Abstract
Chytridiomycosis is an emerging infectious disease affecting amphibians globally and it is caused by the fungal pathogen Batrachochytrium dendrobatidis (Bd). Chytridiomycosis has caused dramatic declines and even extinctions in wild amphibian populations in Europe, Australia, Central and North America. Spanning over two and a half decades, extensive research has led to discovery of epizootic and enzootic lineages of this pathogen. However, the Bd–amphibian system had garnered less attention in Asia until recently when an ancestral Bd lineage was identified in the Korean peninsula. Amphibians co-exist with the pathogen in Asia, only sub-lethal effects have been documented on hosts. Such regions are ‘coldspots’ of infection and are an important resource to understand the dynamics between the enzootic pathogen—Bd and its obligate host—amphibians. Insights into the biology of infection have provided new knowledge on the multi-faceted interaction of Bd in a hyperdiverse Asian amphibian community. We present the findings and highlight the knowledge gap that exists, and propose the ways to bridge them. We emphasize that chytridiomycosis in Asia is an important wildlife disease and it needs focussed research, as it is a dynamic front of pathogen diversity and virulence.
Introduction
Amphibians are declining throughout the world and one of the major drivers of this is an ancient, non-hyphal aquatic fungus1,2. Batrachochytrium dendrobatidis (Bd) is a chytridiomycetes fungal pathogen that infects only amphibian hosts3. In the early part of the twentieth century, frog die-offs in pristine and protected areas in Central America, the Carribean and Australia triggered an alarm. These were called ‘enigmatic declines’ as there was no evidence of a causal factor, until it was discovered that a chytrid fungus was responsible for causing mortality in frog populations. As reports of chytridiomycosis emerged from several continents, the true magnitude of the impact it had caused on frog populations was understood. This event earned an opprobrious title as ‘Amphibian Apocalypse’ and it has been “one of the worst wildlife diseases that has emerged in the twentieth century in terms of the number of species impacted and also its propensity to drive the species to extinction”4. Until now, Bd has been linked to population declines in almost 500 species amphibians, with over 90 extinctions globally, across its three extant Orders5.
In amphibians, skin is an organ of respiration, thermoregulation, and exchange of electrolytes with their environment, and therefore, skin infections could lead to fatality in them. The chytrid fungus has a uni-flagellated, ophisthokont zoospore that swims with a characteristic ‘whiplash’ movement in the aquatic environment6. Once this zoospore finds suitable substrates like the keratinous layer of the amphibians’ skin, it develops a thallus and grows in the stratum corneum of the epidermis3,6. Here it forms a zoosporangium that increases in size and develops discharge papillae that allows motile zoospores to exit the host and latch on to other hosts. The susceptible host finally dies due to an osmotic shock within a maximum of 35 days after onset of infection7. This fungus belongs to the family Chytridiomycota which is an ancient fungal family. Most members of this family of fungi are not pathogenic8.
Since chytridiomycosis is classified as an emerging infectious disease (EID), many theories have been offered to explain the emergence of the disease. A dominant theory posits that disequilibrium between the host, pathogen, and environment causes EIDs to emerge9. The factors contributing to the disequilibrium could be several, including a change in abiotic conditions that might have altered host–pathogen interactions. The emerging nature of the disease has piqued scientific investigations globally, as disease spread of such nature is rare in wildlife. Two major hypotheses were advanced to explain the emergence of chytridiomycosis10. The novel pathogen hypothesis (NPH) states that the disease emerged independently in different continents as a result of global trade and infects naïve hosts. This has been validated by studies that showed epizootic episodes of Bd on the global trade routes2,11–13. Genetic studies using multi-locus sequence typing (MLST) suggest no genetic structuring or pronounced genetic diversity in the samples of Bd collected14. This has been attributed to recent expansion from a singular infection epicentre. The endemic pathogen hypothesis (EPH) posits that Bd is a longstanding commensal on the amphibian skin microbiome and the most recent shifts in climate, habitats and even host factors like, loss of resilience might have distorted the equilibrium between the host and the commensal. This hypothesis receives support from the discovery of Bd from skin swabs of ancient museum specimens of amphibians from South America and Africa10. Over time, evidence has been assembled to join the dots between sudden emergence of chytridiomycosis, the dynamics of amphibian populations and climate change impacts15,16. A strong support for this theory has come from Litoria wilcoxii populations that showed varied responses to Bd infection across an altitudinal or latitudinal range and across seasons17–19. Such a continuum of host responses to various biotic and abiotic factors suggest that EPH might be a tenable explanation for Bd pathogenesis on frogs2,20. However, the most recent evidence from 234 chytrid isolates acquired over a span of 20 years from different continents re-defined our understanding of the origin of the pathogen. This study revealed a distinct Bd ASIA-1 lineage. This lineage with high genetic diversity from Asia might be the ancestral to Bd GPL (Global Panzootic Lineage), Bd ASIA 2/Bd BRAZIL and Bd CAPE21. Asia has been a major exporter of frog legs which might have spread Bd into other continents, and this explains the possible origin of Bd from Asia.
There is enormous disparity in our knowledge on chytridiomycosis in different parts of the world. The continents with Bd hotspots such as, the Americas and Australia, have channelled more resources and published work, which has led to extensive standardisation of diagnostic and monitoring protocols for Bd infections in wild and captive frogs. In Africa and Asia, mortalities have been poorly or not documented. These efforts led to a Global Bd mapping project where regional surveillance efforts have been compiled in the form of an interactive database22. These countries with Bd hotspots have collaborated to acquire and archive cultures for research on Bd. The European Union project RACE (Risk Assessment for Chytridiomycosis to European Amphibian Diversity) is a good example of this, and it has standardised protocols for culturing Bd championed by Joyce Longcore23. This effort witnessed a vast collective of researchers working across 5 continents, 23 countries and 62 extant amphibian species24.
First reports of chytridiomycosis from Asia came as late as 2008 from Japan25. In Giant Japanese Salamander, Andrias japonicas a museum specimen from 1902 had Bd on its skin26. After this, a series of efforts were made to document prevalence of Bd infection from different parts of Asia27–38. Lethal outbreak of the infection has so far not been recorded from Asia and therefore, it is referred to as a ‘coldspot’ of Bd infection. The reason for tolerance of hosts to Bd infections in coldspots is not clearly understood. With more than 500 amphibian species and over 60% endemic to South Asia, this region presents an important region for studies on Chytridiomycosis. This review is an attempt at recapitulating previous studies on the Bd–frog system from Asia to acknowledge the existence of certain crucial research gaps and also emphasize the need for a directed and concerted effort in these ‘coldspots’ to tackle an important wildlife pathogen. In this review, we have tried to understand the course of this host–pathogen system in Asia in the context of parallel advances in this field across other parts of the world, which makes this review different from some previous reviews on chytrid from Asia. This puts our work in perspective and offers wider opportunities to design future trajectory of this dynamic host–pathogen system in Asian coldpots.
Why ‘Coldspots’ of Chytridiomycosis?
Coldspots of infection are geographic regions with abundant host population that do not have the pathogen or areas where the pathogen is present in high or low prevalence with low loads of infection per host39. Initial studies on chytridiomycosis were almost singularly focussed on the frog population that experienced mortalities. This effort did not reveal the existence of genetic structuring in the pathogen. Goka et al.26 study was among the first to highlight that genetic homogeneity observed in Bd haplotypes was due to sampling from the epizootic fronts of the disease. As the Bd sampling expanded to represent different regions including coldspots, many endemic strains were revealed. Interestingly, sampling in ‘coldspots’ suggested that there were some unexpected patterns, such as the existence of endemic strains restricted to certain continents, and the panzootic lineage was found globally. The first extensive study in Asia (along with Papua New Guinea), involved sampling 3363 amphibians belonging to three orders (Anurans, Caudates and Gympniophona) that were swabbed for Bd40. It pointed out low prevalence (2.2%) of Bd and majority of the infection loads lower than the threshold of infection for frogs41. The areas surveyed for Bd infection40 were chosen based on a species distribution model (SDM) of global geographic distribution42.
