Abstract
Many US states now embrace the medical and recreational use of Cannabis. Changes in the laws have heightened interest and encouraged research into both cannabinoid products and the potential harms of Cannabis use, addiction and intoxication. The major active ingredient of Cannabis sativa (marijuana), Δ9-tetrahydrocannabinol (THC) and it powerfully stimulates the type-1 cannabinoid (CB1) receptor. When used in the form of the plant marijuana, because of the many compounds that exist in the plant form they could inhibit the activity of the CB1 receptor thereby reducing many of the effects of THC. While this mechanism seems correct, in our opinion, Vallee., et al. incorrectly suggest that blocking CB1 receptors could open unforeseen approaches to the treatment of cannabis intoxication and addiction. We caution the scientific community that, other CB1 receptor blockers, such as, Rimonabant (SR141718) have been pulled off the market in Europe. In addition, CB1 receptor blockers were rejected by the FDA due to mood changes including suicide ideation. We argue that one issue facing the scientific community, has to do with the increasing legalization of Cannabis products in many states across America. We are in favor of some reform in terms of either decriminalization or restrictive legalization especially in control of legal limits of THC. Like other psychoactive compounds at high doses, it is our hypothesis that chronic use of these drugs including high THC content in its various forms (wax, smoke or vapor) resulting in brain reward dysfunction induces an imbalance of neurotransmission and subsequent hypodopaminergia and lead to aberrant substance and non-substance (behavioral) addictions. It is further proposed that in order to overcome THC and even other psychoactive drugs of abuse induced anhedonia the coupling of genetic risk testing and pro dopamine regulation is warranted.
Keywords: Cannabis Use Disorder (CUD), Cannabis sativa, Δ9-tetrahydrocannabinol (THC), Type-1 Cannabinoid (CB1) Receptor
Introduction
Over the years, the regular use of cannabis has substantially increased among young adults with the development of cannabis use disorder (CUD) with an estimated prevalence of 8.3% in the United States [1]. Research shows that exposure to cannabis is associated with hypodopaminergic anhedonia (depression) cognitive decline, poor memory, inattention, impaired learning performance, reduced dopamine brain response-associated emotionality and increased severity of addiction in young adults [2]. There is increasing concern by the addiction medicine community that because of the high content of delta-9 tetrahydrocannabinol (THC), currently found in oral and vaping cannabis products, the cognitive effects of cannabis may become more pronounced in young adults who use the cannabis edibles and vaping products with high THC content [3].
However, addiction physicians may be able to clinically restore the dopaminergic dysfunction by inducing dopamine homeostasis’ and normalize the behavior in chronic cannabis users. Blum’s group have developed the first patented genetic addiction risk severity (GARS) test as evidenced from studies linked to clinical predictive outcomes using the Addiction Severity Index (ASI). We have tested over 1,000, patients [4,5]. Understanding the common neuromodulating aspects of neurotransmission and its disruption via chronic exposure of drugs like cannabis and or even opioids and behavioral addictions, requires a known approach involving “dopamine homeostasis.” Specifically, published studies illustrate the coupling of GARS with KB220Z variants utilizing a semi-customized precision Pro- Dopamine Regulation (PDR) matched to one’s GARS. Our group showed many beneficial effects of KB220 variants (i.e. attenuation of anhedonia) in over 48 peer reviewed published clinical trials including triple blind placebo control [6].
Figure 1 shows the brain reward cascade (BRC) showing the interaction of many neurotransmitters leading to the neuronal release of dopamine at the N. Accumbens (NAc) [6].
