Abstract
The association of Helicobacter pylori with chronic duodenal ulceration was a seminal observation in the short history of gastroenterology. However, H. pylori is now known to be an ancient bacterium, whereas there is persuasive evidence that the epidemic of duodenal ulceration began in the second half of the 19th century and continued into the second half of the 20th century. Possible explanations for the epidemic include genomic changes in the organism and environmental or other influences on the human host. While genomic changes resulted in the appearance of virulence factors, these seem likely to have appeared thousands of years ago with minimal effects on gastritis because of coexisting suppression of gastric immunity. In contrast, the emergence of duodenal ulceration is best explained by a change in the pattern of gastritis from inflammation involving the antrum and body in most individuals to a significant minority (10–20%) with antral gastritis but with relative sparing of the body of the stomach. In the latter group, the increase in serum gastrin (particularly G17) associated with antral gastritis had trophic effects on gastric parietal cells with an increase in the parietal cell mass and hypersecretion of gastric acid. Hypersecretion of acid is seen as the major risk factor for duodenal ulceration with significant contributions from environmental factors including smoking and use of nonsteroidal, anti‐inflammatory drugs. Host factors favoring changes in the pattern of gastritis include delayed acquisition of infection and improved nutrition; both with enhancing effects on mucosal immunity.
Keywords: chronic duodenal ulcer, gastric acid secretion, gastritis, Helicobacter pylori, ulcer epidemic
The epidemic of chronic duodenal ulcer could be related to genomic changes in Helicobacter pylori or to environmental or other changes in the host. This article favors the latter explanation and attributes the epidemic to the emergence of gastritis largely restricted to the antrum of the stomach. This is likely to have occurred because of enhanced mucosal immunity due to delay in the acquisition of infection and improved nutrition.

Introduction
Helicobacters have been identified in the gastrointestinal tract of most mammals and birds, usually with restriction of particular species to particular hosts (i.e. host specificity). 1 The helicobacter of humans has been called H. pylori and appears to have infected modern humans since their migration from East Africa. 2 Yet duodenal ulceration appeared to be rare prior to the latter part of the 19th century. Subsequently, an increasing number of cases were reported from the United Kingdom and other parts of Europe, and an English surgeon, Berkeley Moynihan, became a celebrity after developing treatment by gastroenterostomy. His book, published in 1910, described the outcomes of 186 patients treated by surgery, most of whom were men (74%) in the age range of 20–60 years. 3
The epidemic of duodenal ulceration appeared to peak in about 1920. This was supported by a study that examined the frequency of perforated peptic ulcer in the United Kingdom and Sweden from late in the 19th century. The papers, published in 1940, documented declining rates in young women but increasing rates in younger and middle‐aged men, particularly after World War 1. 4 These studies were supported by data on mortality from peptic ulceration in England and Wales between 1900 and 1977 and mortality data from various parts of Europe between 1921 and 2004. 5 , 6 A consistent finding was that mortality increased in generations born during the second half of the 19th century but then decreased in all subsequent generations. However, even around the year 2000, a population‐based study from Sweden that included endoscopy revealed duodenal ulcers in 2% of participants. 7
In the current century, incidence rates for duodenal ulcer have substantially declined in most countries, particularly in high income countries. Reasons for this change have been discussed in detail elsewhere but include lower rates of acquisition of H. pylori in children and young adults, eradication of H. pylori with antibiotics, and the widespread use of effective medication to reduce gastric acid secretion. 8 , 9
This review examines the two major hypotheses that have been proposed as explanations for the epidemic of duodenal ulceration; namely that the epidemic was caused by genomic changes in the bacterium or, alternatively, that the epidemic was related to responses to the infection by the human host. The review promotes the latter view and attributes the increased risk of duodenal ulceration to changes in the pattern of gastritis associated with improvements in hygiene and nutrition.
The bacterial hypothesis
H. pylori and humans
H. pylori is a gram negative bacterium that can be transmitted from person‐to‐person by the oral–oral or fecal–oral routes. 10 The organism is not an acidophile (acid‐loving) but is able to colonize the hostile gastric environment using a variety of mechanisms including motility, adhesion proteins, chemotaxis, urease production, and the uptake of nickel into metalloenzymes. 11 Although there are case reports of spontaneous resolution of infection, 12 , 13 most individuals develop a chronic infection with variation in the site and histologic features of changes in the gastric mucosa.
