Abstract
Gene therapy has been experiencing a breakthrough in recent years, targeting various specific cell groups in numerous therapeutic areas. However, most recent clinical studies maintain the use of traditional viral vector systems, which are challenging to manufacture cost-effectively at a commercial scale. Non-viral vectors have been a fast-paced research topic in gene delivery, such as polymers, lipids, inorganic particles, and combinations of different types. Although non-viral vectors are low in their cytotoxicity, immunogenicity, and mutagenesis, attracting more and more researchers to explore the promising delivery system, they do not carry ideal characteristics and have faced critical challenges, including gene transfer efficiency, specificity, gene expression duration, and safety. This review covers the recent advancement in non-viral vectors research and formulation aspects, the challenges, and future perspectives.
KEY WORDS: formulation, gene therapy, lipid, non-viral vector, polymer, transfection
INTRODUCTION
Twenty-twenty marks another milestone in the advancement of the gene therapy field. The first cell-based gene therapy with a white blood cell enrichment step, Tecartus, was approved by the FDA in July (1). From the momentum of the first successful clinical trial of gene therapy in 1990 to the tragic death at the University of Pennsylvania in 1999 that caused a cool-down of work on the technology, gene therapy went a long way to rise again in 2017 with three FDA approvals in 1 year. At present, there are a total of ten gene therapy products on the U.S. market and more than 100 clinical trials recruiting patients in clinicaltrials.gov. The turbulence of gene therapy in the past 30 years has built many lessons-learned opportunities and paved a solid foundation for the recently renewed excitement about the ability of gene delivery systems to tackle some of the most devastating diseases. While a few recent clinical studies have applied non-viral vectors, most recent clinical studies maintain traditional viral vector systems, which are challenging to manufacture cost-effectively at a commercial scale (2, 3). As we know, a vector is a carrier for the genetic material to reach its targeted destination. It plays a vital role in the efficacy and safety of the product. Using a viral vector has historically been controversial. Even though viral vectors are not known to cause any harm to patients, the small potential to trigger immunogenic responses and transgene mis-insertion risks, etc., has led many in the field to keep seeking a non-viral delivery system (4, 5).
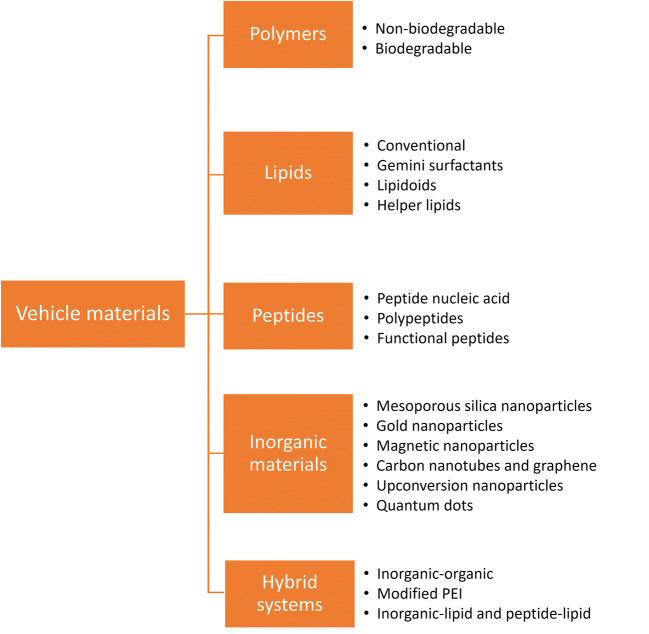
In recent years, the frequently researched non-viral vectors are polymers, lipids, inorganic particles, or combinations of different types (Fig. 1). Compared with a viral vector, non-viral vectors are low in their cytotoxicity, immunogenicity, and mutagenesis, attracting more researchers to explore the promising delivery system and move the gene therapy field forward. However, non-viral vectors do not carry ideal characteristics and have faced critical challenges, such as gene transfer efficiency, specificity, gene expression duration, and safety. Non-viral vectors have been a fast-paced research topic in gene delivery. The review covers the recent advancement in non-viral vectors research and formulation aspects. The challenges and future perspectives are also discussed (4–6).
Fig. 1.
Vehicle materials
MATERIALS
Polymers
Gene therapy comprises the delivery of the genetic material into cells, transfection, and modulation of gene expression. Cationic polymers have been an essential type of non-viral gene therapy vector and have appealed to researchers over the years due to their versatile chemical structure and potential high loading capacity. They can neutralize the negatively charged genetic material to form a complex (polyplex) and carry the payload to targeted cells.
Non-biodegradable Polymers
Polyethylenimine (PEI) was the first polycationic polymer, synthesized both in linear and branched forms for gene therapy in 1995. It has uniquely arranged amine groups on the backbone of the polymer chain, allowing only partial protonation in the physiological pH range. However, in a more acidic compartment of the endosome, additional amine groups are protonated. The presence of the charged PEI generates an osmotic effect (“proton sponge effect”) to induce endosome burst, which is believed to enhance the transfection efficiency. The high buffer capacity of PEI is also beneficial for the endosomal escape of the gene payload (7). Today, PEI remains the gold standard for measuring the transfection efficiency of non-viral vectors (8).
Despite being considered a high transfection non-viral vector, PEI may still suffer from insufficient specificity and transfection. In addition, it is a non-biodegradable polymer that accumulates around the cell and triggers cytotoxicity. In the past several years, scientists have made significant progress in understanding the mechanisms behind those limitations. The ability of PEI to escape from late endosomal vesicles during intracellular delivery, including its interaction with endosomal lipids under osmotic stress, were studied by Clark et al. using model systems of monolayers and vesicles derived from a mixture of neutral and negative lipids 1,2-dipalmitoylphosphatidylcholine (DPPC) and bis(monoacylglycerol)phosphate (BMP), respectively (9). The results confirmed the adsorption of PEI to DPPC/BMP membranes, an important factor contributing to the endosomal escape of polyplexes. The model has provided a new tool to study the subsequent effects of the non-viral vectors on the stability and permeability of the membranes.
In addition to PEI, amine-terminated PAMAM is another cationic dendrimer. In fact, PAMAM is one of the most used dendritic carriers in biological applications and the first to be used for gene delivery. A major disadvantage of those common dendrimers is their toxicity, associated mainly with the chemistry of the surface amine groups. Furthermore, polymethacrylates and polymethacrylamides are the two large varieties of important synthetic vinyl-based cationic polymers with the ability to mimic the pH sensitivity, proton sponge theory, and buffering properties of PEI (10). They have been continually modified over the past 20 years in an attempt to improve gene delivery efficiency and lower toxicity (8, 11–13). Although polymethacrylates are less cytotoxic than PEI, their application in gene therapy is currently limited due to their low ability to interact with membranes (5, 14).
Poly(vinylimidazole) (PVI) is a water-soluble polymer synthesized as poly(1-vinylimidazole) and poly(4-vinylimidazole). Its imidazole group is protonated at acidic pHs and alters the conformation of PVI chains. PVI has additional properties of biocompatibility, non-toxicity, and the ability to escape the endosome by activating the proton sponge mechanism, which makes it an emerging non-viral vector (15). Recently, the alkylated poly(1-vinylimidazole) with different chain lengths has been investigated for DNA complexation and transfection. Among them, butylated PVI was found to be the most effective in HepG2 liver cancer cells. Furthermore, a folic acid–conjugated amine-containing poly(1-vinylimidazole) was also discovered to effectively complex DNA and transfect cancer cells (16).
Biodegradable Polymers
Given that repeated administration is often necessary for gene therapy and less cytotoxicity is preferred, biodegradable polymeric vectors, either synthetic or natural, have a definitive advantage over non-biodegradable ones. The synthetic polymers have excellent chemical structure versatility and batch consistency, but may lack sufficient interaction with cells (17). In contrast, the natural polymers have high biocompatibility but pose batch to batch variation problems due to the origin difference. Hence, to assure product quality, control strategies on the key attributes of natural polymers need to be put in place. The excipient companies sometimes use blending to meet the excipient specifications. In addition, appropriate tests and specifications are used to ensure consistent quality and reliable performance. For example, gel permeation/size exclusion chromatography (GPC/SEC) may be applied to measure the characteristics of the polymers. It will take a collaborative effort among pharma companies, excipient suppliers, the US Pharmacopeial Convention (USP), regulators, and the International Pharmaceutical Excipients Council (IPEC) to control, reduce, or minimize the possible negative impact of excipient variability on the natural excipients, including polymers (18).