Figure 1:
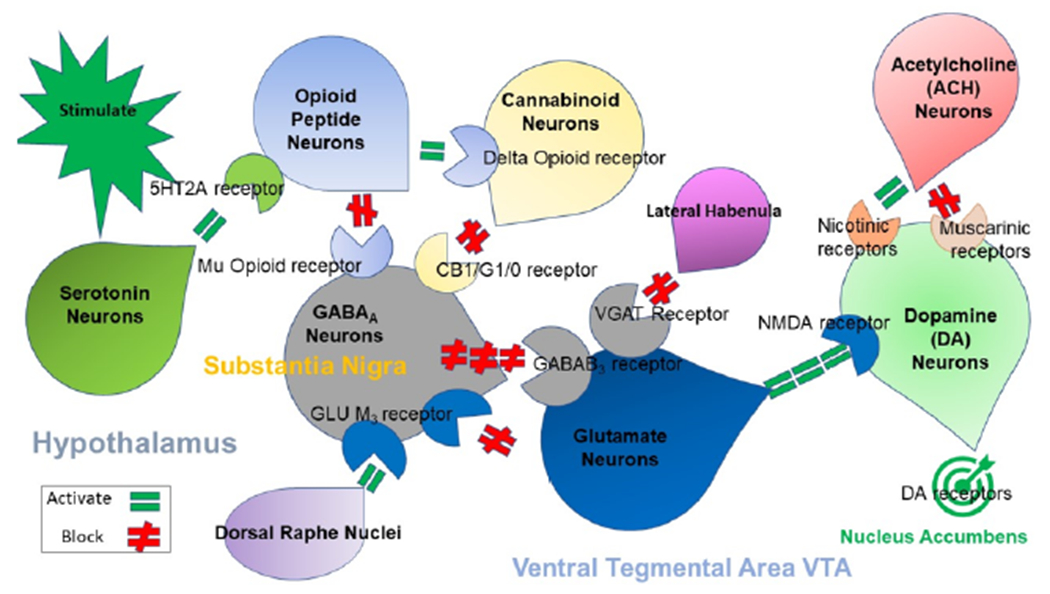
Illustrates the interaction of at least 6 major neurotransmitter pathways involved in the Brain Reward Cascade (BRC). In the hypothalamus environmental stimulation causes the release of serotonin which in turn via, for example, 5HT-2a receptors activate (green equal sign) the subsequent release of opioid peptides from opioid peptide neurons, also in the hypothalamus. Then in turn the opioid peptides having two distinct effects possibly via two different opioid receptors: A) inhibits (red hash sign) through the mu opioid receptor (possibly via enkephalin) and projecting to the Substania Nigra to GABAA neurons B) stimulates (green equal sign) Cannabinoid neurons (e.g. Anandamide and 2-archydonoglcerol) through Beta –Endorphin linked delta receptors, which in turn inhibits GABAA neurons at the substania nigra. Cannabinoids primarily 2-archydonoglcerol, when activated can also indirectly disinhibit (red hash sign) GABAA neurons in the Substania Nigra through activation of G1/0 coupled to CB1 receptors. Similarly. Glutamate neurons located in the Dorsal Raphe Nuclei (DRN) can indirectly disinhibit GABAA neurons in the Substania Nigra through activation of GLU M3 receptors (red hash sign), GABAA neurons when stimulated will in turn powerfully (red hash signs) inhibit VTA glutaminergic drive via GABAB 3 neurons. It is also possible that stimulation of ACH neurons that at the Nucleus Accumbens ACH can stimulate both muscarinic (red hash) or Nicotinic (green Hash). Finally, Glutamate neurons in the VTA will project to dopamine neurons through NMDA receptors (green equal sign] to preferentially release dopamine at the Nucleus Accumbens (NAc) shown as a bullseye indicating euphoria (a wanting response). The end result is that as Dopamine is released (low = unhappiness- Endorphin Deficiency) and (normal = Happiness) depends on happiness tonic set point. With permission [7].
Dopaminergic reward dysfunction in addictive behaviors is well supported in the literature [7]. There is evidence that alterations in synchronous neural activity between brain regions subserving reward and various cognitive functions may significantly contribute to substance-related disorders. One study from our laboratory published in PLOSONE presented the first evidence showing that a pro-dopaminergic nutraceutical (KB220Z) significantly enhances, above placebo, functional connectivity between reward and cognitive brain areas in the rat. These include the nucleus accumbens, anterior cingulate gyrus, anterior thalamic nuclei, hippocampus, prelimbic and infralimbic loci. Significant functional connectivity, increased brain connectivity volume recruitment (potentially neuroplasticity) and dopaminergic functionality were found across the brain reward circuitry [8].
