Abstract
Physical properties of the extracellular matrix (ECM) affect cell behaviors ranging from cell adhesion and migration to differentiation and gene expression; a process known as mechanotransduction. While most studies have focused on the impact of ECM stiffness, using linearly elastic materials such as polyacrylamide gels as cell culture substrates, biological tissues and ECMs are viscoelastic, which means they exhibit time-dependent mechanical responses and dissipate mechanical energy. Recent studies have revealed ECM viscoelasticity, independent of stiffness, as a critical physical parameter regulating cellular processes. These studies have used biomaterials with tunable viscoelasticity as cell-culture substrates, with alginate hydrogels being one of the most commonly used systems. Here, we detail the protocols for three approaches to modulating viscoelasticity in alginate hydrogels for 2D and 3D cell culture studies, as well as the testing of their mechanical properties. Viscoelasticity in alginate hydrogels can be tuned by varying the molecular weight of the alginate polymer, changing the type of crosslinker – ionic vs covalent, or by grafting short poly(ethylene-glycol) (PEG) chains to the alginate polymer. As these approaches are based on commercially available products and simple chemistries, these protocols should be accessible for scientists in the cell biology and bioengineering communities.
Keywords: Viscoelasticity, alginate hydrogels, 3D cell culture, mechanotransduction
INTRODUCTION:
Living cells continuously interact with the surrounding extracellular matrix (ECM) through biochemical and physical interactions. Over recent decades, studies have revealed the importance of ECM mechanical properties in organism development, tissue homeostasis, and disease progression. Matrix stiffness is now widely recognized as a key mediator of cell behaviors from proliferation, apoptosis, and migration to stem cell differentiation and cancer progression (Discher, Janmey, & Wang, 2005; Janmey, Fletcher, & Reinhart-King, 2020; Vogel & Sheetz, 2006).
Many of these fundamental discoveries on the impact of stiffness on cells were made using biomaterials with tunable stiffness. Typically, the biomaterials used are linearly elastic substrates, often collagen or fibronectin coated polyacrylamide hydrogels. Linearly elastic materials behave like a spring, instantaneously deforming by an amount proportional to an applied load and returning to their original shape upon unloading. However, biological tissues behave like viscoelastic rather than linearly elastic materials, in that they respond to stress or strain in a time-dependent manner and dissipate mechanical energy like viscous liquids, behaving more like a combination of springs and dashpots (dampers that resist motion via viscous friction) (Chaudhuri, Cooper-White, Janmey, Mooney, & Shenoy, 2020). These viscoelastic features can be characterized by examining stress relaxation in response to deformation, creep in response to loading, and energy dissipation or loss in rheological studies. Most extracellular matrices and tissues exhibit substantial stress relaxation in response to a deformation over a timescale of 10 – 1,000 seconds, and exhibit a loss modulus, a measure of viscous energy dissipation, that is about 10% of the storage modulus, a measure of elasticity (Chaudhuri et al., 2020). The emergence of biomaterials with independently tunable viscoelasticity has enabled studies of how these properties impact cell-matrix interactions and associated downstream effects. Recent studies have demonstrated that viscoelasticity can regulate cell spreading (Cameron, Frith, & Cooper-White, 2011; Charrier, Pogoda, Wells, & Janmey, 2018; Chaudhuri et al., 2015; Gong et al., 2018; McKinnon, Domaille, Cha, & Anseth, 2014), cell migration (Wisdom et al., 2018), cell-substrate adhesions (Chaudhuri et al., 2015, 2016; Lou, Stowers, Nam, Xia, & Chaudhuri, 2018), cell-cycle progression and mitosis (Nam & Chaudhuri, 2018; Nam, Gupta, et al., 2019), stem cell differentiation (Cameron, Frith, Gomez, Yap, & Cooper-White, 2014; Chaudhuri et al., 2016; Lee, Stowers, & Chaudhuri, 2019), extracellular matrix deposition (Chaudhuri et al., 2016; Lee, Gu, Mooney, Levenston, & Chaudhuri, 2017), and gene expression (Darnell et al., 2018; Lee et al., 2017).
Alginate hydrogels have been used for many of the seminal studies on the impact of substrate viscoelasticity. Many of the common gel formulations for alginate are based on commercially available products and simple chemistries. Alginate is a polymer derived from brown algae that can be crosslinked into a three-dimensional hydrogel with divalent cations such as calcium. Alginate itself is inert to cells, so that cell adhesion to the gels can be controlled by varying the density of cell-adhesion ligands coupled to the alginate. Alginate is not susceptible to degradation by mammalian proteases, though alginate chains can be modified to accommodate degradability (Bouhadir et al., 2001). Further, alginate hydrogels are nanoporous, providing a homogenous mechanical microenvironment for cells, and the porosity of the hydrogels is not altered by varied calcium crosslinking (Huebsch et al., 2010).
In this article, we describe a set of protocols for tuning the viscoelasticity of alginate hydrogels independently of stiffness and ligand density to mimic key aspects of the native cellular microenvironment for 2D and 3D cell culture studies. In particular, we describe three complementary methods for tuning viscoelasticity of alginate hydrogels. These methods consist of varying the molecular weight of the alginate polymer (Basic Protocol 1), varying crosslinking type – covalent vs. ionic – of the hydrogels (Basic Protocol 2), or covalently coupling poly(ethylene-glycol) (PEG) spacers with varying length and density to the alginate polymer (Basic Protocol 3). We also outline how to perform mechanical characterization of the viscoelastic properties of alginate hydrogels with unconfined compression and shear rheology testing (Support Protocol 1), and finally we describe how to conjugate RGD (Arg-Gly-Asp) peptides to alginate to facilitate cell adhesion and spreading (Support Protocol 2). With these methods, users can achieve a range of physiologically relevant hydrogel stiffnesses and timescales of stress relaxation or loss moduli. These tunable alginate hydrogels can be used for 2D or 3D cell culture to study cell-matrix interactions and as biomaterials for regenerative medicine.
STRATEGIC PLANNING
Before purchasing materials and preparing alginate hydrogels, you should first assess your experimental needs. For example, will you culture cells on top of gels (2D) or encapsulate cells within gels (3D)? This decision could be based on the physiological context of the question you are interested in, and whether 2D or 3D microenvironments are more relevant for this context. Another key question is what range of stiffness and stress relaxation times will you target? This decision could be based on the known mechanical properties of the tissue of interest (e.g., in a healthy or diseased state), or based on what range of stiffness and stress relaxation have been previously shown to impact the relevant cell behavior. You should also consider whether the gels need to include cell adhesion ligands, and if so, whether cell-adhesion peptide motifs such as RGD will be sufficient, or whether full length proteins are required.
Varying alginate molecular weight (Basic Protocol 1) is the default choice for tuning viscoelasticity of alginate hydrogels. This well-cited method is the most robust and is the simplest for 3D cell culture. Comparing results from culture on/in alginate gels covalently crosslinked with 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (Basic Protocol 2), which are elastic, versus ionically crosslinked alginate gels, which are viscoelastic, is typically limited to 2D culture but can explore a different range of stress relaxation timescales, and more directly compares with studies using elastic materials such as polyacrylamide gels. Basic Protocol 3 (coupling commercially available PEG spacers to alginate) is similar to Basic Protocol 1 but can produce hydrogels with faster relaxation times than in Basic Protocol 1.
The overall workflow is as follows: 1) choose hydrogel formulations (molecular weight of alginate polymer, ionic or covalent crosslinker concentrations, amount of added PEG, cell-adhesive ligand density); 2) Make hydrogels using one of the three basic protocols; 3) Test the mechanical properties of alginate gels with given formulation(s) following Support Protocol 1; 4) Use alginate gels for cell culture studies.
BASIC PROTOCOL 1
TUNING VISCOELASTICITY BY VARYING ALGINATE MOLECULAR WEIGHT
Introductory paragraph:
One method for tuning the viscoelasticity of ionically crosslinked alginate hydrogels is to vary the molecular weight distributions of the alginate polymers used to form the alginate hydrogels (Chaudhuri et al., 2016). Note that any commercially available alginate product will have a wide distribution in molecular weights in any given batch, but products with distinct distributions and average molecular weights can be purchased. Higher molecular weight alginate will produce slower-relaxing hydrogels as measured with a stress relaxation test and a lower loss modulus, while lower molecular weight alginate will produce faster-relaxing hydrogels and a higher loss modulus (see Commentary section for theory). Mixing different ratios of high and low molecular weight alginate will result in intermediate levels of stress relaxation (Lee et al., 2017). To form a hydrogel, alginate is rapidly mixed with calcium and media (Figure 1, Video 1). The amount of calcium determines the extent of ionic crosslinking and therefore the stiffness of the resulting hydrogel. For 3D cell culture, cells are encapsulated inside the hydrogel at the time of crosslinking.
Figure 1: Overview of the basic steps for preparing ionically crosslinked alginate hydrogels.
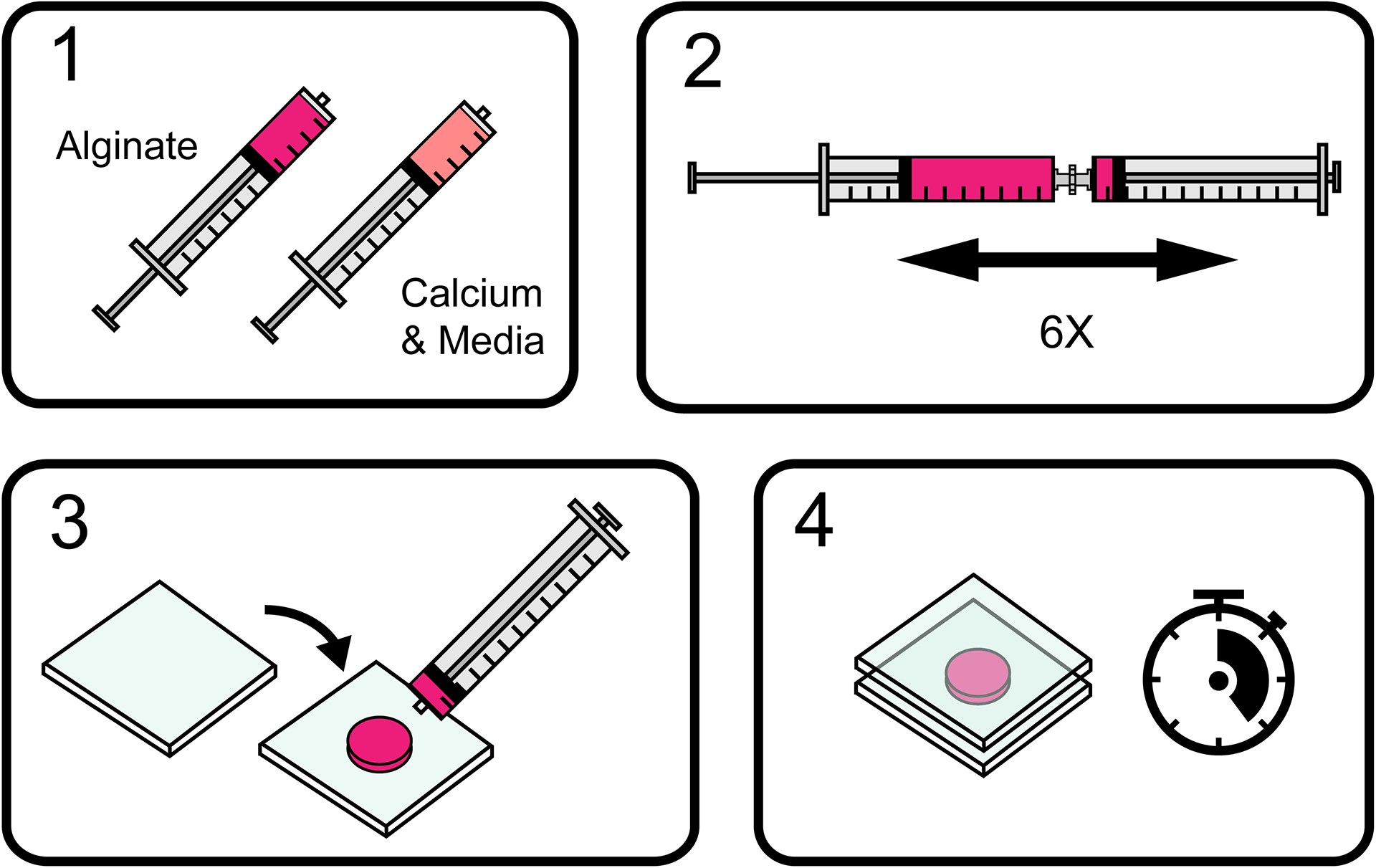
Alginate is rapidly mixed with calcium and media and allowed to gel into a three-dimensional polymer network.