There is persuasive evidence that H. pylori has coevolved with humans for at least 60 000 years and probably longer. This conclusion is based on a comparison of nucleotide sequences of different strains and measurement of mutation rates over time in subjects with two or more positive cultures. 14 There is also evidence that a minority of individuals can have different strains of H. pylori in different regions of the stomach. 15 Whether H. pylori was acquired from a nonhuman host remains unclear but, surprisingly, helicobacters have not been identified in chimpanzees. 2
DNA fingerprinting and sequencing of gene fragments indicate that H. pylori has greater genetic diversity than most other bacterial species. 16 This may reflect a lack of selection for genotypes showing better adaption for humans or, alternatively, that bacteria are adapting in different ways to heterogeneous inflammatory and immune responses by the human gastric mucosa. This genomic diversity has been used to identify differences between strains from different parts of the world and as a tool for tracking human migration. 17 , 18
The diverse clinical presentation of H. pylori has led to a search for bacterial factors that might influence colonization, inflammatory responses, immunologic responses, or carcinogenesis. These have been called virulence factors and were initially focused on variation in genes for the cag pathogenicity island (cagA and others) and vacuolating cytotoxin (vacA). 19 Subsequently, additional virulence factors were identified and categorized as factors affecting colonization, immune responses, and disease induction. Some virulence factors such as CagA, VacA, outer inflammatory protein A (OipA), and blood group binding adhesion A (BabA) have activities in more than one category. 20
Genes for most virulence factors are polymorphic with variation in the secretion of toxins (CagA and VacA), variation in adhesion characteristics (BabA and OipA), and variation in bacterial antigens linked to immune responses. This complexity is amplified by variation within regions of the genes such that there are multiple alleles that may result in higher or lower expression of the virulence protein. Some of this complexity has been unraveled for CagA, VacA, and BabA but information on other virulence factors is limited. Additional considerations include variation in the frequency of virulence factors in different parts of the world, apparent linkage between virulence factors and methodological differences between studies. Although toxin‐producing strains of H. pylori might be expected to evoke a greater inflammatory response, the net effect of toxins on inflammation remains unclear as the bacterium has evolved elaborate strategies to evade and subvert the gastric immune response, some mediated by the same toxin (e.g. VacA). 21
H. pylori strains and duodenal ulcer
Many studies have examined the possibility of associations between duodenal ulcer and the presence of virulence factors. Typically, these have been case–control studies comparing the frequency of virulence factors in patients with H. pylori and duodenal ulcer to controls with H. pylori but without ulcers. Although weak associations have been found for CagA seropositivity, vacA genotypes, and babA2 genotypes in several countries, this does not apply in East Asia where most isolates are positive for CagA, and the riskier genotypes of vacA and babA. In contrast, weak associations have been found for dupA genotypes in Asian countries but not in Western countries. 20
Although the presence of virulence factors may have a minor influence on risks for duodenal ulceration, it seems highly unlikely that the appearance of virulence factors resulted in the epidemic of duodenal ulceration late in the 19th century. Much more likely is the appearance of virulence factors many thousands of years ago when the bacterium was under higher selective pressure. This is supported by observations in a 5300‐year‐old mummy (“Iceman”) where genotyping of H. pylori showed positive results for cagA and a genotype of vacA with enhanced vacuolation activity. 22
The host and environment hypothesis
This review promotes the hypothesis that the epidemic of duodenal ulceration was caused by changes in the pattern of gastritis with the emergence of gastritis in the antrum with only mild inflammation in the superficial layer (foveolar component) of the body. Prior to the period, 1850–1900, it seems likely that H. pylori infections were almost universal and were mostly acquired during the first year of life. This eventually resulted in antral gastritis with more prominent inflammation in the body of the stomach (with or without atrophy) that restricted the development of hypersecretion of gastric acid. This section documents the presence of antral‐predominant gastritis and includes a contemporary view of how a pathologic change resulted in a substantial increase in numbers of patients with duodenal ulceration. Aspects of this hypothesis have been published previously 23 , 24 , 25 but there are only limited attempts to place these observations in an historical context. A diagrammatic representation of the major steps influencing risk for duodenal ulcer are shown in Figure 1 while environmental and minor factors influencing risk are listed in Table 1.
Figure 1.
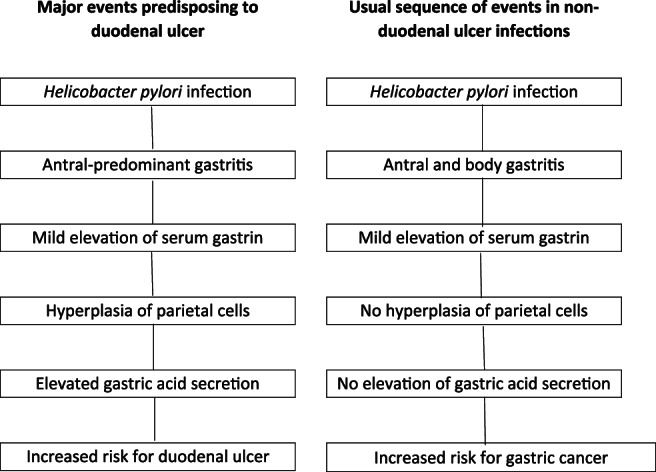
Proposed sequence of major events in duodenal ulcer patients (L column) when compared with non‐duodenal ulcer patients (R column).
Table 1.
