Abstract
Context
Maternal oxidative stress in pregnancy can arise through a multitude of sources and may have lifelong consequences for the child. Animal studies suggest that prenatal oxidative stress may contribute to metabolic dysfunction and excessive weight gain in the offspring. However, this relationship has been studied minimally in humans.
Objective
Determine the association between prenatal oxidative stress biomarkers and child weight and body mass index (BMI) z-scores from birth to age 6.
Methods
Within The Infant Development and the Environment Study (TIDES) prospective pregnancy cohort, we calculated age- and sex-specific Z-scores for child weight and BMI, measured between birth and age 6 (N = 736). Three oxidative stress biomarkers were quantified in third-trimester urine, including 8-iso-prostaglandin F2α (8-iso-PGF2α), its primary metabolite, and prostaglandin F2α (PGF2α). We examined associations between each biomarker and Z-scores using linear regression as well as group-based trajectory modeling.
Results
Prenatal 8-iso-PGF2α and its metabolite were associated with lower birth weight and higher weight at age 4. For example, an ln-unit increase in 8-iso-PGF2α was associated with 0.17 SD higher weight at age 4 (95% CI 0.01, 0.33). These biomarkers were also associated with higher BMI at age 4. Finally, within 4 unique weight trajectories (low, normal, high, and low–high), children of mothers with higher 8-iso-PGF2α were 2.56 times more likely (95% CI 1.22, 5.41) to be in the low–high trajectory than children in the normal group.
Conclusion
We observed associations between third-trimester oxidative stress and lower birth weight as well as higher early childhood weight and BMI. These findings have important implications for understanding the developmental origins of childhood weight gain and metabolic disease.
Keywords: oxidative stress, childhood, weight, trajectory, pregnancy, developmental origins of health and disease
The prenatal environment is a sensitive developmental period that can play a major role in programming future metabolic and other health outcomes. Oxidative stress, originating from a variety of sources, could play an important role in this process (1). Environmental exposures—such as air pollution, pesticides, or toxic metals—can cause the body to increase production of free radicals or decrease antioxidant production (2). Other factors that can contribute to oxidative stress are unhealthy lifestyle factors such as a diet lacking antioxidants, cigarette smoke, and stress in day to day life (2).
Animal models indicate that prenatal oxidative stress may increase the risk of obesity and metabolic syndrome in the offspring postnatally (3). In humans, prenatal oxidative stress in pregnancy has been linked to a reduction in fetal weight and birth weight (4-6), which can result in catchup growth in childhood that is accompanied by excess adipose gain (7). However, studies examining prenatal oxidative stress in relation to early childhood growth have been limited to investigation of child weight within the first 3 years of life and have had mixed findings (5, 8).
Utilizing data from The Infant Development and the Environment Study (TIDES), we evaluated the relationship between maternal oxidative stress biomarkers during pregnancy and offspring weight as well as body mass index (BMI) measured longitudinally from birth to 6 years of age. In addition, we investigated associations with growth trajectories over this time frame by categorizing children based on weight over time and estimating associations with prenatal oxidative stress. We hypothesized that higher oxidative stress biomarker concentrations in utero would be associated with lower birth weight but higher weight in childhood.
Materials and Methods
Study population
TIDES is a prospective pregnancy cohort, which was created to investigate the impact of environmental exposures during pregnancy on childhood health and development. Women were recruited in the United States between 2010 and 2012 during first-trimester prenatal care visits from academic health centers in the following cities: San Francisco, CA; Minneapolis, MN; Rochester, NY; and Seattle, WA. Pregnant women were eligible for inclusion if they were planning to deliver at one of the 4 study hospitals, were less than 13 weeks pregnant, over 18 years old, did not have a serious threat to the pregnancy, and spoke English (9). In study visits during each trimester, participants completed questionnaires, providing demographic information and information on pregnancy-related factors. Women provided urine samples in each trimester as well; sterile specimen cups were used to collect urine and samples were stored at –80°C. All women who were enrolled in the study provided signed informed consent and institutional review boards at all 4 study sites, as well as the coordinating center at the Icahn School of Medicine at Mount Sinai, approved the research study. The analysis of these data was deemed to not be human subjects research by the institutional review board of the National Institute of Environmental Health Sciences. Of the 787 women who delivered live births, we included 736 women in the present analysis who had singleton births as well as oxidative stress measurements from the third-trimester visit and birth weight at delivery.