Video 1 Filename: Preparing ionically crosslinked alginate hydrogels,
Step-by-step instructions for preparing ionically crosslinked alginate hydrogels. Includes loading alginate and calcium solutions into syringes, removing bubbles, mixing between syringes, depositing alginate between plates, and punching out gels with a biopsy punch.
Materials:
Reconstituted alginate, 3% w/v in DMEM (see recipe in Reagents and Solutions)
Serum-free media (e.g., DMEM)
Calcium stock, 488 mM (see recipe in Reagents and Solutions)
Cell suspension
Glass plates (e.g., McMaster-Carr, cat. no. 84815K47, or window glass)
Luerlock connectors (e.g., Value plastics, cat. no. FTLLC-1)
Luerlock syringe. Protocol has been tested primarily in 1 ml or 3 ml syringes (e.g., BD, cat. No. 309628)
1-millimeter-thick glass slides
Biopsy punch (e.g., Integra, cat. no. 3336)
Spatula
Protocol steps — Step annotations:
Materials preparation:
-
1
Obtain alginates with different distributions of alginate molecular weight and prepare sterile, lyophilized alginate for use as described in Reagents and Solutions.
Dupont/Novamatrix sell Pronova Ultrapure MVG (> 200kDa) and Pronova Ultrapure VLVG (<75kDa), which work well as high molecular weight or low molecular weight alginates, respectively. Hydrogels formed with low molecular weight alginate exhibit faster stress relaxation than those formed from higher molecular weight alginate. Note that intermediate ranges of stress relaxation can be obtained by mixing high and low molecular weight alginate in different ratios.
There are also other commercially available sources of alginate available, with varying molecular weight distributions, degrees of purification, and different ratios of guluronic acid to mannuronic acid residues. For alginate with high levels of impurities (e.g., food-grade alginate), we recommend purifying the alginate first as described in Basic Protocol 3.
-
2
At least one day before preparing hydrogels, prepare reconstituted alginate and calcium slurry as described in Reagents and Solutions.
-
3
Autoclave glass plates and Luerlock connectors.
-
4
Calculate volumes of alginate, DMEM, calcium stock, and cell suspension to use.
See Table 1 for sample formulations. Note that the specific properties will vary depending on the specific batch of alginate used for high-MW and low-MW gels and the user will likely need to adjust the calcium crosslinking concentration to achieve the appropriate elastic modulus accordingly.
A 1000 microliter gel typically gives approximately 8 alginate disks of 6-millimeter diameter and 2-millimeter thickness. Note that alginate is reconstituted at 3% weight/volume (or 30 mg/ml) so that the final hydrogel will have alginate at 2% (or 20 mg/ml) concentration.
You may want to check 3D culture papers in your field for help on deciding final cell concentrations used for a specific study. Final cell concentrations in the gel typically range from 50,000 cells/ml up to 20 million cells/ml. If you are doing molecular biology assays (RNA-seq, etc.), you may want higher cell concentrations. If you wish to incorporate fluorescent microbeads into the alginate to track substrate displacements, you can add these to syringe 1 along with the alginate and cell suspension.
Table 1:
Formulations for ~3kPa and ~20kPa ionically crosslinked hydrogels with high and low molecular weight alginate.
| Syringe 1 | Syringe 2 | Final concentrations | Expected mechanical properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Avg. MW of alginate (kDa) | Total gel volume (μL) | Volume of 3% wt/vol alginate (μL) | Volume of cell suspension (μL) | Volume of 488mM calcium stock (μL) | Volume of media (μL) | Crosslinker concentration (mM) | Alginate concentration (wt/vol, %) | Initial elastic modulus (kPa, mean +/− s.d.) | Stress relaxation halftime, τ1/2 (seconds, mean +/− s.d.) | Loss tangent (mean +/− s.d.) |
| High MW low stiffness | 280 | 1200 | 800 | 120 | 24 | 256 | 9.8 | 2 | 3.0 +/− 1.1 | 7058 +/− 1416 | 0.054 +/−0.007 |
| Low MW low stiffness | 35 | 1200 | 800 | 120 | 69 | 211 | 28.1 | 2 | 2.7 +/− 1.6 | 478 +/− 97 | 0.111+/−0.006 |
| High MW high stiffness | 280 | 1200 | 800 | 120 | 60 | 220 | 24.4 | 2 | 18.5 +/− 3.8 | 1007 +/− 163 | - |
| Low MW high stiffness | 35 | 1200 | 800 | 120 | 135 | 145 | 54.9 | 2 | 19.0 +/− 4.5 | 68 +/− 8 | - |
(Adapted with permission from Lee et al., 2017)
Hydrogel preparation:
(See Video 1 for a video illustration of steps 5 through 17).
-
5
Load alginate into syringe 1.
High molecular weight alginate is very viscous and cannot be accurately pipetted with a normal air displacement pipette. A positive displacement pipette may be used, if available. Otherwise, use the syringe to directly draw alginate from the vial, remove bubbles and then dispense excess alginate to reach the desired volume. Since the syringe markings do not account for the volume of the syringe tip, pipette the volume of any liquid matching the desired volume of alginate into an extra syringe to visually confirm/calibrate where the plunger stops. Warming up the alginate to room temperature can also make it easier to work with.
Low molecular weight alginate is less viscous and can be pipetted directly. When transferring solutions (including alginate) from the pipette tip to the syringe, pull back on the syringe plunger to pull the alginate into the syringe rather than pushing down on the pipette plunger.
-
6
Remove any air bubbles trapped within the alginate.
For more viscous alginate (high molecular weight): pull the syringe plunger all the way down and then back up several times to remove bubbles. Air bubbles introduced into the hydrogel could interfere with cell experiments or affect local mechanical properties.
For less viscous alginate (low molecular weight): pull the syringe plunger down, cover the syringe opening with a gloved finger, and firmly tap the syringe (e.g., with a marker). Bubbles should become unstuck and rise to the surface. After removing your finger from the syringe opening, pull down on the syringe plunger first to avoid expelling any droplets of alginate at the tip.
-
7
Load cell suspension into syringe 1.
Note that if you are incorporating fluorescent microbeads, load and mix the fluorescent microbeads with alginate in syringe 1, before adding cells. Most commercially available fluorescent microbeads come in a solution containing sodium azide which is toxic to mammalian cells. Hence, it is important to avoid exposure of cells to high concentrations of sodium azide.
-
8
Mix alginate and cell suspension thoroughly within syringe 1 by moving the syringe plunger up and down several times.
While mixing solutions within a syringe, it is helpful to hold a finger against the barrel flange to prevent accidentally pushing up too far.
-
9
Attach a Luerlock connector to syringe 1 and push the alginate solution meniscus to the top of the open Luerlock connector.
-
10
Load DMEM into syringe 2.
-
11
Load calcium stock into syringe 2.
The calcium sulfate settles quickly in DMEM. To ensure a homogenous mixture and therefore an accurate calcium concentration in the final hydrogel, shake the conical tube containing the calcium stock vigorously by hand. While still swirling the tube, quickly pipette out the desired amount of calcium stock. Make sure that there are no large clumps of calcium blocking the pipette tip.
-
12
Remove any air bubbles in syringe 2.
-
13
Mix DMEM and calcium stock within syringe 2, and then bring liquid meniscus to the opening of the syringe.
Proceed to the next step quickly to prevent the calcium from settling.
-
14
Attach syringe 1 to syringe 2 with the Luerlock connector, mix 6 times (push the plunger all the way to each side 3 times), and quickly deposit onto a glass plate. Cover with a second glass plate, separated by spacers (e.g., 1-millimeter thick glass slides).
These steps must be performed in quick succession to form uniformly mixed gels, and it typically takes time for users to master this method. The glass plates and spacers should be set up easily within reach before connecting the syringes. When connecting the syringes, be sure that the meniscus of each solution is close enough to the opening to avoid introducing air bubbles.
-
15
Allow alginate to gel for at least 20 minutes before removing top plate.
To prevent the gel from sticking to the plate, it is sometimes helpful to slide the top plate off the gel or lift one side of the plate up first rather than lifting straight up. To make the glass plates hydrophobic, periodically re-coat the glass with a siliconizing reagent (e.g., Sigmacote, Sigma-Aldrich). Note that window glass can also work well for the gel plates.
Check alginate to make sure it looks uniform and well-gelled. Calcium sulfate crystals sometimes remain after gelation, but these should dissolve quickly in media. Note that it could take longer for your formulation of alginate to fully form a gel. You can determine the time you need for the alginate to form a gel through mechanical testing (see Support Protocol 1).
-
16
Punch out 6-millimeter disks with a biopsy punch.
Larger disks (e.g., 8-millimeter) are sometimes easier to handle for mechanical testing. Alternatively, the alginate-calcium mixture can be directly deposited directly into a chamber slide for imaging experiments.
-
17
Carefully separate disks with a spatula.
Alginate disks will be fragile. Punching out and separating the disks without breaking apart the hydrogel may be tricky at first but will get easier with practice.
-
18
Transfer alginate disks to growth media and begin experiments.
Sample Data
(See Figure 2)
Figure 2: Tuning stress relaxation in 3kPa ionically crosslinked hydrogels by varying alginate molecular weight.
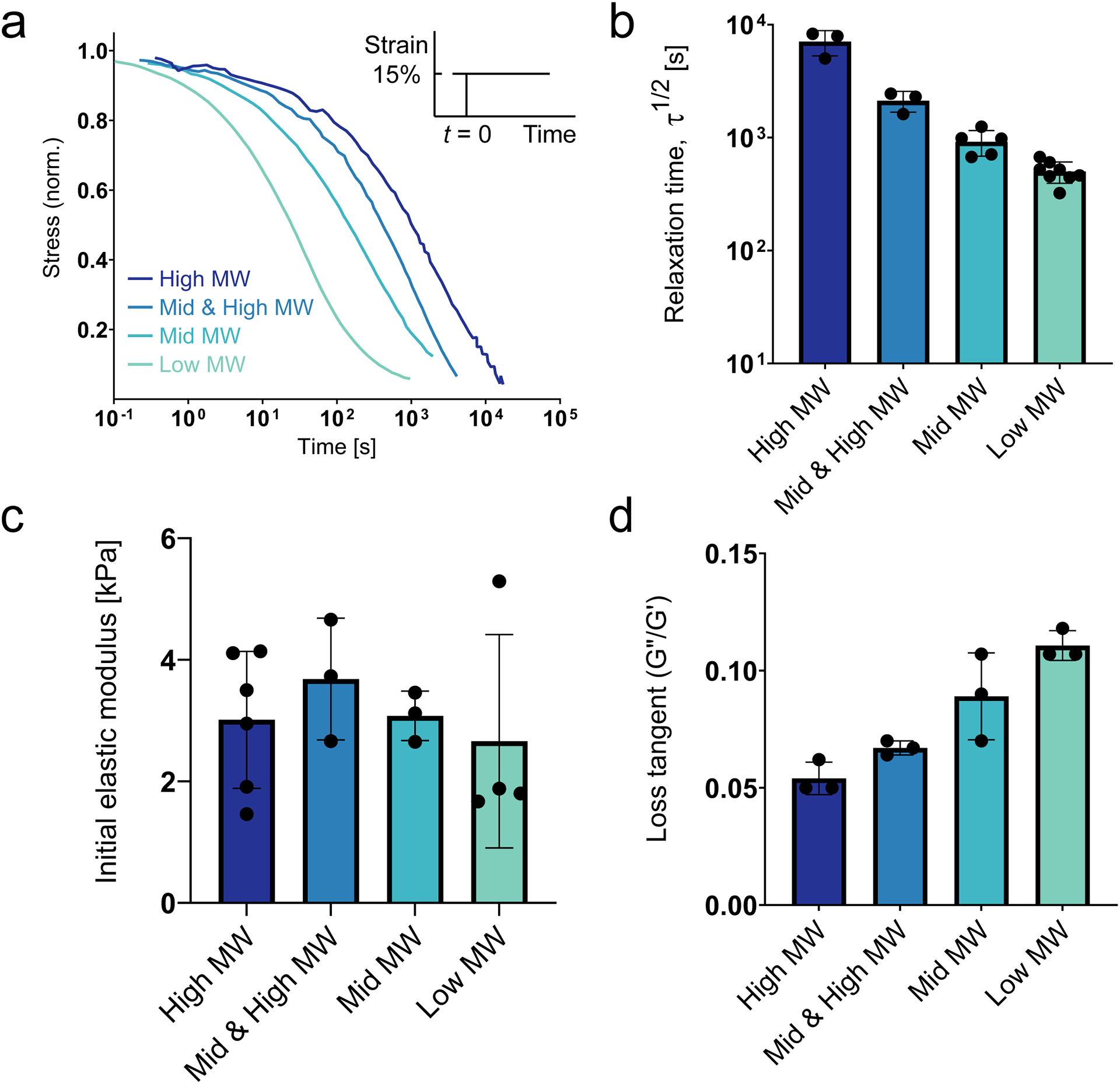
(Adapted with permission from Lee et al., 2017) (a) Representative stress relaxation profiles for hydrogels with varying alginate molecular weight. (b) Characteristic timescale of relaxation for the different alginate hydrogels. (c) Initial elastic modulus (stiffness) for the different alginate hydrogels. (d) Loss tangent (ratio of loss modulus to storage modulus) for the different alginate hydrogels. All mechanical tests were conducted in unconfined compression. All data are shown as mean ± std. dev.