Environmental and other factors known to influence risk for duodenal ulcer
| Environmental factors |
| Cigarette smoking |
| Medication with NSAIDs |
| Other factors |
| Genetic: Non‐secretion of ABO blood group antigens |
| Lewis blood group phenotype (a+) |
| Gender: Male (? Protective effects of hormones in women) |
| Metabolic: Hypercalcemia |
| Factors of uncertain significance |
| Variation in pepsin secretion |
| Gastric metaplasia in duodenal bulb |
H. pylori and gastritis
Although the stomach supports a microbial population of approximately 150 different species, H. pylori is usually the dominant species in H. pylori infections. 26 Furthermore, in most settings, H. pylori induces an inflammatory response in the gastric mucosa (gastritis) that becomes chronic because inflammatory and immune responses fail to eliminate the infection. However, there is variation in the characteristics of gastritis that include severity, location, histologic features, and longer‐term outcomes. These histologic changes have been classified by the Sydney System 27 and updated by an International Workshop. 28
There are only a limited number of reports of endoscopy and gastric biopsies in acute H. pylori gastritis. In adults, early endoscopy has occasionally been performed because of symptoms such as vomiting and epigastric pain. 12 Typical features include inflammatory changes with erosions that are usually more prominent in the antrum than in the body of the stomach. Histologic features include hyperemia and edema with an intense infiltrate of inflammatory cells, particularly neutrophils. Documentation of acute gastritis in children appears to be rare but at least one case has been reported in a child aged 8. 29 Spontaneous resolution of infection does occur but whether it is more frequent in symptomatic infections with severe gastritis remains unclear.
In contrast, gastric biopsies have been performed on infants who died at a young age (usually <6 months) because of trauma or sudden infant death syndrome. Some, particularly those with sudden infant death syndrome, had H. pylori infection but gastritis was either absent or minimal. 30 This probably reflects a degree of immaturity of the immune system that involves both the innate and adaptive (B and T cell) response.
For most individuals, the age of acquisition of H. pylori remains unclear although there is persuasive evidence that the infection is acquired during infancy or childhood in most populations. 9 At the time of diagnosis, the infection is almost always chronic with histologic changes in both the antrum and body. However, the degree of inflammation as assessed histologically is normally greater in the antrum. 31 In the body of the stomach, the degree of inflammation is variable ranging from mild inflammation in the superficial layers (foveolar compartment) to at least moderate inflammation throughout all layers of the mucosa. In the former, gastric (oxyntic) glands are retained along with parietal cells, chief cells, and scattered endocrine cells. In the latter, gastric glands are damaged and lost resulting in variable degrees of gastric atrophy (atrophic gastritis). In some patients with atrophic gastritis, lost gastric glands are replaced by immature glandular elements, usually of the intestinal type (intestinal metaplasia). This subgroup of patients is at higher risk for dysplasia and cancer. A comparison of histologic features of H. pylori in patients with and without duodenal ulcers showed that the severity of gastritis was similar in the antrum but that the ulcer group had milder gastritis in the body with some biopsies categorized as histologically normal. 32 Atrophic gastritis restricted to the antrum of the stomach appears to be rare. Although difficult to study, virulence factors of H. pylori do not appear to determine the overall pattern of gastritis. 33
Endoscopic, histologic, and gastric cytokine changes induced by H. pylori have been compared between children and adults. At endoscopy, antral nodularity is more frequent in children (40–90%) than in adults and is usually due to lymphoid hyperplasia. 34 With histology, children have fewer neutrophils in the gastric mucosa but similar numbers of chronic inflammatory cells including lymphocytes and plasma cells. Studies of lymphocyte populations have shown that children have an increased density of gastric regulatory T cells (Treg Foxp3+) and a reduced density of pro‐inflammatory T helper type 17 (Th17) cells. These cellular changes are corroborated by higher mucosal levels of IL‐10, and Foxp3 mRNA and lower levels of IL‐17 mRNA. In addition, lower mucosal levels of interferon‐G (IFN‐G) in children are consistent with a reduced Th1 response to the infection. 35 , 36 Overall, there is persuasive evidence that immune responses to H. pylori are downregulated in children when compared to adults.
H. pylori and gastrin
The hormone, gastrin, is a major regulator of gastric acid secretion and is released from gastrin‐expressing cells (G cells), predominantly located in the gastric antrum. 37 Physiological stimulants of circulating gastrin include protein meals, antral distension, vagal stimulation, and gastrin‐releasing peptide while inhibitors include a low gastric pH (3 or lower) and levels of the inhibitory hormone, somatostatin. Circulating gastrin exists in three forms: G34 (big gastrin), G17 (little gastrin), and G14 (small gastrin). The latter has no bioactivity. G34 and G17 have different rates of clearance from the circulation such that G34 predominates during fasting and G17 predominates after meals. Gastrin stimulates acid secretion via activation of cholecystokinin receptors (CCK2) on enterochromaffin‐like cells in the body mucosa and, to a lesser extent, by direct stimulation of parietal cells.
Although serum levels of gastrin show some variation with different radioimmunoassays or ELISA kits, there is persuasive evidence that H. pylori gastritis is associated with higher serum levels of gastrin, particularly G17. In one study, a comparison of G17 levels after meals in patients before and after eradication of infection showed that H. pylori was associated with a twofold increase in serum gastrin. In contrast, the levels of G34 were largely unaffected by either meals or H. pylori. 38 In a different study, serum gastrin levels were higher in patients with more severe gastric inflammation. 39 The rise in gastrin has been attributed to a fall in the antral density of D cells and the antral content of somatostatin; both returning toward normal with elimination of the infection. 40
Gastrin levels have also been compared in patients with H. pylori, with and without duodenal ulceration. Similar levels of gastrin have been reported in the two groups. 41 However, gastrin seems likely to have different trophic effects with an increase in the parietal cell mass in individuals with duodenal ulcer and an absence of trophic effects in individuals with greater inflammation in the body mucosa. 42 Whether mild hypergastrinemia increases parietal cell mass slowly or more rapidly remains unclear but the interval between acquisition of infection (usually childhood) and symptomatic duodenal ulceration (usually between 25 and 40 years) suggests that the interval can be two decades or more.