In this animal study to help the reader understand our experimental design we are providing herein a detailed reiteration in short form.
Ten male Long Evans rats (350 - 400 grams) were obtained from Charles River Laboratories (Wilmington, MA) and housed in pairs in a temperature and humidity-controlled room (12 hr light cycle with lights off at 1900 hr). Water and Purina rat chow were provided ad libitum.
Composition of and preparation of KB220Z and placebo
The most recent variant of KB220Z (powdered form) used in the present study is comprised of the following ingredients: Thiamine, 15 mg (1033% of Daily Value); Vitamin B6, 10 mg (500%); Chromium poly nicotinate 200 mcg (166%) and a fixed dose of Synaptose. Synaptose is a combination of amino acids and herbs that contains DL-Phenylalanine, L-Tyrosine, Passion Flower Extract; a Complex containing Arabinogalactans, N-Acetylglucosamine, Astragalus, Aloe Vera, Frankincense Resin, White Pine Bark Extract and Spirulina; Rhodiola; L-Glutamine; 5-Hydroxytryptophan (5-HTP); Thiamine Hydrochloride; Pyroxidal-5-phosphate and Pyridoxine HCl [9]. The powder was manufactured by Cephram, Inc. (New Jersey). Fresh solutions were prepared in double distilled water prior to imaging and delivered at a total concentration 33 mg/ml (based on weighed powder) and delivered in a total volume of 0.1ml over 30 seconds.
Functional MRI datasets were collected on a 1H 470.7MHz (11.1 Tesla) MRI scanner (Magnex Scientific) with high-performance gradients (Resonance Research Inc.; Gmax = 1500 mT/m at 150A and 130 us risetime) and controlled by a VnmrJ 3.1 console (Agilent, Palo Alto, CA). A quadrature transceive 1H surface coil with uniform B1 coverage for most of the rat brain (2.5 x 3.5cm) was used for radiofrequency signal transmission and detection (Figure 2).
Figure 2:
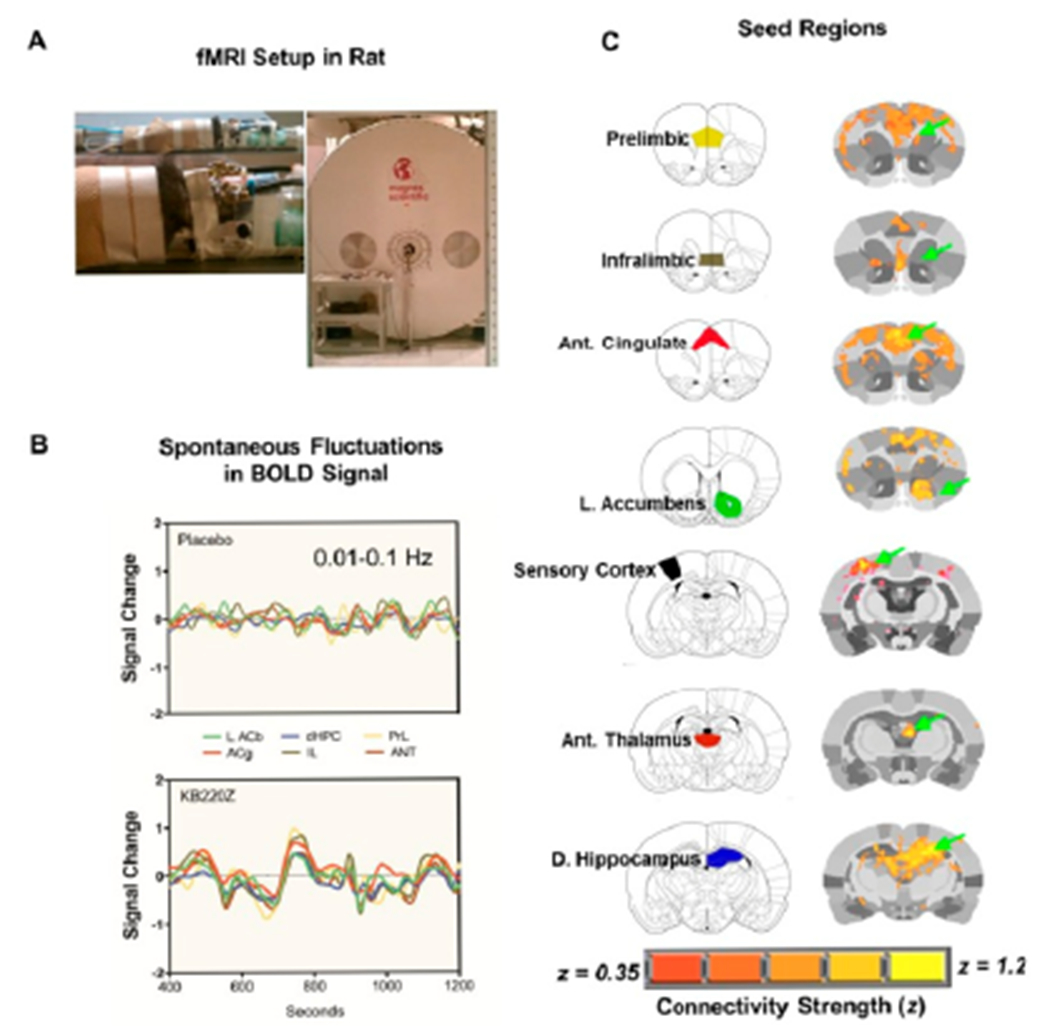
The experimental setup used for data acquisition and analysis of rat rsfMRI. (A) Experimental setup for fMRI data acquisition on a 470-MHz MRI using a 1H transmit/receive head surface coil. (B) Processed Blood-Oxygen-level Dependent (BOLD) signals from regions of the reward system. After skull stripping, atlas registration, motion and drift correction and intensity normalization, images