Abstract
Purpose
Preterm infants are at increased risk of systemic hypertension compared to term infants. Bronchopulmonary dysplasia (BPD) has been shown to be associated with hypertension in preterm infants albeit with no causation reported. BPD is characterized by abnormal pulmonary function tests (PFTs), specifically elevated passive respiratory resistance (Rrs), decreased passive respiratory compliance (Crs) and decreased functional residual capacity (FRC). There have been no studies comparing PFTs in very low birth weight (VLBW) infants with and without hypertension. We hypothesized that stable VLBW infants with hypertension will have altered PFTs.
Patients and Methods
Retrospective cohort study of infants < 1500 grams at birth (VLBW) who had PFTs performed near 34–36 weeks of corrected gestational age (CGA). We excluded infants with congenital anomalies, known hypertensive disorders or those at risk of medication-induced hypertension. Data obtained included PFT parameters (Rrs, Crs, FRC) and mean systolic blood pressure (SBP).
Results
59 VLBW infants were identified for analysis, 14 with and 45 without hypertension. Hypertensive and normotensive patients were similar in terms of mean gestational age (26.6 vs 27.4 weeks), mean CGA at PFTs (36.1 vs 34.6 weeks) and proportion of BPD (36% vs 36%). The Rrs was significantly higher in hypertensive versus normotensive patients [median Rrs of 0.080 (0.069, 0.090) versus 0.066 (0.054, 0.083) cmH2O/mL/sec; p = 0.04]. There was no difference in systolic blood pressure in the infants with and without BPD.
Conclusion
In this cohort of contemporary VLBW infants, those with hypertension had increased Rrs. This finding warrants a prospective study with a larger sample size and long-term follow-up.
Keywords: hypertension, bronchopulmonary dysplasia, pulmonary function test, neonate
Plain Language Summary
Prior work has linked bronchopulmonary dysplasia (a chronic lung disease that is relatively common in the most premature of neonates) to systemic hypertension, however this link has not been fully elucidated. To add to the limited fund of knowledge surrounding this association, our group compared objective pulmonary function test parameters of premature neonates with and those without systemic hypertension. The findings of our work demonstrate that there is commonly an increase in the airway resistance in those with systemic hypertension compared to those without. We speculate that since adequate vascular development within the lungs and kidneys promotes growth, that there could be a common developmental issue within the lung and kidney causing the altered lung mechanics and elevated blood pressure but this will require further study.
Introduction
Systemic hypertension in the neonatal population occurs rarely, with incidence estimates ranging from 0.2% to 3.0%,1 but has important complications including congestive heart failure, seizures, hypertensive retinopathy, and failure to thrive.2 The causes of hypertension in neonates are numerous, including renovascular disease, renal parenchymal disease, hyperthyroidism, congenital adrenal hyperplasia, hyperaldosteronism and coarctation of the aorta.3–5 In addition, there is a population of neonates (both term and preterm) with systemic hypertension without diagnostic causality.6–8 The preterm neonatal population appears to be at an increased risk compared with term infants.7 The factors that are attributed to this increased risk in preterm neonates include, but are not limited to, intraventricular hemorrhage (IVH), patent ductus arteriosus (PDA) and bronchopulmonary dysplasia (BPD).7–10
Bronchopulmonary dysplasia is a chronic lung condition seen in preterm infants that results in significant morbidity and mortality, including neurodevelopmental disabilities.11,12 It is most commonly defined as the requirement for oxygen supplementation at 36 weeks of corrected gestational age (CGA).13 The diagnostic dilemma with BPD is that its diagnosis is largely based on therapeutic management and the status of an oxygen challenge test at 36 weeks of CGA, which may not accurately reflect the underlying pathophysiology. Previous research has demonstrated that patients with BPD have altered pulmonary function tests (PFTs), allowing for an objective physiologic measure of BPD. In particular, BPD is characterized by an increase in passive respiratory resistance (Rrs), a decrease in passive respiratory compliance (Crs) and a decrease in functional residual capacity (FRC).13–15 In contrast, preterm infants without clinical lung disease have been shown to have FRC measurements corrected for body weight that are comparable to FRC measurements in term healthy infants.16
Literature from two decades ago suggests an association between neonatal systemic hypertension and BPD,17–19 however this potential association has not been well defined and the mechanism is not understood. Previously, the mechanism of the association between systemic hypertension and BPD was postulated to be related to increased systemic vascular resistance secondary to hypoxia or hypercarbia.17 There is evidence to suggest that abnormal antenatal/perinatal vascular development within the lung vasculature can impair alveolar lung growth.20–22 The potential relationship between systemic hypertension and BPD with altered PFTs may stem from this impaired angiogenesis or other developmental dysmorphology. Quantifying the potential relationship between systemic hypertension and BPD with its altered PFTs may improve our understanding of the pathophysiology leading to advancements in preventative strategies and therapeutic management of these disorders.
There have been no previous studies evaluating the potential association between blood pressure measurements and pulmonary function tests in very low birth weight (VLBW) infants. The objectives of this study were (1) to evaluate the relationship between neonatal blood pressure measurements and pulmonary function measurements at 34 – 36 weeks in patients < 1500 grams (VLBW) and (2) to investigate the potential association between BPD and systemic hypertension in a contemporary population of VLBW infants. We hypothesize that altered lung function, specifically elevated passive respiratory resistance and a clinical diagnosis of BPD, will be correlated with elevated systemic blood pressure measured at 34 – 36 weeks of CGA.