Oxidative Stress Biomarkers
Third-trimester oxidative stress measures were used in this analysis, as the original objective of this study was to investigate oxidative stress in pregnancy in association with gestational age at delivery (10). The third-trimester urine samples were selected because we hypothesized that oxidative stress in this window would be most biologically relevant for an association with preterm birth. The following biomarkers were measured in urine samples provided at the third-trimester visit: free 8-iso-prostaglandin F2α (8-iso-PGF2α); 2,3-dinor-5,6-dihydro-15-F2t-isoprostane, the primary metabolite of 8-iso-PGF2α; and prostaglandin F2α (PGF2α). 8-Iso-PGF2α reflects arachidonic acid peroxidation by free radicals, and is recognized as a strong biomarker of oxidative stress because of its stability, specificity, and reproducibility of generation in response to prooxidant stimulation (11). The 8-iso-PGF2α metabolite may be a more sensitive biomarker than the parent compound (12). PGF2α is also produced from arachidonic acid, primarily through an enzymatic (cyclooxygenase) pathway; however, in human urine concentrations may also reflect nonenzymatic peroxidation (ie, oxidative stress) (13). Samples were analyzed using gas chromatography negative ion chemical ionization mass spectrometry with stable isotope dilution at the Eicosanoid Core Laboratory at Vanderbilt University Medical Center (Nashville, TN), described in detail elsewhere (14). Measurements which were below the limit of detection (LOD) of 0.101 ng/mL were imputed with the value of the LOD divided by the square root of 2. The oxidative stress biomarkers were detected in almost all samples, with the exception of 8-iso-PGF2α, which was below the LOD in 1 sample.
Specific gravity was measured in all urine samples using a hand-held refractometer as an indicator of urine dilution (15). All oxidative stress measurements were corrected for specific gravity using the following formula: OSc = OS ([1.014−1]/[SG–1]), where OSc is the oxidative stress biomarker concentration corrected for specific gravity, OS is the uncorrected biomarker concentration, SG is the specific gravity measurement in the sample, and 1.014 is the mean specific gravity of all samples in the study cohort (15). Oxidative stress biomarker concentrations were right-skewed and ln-transformed for analyses to improve model fit.
Childhood Weight and Body Mass Index Measures
Measurements of weight were collected at study visits from birth to age 6 years. Birth weight was recorded from the official medical record of each of the study hospitals. At an in-person visit for boys at age 1, a trained examiner measured weight (to the nearest 10 g) using a Seca Infant Scale Model #334 and length (to the nearest 0.1 cm) using a Seca Infantometer Model #416. At child age 3, weight and height were reported by the mother via a mailed questionnaire. At ages 4 and 6, trained research staff collected weight and height measurements at an in-person visit following a detailed protocol. Two weight and height measurements were performed, along with a third measurement if the first 2 differed by more than 0.2 kg or 0.5 cm, respectively, or were outside of the reference range. The average of the measurements was used for all analyses. Before each study visit, examiners participated in an in-person standardized training for all measurements.
Height and weight measures were used to calculate BMI in units of kg/m2. Birth weight examined in the present study has also been used in previous TIDES analyses (16, 17); however, this is the first TIDES study to incorporate the childhood weight measurements gathered from TIDES.
Sex-specific Z-scores for birth weight for gestational age were calculated from the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) standard (18). World Health Organization growth charts were used to compute sex-specific Z-scores for weight and BMI for study visits below 2 years of age, including the age 1 visit (boys only) and the age 3 visit for the children who were less than 2 at that time (19). Centers for Disease Control and Prevention growth charts were used to compute sex-specific Z-scores for childhood measures from visits in which the child was 2 years old or older (20). Per guidelines from each growth chart (19, 20), implausible Z-scores were identified and removed for analyses (n = 1 for weight measurements and n = 6 for BMI measurements).
Statistical Analysis
All analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC). Descriptive statistics, including distributions of demographic characteristics of the study participants and outcome measurements, were tabulated. Unadjusted and adjusted associations between oxidative stress biomarkers and weight and BMI Z-scores from each study visit were assessed using linear regression. Models were adjusted for confounders identified through a directed acyclic graph and included the following variables: maternal age (continuous), maternal race (white, black, or other), maternal education level (high school or less, any technical school/college, or graduate work), and prepregnancy BMI (continuous). Prepregnancy diabetes (yes, no), smoking during pregnancy (yes, no), alcohol use during pregnancy (yes, no), and prenatal vitamin use during pregnancy (yes, no) were also suspected confounders but had a minimal influence (<10% difference) on effect estimates and were thus excluded from primary analyses. Gestational age at delivery was considered to be on the causal pathway and thus was not included as an adjustment factor. Missing covariate data (shown in Table 1) were imputed 10 times with the Markov chain Monte Carlo method using the MI procedure in SAS. Results were then averaged across all 10 imputed datasets using the MIANALYZE procedure in SAS. As sensitivity analyses, we examined models additionally adjusted for study site or for child sex. Additionally, to explore effect modification by sex, we created models with interaction terms between the oxidative stress marker and sex in the adjusted models and examined models stratified by sex.
Table 1.