The key findings from the extensive Bd surveillance effort from Asia prompted three major hypotheses: (1) Bd GPL has not yet emerged or dispersed into Asian frogs; (2) Bd in Asia is endemic, therefore, native amphibians have coevolved and are not impacted fatally; (3) various biotic and abiotic factors in Asian countries are unfavourable for Bd to emerge as a pathogen. The spatio-temporal pattern in Asia seems to deviate from the expectation of an emerging pathogen. A wave-like pattern of spread is expected when Bd infects a naïve amphibian population41,43. It will result in immediate impacts, such as dwindling of frog populations and eventually a collapse of the amphibian community. It will advance in the direction of more susceptible host species in suitable climatic regimes41,43. Infected frog populations in Asia do not follow this geographic pattern of spread. Museum specimens from 1902 revealed Bd, suggesting that it could have been present in Asian frog populations since long26. However, museum specimens representing a geographic area or a period have not been studied in Asia, so the time since Bd infections for Asian frog populations is not known. For the second hypothesis, recent evidence reveals that several haplotypes of Bd are involved in causing asymptomatic infection in frogs26,28,37. The third hypothesis about biotic and abiotic factors might be difficult to test, because of the dearth in understanding of the pathogen in Asia. SDMs predict suitable areas for the pathogen to thrive and infect frogs in Asia. There are speculations that in coldspots, ‘Ghosts of Infection Past’ could have played a role. This means the community of frogs have resisted and recovered from epizootic waves of Bd infection in the historical past39. Coldspots with a high prevalence and low infection loads, imply stable coexistence between the host and pathogen29. Therefore, coldspots of infection should be investigated to gain an in-depth understanding of the limits of pathogen virulence39.
Evolutionary History of Bd and the Importance of Studies from Coldspots
The debate on the origin of Bd centred around Africa12, Japan26, East Asia29, South America44 and North America45. There was ambiguity on the time of divergence of Bd from the most recent ancestor. Age of Bd was estimated to range from 100 years46 to 25,000 years47. Bd ASIA-1 isolated from Korea had the signatures of an ancient lineage and was basal to all the other known lineages in the global phylogeny. Bd CH (Switzerland) grouped with the clade belonging to Bd ASIA-1 and a lineage Bd ASIA-2 that was isolated from introduced North American Bullfrogs in Korea. It was closely related to Bd BRAZIL, and hence it was named as Bd ASIA-2/BRAZIL. These inferences were made using high-throughput sequencing of whole mitogenomes of the Bd isolates to establish the phylogenetic relationship between the various lineages21. Seven South Korean frogs that yielded the isolates of Bd ASIA-1 in this study showed no clinical infections. This was noteworthy, because it points at the need for focussed surveys in Asia where there are no mortalities, because there could be infection by hitherto unknown enzootic Bd lineages. This work constitutes an important landmark in global chytrid research, as it established the link between field surveys and culturing chytrid from coldspots of infection to answer the most basic question that has eluded us for nearly 20 years—where did this pathogen originate?
Extensive use of gene sequences to diagnose infections and draw phylogenies of Bd led to a thorough investigation of the limitations of different genetic markers employed. The use of ITS (Internal Transcribed Spacer region), a nuclear genome element for phylogenetic studies, resulted in ambiguous classification of the isolates, though it is touted as an excellent diagnostic marker for Bd21. Mitogenomes have been used to establish phylogenetic relationships and ascertaining lineages of Bd. Bd GPL diverged from the other lineages 120 years ago which coincided with the global expansion of amphibian pet trade, food and medicinal purposes21. Some of these amphibians might have been infected with the enzootic lineage in Asia and spread into the naïve frog populations in America and Australia.
There is evidence of sexual reproduction in Bd48. Hybridization between lineages upon contacting is possible and it has been reported21,49,50. O’Hanlon et al.21, has also reported three hybrid genotypes, two of them between Bd GPL and Bd CAPE from South Africa. In 2019, Byrne et al.50 reported a new diverged lineage of Bd from Asia, named Bd ASIA-3 which was commonly reported in samples from Philippines, Indonesia and parts of China. In these countries, Bd ASIA-3 is known to co-occur with Bd GPL50. In this study, a sequence from the Philippines could not be classified into either of the two lineages. However, there were no signs of hybridization, and it was concluded to belong to an early branching event caused by a new lineage from Asia50.
With over 7800 amphibians described till date21,51, approximately 700 species were infected with Bd out of only 1300 species screened22, and a thriving global trade of live frogs involving over 450 species52–55, it is huge a challenge to tease apart the cryptic diversity of the pathogen in Asia, where two new lineages have been identified in the last two years! This signals Asia as a coldspot of infection and amphibians here should be monitored for new and hybrid chytrid lineages. These could be potentially new strains that are more virulent than the parent lineages56. Since this region might harbour hybrid lineages, it should be the focus for intensive monitoring; as they might serve as spawning grounds for potent Bd strains to evolve with stronger pathogenic traits than their parents and cause outbreaks in naïve frog populations globally. We already know that the transition from colonising an amphibian to actually causing a disease is context-dependent57–59. Therefore, individual and population level outcomes of Bd infections might depend on various factors irrespective of whether the infection is caused by an enzootic or panzootic lineage. All these factors make future studies in coldspots indispensable to understand the biology of this amphibian-specialist pathogen.
Current Understanding of Chytridiomycosis from Asian Coldspots
Research investments on chytridiomycosis in coldspots ranges from, no knowledge of the pathogen in amphibian populations to regular monitoring of the host, the pathogen, and culturing of Bd isolates. Disparity in research investments has caused large gaps in knowledge about the dynamics of the pathogen. In order to bridge these gaps, they need to be highlighted and filled in due course of time. We present the case for India and the countries that it shares borders with. This geographic area is located at the intersection of global biogeographic realms. As a consequence of this, the phylogenetic richness of amphibians is high. In India alone, there are 406 species of frogs (Anurans), 39 known species of caecilians (Gymnophiona) and 2 known species of salamanders (Caudata)60. Thirty five species of caecilians are endemic to India, making it a hotspot of caecilian diversity61. Amphibian biodiversity in this region had not been surveyed for chytridiomycosis until recently. There has been no amphibian die off events in India except for some incidents linked to unknown causes76. The first report of chytridiomycosis came in 2011 from the Western Ghats, where 180 frogs belonging to the endemic genera Indirana were screened for Ranavirus (a viral disease affecting amphibians) and chytridiomycosis33. While their survey showed all samples negative for Ranavirus, one sample from Indirana brachytarsus, was tested positive for Bd. The infection load reported using quantitative Polymerase Chain Reaction (qPCR) assay for Bd was low (ranged from 0.3 to 3) and the frog did not show any clinical symptoms of chytridiomycosis. This was followed by surveys by Dahanukar et al.34 in the northern Western Ghats, where samples were screened for Bd using nested PCR and qPCR from swab and tissue samples from Nyctibatrachus humayuni, Indirana leithii, and Raorchestes bombayensis which were also subjected to histopathological examination. In this study, 32 frogs belonging to 5 genera (Nyctibatrachus humayuni, Indirana leithii, Raorchestes bombayensis, Euphlyctis cyanophlyctis, and Fejervarya caperata) were swabbed and 8 tested positive for Bd infection.The haplotypes identified on the frogs were closely related to Asia-specific haplotypes like B and K from Japan27 and CN30 from China26. An interesting observation made through this study was—Bd positive frogs showed no symptoms of infection, except for one N. humayuni that showed symptoms such as, browning of skin and lesions on the limbs. A common, albeit mild symptom that was recorded in all the three species was inflammation on the digits. However, the load of infection on the frogs was low. In 2015, around 497 frogs were sampled across the entire Western Ghats, which spans a wide altitudinal, latitudinal range36. They found 8 Bd positive from 497 samples. This study documented a higher infection load ranging from 6 to 785 zoopsore equivalents as opposed to 0.3 to 333 and 2 to 1334. They recorded strains which were identical to that of the previous studies recorded from India, and were closely related to other recorded Asian strains. This suggested an endemic clade of Bd from Asia involved in infections of Indian amphibians with a low prevalence, low individual load of infection. However, it was not clear how widespread the disease was.