Chitosan (CS) is a linear polysaccharide and one of the most abundant natural carbohydrate polymers. It is highly biodegradable, biocompatible, and non-toxic. With an apparent pKa of 6.5, it is only soluble in acidic conditions where most of the amino groups are protonated to form a complex with the genetic material. It was found that chitosan with a high degree of polymerization (>50) could induce a significant opening of the tight junction between cells (19). The surface of a chitosan carrier can also be modified or decorated with ligands to enhance cellular entry and specificity (20). These properties have made chitosan an attractive non-viral vector for gene therapy. In recent years, chitosan-coated nanoparticles are actively studied as carriers for brain cancer gene therapy, where enhanced particle uptake was evidenced by human blood-brain barrier cerebral microvessel endothelial cells (hCMECs) via receptor-mediated endocytosis (21).
Poly(β-amino ester)s (PBAEs) are a class of evolving non-viral vectors that have made significant advancements in the past 20 years. The class was first developed into linear PBAEs in 2000, but the development of this class transitioned to branched PBAEs in 2016 (22, 23). These amphiphilic polymers have shown robust transfection capabilities under challenging conditions as well as efficient endosomal escape properties. However, their application is limited due to forming self-assembled nucleic acid nanoparticles. Thus, they are insufficient to encapsulate proteins of various surface charges. In 2019, Green et al. synthesized a new hyperbranched PBAE containing both cationic and anionic charges. The structural change has offered the differentiation of polymer end-group hydrophobicity, affected protein complexation capabilities, as well as nanoparticle internationalization, and endosomal escape (24). In the same year, Liu et al. synthesized highly branched PBAE containing biodegradable disulfide units in the HPAE backbone and guanidine moieties at the extremities. Those polymers delivered a minicircle DNA to multipotent adipose-derived stem cells and astrocytes, achieving high transfection efficiency (25).
Traditionally, polylactide (PLA) is a synthetic biodegradable polymer extensively applied to drug delivery. Its carboxylic acid hydrolyzes into lactic acid in vivo and rapidly converts to glucose eliminated from the body without adverse effects. In 2013, Jones et al. synthesized a cationic polylactide with tertiary amines to make it suitable for gene therapy (26). Nowadays, PLA draws continued interest in targeted delivery through continual structure modifications (27, 28).
The full potential of polymer-based delivery systems has yet to be realized. In 2020, aminoglycosides, a class of naturally occurring and semi-synthetic antibiotics, have been investigated as new cationic polymeric vectors to facilitate the transfer of genes into cells (29).
Lipids
Lipids have been used to deliver genes for a long time. Most lipids consist of positively charged headgroups which bind with the anionic phosphate groups of nucleic acids via electrostatic interactions to form lipoplexes. Due to the self-assembling lipid tail structures, lipoplexes are often present as liposomes, solid lipid nanoparticles, or lipid emulsions. Compared with other carrier materials, lipids are biodegradable, less toxic, and can incorporate hydrophilic and hydrophobic substances. The first FDA-approved small interfering ribonucleic acid (siRNA) treatment (Onpattro) utilized a lipid-based vector (30). Another promising lipid-based siRNA therapy (inclisiran) for hyperlipidemia treatment was approved in the EU in December 2020 (31, 32). Phase 3 clinical trials have shown that inclisiran lowered the low-density lipoprotein cholesterol levels by 50% by subcutaneous administration every 6 months (33).
Conventional Lipids
Conventional lipids possess one head group on each molecule, which can be permanently or temporarily charged. The common head groups are ammonium, imidazolium, pyridinium, lysine, or arginine, etc. Meanwhile, the hydrophobic tails can be two saturated or unsaturated hydrocarbon chains or steroids (31). The ability of hydrocarbon chain lipids to deliver nucleic acids has been widely explored, especially those with ammonium as head groups. Common examples include monovalent lipids such as N-(1-(2,3-dioleyloxy)propyl)-N,N,N-trimethylammonium (DOTMA), 2,3-bis[[(Z)-octadec-9-enoyl]oxy]propyl-trimethylazanium (DOTAP), 2,3-di(tetradecoxy)propyl-(2-hydroxyethyl)-dimethylazanium (DMRIE), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), or multivalent lipids such as 2,5-bis(3-aminopropylamino)-N-[2-[di(heptadecyl)amino]-2-oxoethyl]pentanamide (DOGS). While those lipids remain dominant as gene carriers due to their positive charges, they are relatively toxic and exhibit less than optimal in vivo behavior, e.g., short half-life. Therefore, the surface-modified ionizable lipids, such as 1,2-dioleoyloxy-3-(dimethylamino)propane (DODAP) or 1,2-dilinoleyloxy-3-dimethylaminopropane (DLin-DMA), were developed to overcome those shortcomings and achieve better efficacy. Those materials are neutral at physiological pH, allowing systemic delivery but can be positively charged to facilitate lipoplex formation with DNA and promote endosomal escape. It is worth noting that heptatriaconta-6,9,28,31-tetraen-19-yl 4-(dimethylamino)butanoate (DLin-MC3-DMA) is the “gold standard” for siRNA delivery because of its superior gene silencing activity relative to its comparators (34) and was successfully applied in Onpattro, the first FDA-approved siRNA treatment (30). In addition to those hydrocarbon chain lipids, cholesterol and its derivatives are another category of lipids that can be used for gene delivery. One of the derivatives, DC-Chol, is now commercially available and has been utilized for cancer gene therapy in clinical trials (35).
Gemini Surfactants
Gemini surfactants have more recently emerged as a group of lipids useful for gene delivery. They are two surfactant monomers with head groups linked by a spacer group via covalent bonds. Gemini surfactants typically have lower CMC than corresponding surfactant monomers, resulting in lower surface tension, higher solubilization capacity, etc. Therefore, the amount of this carrier needed in the delivery system is reduced, and correspondingly so is the toxicity. Recently, research has been focused on the elucidation of the structure of gemini surfactant in relation to bioactivity. For example, Jin et al. has compared three structurally similar gemini surfactants, 16-3-16, 16(Py)-S-2-S-16(Py), and 16-7N(G.K.)-16, which all possess the same number of carbon in their tails (36). It was found that with the head group being a glycyl-lysine di-peptide, 16-7N(G.K.)-16 exhibited much higher transfection efficiency than either the unsubstituted 16-3-16 or the pyridinium containing 16(Py)-S-2-S-16(Py).
Lipidoids
Lipidoids are lipid-like materials synthesized by conjugation of amines with lipophilic acrylates, acrylamides, or epoxide (37). Lipidoids have become attractive due to their solvent- and catalyst-free synthetic process. This rapid and simple synthesis allows for the screening of an extensive library of lipidoids with diverse structures. Early in 2008, Aknic et al. synthesized over 1200 lipidoids and demonstrated effective gene transfer and expression in mice, rats, and nonhuman primates (38). Recently, Molla et al. prepared 13 lipidoids by applying the one-pot synthetic methodology using thiolactone chemistry. Those materials were formulated as liposomes to deliver siRNA and tested on HeLa-GFP cells. Five of the formulations showed superior knock-down efficiency than the commercial reagents, emphasizing the importance of lipidoid structure for transfection capability as well as the liposome formulation attributes such as particle size (39). These findings may pave a foundation for further exploration of lipidoids for gene delivery in the future.
Helper Lipids
Lipid-based gene delivery systems oftentimes utilize “helper lipids” to enhance transfection efficiency, stabilize particles, or improve intracellular trafficking (40, 41). In contrast with cationic and ionizable lipids, helper lipids are neutral materials. 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) is one of the most used helper lipids due to its cone-like molecular shape, favoring membrane fusion and/or bilayer disruption. In addition, cholesterol is also used as a helper lipid because it can enhance cell membrane fluidity and stabilize bilayer lipids in liposome formulations, resulting in improved efficacy and stability (40). Sasayama et al. have employed cholesterol to develop a nanoparticle formulation LNPK15 based on 1,2-distearoyl-sn-glycero-3-phosphatidylethanolamine-N-(poly-ethyleneglycol-2000). This formulation showed extended half-lives in mice and monkeys as 15.2 h and 27.0 h, respectively, and also exhibited potent knock-down efficiency (42).
Peptides
Peptides are short chains of 2–50 amino acid residues linked via peptide bonds. They are biocompatible and biodegradable and also can be rationally designed to serve as building blocks for self-assembling nanoscale structures (43). The genetic material interacts with peptides either via conjugation or electrostatic forces to form peptiplexes which facilitates delivery.
Peptide nucleic acid (PNA) conjugates are comprised of peptide moieties and nucleic acid moieties linked via covalent bonds. They are stable uncharged molecules, able to resist nuclease degradation and less labile to acidic and basic pH, as well as high temperature. Recently, Altrichter and Seitz prepared an antisense module based on peptide nucleic acids comprising a Smac mimetic compound (SMC) (44). By incorporating cell-penetrating peptides, the SMC-PNA resulted in nearly complete downregulation of the cellular FLICE-like protein.