Material and Methods
We performed a retrospective cohort study of infants less than 1500 grams at birth born between January 1, 2014 and August 31, 2016 who had PFTs performed at 34 – 36 weeks CGA. This testing is routinely done in our NICU in VLBW infants at this CGA as part of our clinical respiratory consensus guidelines in infants at risk for BPD. If an infant had multiple PFTs performed during the window of 34 – 36 weeks of CGA, the PFT performed closest to discharge was utilized as the index PFT. We excluded infants who had a major anomaly, a known hypertensive disorder, were intubated at the time of the PFT (since the presence of an endotracheal tube is known to falsely elevate the measurement of passive respiratory resistance), were receiving a short course of a diuretic defined as less than five consecutive days of medication therapy (as this can lead to iatrogenic hypotension), were receiving vasoactive medications or had systemic steroid administration within two weeks of the BP measurements and PFTs (as these often lead to iatrogenic hypertension).
The infants were all cared for in the level IV neonatal intensive care unit in Doernbecher Children’s Hospital at Oregon Health and Science University (Portland, OR).
Sociodemographic and clinical information was extracted by two study personnel from two different sources: our institutional electronic medical record and a separate clinical database of pulmonary function tests. Data obtained included gestational age at birth, singleton or multiple gestation, sex, maternal race, maternal age, maternal tobacco use, mode of delivery, birthweight, size for gestation, prenatal steroid exposure, diagnosis and severity of BPD, number of ventilator days, receipt of antihypertensive medications, symptomatic PDA, history of umbilical artery catheterization, severe IVH, extracorporeal membrane oxygenation (ECMO), diagnosis of neonatal abstinence syndrome (NAS) and corrected gestational age at discharge. For each PFT, the patient’s day of life, CGA and current respiratory support (room air, nasal cannula, CPAP or invasive ventilation) was documented. Each patient had only one PFT measurement during the window near 34 – 36 weeks of corrected gestational age to be used as the index PFT.
Pulmonary Function Measurements
Pulmonary function tests were performed with a computerized infant pulmonary function cart (SensorMedics 2600; SensorMedics Inc., Yorba Linda, CA). The Crs, Rrs and FRC measurements were performed utilizing previously described methodology.23 Briefly, however, Rrs and Crs were measured with the single-breath occlusion technique while the FRC was measured with the nitrogen wash out technique. Acceptable readings were approved and recorded based on prevailing criteria.24,25
Blood Pressure Measurements
Blood pressure measurements were monitored by a peripheral oscillometric device with appropriately sized cuffs per clinical standards with infant lying in prone or supine position, prior to a feed, ideally in a quiet or asleep state per standards outlined in Nwanko et al.26 Blood pressure measurements are known to be influenced by clinical factors such as whether the patient is calm or upset. To account for this and the uncertainties associated with the retrospective nature of this study, we averaged the blood pressure measurements taken within two days of the PFT. Specifically, we averaged systolic and diastolic blood pressure measurements taken during the 5-day period surrounding the date of the PFT.
Systemic hypertension was defined as a mean systolic blood pressure (SBP) measurement greater than the 95th percentile for corrected gestational age or by receipt of an antihypertensive medication. Normative values of systolic and diastolic blood pressure were based on the work of Dionne et al.1,27 Ventilator days were counted from birth to hospital discharge and included the maximum respiratory support during the 24-hour period, excluding brief ventilator support (less than 60 minutes) for the sole purpose of surfactant administration. Severe intraventricular hemorrhage was defined as Grade III or IV in the Papile classification.28 A symptomatic PDA was defined as receipt of medical or surgical treatment for a PDA prior to discharge. Sepsis was defined by blood culture positive infection or blood culture negative infection as indicated by clinical team based on chart review. BPD was defined as need for supplemental oxygen and/or positive pressure ventilation including continuous positive airway pressure (CPAP) at 36 weeks of CGA. Severe BPD was defined as a need for supplemental oxygen ≥ 30% and/or positive pressure ventilation including CPAP at 36 weeks of corrected gestational age.13
Analysis
Maternal and infant demographic and clinical characteristics were compared between infants with hypertension (as defined by the criteria above) to those without hypertension. For binary and categorical variables, frequencies and percentages were reported for each group defined by hypertensive status, and Fisher’s exact test was used to test whether the distribution was the same between groups. For continuous variables, means and standard deviations were reported for each group, and a t-test with unequal variances was used to test whether the means were equal between groups. For the number of ventilator days, the median and interquartile range were reported for each group, and the Wilcoxon rank-sum test was used to test whether the distribution was equal between groups.
To investigate the relationship between blood pressure and PFT parameters, quartiles of each PFT parameter were compared between hypertensive and normotensive infants. Medians and interquartile ranges were reported, and the Wilcoxon rank-sum was used to compare the distribution of each PFT parameter between the groups. This nonparametric approach was taken since the distribution of the PFT parameters were skewed and the groups were small. To examine the relationship between systolic blood pressure and PFT parameters (specifically Rrs) at a more granular level, scatterplots of each of the PFT parameters and systolic blood pressure were created and Pearson’s correlation coefficients were calculated. Finally, to investigate the relationship between a BPD diagnosis and systemic hypertension, the proportion of infants with hypertension was compared between those with and without a BPD diagnosis. Additionally, blood pressure measurements were compared between those with and without a diagnosis of BPD.
All statistical analyses were performed with Stata Statistical Software Release 15 (StataCorp LLC, 2017, College Station, TX). Since this was a pilot study to examine possible associations for future investigation, a convenience sample was used; this study was not powered to detect a given effect.
