SUMMARY
Early in the COVID-19 pandemic, models predicted hundreds of thousands of additional TB deaths as a result of health service disruption. To date, empirical evidence on the effects of COVID-19 on TB outcomes has been limited. Here we summarise the evidence available at a country level, identifying broad mechanisms by which COVID-19 may modify TB burden and mitigation efforts. From the data, it is clear that there have been substantial disruptions to TB health services and an increase in vulnerability to TB. Evidence for changes in Mycobacterium tuberculosis transmission is limited, and it remains unclear how the resources required and available for the TB response have changed. To advocate for additional funding to mitigate the impact of COVID-19 on the global TB burden, and to efficiently allocate resources for the TB response, requires a significant improvement in the TB data available.
Keywords: TB, health services, vulnerability, transmission, resource
Abstract
Au début de la pandémie de COVID-19, les modèles prévoyaient des centaines de milliers de décès supplémentaires dus à la TB en raison des perturbations des services de santé. Jusqu’à présent, les preuves empiriques des effets de COVID-19 sur les résultats de la TB ont été limitées. Nous résumons ici les preuves disponibles au niveau national, en identifiant les mécanismes généraux par lesquels COVID-19 pourrait modifier la charge de la TB et les efforts d’atténuation. Lorsque les données sont disponibles, il est clair qu’il y a eu d’importantes perturbations des services de santé de la TB et une augmentation de la vulnérabilité à la TB. Les preuves de changements dans la transmission de Mycobacterium tuberculosis sont limitées, et l’évolution des ressources nécessaires et disponibles pour la réponse à la TB n’est pas claire. Une amélioration significative des données sur la TB est nécessaire pour obtenir des fonds supplémentaires afin d’atténuer l’impact de COVID-19 sur le fardeau mondial de la TB et pour allouer efficacement les ressources à la réponse à la TB.
GIVEN CONCERNS FOR MAINTAINING TB CARE and prevention services during the COVID-19 pandemic,1 mathematical modellers have attempted to estimate the potential impact on TB incidence and mortality.2–5 Despite the use of different methods and assumptions about the future of the pandemic, as well as modelling for a variety of settings (including India, China, South Africa, Kenya, Ukraine and Brazil), these analyses reached broadly similar conclusions. Specifically, TB incidence, and especially TB mortality, are projected to increase by around 5–15% over the next 5 years, amounting to hundreds of thousands of additional TB deaths worldwide. Indeed, the WHO now estimates that half a million more people may have died from TB in 2020 alone.6 These early modelling analyses, however, relied on a number of assumptions, which should ideally be reevaluated in the context of empirical data. Since these analyses were produced, little evidence has been systematically collected to quantify the impact of COVID-19 on TB burden. A data-driven understanding of this impact is necessary to support efforts to mitigate it, revise the implementation of TB services and allocate resources to different TB interventions. To implement and prioritise effectively, it is essential to understand the current situation.
We expect COVID-19 to affect TB outcomes differently by setting. For example, countries with a large TB burden, such as India and Viet Nam, have experienced very different COVID-19 incidence.7 Countries with a similar COVID-19 burden, such as Brazil and Argentina, have experienced different levels of health system disruption.8 Indeed, within individual countries the impact will further vary between rural and urban areas, by socio-economic status, and as response measures vary spatially. With all of this variation, it is therefore vital to focus on the measurement of setting-specific impact. It is also important to identify when the impact was measured, as the temporal effect of the pandemic varies between countries.
Here we review the evidence available, to inform how the implementation and allocation of resources by TB programmes could be revised. We identify where country-specific data and evidence can be found to quantify the impact of COVID-19 on TB outcomes, and the costs of any mitigation. In Figure 1, we outline the conceptual framework for our narrative review, specifying how COVID-19 may impact across the TB care cascade, identifying disruption to TB health service delivery and changes in demand, alterations in vulnerability to TB (including comorbidities and risk factors) and opportunities for Mycobacterium tuberculosis transmission. We then identify data on the impact of COVID-19 on both availability and requirements of TB resources, and collate this evidence in the Table. We end by highlighting knowledge gaps that should be prioritised for study.
Figure 1.
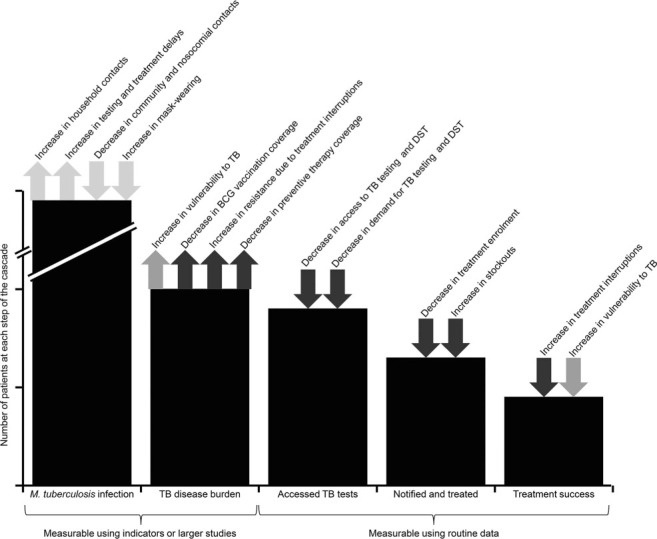
Potential impact of COVID-19 on the TB care cascade. Arrows indicate an increase or a decrease in number of patients at that point of the cascade, including the logic behind the change. black arrows indicate an impact on health service delivery and demand, grey arrows indicate an impact on vulnerability to TB, and light grey arrows indicate an impact on M. tuberculosis transmission. BCG = bacilli Calmette-Guérin; DST = drug susceptibility testing.
Table.