Between 2012 and 2017 extensive Bd surveys were made from all the biodiversity hotspots in India by Mutnale et al.37. A large number of frogs from 147 locations across these hotspots, to be the first study from the country, to screen 9 families, 33 genera and 111 species of frogs from these areas. They recorded 158 samples with Bd infection out of the 1870 swabs collected, and they assessed ITS region haplotype diversity. Out of 57 haplotypes retrieved 46 were unique to India. Some frogs had more than one haplotype of Bd on them. Thirty-three haplotypes were unique to the mainland of India and 19 to the Andaman and Nicobar islands. After their initial experiments showed poor performance of TaqMan quantitative PCR (qPCR) assay, a gold standard diagnostic assay for chytridiomycosis globally, they used Nested PCR assay following the protocol by Goka et al.26. Four novel haplotypes sequenced in this study had insertion–deletion mutations in the Bd diagnostic TaqMan probe binding sites and in the reverse priming site of the qPCR primers. They also went on to predict that the region could have Bd with 160 haplotypes. With high haplotype diversity and poor diagnostic assays, the study documented some patterns that were unprecedented and it drew the attention of researchers globally.
Three major clades of Asian ITS haplotypes have been identified, one that has pan-global distribution (India, China, Japan, Italy, South Africa, and USA); second clade with haplotypes from Korea, Japan, and Brazil; third clade consisted of haplotypes with mutations at the TaqMan probe binding site confined to India, China, and Japan. There were some that did not show any specific association. The most common haplotype IN02 from India formed a cluster with haplotypes from Italy, South Africa, USA, China, and Japan. Haplotypes IN14 and CN13 from India and China, respectively, clustered with global pandemic strain Bd JEL 423. It became clear that Asia has several haplotypes and they are closely related to those found in other continents having chytridiomycosis hotspots.
Scattered evidence from India and other Asian coldspots prompts the following hypotheses: (1) majority of the strains of Bd involved in infection in Asia are enzootic; (2) improved detection assays for Asian Bd would lead to improved understanding of infection biology; (3) lack of die-offs, low prevalence and infection load and high haplotype diversity are common in coldspots and we coin the term “coldspot syndrome” to address this unified phenomenon, and the mechanisms causing them might have a common explanation.
Research Gaps and Challenges in Studies on Chytridiomycosis in Asia
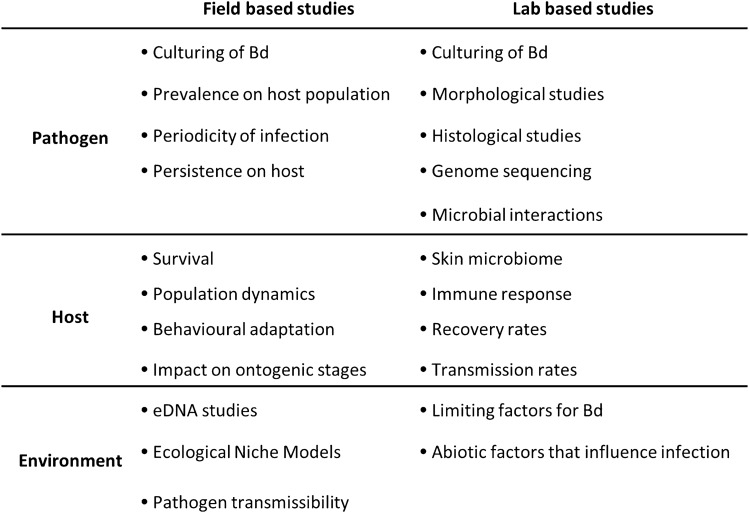
Some significant pointers on chytridiomycosis from coldspots: (1) diagnostic measures for Bd in Asia should be re-visited, because the TaqMan-based qPCR62 (the gold standard detection assay for Bd), did not facilitate detection of some mutant Bd haplotypes from India; (2) There is an increasing trend in prevalence values of the infection from the first report. This might be because of increased sampling size which includes higher species richness as well as a shift from using TaqMan qPCR to Nested PCR which could have helped identification of Bd positive samples with better specificity; (3) Bd haplotypes causing infections in Asia are associated with Bd GPL. There is unrecognized and under-estimated Bd genetic diversity in Asia. There is also an existing void from many Asian countries like Bangladesh and Nepal and also regional biases from within even well-monitored countries63. These leads need to be followed up with further research. The different approaches that could be employed to improve our understanding of Bd infections from ‘coldspots’ are summarised in Fig. 1.
Figure 1:
Different field and lab based approaches to understand the amphibian–Bd interaction in ‘coldspots’.
Develop Efficient Detection Assays
Global standards for efficiency of Bd detection assays are based on universality, affordability and accuracy. Genetic diversity in Bd strains revealed in coldspots has challenged the standards set for genetic markers previously identified. The first step towards improved understanding of Bd is investment in development of new and efficient markers that are truly universal. With asymptomatic infections common in coldspots and low cost involved in screening large samples, genetic tools are indispensable as detection assays (Table 1). Other assays have inherent advantages and disadvantages and they could be employed in specific conditions (Table 1). Nested PCR is a reliable method for detection, but it is more expensive than qPCR. A challenge would be to develop a new qPCR marker that would detect Asian strains reliably.
Table 1:
Comparison of different diagnostic methods used to detect Batrachochytrium dendrobatidis.
| S. no. | Method | Advantages | Disadvantages |
|---|---|---|---|
| 1 | Histological examination with hematoxylin/eosin stain99–101 | 1. Detection of zoospores and zoosporangia directly |
1. Sensitivity is poor, because it is difficult to detect from newly infected amphibians 2. Requires a certain level of expertise to detect the infection from tissues or skin lesions, affecting specificity of the assay100, 102, 103 3. Invasive, if toe-clips are used from live frogs104 4. Not a quick method of detection |
| 2 | Immunoperoxidase (IPX) staining and use of polyclonal antibodies105 | 1. Higher sensitivity and specificity than H/E staining technique105, 106 | Not been widely used for wild collected samples and a large proportion of the infected samples might go undetected because of the above reasons |
| 3 | Co-localisation staining method for staining keratin and chytrid fungus101 | 1. Enhanced staining technique wherein you can detect the infection even from extremely infected hyperkeratotic frogs where the zoosporangium sloughs off along with the skin101 |
1. Advanced expertise required for this staining procedure 2. Can only detect once the infection is advanced and not in individuals newly infected or with mild infection 3. Duration from the collection of the specimen to detection of infection is long 4. Invasive if toe-clips are used107 5. Not standardised for field samples107 |
| 4 | Quantitative polymerase chain reactions assays-qPCR62 |
1. Highly sensitive—detects low levels of infection 2. Provides a measure of the load of infection 3. Specific to Bd within Chytridiomycota, cannot amplify even 5 other closely related chytrid species62 4. Detects infection 7–14 days prior to detection by histological methods 5. A specialist is not required to conduct the assays 6. It is widely used to detect infections in wild collected samples 7. Quick and inexpensive |
1. Swabs collected from the field, might need to be diluted, and in case of a mild infection, it might lead to poor detection, i.e., there will be no detection in one or two well in triplicate assays107 2. More reliable on swabs from infected laboratory frogs than those from the field 3. Use of TaqMan probe, is expensive 4. The specificity of both the probe and the primers are necessary in TaqMan, otherwise there are chances of false negatives |
| 5 | Nested PCR26 |
1. In cases where a TaqMan probe might not work37, nested PCR ensures added specificity 2. Efficient when working with contaminated field samples 3. Have been proven to be efficient in detecting Bd strains that have variable allele copy numbers108 4. Detects Bd DNA as less as 0.001 pg1026 5. Quicker than the histological methods but slower than qPCR method |
1. Requires more time for rapid large scale screening of amphibian samples from the field 2. Cannot provide a measure of the load of infection 3. Expensive when compared with SYBR green qPCR assay 4. Sensitivity is less than in qPCR and hence might not be suitable for regions with low infection load |
| 6 | Environmental DNA samples109 |
1. Efficient as an early monitoring system in environments around amphibians109 2. Ability to collect ample amount of sample in the form of water or soil 3. No handling of animals required, the most non-invasive method 4. Can be used in regions where there are threatened/species of conservation priority, to be informed of possible outbreaks |
1. Use of sophisticated and expensive equipment to collect DNA from environmental samples 2. Where there is a diverse community of amphibians, it cannot tell which is the species that is more susceptible/resistant to infection as it will only give information regarding the level of Bd in the environment109 |
| 7 | Genotyping assay from swabs110 |
1. Accurately discriminates between different lineages of Bd from just swab samples110 2. Helpful in regions where a culture of the fungus and whole genome sequence is not available 3. Efficient method for addressing spatial and temporal distributions of different Bd strains, other than just prevalence and load of infection |
1. More expensive than PCR assays 2. Requires skin swab samples from frogs to be preserved in specific storage conditions, as it cannot yield accurate results with degraded DNA 3. Needs a threshold of 150 Bd genomic equivalents for the assay to perform well110 4. Not suitable for regions with low infection load |