H. pylori, parietal cell mass, and gastric acid secretion
Prior to reports linking H. pylori to gastritis and duodenal ulceration, abnormalities in the secretion of gastric acid were widely considered to be central to the pathogenesis of ulcer disease. Common investigations included passage of a nasogastric tube and measurement of acid secretion in the basal state (usually for 1 h) and after use of an acid stimulant for a similar period. These stimulants included a derivative of histamine (Histalog), pentagastrin, gastrin‐releasing peptide, and standardized meals. Currently, the pentagastrin test can be used to determine maximal or peak acid output. Although mean levels of basal or peak acid output are higher in patients with duodenal ulcer, there is considerable overlap between the two groups. 43 A lesser degree of overlap can be demonstrated if gastric acidity is measured over 24 h with higher levels in ulcer patients, particularly in the evening and at night. 44 Hypersecretion of acid has also been demonstrated using an endoscopic gastrin test 45 and by prolonged acid secretion after a standard meal. 46
Reasons for hypersecretion of acid could include an increase in the number of parietal cells in the body of the stomach or an increase in the sensitivity of parietal cells to endogenous gastrin. There is persuasive evidence in support of the former possibility. For example, a publication in 1952 showed a twofold increase in the number of parietal cells in duodenal ulcer patients when compared with non‐ulcer controls. In contrast, when gastric ulcer patients were compared with the controls, there was a minor but nonsignificant fall in numbers of parietal cells. 47 These conclusions have been corroborated by others. The sensitivity of parietal cells to gastrin is difficult to assess because of variation in the parietal cell mass but may be relevant to hormonal effects on acid secretion in women. 48
In most studies, there has been a good correlation between parietal cell mass and peak acid output. In addition, patients with recurrent ulcers after H. pylori eradication appear to have higher acid secretion than non‐ulcer controls. 49 The cause of duodenal ulcers (often with hypersecretion of acid) that occur in the absence of H. pylori is poorly understood but may be related to vagal overactivity or to functional abnormalities of antral G cells.
H. pylori, gastric acid, and duodenal ulcers
Although there is a strong association between H. pylori and duodenal ulceration, the major mediator of this effect is hypersecretion of gastric acid. For example, hydrochloric acid can induce inflammation and ulcers in the gastrointestinal tract in a variety of experimental settings. 50 There is also incontrovertible evidence that a reduction in gastric acid secretion, either medically or surgically, not only heals ulcers but prevents their recurrence. In addition, the presence of H. pylori does not increase the severity of ulcers in patients with gastrinoma (Zollinger–Ellison syndrome). 51
In individuals with H. pylori but without ulcers, gastric acid secretion is either similar or lower than that in uninfected control subjects. Since this group has similar levels of gastrin, normal or lower acid secretion could be due to gastric atrophy or to functional impairment of parietal cells because of corpus inflammation. The former may apply in older adults but the latter seems likely to be relevant in children and younger adults. For example, eradication of H. pylori increased basal and stimulated acid secretion in children in Bangladesh 52 and in some younger and middle‐aged adults from Western countries. 53
Other factors influencing risk for duodenal ulcer
There is good evidence that risk for duodenal ulcer is influenced by smoking and use of nonsteroidal anti‐inflammatory drugs (NSAID's), including aspirin. Smoking is associated with a twofold risk, perhaps because the proximal duodenum has lower prostaglandin concentrations or reduced secretion of bicarbonate. A similar risk is associated with regular use of NSAID's. This has been attributed to inhibition of prostaglandin synthesis that impairs mucus and bicarbonate secretion, mucosal blood flow, and mucosal proliferation. 54
Minor factors influencing risk involve genetic factors, gender effects, pepsin secretion, and the presence of gastric metaplasia in the duodenal cap. Genome‐wide association studies have shown an association between a toll‐like receptor locus and seropositivity for H. pylori but a relationship with duodenal ulcer was not explored. 55 There is also interest in possible associations between ulcers and the linked genes affecting ABO and Lewis blood groups and secretion of blood group antigens. These genes do not influence susceptibility for H. pylori but non‐secretion of ABO blood group antigens and the Lewis (a+) phenotype may increase the risk for ulcers. 56 These weak associations may be related to interactions between bacterial adhesins, epithelial receptors, and gastric glycoproteins.