Ethical Considerations
The protocol was approved by the Oregon Health and Science University Institutional Review Board and need for consent waived given there was no more than minimal risk to the privacy of the subjects with the study. Standard institutional practices were followed to maintain confidentiality and security of the data collected. All procedures followed were in accordance with the Declaration of Helsinki.
Results
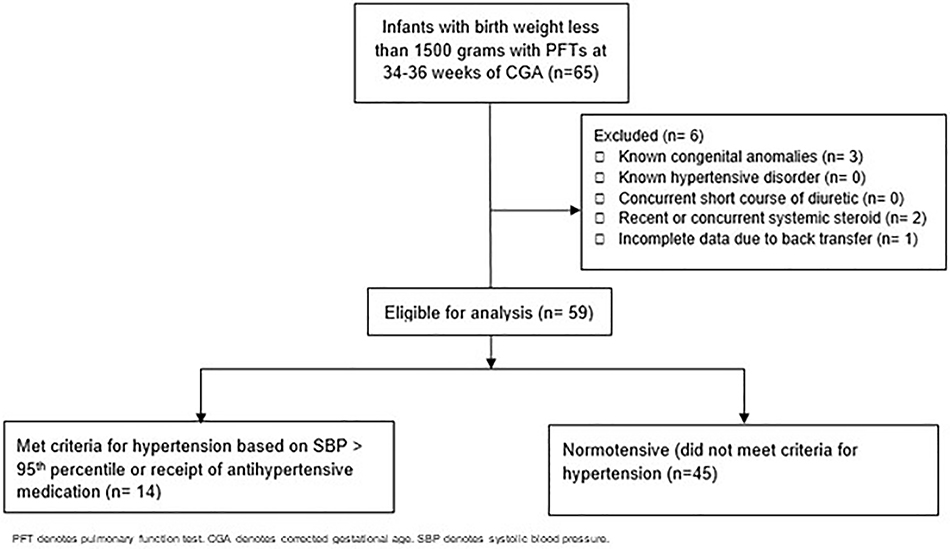
We identified 65 preterm infants born between January 1, 2014 and August 31, 2016 weighing less than 1500 grams at birth who were not intubated and had PFTs performed at 34 – 36 weeks of CGA during their hospital course. Of those, three patients were excluded due to known congenital anomalies, none were excluded due to having a known hypertensive disorder, none were excluded for concurrent receipt of a short course of diuretic, two were excluded for recent or concurrent systemic steroid administration and one was excluded due to incomplete data due to hospital back transfer. Congenital anomalies were the exclusion criteria of three patients, one patient had ambiguous genitalia, a cleft palate, facial dysmorphism without renal anomalies, one patient was diagnosed with pulmonary interstitial glycogenosis and one patient was diagnosed clinically with VACTERL (which stands for vertebral defects, anal atresia, cardiac defects, trachea-esophageal fistula, renal anomalies and limb abnormalities) which included left renal cystic dysplasia. Concurrent steroid administration was the exclusion criteria for two patients, one patient required dexamethasone to facilitate weaning of respiratory support and the other patient required hydrocortisone for adrenal insufficiency.
The remaining 59 infants were included in the analysis. None of the patients had more than one PFT performed in the specified period. A mean of 5.4 ± 2.0 blood pressure measurements for each neonate were obtained in our cohort during the 5-day period. Fourteen met our criteria for hypertension (based on a SBP > 95th percentile or receipt of an antihypertensive medication) and 45 had normal blood pressure status (Figure I).
Figure I.
Cohort selection process. A total of 65 very low birth weight patients were identified as having PFTs performed during our study period. Six patients were excluded because they did meet the criteria for exclusion. Allocation to the two groups were based on blood pressure status according to methods described. In the hypertensive group, there were 14 patients included in the analysis. In the normotensive group, there were 45 patients included in the analysis.
When comparing infants with hypertension to those without, there were no significant differences in mean gestational age at birth (26.6 vs 27.4 weeks, p = 0.19), sex (64% vs 60% male, p = 1.0) or history of placement of an umbilical artery catheter, UAC (86% vs 67%, p = 0.31; Table I). No patients in our cohort were managed on extracorporeal membrane oxygenation (ECMO) or diagnosed with neonatal abstinence syndrome (NAS). The mean CGA at the time of PFTs was similar between the two groups, 36.1 weeks in those with hypertension versus 34.6 weeks in those without (p = 0.08). There were no differences in the rates of sepsis, BPD, IVH or small for gestational age (SGA). The presence of a symptomatic PDA (either medically or surgically treated) was more common in those with defined systemic hypertension compared to those without (43% vs 13%, p = 0.03). There were 12 patients treated for a symptomatic PDA, seven patients received medical management (one with ibuprofen, six with indomethacin), four patients were surgically treated with ligation and one patient was treated with indomethacin followed by surgical ligation. The median number of days between the final dose of NSAID therapy and subsequent PFT measurement was 46 days with the shortest time interval being 27 days.
Table I.