Available or upcoming data on the impact of COVID-19 on TB by country for WHO high TB, TB-HIV and multidrug-resistant TB burden countries13
| Country | Health services data | Vulnerability data | Transmission data | Resource data | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Treatment | Prevention | HIV | Poverty | No control measures | Under control measures | Required | Available | ||||||||||
| Cases | Testing | DST | Delays | Outcomes | BCG coverage | Preventive therapy | Testing | ART | Patient costs | Household transmission | Contacts | Contacts | Mobility | Mask-wearing | Resource utilisation | Prices | Budgets | |
| Angola | 32 | 76 | 97 | 99 | 118 | |||||||||||||
| Azerbaijan | 76 | 99 | 118 | |||||||||||||||
| Bangladesh | 32 | 13 | 107 | 76 | 97 | 99 | 118 | |||||||||||
| Belarus | 32 | 76 | 97 | 99 | 118 | |||||||||||||
| Botswana | 70 | 70 | 107 | 76 | 97 | 99 | 118 | |||||||||||
| Brazil | 23,25,32 | 44 | 44 | 44 | 25 | 70 | 13 | 107,108 | 76 | 97 | 99 | |||||||
| Cambodia | 13,32 | 70 | 76 | 97 | 99 | |||||||||||||
| Cameroon | 70 | 13 | 76 | 97 | 99 | 118 | ||||||||||||
| Central African Republic | 76 | 99 | 118 | |||||||||||||||
| Chad | 76 | 99 | 118 | |||||||||||||||
| China | 13–17,30 | 15–17 | 17 | 16,17 | 76,77,80,81 | 94 | 100 | |||||||||||
| Congo | 76 | 99 | 118 | |||||||||||||||
| DPR Korea | 32 | 76 | 99 | |||||||||||||||
| DR Congo | 32 | 76 | 99 | 118 | ||||||||||||||
| Eswatini | 76 | 99 | 118 | |||||||||||||||
| Ethiopia | 32 | 46 | 70 | 70 | 13 | 76 | 99 | 118 | ||||||||||
| Ghana | 76 | 97 | 99 | 118 | ||||||||||||||
| Guinea-Bissau | 107 | 76 | 97 | 99 | ||||||||||||||
| India | 13,20,21–25,27, 28 31,32,35 | 31,51 | 13 | 107 | 76,77 | 97 | 100 | 118 | ||||||||||
| Indonesia | 13,32 | 70 | 70 | 13 | 107 | 76 | 97 | 100 | ||||||||||
| Kazakhstan | 32 | 76 | 97 | 99 | 118 | |||||||||||||
| Kenya | 13,24,25,32 | 43 | 43 | 24 | 25 | 24,70 | 70 | 104,107 | 76,82,83 | 95 | 97 | 99 | 118 | |||||
| Kyrgyzstan | 32 | 70 | 76 | 97 | 99 | 118 | ||||||||||||
| Lesotho | 32 | 70 | 70 | 76 | 99 | 118 | ||||||||||||
| Liberia | 70 | 76 | 99 | 118 | ||||||||||||||
| Malawi | 24,34 | 43 | 43 | 24 | 24 | 13 | 104,109 | 76,84 | 99 | 118 | ||||||||
| Mozambique | 13,32 | 70 | 70 | 13 | 76 | 97 | 99 | 118 | ||||||||||
| Myanmar | 70 | 70 | 76 | 97 | 99 | 118 | ||||||||||||
| Namibia | 13,32 | 13 | 76 | 97 | 99 | 118 | ||||||||||||
| Nigeria | 18,32 | 40 | 70 | 107 | 76 | 97 | 99 | 118 | ||||||||||
| Pakistan | 22,32,33 | 45 | 52 53 | 76 | 97 | 99 | 118 | |||||||||||
| Papua New Guinea | 32 | 76 | 97 | 99 | 118 | |||||||||||||
| Peru | 32 | 70 | 70 | 13 | 107,110,111 | 76,85 | 97 | 99 | 118 | |||||||||
| Philippines | 13,25,29 32 | 41 | 41 | 41 | 76 | 97 | 100 | 118 | ||||||||||
| Republic of Moldova | 32 | 76 | 97 | 99 | 118 | |||||||||||||
| Russian Federation | 25,32 | 25 | 76,77,86,87 | 99 | ||||||||||||||
| Sierra Leone | 13,19,25 32 | 25 | 70 | 70 | 107 | 76 | 99 | |||||||||||
| Somalia | 99 | 118 | ||||||||||||||||
| South Africa | 13,26,32 | 42 | 42 | 47 | 49 | 70 | 70 | 13 | 104,107,112–115 | 76,77,88–90 | 96,119 | 97 | 99 | 118 | ||||
| Tajikistan | 32 | 70 | 70 | 76 | 97 | 99 | ||||||||||||
| Tanzania | 13,32 | 70 | 76 | 97 | 99 | |||||||||||||
| Thailand | 13,32 | 13 | 76,78 | 97 | 100 | |||||||||||||
| Uganda | 32,120 | 44 | 44 | 70 | 104,107,108,116 | 76,91 | 97 | 99 | 118 | |||||||||
| Ukraine | 32 | 70 | 70 | 76 | 97 | 99 | 118 | |||||||||||
| Uzbekistan | 76 | 99 | 118 | |||||||||||||||
| Viet Nam | 13,32 | 44 | 44 | 107,117 | 76,92 | 97 | 100 | 118 | ||||||||||
| Zambia | 13,32 | 50 | 13 | 76,89,90 | 97 | 99 | 118 | |||||||||||
| Zimbabwe | 24 | 43 | 43 | 24 | 24,70 | 70 | 76,93 | 97 | 99 | 118 | ||||||||
DST = drug susceptibility testing; BCG = bacilli Calmette-Guérin; ART =antiretroviral therapy; DPR = Democratic People’s Republic; DR = Democratic Republic.
SEARCH STRATEGY AND SELECTION CRITERIA
We conducted a narrative and bibliometric review, combining a rapid semi-systematic search and convening a range of experts. For the rapid review, references were identified through searches of PubMed, medRxiv and bioRxiv for articles published from January 2020 to March 2021, using the terms “COVID” or “SARS” or “corona”, and “TB” or “tuberculosis”. In addition, literature relevant to TB vulnerabilities, Mycobacterium tuberculosis transmission and TB resources was identified through the authors’ personal libraries. Additional relevant grey literature was identified through communication with the WHO Global TB Department, as well as through a virtual meeting of the TB Modelling and Analysis Consortium, where a group of TB experts from global agencies, academic institutions and country programmes were invited to identify additional sources of data and to confirm and highlight priority knowledge gaps. Grey literature was included in this instance as it represents a significant proportion of the relevant data available to country-level TB decision makers when making policy choices. Articles resulting from these searches and relevant references cited in those articles were reviewed.
Articles which contained data on country-specific quantitative changes to TB health service indicators, burden of TB vulnerabilities, M. tuberculosis transmission and TB resources for the WHO high TB, TBHIV and multidrug-resistant TB (MDR-TB) burden countries were included, and data extracted from these articles. A summary of sources found by country on each topic is presented in the Table. We provide a narrative synthesis of our findings below.