Demographic characteristics of the TIDES study population (N = 736)
| n (%) or mean (SD) | ||
|---|---|---|
| Study center | ||
| University of California San Francisco | 184 | (25.0) |
| University of Minnesota | 201 | (27.3) |
| University of Rochester Medical Center | 209 | (28.4) |
| University of Washington | 142 | (19.3) |
| Maternal age (years) | 30.5 | (5.5) |
| Maternal race | ||
| White | 503 | (68.4) |
| Black | 95 | (12.9) |
| Other | 137 | (18.6) |
| Maternal education level | ||
| High school or less | 103 | (14.1) |
| Any technical school/college | 316 | (43.3) |
| Graduate work | 311 | (42.6) |
| Prepregnancy BMI (kg/m2) | 25.6 | (6.3) |
| Prepregnancy diabetes | ||
| Yes | 34 | (4.9) |
| No | 665 | (95.1) |
| Ever smoked during pregnancy | ||
| Yes | 53 | (7.9) |
| No | 621 | (92.1) |
| Ever used alcohol during pregnancy | ||
| Yes | 91 | (13.6) |
| No | 580 | (86.4) |
| Ever took prenatal vitamins during pregnancy | ||
| Yes | 704 | (96.4) |
| No | 26 | (3.6) |
Missingness for each covariate was as follows: maternal age (n = 1); maternal race (n = 1); maternal education level (n = 6); pre-pregnancy BMI (n = 9); pre-pregnancy diabetes (n = 37); smoking (n = 62); alcohol use (n = 65); use of prenatal vitamins (n = 6).
Abbreviations: BMI, body mass index; SD, standard deviation.
We created trajectories for child weight Z-scores from birth to age 6 using group-based trajectory modeling in SAS, which allows for clustering individuals based on shared longitudinal data points and can also handle missingness in longitudinal data (21). We evaluated the appropriate number of groups based on substantive knowledge and statistical criteria, including the Bayesian information criterion, average posterior probabilities of class membership, and size of the smallest group (21). We did not examine trajectories for BMI since we had fewer measurements at each time point, and length/height data were not available at birth or age 6. To test the associations between oxidative stress markers and group membership, we created multinomial logistic regression models of group membership in association with oxidative stress biomarker concentrations, adjusted for the same covariates included in the linear regression models. The output can be interpreted as the odds of falling into each group compared with a reference group among individuals with higher oxidative stress biomarker concentrations.
Results
Women in our analysis were 30.5 years of age on average, primarily white (68%), and well educated (Table 1). Most women in the study took prenatal vitamins during pregnancy (96%) and the mean prepregnancy BMI of the study cohort was 25.6 kg/m2. Most babies were born at term (92%) and the mean birth weight was 3.37 kg (Table 2). We observed loss to follow-up in childhood study visits, but 71% of participants included in the analysis had at least 1 weight measurement at ages 3, 4, or 6. The number of observations at each study visit is shown in Table 2. Associations between oxidative stress biomarkers and demographic characteristics have been reported previously (22). Briefly, concentrations were highest in mothers who were nonwhite, had the lowest education and income levels, smoked during pregnancy, and enrolled at the Rochester study site (22).
Table 2.
Percentiles of child weight and body mass index by study visit
| Weight (kg) | BMI (kg/m2) | Sex (n) | ||||
|---|---|---|---|---|---|---|
| Study visit | n | 50th (25th, 75th) | n | 50th (25th, 75th) | Male | Female |
| Birth | 736 | 3.4 (3.1, 3.7) | 353 | 383 | ||
| Age 1 | 263 | 10.1 (9.5, 10.9) | 263 | 16.8 (15.9, 17.6) | 263 | 0 |
| Age 3 | 348 | 14.5 (12.7, 16.0) | 266 | 16.2 (15.4, 17.4) | 165 | 183 |
| Age 4 | 407 | 18.1 (16.5, 19.5) | 404 | 15.6 (14.5, 16.5) | 186 | 221 |
| Age 6 | 336 | 22.3 (20.0, 24.2) | 160 | 176 |
Abbreviations: BMI, body mass index. No data collected for females at age 1.
In adjusted models of child weight Z-scores, 8-iso-PGF2α and its primary metabolite were associated with decreased birth weight and increased weight in childhood (Table 3). A ln-unit increase in 8-iso-PGF2α was associated with a –0.12 decrease in birth weight Z-score (95% CI –0.25, 0.01), which is approximately a 1.5% decrease relative to the mean. The 8-iso-PGF2α metabolite was associated with a similar decrease in birth weight (B = –0.16, 95% CI –0.33, 0.01). Both biomarkers were also associated with increased weight at the age 4 visit. For example, 8-iso-PGF2α was associated with an increase in Z-score of 0.17 (95% CI 0.01, 0.33), corresponding to an approximate 2% increase relative to mean. No associations were observed between weight and PGF2α. Effect estimates for the associations between oxidative stress biomarkers and weight Z-scores were slightly greater in magnitude (ie, further from the null) at both time points in unadjusted models (Supplemental Table 1 (23)). However, results from adjusted models were very similar to those from models with additional adjustment for prepregnancy diabetes, smoking and alcohol use in pregnancy, and prenatal vitamin use (Supplemental Table 2 (23)), in models where the covariates were not imputed (Supplemental Table 3 (23)), in models additionally adjusted for study site (Supplemental Table 4 (23)), and in models additionally adjusted for child sex (Supplemental Table 5 (23)).