Demographic characteristics of full cohort and separated by hypertensive status
| All patients (n=59) | Hypertensive patients (n=14) | Normotensive patients (n=45) | p value† | |
|---|---|---|---|---|
| Maternal Characteristics | ||||
| Age (years), mean ± SD | 29.6 ± 7.0 | 30.7 ± 7.1 | 29.2 ± 7.0 | 0.49 |
| Smoking history, % (n) | 18% (10) | 7% (1) | 22% (9) | 0.42 |
| Antenatal steroids, % (n) | 83% (49) | 93% (13) | 80% (36) | 0.43 |
| Multiple gestation, % (n) | 24% (14) | 21% (3) | 24% (11) | 1.00 |
| Vaginal delivery, % (n) | 25% (15) | 21% (3) | 27% (12) | 1.00 |
| Race, % (n) | 1.00 | |||
| Asian | 3% (2) | 0% (0) | 4% (2) | |
| Black or African American | 2% (1) | 0% (0) | 2% (1) | |
| White or Caucasian | 76% (45) | 86% (12) | 73% (33) | |
| Multiple | 15% (9) | 14% (2) | 16% (7) | |
| Other | 2% (1) | 0% (0) | 2% (1) | |
| Unknown | 2% (1) | 0% (0) | 2% (1) | |
| Infant Characteristics | ||||
| Male sex, % (n) | 61% (36) | 64% (9) | 60% (27) | 1.00 |
| Gestational age at birth (weeks), mean ± SD | 27.2 ± 2.0 | 26.6 ± 2.0 | 27.4 ± 2.0 | 0.19 |
| CGA at discharge, mean ± SD | 40.3 ± 5.0 | 41.1 ± 5.6 | 40.1 ± 4.8 | 0.55 |
| Birth weight (grams), mean ± SD | 968 ± 235 | 853 ± 223 | 1004 ± 230 | 0.04 |
| Small for gestation, % (n) | 10% (6) | 14% (2) | 9% (4) | 0.62 |
| History of UAC, % (n) | 71% (42) | 86% (12) | 67% (30) | 0.31 |
| Number of ventilator days, median (IQR*) | 2 (0,22) | 7 (0,22) | 2 (1,20) | 0.45 |
| CGA at time of PFTs (weeks), mean ± SD | 34.9 ± 3.3 | 36.1 ± 2.5 | 34.6 ± 3.4 | 0.08 |
| Systolic BP at time of PFTs, mean ± SD | 79.0 ± 8.0 | 85.7 ± 10.2 | 76.9 ± 5.9 | 0.01 |
| Comorbidities, % (n) | ||||
| History of Symptomatic PDA | 20% (12) | 43% (6) | 13% (6) | 0.03 |
| Sepsis | 30% (18) | 35% (5) | 29% (13) | 0.74 |
| BPD | 36% (21) | 36% (5) | 36% (16) | 1.00 |
| Severe IVH | 17% (10) | 14% (2) | 18% (8) | 1.00 |
p-value from t-test with unequal variances, Fisher’s exact test, or Wilcoxon rank-sum test, as appropriate
IQR: interquartile range
CGA, corrected gestational age; UAC, umbilical artery catheter; PFT, pulmonary function test; BP, blood pressure; PDA, patent ductus arteriosus; BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage
The hypertensive group had greater passive respiratory resistance than the normotensive group did (median 0.080 vs 0.066 cmH2O/mL/sec, Wilcoxon rank-sum p = 0.04; Table II). As expected, the mean SBP was significantly greater in the hypertensive group compared to the normotensive group (85.7 vs 76.9 mmHg, p = 0.01). Of the fourteen patients in the hypertensive group, six were treated with antihypertensive medications. The remaining eight patients were classified as hypertensive according to the SBP at the time of the PFT but never received medical treatment for their hypertension. Those treated with medications received isradipine (three patients received this medication on an as needed basis while two patients were treated with scheduled doses), spironolactone (one patient received this scheduled medication), chlorothiazide (one patient received this scheduled medication) and hydralazine (one patient received this medication on an as needed basis). For the eight patients who did not receive medical treatment for their hypertension, two patients received a renal ultrasound following up prenatally diagnosed pelviectasis, two patients received a renal ultrasound with VCUG following recurrent urinary tract infections, one patient received laboratory evaluations with plasma renin activity and serum aldosterone due to electrolyte abnormalities, the remaining three patients did not have any diagnostic evaluations for hypertension or renal pathology.
Table II.
Pulmonary function test parameters separated by hypertensive status
| PFT Parameter | Hypertensive patients (n=14) | Normotensive patients (n=45) | p value† |
|---|---|---|---|
| Rrs (cmH2O/mL/sec), median (IQR*) | 0.080 (0.069, 0.090) | 0.066 (0.054, 0.083) | 0.04 |
| Crs (mL/cmH2O/kg), median (IQR) | 0.95 (084, 1.06) | 1.00 (0.82, 1.18) | 0.61 |
| FRC (mL/kg), median (IQR) | 24.2 (23.1, 25.4) | 25.2 (20.0, 27.8) | 0.93 |
p-value from Wilcoxon rank-sum test
IQR: interquartile range
PFT, pulmonary function test; Rrs, passive respiratory resistance; Crs, passive respiratory compliance; FRC, functional residual capacity
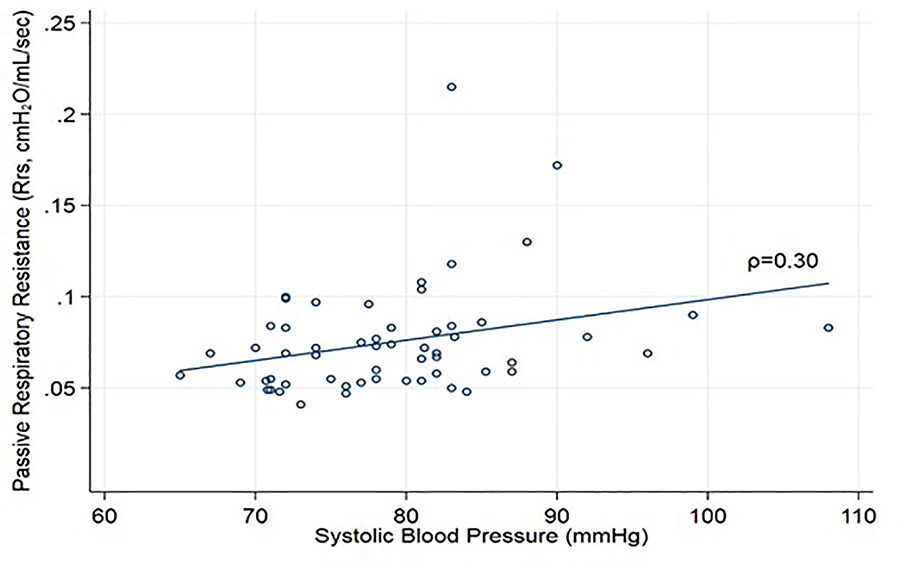
Among the entire group of VLBW infants, there was a weak positive association between Rrs and SBP (Pearson’s correlation coefficient: 0.30, p = 0.03, Figure II). There was no difference in mean systolic blood pressure or rate of systemic hypertension between the groups according to the diagnosis of BPD (Table III). In a secondary analysis of severe BPD (n=16), the proportion of infants with hypertension was similar between those with severe BPD and those with mild, moderate, or no BPD (25% vs 23%, p = 1.00).