Ethical approval was not required for this study as this was a review of existing studies.
TB HEALTH SERVICES
The provision of TB health services (TB diagnosis, care and prevention services), and access to these services, has been severely disrupted by COVID-19.9–11 TB service providers across many high TB burden contexts have faced difficulties in service provision, due to lack of appropriate equipment and capacity, restrictions to movement (affecting health care workers, commodities and stock) and reallocation of resources.10 Meanwhile, individual TB patients have struggled to access TB services, either through fear of SARS-CoV-2 infection, fear of stigma, restrictions to movement, reduced health facility opening hours or reductions in the ability to pay for care or transport.9 Globally, TB diagnosis, care and prevention has been affected as a result. However, nearly a year after these disruptions began, relatively little high-level information is available, focused primarily on reductions in the number of TB patients.12 Most data that are available deal with the first two quarters of 2020, with little data except for patient numbers available for quarters three and four when services might be expected to be somewhat restored.
Most high TB burden countries have observed some changes in TB case numbers or notifications (when TB is diagnosed in a patient and this is reported through the national surveillance system) that have resulted due to COVID-19.13–35 Continuous surveillance systems and current data collection efforts36,37 suggest that additional data may also be forthcoming. In general, TB notifications decreased significantly during the early stages of the pandemic compared to previous years. The United States Agency for International Development (USAID) preliminarily estimates are that over 1 million fewer cases in 24 high TB burden countries alone may have been notified in 2020 as a result of the pandemic, with a 7% relative reduction in Africa, a 15% reduction in Central Asia and Europe, and a 27% reduction in Asia compared to 2019.38 More recent estimates by the WHO,6 the Global Fund to Fight AIDS, Tuberculosis and Malaria (the Global Fund)39 and the Stop TB partnership35 suggest that globally around 20–30% fewer people were notified with TB than in 2019, with 45% fewer tested for MDR-TB. A limited number of countries appear to have either avoided this trend (such as Mozambique and Tanzania) or have seen notifications dip and since recover to pre-pandemic levels (such as China and Viet Nam).13 However, without data on TB testing and positivity rates it is difficult to determine whether this widespread decrease in notifications reflects a true decrease in incidence, or a decrease in access to TB diagnostic services. In several countries where testing data, including for drug susceptibility testing, are available (China,15–17 Nigeria,40 the Philippines41 and South Africa,42 with further studies underway in Kenya, Malawi and Zimbabwe,43 as well as Brazil, Uganda and Viet Nam44), testing decreased. In South Africa, this was accompanied by a corresponding increase in the proportion of TB tests that were positive.42 The implication of this is that there are likely to be large numbers of undiagnosed cases of TB in the community, who may now face poorer treatment outcomes due to delayed diagnosis and treatment.
In addition to reducing TB diagnosis, COVID-19 may have hampered treatment for TB patients due to limited treatment support and medication stockouts. Such disruption could increase the risk of treatment interruption and delay, and decrease treatment adherence, which can be expected to result in worsening TB treatment outcomes. Due to the long duration of TB treatment, definitive data on changes in TB treatment outcomes as a result of COVID-19 may not be available for several months. In brief reports of patients in private-sector centres in Pakistan,45 a Chinese province16 and cities in Ethiopia46 and Zimbabwe,24 treatment outcomes and support have worsened slightly (approximately 5–15% relative reduction). On the other hand, analysis of data from China17 and of a small number of patients in cities in Kenya and Malawi24 did not show strong evidence of a significant reduction in treatment success. Also, non-TB-specific data in a South African province showed that numbers of clinic visits in general did not decline, although there was a significant (but temporary) decrease in child healthcare visits.47 Further studies are underway in Brazil, Uganda and Viet Nam.44 At this point, it is difficult to determine how effective calls for the use of digital technologies, additional medicines to take home and other approaches to ensure adequate treatment48 have been, although many patients have reported feeling insufficiently supported.9
TB prevention services such as routine bacilli Calmette-Guérin (BCG) vaccination, household contact management and preventive therapy are also likely to have been impacted by the COVID-19 pandemic. Routine reporting on these indicators is limited, and this challenges efforts to quantify the impact of COVID-19 on provision of these preventive services. TB centres in Brazil,25 Kenya,25 the Philippines,41 Russia,25 South Africa,49 Sierra Leone25 and Zambia50 reported relative declines in preventive therapy enrolment of 30–70%, although in the Philippines this decline appears to be consistent with pre-pandemic recent trends, and in South Africa as well as one Brazilian centre, preventive therapy enrolment seems to have recovered to pre-COVID levels. Meanwhile, India31,51 and Pakistan52,53 reported major decreases in relative BCG vaccination coverage of up to 60%, with significant potential consequences for paediatric TB mortality in particular.54
VULNERABILITY TO TB
Just as the COVID-19 pandemic has impacted TB burden, it has also impacted global vulnerability to TB, through a general decrease in health care access, an increase in poverty and the potential for post-COVID-19 lung disease. These vulnerabilities could increase progression to TB disease among those with M. tuberculosis infection, as well as worsen treatment outcomes for patients on treatment. Modelling evidence broadly suggests that an increase in these vulnerabilities is likely,4,55,56 but clear evidence of an increase is thus far scarce.
There is growing evidence to suggest that previous or current TB infection or disease are associated with poor COVID-19 outcomes,57–60 including an approximately two- to three-fold increase in mortality (which occurred more quickly) and a 25% relative decrease in the possibility of recovery (which occurred more slowly) for COVID-19 coinfection with current TB disease.61–64 However, while there is little evidence as yet that previous SARS-CoV-2 infection or COVID-19 disease affect either progression to TB disease or TB treatment outcomes,65 the possibility of post-COVID-19 lung damage and subsequent vulnerability to TB is a major concern.12,58,66 A number of studies are underway to investigate this issue.67–69
At the same time, a similar decrease in health care provision to that described above for TB could significantly impact TB vulnerabilities such as HIV and diabetes. Data for HIV health services are available from UNAIDS for many,70 but not all, high TB-HIV burden countries. This includes both testing and treatment data for Botswana, Ethiopia, Indonesia, Kenya, Lesotho, Mozambique, Myanmar, Peru, Sierra Leone, Tajikistan, Ukraine and Zimbabwe, testing data only for Brazil, Cambodia, Liberia, Uganda and Tanzania, (as well as the capital cities of Kenya, Malawi and Zimbabwe24) and treatment data only for Cameroon, Kyrgyzstan and Nigeria. Broadly, HIV testing has declined significantly due to COVID-19, particularly in the early stages of the pandemic. However, in many settings this has recovered somewhat, through HIV self-testing.70 In addition, the proportion of tests that are positive has generally not changed, suggesting that there has likely been relative stability in testing practices, if not coverage. Meanwhile, although numbers on treatment have been less affected, numbers initiating treatment have declined precipitously and generally not returned to pre-COVID-19 levels.70 However, it is not yet clear how the actual burden of HIV, diabetes and other TB vulnerabilities has increased due to COVID-19.