Polypeptides are a major class of peptides used for gene delivery, including poly(L-lysine) (PLL), poly(L-arginine) (PLR), and poly(L-glutamate) (PGA). The most widely used PLL possesses a high charge density of its side chains, allowing effective DNA condensation. The easy uptake of PLL/plasmid DNA (pDNA) complex by endocytosis has been reported with a size of around 100 nm (45). However, its low transfection efficiency and suboptimal endosomal escape have limited its application. PLL has been modified with various functional groups, such as PEI or palmitic acid, to improve efficacy. It has also been combined with other peptides to overcome the poor endosomal escape. Zhang et al. prepared the PLL/DNA polyplex with a glutamic acid–modified peptide (AR-23), promoting endosomal escape and enhancing the lytic activity (46).
Polypeptides can further be designed into dendrimers, which use amino acids as building blocks in the core, the branches, the dendrimer surface, or any combination of the three units. Peptide dendrimers may provide the necessary positively charged groups to complex with the genetic material, the likelihood to pass the cellular membrane, and the buffering capacity needed to escape endosomes. For example, a PLL dendrimer may utilize the flexible branched dendrimer structure and the amino acid lysine in its core (47). Recent studies have expanded the choice of amino acid from lysine to arginine or other substitutions, bringing additional benefits to the PLL dendrimer as a gene delivery vector. These efforts have changed the flexibility and charge distribution of the dendrimer, providing additional interactions with nucleic acids and increasing cellular uptake (48). Dendrimer systems can also incorporate lipid or polymers to obtain greater efficacy. It has been demonstrated that transfection was enhanced by the addition of a polyol to the lipid/dendrimer hybrid (49) or a polymer excipient, such as Polyvinylalcohol 18 (PVA 18) (50).
In addition to polypeptides, a number of functional peptides have also been developed. These peptides possess certain sequences in their structure, resulting in various benefits such as enhanced cell penetration or targeting. For instance, cell-penetrating peptides (CPP) are small peptides that can easily move across cell membranes and facilitate genetic material transport. As a commonly used CPP, trans-Activator of Transcription (TAT) protein was recently evaluated on solid tumors using the multicellular tumor spheroids as cell models. It showed that higher TAT concentrations significantly increased peptide uptake (51). In addition to TAT, penetratin, GALA, transportan, and its derivatives such as PepFect and NickFect have also received attention for their cell penetration abilities (52). Some other peptides can target specific cells by recognizing receptors at the cell surface, resulting in enhanced efficiency and reduced toxicity. Various targeting peptides have also been discovered or synthesized, such as the RGD peptide and transferrin (Tf). Additional Tf receptor–binding peptides are drawing interest to improve targeting capability, such as the T7 peptide. Gu et al. applied T7-modified polypeptide nanoparticles, CRD-PEG-T7, to deliver the pDNA pPMEPA1 for bone metastatic prostate cancer treatment. They found the incorporation of T7 inhibited tumor growth and extended survival time of tumor-bearing mice (53).
Inorganic Materials
Inorganic materials are more stable than organic materials and have also been used as gene carriers. In fact, the first reported non-viral gene delivery was based on calcium phosphate, back in the 1960s (54). Nowadays, the more commonly reported inorganic carriers include silica-based systems, such as mesoporous silica nanoparticles, gold nanoparticles, magnetic nanoparticles, carbon nanotubes, graphene, upconversion nanoparticles, and quantum dots (55, 56). Gold nanoparticles are relatively less toxic and can be prepared with polymeric and lipid carriers. Liu et al. constructed a gold nanoparticle composite for Parkinson’s disease treatment, where pDNA was adsorbed onto the surface of positively charged gold nanoparticles and encapsulated into liposomes, followed by attaching a targeting NGF and DHA. It was noticed that this system exhibited significant neuroprotective effects for mice by improving both motor and non-motor dysfunction (57). Carbon nanotubes are another attractive carrier, composed of single or multiple graphene sheets that can range in size from hundreds of nanometers to tens of microns. Carbon nanotubes facilitate gene penetration through the cell membrane independent of the endocytosis process of mammalian cells. With the help of molecular dynamics simulation, Liang et al. found that carbon nanotubes assisted nucleotide penetration through a lipid membrane by decreasing the free energy of this process (58). In 2020, a single-walled carbon nanotube linked with siRNA from Caspase3 was synthesized to treat cardiovascular diseases. This gene carrier significantly improved transfection efficiency, resulting in greater Caspase3 gene silencing (59).
HYBRID VECTOR SYSTEMS FOR TRANSFECTION ENHANCEMENT AND CYTOTOXICITY REDUCTION
Non-viral vectors having an efficient gene transfection and low cytotoxicity have been a double-edged sword at the forefront of gene delivery. Some of these recent attempts to enhance transfection efficiency while lowering the cytotoxicity include inorganic-organic hybrid vectors, modified PEI vectors, inorganic-lipid vectors, and peptide-lipid vectors.
Inorganic-Organic Hybrid Vectors
Inorganic-organic hybrid vectors have been identified as an increasing trend in the new class of non-viral vectors, with either a platform or targeted gene deliveries, to various cell types. One attempt was to incorporate a mannitol-group into the vector. In 2020, Ma et al. were inspired by multi-hydroxyl compound mannitol being used as an osmolyte in the clinic (60). They constructed biomimetic non-viral vectors with a controlled cellular uptake and consequent intracellular trafficking for the gene delivery and introduced for the first time mannitol-based calcium phosphate mannitol-alendronate (CaP-MA) organic-inorganic non-viral vectors. The new vectors with mannitol groups may simulate caveolae-mediated cellular update and transfer the genetic payload in a non-destructive pathway and subsequently avoid gene degradation in the lysosome. As a result, these vectors are shown to be superior to the unmodified CaP nanoparticles in transfection, biocompatibility, and toxicity.
Another attempt at cytotoxicity reduction was to use amine-free vectors to counter amine-associated toxicity (61). Choi et al. have developed a non-conventional non-viral vector, using mesoporous silica nanoparticles (MSNs) as a biocompatible agent, to load siRNA in a sequential, cumulative, and directional way. It was established through calcium ion–mediated interconnection (calcium gluing) between phosphates of siRNA and the non-positively charged bare surface of MSN. The results suggested the potential of the non-positively charged MSN-based calcium-gluing strategy as a general non-viral vector platform for RNAi delivery, which may open the door for additional investigation of its application in gene delivery.
Modified PEI Vectors
The most significant challenge for PEI vectors is cytotoxicity due to the presence of non-degradable amide bonds, causing accumulation and disruption of metabolic activity in normal cells. It is also known that the extent of cytotoxicity has a positive relationship with polymer molecular weight. Hence, a promising strategy to reduce toxicity lies in ligand-modified low molecular weight PEIs, such as those incorporating folic acid (62) or various functional peptides (63). Yu et al. have demonstrated that self-assembled nanoparticles (SNPs) prepared from cyclodextrin-grafted low molecular weight PEI (CD-PEI800) presented low cytotoxicity and a high transfection efficiency to Jurkat cells. They postulated that the cationic hydrogel generated from CD-PEI800 contributed to SNPs’ enhanced gene encapsulation efficiency (64).
The first trisaminocyclopropenium-crosslinked linear PEI (PEI/TAC) nanoparticle vector was synthesized by Steinman et al. to lower the PEI toxicity in 2020 (65). The vector particles with DNA were found to be smaller than those prepared with the unmodified PEI, which positively influenced cellular uptake. The cross-linking by TAC increased the cationic charge of the polymer, allowing binding to the genetic material and promoting endosomal escape, and resulting in enhanced overall transfection efficiency. Meanwhile, the decrease in the amount of polymer introduced to the cell further reduced the toxicity effects.
Another way to keep the PEI biocompatible and lower toxicity is through a combination of poly lactic-co-glycolic acid (PLGA) nanoparticles with arginine-modified PEI polymers (AnPn) (66). The addition of AnPn significantly improved the nuclear localization of pDNA and successful gene expression in primary human astrocytes.
Inorganic-Lipid or Peptide-Lipid Vectors
One challenge of using a lipoplex is the low transfection efficiency. Lebrón et al. generated cationic metallo-liposomes of Ruthenium (Ru) and phospholipid complexes to increase polycation and enhance transfection (67). They also discovered that transfection was impacted by the length of the Ru-lipid and hydrophobicity. A positive transfection efficiency obtained with those vectors indicated a possibility of using them as nano-vectors in gene therapy. Additionally, their size and superficial charge impacted compaction using different mass ratios of lipid to DNA, further preventing the nucleic acid from degradation. Interestingly, the Ru-lipid vectors exhibited less cytotoxicity to normal cells than cancer cells, which shows promise for targeted gene therapy.