Fig II.
Systolic blood pressure (mmHg) versus passive respiratory resistance (Rrs, cmH2O/mL/sec) of a cohort (n=59) of very low birth weight infants who had pulmonary function tests performed between 34 – 36 weeks of corrected gestational age. Pearson’s correlation coefficient is 0.30 at a significance value of p-value of 0.03.
Table III.
Blood pressure data of patients separated by BPD diagnosis
| BPD Diagnosis (n= 21) | No BPD Diagnosis (n=38) | p value† | |
|---|---|---|---|
| Blood Pressure data | |||
| CGA at time of PFT (weeks), mean ± SD | 36.3 ± 3.4 | 34.1 ± 3.0 | 0.02 |
| Systolic BP at time of PFT, mean ± SD | 79.5 ± 5.7 | 78.8 ± 9.1 | 0.72 |
| Hypertension diagnosis, % (n) | 24% (5) | 24% (9) | 1.00 |
p-value from t-test with unequal variances or Fisher’s exact test, as appropriate
BPD, bronchopulmonary dysplasia; CGA, corrected gestational age; BP, blood pressure
Discussion
The purpose of this study was to evaluate the potential relationship between pulmonary function measurements and systemic blood pressure in stable premature infants with birth weights less than 1500 grams and to investigate the potential association between BPD and systemic hypertension in these patients. Results from this cohort of VLBW infants demonstrated a significant increase in passive respiratory resistance in hypertensive patients compared to normotensive patients on PFTs prior to discharge at 34 – 36 weeks of CGA. There were no differences in the measurements of passive respiratory compliance or functional residual capacity. There were no differences in blood pressure according to BPD status in this cohort of infants.
Literature has highlighted numerous postnatal risk factors for neonatal hypertension in premature infants including, but not limited to, IVH, SGA, BPD and placement of a UAC.7–10 When comparing the infants with hypertension to those who were normotensive in our cohort, there were no differences in the incidence of these risk factors. The similarities between the groups is advantageous as it allows us to evaluate the objective PFT parameters without other potential confounders. There were two important differences between the groups. First, the hypertensive cohort had a lower birth weight compared to the normotensive group. Although this finding is statistically different, the clinical implications are potentially less important given the small weight difference of 151 grams and the similar incidence of SGA in the two groups. The second difference was a higher incidence of symptomatic PDA in the hypertensive group. It is well documented in the literature that neonates with a PDA require prolonged ventilation.29–31 Correspondingly, in our cohort, patients with symptomatic PDA required prolonged mechanical ventilation compared to those without a PDA. Interestingly, however, there was no difference in duration of mechanical ventilation in those with a PDA when comparing the hypertensive group to the normotensive group. With this variation, we can only speculate that a longer time receiving mechanical ventilation could contribute to airway remodeling with subsequent increased passive respiratory resistance in our cohort. Although the presence of a PDA is linked to systemic hypertension, its linkage to altered pulmonary function tests remains unclear. Chen et al recently published an observational study evaluating FRC in patients with and those without PDA at the time of discharge and did not identify any differences in pulmonary function.32 McCurnin reported improved compliance following PDA closure in baboons.33 This heterogeneity in the literature evaluating the relationship between pulmonary function and PDA makes it difficult to understand the importance of higher rates of symptomatic PDA in our hypertensive group in terms of its impact on pulmonary function test parameters. The prolonged distance in time between treatment of the ductus and PFT, several weeks between, renders this relationship more historical in nature rather than likely of clinical influence.