Another area of interest is the higher frequency of duodenal ulcers in men when compared to women, particularly in studies performed prior to the widespread use of NSAID's. This can be partly explained by the higher prevalence of smoking by men in most populations. However, very low frequencies of ulcer disease during pregnancy 57 raise the possibility of hormonal effects on gastric acid secretion. Although few studies have been performed during pregnancy, comparative studies between women (nonpregnant) and men have shown that women have a higher basal gastric pH, higher levels of G17 after meals, and reduced sensitivity of parietal cells to gastrin. 48
The role of pepsin in the etiology of duodenal ulcer is difficult to study because the activity of pepsin is largely determined by gastric acid (pH). Elevated levels of pepsinogen 1 were initially thought to be related to genetic factors but are now known to be secondary to H. pylori infection. 58 Nevertheless, studies in animal models indicate that the combination of acid and pepsin results in greater mucosal damage than acid alone. 59
There is also debate about the significance of gastric metaplasia in the duodenal bulb and susceptibility to duodenal ulcer. Gastric metaplasia can occur in the absence of H. pylori but is more frequent and more extensive in the presence of infection. Furthermore, bacteria in the duodenal cap are largely confined to areas of gastric metaplasia and are usually associated with a polymorphic infiltrate (duodenitis). However, whether gastric metaplasia is a response to duodenitis, acid hypersecretion, numbers of duodenal bacteria, or a combination of factors has not been resolved. 60 It is also unclear whether duodenal ulcers are more likely to be located in areas of gastric metaplasia than areas of normal duodenal mucosa.
A related issue is the association of duodenal ulcer with impaired secretion of mucosal bicarbonate from the proximal duodenum. This was initially thought to be a secretory defect peculiar to ulcer patients, but is now recognized as a phenomenon that resolves after eradication of H. pylori. 61
Why did the pattern of gastritis change?
The second half of the 19th century was associated with major improvements in education, sanitation, and nutrition as well as limitations on child labor, the evolution of unions, and improved wages and conditions for many workers. These developments lifted many families from poverty and resulted in significant improvements in health and longevity. In England, for example, public health measures included the development of sewerage systems, public waste collection, and the provision of clean water to homes rather than public pumps. There was also increasing acceptance of the germ theory of disease; an idea that began early in the 19th century and gained traction from John Snow and others in the mid‐19th century. One well‐publicized event by John Snow (1854) was the control of an outbreak of cholera in London by removing the handle of the Broad Street pump. However, wide acceptance of the germ theory did not occur until late in the century following the work of Casimir Davaine (1812–1882) and Louis Pasteur (1822–1895) in France and Robert Koch (1843–1910) in Germany. Their work led to renewed interest in national and local legislation related to pollution and food safety as well as a greater focus on domestic and personal hygiene. 62
The hypothesis developed in this article attributes changes in the pattern of gastritis to environmental factors that evolved in the second half of the 19th century. As a result of improvements in hygiene, one important factor seems likely to be delay in the acquisition of infection to an age where the mucosal immune response is more robust. This response fails to eliminate the infection in most individuals but does appear to restrict the proliferation of bacteria, particularly in the body of the stomach where colonization is less favorable because of a lower pH. In contrast, there is persuasive evidence that acquisition of infection in infancy is associated with an impaired mucosal immune response because of immaturity of the immune system and the promotion of immunosuppression, partly mediated by the organism and partly mediated by anti‐inflammatory activities in the host. Some of these issues have been addressed in a comparison of gastric responses to infection between adults and children. Most studies show that children have milder gastritis, greater numbers of gastric bacteria, more gastric lymphoid follicles, and regulatory T‐cells, and perhaps increases in the activity of anti‐inflammatory M2 macrophages. Although maternal milk may contain H. pylori antibodies, breast feeding is unlikely to have a significant effect on the infection 63 as animal studies show that the severity of gastritis is mediated by helper (CD4+) T‐cells and correlates inversely with numbers of bacteria.
An alternative hypothesis attributes changes in the pattern of gastritis to improvements in nutrition. 64 In particular, better wages were associated with a reduction in childhood malnutrition in many parts of the world while developments in farming and transportation resulted in diets that were more varied and more likely to contain fresh fruit and vegetables. Potential benefits of nutritional factors include enhanced mucosal immunity and delays in the development of gastric atrophy and/or intestinal metaplasia. Factors that enhance mucosal immunity include vitamin A (largely derived from milk, milk products, and eggs) and vitamin D (largely derived from endogenous synthesis by the skin in response to sunlight). 65 However, there are no experimental studies on the effects of vitamin A and D deficiency on gastritis induced by helicobacters. Another nutritional factor of potential relevance is vitamin C; a vitamin largely contained in fresh fruit and fruit juices. When associated with H. pylori, deficiency of vitamin C appears to aggravate gastritis and may accelerate the development of gastric atrophy. 66 An argument in favor of the nutritional hypothesis is the prevalence of duodenal ulcers in lower‐income countries such as India and Bangladesh where most infections seem likely to be acquired in infancy. However, whether improved nutrition in children or young adults can promote the immune response to an infection established early in life remains unclear. The nutritional hypothesis also raises the possibility that diet may delay the onset of gastric atrophy induced by H.pylori in some populations, leaving individuals at risk for duodenal ulcer, perhaps at a younger age. This is not supported by historical data indicating that duodenal ulcers are diagnosed at a similar age in low and high‐income countries.
The above hypotheses are not mutually exclusive and both may contribute to emergence of the epidemic. In particular, the effects of delay in the acquisition of infection could be augmented by improvements in nutrition that further enhance mucosal immunity and minimize the development of atrophy in the body of the stomach. These issues might be clarified by the use of animal models but, thus far, various models for the human infection have not been entirely satisfactory.