Table 3.
Adjusteda change in child weight Z-scores (95% CI) per ln-unit increase in specific gravity-corrected urinary oxidative stress biomarker
| 8-iso-PGF2α | 8-iso-PGF2α metabolite | PGF2α | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Δ | 95% CI | P | Δ | 95% CI | P | Δ | 95% CI | P | |
| Birth | 736 | –0.12 | (–0.25, 0.01) | .07 | –0.16 | (–0.33, 0.01) | .06 | –0.03 | (–0.13, 0.07) | .54 |
| Age 1 | 263 | 0.01 | (–0.22, 0.24) | .93 | 0.03 | (–0.25, 0.30) | .84 | 0.07 | (–0.10, 0.23) | .42 |
| Age 3 | 348 | 0.17 | (–0.03, 0.37) | .10 | 0.15 | (–0.11, 0.40) | .25 | 0.11 | (–0.03, 0.26) | .13 |
| Age 4 | 407 | 0.17 | (0.01, 0.33) | .04 | 0.18 | (–0.03, 0.38) | .09 | 0.07 | (–0.05, 0.19) | .24 |
| Age 6 | 336 | 0.11 | (–0.07, 0.29) | .24 | 0.11 | (–0.11, 0.33) | .34 | 0.04 | (–0.10, 0.18) | .58 |
No data collected for females at age 1.
Abbreviations: 8-iso-PGF2α, 8-iso-prostaglandin F2α; PGF2α, prostaglandin F2α. Models include imputed covariates.
a Models adjusted for maternal age, race, education level, and prepregnancy body mass index.
In investigating effect modification by child sex, we found that, for the most part, there were no statistically significant interactions (Supplemental Table 6 (23)). We did note, however, that the association between 8-iso-PGF2α and weight at age 4 was greater in magnitude in girls than in boys (P for interaction = 0.01). In girls, an ln-unit increase in 8-iso-PGF2α was associated with a 0.29 increase in weight Z-score (95% CI 0.09, 0.49), whereas, in boys, the association was null (change in Z-score = –0.06, 95% CI –0.33, 0.20).
For BMI, we similarly observed associations between 8-iso-PGF2α and its primary metabolite and childhood BMI at age 4 (Table 4). For example, an ln-unit increase in 8-iso-PGF2α was associated with an increase in BMI Z-score of 0.36 (95% CI 0.13, 0.59), corresponding to an approximate 3% increase relative to mean. These associations were also similar in unadjusted models (Supplemental Table 7 (23)), in models additionally adjusted for prepregnancy diabetes, smoking and alcohol use in pregnancy, and prenatal vitamin use, in models without imputed data, and in models additionally adjusted for child sex (data not shown). We did note an attenuation of associations between 8-iso-PGF2α and its primary metabolite and child BMI at age 4 in models additionally adjusted for study site (Supplemental Table 8 (23)). The change in child BMI Z-score in association with an ln-unit increase in 8-iso-PGF2α decreased to 0.19 (95% CI –0.01, 0.39), and the association with the 8-iso-PGF2α metabolite decreased to 0.27 (95% CI 0.02, 0.53). We did not observe interactions between oxidative stress markers and child sex in the associations with BMI (Supplemental Table 9 (23)).
Table 4.
Adjusteda change in child body mass index Z-scores (95% CI) per ln-unit increase in specific gravity-corrected urinary oxidative stress biomarker
| n | 8-iso-PGF2α | 8-iso-PGF2α metabolite | PGF2α | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δ | 95% CI | P | Δ | 95% CI | P | Δ | 95% CI | P | ||
| Age 1 | 263 | 0.06 | (–0.17, 0.29) | .62 | –0.03 | (–0.31, 0.24) | .82 | 0.02 | (–0.15, 0.18) | .84 |
| Age 3 | 266 | 0.05 | (–0.24, 0.33) | .75 | 0.28 | (–0.07, 0.62) | .12 | 0.04 | (–0.16, 0.25) | .66 |
| Age 4 | 404 | 0.36 | (0.13, 0.59) | <.01 | 0.43 | (0.14, 0.72) | <.01 | 0.08 | (–0.09, 0.25) | .37 |
No data collected for females at age 1.