were band-pass filtered between 0.01-0.1Hz. (C) Anatomical location of selected seed regions within the reward system that were used for cross-correlation analysis (to generate voxel-wise maps of Pearson’s r coefficient that was later z-transformed prior to group comparisons). Shown are both a standard anatomical atlas and high-resolution MRI-based atlas maps. Green arrows highlight the seed region. Overlays on the rat brain atlas show connectivity with corresponding seed regions based on Fisher’s z-transformed r coefficient values (r ≥ 0.35). With permission [8].
Regions of interest were selected within each hemisphere and analyzed separately to determine cross-hemispheric symmetrical patterns of connectivity and to avoid averaging signals from distant seeds, like for example, left and right dorsal hippocampus. Individual seed regions of interest (ROI) were chosen from the brain reward system based on rat brain atlas. These regions included the NAc, anterior cingulate cortex, dorsal hippocampus, amygdala, lateral hypothalamus and mediodorsal thalamus. From these individual time series, signals were extracted and used for correlating with the rest of the brain on a voxel-by-voxel basis using Analysis of Functional NeuroImages AFNI.
The first 5-minutes of baseline scan were not utilized in the cross-correlation procedure. Fisher’s z-transformed images were group-analyzed using a t-test. AFNI’s 3dClustSim program was used to determine an adequate voxel cluster size for a given p-value. The resultant voxel cluster size at p < 0.05 was used to limit the chance of noise voxels in the functional connectivity maps to below 5%. Correlation coefficient values representing functional connectivity between pairs of brain regions were exported for each seed ROI for further detailed analyses comparing KB220Z rats to controls using a t-test (corrected for multiple comparisons using the Holm-Sidak method). This initial analysis revealed that KB220Z produced increases in the spatial extent of functionally connected ROI. Consequently, (using the AFNI tool 3dROIstats) per each ROI the number of nonzero voxels surviving a z threshold of 0.3 were exported and these results analyzed as functional connectivity volume.