Epidemiological Studies
Assessments on Bd prevalence in several parts of Asia are descriptive. They do not focus on aspects of the host or pathogen biology. Information regarding Bd pathogenesis that we presently know is because of studies on individual amphibians in laboratory conditions3,6. These approaches should be used to understand the ‘coldspot syndrome’ in Asia and elsewhere.A good example of such studies is in Bovo et al.64, where three species of Brazilian frogs were infected with an enzootic strain of Bd. This study showed that enzootic strains might cause only sub-lethal infections; however, the skin resistance of the affected amphibians increased. This study also pointed out that sub-lethal infections varied in intensity by species. This probably means that while enzootic infections might not cause a staggering effect on the individual, it could affect the host’s fitness and thereby influence survival. While knowledge on the fate of a host exposed to infection is important, the setting of such host–pathogen interactions would be most informative. To understand such factors, studies focussing on interactions between hosts showing a range of responses are required. At present, we have a poor understanding of the factors influencing survival of infected frogs in coldspots. Increased host diversity could lead to reduced impacts of the disease because of ‘dilution effect’65. High host species richness might impede pathogen transmission as different species have specific responses to infection, creating a heterogeneous host assemblage for the pathogen to thrive on. Some studies of dilution effect in the amphibian–Bd system have shown that host diversity decreased risks of chytridiomycosis66–68, while others have showed that host diversity increased risks for infection69. Heterogeneity in resilience of Bd infection in frog species is not well understood. Some species that are ‘super-shedders’ of the chytrid zoospores, might facilitate a higher prevalence of infection and disease risks in that community. An example of this is Atelopus zeteki, which is highly susceptible to chytridiomycosis, that sheds chytrid zoospores in the environment, thus exposing sympatric species to infection risks7. Some frog species are known to serve as reservoirs of chytrid zoospores, like Lithobates catesbeianus and Xenopus laevis70,71. To understand the scale of infection in frog populations, infection histories of individual frogs, overall prevalence in the frog populations and recovery rates in infected individuals are vital parameters that need to be considered.
Monitoring Host Populations
Several amphibian population monitoring programmes are being actively pursued globally. Many amphibian monitoring programmes are designed for short-term or focused on single species. Asymptomatic infected frogs showed reduced fitness, skipped breeding events, or experienced die-offs during metamorphosis of infected larvae, causing population declines72–75. Tadpoles are especially vulnerable to Bd infection because their adaptive immune system shuts down during metamorphosis and a recovery from chytrid infection during this time could be difficult76. Smaller frogs showed greater vulnerability to infection than large ones18. Latitude, elevation, and seasonality have varied influences prevalence and load of Bd in hotspots of infection17–19. In coldspots, seasonal fluctuation in Bd prevalence has been recorded29,30,38. Detection of these patterns at a population level and inferring infection by the pathogen requires appropriate designs of amphibian monitoring programs. While efforts are afoot in hotspots of infection, the need to establish such programs in coldspots of infection has not taken off. Both NPH and EPH predict that places with uncharacterised pathogen, and host diversity could become a dynamic front for evolution of new hybridised lineages of the pathogen which could have unpredictable disease outcomes21,50. With climate change impacts sweeping through natural ecosystems, there could be a spate of frog species that might need to be rescued by ex-situ interventions in future. Therefore, understanding susceptibility to infection in the context of the host community, pathogen strains, habitat, host life history, habitats and landscape parameters become necessary precursors for establishing ex-situ programmes84–86. Repeated observations made during monitoring have revealed behaviours in some frogs that resulted in recovery from chytrid infection37,87. Long-term population monitoring programmes should become an indispensable part of chytrid research, equitably in all regions of the world.
Understanding Frog Defences
There is also a growing body of research on amphibian skin microbiome and its impacts on disease persistence59,77–79. The skin microbiome forms the first line of defence by the innate immune system and the diversity of the microbial community helps clear infections before they cause pathogenesis by also producing antimicrobial peptides (AMPs)80,81. There are few studies from cold spots integrating the skin microbiome to chytridiomycosis research. Study on Bombina orientalis, found that there are diverse communities of microbes present on the ventral and dorsal surfaces of the frog skin which are different between the captive and wild toads78. Amphibians undergo a dramatic morphological and physiological change during metamorphosis. From a tadpole to an adult, there was a turnover of skin microbiomes which might have important survival outcomes against disease82. Amphibian species in communities occupy different niches and have diverse reproductive strategies. This presents a unique opportunity to explore the mechanisms that impart heterogeneity in host resilience. Coldspots from India have reported elevated levels of anti-Bd microbes on the skin of uninfected frogs that were terrestrial, arboreal, and aquatic83. This study shows a predominance of anti-Bd bacterial communities on six frogs (51.7%), suggest the role of microbiome in offering resistance to Bd infections in them. This study spanned a narrow sample size, and more such studies covering more samples, both uninfected and mildly infected will throw more light on whether the presence of a mild Bd infection alters the microbiome at all. Such a knowledge gap compounds the already present voids in our comprehension of Bd’s pathogenesis in cold spots. At population levels, the host genotype also plays a major role in deciding disease impacts. For example, the alleles associated with the Major Histocompatibility Complex (MHC) which contribute to the adaptive immune response of the hosts, was reported to affect the resistance and survival to chytridiomycosis in Lithobates yavapaiensis32and Littoria verreauxii84. These are yet unexplored though important areas to examine in communities of amphibians hosts in cold spots.
Culturing of the Pathogen
The most important step for in-depth studies of any disease is culturing and maintaining pathogens in controlled conditions. This is relatively easy when infected hosts are detected easily. Bd cultures are of considerable research value, as they have catalysed our understanding of origin, pathogenesis, virulence factors, phenotypic characters of the pathogen and other epidemiological parameters of the disease85–93.There is a disproportionately large number of Bd strains isolated and cultured in hotspots of infection than in Asian coldspots23.However, in cold spots, this task is challenging because infection on the hosts is not easily detected and the pathogen load in a frog is low. With few viable zoospores and the possibility of contamination with other fast-growing fungus, culturing attempts in Asian coldspots have often failed. Culturing protocol outlined for Bd by Longcore et al.3 relies on finding evidently infected hosts and also euthanizing them for isolation. This might not be a practical approach in coldspots because finding infected frogs is not easy. This is difficult in cold spots where you might have to sacrifice healthy individuals sometimes, because of their unidentified infection status. To confront this issue, Fisher 2012, designed non-lethal protocols for isolation which included collecting toe-clips and tissue specimens of individuals from the wild and using tadpoles as baits to isolate the chytrid fungus 23,94. Tadpoles with a prolonged larval phase have a higher burden of infection95. Infection in tadpoles are recognized by hyperkeratosis and depigmentation especially in the keratinous jawsheaths of infected tadpoles74,96–98. Infected tadpoles can be used to extract the mouth parts while uninfected tadpoles can be used as live baits for infection, by co-housing with infected adult individuals29. This technique should be used in coldspots, as this method amplifies the number of zoospores. It is also important to perform repeated culturing experiments in different parts of coldspots. Oral deformities have been observed in Nasikabatrachus sahyadrensis larvae (Vasudevan personal communication), but such field observations on other species are not known. We suggest that reports from coldspots are important and it could provide valuable leads to successfully culture the enzootic Bd.