Abbreviations: 8-iso-PGF2α, 8-iso-prostaglandin F2α; PGF2α, prostaglandin F2α. Models include imputed covariates.
a Models adjusted for maternal age, race, education level, and prepregnancy body mass index.
Weight Trajectories
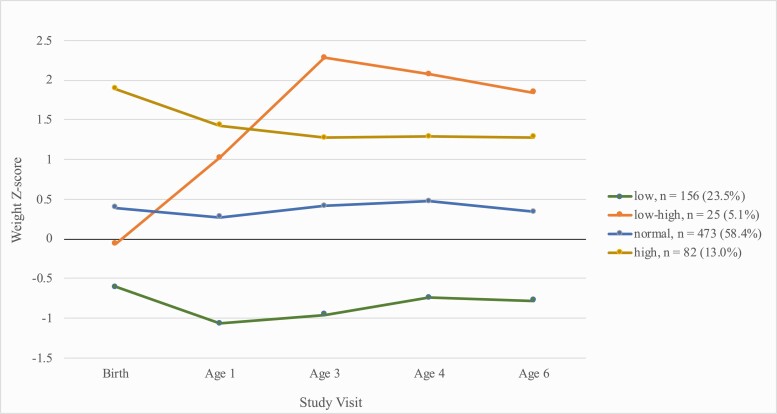
We identified 4 unique weight trajectories among children in TIDES. The 4-trajectory solution was selected as these groups were the most interpretable; however, this solution also had the lowest Bayesian information criterion among all possible solutions that maintained an average posterior probability of >0.80 (Supplemental Table 10 (23)). The 4 trajectories showed the following qualitative patterns (Fig. 1). The “low” trajectory (n = 156, 24%) included babies born lighter than average (mean 2.96 kg vs mean 3.37 kg in the overall population) who remained lighter through age 6 (mean 18.8 kg vs mean 22.3 kg in the overall population). The “low–high” trajectory (n = 25, 5%) included babies born slightly lighter than average (3.08 kg) and who had the highest weights at age 6 (mean 30.7 kg). The “normal” trajectory (n = 473, 58%) was the most common, and included babies with average weight from birth (3.38 kg) through age 6 (22.7 kg). Finally, the “high” trajectory (n = 82, 13%) included babies born heavier than average (4.17 kg) who remained heavy through age 6 (26.3 kg). Demographic characteristics differed slightly across groups (Supplemental Table 11 (23)). Compared with women with children in the normal trajectory, mothers of children in the low–high trajectory were more likely to be black (20% vs 13%), had lower education levels, had slightly higher prepregnancy BMI (30 kg/m2 vs 26 kg/m2), and were more likely to be from the Rochester study site (52% vs 27%) (Supplemental Table 11 (23)). Missingness in weight measurements by visits was evenly distributed across groups, with the exception of age 4 where children in the low–high trajectory had slightly lower missingness (12%) than other groups (40-48%) (Supplemental Table 11) (23)).
Figure 1.
Median weight Z-scores by visit from birth to age 6 by child growth trajectories.
Pregnant women with higher urinary concentrations of 8-iso-PGF2α and its primary metabolite were more likely to have children falling in the low–high growth trajectory than in the normal weight trajectory (Table 5). For example, an ln-unit increase in urinary 8-iso-PGF2α was associated with 2.56 times the odds (95% CI 1.22, 5.41) of having a child in the low–high trajectory relative to the normal weight trajectory, after adjusting for relevant covariates. These results were also similar with additional adjustment for study site or child sex (data not shown).
Table 5.
Adjusteda OR of weight trajectory membership (95% CI) per ln-unit increase in specific gravity-corrected urinary oxidative stress biomarker
| n | 8-iso-PGF2α | 8-iso-PGF2α metabolite | PGF2α | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||
| Normal | 473 | reference | reference | reference | ||||||
| Low | 156 | 1.11 | (0.80, 1.54) | .52 | 1.34 | (0.88, 2.05) | .17 | 1.11 | (0.88, 1.42) | .38 |
| Low-high | 25 | 2.56 | (1.22, 5.41) | .01 | 3.04 | (1.27, 7.28) | .01 | 1.41 | (0.82, 2.43) | .22 |
| High | 82 | 1.02 | (0.67, 1.56) | .91 | 0.99 | (0.56, 1.72) | .96 | 1.05 | (0.77, 1.43) | .76 |
Abbreviations: 8-iso-PGF2α, 8-iso-prostaglandin F2α; OR, odds ratio; PGF2α, prostaglandin F2α. Models include imputed covariates.
a Models adjusted for maternal age, race, education level, and prepregnancy body mass index.