Increases in functional connectivity were specific to these regions and were not broadly distributed across the brain. Figure 3 shows that KB220z induces Bold activation and increases resting state functional connectivity in naive rodents specific to the brain reward circuitry. For details of this experiment along with all reported data the reader is encouraged to see Febo., et al. [8]. In order to assist the reader in understanding a high level summary of these results we provide the previously published composite three-dimensional functional connectivity maps comparing KB220Z and placebo. We encourage the readership to review the entire study published in PLOSONE to understand the experimental design.
Figure 3:
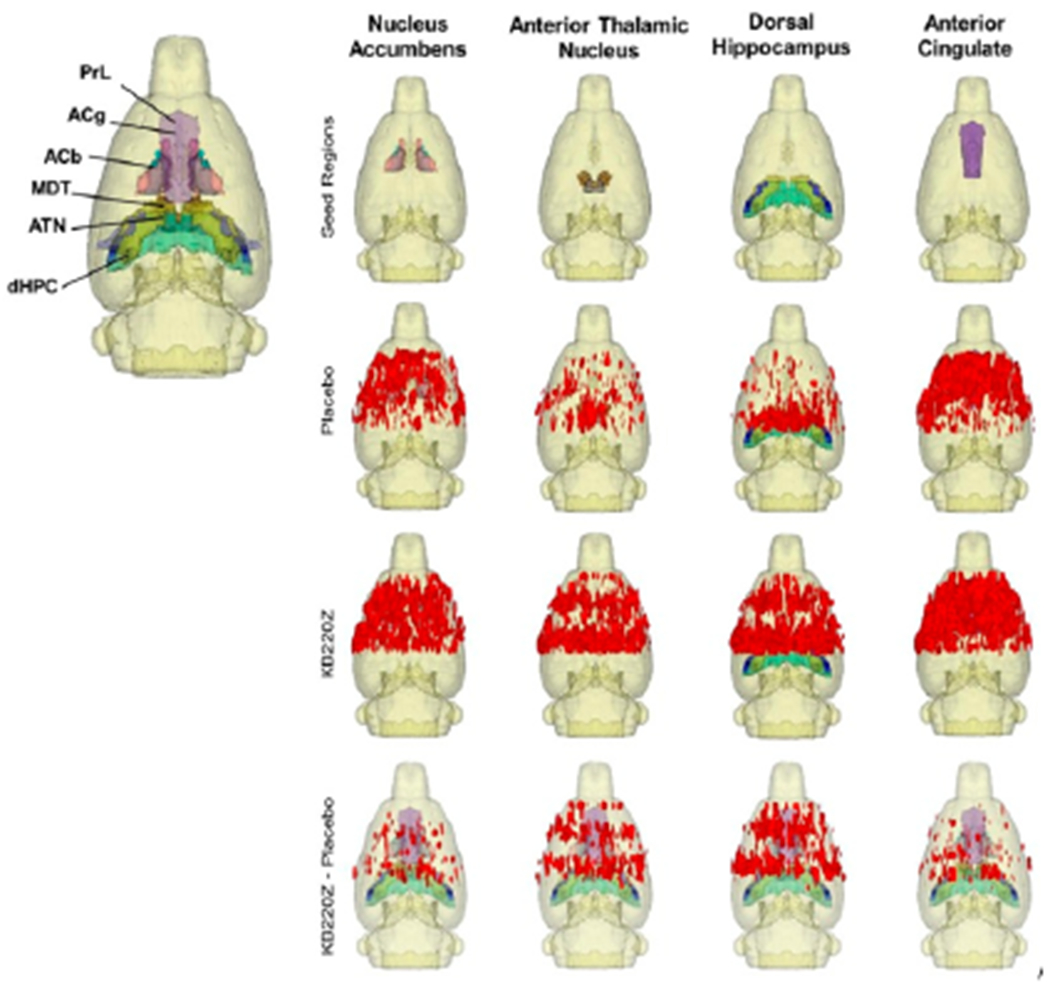
Composite three-dimensional functional connectivity maps comparing KB220Z and placebo. The top row shows the segmented 3D ROI used as seed for the placebo and KB220Z maps seen below them. High clustering of voxels occurs within the seed regions for both placebo and KB220Z groups. Greater connectivity based on the number of voxels showing high correlation coefficient values is observed in the KB220Z maps. Difference maps (KB220Z minus placebo) are shown in the bottom row. Maps are set at a lower statistical threshold of p < 0.005 (voxel cluster size corrected). With permission [8].