The majority of studies demonstrating an association between BPD and systemic hypertension were published when the pattern of lung injury was heterogeneous due to damage of the alveolar structure (which has been termed “classic” BPD) from mechanical ventilation and lack of surfactant.34 With the scientific advancements that have improved our respiratory management of premature neonates (antenatal steroids for lung maturation, CPAP, exogenous surfactant) there has been a shift to a new pattern of lung injury (“new” BPD) which is characterized by impaired lung development from extreme prematurity.35 The previous reports from decades ago described patients with “classic” BPD being at risk for systemic hypertension of unclear mechanistic etiology. In these studies, the diagnosis of BPD-associated hypertension was made after other etiologies had been excluded. Studies have worked to characterize the “old” BPD-associated hypertension. Alagappan et al found that systemic hypertension was more common in BPD patients with prolonged hospital stays, prolonged use of oxygen therapy and a longer use of aminophylline therapy.18 Anderson et al found that hypertensive infants had greater respiratory needs including increased use of bronchodilators, diuretics and home oxygen along with increased mortality.19 It is possible that the pathophysiologic mechanism for hypertension would be different in the “new” BPD. Based on the work by Alagappan and Anderson, it could be speculated that severe lung disease could be causative towards development of systemic hypertension. However, when evaluating “new” BPD, recent work has demonstrated the similarities in the clinical characteristics of hypertensive neonates with and those without this “new” BPD.10 This dichotomy could suggest that the etiology of hypertension is unrelated to the severity of lung disease but rather potentially related to impaired renal and vascular development from extreme prematurity. Overall, however, given the unclear etiology and rarity of neonatal hypertension secondary to BPD, it is difficult to make conclusions to the fidelity of the relationship between the two diagnoses. In our current cohort, there was no difference in the diagnosis of hypertension in patients with BPD compared to those without. The sample size may have contributed to this lack of association as well as our strict exclusion criteria that likely excluded those with the more severe lung pathology and could be contributing to the lack of difference in hypertension rates.
Another consideration for understanding hypertension in our cohort as well as in other neonatal ICUs would be evaluating the exposure to environmental phthalates. Phthalates are common chemicals used as plasticizers in medical equipment (including some intravenous tubing and respiratory equipment tubing). There has been associations of phthalate exposure and increased blood pressure in children, including mention of the association in the recent clinical practice guideline released by the American Academy of Pediatrics about screening and management of high blood pressure.36–39
The strengths of our study are several. Our laboratory is experienced in performing reproducible PFTs and correlating them with clinical outcomes.40,41 This study is the first to evaluate a comparison between an objective measure of PFTs in relation to the elevated systemic blood pressure in VLBW infants. PFTs have provided important physiologic data in the field of neonatology during the study of a number of pharmacologic agents such as surfactant and postnatal steroids.42 Our laboratory has shown that a single course of antenatal steroids causes a significant improvement in compliance and FRC in premature infants and those changes correlate with less need for surfactant and less oxygen need.43 Another strength is the contemporary evaluation of the association between pulmonary function and systemic hypertension, as most of the published literature linking BPD and systemic hypertension is several decades old. Studies have demonstrated that small changes in pulmonary function can translate into large clinical benefits as the infant ages over time.40 We have previously reported Rrs measurements of 0.043 cmH2O/mL/sec in 31 infants delivered at 40 weeks gestation without any respiratory disease.16 This gives context and highlights the clinical impact in our cohort of the Rrs measurements of the hypertensive and normotensive groups of VLBW infants of 0.080 and 0.066 cmH2O/mL/sec respectively. As expected, both of these latter groups had increased Rrs measurements compared to those previously reported in healthy term infants. In our cohort, there was a slight difference in the corrected gestational age at the time of the PFT between hypertensive and normotensive infants (36.1 weeks vs 34.6 weeks, p = 0.08). Although there is no direct literature highlighting the expected Rrs by week of corrected gestational age, we do know that Rrs should decrease with time, typically over months. Therefore, in our cohort, the resistance in the slightly younger, hypertensive group was higher which suggests that the gestational age was likely not impactful of these results. Interestingly, in the entire group of VLBW infants, we demonstrated a weak positive relationship (correlation coefficient of 0.30; p = 0.03) between SBP and Rrs at 34 – 36 weeks of CGA. Since lung vascular development precedes and promotes alveolar growth, we could speculate that common alterations in vascular development may be affecting SBP and lung development but this will require further study.
Our study has several limitations. Ideally, we would have prospectively followed VLBW infants to identify those with elevated SBP and then performed the PFTs. However, given the routine nature of obtaining PFTs as a part of our clinical respiratory consensus guidelines at a consistent CGA in VLBW neonates, we offer a unique consistency potentially reducing the need for a prospective evaluation. Our sample size is small making broad generalizations difficult, so the difference in Rrs between the hypertensive and normotensive patients may due to a type I error. However, the patients who developed hypertension tended to be smaller, more premature babies so the increased Rrs may be due to the earlier disruption of lung development with the need for increased days of mechanical ventilation which may have led to airway remodeling with an increased Rrs demonstrated by PFT. Neonatal hypertension is a relatively rare condition, particularly when data is gathered at a single NICU. We were only able to identify fourteen patients with hypertension, which is a limited sample size given the variety of factors that may contribute to the development of hypertension. Our sample size was also limited by excluding infants who had received recent postnatal steroids (given the risk of iatrogenic hypertension) and those who were intubated (given the falsely elevated respiratory resistance created by the endotracheal tube). Since infants most at risk for BPD are treated with short courses of postnatal steroids44 and those who are intubated at 34 – 36 weeks will likely carry a diagnosis of BPD, we likely eliminated those at highest risk of moderate to severe BPD. Another potential limitation is the fact that some of the hypertensive infants were managed with various medications. However, these medications have not been shown to impact pulmonary function testing parameters.45–47 Although PFTs were performed following a standardized protocol, the performance of blood pressure measurements in small infants can be challenging and this may have affected the quality of this data. To reduce this potential confounding factor, an average blood pressure was obtained over multiple days to reduce the variation. In addition, we measured passive respiratory resistance which primarily measures the resistance in the large airways, as opposed to the small airways, which are more likely to be altered in “new” BPD. Lastly, since this was a pilot study, we did not have a predetermined sample size and, therefore, the study was likely underpowered.