Poverty is expected to increase due to COVID-19,55 and surveys show it is driving people with TB into poverty and increasing inequities.9 Although data on changes to costs faced by TB patients are not yet available, national surveys are already underway or planned in 13 of the 48 high TB, TB-HIV or MDRTB burden countries.13 In particular, one survey recently completed in India contains samples from both pre- and mid-pandemic periods. The effects of an increase in poverty and inequality include a likely increase in catastrophic costs (>20% of household annual income) faced by TB patients and a resulting inability to access TB health services as discussed above.71 Increases in poor living conditions and malnutrition can also drive increases in TB.72,73 With as much as 30–50% of TB incidence attributable to malnutrition, the potential longer-term consequences for these economic effects on the TB epidemic will be important to investigate.74
MYCOBACTERIUM TUBERCULOSIS TRANSMISSION
We do not yet know how M. tuberculosis transmission has been affected by COVID-19 and the use of interventions to reduce SARS-CoV-2 transmission. A reduction in respiratory contacts in the community and healthcare settings, in addition to the widespread use of masks, may reduce transmission of M. tuberculosis, as has been observed for influenza.75 However, a potential increase in contact within household settings, and the long duration of latent TB infection and TB disease as compared to COVID-19, may increase transmission in these settings. This effect could be compounded if decreasing access to TB health services increases the duration of TB infectiousness and increasing vulnerabilities lead to greater risk of TB disease.
Studying TB transmission is challenging. One approach to estimate potential changes in M. tuberculosis transmission is to consider changes in contacts in different social settings over time, particularly as these data are collected elsewhere to understand changes to SARS-CoV-2 transmission. Unfortunately, for most high TB burden countries, contact surveys are limited. While synthetic contact matrices are available for all high TB burden countries except Somalia,76–79 only 10 high TB, TB-HIV or MDR-TB burden countries have contact surveys available from before the pandemic.80–93 Furthermore, only China,94 Kenya95 and South Africa96 have contact surveys available from during the pandemic (with a survey currently underway in Pakistan), showing a marked decrease in contacts outside of the household.
New sources of mobility data, for example, from Google97 or mobile phone providers, suggest massive, time-varying changes in population movements as a result of COVID-19. Although this does not provide information on how contacts have changed, it does allow for a better understanding of locations (such as public transport or places of worship), where contacts have decreased. This can be used, alongside contact surveys where the location of contact was recorded, to estimate likely reductions in contacts. A major caveat is that those surveyed include mobile phone owners only, which may underrepresent both TB patients98 and potentially those unable to practice physical distancing.
As a result of efforts to understand the pandemic, data on mask-wearing are widely available for all high TB burden countries, and shows a major increase,99,100 which has the potential to be of great benefit to the TB response.101 Although the impact of mask use on M. tuberculosis transmission is poorly understood,102 it may be significant in some settings, particularly if sustained for significant time periods.103
The impact on M. tuberculosis transmission of changes in contacts or mask-wearing in particular locations is dependent on the extent to which transmission occurs in those locations and the potential for changes in per-contact risk to affect overall risk of transmission. Studies from before the pandemic suggest that even for children only 10–30% of population-attributable transmission is due to household exposure.104,105 Presuming contact saturation within the home limits the amount of additional transmission that could occur as a result of increased time spent there,106 decreased community contact and mask-wearing could significantly reduce overall M. tuberculosis transmission per person with TB disease. The relative importance of this reduction in community transmission is likely to be dependent on the extent to which transmission occurs outside of the home. Some evidence of the proportion of M. tuberculosis transmission attributable to the household or other locations is available for a number of countries, where this may depend in part on the burden of disease.104,107–117
TB RESOURCES
To understand and mitigate the consequences of COVID-19 on TB interventions and outcomes, it is necessary to understand how the resource needs of TB services have changed, and the impact of COVID-19 on the resources available. First, approaches to delivering TB interventions are likely to have changed, either through design (such as an increased need for personal protective equipment, or additional staff time required for infection control and physical distancing measures), or through shortages or constraints to some inputs (such as staffing and diagnostic capacity).48 Second, prices for different intervention inputs could change substantially as demand increases. Third, the costs of providing services are linked to service volumes (for example, a short-term reduction in demand may result in temporary over capacity of some TB focused resources). Finally, the available budget for supporting TB services may be lower, with resources diverted to COVID-19 care or mitigation. Indeed, nearly half of high TB burden countries reported reallocation of TB funding to the COVID-19 response,13 with TB funding decreasing significantly.9 Although additional funding to many countries (apart from Brazil, Cambodia, China, DPR Korea, Guinea-Bissau, Indonesia, Russian Federation, Sierra Leone, Tajikistan, Thailand and Tanzania) has been made available (e.g., by funders such as the Global Fund),118 this is aimed at mitigating the impact on the HIV, TB and malaria programmes in general, and does not shed light on any changes to the budget available to the TB programme. We found no country-level quantitative data currently publicly available on the impact of COVID-19 on the resources available to (or required for) the TB response. During the expert meeting, researchers confirmed that in the main, cost data collection had been suspended during the COVID-19 period.
