini A: Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 346: 77–84, 2002. 10.1056/NEJMoa010369 [DOI] [PubMed] [Google Scholar]
- 72.Borghi L, Meschi T, Amato F, Briganti A, Novarini A, Giannini A: Urinary volume, water and recurrences in idiopathic calcium nephrolithiasis: A 5-year randomized prospective study. J Urol 155: 839–843, 1996. 10.1016/S0022-5347(01)66321-3 [DOI] [PubMed] [Google Scholar]
- 73.Toussi R, Fujioka K, Coleman KJ: Pre- and postsurgery behavioral compliance, patient health, and postbariatric surgical weight loss. Obesity (Silver Spring) 17: 996–1002, 2009. 10.1038/oby.2008.628 [DOI] [PubMed] [Google Scholar]
- 74.Hood MM, Corsica J, Bradley L, Wilson R, Chirinos DA, Vivo A: Managing severe obesity: Understanding and improving treatment adherence in bariatric surgery. J Behav Med 39: 1092–1103, 2016. 10.1007/s10865-016-9772-4 [DOI] [PubMed] [Google Scholar]
- 75.Pang R, Linnes MP, O’Connor HM, Li X, Bergstralh E, Lieske JC: Controlled metabolic diet reduces calcium oxalate supersaturation but not oxalate excretion after bariatric surgery. Urology 80: 250–254, 2012. 10.1016/j.urology.2012.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Curhan GC, Willett WC, Speizer FE, Spiegelman D, Stampfer MJ: Comparison of dietary calcium with supplemental calcium and other nutrients as factors affecting the risk for kidney stones in women. Ann Intern Med 126: 497–504, 1997. 10.7326/0003-4819-126-7-199704010-00001 [DOI] [PubMed] [Google Scholar]
- 77.Taylor EN, Curhan GC: Dietary calcium from dairy and nondairy sources, and risk of symptomatic kidney stones. J Urol 190: 1255–1259, 2013. 10.1016/j.juro.2013.03.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Penniston KL, Nakada SY: Effect of dietary changes on urinary oxalate excretion and calcium oxalate supersaturation in patients with hyperoxaluric stone formation. Urology 73: 484–489, 2009. 10.1016/j.urology.2008.10.035 [DOI] [PubMed] [Google Scholar]
- 79.Nordenvall B, Backman L, Burman P, Larsson L, Tiselius HG: Low-oxalate, low-fat dietary regimen in hyperoxaluria following jejunoileal bypass. Acta Chir Scand 149: 89–91, 1983 [PubMed] [Google Scholar]
- 80.Andersson H, Jagenburg R: Fat-reduced diet in the treatment of hyperoxaluria in patients with ileopathy. Gut 15: 360–366, 1974. 10.1136/gut.15.5.360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Espino-Grosso PM, Canales BK: Kidney stones after bariatric surgery: Risk assessment and mitigation. Bariatr Surg Pract Patient Care 12: 3–9, 2017. 10.1089/bari.2016.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pearle MS, Goldfarb DS, Assimos DG, Curhan G, Denu-Ciocca CJ, Matlaga BR, Monga M, Penniston KL, Preminger GM, Turk TM, White JR; American Urological Assocation: Medical management of kidney stones: AUA guideline. J Urol 192: 316–324, 2014. 10.1016/j.juro.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 83.Sakhaee K, Pak C: Superior calcium bioavailability of effervescent potassium calcium citrate over tablet formulation of calcium citrate after Roux-en-Y gastric bypass. Surg Obes Relat Dis 9: 743–748, 2013. 10.1016/j.soard.2011.11.011 [DOI] [PubMed] [Google Scholar]
- 84.Sakhaee K, Griffith C, Pak CYC: Biochemical control of bone loss and stone-forming propensity by potassium-calcium citrate after bariatric surgery. Surg Obes Relat Dis 8: 67–72, 2012. 10.1016/j.soard.2011.05.001 [DOI] [PubMed] [Google Scholar]