Peptide-based cationic lipids are another class of promising non-viral vectors for nucleic acid delivery into cells. Zhao et al. synthesized two peptide lipids containing a tri-ornithine head (LOrn3) and a mono-ornithine head (LOrn1) and studied the interaction kinetics of the liposome-mediated gene delivery (68). Both lipoplexes were used as vectors to deliver the green fluorescent protein (GFP) into Hela and Hep-2 cells. The transfection efficiency was significantly impacted by the charge ratios of the peptide lipids. LOrn1 and LOrn3 had the highest transfection efficiency and lowest toxicity to Hela cells at charge ratios of 4:1 and 3:1, respectively. Although both peptide-lipid vectors have similar chemical structures, LOrn3 contained more amino groups, which produced both cellular internalization and higher proton buffering capacity. As a result, LOrn3 led to enhanced endosome escape and DNA release and more efficient transfection than LOrn1. The study provided theoretical insight into further research directions to advance peptide-lipid vectors in the future.
EFFECTS OF GENE VECTOR PROPERTIES AND FORMULATION FACTORS ON IN VITRO AND IN VIVO PERFORMANCE
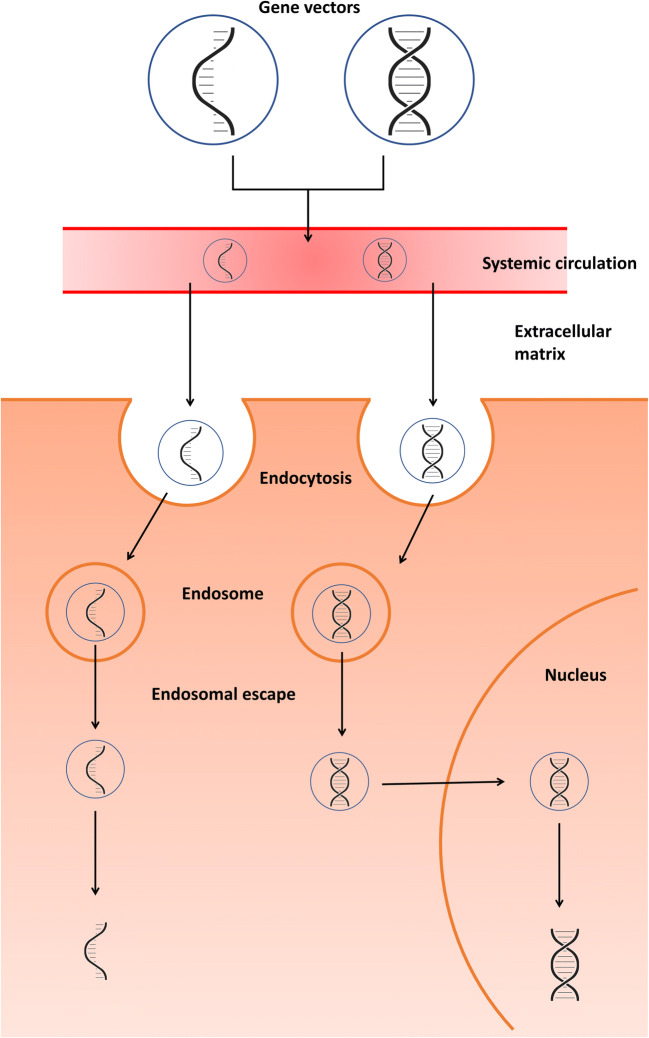
In order to have a therapeutic effect in target cells, gene delivery systems need to be stable enough in blood vessels to be transported to the intercellular site of action. Subsequently, they must attach to the cell membrane, enter the cell, escape from endosomes into the cytoplasm, and release the therapeutic gene from vehicle material (Fig. 2). In addition, if the pDNA is delivered, it must also diffuse through the cytoplasm and enter the nucleus. Therefore, to design a proper gene delivery system with sufficient efficacy, it is critical to understand the effects of the properties of gene vehicle and delivery systems on gene transfection efficiency (Fig. 3).
Fig. 2.
Delivery mechanism for non-viral gene vectors
Fig. 3.
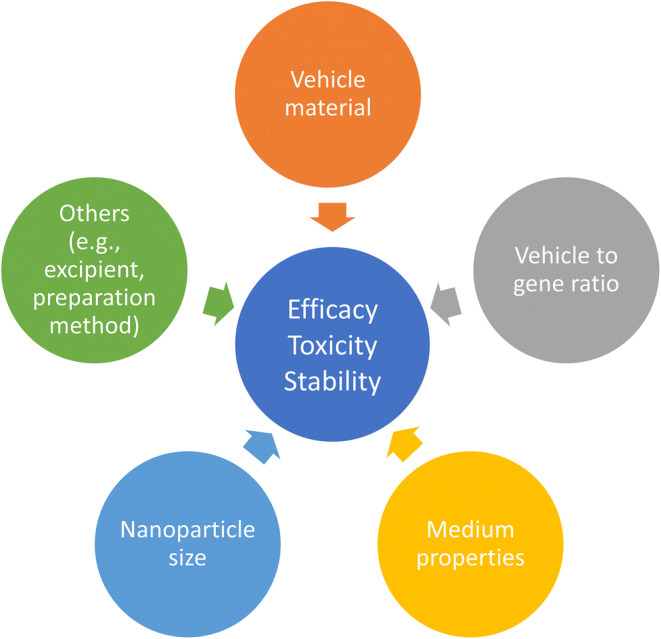
Effects of gene vector properties and formulation factors on non-viral gene delivery systems
Vehicle Material
Chemical structures and properties of vehicle materials are major considerations for successful gene delivery. Interest has been drawn to establish the structure activity relationship (SAR) to understand the effects of molecular structures and facilitate the design of gene vectors. There have been investigations on SAR for polymer and lipid materials. For example, Buck et al. combined short-chain aminolipids to DOTAP:chol-based lipoplexes, finding that the longer lipid tails (C12) improved transfection efficiency of minicircle DNA as compared with the shorter ones (C10) (69). By constructing a series of α-cyclodextrin-threaded polyrotaxanes using polyethylene glycols (PEG) with different molecular weights, Ghodke et al. exhibited that the composite containing the largest PEG in the study (PEG 1500) had the least silencing efficiency on Turbo GFP siRNA as compared with smaller PEG (PEG 200, 400, and 600) (70). Ionization status is one of the critical properties of vehicle material. Lipid materials having pKa of 5.5–6.5 are favored for in vivo potency (40). For polymer-based vectors, a generalized rule has not been established, as investigations are ongoing. For instance, Routkevitch et al. recently reported that use of poly(β-amino esters) (PBAE) with a narrower buffering range led to transfection levels that were more sensitive to pH (71).
The Ratio of Vehicle Material to Gene
The ratio of vehicle material to gene plays a crucial role in transfection efficiency, since it can affect the complexation ability and physicochemical properties of the vehicle-gene complexes. Neves et al. have characterized the peptide RALA/p53 encoding pDNA vectors at different nitrogen to phosphate groups (N/P) ratios. They found that, in comparison with N/P as 2, systems with N/P from 5 to 10 had smaller particle size, more positively charged surfaces, and higher pDNA encapsulation capacity (72), which in turn led to better cell internalization, higher p53 protein expression levels, and large extent of apoptosis when tested using HeLa cells. Sainz-Ramos et al. have developed 2,3-di(tetradecyloxy)propan-1-amine (DTPA)-based nioplex formulations with three different lipid to DNA ratios to deliver the CFTR gene for treating cystic fibrosis (73). They indicated that the formulation with the lipid to DNA ratio as 5/1 exhibited much greater transfection efficiency than either 2/1 or 10/1.
Nanoparticle Size
The nanoparticle size is a key factor that affects all steps in the delivery process. Generally, a larger size of around 100 nm is favored for long circulation time in blood, but a relatively smaller vector can more efficiently permeate cells. Particles less than 5 nm can be cleared by renal filtration, while those greater than 200 nm are cleared by spleen and liver filtration (74). Particle size can also affect the cellular uptake mechanism and rate. For example, particles larger than 1 μm are taken up by phagocytosis, while those around 200 nm are more prone to the clathrin- or caveolae-mediated uptake pathways (40). However, it is difficult to define a particle size range for optimal transfection efficiency because the size effect is highly dependent on the vector type and cellular uptake pathway. Xia et al. compared cellular uptake, cytotoxicity, and biodistribution for commercially available gold nanoparticles with a size range of 5–50 nm. It was observed that the increase in particle size led to greater cellular uptake by the HepG2 cancer cells but less by L02 normal cells. Gold nanoparticles of 5 nm showed the highest toxicity, while 50 nm nanoparticles sustained the longest circulation in the blood (75). Additionally, Haddick et al. prepared various mesoporous silica nanoparticles with a size range of 60–160 nm. They noted that the nanoparticles with a size of 160 nm displayed the fastest cellular internalization and best knock-down efficacy (76). It was postulated that the larger size could result in greater contact surface area between particle and cell membrane, leading to greater endocytosis.