CONCLUSION
In general, where data are available, TB health services appear to have decreased significantly in most settings due to COVID-19. Numbers of patients, as well as testing and prevention coverage, have decreased more noticeably than treatment outcomes, although few data are available on the latter. Ensuring adequate treatment for known TB patients, through provision of additional medicine and digital treatment support, appears to be more amenable to physical distancing than TB diagnosis, which typically requires direct contact between individuals. Meanwhile, vulnerability to TB has widely increased. HIV services appear to have recovered somewhat, although the potential for COVID-19-related lung damage to lead to widespread vulnerability to TB is still unknown, as are the impacts of changes in other vulnerabilities such as diabetes and malnutrition. Data on the impact of an increase in poverty on TB patient costs are currently unavailable, although many studies are underway to address this. Unlike TB health services, which have in a number of cases been restored, vulnerabilities are likely to continue to increase despite COVID-19 vaccines being available, as widespread poverty remains and SARS-CoV-2 infections continue to increase. Although community transmission of M. tuberculosis has likely decreased, the effect of household transmission and a potential increase in cases means that it is difficult to draw any conclusions on changes in M. tuberculosis transmission. Indeed, this may never be possible, although the location of transmission events is likely to have shifted. Finally, while some additional funding has been allocated by global agencies to countries for their TB response, it remains unclear how overall health system resource constraints and the changing resources of service delivery are impacting TB. Although it is difficult to draw any conclusions on the geographic availability of data, we note that little appear to be available for the high MDR-TB burden countries of Central Asia, while many smaller studies are available for countries in sub-Saharan Africa. In general, only a limited number of countries (such as China and South Africa) have good data available across a range of indicators.
When identifying priority gaps that remain for understanding and mitigating the impact of COVID-19 on TB, it is important to be clear on what these data will be used for. We suggest that this should primarily be to allocate TB resources more efficiently and to help advocate for additional resources for the TB response. The first of these requires a good understanding of the effect on health services, and the resources available and required to restore these to at least pre-pandemic levels. In addition, the second point requires an understanding of how vulnerability to TB and M. tuberculosis transmission have changed. In an online meeting of 60 TB experts (TB Modelling and Analysis Consortium meeting on the impact and mitigation of COVID-19 on TB, held on 12 January 2021), a range of priorities were identified from across the four broad areas identified above and these are outlined in Figure 2. There was strong support for data on delays to diagnosis and treatment, changes to patient costs of TB services, the impact of COVID-19 infection and disease on vulnerability to TB and mortality, and the effect of changing contacts and mobility on household and community transmission of M. tuberculosis. A key priority was the longer-term requirement for more responsive TB information systems. While this has not been as much of a problem in the past, the rapid nature of the COVID-19 pandemic has highlighted the need for frequently reported, disaggregated TB health service availability and use data, to allow for an appropriate response. A lack of real-time data to make decisions suggests that investment in a change to TB information and reporting systems to enhance real-time empirical evidence (as can be seen for COVID-19) is required. Data collation and monitoring efforts, by an appropriate global stakeholder, should additionally be strengthened.
Figure 2.
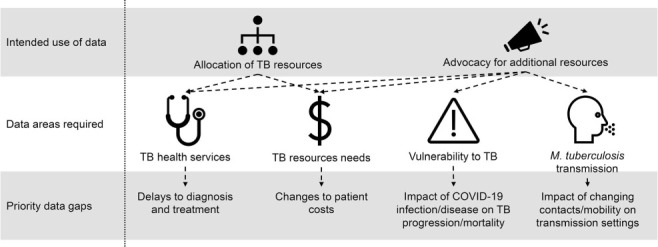
Outline of priority gaps that remain for understanding and mitigating the impact of COVID-19 on TB.
In conclusion, while the numbers of TB patients have declined globally, it is not yet possible to determine the key causes for these declines, what they represent in terms of changing TB burden and what action is required to mitigate this. Advocating for additional funding to mitigate the impact of COVID-19 on the global TB burden, and allocating available resources efficiently for the TB response, will require a significant improvement in the availability of TB data.
Acknowledgements
CFM was funded by the Bill and Melinda Gates Foundation (BMGF), Seattle, WA, USA (TB Modelling and Analysis Consortium OPP1135288) and the Unitaid Adherence Support Coalition to End TB (ASCENT) project (grant agreement number: 2019-33-ASCENT). RGW is funded by the Wellcome Trust, London, UK (218261/Z/19/Z); the National Institutes of Health, Bethesda, MD, USA (1R01AI147321-01), the European & Developing Countries Clinical Trials Partnership, The Hague, The Netherland (RIA208D-2505B); UK Medical Research Council, London, UK (CCF17-7779 via SET Bloomsbury), Economic and Social Research Council, Swindon, UK (ES/P008011/1), BMGF (OPP1084276, OPP1135288 & INV-001754), and the WHO, Geneva, Switzerland (2020/985800-0).
Conflicts of interest: none declared.
References
- 1.Wingfield T, et al. Tackling two pandemics: a plea on World Tuberculosis Day. Lancet Respir Med. 2020;8(6):536–538. doi: 10.1016/S2213-2600(20)30151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glaziou P. Predicted impact of the COVID-19 pandemic on global tuberculosis deaths in 2020. medRxiv. 2020 2020.04.28.20079582. [Google Scholar]
- 3.McQuaid CF, et al. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J. 2020;56:2001718. doi: 10.1183/13993003.01718-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hogan AB, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. The Lancet Global Health. 2020;8(9):e1132–e41. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cilloni L, et al. The potential impact of the COVID-19 pandemic on the tuberculosis epidemic a modelling analysis. EClinicalMedicine. 2020;28:100603. doi: 10.1016/j.eclinm.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Geneva, Switzerland: WHO; 2021. Impact of the COVID-19 pandemic on TB detection and mortality in 2020. https://www.theglobalfund.org/en/news/2021-03-24-tb-testing-in-2020-dropped-drastically-due-to-covid-19/ Accessed March 2021. [Google Scholar]
- 7.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hale T, et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker) Nat Hum Behav. 2021 Mar 8; doi: 10.1038/s41562-021-01079-8. doi. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Stop TB Partnership Civil society-led TB/COVID-19 Working Group. The impact of COVID-19 on the TB epidemic: a community perspective. Geneva, Switzerland: Stop TB Partnership, 2020. http://www.stoptb.org/assets/documents/resources/publications/acsm/Civil%20Society%20Report%20on%20TB%20and%20COVID.pdf?fbclid=IwAR3SOY4kyBs5a_35HIeUhcvwRIWspePA4vVHESqcQxio7G4irivJ90cSU8k Accessed November 2020.