BASIC PROTOCOL 2
TUNING VISCOELASTICITY WITH IONIC VS. COVALENT CROSSLINKING
Introductory paragraph:
Ionically crosslinked alginate gels (Basic Protocol 1) are necessarily viscoelastic, because ionic crosslinks are weak bonds which break and reform under stress. However, covalent crosslinks are permanent and lead to elastic gels. Alginate may be covalently crosslinked through using click chemistry (Desai, Koshy, Hilderbrand, Mooney, & Joshi, 2015) or methacrylation (Jeon, Bouhadir, Mansour, & Alsberg, 2009), but the simplest approach is with carbodiimide chemistry (Basic Protocol 2). In this method, alginate is mixed with EDC, which creates crosslinks between carboxyl groups and primary amines. Since alginate does not contain amine groups, a diamine such as adipic acid dihydrazide (AAD) is added to link carboxyl groups on different alginate chains. Crosslinking with EDC is primarily recommended for 2D cell culture studies, where cells are plated on top of hydrogels after the hydrogels have been rinsed of excess EDC, as EDC can be cytotoxic. Click crosslinking, which is highly specific and bio-orthogonal, might be preferable for 3D culture studies and is described elsewhere (Desai et al., 2015). However, EDC crosslinking has been used to encapsulate mesenchymal stromal cell spheroids in 3D (Whitehead et al., 2021). With these methods users can create distinct alginate hydrogels with equivalent stiffness, but with one gel ionically crosslinked and viscoelastic (Basic Protocol 1) and the other gel covalently crosslinked and elastic (Basic Protocol 2).
Materials:
MES Buffer (see recipe in Reagents and Solutions)
Reconstituted alginate, 3% w/v in MES buffer (see recipe in Reagents and Solutions)
AAD/HOBT solution (see recipe in Reagents and Solutions)
EDC solution (see recipe in Reagents and Solutions)
Serum free media (e.g., DMEM)
Luerlock syringe (e.g., BD, cat. No. 309618)
Luerlock connectors (e.g., Value plastics, cat. no. FTLLC-1)
Glass plates (e.g., McMaster-Carr, cat. no. 84815K47)
1-millimeter-thick glass slides
Biopsy punch (e.g., Integra 3336)
Spatula
Protocol steps — Step annotations:
-
Calculate volumes of alginate, AAD/HOBT solution, MES buffer, and EDC to use. (See Table 2)
For covalently crosslinked gels, alginate is reconstituted in MES rather than DMEM because the EDC reaction works best at pH 6.5.
Users will likely want to match the molecular weight of alginate used for ionically crosslinked gels (Basic Protocol 1).
-
Load alginate in syringe 1.
High molecular weight alginate is very viscous and cannot be accurately pipetted with a normal air displacement pipette. A positive displacement pipette may be used, if available. Otherwise, use the syringe to directly draw alginate from the vial, remove bubbles and then dispense excess alginate to reach the desired volume. Since the syringe markings do not account for the volume of syringe tip, pipette the volume of any liquid matching the desired volume of alginate into an extra syringe to visually confirm/calibrate where the plunger stops. Warming up the alginate to room temperature can also make it easier to work with.
Low molecular weight alginate is less viscous and can be pipetted directly. When transferring solutions (including alginate) from the pipette tip to the syringe, pull back on the syringe plunger to pull the alginate into the syringe rather than pushing down on the pipette plunger.
-
Remove any air bubbles trapped within the alginate.
For more viscous alginate (high molecular weight): pull the syringe plunger all the way down and then back up several times to remove bubbles. Air bubbles introduced into the hydrogel could interfere with cell experiments or affect local mechanical properties.
For less viscous alginate (low molecular weight): pull the syringe plunger down, cover the syringe opening with a finger, and tap the syringe (e.g., with a marker). Bubbles should become unstuck and rise to the surface. After removing your finger from the syringe opening, pull down on the syringe plunger first to avoid expelling any droplets of alginate at the tip.
Load AAD/HOBT solution and MES buffer into syringe 2.
Attach syringe 1 to syringe 2 with a Luerlock connecter and mix thoroughly.
Shift the alginate and AAD mixture back to syringe 1 and remove air bubbles.
Load EDC solution into syringe 3.
-
Attach syringe 1 (alginate and AAD solution) to syringe 3 (EDC solution) with a Luerlock connector, mix, and deposit onto a glass plate. Cover with a second glass plate, separated by spacers (e.g., 1- or 2-millimeter stacks of glass slides).
For 2D studies, you may wish to directly deposit the alginate into a mold or chamber slide.
-
Allow the alginate to gel for 3–12 hours before removing top plate.
Prevent evaporation by covering the entire assembly.
Punch out disks with a biopsy punch of the desired size.
-
Carefully separate disks with a spatula.
Alginate disks will be fragile. Punching out and separating the disks without breaking apart the hydrogel may be tricky at first but will get easier with practice.
-
Wash disks 4 to 5 times with serum-free DMEM over 1 to 2 days.
Washing the hydrogels allows excess EDC to diffuse out before cell seeding. Note that the covalently crosslinked alginate hydrogels might swell at very low crosslinking densities.
-
Transfer alginate disks to growth media and begin experiments.
For 2D studies, seed cells on top of the alginate after washing out excess EDC. Note that for 2D studies, you will likely require RGD or another adhesive ligand.
Table 2:
Hydrogel formulations for covalently crosslinked alginate. (Adapted with permission from Chaudhuri et al., 2015)
| Syringe 1 | Syringe 2 | Syringe 3 | Final concentrations | Expected mechanical properties | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Avg. MW of alginate (kDa) | Total gel volume (μL) | Volume of 3% wt/vol alginate (μL) | AAD/HOBT concentration | Volume of AAD/HOBT solution (μL) | Volume of MES buffer (μL) | Volume of EDC solution (μL) | AAD crosslinker concentration (mM) | Alginate concentration (wt/vol, %) | Initial elastic modulus (kPa) | Stress relaxation |
| Covalently crosslinked, high stiffness | 280 | 1200 | 800 | 1X AAD | 120 | 160 | 120 | 5.74 | 2 | 9.7 | None |
| Covalently crosslinked, inter. Stiffness | 280 | 1200 | 800 | 0.2X AAD | 120 | 160 | 120 | 1.15 | 2 | 3.4 | None |
| Covalently crosslinked, low stiffness | 280 | 1200 | 800 | 0.2X AAD | 60 | 220 | 120 | 0.57 | 2 | 1.5 | None |
Sample Data
(See Figure 3)
Figure 3: Mechanical properties for ionically vs covalently crosslinked alginate hydrogels.
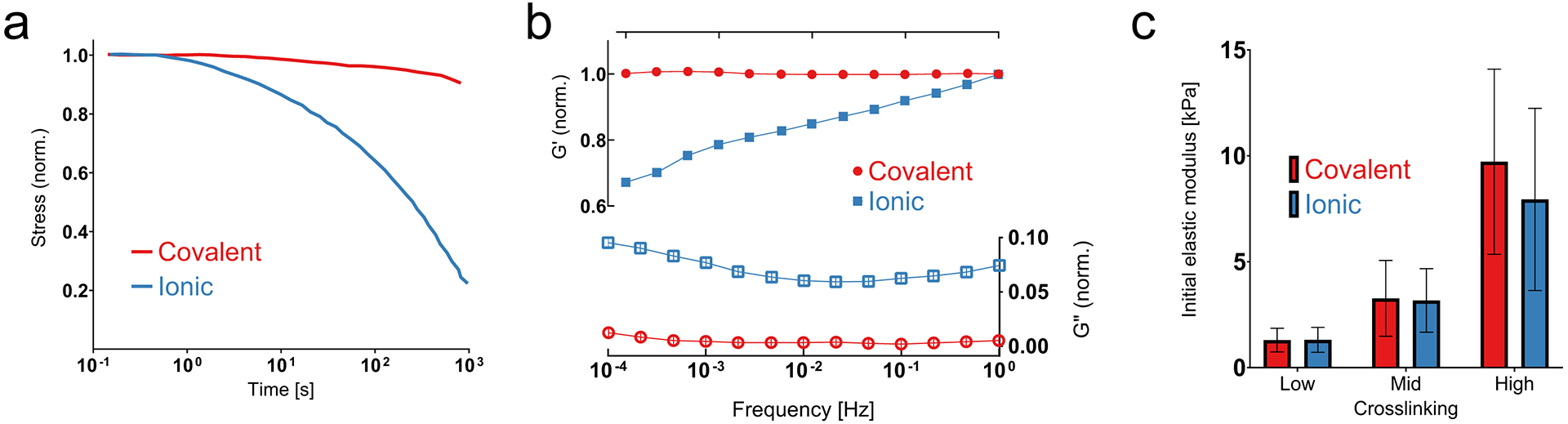
(Adapted with permission from Chaudhuri et al., 2015) (a) Representative stress relaxation profiles for covalently vs ionically crosslinked alginate hydrogels. (b) Storage modulus (G’) and loss modulus (G”) as a function of frequency for covalently vs ionically crosslinked alginate hydrogels. All values are normalized by the storage modulus at 1Hz. (c) Initial elastic modulus (stiffness) for covalently vs ionically crosslinked alginate hydrogels, as measured using atomic force microscopy, with different levels of crosslinking (see Table 2). Data are shown as mean ± std. dev., n = 3 independent gels for each condition.
BASIC PROTOCOL 3
TUNING VISCOELASTICITY BY ADDING PEG SPACERS TO ALGINATE CHAINS
Introductory paragraph:
The viscoelasticity of ionically crosslinked alginate hydrogels can also be tuned by adjusting the amount of PEG covalently coupled to the alginate (Chaudhuri et al., 2016). Commercially available PEG-amines of varying molecular weights can be covalently coupled to the alginate polymer backbone at varying concentrations with simple carbodiimide chemistry. Stress relaxation of alginate-PEG hydrogels is determined by the total mass amount of added PEG rather than the molecular weight or concentration of PEG alone, with more PEG leading to faster stress relaxation, enhanced creep, and a higher loss modulus (Nam, Stowers, Lou, Xia, & Chaudhuri, 2019). This approach enables precise tuning of stress relaxation over a wide range while using the same molecular weight of commercially available alginate. A key advantage of this approach is that addition of PEG to the alginate leads to hydrogels with faster stress relaxation and a higher loss modulus, extending the range of accessible stress relaxation times relative to alginate-only hydrogels (Chaudhuri et al., 2016). One limitation is that varying the PEG amount will likely affect the pore size of the alginate hydrogel. In addition, it is possible that adding large amounts of PEG could limit the availability of sites to conjugate RGD or other adhesion ligands and that RGD ligands close to PEG chains could be less accessible to cells for adhesion.