Conclusion
Chytridiomycosis-induced amphibian declines have posed an unenviable challenge to batrachologists and conservation biologists globally. While the pathogen has swept through continents causing devastating impacts on amphibian populations, conservation biologists have been able to provide insight into the magnitude of the impact and suggest ways to ameliorate it. Some salient points have emerged from a large body of knowledge amassed over two and half decades. Amphibians living in the presence of Bd in the environment or on their body do not necessarily suffer from chytridiomycosis. After the onset of the disease, the desired outcome could be complete elimination of Bd from the frog population. It is not practical, as there are chances of pathogen re-emergence, which might cause far more serious impacts on the amphibian populations than in the first instance of emergence. It is important to understand the multi-faceted interaction between Bd and the amphibian, and to maintain a viable host population, even if they are Bd-colonised85. Coldspots of infection present a scenario of a natural experiment unfolding where Bd is under selection pressure to cause infection and the hosts are under similar pressure to survive. Due attention must be given to understand the dynamics between the host and the pathogen. In coldspots, focussing on the role of ecological immunity through long-term monitoring of amphibian population is more important than on clearing infections. Leveraging research findings from coldspots of Bd infection is part of the grand challenge biologists are faced with at present. This requires large scale collaborative efforts. In the context of low number of systematic chytridiomycosis assessments from a highly diverse amphibian area, we have tried to highlight the caveats in existing methodologies and the emphasis on research in coldspots should be on: (1) ramping up culturing and genome sequencing of enzootic strains of Bd; (2) understanding mechanisms that cause and maintain ‘coldspot syndrome’ in populations; (3) understanding the environmental, host defence and life history factors that clear infections or reduce zoospore loads on amphibians (4) establishing long-term monitoring stations in coldspots to gather longitudinal data on host–pathogen dynamics.
Acknowledgements
We extend our sincere thanks to University Grants Commission for research fellowship to GS; Council for Scientific and Industrial Research institute Centre for Cellular and Molecular Biology-Hyderabad for generously supporting chytridiomycosis work at the Laboratory for the Conservation of Endangered Species. Wildlife Institute of India, Dehradun provided KV support for several projects in the field that allowed him to make observations on frogs throughout India.
Biographies
Gayathri Sreedharan
finished her graduate and postgraduate studies in Microbiology from Amrita Vishwa Vidyapeetham, Kollam, Kerala, between 2010 and 2015. She has been keen on ecological and evolutionary questions pertaining to animal societies from her masters. She chose to work on the host–pathogen dynamics of Batrachochytrium dendrobatidis (Bd) and amphibians in Northern Western Ghats for her Ph.D. This work is among the first in the country to investigate individual and population-level outcomes of an enzootic pathogen using capture–mark–recapture models on frog populations.

Karthikeyan Vasudevan
is interested in biology of amphibians and has spent two decades on different aspects of research on the ecology of south Asian amphibians. He is the Co-Chair of IUCN’s Amphibian Specialist Group for south Asia region and contributes to conservation efforts. Asian amphibian chytridiomycosis is a topic of research in his lab where he is working to reveal interesting aspects of the pathogen–host–environment interactions.

Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stuart SN, Chanson JS, Cox NA, Young BE, Rodrigues ASL, Fischman DL, et al. Status and trends of amphibian declines and extinctions worldwide. Science (New York, NY) 2004;306:1783–1786. doi: 10.1126/science.1103538. [DOI] [PubMed] [Google Scholar]
- 2.Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Longcore JE, Pessier AP, Nichols DK. (1999) Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. doi: 10.1080/00275514.1999.12061011. [DOI] [Google Scholar]
- 4.Gascon C, Collins JP, Moore RD, Church DR, Mckay JE, Mendelson III JR (2005) Amphibian conservation action plan. In: IUCN/SSC Amphibian Specialist Group. Gland, Switzerland and Cambridge, UK, p 64
- 5.Scheele BC, Pasmans F, Skerratt LF, Berger L, Martel A, Beukema W, et al. Amphibian fungal panzootic causes catastrophic and ongoing loss of biodiversity. Science. 2019;363:1459–1463. doi: 10.1126/science.aav0379. [DOI] [PubMed] [Google Scholar]
- 6.Berger L, Hyatt AD, Speare R, Longcore JE. Life cycle stages of the amphibian chytrid Batrachochytrium dendrobatidis. Dis Aquat Org. 2005;68:51–63. doi: 10.3354/dao068051. [DOI] [PubMed] [Google Scholar]
- 7.DiRenzo GV, Langhammer PF, Zamudio KR, Lips KR. Fungal infection intensity and zoospore output of Atelopus zeteki, a potential acute chytrid supershedder. PLoS ONE. 2014;9:1–6. doi: 10.1371/journal.pone.0093356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature. 2006;443:818–822. doi: 10.1038/nature05110. [DOI] [PubMed] [Google Scholar]
- 9.Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, Phillott AD, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. doi: 10.1007/s10393-007-0093-5. [DOI] [Google Scholar]
- 10.Rachowicz LJ, Hero JM, Alford RA, Taylor JW, Morgan JAT, Vredenburg VT, et al. The novel and endemic pathogen hypotheses: competing explanations for the origin of emerging infectious diseases of wildlife. Conserv Biol. 2005;19:1441–1448. doi: 10.1111/j.1523-1739.2005.00255.x. [DOI] [Google Scholar]
- 11.Mazzoni R, Cunningham AA, Daszak P, Apolo A, Perdomo E, Speranza G. Emerging pathogen of wild amphibians in frogs (Rana catesbeiana) farmed for international trade. Emerg Infect Dis. 2003;9:995–998. doi: 10.3201/eid0908.030030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weldon C, du Preez LH, Hyatt AD, Muller R, Speare R. Origin of the amphibian chytrid fungus. Emerg Infect Dis. 2004;10:2100–2105. doi: 10.3201/eid1012.030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garner TWJ, Perkins MW, Govindarajulu P, Seglie D, Walker S, Cunningham AA, et al. The emerging amphibian pathogen Batrachochytrium dendrobatidis globally infects introduced populations of the North American bullfrog, Rana catesbeiana. Biol Lett. 2006;2:455–459. doi: 10.1098/rsbl.2006.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morehouse EA, James TY, Ganley ARD, Vilgalys R, Berger L, Murphy PJ, et al. Multilocus sequence typing suggests the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol. 2003;12:395–403. doi: 10.1046/j.1365-294X.2003.01732.x. [DOI] [PubMed] [Google Scholar]
- 15.Reading CJ. Linking global warming to amphibian declines through its effects on female body condition and survivorship. Oecologia. 2007;151:125–131. doi: 10.1007/s00442-006-0558-1. [DOI] [PubMed] [Google Scholar]
- 16.Bosch J, Martínez-Solano I. Chytrid fungus infection related to unusual mortalities of Salamandra salamandra and Bufo bufo in the Peñalara Natural Park, Spain. Oryx. 2006;40:84–89. doi: 10.1017/S0030605306000093. [DOI] [Google Scholar]
- 17.Kriger KM, Hero JM. Survivorship in wild frogs infected with chytridiomycosis. EcoHealth. 2006;3:171–177. doi: 10.1007/s10393-006-0027-7. [DOI] [Google Scholar]