Conclusion
It seems almost certain that H.pylori has infected humans for many thousands of years. Yet, peptic ulceration appeared to be rare prior to the latter half of the 19th century despite the earlier recognition of gastric cancer. Reasons for the appearance of duodenal ulceration are explored in this article including genomic changes in the bacterium and environmental changes to the host. The evidence supports the view that environmental changes including delayed acquisition of infection and improved nutrition led to the emergence of antral gastritis with minimal inflammation in the body of the stomach rather than gastritis involving the antrum and body that prevented the development of hypersecretion of gastric acid. Hypersecretion of gastric acid is viewed as the central risk factor for duodenal ulceration but other risk factors include smoking, medication, gender, and genes. Whether risk is also influenced by variation in pepsin secretion or the presence of gastric metaplasia in the duodenal bulb has not been clarified.
Acknowledgments
There are thousands of references on the subject of H. pylori and peptic ulceration. In this article, the number of references has been pruned in order to meet journal requirements.
Declaration of conflict of interest: None.
References
- 1. Fox JG. The expanding genus of Helicobacter: pathogenic and zoonotic potential. Semin. Gastrointest. Dis. 1997; 8: 124–41. [PubMed] [Google Scholar]
- 2. Moodley Y, Linz B, Bond RP et al. Age of the association between Helicobacter pylori and man. PLoS Pathog. 2012; 8: e1002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moynihan BGA. Duodenal ulcer. Initially printed by WB Saunders Co in 1910 and reprinted for The Classics of Medicine Library, 1991.
- 4. Jennings D. Perforated peptic ulcer. Changes in age‐incidence and sex‐distribution in the last 150 years. Lancet. 1940; 1: 395–8 444–7. [Google Scholar]
- 5. Susser M. Period effects, generation effects and age effects in peptic ulcer mortality. J. Chronic Dis. 1982; 35: 29–40. [DOI] [PubMed] [Google Scholar]
- 6. Sonnenberg A. Time trends of ulcer mortality in Europe. Gastroenterology. 2007; 132: 2320–7. [DOI] [PubMed] [Google Scholar]
- 7. Aro P, Storskrubb T, Ronkainen J et al. Peptic ulcer in a general adult population: the Kalixanda study: a random population‐based study. Am. J. Epidemiol. 2006; 163: 1025–34. [DOI] [PubMed] [Google Scholar]
- 8. Sung JJY, Kuipers EJ, El‐Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment. Pharmacol. Ther. 2009; 29: 938–46. [DOI] [PubMed] [Google Scholar]
- 9. Roberts‐Thomson IC. Rise and fall of peptic ulceration: a disease of civilization? J. Gastroenterol. Hepatol. 2018; 33: 1321–6. [DOI] [PubMed] [Google Scholar]
- 10. Brown LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol. Rev. 2000; 22: 283–97. [DOI] [PubMed] [Google Scholar]
- 11. Camilo V, Sugiyama T, Touati E. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2017; 22 (Suppl. 1). 10.1111/hel.12405. [DOI] [PubMed] [Google Scholar]
- 12. Rocha GA, Queiroz DMM, Mendes EN, Barbosa AJA, Lima GF, Oliveira CA. Helicobacter pylori acute gastritis: histological, endoscopical, clinical and therapeutic features. Am. J. Gastroenterol. 1991; 86: 1592–5. [PubMed] [Google Scholar]
- 13. Malaty HM, El‐Kasabany A, Graham DY et al. Age at acquisition of Helicobacter pylori infection: a follow‐up study from infancy to adulthood. Lancet. 2002; 359: 931–5. [DOI] [PubMed] [Google Scholar]
- 14. Falush D, Kraft C, Taylor NS et al. Recombination and mutation during long‐term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc. Natl. Acad. Sci. U. S. A. 2001; 98: 15056–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cellini L, Allocati N, Campli ED, Masulli M, DiBartolomeo S, Dainelli B. Helicobacter pylori isolated from stomach corpus and antrum: comparison of DNA patterns. J. Infect. 1996; 32: 219–21. [DOI] [PubMed] [Google Scholar]
- 16. Akopyants NS, Fradkov A, Diatchenko L et al. PCR‐based subtractive hybridization and differences in gene content among strains of Helicobacter pylori . Proc. Natl. Acad. Sci. U. S. A. 1998; 95: 13108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kersulyte D, Mukhopadhyay AK, Velapatino B et al. Differences in genotypes of Helicobacter pylori from different human populations. J. Bacteriol. 2000; 182: 3210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yamaoka Y. Helicobacter pylori typing as a tool for tracking human migration. Clin. Microbiol. Infect. 2009; 15: 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nat. Rev. Gastroenterol. Hepatol. 2010; 7: 629–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang W‐L, Yeh Y‐C, Sheu B‐S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. J. Biomed. Sci. 2018; 25: 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Atherton JC, Blaser MJ. Coadaption of Helicobacter pylori and humans: ancient history, modern implications. J. Clin. Invest. 2009; 119: 2475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maixner F, Krause‐Kyora B, Turaev D et al. The 5300‐year‐old Helicobacter pylori genome of the iceman. Science. 2016; 351: 162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Graham DY. Helicobacter pylori: its epidemiology and its role in duodenal ulcer disease. J. Gastroenterol. Hepatol. 1991; 6: 105–13. [DOI] [PubMed] [Google Scholar]