Materials:
Alginate powder from vendor
PEG-amine (e.g., Laysan Bio, 2kDa-20kDa)
MES buffer (see recipe in Reagents and Solutions)
EDC (Sigma-Aldrich, cat. no. E6383)
Sulfo-NHS (Thermo Scientific, cat. no. 24510)
Hydroxylamine hydrochloride (Sigma-Aldrich, cat. no. 255580)
Sodium chloride
Deionized water
Dialysis Tubing, 3.5 kDa MWCO (Spectrum 132592)
Nalgene bucket, 4 liters (Thermo Scientific, cat. no. 1201–4000)
Clamps (e.g., WeLock PA 70, 1207001)
Parafilm
Stericup Quick Release Sterile Vacuum Filtration System (MilliporeSigma, cat. no. S2GPU05RE)
Steriflip Sterile Disposable Vacuum Filter Units (MilliporeSigma, cat. no. SCGP00525)
Lyophilizer
Stir plate
Stir bars
Lab tape
Glass beakers
Protocol steps—Step annotations:
Conjugate PEG to alginate
Note: conjugating PEG to alginate follows the same basic process as the RGD-coupling protocol (Support Protocol 2).
-
1
Select alginate molecular weight.
The alginate molecular weight sets the starting point for stress relaxation. Adding PEG will give faster-relaxing gels, so you should choose the molecular weight accordingly. (See Basic Protocol 1 for more information on alginate selection).
-
2
Select the mass mount of PEG to add for each hydrogel formulation (See Table 3 for examples).
Degree of substitution (DS) indicates the number of PEG chains added per alginate chain. The total mass of PEG per chain is equal to the PEG molecular weight multiplied by the DS. The amount of added PEG is based on the target DS and an estimated coupling efficiency of 60% (Rowley, Madlambayan, & Mooney, 1999). All alginate samples in Table 3 are high molecular weight (280 kDa).
-
3
Dry alginate from vendor overnight on lyophilizer.
The alginate is lyophilized to remove water and ensure that dry weight measurements are accurate.
-
4
While stirring on a stir plate, slowly add 1 gram of lyophilized alginate per 100 milliliters of MES buffer.
-
5
Cover the beaker with parafilm. Wait overnight until all the alginate has fully dissolved.
-
6
Calculate the masses of sulfo-NHS, EDC, and PEG to use, and weight out into small weighing trays (Table 3).
-
7
Mixing quickly, add the PEG, sulfo-NHS, and then EDC.
Add a small amount of MES buffer into the weighing tray and then pour into the alginate mixture. Flush out any remaining powder into the alginate mixture using a pipette.
-
8
Cover the beaker with parafilm. Allow the reaction to proceed for 20 hours.
-
9
While stirring, quench the reaction with 18 milligrams of hydroxylamine hydrochloride per gram of alginate.
-
10
Cut several segments of dialysis tubing. Soak the tubing in hot water for several minutes.
Cut the tubing segments to be slightly longer than the height of the bucket. For a 4-liter bucket, cut approximately 4 segments per 100 milliliters of alginate mixture.
-
11
Clamp one end of each segment of tubing. Fill each segment with approximately 25 milliliters of alginate mixture. Clamp the opposite end of the tubing, leaving some air at the top. Tape one end of each segment to the edge of the 4-liter bucket so that the tubing hangs down into the bucket.
Make sure that the clamps will not open and release the alginate solution. The clamps should be heavy enough to weigh down the bottom of the tubing once the bucket is filled with water.
-
12
Add 30 grams of sodium chloride to the bucket and fill with 4 liters of deionized water. Stir on a stir plate for approximately 6 hours.
When adding water, avoid pouring too strongly or directly onto the tubing to avoid accidentally opening the clamps.
-
13
Repeat this process (from the previous step) to dialyze the alginate in several decreasing salt solutions of deionized water and sodium chloride for 3 days. Change the salt solution 2–3 times per day (e.g., morning, afternoon, evening).
NaCl per 4L deionized water: 30g-25g-20g-15g-10g-5g-0g-0g-0g-0g
For coupling reactions, dialysis is critical to remove all the reaction products and unreacted components.
-
14
Pour the dialyzed alginate solution from each segment of tubing into a beaker. Sterile filter the alginate solution using a large bottle filter unit (Stericup system).
Ultrapure research grade alginate does not require additional cleaning, but food grade alginate should be cleaned with activated charcoal prior to sterile filtering to remove impurities. If necessary, add 0.5 grams of activated charcoal per gram of alginate, stir for 30 minutes, stop stirring and let the activated charcoal settle, then sterile filter.
-
15
Unscrew the filter top of a 50-milliliter Steriflip unit. Pour approximately 35–40 milliliters of sterile alginate solution into the Steriflip and replace the filter top. Repeat for the entire volume of alginate.
For 50-milliliter Steriflip tubes, only fill to approximately 35–40 milliliters to prevent the tube from cracking when the alginate freezes. The filter top is used to maintain sterility while allowing sublimation of ice to occur during lyophilization.
-
16
Freeze the alginate overnight at −20/−30C.
We recommend placing the tubes at a slight angle in the freezer to prevent cracking. If a tube cracks, thaw the alginate, refilter, and refreeze in a new Steriflip tube.
-
17
Lyophilize for approximately 3 days until the alginate is completely dry.
-
18
Store lyophilized alginate at −20/−30C for up to two years.
Table 3:
Formulations for coupling 5kDa and 20kDa PEG to alginate with varying degrees of substitution. Note that the molecular weight of all alginate samples here is 280kDa.
| Sample | Alginate (mg) | PEG (mg) | Sulfo-NHS (mg) | EDC (mg) |
|---|---|---|---|---|
| 5kDa-DS16 | 200 | 95.2 | 43.8 | 77.5 |
| 5kDa-DS32 | 200 | 190.5 | 87.7 | 154.9 |
| 20kDa-DS4 | 200 | 95.2 | 11 | 19.4 |
| 20kDa-DS8 | 200 | 190.5 | 21.9 | 38.7 |
(Adapted with permission from Nam, Stowers, et al., 2019).
Prepare hydrogels
-
19
Calculate volumes of alginate, calcium stock, DMEM, and cell suspension to use (See Table 4 for examples).
Note that conjugated PEG will contribute to the total dry mass of lyophilized alginate-PEG, and so the 3% wt/vol alginate stock will need to be prepared based on the expected fraction of alginate in the lyophilized alginate-PEG. For example, 100 kDa alginate with 50 kDa of PEG added per chain would need to be reconstituted at 4.5% total polymer wt/vol to achieve 3% wt/vol of alginate.
-
20
Prepare alginate hydrogels as described in Basic Protocol 1, beginning with step 2, using PEG-conjugated alginate as the starting material.
Table 4:
Hydrogel formulations for ionically crosslinked alginate with varying amounts of coupled PEG.
| Syringe 1 | Syringe 2 | Final concentrations | Expected mechanical properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (PEG MW & DS) | Avg. MW of alginate (kDa) | Total gel volume (μL) | Volume of 3% wt/vol alginate (μL) | Volume of cell suspension (μL) | Volume of 488mM calcium stock (μL) | Volume of media (μL) | Crosslinker concentration (mM) | PEG concentration (wt/vol, %) | Alginate concentration (wt/vol, %) | Initial elastic modulus (kPa) | Stress relaxation halftime, τ1/2 (seconds) | Loss tangent |
| Unmodified alginate | 280 | 1200 | 800 | 120 | 26 | 254 | 10.6 | 0 | 2 | 2.5 | 18,500 | 0.023 |
| 5kDa-DS16 | 280 | 1200 | 800 | 120 | 24 | 256 | 9.8 | 0.57 | 2 | 3.7 | 1392 | 0.084 |
| 5kDa-DS32 | 280 | 1200 | 800 | 120 | 34 | 246 | 13.9 | 1.14 | 2 | 4.5 | 30 | 0.053 |
| 20kDa-DS4 | 280 | 1200 | 800 | 120 | 29 | 251 | 11.8 | 0.57 | 2 | 4.7 | 3150 | 0.044 |
| 20kDa-DS8 | 280 | 1200 | 800 | 120 | 40 | 240 | 16.2 | 1.14 | 2 | 3.5 | 1074 | 0.067 |
(Adapted with permission from Nam, Stowers, et al., 2019).
Sample Data
(See Figure 4)
Figure 4: Increasing the amount of PEG grafted to alginate increases stress relaxation and loss modulus in hydrogels.
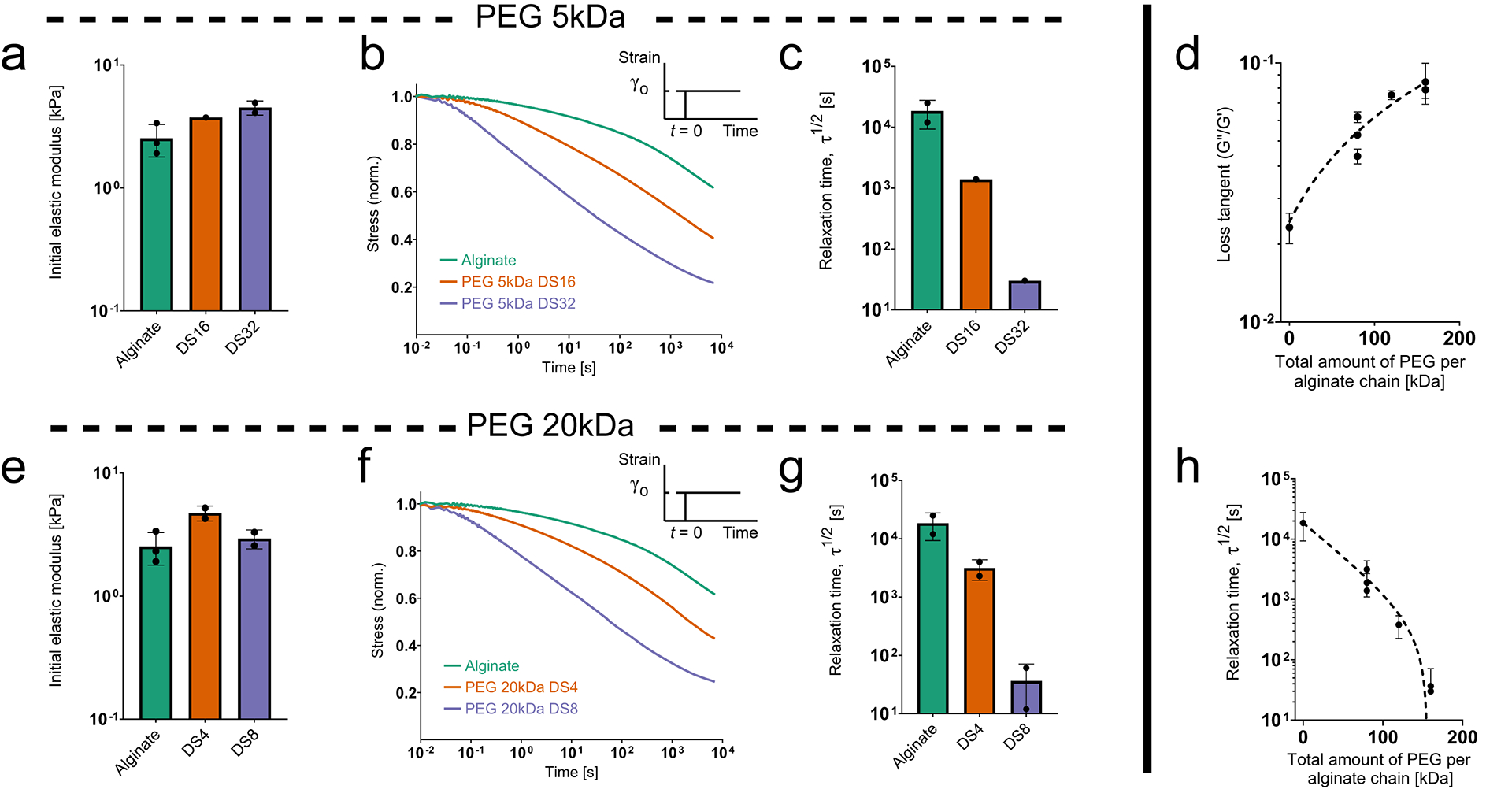
(Adapted with permission from Nam, Stowers, et al., 2019) (a) Initial elastic modulus for hydrogels made from 280kDa alginate grafted with varying degrees of substitution (DS) of 5kDa PEG. (b) Representative stress relaxation profiles for the same set of hydrogels. (c) Characteristic relaxation timescale for the same set of hydrogels. (d) Loss tangent as a function of the total amount of PEG per alginate chain. (e) Initial elastic modulus for hydrogels made from 280kDa alginate grafted with varying degrees of substitution (DS) of 20kDa PEG. (f) Representative stress relaxation profiles for the same set of hydrogels. (g) Characteristic relaxation timescale for the same set of hydrogels. (h) Characteristic relaxation timescale as a function of the total amount of PEG per alginate chain.