Medium pH and Ionic Strength
When the carrier-gene complexes are formed based on their electrostatic interactions, the medium pH and ionic strength can shift the complexation equilibria. Therefore, the complex physicochemical properties and their efficacies can be greatly affected. Chivu et al. evaluated the poly(hexamethylene biguanide)/pDNA nanoparticles prepared in either pH 7.4 or 12 phosphate buffer (77). Their findings revealed that nanoparticles had higher zeta potential at pH 7.4 due to the higher charge density of the biguanide group (pKa ≈ 12). As a result, when loaded with fluorescently labeled oligodeoxynucleotides, the polyplex presented enhanced cellular uptake by the HepG2, HEK293T, HeLa cell lines at pH 7.4 than that at pH 12. Chroni et al. synthesized a magnetopolyplex incorporating a diblock copolymer, hydrophilic anionic magnetic nanoparticles, and short deoxyribonucleic acid (78). The intensity of this magnetopolyplex measured through dynamic light scattering was constant at low NaCl concentrations followed by a gradual decrease as ionic strength increased because a higher ionic strength produced a weaker interaction between DNA and carrier, reducing the amount of complexes.
Other Effects
The excipients used in a drug delivery system are anticipated to affect formulation performance. Keil et al. designed a nano-embedded microparticle (NEM) powder of gene therapy for localized pulmonary disease treatment (79). The NEM was prepared by spray drying the polyplex containing PEI and nucleic acids in inert excipients, mannitol, or trehalose. Mannitol was found to produce dried particles with a much larger aerodynamic diameter and also to increase the particle size of the polyplex after reconstitution. Moreover, compared with trehalose, mannitol-containing dry powders exhibited low solubility, preventing evaluation of its biological properties. Wickline et al. recently discovered that the use of hyaluronic acid unexpectedly provided a more uniform nanoparticle size distribution without aggregation to a cationic amphipathic peptide and RNA formulation (80). The novel hyaluronic acid nanoparticles also offered a more potent effect than other nanoparticle formulations, as demonstrated by the ability of a 10-fold lower dose of siRNA to suppress abdominal aortic aneurysms. Additionally, the hyaluronic acid nanoparticles were also complexed with mRNA structures to form stable nanoparticles that are small enough to penetrate cartilage, thus enabling delivery and translation of the mRNA in that tissue. Collectively, the evidence may indicate that the selection of formulation compositions is another aspect to investigate for improved transfection to the cells and improved stability in gene delivery systems.
In addition to formulation composition, the nanoparticle preparation method can also impact its properties. Pezzoli et al. applied two approaches to preparing the PEI-pDNA polyplexes (81). One is the DROPPING mode, where pDNA was added to the polymer solution as one single drop followed by leaving the complex solution standing. The other is the MIXING mode, where pDNA was mixed with the polymer solution by vigorous pipetting up and down. The study demonstrated that the DROPPING mode generated larger nanoparticles, resulting in remarkably higher transfection efficiency.
CHALLENGES AND PROSPECTS
Gene therapy has been experiencing a breakthrough in recent years, targeting a variety of specific cell groups in numerous therapeutic areas. The mechanistic challenges of delivering gene therapy using a non-viral vector have been well-reviewed by Yin and Anderson et al. in 2014 (82). While a non-viral vector needs to achieve the characteristics of protecting the genetic material from endonuclease degradation to provide transport to the nucleus and nuclear uptake, and vector unpacking, it often requires a customized system. Also, the delivery systems should be designed according to different mechanisms of action for therapeutic modalities. The transport of DNA into the nucleus is necessary for its therapeutic effect, so the relatively larger particle size poses an additional challenge for efficacy. RNA does not need to enter the cell nucleus for expression, but it is less stable compared with DNA. Furthermore, the interaction between cell and vector varies with different modalities, which greatly affects transfection efficiency. To date, a one size fits all approach to select a non-viral vector delivery system has not been feasible. As presented in all three recently FDA-approved or emergency use authorized gene delivery products that incorporated lipid nanoparticles as the vector, each product customized its own lipid system (Table I), even though both Moderna and Pfizer/BioNTech delivered an mRNA vaccine for the same purpose of COVID-19 prevention. Moreover, the vector systems can be quite complicated. For example, Pfizer/BioNTech vaccine had to use a package of 4 lipids; an ionizable cationic lipid to encapsulate the negatively charged mRNA, a PEGylated lipid to help control particle size, distearoylphosphatidylcholine (DSPC), a phospholipid, and cholesterol to contribute to forming the structure of the lipid nanoparticles (83). Due to the complexity of vector formulations, it takes a tremendous effort to screen a successful vector. It is reported that more than 300 ionizable lipids had to be screened during the development of patsiran, an siRNA drug (84). Recently, GuidTx has developed a high throughput technology to screen lipid nanoparticle systems, which would potentially improve the efficiency of selecting non-viral vectors for gene delivery with improved precision (85).
Table I.
Current Approved/Emergency Use Authorized Genetic Material Products in the USA with Non-viral (Lipid) Vectors
| Generic name | Genetic material | Non-viral vector composition |
|---|---|---|
| Patisiran | siRNA |
DLin-MC3-DMA, PEG2000-C-DMG |
| Pfizer/BioNTech Covid-19 vaccine | mRNA |
(4-hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis (ALC-3015), (2- hexyldecanoate),2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide (ALC-0159), 1,2-distearoyl-snglycero-3-phosphocholine (DPSC), cholesterol |
| Moderna COVID-19 vaccine | mRNA |
SM-102, 1,2-dimyristoyl-rac-glycero3-methoxypolyethylene glycol-2000 (PEG2000-DMG), 1,2-distearoyl-snglycero-3-phosphocholine (DSPC), cholesterol |
The development of an effective and safe gene vector requires comprehensive knowledge of physicochemical and biological properties of the genetic material and carrier, the physiology of the target cells, and a mechanistic understanding of vector-induced transfection at the molecular level. For example, differences between cell membrane electrical charge among various targeted cell types create many research opportunities to investigate and modify non-viral vectors for a specific genetic payload to achieve the desired efficacy in a particular cell type. In addition to efficacy, developers should also pay attention to toxicity. While non-viral gene vectors possess lower immunotoxicity than viral vectors, it should not be neglected that the nanoparticles can trigger immune reactions (86). For example, chitosan was found to induce the IL-1β response in mouse dendritic cells, peritoneal macrophages, and human peripheral blood mononuclear cells (87). Silica nanoparticles led to an increase in pro-inflammatory cytokine secretion. They were also shown to activate macrophages to secrete IL-1β (88). The immunotoxicity depends on vector materials and cells. Hence, it needs to be evaluated on a case by case basis.
It is not surprising that most of the recent research publications on new non-viral vectors have focused on the improvement of vector performance characteristics, such as size, surface charge, transfection, cytotoxicity, and inter- and intra-cellular trafficking. Meanwhile, fewer publications reported research from a product development perspective, especially relating to a vector’s physical and chemical stability during formulation preparation and product storage. For instance, it would be worth investigating the effect of ionic strength and pH of the formulation buffer on the stability of non-viral vectors, since those factors could impact the transfection efficiency of the vector or alter its potential clinical applications. Furthermore, freezing or refrigeration is commonly used to store gene therapy formulations. Thus, the freeze-thaw stress and impact of temperature elevations before administration may impair a gene therapy’s effectiveness, such as by triggering changes in the nanoparticle size distributions, aggregation potential, or chemical degradation. On the other hand, stability-indicating techniques for gene therapy product characterization have not been well established, limiting the tools available for research in this area (89).
Moreover, the non-viral gene delivery systems also present complexity in bringing the medicine from bench to bedside. In light of quality-by-design principles, it is important to define the appropriate critical quality attributes and critical process parameters not only to assure product quality but also to accelerate product development (90). Since the gene vectors inherently are variable and the manufacturing process involves living cell, it is challenging to control material impurity and ensure lot-to-lot consistency. When establishing specification acceptance criteria, it is also necessary to understand the relationship between product attributes and their safety and efficacy (91). Lastly, the formulation design of a non-viral vector gene therapy is still in its infancy. Various approaches are quickly evolving. Chen et al. recently incorporated hydrophobic prodrugs into a lipid-based nanoparticle gene delivery system, which provided a convenient way to avoid the immunostimulatory consequences of systemic administration of genetic drug formulations (92). Many more research opportunities lie ahead to investigate and understand the properties and impacts of non-viral vector gene therapy formulations on clinical applications.
Among the 110 upcoming gene therapy trials currently registered at ClinicaltTrials.gov under either recruiting or not yet recruiting status, viral vectors are still the dominant gene delivery systems in those trials; only one phase I/II trial by Sigilon Therapeutics applies a non-viral vector to deliver a genetically modified cell line (93). Meanwhile, there are many preclinical research and publications on various non-viral vectors in recent years, which undoubtedly demonstrated a quickly evolving field in gene delivery.
Despite many challenges, both the Moderna and Pfizer-BioNTech emergency use COVID-19 vaccines successfully used mRNA-based lipid nanoparticles and reached 94.5% and 95% effectiveness in preventing COVID-19, respectively (94, 95). Significantly, both were developed under such a short timeline. These recent successes have ushered in excitement and hope for discovering additional effective and safe non-viral vector-based gene therapy products in the future.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/tecartus-brexucabtagene-autoleucel. Accessed 11 Dec 2020.