In conclusion, in this cohort of very low birth weight infants, those with elevated systolic blood pressure had altered pulmonary function test parameters, specifically an increased respiratory resistance, compared with those with normal systolic blood pressure. By adding to the growing body of literature investigating the link between altered lung function and systemic hypertension in preterm neonates we hope to spur further research in this important clinical area. In particular, the clinical implication of the resistive difference remains unclear necessitating larger prospective studies to evaluate the relationship between pulmonary function and systemic hypertension in neonates, its evolution over time, and investigation of mechanisms of this potential association.
Conclusion
In this cohort of contemporary VLBW infants, those with hypertension had altered pulmonary function test parameters, specifically increased respiratory resistance, compared to those with normal systolic blood pressure. This finding warrants a prospective study with a larger sample size and long-term follow-up.
Acknowledgements
The authors thank the neonates and families for allowing us the opportunity to be involved in their care. This study was performed with the support of the Tartar Family Trust.
Footnotes
Disclosure
The authors declare that they have no conflict of interest in this work.
References
- 1.Dionne JM, Abitbol CL, Flynn JT. Hypertension in infancy: diagnosis, management and outcome. Pediatric nephrology (Berlin, Germany). 2012;27(1):17–32. [DOI] [PubMed] [Google Scholar]
- 2.Sharma D, Farahbakhsh N, Shastri S, Sharma P. Neonatal hypertension. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2017;30(5):540–550. [DOI] [PubMed] [Google Scholar]
- 3.Flynn JT. Hypertension in the neonatal period. Current opinion in pediatrics. 2012;24(2):197–204. [DOI] [PubMed] [Google Scholar]
- 4.Flynn JT. Neonatal hypertension. In: Pediatric Hypertension. Vol 2nd Ed. New York, NY: Humana Press Inc; 2011:375–396. [Google Scholar]
- 5.Blowey DL, Duda PJ, Stokes P, Hall M. Incidence and treatment of hypertension in the neonatal intensive care unit. Journal of the American Society of Hypertension : JASH. 2011;5(6):478–483. [DOI] [PubMed] [Google Scholar]
- 6.Sahu R, Pannu H, Yu R, Shete S, Bricker JT, Gupta-Malhotra M. Systemic hypertension requiring treatment in the neonatal intensive care unit. The Journal of pediatrics. 2013;163(1):84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seliem WA, Falk MC, Shadbolt B, Kent AL. Antenatal and postnatal risk factors for neonatal hypertension and infant follow-up. Pediatric nephrology (Berlin, Germany). 2007;22(12):2081–2087. [DOI] [PubMed] [Google Scholar]
- 8.Singh HP, Hurley RM, Myers TF. Neonatal hypertension. Incidence and risk factors. American journal of hypertension. 1992;5(2):51–55. [DOI] [PubMed] [Google Scholar]
- 9.Al Awad EYK, Soraisham A, Obaid H, Sundaram A, Samedi V, Akierman A. Transient hyperaldosteronism and neonatal hypertension: Case series and literature review. J Clin Neon. 2018;7(3):185–189. [Google Scholar]
- 10.Jenkins RD, Aziz JK, Gievers LL, Mooers HM, Fino N, Rozansky DJ. Characteristics of hypertension in premature infants with and without chronic lung disease: a long-term multi-center study. Pediatric nephrology (Berlin, Germany). 2017. [DOI] [PubMed] [Google Scholar]
- 11.Singer LT, Siegel AC, Lewis B, Hawkins S, Yamashita T, Baley J. Preschool language outcomes of children with history of bronchopulmonary dysplasia and very low birth weight. Journal of developmental and behavioral pediatrics : JDBP. 2001;22(1):19–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105(6):1216–1226. [DOI] [PubMed] [Google Scholar]
- 13.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American journal of respiratory and critical care medicine. 2001;163(7):1723–1729. [DOI] [PubMed] [Google Scholar]
- 14.Tepper RS, Morgan WJ, Cota K, Taussig LM. Expiratory flow limitation in infants with bronchopulmonary dysplasia. The Journal of pediatrics. 1986;109(6):1040–1046. [DOI] [PubMed] [Google Scholar]
- 15.Gerhardt T, Hehre D, Feller R, Reifenberg L, Bancalari E. Serial determination of pulmonary function in infants with chronic lung disease. The Journal of pediatrics. 1987;110(3):448–456. [DOI] [PubMed] [Google Scholar]
- 16.McEvoy C, Venigalla S, Schilling D, Clay N, Spitale P, Nguyen T. Respiratory function in healthy late preterm infants delivered at 33–36 weeks of gestation. The Journal of pediatrics. 2013;162(3):464–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abman SH, Warady BA, Lum GM, Koops BL. Systemic hypertension in infants with bronchopulmonary dysplasia. The Journal of pediatrics. 1984;104(6):928–931. [DOI] [PubMed] [Google Scholar]
- 18.Alagappan A, Malloy MH. Systemic hypertension in very low-birth weight infants with bronchopulmonary dysplasia: incidence and risk factors. American journal of perinatology. 1998;15(1):3–8. [DOI] [PubMed] [Google Scholar]
- 19.Anderson AH, Warady BA, Daily DK, Johnson JA, Thomas MK. Systemic hypertension in infants with severe bronchopulmonary dysplasia: associated clinical factors. American journal of perinatology. 1993;10(3):190–193. [DOI] [PubMed] [Google Scholar]
- 20.Janer J, Andersson S, Kajantie E, Lassus P. Endostatin concentration in cord plasma predicts the development of bronchopulmonary dysplasia in very low birth weight infants. Pediatrics. 2009;123(4):1142–1146. [DOI] [PubMed] [Google Scholar]