Recently, Willuhn., et al. [9] reported that cocaine misuse and even non-substance-related addictive behavior increases as dopaminergic function is reduced. Chronic cocaine exposure has been associated with decreases in D2/D3 receptors and was also associated with lower activation of cues in occipital cortex and cerebellum, in a recent PET study by Tomasi., et al [10].
Treatment strategies, like dopamine agonist therapy, that might conserve dopamine function may be an interesting approach to relapse prevention in psychoactive drug and behavioral addictions. To this aim, in a previously published study, Blum., et al. [11] evaluated the effect of KB220Z on reward circuitry of 10 heroin addicts undergoing protracted abstinence (average 16.9 months). In a randomized placebo-controlled crossover study of KB220Z, five subjects completed a triple-blinded experiment in which the subject, the person administering the treatment and the person evaluating the response to treatment were blinded to the treatment that any particular subject was receiving. In addition, nine subjects were genotyped utilizing the GARS test. Blum., et al. [10] preliminarily report that KB220Z induced an increase in BOLD activation in caudate-accumbens-dopaminergic pathways compared to placebo following 1-hour acute administration.
Furthermore, KB220Z also reduced resting-state activity in the cerebellum of abstinent heroin addicts. In the second phase of this pilot study of all 10 abstinent heroin-dependent subjects, we observed that three brain regions of interest were significantly activated from resting state by KB220Z compared to placebo (p < 0.05). Increased functional connectivity was observed in a putative network that included the dorsal anterior cingulate, medial frontal gyrus, nucleus accumbens, posterior cingulate, occipital cortical areas and cerebellum. These results and other quantitative electroencephalogy (qEEG) study results suggest a putative anti-craving/anti-relapse role of KB220Z in addiction by direct or indirect dopaminergic interaction. Due to small sample size, we caution definitive interpretation of these preliminary results and confirmation with additional research and ongoing rodent and human studies of KB220Z is required [12–14].
Figure 4 shows rsfMRI in abstinent heroin addicts following one-hour post KB220z oral administration revealing induction of “dopamine homeostasis” [10]
Figure 4:
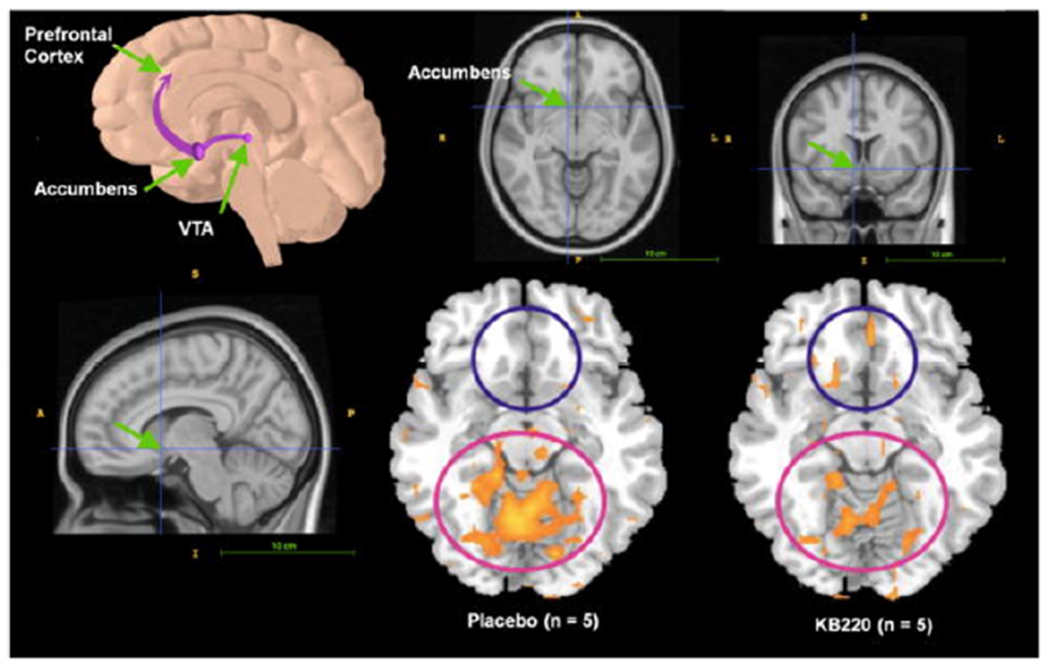
Location of the nucleus accumbens in human CNS. Resting fMRI Data Analysis in the heroin users (n =5) before and after KB220Z™ (Synaptose™) and placebo. Standard atlas shows the location of the nucleus accumbens (surrounding area) with significant increases in rsfMRI response with KB220Z. With Permission [11].