SUPPORT PROTOCOL 1
TESTING MECHANICAL PROPERTIES OF ALGINATE HYDROGELS
Introductory paragraph:
While we have provided sample mechanical properties of alginate hydrogels made using specific formulations for each protocol (Table 1, Table 2, Table 4), these numbers should be considered a ballpark estimate for initial guidance and reference. Users should conduct their own mechanical measurements. It is important to do mechanical testing of the alginate hydrogels you prepare in your own hands, due to batch-to-batch variations in the alginate molecular weight or chain structure and resulting mechanical properties, and because the hydrogel preparation is a manual process. Here we describe two different methods of mechanical testing – unconfined compression and shear rheology. Unconfined compression is more typical historically and involves compressing a cylindrical gel disk between two plates, with the gel free to expand in the radial direction, while the vertical load and displacement are measured and used to calculate mechanical stress and strain. In a shear rheometer, a sample is adhered between two parallel discs which rotate relative to one another to shear the sample while controlling and/or monitoring torque and angular displacement, in order to calculate shear stress and shear strain.
With unconfined compression you need to consider poroelastic stress relaxation due to water flow out of gels (Zhao et al 2010), whereas with rheology this is not a concern since shear is volume-conserving. An advantage of unconfined compression is that gels can be equilibrated in media prior to testing and can be measured at different timepoints, for example to look at how cell activities modulate the mechanical properties of the cell-gel construct and to characterize gel degradation over time. In contrast, for shear rheology the gel needs to stick to the rheometer plates. This typically requires the gels to be formed directly in the rheometer, without any equilibration time. On the other hand, shear rheology can be used to monitor gelation over time as well as obtain frequency-dependent rheology and stress relaxation measurements. We recommend using both unconfined compression and shear rheology in order to fully characterize your gels. The required equipment are a basic mechanical tester for unconfined compression studies (e.g., Instron 5848 with a 10 Newton load cell) and a parallel plate rheometer for shear rheology studies (e.g., TA Instruments DHR-2). These instruments are commonly found in engineering departments or material characterization core facilities.
Materials:
Alginate hydrogels (prepared using basic protocols, not yet mixed for rheology, formed into disks for unconfined compression)
Mineral oil (Sigma Aldrich)
Material testing system for compression testing (e.g., Instron 5848 material testing system with 10 Newton load cell)
Rheometer (e.g., DHR-2 stress controlled rheometer, TA Instruments) with 20mm plate
Protocol steps—Step annotations:
Method 1: Unconfined compression
Prepare alginate disks with a 2-millimeter thickness and 8-millimeter diameter as described in Basic Protocols 1, 2 or 3.
Allow gels to equilibrate in media for 24 hours.
-
Using a flat cylindrical crosshead larger than the alginate disk, compress the disks at 1 millimeter/minute up to a compressional strain of 15%. Calculate the initial elastic modulus (stiffness) as the slope of the stress-strain curve between 5% and 10% strain.
It is important to match strain rate (not extension rate) for all experiments, since alginate stiffness is strain-rate dependent (e.g., a 2-millimeter-thick gel with 1 millimeter/minute extension rate corresponds to a 1-millimeter-thick gel with 0.5 millimeter/minute extension rate).
Hold the strain at 15% while measuring stress over time. Calculate the time taken for stress to relax to one half of the peak stress at the initial hold. This is the stress relaxation half time (τ½) and is a useful empirical measure of stress relaxation rate.
Method 2: Shear rheology
Prepare alginate and crosslinker syringes as described in Basic Protocols 1, 2 or 3.
Immediately after mixing, deposit 500 microliters of alginate directly onto the bottom plate of the rheometer.
Immediately bring down a 20-millimeter top plate to contact the alginate, forming a 20-millimeter gel disk with a thickness of 1600 μm (for a different volume of alginate deposited, calculate the gap between plates or thickness of the gel by using the formula for the volume of a cylinder of 20 mm diameter. Gently spinning the rheometer head as it is brought down can help form a uniform gel disk between the plates.
Add mineral oil around the exposed hydrogel surface to prevent dehydration.
-
Measure the mechanical properties over time until the storage and loss moduli reach an equilibrium value.
Measure the storage and loss moduli at 1% strain and 1rad/s periodically for 60 minutes. Equilibrium loss tangent can be measured as the ratio of loss modulus to storage modulus at the end of the oscillation test.
-
Downstream rheological tests:
Note that it is not recommended to perform more than one downstream rheological test on a given sample. Prepare a new sample for each new test.-
6a)Strain sweep to confirm that the strain amplitude used for the oscillation test (Step 5) is within the linear elastic regime.Measure the storage and loss moduli at 1 rad/s from 0.5% to 50% strain.
-
6b)Frequency sweep to confirm that the angular frequency used for the oscillation test (Step 5) is within the linear elastic regime.Measure the storage and loss moduli at 1% strain from 0.01 to 100 rad/s.
-
6c)Stress relaxation testApply a step shear strain of 10% and measure shear stress over 1–2 hours or longer (until stress relaxes to about 20% of the initial peak stress). Calculate the time taken for the stress to relax to 50% of the initial peak stress – this is the characteristic stress relaxation time τ ½.
-
6a)
Note that the elastic modulus (i.e., Young’s modulus) can be related to the shear storage and loss moduli through the equation
Where the Poisson ratio (ν) is typically assumed to be 0.5 for alginate gels, and where the complex shear modulus G* is calculated from the storage and loss moduli using
Sample Data
(See Figure 5)
Figure 5: Sample mechanical testing for alginate hydrogels with unconfined compression and shear rheology.
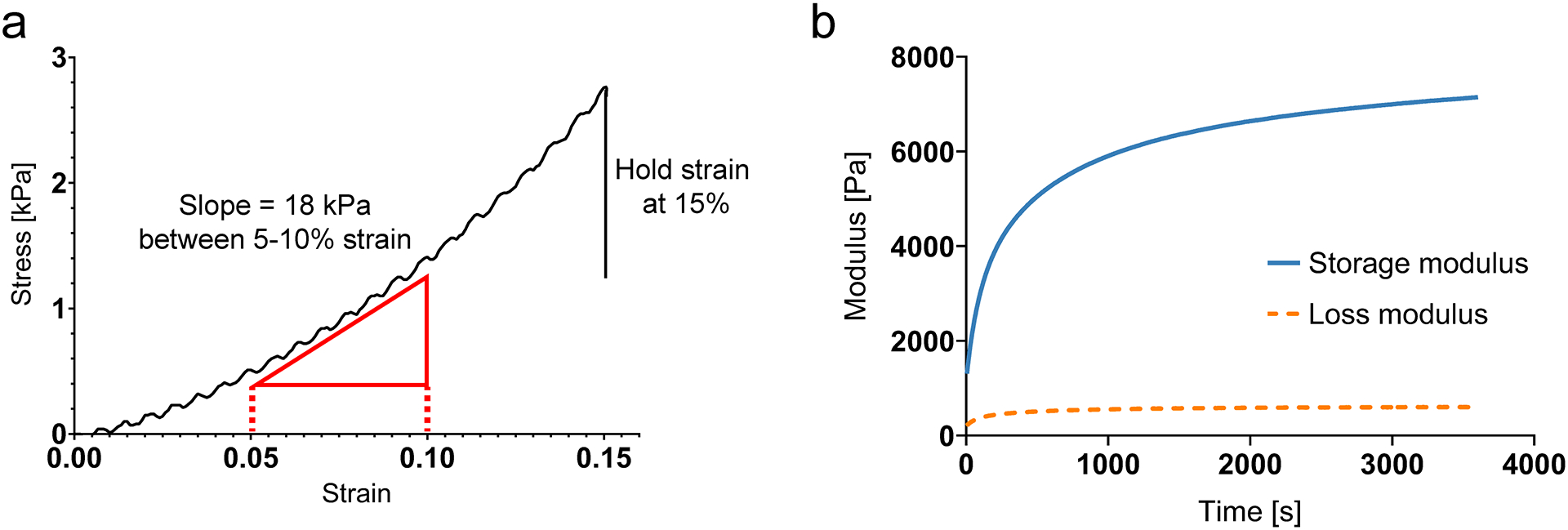
(a) Sample unconfined compression test of ionically crosslinked alginate. Elastic modulus (stiffness) is measured as the slope of the stress-strain curve between 5% and 10% strain. For stress relaxation tests, strain is held at 15% until stress drops below one-half of the peak value. (b) Sample shear rheometer test of ionically crosslinked alginate. Storage and loss modulus are measured until the values reach equilibrium. Gel is deposited into the rheometer just before time t = 0s.
SUPPORT PROTOCOL 2
CONJUGATING CELL-ADHESION PEPTIDE RGD TO ALGINATE
Introductory paragraph:
Unmodified alginate does not present binding sites for mammalian cell adhesion. However, we can covalently couple the cell-adhesion peptide motif RGD (derived from the ECM protein fibronectin) to the alginate chain to promote integrin-based cell adhesion using a well-established and long documented protocol based on carbodiimide chemistry (Rowley et al., 1999). This protocol (Rowley et al., 1999) should be cited for coupling of RGD to alginate and is only described here for convenience. Alternatively, you can purchase RGD-coupled alginate directly (Dupont/Novamatrix). Note that this same protocol can be used to couple other cell-adhesion peptide motifs to the alginate. Note that an alternative approach for promoting cell adhesion is forming an interpenetrating network of alginate with collagen (Branco da Cunha et al., 2014; Vining, Stafford, & Mooney, 2019) or reconstituted basement membrane matrix (e.g., the commercial product Matrigel) (Chaudhuri et al., 2014; Wisdom et al., 2018).
Materials:
Alginate powder from vendor
MES buffer (see recipe in Reagents and Solutions)
EDC (Sigma-Aldrich, cat. no. E6383)
Sulfo-NHS (Thermo Scientific, cat. no. 24510)
GGGGRGDSP peptide, lyophilized (Peptide 2.0 custom order)
Hydroxylamine hydrochloride (Sigma-Aldrich, cat. no. 255580)
Sodium chloride
Deionized water
Dialysis Tubing, 3.5 kDa MWCO (Spectrum 132592)
Nalgene bucket, 4 liters (Thermo Scientific, cat. no. 1201–4000)
Clamps (e.g., WeLock PA 70, 1207001)
Parafilm
Stericup Quick Release Sterile Vacuum Filtration System (MilliporeSigma, cat. no. S2GPU05RE)
Steriflip Sterile Disposable Vacuum Filter Units (MilliporeSigma, cat. no. SCGP00525)
Lyophilizer
Stir plate
Stir bars
Lab tape
Glass beakers
Protocol steps — Step annotations:
-
Dry the powdered alginate from the vendor overnight on a lyophilizer.
The alginate is lyophilized to remove water and ensure that dry weight measurements are accurate.
While stirring on stir plate, slowly add 1 gram of lyophilized alginate per 100 milliliters of MES buffer.
Cover the beaker with parafilm. Wait overnight until all the alginate has fully dissolved.
-
Calculate the masses of sulfo-NHS, EDC, and RGD powder to use, and weigh out into small weighing trays. (See Table 5 for examples).
The RGD listed has amine groups already added. Sulfo-NHS is a catalyst.
-
Mixing quickly, add in order the sulfo-NHS, EDC, and then RGD.
Add a small amount of MES buffer into the weighing tray and then pour into the alginate mixture. Flush out any remaining powder into the alginate mixture using a pipette.
Cover the beaker with parafilm. Allow the reaction to proceed for 20 hours.
While stirring, quench the reaction with 18 milligrams of hydroxylamine hydrochloride per gram of alginate.
-
Cut several segments of dialysis tubing. Soak the tubing in hot water for several minutes.
Cut the tubing segments to be slightly longer than the height of the bucket. For a 4-liter bucket, cut approximately 4 segments per 100 milliliters of alginate mixture.
-
Clamp one end of each segment of tubing. Fill each segment with approximately 25 milliliters of alginate mixture. Clamp the opposite end of the tubing, leaving some air at the top. Tape one end of each segment to the edge of the 4-liter bucket so that the tubing hangs down into the bucket.