- 2.Goswami R, Subramanian G, Silayeva L, Newkirk I, Doctor D, Chawla K, Chattopadhyay S, Chandra D, Chilukuri N, Betapudi V. Gene therapy leaves a vicious cycle. Front Oncol. 2019;9:297. doi: 10.3389/fonc.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shahryari A, Saghaeian Jazi M, Mohammadi S, Razavi Nikoo H, Nazari Z, Hosseini ES, Burtscher I, Mowla SJ, Lickert H. Development and clinical translation of approved gene therapy products for genetic disorders. Front Genet. 2019;10:868. doi: 10.3389/fgene.2019.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foldvari M, Chen DW, Nafissi N, Calderon D, Narsineni L, Rafiee A. Non-viral gene therapy: gains and challenges of non-invasive administration methods. J Control Release. 2016;240:165–190. doi: 10.1016/j.jconrel.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Ramamoorth M, Narvekar A. Non viral vectors in gene therapy-an overview. J Clin Diagn Res. 2015;9(1):GE01–GE06. doi: 10.7860/JCDR/2015/10443.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung YK, Kim SW. Recent advances in the development of gene delivery systems. Biomater Res. 2019;23(1):8. doi: 10.1186/s40824-019-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwarz B, Merkel OM. Functionalized PEI and its role in gene therapy. Mater Matters. 2017;12:2. [Google Scholar]
- 8.Gallops C, Ziebarth J, Wang Y. Polymers in therapeutic delivery. 2020. A polymer physics perspective on why PEI is an effective nonviral gene delivery vector; pp. 1–12. [Google Scholar]
- 9.Clark SR, Lee KY, Lee H, Khetan J, Kim HC, Choi YH, Shin K, Won YY. Determining the effects of PEI adsorption on the permeability of 1, 2-dipalmitoylphosphatidylcholine/bis (monoacylglycero)phosphate membranes under osmotic stress. Acta Biomater. 2018;65:317–326. doi: 10.1016/j.actbio.2017.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Dubruel P, Schacht E. Vinyl polymers as non-viral gene delivery carriers: current status and prospects. Macromol Biosci. 2006;6(10):789–810. doi: 10.1002/mabi.200600110. [DOI] [PubMed] [Google Scholar]
- 11.Christiaens B, Dubruel P, Grooten J, Goethals M, Vandekerckhove J, Schacht E, Rosseneu M. Enhancement of polymethacrylate-mediated gene delivery by penetratin. Eur J Pharm Sci. 2005;24(5):525–537. doi: 10.1016/j.ejps.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Barua S, Ramos J, Potta T, Taylor D, Huang HC, Montanez G, Rege K. Discovery of cationic polymers for non-viral gene delivery using combinatorial approaches. Comb Chem High Throughput Screen. 2011;14(10):908–924. doi: 10.2174/138620711797537076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhatt P, Khatri N, Kumar M, Baradia D, Misra A. Microbeads mediated oral plasmid DNA delivery using polymethacrylate vectors: an effectual groundwork for colorectal cancer. Drug Deliv. 2015;22(6):849–861. doi: 10.3109/10717544.2014.898348. [DOI] [PubMed] [Google Scholar]
- 14.Arya G, Kumari RM, Sharma N, Gupta N, Chandra R, Nimesh S. Polymeric nanocarriers for site specific gene therapy. In: Drug Targeting and Stimuli Sensitive Drug Delivery Systems: William Andrew Publishing; 2018. p. 689–714.
- 15.Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4(7):581–593. doi: 10.1038/nrd1775. [DOI] [PubMed] [Google Scholar]
- 16.Kandasamy G, Danilovtseva EN, Annenkov VV, Krishnan UM. Poly (1-vinylimidazole) polyplexes as novel therapeutic gene carriers for lung cancer therapy. Beilstein J Nanotechnol. 2020;11(1):354–369. doi: 10.3762/bjnano.11.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CK, Huang PK, Law WC, Chu CH, Chen NT, Lo LW. Biodegradable polymers for gene-delivery applications. Int J Nanomedicine. 2020;15:2131–2150. doi: 10.2147/IJN.S222419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.https://www.pharmtech.com/view/understanding-how-excipients-affect-drug-quality-1. Accessed 16 Mar 2021.
- 19.Holme H, Hagen A, Dornish M. Influence of chitosans on permeability of human intestinal epithelial (Caco-2) cell: the effect of molecular weight and degree of deacetylation and exposure time. Advan. Chitin Sci. 2000;4:259–65.
- 20.Cao Y, Tan YF, Wong YS, Liew MW, Venkatraman S. Recent advances in chitosan based carriers for gene delivery. Marine Drugs. 2019;17(6):381. doi: 10.3390/md17060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lara-Velazquez MA, Al-kharboosh RM, Norton ES, Ramirez-Loera C, Freeman WD, Guerrero-Cazares H, Forte AJ, Quiñones-Hinojosa A, Sarabia ER. Chitosan-based non-viral gene and drug delivery systems for brain cancer. Front Neurol. 2020;11:740. doi: 10.3389/fneur.2020.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chintakunta R, Buaron N, Kahn N, Moriah A, Lifshiz R, Goldbart R, Traitel T, Tyler B, Brem H, Kost J. Synthesis, characterization, and self-assembly with plasmid DNA of a quaternary ammonium derivative of pectic galactan and its fluorescent labeling for bioimaging applications. Carbohydr Polym. 2016;150:308–318. doi: 10.1016/j.carbpol.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Zhou D, Cutlar L, Gao Y, Wang W, O’Keeffe-Ahern J, McMahon S, Duarte B, Larcher F, Rodriguez BJ, Greiser U, Wang W. The transition from linear to highly branched poly (β-amino ester)s: branching matters for gene delivery. Sci Adv. 2016;2(6):e1600102. doi: 10.1126/sciadv.1600102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rui Y, Wilson DR, Choi J, Varanasi M, Sanders K, Karlsson J, Lim M, Green JJ. Carboxylated Branched Poly (β-amino ester) Nanoparticles enable robust cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci Adv. 2019;5(12):eaay3255. doi: 10.1126/sciadv.aay3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Gao Y, Zhou D, Zeng M, Alshehri F, Newland B, Lyu J, O’Keeffe-Ahern J, Greiser U, Guo T, Zhang F. Highly branched poly (β-amino ester) delivery of minicircle DNA for transfection of neurodegenerative disease related cells. Nat Commun. 2019;10(1):1–4. doi: 10.1038/s41467-019-11190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones CH, Chen CK, Jiang M, Fang L, Cheng C, Pfeifer BA. Synthesis of cationic polylactides with tunable charge densities as nanocarriers for effective gene delivery. Mol Pharm. 2013;10(3):1138–1145. doi: 10.1021/mp300666s. [DOI] [PubMed] [Google Scholar]
- 27.Rai R, Alwani S, Badea I. Polymeric nanoparticles in gene therapy: new avenues of design and optimization for delivery applications. Polymers. 2019;11(4):745. doi: 10.3390/polym11040745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roacho-Perez JA, Gallardo-Blanco HL, Sanchez-Dominguez M, Garcia-Casillas PE, Chapa-Gonzalez C, Sanchez-Dominguez CN. Nanoparticles for death-induced gene therapy in cancer. Mol Med Rep. 2018;17(1):1413–1420. doi: 10.3892/mmr.2017.8091. [DOI] [PubMed] [Google Scholar]
- 29.Bellucci MC, Volonterio A. Aminoglycosides: from antibiotics to building blocks for the synthesis and development of gene delivery vehicles. Antibiotics. 2020;9(8):504. doi: 10.3390/antibiotics9080504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng Y, Xiao H, Zhang J, Liang XJ, Huang Y. RNAi therapeutic and its innovative biotechnological evolution. Biotechnol Adv. 2019;37(5):801–825. doi: 10.1016/j.biotechadv.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 31.https://www.novartis.com/news/media-releases/novartis-receives-positive-chmp-opinion-leqvio-inclisiran-potential-first-class-sirna-treatment-high-cholesterol. Accessed 7 Dec 2020.
- 32.https://www.novartis.com/news/media-releases/novartis-receives-eu-approval-leqvio-inclisiran-first-class-sirna-lower-cholesterol-two-doses-year. Accessed 16 Dec 2020.