While there is plethora of research supporting high dose THC and alterations of both anatomical and neurophysiological unwanted brain changes, there are some positive effects of for example CBD and or even lower doses of THC to help attenuate anxiety [15] having heuristic value for prophylaxis. Certainly, based on what we know about the co-localization of CB1 and delta/mu opioid receptors, when activated by for example THC and subsequent enkephalin induced inhibition of GABA leads to required dopamine release at NAc [16].
Early [70s] studies from Blum’s group4 showed the importance of THC in reducing alcohol withdrawal and confirmed by more recent published reports, provide rational to carefully investigate CBD with THC to potentially help reduce unwanted aberrant heroin seeking behavior [17–19].
Understanding these psychological, neurobiological, anatomical, genetic and epigenetic data provides indisputable evidence that must be utilized in decisions concerned with cannabis legalization and medical utilization of CBD products.
Summary
Although the prevalence of recreational cannabis users at high risk for developing anhedonia and depression is unknown, the amount of cannabis used (dose of THC) seems to be an important factor. Chronic use of high THC content cannabis, either by oral ingestion or vaping, results in reversible neuroanatomic alterations in the mesolimbic and cortical brain regions with subsequent hypodopaminergia and associated depression/anhedonia. Cannabis use among young adults causes these neuroanatomical and psychological changes, magnified by DNA polymorphisms in pro-dopamine reward genes (like DAT1, DRD2, DRD4, COMT) [14]. These DNA polymorphisms can be measured either before cannabis use (prophylaxis) or positive (epigenetic). The most recent reiteration of these reward genes are constituents of the Genetic Addiction Risk Severity (GARS) test.
Treatment should involve the induction of dopamine homeostasis via pro-dopamine regulation and thereby ameliorate anhedonia. No FDA-approved therapies are currently available to treat CUD or any comorbidities, such as depression or cognitive decline.
Using a dopamine up-regulator such as KB220Z to restore brain dopamine in hypodopaminergia until an FDA-approved therapy is available could be considered for chronic cannabis users with CUD. The development of an appropriate policy regarding the legalization of cannabis and cannabis products and decriminalization is needed [2].
Acknowledgments
Funding
RDB is the recipient of NIH R01NS073884 and KB along with Marjorie Gondre Lewis (Howard University) are the recipients of R41 MD012318/MD/NIMHD NIH HHS/United States.
Footnotes
Conflict of Interest
Dr Blum is the patent holder and licensee of GARS and KB220 and has ownership in various companies involved in commercialization. Joseph Morgan owns equity in Geneus Health LC of San Antonio along with Dr. Blum. There are no other conflicts to report.