Make sure that the clamps will not open and release the alginate solution. The clamps should be heavy enough to weigh down the bottom of the tubing once the bucket is filled with water.
-
Add 30 grams of sodium chloride to the bucket and fill with 4 liters of deionized water. Stir on a stir plate for approximately 6 hours.
When adding water, avoid pouring too strongly or directly onto the tubing to avoid accidentally opening the clamps.
-
Repeat this process (from the previous step) to dialyze the alginate in several decreasing salt solutions of deionized water and sodium chloride for 3 days. Change the salt solution 2–3 times per day (e.g., morning, afternoon, evening).
NaCl per 4L deionized water: 30g-25g-20g-15g-10g-5g-0g-0g-0g-0g
For coupling reactions, dialysis is critical to remove all the reaction products and unreacted components.
-
Pour the dialyzed alginate solution from each segment of tubing into a beaker. Sterile filter the alginate solution using a large bottle filter unit (Stericup system).
Ultrapure or research grade alginate does not require additional purification, but food grade alginate should be purified with activated charcoal prior to sterile filtering to remove impurities. For the latter case, add 0.5 grams of activated charcoal per gram of alginate, stir for 30 minutes, stop stirring and let the activate charcoal settle, then sterile filter.
-
Unscrew the filter top of a 50-milliliter Steriflip unit. Pour approximately 35–40 milliliters of sterile alginate solution into the Steriflip and replace the filter top. Repeat for the entire volume of alginate.
For 50-milliliter Steriflip tubes, only fill to approximately 35–40 milliliters to prevent the tube from cracking when the alginate freezes. The filter top is used to maintain sterility while allowing sublimation of ice to occur during lyophilization.
-
Freeze the alginate overnight at −20/−30C.
We recommend placing the tubes at a slight angle in the freezer to prevent cracking. If a tube cracks, thaw the alginate, refilter, and refreeze in a new Steriflip tube.
Lyophilize for approximately 3 days until the alginate is completely dry.
Store lyophilized alginate at −20/−30C for up to two years.
Table 5:
Formulations for coupling RGD peptide to alginate with varying degrees of substitution (DS). Note that the molecular weight of all alginate samples here is 280kDa.
| Sample | Alginate (mg) | RGD (mg) | Sulfo-NHS (mg) | EDC (mg) | Final RGD concentration for 2% alginate [μm] |
|---|---|---|---|---|---|
| DS2 | 200 | 2.87 | 5.64 | 9.97 | 150 |
| DS20 | 200 | 28.71 | 56.44 | 99.75 | 1500 |
REAGENTS AND SOLUTIONS:
AAD/HOBT solution
For 1X AAD/HOBT solution, mix 100 milligrams AAD (Adipic acid dihydrazide, Sigma-Aldrich A0638) and 100 milligrams of HOBT (1-Hydroxybenzotriazole hydrate, Sigma-Aldrich 54802 or Advanced ChemTech RC8201) in 10 milliliters of MES buffer (see recipe below). For 0.2X AAD/HOBT solution, mix 20 milligrams AAD and 100 milligrams of HOBT in 10 milliliters of MES buffer. Sterile filter and store at 4C for 6–12 months.
Calcium slurry, 1.22 M
Mix 8.4 grams of calcium sulfate dihydrate (Sigma-Aldrich, C3771) per 40 milliliters of deionized water. The calcium settles rapidly, so place a stir bar in the bottle before autoclaving to break up clumps and ensure an even dispersion before using. Autoclave and store indefinitely at room temperature. We prefer to use calcium sulfate instead of calcium chloride for introducing calcium to crosslink the hydrogels. Calcium chloride is highly soluble so that it rapidly crosslinks alginate, making it difficult to form a homogenously crosslinked gel. Calcium sulfate is less water-soluble than calcium chloride and therefore provides a slower delivery of calcium to the alginate, reducing the speed of gelation and allowing manual mixing of the crosslinker with alginate to form a homogenous hydrogel.
Calcium stock working solution, 488mM
Mix autoclaved calcium slurry well using the stir bar. In a 15-milliliter conical tube, add 400 microliters of calcium slurry to 600 microliters of DMEM. When pipetting from the calcium slurry, watch out for clumps of calcium which can clog the pipette tip and produce inaccurate volumes. Store at 4C for 1–2 weeks.
EDC solution
Allow EDC (Sigma-Aldrich E6383) to warm to room temperature (15–20 minutes) before opening the bottle. We recommend purchasing EDC in small bottles as it is extremely hygroscopic and will lose activity upon repeated exposure to moisture. Add 100 milligrams of EDC per 1 milliliter of MES buffer, then sterile filter. EDC solution must be freshly prepared just before use in crosslinking reactions.
MES buffer (0.1M MES, 0.3M NaCl)
Add 9.76 grams of MES hydrate (Sigma M8250) and 8.77 grams of sodium chloride (Fisher, S671) to 500 milliliters of deionized water. Bring to pH 6.5 with 10N sodium hydroxide (Sigma-Aldrich S8045).
Reconstituted alginate stock, 3% w/v
Begin with unmodified alginate that has been sterile-filtered and lyophilized (see recipe below), or alginate which has been modified with PEG (Basic Protocol 3) or RGD (Support Protocol 2) and lyophilized. Add serum-free DMEM (for ionically crosslinked hydrogels) or MES buffer (for covalently crosslinked hydrogels) to lyophilized alginate to bring the total polymer concentration to 3% weight/volume, or 30 milligrams alginate per 1 milliliter of DMEM/MES buffer. Dissolve using a sterile, autoclaved stir bar. For high molecular weight alginate, leave on the stir plate overnight to ensure complete mixing. Store at 4C for 1–2 months.
Sterile-filtered and lyophilized alginate
Begin with alginate powder from vendor (e.g., Pronova Ultrapure MVG (> 200kDa) from Dupont/Novamatrix for high molecular weight, Pronova Ultrapure VLVG (<75kDa) for low molecular weight). While stirring on a stir plate, add 1 gram of alginate powder per 100 milliliters of deionized water. Cover with parafilm and wait until the alginate has fully dissolved (several hours to overnight).
Dialysis is not strictly necessary for ultrapure unmodified alginate but will help to filter out lower molecular weight alginate fragments. Dialyze the alginate against DI water with 5 washes over 3 days (see Basic Protocol 3 steps 10 through 13 for detailed dialysis instructions but dialyze with DI water rather than sodium chloride solution). If you choose not to dialyze the alginate, proceed directly to sterile filtering after dissolving the alginate powder in water.
Sterile filter, freeze, and lyophilize the alginate solution (see Basic Protocol 3 steps 14 through 18 for detailed instructions). Store lyophilized alginate at −20/−30C for up to two years.
COMMENTARY
BACKGROUND INFORMATION:
Studies over the last two decades have indicated that cells sense and respond to the stiffness of ECMs. Mechanistically, the current thinking is that stiff substrates resist deformation by cell-generated contractions more strongly than soft substrates, resulting in higher forces carried by the adhesions and cytoskeleton, and consequently, altered cellular signaling and behavior through various molecular mechanisms (Discher et al., 2005; Janmey et al., 2020; Vogel & Sheetz, 2006). However, these experiments often seeded cells on linearly elastic 2D substrates (i.e., materials that behave like a simple spring). In contrast, most biological tissues and reconstituted extracellular matrices are viscoelastic, meaning they exhibit time-dependent mechanical responses and the ability to dissipate energy (Chaudhuri et al., 2020). Viscoelastic materials relax stresses under a constant applied deformation or increase deformation (“creep”) over time under a constant applied stress, as opposed to linearly elastic materials in which stress is always proportional to strain. Growing evidence demonstrates that cells can sense and respond to changes in substrate viscoelasticity, suggesting that a refined picture of mechanotransduction is needed (Chaudhuri et al., 2020).
Viscoelasticity in extracellular matrices and tissues can originate from several different mechanisms (Chaudhuri, 2017). Weak non-covalent bonds in fibrous biopolymer networks (e.g., collagen and fibrin) can break and reform to allow viscoelastic matrix flow (Nam, Hu, Butte, & Chaudhuri, 2016). The release of entangled polymers and the unfolding of proteins can similarly dissipate energy under loading (A. E. X. Brown, Litvinov, Discher, Purohit, & Weisel, 2009; Zhao, 2014). Viscous dissipation can also result from the flow of water through porous networks (i.e., poroelasticity) (Zhao, Huebsch, Mooney, & Suo, 2010). The importance of these mechanisms varies between different tissues and ECMs.
Biomaterials with tunable viscoelasticity will likely play a key role in furthering our understanding of cell-matrix interactions and advancing the field of mechanobiology. Natural ECM-based materials such as collagen and reconstituted basement membrane matrix (e.g., Matrigel) are naturally viscoelastic (Nam, Hu, et al., 2016) and readily support cell adhesion but are not easily tunable, making it difficult to decouple the effects of stiffness, porosity, degradability, ligand density, and stress relaxation on cell behaviors. Further, these natural ECM based materials tend to be relatively soft, with elastic moduli lower than 100 Pa, much softer than many soft tissues, which can have moduli ranging from 100s of Pa to 10s of kPa (Chaudhuri et al., 2020). Synthetic approaches have been developed to vary viscoelastic properties independently of the initial elastic modulus. Viscoelastic polyacrylamide gels with similar stiffness but different levels of creep and loss moduli (a measure of viscous energy dissipation) have been created by partial crosslinking (Cameron et al., 2011) or by entrapping linear chains of polyacrylamide within a crosslinked network (Charrier et al., 2020, 2018). Polyacrylamide substrates can support cell adhesion with the addition of ECM coatings (e.g., collagen or fibronectin), but these materials are limited to 2D studies, due to the toxicity of bisacrylamide crosslinker used to form the polyacrylamide gels. Viscoelastic polyethylene glycol (PEG)-based hydrogels with dynamic covalent bonds that unbind and rebind under cell-generated stresses have been developed for 3D cell culture (T. E. Brown et al., 2018; McKinnon et al., 2014; Richardson, Wilcox, Randolph, & Anseth, 2019; Tang et al., 2018). Other materials used to vary viscoelasticity include hyaluronic acid-based hydrogels (Loebel, Mauck, & Burdick, 2019; Lou et al., 2018) and protein networks. (Dooling, Buck, Zhang, & Tirrell, 2016) While many of these approaches use different crosslinker chemistries or polymer concentrations to vary stress relaxation properties, the alginate system here uses a single type of crosslinker and keeps polymer concentration constant while remaining easily amenable to 3D cell culture. Alginate, along with synthetic polymers such as PEG, are non-degradable by mammalian enzymes and bioinert, but cell-adhesion can be enabled by adding defined densities of ligands such as RGD.
The molecular structure of alginate and interactions with crosslinkers give rise to the unique mechanical properties of alginate-based hydrogels. Alginate is a naturally derived polysaccharide made up of monomers of mannuronic acid (M-units) and guluronic acid (G-units). An alginate molecule contains segments of repeating M-units (M blocks) and repeating G-units (G blocks) as well as blocks of alternating M- and G-units. Divalent cations (e.g., calcium) can bridge two G blocks to form an ionic crosslink between different alginate chains and lead to gelation of a three-dimensional polymer network (Rowley et al., 1999). These weak ionic crosslinks can break under stress and then reform, allowing local matrix flow to dissipate stress and resulting in macroscopic stress relaxation of the hydrogel under an applied load (Zhao et al., 2010). The length of the G blocks is expected to control the length of the ionic crosslink zone (Kong, Wong, & Mooney, 2003), with the number of calcium ions packed between G-blocks controlling the strength of the bond (egg-box model) (Grant, Morris, Rees, Smith, & Thom, 1973). Once the G-blocks are lined up with some minimum level of crosslinking, additional crosslinks fill in these crosslink junctions and do not change the pore structure. As a result, the pore size of ionically crosslinked alginate hydrogels is not altered by increased crosslinking, as has been established by diffusion studies (Boontheekul, Kong, & Mooney, 2005; Huebsch et al., 2010). We have similarly found that varying the molecular weight of the constituent alginate to vary viscoelasticity also does not change the pore structure, based on diffusion studies (Agarwal et al., 2021). The gel swelling ratio, typically defined as the ratio of the wet gel mass to the dried gel mass, also does not vary greatly between gel formulations (Lee et al., 2017).