- 18.Kriger KM, Pereoglou F, Hero JM. Latitudinal variation in the prevalence and intensity of chytrid (Batrachochytrium dendrobatidis) infection in eastern Australia. Conserv Biol. 2007;21:1280–1290. doi: 10.1111/j.1523-1739.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 19.Kriger KM, Hero JM. Altitudinal distribution of chytrid (Batrachochytrium dendrobatidis) infection in subtropical Australian frogs. Aust Ecol. 2008;33:1022–1032. doi: 10.1111/j.1442-9993.2008.01872.x. [DOI] [Google Scholar]
- 20.Drew A, Allen EJ, Allen LJS. Analysis of climatic and geographic factors affecting the presence of chytridiomycosis in Australia. Dis Aquat Org. 2006;68:245–250. doi: 10.3354/dao068245. [DOI] [PubMed] [Google Scholar]
- 21.O’Hanlon SJ, Rieux A, Farrer RA, Rosa GM, Waldman B, Bataille A, et al. Recent Asian origin of chytrid fungi causing global amphibian declines. Science. 2018;360:621–627. doi: 10.1126/science.aar1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olson DY, Ronnenberg K. Mapping project: 2014 update. FrogLog. 2014;22:17–21. [Google Scholar]
- 23.Fisher MC, Ghosh P, Shelton JMG, Bates K, Brookes L, Wierzbicki C, et al. Development and worldwide use of non-lethal, and minimal population-level impact, protocols for the isolation of amphibian chytrid fungi. Sci Rep. 2018;8:4–11. doi: 10.1038/s41598-018-24472-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher MC, Garner TWJ. Chytrid fungi and global amphibian declines. Nat Rev Microbiol. 2020;18:332–343. doi: 10.1038/s41579-020-0335-x. [DOI] [PubMed] [Google Scholar]
- 25.Une Y, Kadekaru S, Tamukai K, Goka K, Kuroki T. First report of spontaneous chytridiomycosis in frogs in Asia. Dis Aquat Org. 2008;82:157–160. doi: 10.3354/dao02006. [DOI] [PubMed] [Google Scholar]
- 26.Goka K, Yokoyama J, Une Y, Kuroki T, Suzuki K, Nakahara M, et al. Amphibian chytridiomycosis in Japan: distribution, haplotypes and possible route of entry into Japan. Mol Ecol. 2009;18:4757–4774. doi: 10.1111/j.1365-294X.2009.04384.x. [DOI] [PubMed] [Google Scholar]
- 27.Bai C, Garner TWJ, Li Y. First evidence of batrachochytrium dendrobatidis in China: discovery of chytridiomycosis in introduced American bullfrogs and native amphibians in the Yunnan Province, China. EcoHealth. 2010;7:127–134. doi: 10.1007/s10393-010-0307-0. [DOI] [PubMed] [Google Scholar]
- 28.Bai C, Liu X, Fisher MC, Garner TWJ, Li Y. Global and endemic Asian lineages of the emerging pathogenic fungus Batrachochytrium dendrobatidis widely infect amphibians in China. Divers Distrib. 2012;18:307–318. doi: 10.1111/j.1472-4642.2011.00878.x. [DOI] [Google Scholar]
- 29.Bataille A, Fong JJ, Cha M, Wogan GOU, Baek HJ, Lee H, et al. Genetic evidence for a high diversity and wide distribution of endemic strains of the pathogenic chytrid fungus Batrachochytrium dendrobatidis in wild Asian amphibians. Mol Ecol. 2013;22:4196–4209. doi: 10.1111/mec.12385. [DOI] [PubMed] [Google Scholar]
- 30.Fong JJ, Cheng TL, Bataille A, Pessier AP, Waldman B, Vredenburg VT. Early 1900s detection of Batrachochytrium dendrobatidis in Korean amphibians. PLoS ONE. 2015;10:1–8. doi: 10.1371/journal.pone.0115656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kusrini MD, Skerratt LF, Garland S, Berger L, Endarwin W. Chytridiomycosis in frogs of Mount Gede Pangrango, Indonesia. Dis Aquat Org. 2008;82:187–194. doi: 10.3354/dao01981. [DOI] [PubMed] [Google Scholar]
- 32.Savage AE, Sredl MJ, Zamudio KR. Disease dynamics vary spatially and temporally in a North American amphibian. Biol Conserv. 2011;144:1910–1915. doi: 10.1016/j.biocon.2011.03.018. [DOI] [Google Scholar]
- 33.Nair A, Olivia D, Gopalan SV, George S, Kumar SK, Merila J, et al. Herpetological review infectious disease screening of Indirana frogs from the. Herpetol Rev. 2011;42:554–557. [Google Scholar]
- 34.Dahanukar N, Krutha K, Paingankar MS, Padhye AD, Modak N, Molur S. Endemic Asian chytrid strain infection in threatened and endemic anurans of the Northern Western Ghats, India. PLoS ONE. 2013;8:1–8. doi: 10.1371/journal.pone.0077528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rowley JJL, Alford RA. Hot bodies protect amphibians against chytrid infection in nature. Sci Rep. 2013;3:1515. doi: 10.1038/srep01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molur S, Krutha K, Paingankar MS, Dahanukar N. Asian strain of Batrachochytrium dendrobatidis is widespread in the Western Ghats, India. Dis Aquat Org. 2015;112:251–255. doi: 10.3354/dao02804. [DOI] [PubMed] [Google Scholar]
- 37.Mutnale MC, Anand S, Eluvathingal LM, Roy JK, Reddy GS, Vasudevan K. Enzootic frog pathogen Batrachochytrium dendrobatidis in Asian tropics reveals high ITS haplotype diversity and low prevalence. Sci Rep. 2018;8:10125. doi: 10.1038/s41598-018-28304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thorpe CJ, Lewis TR, Fisher MC, Wierzbicki CJ, Kulkarni S, Pryce D, et al. Climate structuring of Batrachochytrium dendrobatidis infection in the threatened amphibians of the northern Western Ghats, India. R Soc Open Sci. 2018;5:180211. doi: 10.1098/rsos.180211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.James TY, Toledo LF, Rödder D, Silva Leite D, Belasen AM, Betancourt-Román CM, et al. Disentangling host, pathogen, and environmental determinants of a recently emerged wildlife disease: lessons from the first 15 years of amphibian chytridiomycosis research. Ecol Evol. 2015;5:4079–4097. doi: 10.1002/ece3.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swei A, Rowley JJL, Rödder D, Diesmos MLL, Diesmos AC, Briggs CJ, et al. Is chytridiomycosis an emerging infectious disease in Asia? PLoS ONE. 2011;6:e23179. doi: 10.1371/journal.pone.0023179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vredenburg VT, Knapp RA, Tunstall TS, Briggs CJ. Dynamics of an emerging disease drive large-scale amphibian population extinctions. Proc Natl Acad Sci USA. 2010;107:9689–9694. doi: 10.1073/pnas.0914111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rödder D, Kielgast J, Lötters S. Future potential distribution of the emerging amphibian chytrid fungus under anthropogenic climate change. Dis Aquat Org. 2010;92:201–207. doi: 10.3354/dao02197. [DOI] [PubMed] [Google Scholar]
- 43.Lips KR, Diffendorfer J, Mendelson JR, Sears MW. Riding the wave: reconciling the roles of disease and climate change in amphibian declines. PLoS Biol. 2008;6:0441–0454. doi: 10.1371/journal.pbio.0060072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez D, Becker CG, Pupin NC, Haddad CFB, Zamudio KR. Long-term endemism of two highly divergent lineages of the amphibian-killing fungus in the Atlantic Forest of Brazil. Mol Ecol. 2014;23:774–787. doi: 10.1111/mec.12615. [DOI] [PubMed] [Google Scholar]
- 45.Talley BL, Muletz CR, Vredenburg VT, Fleischer RC, Lips KR. A century of Batrachochytrium dendrobatidis in Illinois amphibians (1888–1989) Biol Conserv. 2015;182:254–261. doi: 10.1016/j.biocon.2014.12.007. [DOI] [Google Scholar]
- 46.Farrer RA, Weinert LA, Bielby J, Garner TWJ, Balloux F, Clare F, et al. Multiple emergences of genetically diverse amphibian-infecting chytrids include a globalized hypervirulent recombinant lineage. Proc Natl Acad Sci USA. 2011;108:18732–18736. doi: 10.1073/pnas.1111915108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenblum EB, James TY, Zamudio KR, Poorten TJ, Ilut D, Rodriguez D, et al. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proc Natl Acad Sci USA. 2013;110:9385–9390. doi: 10.1073/pnas.1300130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan JAT, Vredenburg VT, Rachowicz LJ, Knapp RA, Stice MJ, Tunstall T, et al. Population genetics of the frog-killing fungus Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2007;104:13845–13850. doi: 10.1073/pnas.0701838104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schloegel LM, Toledo LF, Longcore JE, Greenspan SE, Vieira CA, Lee M, et al. Novel, panzootic and hybrid genotypes of amphibian chytridiomycosis associated with the bullfrog trade. Mol Ecol. 2012;21:5162–5177. doi: 10.1111/j.1365-294X.2012.05710.x. [DOI] [PubMed] [Google Scholar]