Discussion
In a prospective study of women and their children from 4 US sites, we observed that urinary markers of prenatal oxidative stress were associated with decreased birth weight as well as increased childhood weight at age 4. Prenatal oxidative stress biomarkers were also associated with higher childhood BMI at age 4. Additionally, results from our study show that prenatal oxidative stress is associated with trajectories of weight from birth to age 6 in children. Higher oxidative stress levels were observed among children who had a weight trajectory characterized by being born smaller than average but who were larger than average at age 6. This study demonstrates a potential link between in utero oxidative stress exposure and an adverse profile of weight gain in childhood which has not been shown previously.
Previous studies of prenatal oxidative stress and birth weight have had inconsistent results (4-6, 8, 24, 25). These studies have used a mix of biomarkers, including 8-iso-PGF2α but also 8-hydroxydeoxyguanosine (8-OHdG), malondialdehyde (MDA), and others, have measured concentrations in plasma as well as urine using varying laboratory methods, have all had smaller sample sizes (≤500), and have primarily been in racially homogeneous populations. Our study may have been better suited to detect associations between oxidative stress markers and birth weight for several reasons. First, our study had the largest sample size available for addressing this question. Second, the oxidative stress biomarkers that we measured in our study are relatively stable across the course of pregnancy (26), and we performed measurements using mass spectrometry, which is very specific (11, 12, 14, 26). MDA may be more sensitive to dietary sources than 8-iso-PGF2α, and 8-OHdG can arise through sources other than oxidative DNA damage and is thus less specific (27).
Fewer studies have examined the association between prenatal oxidative stress biomarkers and childhood weight or BMI. One previous study observed null associations between prenatal oxidative stress, as indicated by MDA and 8-OHdG, and measures of weight from birth to age 3 (8). Another observed positive associations between maternal blood DNA damage, measured by comet assay, and infant weight measured at birth, 2 months, 6 months, and 1 year, showing that an increase in oxidative stress markers were associated with decreased infant weight at all time points (5). Our study builds on these findings by following children further to age 6. Interestingly, our results show that associations between prenatal oxidative stress and child weight or BMI may not be detectable until later in childhood.
We hypothesized that, in addition to associations with weight at individual time points, prenatal oxidative stress would be associated with an abnormal trajectory of weight characterized by low weight at birth and high weight in childhood. This pattern of growth has been identified in previous studies and is associated with greater risk of obesity and heart conditions later in life (28, 29). Children born at lower birth weights frequently exhibit this pattern (30). Risk factors for the trajectory are thus similar to those for lower birth weight. It may be difficult to disentangle whether the child growth trajectory is independent of birth weight; however, at least in our analysis, there was stronger evidence for some association between prenatal oxidative stress and the low-high growth trajectory compared with the association for birth weight by itself.
During pregnancy, maternal exposures to toxic substances in the environment, malnutrition, as well as gestational hypoxia and other gestational complications, can lead to oxidative stress and fetal distress (1). This can lead to lower birth weight, setting the course for the growth trajectory described above. In addition, oxidative damage to macromolecules, such as proteins, carbohydrates, and lipids, has the ability to disrupt cell defense mechanisms, metabolism, and cell growth in utero, which can subsequently have impacts that extend beyond what can be observed at birth (1, 31-33). For example, oxidative stress can damage the mitochondrial DNA of the fetus, which can lead to mitochondrial dysfunction and altered metabolism later in life (1). Studies have also shown that oxidative stress may be a key component of epigenetic changes, impacting fetal programming (34), which could contribute to the development of metabolic disease later in life (34, 35).
Our study has several limitations. First, while we had a larger sample size than previous studies of maternal oxidative stress and childhood growth, we observed some loss to follow-up in the childhood visits. However, the characteristics of mothers whose children participated in the final age 6 visit were very similar to those of mothers overall (Supplemental Table 12 (23)). Second, our age 1 visit was only performed in boys, and, furthermore, BMI estimates in this time frame may not be clinically meaningful (36). Third, there may have been several issues regarding trajectory groups. Selection of trajectory groups is not automated, but rather is based on substantive knowledge in combination with statistical criteria. Sample sizes for some of the trajectory groups selected in our study were also small, particularly for the low–high trajectory group. This could have resulted in some spurious associations. Also, in regard to trajectory groupings, attrition over time could have impacted categorization, where individuals with more missing data would be more likely to be grouped in the classes where size is consistent over time. Lastly, though we adjusted for the most prominent confounders throughout our analysis, there may be additional factors which introduce residual confounding and subsequent bias into the results. Overall it is important to note that the findings from our study do not indicate a causal relationship between oxidative stress and childhood growth, but rather suggest an association between the 2.