In addition to being viscoelastic, ionically crosslinked alginate hydrogels can undergo plastic, or irreversible, deformations in response to mechanical loading. Mechanical plasticity can be characterized with a creep-recovery test (Nam, Lee, Brownfield, & Chaudhuri, 2016). After applying a constant stress to a material for some length of time, the stress is removed, and the material relaxes. For viscoplastic materials (viscoelastic and plastic), there will be an elastic component of deformation which is recovered as well as a plastic component of deformation which is permanent. Matrix plasticity may mediate some of the effects of material properties on cell behavior in 2D and 3D and should be considered in addition to viscoelasticity (Chaudhuri et al., 2020). For example, increased loss modulus or stress relaxation on viscoelastic and viscoplastic substrates promoted fibroblast spreading, (Chaudhuri et al., 2015) whereas increased loss modulus or stress relaxation on viscoelastic, but not viscoplastic, substrates restricted fibroblast spreading (Charrier et al., 2018). Similarly, a more recent approach for decoupling plasticity from stress relaxation in alginate-PEG hydrogels suggests an important role for plasticity in MSC spreading and downstream signaling pathways (Grolman, Weinand, & Mooney, 2020). The ability of alginate-based hydrogels to be plastically deformed has been implicated in 3D cell migration, with cells plastically opening up micron-size channels in order to migrate through the gels (Wisdom et al., 2018). Thus, it may be important to consider the plasticity of the gels when trying to ascertain mechanisms, particularly in 3D studies.
Each of the three Basic Protocols described in this article rely on different methods for tuning viscoelasticity by altering the structure and strength of interactions between alginate polymer chains (Figure 6). Decreasing the length (i.e., molecular weight) of the alginate molecule (Basic Protocol 1) is thought to increase chain mobility and thus enhance stress relaxation, whereas increasing alginate molecular weight will decrease chain mobility and impede stress relaxation (Chaudhuri et al., 2016). Alternatively, alginate chains can be covalently crosslinked (Basic Protocol 2) by linking carboxylic groups in the alginate molecule via a crosslinker molecule (e.g., a diamine) through carbodiimide chemistry (Rowley et al., 1999). Covalently crosslinked alginate hydrogels are elastic and show minimal stress relaxation at the length and timescales most relevant to cell behaviors (Chaudhuri et al., 2015). Lastly, the coupling of short PEG spacers to alginate chains (Basic Protocol 3) enhances stress relaxation of ionically crosslinked hydrogels at a given alginate molecular weight (Nam, Stowers, et al., 2019). This enhanced stress relaxation is likely a result of the PEG spacers physically interfering with crosslinking sites (i.e., steric hindrance) to alter the strength of chain connections (Chaudhuri et al., 2016).
Figure 6: Tuning stress relaxation in alginate hydrogels by varying the type of crosslinking and alginate molecular weight.
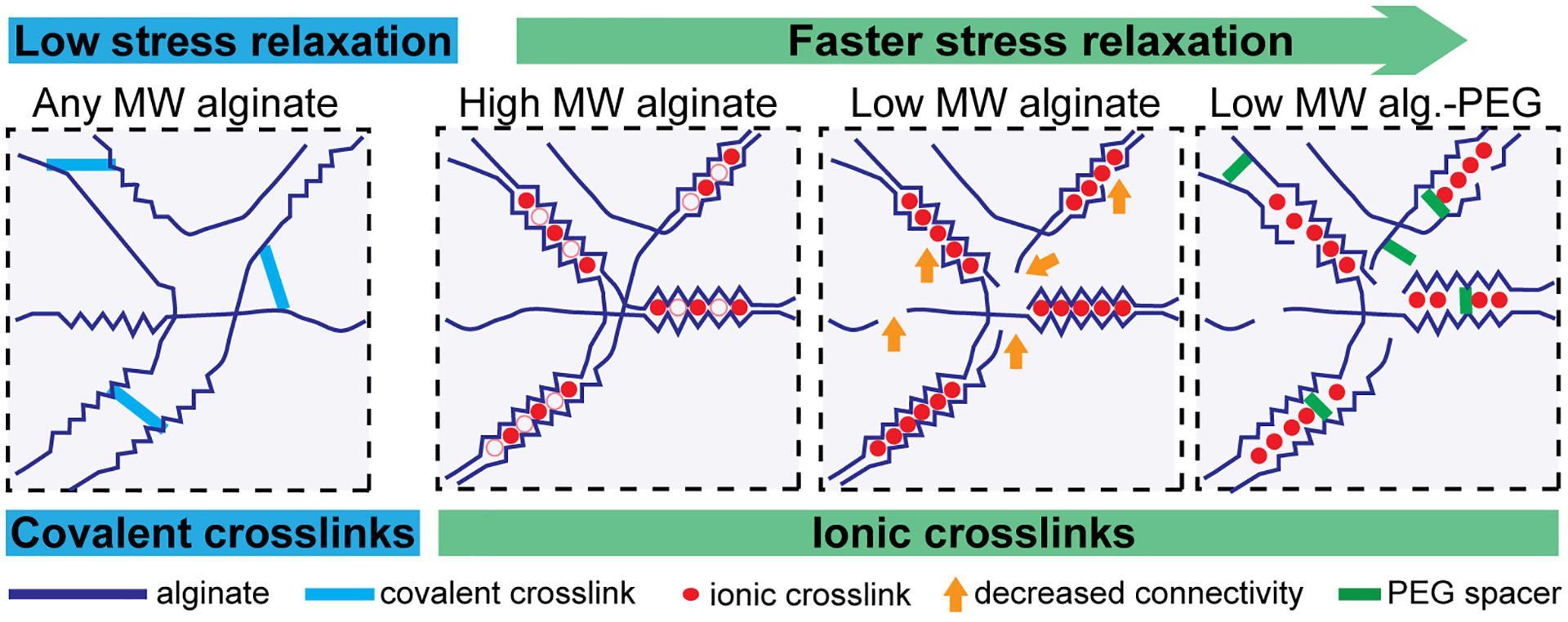
(Modified with permission from Chaudhuri et al., 2016). Decreasing the alginate molecular weight or adding PEG spacers to alginate enhances stress relaxation in ionically crosslinked alginate hydrogels. Covalently crosslinked alginate hydrogels exhibit minimal stress relaxation.
The tunable alginate system described here has been used to study the impact of viscoelasticity on cell behavior in a number of 2D and 3D contexts. Ionically crosslinked alginate substrates with stress relaxation promoted greater 2D cell spreading and activation of mechanosensitive proteins (i.e., YAP) than elastic, covalently crosslinked alginate substrates with the same stiffness (Chaudhuri et al., 2015). Faster stress relaxation in 3D alginate hydrogels promoted MSC spreading, proliferation, and osteogenic differentiation, as well as formation of an interconnected bone-like matrix by osteogenically differentiated MSCs (Chaudhuri et al., 2016; Lee et al., 2019). Similarly, faster stress relaxation enhanced new bone growth for implanted alginate hydrogels (Darnell et al., 2017), demonstrating the importance of viscoelasticity as a design parameter for biomaterials in regenerative medicine. Faster stress relaxation in alginate hydrogels also reduced cell death and increased matrix formation by encapsulated chondrocytes (Lee et al., 2017). Neural progenitor cells (NPCs) in viscoelastic, ionically crosslinked alginate hydrogels were able to remodel the matrix and maintain expression of NPC stemness markers, whereas NPCs in elastic, covalently crosslinked alginate were unable to spread and maintain stemness (Madl et al., 2017). Faster stress relaxation promoted cell cycle entry and tumor spheroid growth for breast cancer cells encapsulated in alginate hydrogels with no adhesive ligands (Nam, Gupta, et al., 2019).
These initial studies have started to uncover some of the mechanisms by which cells sense ECM viscoelasticity in different contexts, some of which were not implicated in stiffness-sensing and might be unique to sensing of viscoelasticity. The clustering of adhesive ligands and bound integrins has been implicated in the enhanced spreading of cancer cells on faster-relaxing 2D alginate (Chaudhuri et al., 2015), and in the osteogenic differentiation of MSCs in viscoelastic 3D alginate gels (Chaudhuri et al., 2016; Huebsch et al., 2010). A 2D molecular clutch model predicted that maximal cell spreading on soft viscoelastic substrates occurs at an intermediate viscosity, when the timescale of material relaxation is between the timescale of clutch binding and focal adhesion lifetime (Gong et al., 2018). This finding was validated in three different tunable material systems (polyacrylamide, hyaluronic acid, and alginate) providing strong support for this finding. An alternative adhesion-independent mechanism for sensing viscoelasticity in 3D mechanically confining environments is by cell volume expansion. Cells can grow to expand their volume in faster-relaxing matrices but are restricted in slower-relaxing or elastic matrices. The restriction of chondrocyte expansion in slower-relaxing alginate hydrogels lacking adhesive ligands led to increases in pro-inflammatory signaling and an osteoarthritic phenotype, whereas faster relaxation promoted increased formation of cartilage matrix (Lee et al., 2017). Similarly, MSC volume expansion during cell spreading activated TRPV4 mechanosensitive ion channels to promote osteogenic differentiation (Lee et al., 2019), and cancer cell growth during G1 phase activated a TRPV4-PI3K/Akt-p27 pathway to promote cell cycle progression (Nam, Gupta, et al., 2019). In the case of chondrocyte expansion and cancer cell growth, stiffness did not have as much of an impact, at least over the range of stiffness probed (3 kPa – 20 kPa). Thus, matrix viscoelasticity appears to relate strongly to the concept of confinement, and mechanisms for sensing of confinement appear to be distinct from mechanisms of stiffness-sensing. These discovered mechanisms of integrin clustering, the molecular clutch, and volume expansion coupled with activation of mechanosensitive ion channels provide a good starting point for probing mechanism.
CRITICAL PARAMETERS:
Users should consider the desired hydrogel mechanical properties for a given experiment before conducting these protocols. The alginate molecular weight, alginate concentration (% w/v), type or amount of crosslinker (Ca2+ or EDC), and amount of added PEG are critical parameters for determining the hydrogel stiffness and the timescale of stress relaxation. While we have provided sample mechanical testing data for specified combinations of these parameters (Table 1, Table 2, Table 4), these values should only serve as an initial reference point. The actual mechanical properties of a given hydrogel may depend on batch-to-batch variations in the alginate starting material, such as the distribution of molecular weights.
We recommend that users practice making gels until they can confidently obtain repeatable mechanical properties for gels made on separate occasions but from the same batch of alginate. This allows the user to characterize the stiffness and relaxation time of a given set of gels and assume they will be representative of a separate set of gels used for cell experiments. Users may wish to include calcium in the media at higher levels (e.g., ~ 1 mM or higher) to prevent a possible reduction in hydrogel mechanics over time due to calcium crosslinker slowly diffusing out of the gel.
In addition to mechanical properties, the RGD ligand density can have a large impact on cellular behavior in some contexts, e.g., in processes dependent on cell-adhesion. Users may wish to conduct pilot experiments to determine an optimal ligand density for their experiments. We typically include RGD in the range of 150 to 1500 μM for the final gel concentration.
Finally, users should conduct calcium control studies to ensure that the calcium crosslinker itself is not impacting cells. This can be done in various ways including: (1) conducting select sets of studies in a separate gel system with added calcium (e.g., collagen, Matrigel, or agarose); (2) plotting a key output variable vs. calcium crosslinking concentration for studies with hydrogels with a range of stiffnesses and stress relaxation and seeing if there is a strong correlation with calcium; (3) varying viscoelasticity using orthogonal approaches (e.g., Basic Protocol 1 and Basic Protocol 3). Note that while calcium crosslinking concentrations can be as high as 20 mM, most of the calcium is sequestered in crosslinks and not available to cells. Thus, the available calcium concentration is likely to be closer to 1 – 2 mM, which is similar to the range found in some media formulations. In our studies, varied calcium crosslinking levels have not been found to impact cell behaviors through altered biological calcium signaling.
TROUBLESHOOTING:
Issue: gelation in syringe - After mixing the alginate and calcium between syringes, the gel should flow as a viscous liquid. If the mixture begins to gel inside the syringe, the steps of mixing and depositing into the mold may not be quick enough. It can take some practice to perform these steps quickly.