- 10.Khan MS, et al. Mitigating the impact of COVID-19 on tuberculosis and HIV services: a cross-sectional survey of 669 health professionals in 64 low and middle-income countries. PLoS One. 2021;16(2):e0244936. doi: 10.1371/journal.pone.0244936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Geneva, Switzerland: WHO; 2020. Pulse survey on continuity of essential health services during the COVID-19 pandemic: interim report, 27 August 2020. [Google Scholar]
- 12.Visca D, et al. Tuberculosis and COVID-19 interaction: a review of biological, clinical and public health effects. Pulmonology. 2021;27(2):151–165. doi: 10.1016/j.pulmoe.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Geneva, Switzerland: WHO; 2020. Global Tuberculosis Report 2020. [Google Scholar]
- 14.Chen H, Zhang K. Insight into impact of COVID-19 epidemic on tuberculosis burden in China. Eur Respir J. 2020;56:2002710. doi: 10.1183/13993003.02710-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z, et al. Impact of the COVID-19 pandemic on the detection of TB in Shanghai, China. Int J Tuberc Lung Dis. 2020;24(10):1122–1124. doi: 10.5588/ijtld.20.0539. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, et al. Collateral impact of the coronavirus disease 2019 (COVID-19) pandemic on tuberculosis control in Jiangsu Province, China. Clin Infect Dis. 2020 Aug 28; doi: 10.1093/cid/ciaa1289. doi. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fei H, et al. The impact of the COVID-19 epidemic on tuberculosis control in China. Lancet Regional Health Western Pacific. 2020;3:100032. doi: 10.1016/j.lanwpc.2020.100032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adewole OO. Impact of COVID-19 on TB care: Experiences of a treatment centre in Nigeria. Int J Tuberc Lung Dis. 2020;24(9):981–982. doi: 10.5588/ijtld.20.0418. [DOI] [PubMed] [Google Scholar]
- 19.Buonsenso D, Iodice F, Sorba B, iala J, Goletti D. COVID-19 effects on tuberculosis care in Sierra Leone. Pulmonology. 2020;27(1):67–69. doi: 10.1016/j.pulmoe.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.India Ministry of Health and Family Welfare. New Delhi, India: MoHFW; 2020. Nikshay Reports, 2020. [Google Scholar]
- 21.Behera D. TB control in India in the COVID era. Indian J Tuberc. 2020;68(1):128–133. doi: 10.1016/j.ijtb.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pakistan National TB Control Program. Islamabad, Pakistan: Pakistan NTP; 2020. Rapid assessment – impact of outbreak of COVID-19 on TB care services in Pakistan, 2020. [Google Scholar]
- 23.de Souza CDF et al. Impact of COVID-19 on TB diagnosis in Northeastern Brazil. Int J Tuberc Lung Dis. 2020;24(11):1220–1222. doi: 10.5588/ijtld.20.0661. [DOI] [PubMed] [Google Scholar]
- 24.Harries A. Mid-term report on impact of COVID-19 on TB and HIV in Africa. Paris, France: International Union Against Tuberculosis and Lung Disease, 2020. https://theunion.org/news/the-union-shares-mid-term-report-on-impact-of-covid-19-on-people-with-tb-and-hivaids-in-africa Accessed December 2020.
- 25.Migliori GB, et al. Worldwide effects of coronavirus disease pandemic on tuberculosis services, January–April 2020. Emerg Infect Dis. 2020;26(11):2709–2712. doi: 10.3201/eid2611.203163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebina L, et al. Trends in paediatric tuberculosis diagnoses in two South African hospitals early in the COVID-19 pandemic. S Afr Med J. 2020;110(12):1149–1150. doi: 10.7196/SAMJ.2020.v110i12.15386. [DOI] [PubMed] [Google Scholar]
- 27.Datta B, et al. The untimely demise of the TB Free block model in the wake of coronavirus disease 2019 in India. Trans R Soc Trop Med Hyg. 2020;114(11):789–791. doi: 10.1093/trstmh/traa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Behera D. Tuberculosis, COVID-19, and the End Tuberculosis strategy in India. Lung India. 2020;37(6):467–472. doi: 10.4103/lungindia.lungindia_544_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chiang C-Y, et al. The impact of COVID-19 and the restoration of tuberculosis services in the Western Pacific Region. Eur Respir J. 2020;56(4):2003054. doi: 10.1183/13993003.03054-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen X, et al. Continuity of TB services during the COVID-19 pandemic in China. Int J Tuberc Lung Dis. 2021;25(1):81–83. doi: 10.5588/ijtld.20.0632. [DOI] [PubMed] [Google Scholar]
- 31.Shrinivasan R, Rane S, Pai M. India’s syndemic of tuberculosis and COVID-19. BMJ Global Health. 2020;5(11):e003979. doi: 10.1136/bmjgh-2020-003979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Geneva, Switzerland: WHO; 2021. High TB burden countries and other regional priority countries that reported at least one case in the final reporting period of 2020. https://worldhealthorg.shinyapps.io/tb_pronto/ Accessed March 2021. [Google Scholar]
- 33.Fatima R, et al. Building better tuberculosis control systems in a post-COVID world: learning from Pakistan during the COVID-19 pandemic. Int J Infect Dis. 2021 Mar 17; doi: 10.1016/j.ijid.2021.03.026. doi. [Online ahead of print.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nzawa R, et al. Impact of COVID-19 on tuberculosis notifications in Blantyre Malawi: an interrupted time series analysis and qualitative study with healthcare workers. medRxiv. 2021 2021.03.15.21253601. [Google Scholar]
- 35.Stop TB Partnership. Geneva, Switzerland: Stop TB Partnership; 2021. 12 months of COVID-19 eliminated 12 years of progress in the global fight against tuberculosis. http://www.stoptb.org/news/stories/2021/ns21_011.html Accessed March 2021. [Google Scholar]
- 36.European Society of Clinical Microbiology and Infectious Diseases Study Group for Mycobacterial Infections Geneva, Switzerland: ESCMID; 2020. COVIDxTB Survey on the impact of SARS-CoV-2 pandemic on laboratory diagnosis of tuberculosis. https://www.surveymonkey.com/r/COVIDxTB Accessed January 2021. [Google Scholar]
- 37.Joint Special Programme for Research and Training in Tropical Diseases & World Health Organization Regional Office for Europe Copenhagen, Denmark: WHO; 2020. Small grants scheme for operational/implementation research to ensure continuity of essential tuberculosis services during the COVID-19 pandemic. https://who.force.com/etdr/s/gs-solicitation/a0p3X00000ZpYivQAF/ca200002 Accessed January 2021. [Google Scholar]
- 38.Geneva, Switzerland: Friends of the Global Fight Against AIDS, Tuberculosis and Malaria; 2021. Friends of the Global Fight Against AIDS, Tuberculosis and Malaria. How COVID-19 is affecting the global response to AIDS, tuberculosis and malaria. https://www.theglobalfight.org/covid-aids-tb-malaria/ Accessed February 2021. [Google Scholar]