- 21.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. American journal of respiratory and critical care medicine. 2007;175(10):978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gien J, Kinsella JP. Pathogenesis and treatment of bronchopulmonary dysplasia. Current opinion in pediatrics. 2011;23(3):305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McEvoy C, Schilling D, Peters D, et al. Respiratory compliance in preterm infants after a single rescue course of antenatal steroids: a randomized controlled trial. American journal of obstetrics and gynecology. 2010;202(6):544.e541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gappa M, Colin AA, Goetz I, Stocks J. Passive respiratory mechanics: the occlusion techniques. The European respiratory journal. 2001;17(1):141–148. [DOI] [PubMed] [Google Scholar]
- 25.Morris MG, Gustafsson P, Tepper R, Gappa M, Stocks J. The bias flow nitrogen washout technique for measuring the functional residual capacity in infants. ERS/ATS Task Force on Standards for Infant Respiratory Function Testing. The European respiratory journal. 2001;17(3):529–536. [DOI] [PubMed] [Google Scholar]
- 26.Nwankwo MU, Lorenz JM, Gardiner JC. A standard protocol for blood pressure measurement in the newborn. Pediatrics. 1997;99(6):E10. [DOI] [PubMed] [Google Scholar]
- 27.Zubrow AB, Hulman S, Kushner H, Falkner B. Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study. Philadelphia Neonatal Blood Pressure Study Group. Journal of perinatology : official journal of the California Perinatal Association. 1995;15(6):470–479. [PubMed] [Google Scholar]
- 28.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. The Journal of pediatrics. 1978;92(4):529–534. [DOI] [PubMed] [Google Scholar]
- 29.Clyman R, Cassady G, Kirklin JK, Collins M, Philips JB 3rd. The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: reexamining a randomized controlled trial. The Journal of pediatrics. 2009;154(6):873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cotton RB, Stahlman MT, Kovar I, Catterton WZ. Medical management of small preterm infants with symptomatic patent ductus arteriosus. The Journal of pediatrics. 1978;92(3):467–473. [DOI] [PubMed] [Google Scholar]
- 31.Jacob J, Gluck L, DiSessa T, et al. The contribution of PDA in the neonate with severe RDS. The Journal of pediatrics. 1980;96(1):79–87. [DOI] [PubMed] [Google Scholar]
- 32.Chen HL, Yang RC, Lee WT, et al. Lung function in very preterm infants with patent ductus arteriosus under conservative management: an observational study. BMC pediatrics. 2015;15:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCurnin DC, Yoder BA, Coalson J, et al. Effect of ductus ligation on cardiopulmonary function in premature baboons. American journal of respiratory and critical care medicine. 2005;172(12):1569–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northway WH Jr., Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. The New England journal of medicine. 1967;276(7):357–368. [DOI] [PubMed] [Google Scholar]
- 35.McEvoy CT, Jain L, Schmidt B, Abman S, Bancalari E, Aschner JL. Bronchopulmonary dysplasia: NHLBI Workshop on the Primary Prevention of Chronic Lung Diseases. Annals of the American Thoracic Society. 2014;11 Suppl 3:S146–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flynn JT, Kaelber DC, Baker-Smith CM, et al. ; Subcommittee on Screening and Management of High Blood Pressure in Children. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3):e20171904. Pediatrics. 2018;142(3). [DOI] [PubMed] [Google Scholar]
- 37.Trasande L, Attina TM. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension (Dallas, Tex : 1979). 2015;66(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. Urinary phthalates are associated with higher blood pressure in childhood. The Journal of pediatrics. 2013;163(3):747–753.e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valvi D, Casas M, Romaguera D, et al. Prenatal Phthalate Exposure and Childhood Growth and Blood Pressure: Evidence from the Spanish INMA-Sabadell Birth Cohort Study. Environmental health perspectives. 2015;123(10):1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McEvoy CT, Schilling D, Clay N, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. Jama. 2014;311(20):2074–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jordan BK, Schilling D, McEvoy CT. Pulmonary Function at Hospital Discharge in Preterm Infants Randomized to a Single Rescue Course of Antenatal Steroids. The Journal of pediatrics. 2017;181:62–66.e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Durand M, Mendoza ME, Tantivit P, Kugelman A, McEvoy C. A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics. 2002;109(2):262–268. [DOI] [PubMed] [Google Scholar]
- 43.McEvoy C, Bowling S, Williamson K, Stewart M, Durand M. Functional residual capacity and passive compliance measurements after antenatal steroid therapy in preterm infants. Pediatric pulmonology. 2001;31(6):425–430. [DOI] [PubMed] [Google Scholar]
- 44.Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. The Journal of pediatrics. 2014;165(6):1258–1260. [DOI] [PubMed] [Google Scholar]
- 45.Kao LC, Warburton D, Cheng MH, Cedeno C, Platzker AC, Keens TG. Effect of oral diuretics on pulmonary mechanics in infants with chronic bronchopulmonary dysplasia: results of a double-blind crossover sequential trial. Pediatrics. 1984;74(1):37–44. [PubMed] [Google Scholar]
- 46.Reiterer W, Meisner W. Effect of isradipine and atenolol on lung function in patients with mild essential hypertension. American journal of hypertension. 1991;4(2 Pt 2):197s–199s. [DOI] [PubMed] [Google Scholar]
- 47.Hoffman DJ, Gerdes JS, Abbasi S. Pulmonary function and electrolyte balance following spironolactone treatment in preterm infants with chronic lung disease: a double-blind, placebo-controlled, randomized trial. Journal of perinatology : official journal of the California Perinatal Association. 2000;20(1):41–45. [DOI] [PubMed] [Google Scholar]