- 50.Byrne AQ, Vredenburg VT, Martel A, Pasmans F, Bell RC, Blackburn DC, et al. Cryptic diversity of a widespread global pathogen reveals expanded threats to amphibian conservation. Proc Natl Acad Sci USA. 2019;116:20382–20387. doi: 10.1073/pnas.1908289116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Catenazzi A. State of the World’s amphibians. Annu Rev Environ Resour. 2015;40:91–119. doi: 10.1146/annurev-environ-102014-021358. [DOI] [Google Scholar]
- 52.Carpenter AI, Andreone F, Moore RD, Griffiths RA. A review of the international trade in amphibians: the types, levels and dynamics of trade in CITES-listed species. Oryx. 2014;48:565–574. doi: 10.1017/S0030605312001627. [DOI] [Google Scholar]
- 53.Mohanty NP, Measey J. The global pet trade in amphibians: species traits, taxonomic bias, and future directions. Biodivers Conserv. 2019;28:3915–3923. doi: 10.1007/s10531-019-01857-x. [DOI] [Google Scholar]
- 54.Walker SF, Bosch J, James TY, Litvintseva AP, Oliver Valls JA, Piña S, et al. Invasive pathogens threaten species recovery programs. Curr Biol. 2008;18:853–854. doi: 10.1016/j.cub.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 55.Wombwell EL, Garner TWJ, Cunningham AA, Quest R, Pritchard S, Rowcliffe JM, et al. Detection of Batrachochytrium dendrobatidis in amphibians imported into the UK for the pet trade. EcoHealth. 2016;13:456–466. doi: 10.1007/s10393-016-1138-4. [DOI] [PubMed] [Google Scholar]
- 56.Fu M, Waldman B. Ancestral chytrid pathogen remains hypervirulent following its long coevolution with amphibian hosts. Proc R Soc B Biol Sci. 2019;286:1–9. doi: 10.1098/rspb.2019.0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savage AE, Zamudio KR. Adaptive tolerance to a pathogenic fungus drives major histocompatibility complex evolution in natural amphibian populations. Proc R Socy B Biol Sci. 2016;283:20153115. doi: 10.1098/rspb.2015.3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Refsnider JM, Poorten TJ, Langhammer PF, Burrowes PA, Rosenblum EB. Genomic correlates of virulence attenuation in the deadly amphibian chytrid fungus, Batrachochytrium dendrobatidis. G3 Genes Genomes Genet. 2015;5:2291–2298. doi: 10.1534/g3.115.021808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bates KA, Clare FC, O’Hanlon S, Bosch J, Brookes L, Hopkins K, et al. Amphibian chytridiomycosis outbreak dynamics are linked with host skin bacterial community structure. Nat Commun. 2018;9:1–11. doi: 10.1038/s41467-018-02967-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deepak P, Dinesh KP, Prasad VK, Das A, Ashadevi JS. Distribution status of the Western burrowing frog, Sphaerotheca pashchima in India. Zootaxa. 2020;4894:146–150. doi: 10.11646/zootaxa.4894.1.10. [DOI] [PubMed] [Google Scholar]
- 61.Kamei RG, Gower DJ, Wilkinson M, Biju SD. Systematics of the caecilian family chikilidae (Amphibia: Gymnophiona) with the description of three new species of chikila from northeast India. Zootaxa. 2013;3666:401–435. doi: 10.11646/zootaxa.3666.4.1. [DOI] [PubMed] [Google Scholar]
- 62.Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. (2004) Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis Aquat Org. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- 63.Rahman MM, Badhon MK, Salauddin M, Rabbe MF, Islam MS. Chytrid infection in asia: how much do we know and what else do we need to know? Herpetol J. 2020;30:99–111. doi: 10.33256/hj30.2.99111. [DOI] [Google Scholar]
- 64.Bovo RP, Andrade DV, Toledo LF, Longo AV, Rodriguez D, Haddad CFB, et al. Physiological responses of Brazilian amphibians to an enzootic infection of the chytrid fungus Batrachochytrium dendrobatidis. Dis Aquat Org. 2016;117:245–252. doi: 10.3354/dao02940. [DOI] [PubMed] [Google Scholar]
- 65.Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- 66.Searle CL, Biga LM, Spatafora JW, Blaustein AR. A dilution effect in the emerging amphibian pathogen Batrachochytrium dendrobatidis. Proc Natl Acad Sci USA. 2011;108:16322–16326. doi: 10.1073/pnas.1108490108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guilherme Becker C, Rodriguez D, Felipe Toledo L, Longo AV, Lambertini C, Corrêa DT, et al. Partitioning the net effect of host diversity on an emerging amphibian pathogen. Proc R Soc B Biol Sci. 2014;281:1–7. doi: 10.1098/rspb.2014.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venesky MD, Raffel TR, Mcmahon TA, Rohr JR. Confronting inconsistencies in the amphibian-chytridiomycosis system: Implications for disease management. Biol Rev. 2014;89:477–483. doi: 10.1111/brv.12064. [DOI] [PubMed] [Google Scholar]
- 69.Becker CG, Zamudio KR. Tropical amphibian populations experience higher disease risk in natural habitats. Proc Natl Acad Sci USA. 2011;108:9893–9898. doi: 10.1073/pnas.1014497108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78:103–116. doi: 10.1016/S0001-706X(00)00179-0. [DOI] [PubMed] [Google Scholar]
- 71.Vredenburg VT, Felt SA, Morgan EC, McNally SVG, Wilson S, Green SL. Prevalence of Batrachochytrium dendrobatidis in xenopus collected in Africa (1871–2000) and in California (2001–2010) PLoS ONE. 2013;8:6–9. doi: 10.1371/journal.pone.0063791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Briggs CJ, Vredenburg VT, Knapp RA, Rachowicz LJ. Investigating the population-level effects of chytridiomycosis: an emerging infectious disease of amphibians. Ecology. 2005;86:3149–3159. doi: 10.1890/04-1428. [DOI] [Google Scholar]
- 73.Knapp RA, Morgan JAT. Tadpole mouthpart depigmentation as an accurate indicator of chytridiomycosis, an emerging disease of amphibians. Copeia. 2006;2006:188–197. doi: 10.1643/0045-8511(2006)6[188:TMDAAA]2.0.CO;2. [DOI] [Google Scholar]
- 74.Fellers GM, Green ED, Longcore JE. Oral chytridiomycosis in the mountain yellow-legged frog (Rana muscosa) Copeia. 2001;2001:945–953. doi: 10.1643/0045-8511(2001)001[0945:OCITMY]2.0.CO;2. [DOI] [Google Scholar]
- 75.Blaustein AR, Romansic JM, Scheessele EA, Han BA, Pessier AP, Longcore JE. Interspecific variation in susceptibility of frog tadpoles to the pathogenic fungus Batrachochytrium dendrobatidis. Conserv Biol. 2005;19:1460–1468. doi: 10.1111/j.1523-1739.2005.00195.x. [DOI] [Google Scholar]
- 76.Rollins-Smith LA. Metamorphosis and the amphibian immune system. Immunol Rev. 1998;166:221–230. doi: 10.1111/j.1600-065X.1998.tb01265.x. [DOI] [PubMed] [Google Scholar]
- 77.Rebollar EA, Martínez-Ugalde E, Orta AH. The amphibian skin microbiome and its protective role against chytridiomycosis. Herpetologica. 2020;76:167–177. doi: 10.1655/0018-0831-76.2.167. [DOI] [Google Scholar]
- 78.Bataille A, Lee-Cruz L, Tripathi B, Kim H, Waldman B. Microbiome variation across amphibian skin regions: implications for chytridiomycosis mitigation efforts. Microb Ecol. 2015;71:221–232. doi: 10.1007/s00248-015-0653-0. [DOI] [PubMed] [Google Scholar]
- 79.Vredenburg VT, Briggs CJ, Harris RN. Fungal diseases: an emerging threat to human, animal, and plant health. Washington, DC: National Academy Press; 2011. Host-pathogen dynamics of amphibian chytridiomycosis: the role of the skin microbiome in health and disease; pp. 342–355. [Google Scholar]