- 39.The Global Fund Geneva, Switzerland: Global Fund; 2021. TB testing in 2020 dropped drastically due to COVID-19. https://www.theglobalfund.org/en/news/2021-03-24-tb-testing-in-2020-dropped-drastically-due-to-covid-19/ Accessed March 2021. [Google Scholar]
- 40.Odume B, et al. Impact of COVID-19 on TB active case finding in Nigeria. Public Health Action. 2020;10(4):157–162. doi: 10.5588/pha.20.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philippines Department of Health Manila, The Philippines: DoH; 2020. Race to end TB dashboard. [Google Scholar]
- 42.Ismail N, Moultrie H. Johannesburg, South Africa: National Institute for Communicable Diseases; 2020. Impact of COVID-19 intervention on TB testing in South Africa. [Google Scholar]
- 43.Harries AD. Paris, France: The International Union Against Tuberculosis and Lung Disease; 2020. The Union shares protocol to measure the impact of COVID-19 on people with TB and HIV/AIDS. https://theunion.org/news/the-union-shares-protocol-to-measure-the-impact-of-covid-19-on-people-with-tb-and-hivaids Accessed December 2020) [Google Scholar]
- 44.World Health Organization. Geneva, Switzerland: WHO; 2020. Compendium of ongoing TB/COVID-19 research projects. https://www.who.int/teams/global-tuberculosis-programme/covid-19/compendium Accessed January 2021. [Google Scholar]
- 45.Jamal WZ, et al. COVID-19: ensuring continuity of TB services in the private sector. Int J Tuberc Lung Dis. 2020;24(8):870–872. doi: 10.5588/ijtld.20.0400. [DOI] [PubMed] [Google Scholar]
- 46.Mohammed H, et al. Containment of COVID-19 in Ethiopia and implications for tuberculosis care and research. Infect Dis Poverty. 2020;9(1):131. doi: 10.1186/s40249-020-00753-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siedner MJ, et al. Access to primary healthcare during lockdown measures for COVID-19 in rural South Africa: an interrupted time series analysis. BMJ Open. 2020;10(10):e043763. doi: 10.1136/bmjopen-2020-043763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. Geneva, Switzerland: WHO; 2020. Information note: tuberculosis and COVID-19. [Google Scholar]
- 49.Churchyard G. Johannesburg, South Africa: The Aurum Institute; 2020. COVID-19 and TB preventive therapy: the time to scale up 3HP is now! https://storage.googleapis.com/stateless-bhekisisa-website/wordpress-uploads/2020/06/092065e8-covid19-tpt-in-sa-1-06.08.20pdf Accessed January 2021. [Google Scholar]
- 50.Khunga M. Lusaka, Zambia: Ministry of Health, Zambia; 2020. TPT surge sites in Zambia: aligning TPT with 6MMD ART dispensation. [Google Scholar]
- 51.Rukmini S. Mumbai, India: IndiaSpend; 2020. COVID-19 Disrupted India’s Routine Health Services. https://www. indiaspend.com/covid-19-disrupted-indias-routine-health-services/ Accessed November 2020. [Google Scholar]
- 52.Malik AA, et al. Tuberculosis control and care in the era of COVID-19. Health Policy Plan. 2020;35(8):1130–1132. doi: 10.1093/heapol/czaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chandir S, et al. Impact of COVID-19 pandemic response on uptake of routine immunizations in Sindh, Pakistan: an analysis of provincial electronic immunization registry data. Vaccine. 2020;38(45):7146–7155. doi: 10.1016/j.vaccine.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris RC, Dodd PJ, White RG. The potential impact of BCG vaccine supply shortages on global paediatric tuberculosis mortality. Bmc Med. 2016;14(1):138. doi: 10.1186/s12916-016-0685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lakner C, et al. Washington DC, USA: World Bank; 2020. Updated estimates of the impact of COVID-19 on global poverty: the effect of new data. [Google Scholar]
- 56.Headey D, et al. Impacts of COVID-19 on childhood malnutrition and nutrition-related mortality. Lancet. 2020;396(10250):519–521. doi: 10.1016/S0140-6736(20)31647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gupta N, et al. A profile of a retrospective cohort of 22 patients with COVID-19 and active/treated tuberculosis. Eur Respir J. 2020;56(5):2003408. doi: 10.1183/13993003.03408-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumar MS, et al. Mortality due to TB-COVID-19 coinfection in India. Int J Tuberc Lung Dis. 2021;25(3):250–251. doi: 10.5588/ijtld.20.0947. [DOI] [PubMed] [Google Scholar]
- 59.Motta I, et al. Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26(4):233–40. doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tadolini M, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56(1):2001398. doi: 10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boulle A, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2020 Aug 29; doi: 10.1093/cid/ciaa1198. doi. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis. 2020;52(12):902–907. doi: 10.1080/23744235.2020.1806353. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, et al. medRxiv. 2020. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. 2020.03.10.20033795. [Google Scholar]
- 64.Demkina AE, et al. medRxiv. 2020. Risk factors for outcomes of COVID-19 patients: an observational study of 795 572 patients in Russia. 2020.11.02.20224253. [Google Scholar]
- 65.Stochino C, et al. Clinical characteristics of COVID-19 and active tuberculosis co-infection in an Italian reference hospital. Eur Respir J. 2020;56(1):2001708. doi: 10.1183/13993003.01708-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheerin D, et al. Systematic evaluation of transcriptomic disease risk and diagnostic biomarker overlap between COVID-19 and tuberculosis: a patient-level meta-analysis. medRxiv. 2020 2020.11.25.20236646. [Google Scholar]
- 67.The TB/COVID-19 Global Study Group. TB and COVID-19 co-infection: rationale and aims of a global study. Int J Tuberc Lung Dis. 2021;25(1):78–80. doi: 10.5588/ijtld.20.0786. [DOI] [PubMed] [Google Scholar]
- 68.UK Collaborative on Development Research London, UK: UKCDR; 2021. COVID-19 research project tracker by UKCDR & Global Research Collaboration for Infectious Disease Preparedness. https://www.ukcdr.org.uk/covid-circle/covid-19-research-project-tracker/ Accessed January 2021. [Google Scholar]
- 69.Migliori GB, et al. Tuberculosis, COVID-19 and hospital admission: Consensus on pros and cons based on a review of the evidence. Pulmonology. 2021 Jan 28; doi: 10.1016/j.pulmoe.2020.12.016. doi. 10.1016/j.pulmoe.2020.12.016. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.UNAIDS. Geneva, Switzerland: UNAIDS; 2020. UNAIDS HIV services tracking. https://hivservicestracking.unaids.org/ Accessed December 2020. [Google Scholar]