- 33.Ray KK, Wright RS, Kallend D, Koenig W, Leiter LA, Raal FJ, Bisch JA, Richardson T, Jaros M, Wijngaard PL, Kastelein JJ. Two phase 3 trials of inclisiran in patients with elevated LDL cholesterol. N Engl J Med. 2020;382(16):1507–1519. doi: 10.1056/NEJMoa1912387. [DOI] [PubMed] [Google Scholar]
- 34.Cullis PR, Hope MJ. Lipid nanoparticle systems for enabling gene therapies. Mol Ther. 2017;25(7):1467–1475. doi: 10.1016/j.ymthe.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Zhang L, Zhu W, Guo R, Sun H, Chen X, et al. Barriers and strategies of cationic liposomes for cancer gene therapy. Mol Ther Methods Clin Dev. 2020;18:751–64. [DOI] [PMC free article] [PubMed]
- 36.Jin W, Al-Dulaymi M, Badea I, Leary SC, Rehman J, El-Aneed A. Cellular uptake and distribution of gemini surfactant nanoparticles used as gene delivery agents. AAPS J. 2019;21(5):98. doi: 10.1208/s12248-019-0367-1. [DOI] [PubMed] [Google Scholar]
- 37.Sun S, Wang M, Knupp SA, Soto-Feliciano Y, Hu X, Kaplan DL, Langer R, Anderson DG, Xu Q. Combinatorial library of lipidoids for in vitro DNA delivery. Bioconjug Chem. 2012;23(1):135–140. doi: 10.1021/bc200572w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A. A Combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26(5):561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Molla MR, Chakraborty S, Munoz-Sagredo L, Drechsler M, Orian-Rousseau V, Levkin PA. Combinatorial synthesis of a lipidoid library by thiolactone chemistry: in vitro screening and in vivo validation for siRNA delivery. Bioconjug Chem. 2020;31(3):852–860. doi: 10.1021/acs.bioconjchem.0c00013. [DOI] [PubMed] [Google Scholar]
- 40.Buck J, Grossen P, Cullis PR, Huwyler J, Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13(4):3754–3782. doi: 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- 41.Cheng X, Lee RJ. The role of helper lipids in lipid nanoparticles (LNPs) designed for oligonucleotide delivery. Adv Drug Deliv Rev. 2016;99:129–137. doi: 10.1016/j.addr.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Sasayama Y, Hasegawa M, Taguchi E, Kubota K, Kuboyama T, Naoi T, Yabuuchi H, Shimai N, Asano M, Tokunaga A, Ishii T. In vivo activation of PEGylated long circulating lipid nanoparticle to achieve efficient siRNA delivery and target gene knock down in solid tumors. J Control Release. 2019;311:245–256. doi: 10.1016/j.jconrel.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 43.Tarvirdipour S, Huang X, Mihali V, Schoenenberger CA, Palivan CG. Peptide-based nanoassemblies in gene therapy and diagnosis: paving the way for clinical application. Molecules. 2020;25(15):3482. doi: 10.3390/molecules25153482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Altrichter Y, Seitz O. Simultaneous targeting of two master regulators of apoptosis with dual-action PNA–and DNA–peptide conjugates. Bioconjug Chem. 2020;31(8):1928–1937. doi: 10.1021/acs.bioconjchem.0c00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen J, Guan X, Hu Y, Tian H, Chen X. Polymeric Gene Delivery Systems. Cham: Springer; 2017. Peptide-based and polypeptide-based gene delivery systems; pp. 85–112. [DOI] [PubMed] [Google Scholar]
- 46.Zhang SK, Gong L, Zhang X, Yun ZM, Li SB, Gao HW, Dai CJ, Yuan JJ, Chen JM, Gong F, Tan YX. Antimicrobial peptide AR-23 derivatives with high endosomal disrupting ability enhance poly (l-lysine)-mediated gene transfer. J Gene Med. 2020;9:e3259. doi: 10.1002/jgm.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filipe LC, Machuqueiro M, Darbre T, Baptista AM. Exploring the structural properties of positively charged peptide dendrimers. J Phys Chem B. 2016;120(43):11323–11330. doi: 10.1021/acs.jpcb.6b09156. [DOI] [PubMed] [Google Scholar]
- 48.Gorzkiewicz M, Kopeć O, Janaszewska A, Konopka M, Pędziwiatr-Werbicka E, Tarasenko II, Bezrodnyi VV, Neelov IM, Klajnert-Maculewicz B. Poly (lysine) dendrimers form complexes with siRNA and provide its efficient uptake by myeloid cells: model studies for therapeutic nucleic acid delivery. Int J Mol Sci. 2020;21(9):3138. doi: 10.3390/ijms21093138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saher O, Lehto T, Gissberg O, Gupta D, Gustafsson O, Andaloussi SE, Darbre T, Lundin KE, Smith CI, Zain R. Sugar and polymer excipients enhance uptake and splice-switching activity of peptide-dendrimer/lipid/oligonucleotide formulations. Pharmaceutics. 2019;11(12):666. doi: 10.3390/pharmaceutics11120666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daralnakhla H, Saher O, Zamolo S, Bazaz S, P Bost J, Heitz M, Lundin KE, El Andaloussi S, Darbre T, Reymond JL, Zain R. Lipophilic peptide dendrimers for delivery of splice-switching oligonucleotides. Pharmaceutics. 2021;13(1):116. doi: 10.3390/pharmaceutics13010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ur Rahman A, Khan S, Khan M. Transport of trans-activator of transcription (TAT) peptide in tumour tissue model: evaluation of factors affecting the transport of TAT evidenced by flow cytometry. J Pharm Pharmacol. 2020;72(4):519–530. doi: 10.1111/jphp.13221. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Wagner E. Non-viral targeted nucleic acid delivery: apply sequences for optimization. Pharmaceutics. 2020;12(9):888. doi: 10.3390/pharmaceutics12090888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu Y, Chen X, Zhang H, Wang H, Chen H, Huang S, Xu Y, Zhang Y, Wu X, Chen J. Study on the cellular internalization mechanisms and in vivo anti-bone metastasis prostate cancer efficiency of the peptide T7-modified polypeptide nanoparticles. Drug Deliv. 2020;27(1):161–169. doi: 10.1080/10717544.2019.1709923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szybalska EH, Szybalski W. Genetics of human cell lines, IV. DNA-mediated heritable transformation of a biochemical trait. Proc Natl Acad Sci U S A. 1962;48(12):2026. doi: 10.1073/pnas.48.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Loh XJ, Lee TC, Dou Q, Deen GR. Utilising inorganic nanocarriers for gene delivery. Biomater Sci. 2016;4(1):70–86. doi: 10.1039/C5BM00277J. [DOI] [PubMed] [Google Scholar]
- 56.Vincent M, De Lázaro I, Kostarelos K. Graphene materials as 2D non-viral gene transfer vector platforms. Gene Ther. 2017;24(3):123–132. doi: 10.1038/gt.2016.79. [DOI] [PubMed] [Google Scholar]
- 57.Liu L, Li M, Xu M, Wang Z, Zeng Z, Li Y, Zhang Y, You R, Li CH, Guan YQ. Actively targeted gold nanoparticle composites improve behavior and cognitive impairment in Parkinson’s disease mice. Mater Sci Eng C. 2020;1:111028. doi: 10.1016/j.msec.2020.111028. [DOI] [PubMed] [Google Scholar]
- 58.Liang L, Zhang Y, Kong Z, Liu F, Shen JW, He Z, Wang H. DNA Fragment translocation through the lipid membrane assisted by carbon nanotube. Int J Pharm. 2020;574:118921. doi: 10.1016/j.ijpharm.2019.118921. [DOI] [PubMed] [Google Scholar]
- 59.Li Y, Yu H, Zhao L, Zhu Y, Bai R, Jin Z, Fu Z, Zhang X, Su J, Liu H, Shi X. Effects of carbon nanotube-mediated caspase3 gene silencing on cardiomyocyte apoptosis and cardiac function during early acute myocardial infarction. Nanoscale. 2020;12(42):21599–21604. doi: 10.1039/D0NR05032F. [DOI] [PubMed] [Google Scholar]
- 60.Ma XX, Xu JL, Jia YY, Zhang YX, Wang W, Li C, He W, Zhou SY, Zhang BL. Enhance transgene responses through improving cellular uptake and intracellular trafficking by bio-inspired non-viral vectors. J Nanobiotechnol. 2020;18(1):26. doi: 10.1186/s12951-020-0582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi E, Lee J, Kwon IC, Lim DK, Kim S. Cumulative directional calcium gluing between phosphate and silicate: a facile, robust and biocompatible strategy for siRNA delivery by amine-free non-positive vector. Biomaterials. 2019;209:126–137. doi: 10.1016/j.biomaterials.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Chen Z, Lv Z, Sun Y, Chi Z, Qing G. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J Mater Chem B. 2020;8(15):2951–2973. doi: 10.1039/C9TB02271F. [DOI] [PubMed] [Google Scholar]