Bibliography
- 1.Lapham GT, et al. “The prevalence of Healthcare Effectiveness Data and Information Set (HEDIS) initiation and engagement in treatment among patients with cannabis use disorders in 7 US health systems”. Substance Abuse 40.3 (2019): 268–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blum K, et al. “Cannabis-induced Hypodopaminergic Anhedonia and Cognitive Decline in Humans in the Face of Legalization or Decriminalization: Putative Induction of Dopamine Homeostasis”. Journal: Frontiers in Psychiatry, section Addictive Disorders (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubino T, et al. “Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates”. Neuropsychopharmacology 33.11 (2008): 2760–2771. [DOI] [PubMed] [Google Scholar]
- 4.Blum K, et al. “Genetic addiction risk score (GARS) ™, a predictor of vulnerability to opioid dependence”. Frontiers in Bioscience (2018): 175–196. [DOI] [PubMed] [Google Scholar]
- 5.Blum K, et al. “Biotechnical development of genetic addiction risk score (GARS) and selective evidence for inclusion of polymorphic allelic risk in substance use disorder (SUD)”. Journal of Systems and Integrative Neuroscience (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blum K, et al. “Pro-Dopamine Regulator (KB220) A Fifty Year Sojourn to Combat Reward Deficiency Syndrome (RDS): Evidence Based Bibliography (Annotated)”. CPQ Neurology and Psychology’s 1.2 (2018). [PMC free article] [PubMed] [Google Scholar]
- 7.Gold MS, et al. “Neurological correlates of brain reward circuitry linked to opioid use disorder (OUD): Do homo sapiens acquire or have a reward deficiency syndrome?” Journal of the Neurological Sciences 418 (2020): 117137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Febo M, et al. “Enhanced functional connectivity and volume between cognitive and reward centers of naïve rodent brain produced by pro-dopaminergic agent KB220Z”. PLoS One 12.4 (2017): e0174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Willuhn I, et al. “Excessive cocaine use results from decreased phasic dopamine signaling in the striatum”. Nature Neuroscience 17.5 (2014): 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasi D, et al. “Overlapping patterns of brain activation to food and cocaine cues in cocaine abusers: association to striatal D2/D3 receptors”. Human Brain Mapping 36.1 (2015): 120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blum K, et al. “rsfMRI effects of KB220Z™ on neural pathways in reward circuitry of abstinent genotyped heroin addicts”. Postgraduate Medical Journal 127.2 (2015): 232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blum K, et al. “Introducing Precision Addiction Management of Reward Deficiency Syndrome, the Construct That Underpins All Addictive Behaviors”. Frontiers in Psychiatry 9 (2018): 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blum K, et al. “Overcoming qEEG abnormalities and reward gene deficits during protracted abstinence in male psychostimulant and polydrug abusers utilizing putative dopamine D2 agonist therapy: part 2”. Postgraduate Medical Journal 122.6 (2010): 214–226. [DOI] [PubMed] [Google Scholar]
- 14.Miller DK, et al. “Acute intravenous synaptamine complex variant KB220™ “normalizes” neurological dysregulation in patients during protracted abstinence from alcohol and opiates as observed using quantitative electroencephalographic and genetic analysis for reward polymorphisms: part 1, pilot study with 2 case reports”. Postgraduate Medical Journal 122.6 (2010): 188–213. [DOI] [PubMed] [Google Scholar]
- 15.Blessing EM, et al. “Cannabidiol as a Potential Treatment for Anxiety Disorders”. Neurotherapeutics 12.4 (2015): 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roeckel LA, et al. “CB1 Agonism Alters Addiction-Related Behaviors in Mice Lacking Mu or Delta Opioid Receptors”. Front Psychiatry 9 (2018): 630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum K, et al. “Tetrahydrocannabinol: Inhibition of alcohol induced withdrawal symptoms in mice”. In: Singh JM, Lal H, ed. Drug Addiction; ”. Neurobiology and Influences on Behavior 3 (1974): 39–53. [Google Scholar]
- 18.Hurd YL, et al. “Cannabis and the Developing Brain: Insights into Its Long-Lasting Effects”. The Journal of Neuroscience 39.42 (2019): 8250–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gu B, et al. “Cannabidiol attenuates seizures and EEG abnormalities in Angelman syndrome model mice”. Journal of Clinical Investigation 129.12 (2019): 5462–5467. [DOI] [PMC free article] [PubMed] [Google Scholar]