Despite limitations, our study has many strengths. The primary strength is the prospective longitudinal study design with the availability of repeated measures of weight collected on children from birth to age 6. In addition, the marker of oxidative stress which we used in our study, 8-iso-PGF2α, is believed to be 1 of the best biomarkers for oxidative stress due to its stability and reliability during pregnancy (11). In utilizing oxidative stress biomarkers, such as 8-iso-PGF2α, we are able to capture the overall damage (in this case, to lipids) that results from prooxidant as well as antioxidant activity. Thus, while incorporating antioxidant measures is often thought of as an important consideration for oxidative stress, it is not necessary to measure antioxidant activity or antioxidant intake. Finally, we had representation in our study from 4 US sites which may give our findings more generalizability than single-region studies.
Conclusion
Our study suggests that prenatal oxidative stress is associated with increases in weight and BMI in childhood and also with a growth trajectory of low to high weight from birth to age 6. Although effect sizes were small, these findings have important implications for the developmental origins of childhood obesity and metabolic disease given that even slight shifts of growth trajectories in early life can have a major impact on life-long disease risk. As oxidative stress can be caused by a variety of factors, including various environmental exposures and nutrient intake, particular attention should be given to public health interventions and recommendations in these areas.
Acknowledgments
We would like to thank the participating families for their contributions to the research, study site study coordinators (Garry Alcedo, Sarah Caveglia, Alana Cordeiro, and Stacey Moe) and research assistants for their data collection, and Aria Matthias and Kate Christenbury for their assistance with data management.
Financial Support: This work was supported by the Intramural Research Program, National Institutes of Health, National Institute of Environmental Health Sciences (ZIA103313) ,and the extramural NIEHS grants R01 ES016863-04 and P30 ES005022.
Glossary
Abbreviations
- 8-iso-PGF2α
8-iso-prostaglandin F2α
- 8-OHdG
8-hydroxydeoxyguanosine
- BMI
body mass index
- LOD
limit of detection
- MDA
malondialdehyde
- PGF2α
prostaglandin F2α
- TIDES
The Infant Development and the Environment Study
Additional Information
Disclosures: The authors declare that they have no actual or potential conflicts of interests.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Rodríguez-Rodríguez P, Ramiro-Cortijo D, Reyes-Hernández CG, López de Pablo AL, González MC, Arribas SM. Implication of oxidative stress in fetal programming of cardiovascular disease. Front Physiol. 2018;9:602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aseervatham GS, Sivasudha T, Jeyadevi R, Arul Ananth D. Environmental factors and unhealthy lifestyle influence oxidative stress in humans–an overview. Environ Sci Pollut Res Int. 2013;20(7):4356-4369. [DOI] [PubMed] [Google Scholar]
- 3. Wei Y, Zhang JJ, Li Z, et al. . Chronic exposure to air pollution particles increases the risk of obesity and metabolic syndrome: findings from a natural experiment in Beijing. FASEB J. 2016;30(6):2115-2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hsieh TTA, Chen SF, Lo LM, Li MJ, Yeh YL, Hung TH. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reprod Sci. 2012. doi: 10.1177/1933719111426601 [DOI] [PubMed] [Google Scholar]
- 5. Loy SL, Sirajudeen KN, Hamid Jan JM. The effects of prenatal oxidative stress levels on infant adiposity development during the first year of life. J Dev Orig Health Dis. 2014;5(2):142-151. [DOI] [PubMed] [Google Scholar]
- 6. Weber D, Stuetz W, Bernhard W, et al. . Oxidative stress markers and micronutrients in maternal and cord blood in relation to neonatal outcome. Eur J Clin Nutr. 2014;68(2):215-222. [DOI] [PubMed] [Google Scholar]
- 7. Ibáñez L, Suárez L, Lopez-Bermejo A, Díaz M, Valls C, De Zegher F. Early development of visceral fat excess after spontaneous catch-up growth in children with low birth weight. J Clin Endocrinol Metab. 2008;93(3):925-928. [DOI] [PubMed] [Google Scholar]
- 8. Hong J, Lee HA, Park EA, et al. . Association of mid-pregnancy antioxidative vitamin and oxidative stress levels with infant growth during the first 3 years of life. Food Nutr Res. 2014;58. Published online September 12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrett ES, Sathyanarayana S, Janssen S, et al. ; TIDES Study Team . Environmental health attitudes and behaviors: findings from a large pregnancy cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;176:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosen EM, van ‘t Erve TJ, Boss J, et al. . Urinary oxidative stress biomarkers and accelerated time to spontaneous delivery. Free Radic Biol Med. 2019;130:419-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roberts LJ, Morrow JD. Measurement of F2-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28(4):505-513. [DOI] [PubMed] [Google Scholar]