- 80.Becker MH, Walke JB, Cikanek S, Savage AE, Mattheus N, Santiago CN, et al. Composition of symbiotic bacteria predicts survival in Panamanian golden frogs infected with a lethal fungus. Proc R Soc B Biol Sci. 2015;282:1–9. doi: 10.1098/rspb.2014.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Loudon AH, Venkataraman A, Van Treuren W, Woodhams DC, Parfrey LW, McKenzie VJ, et al. Vertebrate hosts as Islands: dynamics of selection, immigration, loss, persistence, and potential function of bacteria on salamander skin. Front Microbiol. 2016;7:1–11. doi: 10.3389/fmicb.2016.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bataille A, Lee-Cruz L, Tripathi B, Waldman B. Skin bacterial community reorganization following metamorphosis of the fire-bellied toad (Bombina orientalis) Microb Ecol. 2018;75:505–514. doi: 10.1007/s00248-017-1034-7. [DOI] [PubMed] [Google Scholar]
- 83.Mutnale MC, Reddy GS, Vasudevan K. Bacterial community in the skin microbiome of frogs in a coldspot of chytridiomycosis infection. Microb Ecol. 2021 doi: 10.1007/s00248-020-01669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bataille A, Cashins SD, Grogan L, Skerratt LF, Hunter D, McFadden M, et al. Susceptibility of amphibians to chytridiomycosis is associated with MHC class II conformation. Proc R Soc B Biol Sci. 2015;282:20143127. doi: 10.1098/rspb.2014.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Voyles J, Berger L, Young S, Speare R, Webb R, Warner J, et al. Electrolyte depletion and osmotic imbalance in amphibians with chytridiomycosis. Dis Aquat Org. 2007;77:113–118. doi: 10.3354/dao01838. [DOI] [PubMed] [Google Scholar]
- 86.Farrer RA, Martel A, Verbrugghe E, Abouelleil A, Ducatelle R, Longcore JE, et al. Genomic innovations linked to infection strategies across emerging pathogenic chytrid fungi. Nat Commun. 2017 doi: 10.1038/ncomms14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rosenblum EB, Poorten TJ, Joneson S, Settles M. Substrate-specific gene expression in Batrachochytrium dendrobatidis, the chytrid pathogen of amphibians. PLoS ONE. 2012;7:1–10. doi: 10.1371/journal.pone.0049924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Piotrowski JS, Annis SL, Longcore JE. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004;96:9. doi: 10.2307/3761981. [DOI] [PubMed] [Google Scholar]
- 89.Fisher MC, Bosch J, Yin Z, Stead DA, Walker J, Selway L, et al. Proteomic and phenotypic profiling of the amphibian pathogen Batrachochytrium dendrobatidis shows that genotype is linked to virulence. Mol Ecol. 2009;18:415–429. doi: 10.1111/j.1365-294X.2008.04041.x. [DOI] [PubMed] [Google Scholar]
- 90.Becker CG, Greenspan SE, Tracy KE, Dash JA, Lambertini C, Jenkinson TS, et al. Variation in phenotype and virulence among enzootic and panzootic amphibian chytrid lineages. Fungal Ecol. 2017;26:45–50. doi: 10.1016/j.funeco.2016.11.007. [DOI] [Google Scholar]
- 91.Garner TWJ, Walker S, Bosch J, Leech S, Rowcliffe JM, Cunningham AA, et al. Life history tradeoffs influence mortality associated with the amphibian pathogen Batrachochytrium dendrobatidis. Oikos. 2009;118:783–791. doi: 10.1111/j.1600-0706.2008.17202.x. [DOI] [Google Scholar]
- 92.Rosenblum EB, Poorten TJ, Settles M, Murdoch GK. Only skin deep: shared genetic response to the deadly chytrid fungus in susceptible frog species. Mol Ecol. 2012;21:3110–3120. doi: 10.1111/j.1365-294X.2012.05481.x. [DOI] [PubMed] [Google Scholar]
- 93.Ribas L, Li M-S, Doddington BJ, Robert J, Seidel JA, Kroll JS, et al. Expression profiling the temperature-dependent amphibian response to infection by Batrachochytrium dendrobatidis. PLoS ONE. 2009;4:e8408. doi: 10.1371/journal.pone.0008408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fisher MC, Schmeller DS, Bosch J, Aanensen DM, Garner TWJ. (2012) RACE: risk assessment of chytridiomycosis to European amphibian biodiversity. FrogLog. 2012;101:45–47. [Google Scholar]
- 95.Skerratt LF, Berger L, Hines HB, McDonald KR, Mendez D, Speare R. Survey protocol for detecting chytridiomycosis in all Australian frog populations. Dis Aquat Org. 2008;80:85–94. doi: 10.3354/dao01923. [DOI] [PubMed] [Google Scholar]
- 96.Smith KG, Weldon C, Conradie W, Du Preez LH. Relationships among size, development, and Batrachochytrium dendrobatidis infection in African tadpoles. Dis Aquat Org. 2007;74:159–164. doi: 10.3354/dao074159. [DOI] [PubMed] [Google Scholar]
- 97.Navarro-Lozano A, Sánchez-Domene D, Rossa-Feres DC, Bosch J, Sawaya RJ. Are oral deformities in tadpoles accurate indicators of anuran chytridiomycosis? PLoS ONE. 2018;13:1–9. doi: 10.1371/journal.pone.0190955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marantelli G, Berger L, Speare R, Keegan L. Distribution of the amphibian chytrid Batrachochytrium dendrobatidis and keratin during tadpole development. Pac Conserv Biol. 2004;10:173–179. doi: 10.1071/pc040173. [DOI] [Google Scholar]
- 99.Aplin K, Kirkpatrick P (1999) Progress report on investigations into chytrid fungal outbreak in Western Australia.Perth: Western Australia Museaum
- 100.Berger L, Speare R, Daszak P, Green DE, Cunningham AA, Goggin CL, et al. Chytridiomycosis causes amphibian mortality associated with population declines in the rain forests of Australia and Central America. Proc Natl Acad Sci. 1998;95:9031–9036. doi: 10.1073/pnas.95.15.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berger L, Speare R, Hyatt A. Chytrid fungi and amphibian declines: overview, implications and future directions. In: Campbell A, editor. Declines and disappearances of Australian frogs. Canberra: Environment Australia; 1999. pp. 23–33. [Google Scholar]
- 102.Aplin K (2000) Chytridiomycosis in southwest Australia: historical sampling documents the date of introduction, rates of spread and seasonal epidemiology, and sheds new light on chytrid ecology. In: In Getting the Jump! on amphibian disease: conference and workshop compendium. Cairns, August 2000
- 103.Olsen V, Hyatt AD, Boyle DG, Mendez D. Co-localisation of Batrachochytrium dendrobatidis and keratin for enhanced diagnosis of chytridiomycosis in frogs. Dis Aquat Org. 2004;61:85–88. doi: 10.3354/dao061085. [DOI] [PubMed] [Google Scholar]
- 104.McCarthy MA, Parris KM. Clarifying the effect of toe clipping on frogs with Bayesian statistics. J Appl Ecol. 2004;41:780–786. doi: 10.1111/j.0021-8901.2004.00919.x. [DOI] [Google Scholar]
- 105.Berger L, Hyatt A, Olsen V, Hengstberger S, Boyle D, Marantelli G, et al. Production of polyclonal antibodies to Batrachochytrium dendrobatidis and their use in an immunoperoxidase test for chytridiomycosis in amphibians. Dis Aquat Org. 2002;48:213–220. doi: 10.3354/dao048213. [DOI] [PubMed] [Google Scholar]
- 106.Waldman B, Van De Wolfshaar KE. Chytridiomycosis in New Zealand frogs. Surveillance. 2001;28:9–11. [Google Scholar]
- 107.Kriger K, Hines H, Hyatt A, Boyle D, Hero J. Techniques for detecting chytridiomycosis in wild frogs: comparing histology with real-time Taqman PCR. Dis Aquat Org. 2006;71:141–148. doi: 10.3354/dao071141. [DOI] [PubMed] [Google Scholar]
- 108.Shin J, Bataille A, Kosch TA, Waldman B. Swabbing often fails to detect amphibian chytridiomycosis under conditions of low infection load. PLoS ONE. 2014;9:e111091. doi: 10.1371/journal.pone.0111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kamoroff C, Goldberg C. Using environmental DNA for early detection of amphibian chytrid fungus Batrachochytrium dendrobatidis prior to a ranid die-off. Dis Aquat Org. 2017;127:75–79. doi: 10.3354/dao03183. [DOI] [PubMed] [Google Scholar]
- 110.Byrne AQ, Rothstein AP, Poorten TJ, Erens J, Settles ML, Rosenblum EB. Unlocking the story in the swab: a new genotyping assay for the amphibian chytrid fungus Batrachochytrium dendrobatidis. Mol Ecol Resour. 2017;17:1283–1292. doi: 10.1111/1755-0998.12675. [DOI] [PubMed] [Google Scholar]