- 24. McColl KE, el Omar EM, Gillen D. The role of H. pylori infection in the pathophysiology of duodenal ulcer disease. J. Physiol. Pharmacol. 1997; 48: 287–95. [PubMed] [Google Scholar]
- 25. Malfertheiner P. The intriguing relationship between Helicobacter pylori infection and acid secretion in peptic ulcer disease and gastric cancer. Dig. Dis. 2011; 29: 459–64. [DOI] [PubMed] [Google Scholar]
- 26. Bik EM, Eckburg PB, Gill SR et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc. Natl. Acad. Sci. U. S. A. 2006; 103: 732–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Price AB. The Sydney System: histological division. J. Gastroenterol. Hepatol. 1991; 6: 209–22. [DOI] [PubMed] [Google Scholar]
- 28. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the histopathology of gastritis, Houston 1994. Am. J. Surg. Pathol. 1996; 20: 1161–81. [DOI] [PubMed] [Google Scholar]
- 29. Barbosa AJ, Queiroz DM, Mendes EN, Rocha GA, Carvalho AS, Roquete ML. Campylobacter pylori associated acute gastritis in a child. J. Clin. Pathol. 1989; 42: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stray‐Pedersen A, Vege A, Rognum T. Helicobacter pylori antigen in stool is associated with SIDS and sudden infant deaths due to infectious disease. Pediatr. Res. 2008; 64: 405–10. [DOI] [PubMed] [Google Scholar]
- 31. Bayerdorffer E, Lehn N, Hatz R et al. Difference in expression of Helicobacter pylori gastritis in antrum and body. Gastroenterology. 1992; 102: 1575–82. [DOI] [PubMed] [Google Scholar]
- 32. Queiroz DM, Barbosa AJ, Mendes EN et al. Distribution of Campylobacter pylori and gastritis in the stomach of patients with and without duodenal ulcer. Am. J. Gastroenterol. 1988; 83: 1368–70. [PubMed] [Google Scholar]
- 33. Ko JS, Kim KM, Oh YL, Seo JK. cagA, vacA, and iceA genotypes of Helicobacter pylori in Korean children. Pediatr. Int. 2008; 50: 628–31. [DOI] [PubMed] [Google Scholar]
- 34. Lazowska‐Przeorek I, Kotowska M, Banasiuk M et al. Value of antral nodularity for the diagnosis of Helicobacter pylori infection in children. Med. Sci. Monit. 2015; 21: 1827–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sustmann A, Okuda M, Koletzko S. Helicobacter pylori in children. Helicobacter. 2016; 21 (Suppl. 1): 49–54. [DOI] [PubMed] [Google Scholar]
- 36. Razavi A, Bagheri N, Azadegan‐Dehkordi F et al. Comparative immune response in children and adults with H. pylori infection. J. Immunol. Res. 2015; 2015: 315957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ahmed M, Ahmed S. Functional, diagnostic and therapeutic aspects of gastrointestinal hormones. Gastroenterology Res. 2019; 12: 233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mulholland G, Ardill JE, Fillmore D, Chittajallu RS, Fullarton GM, McColl KE. Helicobacter pylori related hypergastrinaemia is the result of a selective increase in gastrin 17. Gut. 1993; 34: 757–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wagner S, Haruma K, Gladziwa U et al. Helicobacter pylori infection and serum pepsinogen A, pepsinogen C, and gastrin in gastritis and peptic ulcer: significance of inflammation and effect of bacterial eradication. Am. J. Gastroenterol. 1994; 89: 1211–8. [PubMed] [Google Scholar]
- 40. Queiroz DM, Moura SB, Mendes EN, Rocha GA, Barbarosa AJ, de Carvalho AS. Effect of Helicobacter pylori eradication on G‐cell and D‐cell density in children. Lancet. 1994; 343: 1191–3. [DOI] [PubMed] [Google Scholar]
- 41. Mossi S, Meyer‐Wyss B, Renner EL, Merki HS, Gamboni G, Beglinger C. Influence of Helicobacter pylori, sex, and age on serum gastrin and pepsinogen concentrations in subjects without symptoms and patients with duodenal ulcers. Gut. 1993; 34: 752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Friis‐Hansen L. Lessons from gastrin and gastrin receptor knockout mice. Scand. J. Clin. Lab. Invest. Suppl. 2001; 234: 41–6. [DOI] [PubMed] [Google Scholar]
- 43. Blair AJ 3rd, Feldman M, Barnett C, Walsh JH, Richardson CT. Detailed comparison of basal and food‐stimulated gastric acid secretion rates and serum gastrin concentrations in duodenal ulcer patients and normal subjects. J. Clin. Invest. 1987; 79: 582–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Feldman M, Richardson CT. Total 24‐hour gastric acid secretion in patients with duodenal ulcer. Comparison with normal subjects and effects of cimetidine and parietal cell vagotomy. Gastroenterology. 1986; 90: 540–4. [DOI] [PubMed] [Google Scholar]