Issue: non-homogenous gels - If the gel is heterogenous (e.g., some regions are gelled whereas others are watery), try using larger syringes to mix more forcefully (e.g., 3-millimeter rather than 1-millimeter syringes). If this does not work, test out different numbers of mixes. Mixing 6 times (i.e., 3 pushes of the syringe plunger to each side) generally works best for typical gel volumes (e.g., 1000 microliter gel and 3-milliliter syringes), but this may vary with gel volumes and syringe sizes. Gel volumes below 500 microliters can be difficult to work with and are not recommended.
If there are still issues with gel formation, check the concentrations and volumes of the calcium slurry and reconstituted alginate, as well as check for contamination. Alginate is resistant to mammalian enzymes but can be degraded by alginate lyase from bacteria. Sodium azide (0.02% w/v) can be added to alginate to prevent contamination of gels that will not be used for cell culture (e.g., practice gels) but it is best to sterilize the alginate and then use aseptic technique. Alginate also degrades rapidly at high pH, so media for reconstitution should be at or below pH 7.2–7.4.
TIME CONSIDERATIONS:
Preparing PEG-conjugated (Basic Protocol 3), RGD-conjugated (Support Protocol 2), or unmodified sterile alginate takes approximately one and a half weeks, which includes several days of dialysis and lyophilization. Lyophilized alginate can be reconstituted in media or buffer overnight. Once the reconstituted alginate and calcium stock are prepared, it takes approximately one hour to prepare ionically crosslinked alginate gels (Basic Protocol 1). Covalently crosslinked alginate gels (Basic Protocol 2) can be prepared in the same amount of time but take several hours to fully gel and should then be rinsed of excess EDC over 1–2 days before use. Mechanical testing of alginate gels (Support Protocol 1) takes several hours, depending on the method and the number of samples being tested.
ACKNOWLEDGEMENTS:
O.C. acknowledges support from a National Science Foundation CAREER award (CMMI 1846367) and a National Institutes of Health National Cancer Institute grant (R37 CA214136). F.C. acknowledges support from a National Science Foundation Graduate Research Fellowship (DGE-1656518). The authors would also like to thank Hong-Pyo Lee, Sungmin Nam, and current members of the Chaudhuri lab for helpful discussions.
Footnotes
CONFLICT OF INTEREST
None
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
LITERATURE CITED:
- Agarwal P, Lee H, Smeriglio P, Grandi F, Goodman S, Chaudhuri O, & Bhutani N (2021). A dysfunctional TRPV4–GSK3β pathway prevents osteoarthritic chondrocytes from sensing changes in extracellular matrix viscoelasticity. Nature Biomedical Engineering. 10.1038/s41551-021-00691-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boontheekul T, Kong HJ, & Mooney DJ (2005). Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials, 26(15), 2455–2465. 10.1016/j.biomaterials.2004.06.044 [DOI] [PubMed] [Google Scholar]
- Bouhadir KH, Lee KY, Alsberg E, Damm KL, Anderson KW, & Mooney DJ (2001). Degradation of partially oxidized alginate and its potential application for tissue engineering. Biotechnology Progress, 17(5), 945–950. 10.1021/bp010070p [DOI] [PubMed] [Google Scholar]
- Branco da Cunha C, Klumpers DD, Li WA, Koshy ST, Weaver JC, Chaudhuri O, … Mooney DJ (2014). Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials, 35(32), 8927–8936. 10.1016/j.biomaterials.2014.06.047 [DOI] [PubMed] [Google Scholar]
- Brown AEX, Litvinov RI, Discher DE, Purohit PK, & Weisel JW (2009). Multiscale mechanics of fibrin polymer: Gel stretching with protein unfolding and loss of water. Science, 325(5941), 741–744. 10.1126/science.1172484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TE, Carberry BJ, Worrell BT, Dudaryeva OY, McBride MK, Bowman CN, & Anseth KS (2018). Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials, 178, 496–503. 10.1016/j.biomaterials.2018.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron AR, Frith JE, & Cooper-White JJ (2011). The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials, 32(26), 5979–5993. 10.1016/j.biomaterials.2011.04.003 [DOI] [PubMed] [Google Scholar]
- Cameron AR, Frith JE, Gomez GA, Yap AS, & Cooper-White JJ (2014). The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials, 35(6), 1857–1868. 10.1016/j.biomaterials.2013.11.023 [DOI] [PubMed] [Google Scholar]
- Charrier EE, Pogoda K, Li R, Park CY, Fredberg JJ, & Janmey PA (2020). A novel method to make viscoelastic polyacrylamide gels for cell culture and traction force microscopy. APL Bioengineering, 4(3), 036104. 10.1063/5.0002750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier EE, Pogoda K, Wells RG, & Janmey PA (2018). Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nature Communications, 9(1). 10.1038/s41467-018-02906-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O (2017). Viscoelastic hydrogels for 3D cell culture. Biomaterials Science. 10.1039/c7bm00261k [DOI] [PubMed] [Google Scholar]
- Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, & Shenoy VB (2020). Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature, 584(7822), 535–546. 10.1038/s41586-020-2612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, … Mooney DJ (2015). Substrate stress relaxation regulates cell spreading. Nature Communications, 6, 6364. 10.1038/ncomms7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, … Mooney DJ (2016). Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nature Materials, 15(3), 326–334. 10.1038/nmat4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri O, Koshy ST, Branco Da Cunha C, Shin JW, Verbeke CS, Allison KH, & Mooney DJ (2014). Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nature Materials, 13(10), 970–978. 10.1038/nmat4009 [DOI] [PubMed] [Google Scholar]
- Darnell M, O’Neil A, Mao A, Gu L, Rubin LL, & Mooney DJ (2018). Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proceedings of the National Academy of Sciences of the United States of America, 115(36), E8368–E8377. 10.1073/pnas.1802568115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell M, Young S, Gu L, Shah N, Lippens E, Weaver J, … Mooney D (2017). Substrate Stress-Relaxation Regulates Scaffold Remodeling and Bone Formation In Vivo. Advanced Healthcare Materials, 6(1), 1601185. 10.1002/adhm.201601185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RM, Koshy ST, Hilderbrand SA, Mooney DJ, & Joshi NS (2015). Versatile click alginate hydrogels crosslinked via tetrazine-norbornene chemistry. Biomaterials, 50(1), 30–37. 10.1016/j.biomaterials.2015.01.048 [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, & Wang YL (2005). Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science, 310(5751), 1139–1143. 10.1126/science.1116995 [DOI] [PubMed] [Google Scholar]
- Dooling LJ, Buck ME, Zhang W Bin, & Tirrell DA (2016). Programming Molecular Association and Viscoelastic Behavior in Protein Networks. Advanced Materials, 28(23), 4651–4657. 10.1002/adma.201506216 [DOI] [PubMed] [Google Scholar]
- Gong Z, Szczesny SE, Caliari SR, Charrier EE, Chaudhuri O, Cao X, … Shenoy VB (2018). Matching material and cellular timescales maximizes cell spreading on viscoelastic substrates. Proceedings of the National Academy of Sciences, 115(12), 201716620. 10.1073/pnas.1716620115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant GT, Morris ER, Rees DA, Smith PJC, & Thom D (1973). Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Letters, 32(1), 195–198. 10.1016/0014-5793(73)80770-7 [DOI] [Google Scholar]
- Grolman JM, Weinand P, & Mooney DJ (2020). Extracellular matrix plasticity as a driver of cell spreading. Proceedings of the National Academy of Sciences of the United States of America, 117(42), 25999–26007. 10.1073/pnas.2008801117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, … Mooney DJ (2010). Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature Materials, 9(6), 518–526. 10.1038/nmat2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janmey PA, Fletcher DA, & Reinhart-King CA (2020). Stiffness Sensing by Cells. Physiological Reviews, 100(2), 695–724. 10.1152/physrev.00013.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon O, Bouhadir KH, Mansour JM, & Alsberg E (2009). Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials, 30(14), 2724–2734. 10.1016/j.biomaterials.2009.01.034 [DOI] [PubMed] [Google Scholar]
- Kong HJ, Wong E, & Mooney DJ (2003). Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules, 36(12), 4582–4588. 10.1021/ma034137w [DOI] [Google Scholar]
- Lee HP, Gu L, Mooney DJ, Levenston ME, & Chaudhuri O (2017). Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nature Materials, 16(12), 1243–1251. 10.1038/nmat4993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HP, Stowers R, & Chaudhuri O (2019). Volume expansion and TRPV4 activation regulate stem cell fate in three-dimensional microenvironments. Nature Communications, 10(1), 529. 10.1038/s41467-019-08465-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebel C, Mauck RL, & Burdick JA (2019). Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels. Nature Materials, 18, 883–891. 10.1038/s41563-019-0307-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou J, Stowers R, Nam S, Xia Y, & Chaudhuri O (2018). Stress relaxing hyaluronic acid-collagen hydrogels promote cell spreading, fiber remodeling, and focal adhesion formation in 3D cell culture. Biomaterials, 154, 213–222. 10.1016/j.biomaterials.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Madl CM, Lesavage BL, Dewi RE, Dinh CB, Stowers RS, Khariton M, … Heilshorn SC (2017). Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nature Materials, 16(12), 1233–1242. 10.1038/nmat5020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinnon DD, Domaille DW, Cha JN, & Anseth KS (2014). Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3d cell culture systems. Advanced Materials, 26(6), 865–872. 10.1002/adma.201303680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, & Chaudhuri O (2018). Mitotic cells generate protrusive extracellular forces to divide in three-dimensional microenvironments. Nature Physics, 14(6), 621–628. 10.1038/s41567-018-0092-1 [DOI] [Google Scholar]
- Nam S, Gupta VK, Lee H, Lee JY, Wisdom KM, Varma S, … Chaudhuri O (2019). Cell cycle progression in confining microenvironments is regulated by a growth-responsive TRPV4-PI3K/Akt-p27 Kip1 signaling axis. Science Advances, 5(8), eaaw6171. 10.1126/sciadv.aaw6171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Hu KH, Butte MJ, & Chaudhuri O (2016). Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proceedings of the National Academy of Sciences, 113(20), 5492–5497. 10.1073/pnas.1523906113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Lee J, Brownfield DG, & Chaudhuri O (2016). Viscoplasticity Enables Mechanical Remodeling of Matrix by Cells. Biophysical Journal, 111(10), 2296–2308. 10.1016/j.bpj.2016.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S, Stowers R, Lou J, Xia Y, & Chaudhuri O (2019). Varying PEG density to control stress relaxation in alginate-PEG hydrogels for 3D cell culture studies. Biomaterials, 200, 15–24. 10.1016/j.biomaterials.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BM, Wilcox DG, Randolph MA, & Anseth KS (2019). Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering. Acta Biomaterialia, 83, 71–82. 10.1016/j.actbio.2018.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley JA, Madlambayan G, & Mooney DJ (1999). Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials, 20(1), 45–53. 10.1016/S0142-9612(98)00107-0 [DOI] [PubMed] [Google Scholar]
- Tang S, Ma H, Tu HC, Wang HR, Lin PC, & Anseth KS (2018). Adaptable Fast Relaxing Boronate-Based Hydrogels for Probing Cell–Matrix Interactions. Advanced Science, 5(9), 1–8. 10.1002/advs.201800638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vining KH, Stafford A, & Mooney DJ (2019). Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials. 10.1016/j.biomaterials.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V, & Sheetz M (2006). Local force and geometry sensing regulate cell functions. Nature Reviews Molecular Cell Biology, 7(4), 265–275. 10.1038/nrm1890 [DOI] [PubMed] [Google Scholar]
- Whitehead J, Griffin KH, Gionet-Gonzales M, Vorwald CE, Cinque SE, & Leach JK (2021). Hydrogel mechanics are a key driver of bone formation by mesenchymal stromal cell spheroids. Biomaterials, 269, 120607. 10.1016/j.biomaterials.2020.120607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom KM, Adebowale K, Chang J, Lee JY, Nam S, Desai R, … Chaudhuri O (2018). Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nature Communications, 9(1). 10.1038/s41467-018-06641-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X (2014). Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter, 10(5), 672–687. 10.1039/c3sm52272e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Huebsch N, Mooney DJ, & Suo Z (2010). Stress-relaxation behavior in gels with ionic and covalent crosslinks. In Journal of Applied Physics (Vol. 107, p. 63509). 10.1063/1.3343265 [DOI] [PMC free article] [PubMed] [Google Scholar]