- 63.Lu S, Bao X, Hai W, Shi S, Chen Y, Yu Q, Zhang M, Xu Y, Peng J. Multi-functional self-assembled nanoparticles for pVEGF-shRNA loading and anti-tumor targeted therapy. Int J Pharm. 2020;575:118898. doi: 10.1016/j.ijpharm.2019.118898. [DOI] [PubMed] [Google Scholar]
- 64.Yu Q, Zhang M, Chen Y, Chen X, Shi S, Sun K, Ye R, Zheng Y, Chen Y, Xu Y, Peng J. Self-assembled nanoparticles prepared from low-molecular-weight PEI and low-generation PAMAM for EGFRvIII-chimeric antigen receptor gene loading and T-cell transient modification. Int J Nanomedicine. 2020;15:483–495. doi: 10.2147/IJN.S229858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steinman NY, Campos LM, Feng Y, Domb AJ, Hosseinkhani H. Cyclopropenium nanoparticles and gene transfection in cells. Pharmaceutics. 2020;12(8):768. doi: 10.3390/pharmaceutics12080768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Proulx J, Joshi C, Vijayaraghavalu S, Saraswathy M, Labhasetwar V, Ghorpade A, Borgmann K. Arginine-modified polymers facilitate poly (lactide-co-glycolide)-based nanoparticle gene delivery to primary human astrocytes. Int J Nanomedicine. 2020;15:3639–3647. doi: 10.2147/IJN.S250865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lebrón JA, Ostos FJ, López-López M, Moyá ML, Sales C, García E, García-Calderón CB, García-Calderón M, Peña-Gómez MJ, Rosado IV, Balestra FR. Metallo-liposomes of ruthenium used as promising vectors of genetic material. Pharmaceutics. 2020;12(5):482. doi: 10.3390/pharmaceutics12050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y, Zhao T, Du Y, Cao Y, Xuan Y, Chen H, Zhi D, Guo S, Zhong F, Zhang S. Interaction kinetics of peptide lipids-mediated gene delivery. J Nanobiotechnol. 2020;18(1):1–4. doi: 10.1186/s12951-020-00707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buck J, Mueller D, Mettal U, Ackermann M, Grisch-Chan HM, Thöny B, Zumbuehl A, Huwyler J, Witzigmann D. Improvement of DNA vector delivery of DOTAP lipoplexes by short-chain aminolipids. ACS Omega. 2020;5(38):24724–24732. doi: 10.1021/acsomega.0c03303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghodke SB, Parkar JN, Deshpande AR, Dandekar PP, Jain RD. Structure–activity relationship of polyester-based cationic polyrotaxane vector-mediated in vitro siRNA delivery: effect on gene silencing efficiency. ACS Appl Bio Mater. 2020;3(11):7500–7514. doi: 10.1021/acsabm.0c00717. [DOI] [PubMed] [Google Scholar]
- 71.Routkevitch D, Sudhakar D, Conge MJ, Varanasi M, Tseng S, Wilson D, Green JJ. Efficiency of cytosolic delivery with poly (beta-amino ester) nanoparticles is dependent on the effective pKa of the polymer. ACS Biomater Sci Eng. 2020;6:3411–3421. doi: 10.1021/acsbiomaterials.0c00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Neves AR, Sousa A, Faria R, Albuquerque T, Queiroz JA, Costa D. Cancer gene therapy mediated by RALA/plasmid DNA vectors: nitrogen to phosphate groups ratio (N/P) as a tool for tunable transfection efficiency and apoptosis. Colloids Surf B: Biointerfaces. 2020;185:110610. doi: 10.1016/j.colsurfb.2019.110610. [DOI] [PubMed] [Google Scholar]
- 73.Sainz-Ramos M, Villate-Beitia I, Gallego I, Qtaish NA, Lopez-Mendez TB, Eritja R, Grijalvo S, Puras G, Pedraz JL. Non-viral mediated gene therapy in human cystic fibrosis airway epithelial cells recovers chloride channel functionality. Int J Pharm. 2020;588:119757. doi: 10.1016/j.ijpharm.2020.119757. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Z, Liu X, Zhu D, Wang Y, Zhang Z, Zhou X, Qiu N, Chen X, Shen Y. Nonviral cancer gene therapy: delivery cascade and vector nanoproperty integration. Adv Drug Deliv Rev. 2017;115:115–154. doi: 10.1016/j.addr.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 75.Xia Q, Huang J, Feng Q, Chen X, Liu X, Li X, Zhang T, Xiao S, Li H, Zhong Z, Xiao K. Size-and cell type-dependent cellular uptake, cytotoxicity and in vivo distribution of gold nanoparticles. Int J Nanomedicine. 2019;14:6957–6970. doi: 10.2147/IJN.S214008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haddick L, Zhang W, Reinhard S, Möller K, Engelke H, Wagner E, Bein T. Particle-size-dependent delivery of antitumoral miRNA using targeted mesoporous silica nanoparticles. Pharmaceutics. 2020;12(6):505. doi: 10.3390/pharmaceutics12060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chivu A, Chindera K, Mendes G, An A, Davidson B, Good L, et al. Cellular gene delivery via poly (hexamethylene biguanide)/pDNA self-assembled nanoparticles. Eur J Pharm Biopharm. 2021;158:62–71. [DOI] [PubMed]
- 78.Chroni A, Forys A, Trzebicka B, Alemayehu A, Tyrpekl V, Pispas S. Poly [oligo (ethylene glycol) methacrylate]-b-poly [(vinyl benzyl trimethylammonium chloride)] based multifunctional hybrid nanostructures encapsulating magnetic nanoparticles and DNA. Polymers. 2020;12(6):1283. doi: 10.3390/polym12061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keil TW, Feldmann DP, Costabile G, Zhong Q, da Rocha S, Merkel OM. Characterization of spray dried powders with nucleic acid-containing PEI nanoparticles. Eur J Pharm Biopharm. 2019;143:61–69. doi: 10.1016/j.ejpb.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wickline SA, Pan H, Pham CT, Yan H. U.S. 16/870,035. 2020. Peptide-polynucleotide-hyaluronic acid nanoparticles and methods for polynucleotide transfection. [Google Scholar]
- 81.Pezzoli D, Giupponi E, Mantovani D, Candiani G. Size matters for in vitro gene delivery: investigating the relationships among complexation protocol, transfection medium, size and sedimentation. Sci Rep. 2017;7:44134. doi: 10.1038/srep44134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15(8):541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 83.McCoy M. Lipids, The unsung COVID-19 vaccine component, get investment. In: Chemical & Engineering News. 2021. https://cen.acs.org/business/outsourcing/Lipids-unsung-COVID-19-vaccine/99/web/2021/02. Accessed 19 Mar 2021.
- 84.Editorial Let’s talk about lipid nanoparticles. Nat Rev Mater. 2021;6:99. doi: 10.1038/s41578-021-00281-4. [DOI] [Google Scholar]
- 85.https://investors.beamtx.com/news-releases/news-release-details/beam-therapeutics-announces-acquisition-guide-therapeutics. Accessed 19 Mar 2021.
- 86.Jones CH, Chen CK, Ravikrishnan A, Rane S, Pfeifer BA. Overcoming nonviral gene delivery barriers: perspective and future. Mol Pharm. 2013;10(11):4082–4098. doi: 10.1021/mp400467x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bueter CL, Lee CK, Wang JP, Ostroff GR, Specht CA, Levitz SM. Spectrum and mechanisms of inflammasome activation by chitosan. J Immunol. 2014;192(12):5943–5951. doi: 10.4049/jimmunol.1301695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pandey RK, Prajapati VK. Molecular and immunological toxic effects of nanoparticles. Int J Biol Macromol. 2018;107:1278–1293. doi: 10.1016/j.ijbiomac.2017.09.110. [DOI] [PubMed] [Google Scholar]
- 89.Crommelin DJ, Mastrobattista E, Hawe A, Hoogendoorn KH, Jiskoot W. Shifting paradigms revisited: biotechnology and the pharmaceutical sciences. J Pharm Sci. 2020;109(1):30–43. doi: 10.1016/j.xphs.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 90.https://www.contractpharma.com/csd/profile/centre-for-commercialization-of-regenerative-medic/view_quality-by-design-approach-to-manufacturing-cell-and-gene-therapies/. Accessed 12 Mar 2021.
- 91.Gavin DK. Advanced topics: successful development of quality cell and gene therapy products. https://www.fda.gov/media/80404/download. Accessed 12 Mar 2021.
- 92.Chen S, Zaifman J, Kulkarni JA, Zhigaltsev IV, Tam YK, Ciufolini MA, Tam YY, Cullis PR. Dexamethasone prodrugs as potent suppressors of the immunostimulatory effects of lipid nanoparticle formulations of nucleic acids. J Control Release. 2018;286:46–54. doi: 10.1016/j.jconrel.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 93.https://clinicaltrials.gov/ct2/show/NCT04541628. Accessed 19 Mar 2021.
- 94.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vvaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.https://investors.modernatx.com/news-releases/news-release-details/moderna-announces-primary-efficacy-analysis-phase-3-cove-study. Accessed 11 Dec 2020.