- 45. Lijima K, Ohara S, Sekine H et al. Changes in gastric acid secretion assayed by endoscopic gastrin test before and after Helicobacter pylori eradication. Gut. 2000; 46: 20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hamlet A, Olbe L. The influence of Helicobacter pylori on postprandial duodenal acid load and duodenal pH in humans. Gastroenterology. 1996; 111: 391–400. [DOI] [PubMed] [Google Scholar]
- 47. Cox AJ. Stomach size and its relation to chronic peptic ulcer. A.M.A. Arch. Pathol. 1952; 54: 407–22. [PubMed] [Google Scholar]
- 48. Feldman M, Richardson CT, Walsh JH. Sex‐related differences in gastrin release and parietal cell sensitivity to gastrin in healthy human beings. J. Clin. Invest. 1983; 71: 715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harris AW, Gummett PA, Phull PS, Jacyna MR, Misiewicz JJ, Baron JH. Recurrence of duodenal ulcer after Helicobacter pylori eradication is related to high acid output. Aliment. Pharmacol. Ther. 1997; 11: 331–4. [DOI] [PubMed] [Google Scholar]
- 50. Gustafson J, Welling D. “No acid, no ulcer”‐100 years later: a review of the history of peptic ulcer disease. J. Am. Coll. Surg. 2010; 210: 110–6. [DOI] [PubMed] [Google Scholar]
- 51. Saeed ZA, Evans DJ Jr, Evans DG et al. Helicobacter pylori and Zollinger‐Ellison syndrome. Dig. Dis. Sci. 1991; 36: 15–8. [DOI] [PubMed] [Google Scholar]
- 52. Sarker SA, Sultana S, Sattar S et al. Influence of Helicobacter pylori infection on gastric acid secretion in pre‐school Bangladeshi children. Helicobacter. 2012; 17: 333–9. [DOI] [PubMed] [Google Scholar]
- 53. Gutierrez O, Melo M, Segura AM, Angel A, Genta RM, Graham DY. Cure of Helicobacter pylori infection improves gastric acid secretion in patients with corpus gastritis. Scand. J. Gastroenterol. 1997; 32: 664–8. [DOI] [PubMed] [Google Scholar]
- 54. Nguyen TN, Barkun AN, Fallone CA. Host determinants of Helicobacter pylori infection and its clinical outcome. Helicobacter. 1999; 4: 185–97. [DOI] [PubMed] [Google Scholar]
- 55. Mayerle J, den Hoed C, Schurmann CM et al. Identification of genetic loci associated with Helicobacter pylori serologic status. JAMA. 2013; 309: 1912–20. [DOI] [PubMed] [Google Scholar]
- 56. Hein HO, Suadicani P, Gyntelberg F. Genetic markers for stomach ulcer. A study of 3,387 men aged 54‐74 years from the Copenhagen male study. Ugeskr. Laeger. 1998; 160: 5045–9. [PubMed] [Google Scholar]
- 57. Cappell MS. Gastric and duodenal ulcers during pregnancy. Gastroenterol. Clin. North Am. 2003; 32: 263–308. [DOI] [PubMed] [Google Scholar]
- 58. Parente F, Maconi G, Sangaletti O, Minguzzi M, Vago L, Bianchi PG. Behaviour of acid secretion, gastrin release, serum pepsinogen 1, and gastric emptying of liquids over six months from eradication of Helicobacter pylori in duodenal ulcer patients. A controlled study. Gut. 1995; 37: 210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Samloff IM, Taggart RT. Pepsinogens, pepsins, and peptic ulcer. Clin. Invest. Med. 1987; 10: 215–21. [PubMed] [Google Scholar]
- 60. Futami H, Takashima M, Furuta T, Hanai H, Kaneko E. Relationship between Helicobacter pylori infection and gastric metaplasia in the duodenal bulb in the pathogenesis of duodenal ulcer. J. Gastroenterol. Hepatol. 1999; 14: 114–9. [DOI] [PubMed] [Google Scholar]
- 61. Hogan DL, Rapier RC, Dreilinger A et al. Duodenal bicarbonate secretion: eradication of Helicobacter pylori and duodenal structure and function in humans. Gastroenterology. 1996; 110: 705–16. [DOI] [PubMed] [Google Scholar]
- 62. Pelling M. Contagion/germ theory/specificity. In: Bynum WF, Porter R (eds). Companion Encyclopedia of the History of Medicine. London: Routledge, 1993; 309–34. [Google Scholar]
- 63. Soltani J, Nikkhoo B, Khormehr J, Ataee P, Hakhamaneshi MS, Gharibi F. Breastfeeding and Helicobacter pylori infection in early childhood: a continuing dilemma. Iran. J. Pediatr. 2014; 24: 745–52. [PMC free article] [PubMed] [Google Scholar]
- 64. Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J. Gastroenterol. 2014; 20: 5191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cantorna MT, Snyder L, Arora J. Vitamin A and vitamin D regulate the microbial complexity, barrier function, and the mucosal immune responses to ensure intestinal homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019; 54: 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aditi A, Graham DY. Vitamin C, gastritis, and gastric disease: a historical review and update. Dig. Dis. Sci. 2012; 57: 2504–15. [DOI] [PMC free article] [PubMed] [Google Scholar]