- 12. Dorjgochoo T, Gao YT, Chow WH, et al. . Major metabolite of F2-isoprostane in urine may be a more sensitive biomarker of oxidative stress than isoprostane itself. Am J Clin Nutr. 2012;96(2):405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yin H, Gao L, Tai HH, Murphey LJ, Porter NA, Morrow JD. Urinary prostaglandin F2α is generated from the isoprostane pathway and not the cyclooxygenase in humans. J Biol Chem. 2007;282(1):329-336. [DOI] [PubMed] [Google Scholar]
- 14. Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2(1):221-226. [DOI] [PubMed] [Google Scholar]
- 15. Swan SH, Sathyanarayana S, Barrett ES, et al. ; TIDES Study Team . First trimester phthalate exposure and anogenital distance in newborns. Hum Reprod. 2015;30(4):963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sathyanarayana S, Grady R, Barrett ES, et al. . First trimester phthalate exposure and male newborn genital anomalies. Environ Res. 2016;151:777-782. [DOI] [PubMed] [Google Scholar]
- 17. Luthra G, Vuckovic I, Bangdiwala A, et al. . First and second trimester urinary metabolic profiles and fetal growth restriction: an exploratory nested case-control study within the infant development and environment study. BMC Pregnancy Childbirth. 2018;18(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Villar J, Ismail LC, Victora CG, et al. . International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857-868. [DOI] [PubMed] [Google Scholar]
- 19. CDC. A SAS Program for the WHO Growth Charts (ages 0 to <2 years). Published 2000. ProMED-mail website. Accessed January 21, 2020. http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 20. National center for Chronic Disease Prevention and Health Promotion. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years). Division of Nutrition Physical Activity, and Obesity. Published 2016. ProMED-mail website. Accessed January 21, 2020. https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 21. Nagin DS, Jones BL, Passos VL, Tremblay RE. Group-based multi-trajectory modeling. Stat Methods Med Res. 2018;27(7):2015-2023. [DOI] [PubMed] [Google Scholar]
- 22. Eick SM, Barrett ES, van ‘t Erve TJ, et al. . Association between prenatal psychological stress and oxidative stress during pregnancy. Paediatr Perinat Epidemiol. 2018;32(4):318-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arogbokun O, Rosen E, Keil AP, et al. . Maternal oxidative stress biomarkers in pregnancy and child growth from birth to age 6. Supplemental Tables 1–12. Fig Share Digital Repository 2020. Deposited 17 December 2020. https://doi.org/10.6084/m9.figshare.12585041.v2
- 24. Ferguson KK, Kamai EM, Cantonwine DE, Mukherjee B, Meeker JD, McElrath TF. Associations between repeated ultrasound measures of fetal growth and biomarkers of maternal oxidative stress and inflammation in pregnancy. Am J Reprod Immunol. 2018;80(4):e13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindström E, Persson LÅ, Raqib R, El Arifeen S, Basu S, Ekström EC. Associations between oxidative parameters in pregnancy and birth anthropometry in a cohort of women and children in rural Bangladesh: the MINIMat-cohort. Free Radic Res. 2012;46(3):253-264. [DOI] [PubMed] [Google Scholar]
- 26. Ferguson KK, McElrath TF, Chen YH, Loch-Caruso R, Mukherjee B, Meeker JD. Repeated measures of urinary oxidative stress biomarkers during pregnancy and preterm birth. Am J Obstet Gynecol. 2015;212(2):208.e1-208.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Palmieri B, Sblendorio V. Oxidative stress tests: overview on reliability and use. Part II. Eur Rev Med Pharmacol Sci. 2007;11(6):383-399. [PubMed] [Google Scholar]
- 28. Jones-Smith JC, Neufeld LM, Laraia B, Ramakrishnan U, Garcia-Guerra A, Fernald LC. Early life growth trajectories and future risk for overweight. Nutr Diabetes. 2013;3(2):e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Law CM, Shiell AW, Newsome CA, et al. . Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105(9):1088-1092. [DOI] [PubMed] [Google Scholar]
- 30. Casey PH. Growth of low birth weight preterm children. Semin Perinatol. 2008;32(1):20-27. [DOI] [PubMed] [Google Scholar]
- 31. Barker DJP, Hales CN, Fall CHD, Osmond C, Phipps K, Clark PMS. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1):62-67. [DOI] [PubMed] [Google Scholar]
- 32. Barnes SK, Ozanne SE. Pathways linking the early environment to long-term health and lifespan. Prog Biophys Mol Biol. 2011;106(1):323-336. [DOI] [PubMed] [Google Scholar]
- 33. Hales CN, Barker DJ, Clark PM, et al. . Fetal and infant growth and impaired glucose tolerance at age 64. BMJ. 1991;303(6809):1019-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson LP, Al-Hasan Y. Impact of oxidative stress in fetal programming. J Pregnancy. 2012;2012:582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ramírez-Vélez R. In utero fetal programming and its impact on health in adulthood. Endocrinol y Nutr (English Ed). 2012;59(6):383-393. [DOI] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention. Using the WHO Growth Standard Charts. Centers for Disease Control and Prevention;2015. https://www.cdc.gov/nccdphp/dnpao/growthcharts/who/index.htm. Accessed February 02, 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.