- 71.Fuady A, Houweling TAJ, Richardus JH. COVID-19 and tuberculosis-related catastrophic costs. Am J Trop Med Hyg. 2021;104(2):436–440. doi: 10.4269/ajtmh.20-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Marais BJ, et al. The burden of childhood tuberculosis: a public health perspective. Int J Tuberc Lung Dis. 2005;9(12):1305–1313. [PubMed] [Google Scholar]
- 73.Lönnroth K, et al. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol. 2010;39(1):149–155. doi: 10.1093/ije/dyp308. [DOI] [PubMed] [Google Scholar]
- 74.Bhargava A, Shewade HD. The potential impact of the COVID-19 response related lockdown on TB incidence and mortality in India. Indian J Tuberc. 2020;67(Suppl):S139–S146. doi: 10.1016/j.ijtb.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Olsen SJ, et al. Decreased Influenza Activity During the COVID-19 Pandemic - United States, Australia, Chile, and South Africa, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1305–1309. doi: 10.15585/mmwr.mm6937a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Prem K, et al. medRxiv. 2020. Projecting contact matrices in 177 geographical regions: an update and comparison with empirical data for the COVID-19 era. 2020.07.22.20159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mistry D, et al. Inferring high-resolution human mixing patterns for disease modeling. Nat Commun. 2021;12(1):323. doi: 10.1038/s41467-020-20544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grefenstette JJ, et al. FRED (A Framework for Reconstructing Epidemic Dynamics): an open-source software system for modeling infectious diseases and control strategies using census-based populations. BMC Public Health. 2013;13(1):940. doi: 10.1186/1471-2458-13-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gallagher S, et al. SPEW: synthetic populations and ecosystems of the world. J Comput Graph Stat. 2018;27(4):773–784. [Google Scholar]
- 80.Read JM, et al. Social mixing patterns in rural and urban areas of southern China. Proc Biol Sci. 2014;281(1785) doi: 10.1098/rspb.2014.0268. 20140268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang J, et al. Patterns of human social contact and contact with animals in Shanghai, China. Sci Rep. 2019;9(1):15141. doi: 10.1038/s41598-019-51609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiti MC, et al. Quantifying age-related rates of social contact using diaries in a rural coastal population of Kenya. PloS One. 2014;9(8):e104786. doi: 10.1371/journal.pone.0104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kiti MC, et al. Quantifying social contacts in a household setting of rural Kenya using wearable proximity sensors. EPJ Data Science. 2016;5(1):21. doi: 10.1140/epjds/s13688-016-0084-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Glynn J, et al. Effect of acute illness on contact patterns, Malawi, 2017. Emerg Infect Dis. 2020;26(1):44. doi: 10.3201/eid2601.181539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Grijalva CG, et al. A household-based study of contact networks relevant for the spread of infectious diseases in the highlands of Peru. PLoS One. 2015;10(3):e0118457. doi: 10.1371/journal.pone.0118457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ajelli M, Litvinova M. Estimating contact patterns relevant to the spread of infectious diseases in Russia. J Theo Bio. 2017;419:1–7. doi: 10.1016/j.jtbi.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 87.Litvinova M, et al. Reactive school closure weakens the network of social interactions and reduces the spread of influenza. P Natl Acad Sci USA. 2019;116(27):13174–13181. doi: 10.1073/pnas.1821298116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Johnstone-Robertson SP, et al. Social mixing patterns within a South African township community: implications for respiratory disease transmission and control. Am J Epidemiol. 2011;174(11):1246–1255. doi: 10.1093/aje/kwr251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dodd P, et al. Age- and sex-specific social contact patterns and incidence of Mycobacterium tuberculosis infection. Am J Epidemiol. 2016;183(2):156–166. doi: 10.1093/aje/kwv160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McCreesh N, et al. Comparison of indoor contact time data in Zambia and Western Cape, South Africa suggests targeting of interventions to reduce Mycobacterium tuberculosis transmission should be informed by local data. BMC Infect Dis. 2016;16:71. doi: 10.1186/s12879-016-1406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.le Polain de Waroux O et al. Characteristics of human encounters and social mixing patterns relevant to infectious diseases spread by close contact: a survey in Southwest Uganda. BMC Infect Dis. 2018;18(1):172. doi: 10.1186/s12879-018-3073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Horby P, et al. Social contact patterns in vietnam and implications for the control of infectious diseases. PloS One. 2011;6(2):e16965. doi: 10.1371/journal.pone.0016965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Melegaro A, et al. Social contact structures and time use patterns in the Manicaland Province of Zimbabwe. PLoS One. 2017;12(1):e0170459. doi: 10.1371/journal.pone.0170459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang J, et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020;368(6498):1481. doi: 10.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quaife M, et al. The impact of COVID-19 control measures on social contacts and transmission in Kenyan informal settlements. BMC Med. 2020;18(1):316. doi: 10.1186/s12916-020-01779-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCreesh N, et al. Impact of social distancing regulations and epidemic risk perception on social contact and SARS-CoV-2 transmission potential in rural South Africa: analysis of repeated cross-sectional surveys. medRxiv. 2020 doi: 10.1186/s12879-021-06604-8. 2020.12.01. 20241877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Google Google COVID-19 community mobility reports. 2020 Google. Mountain View, CA, USA. www.google.com/covid19/mobility/ Accessed December 2020.
- 98.Saunders MJ, et al. Mobile phone interventions for tuberculosis should ensure access to mobile phones to enhance equity – a prospective, observational cohort study in Peruvian shantytowns. Trop Med Int Health. 2018;23(8):850–859. doi: 10.1111/tmi.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fan J, et al. College Park, MD, USA: University of Maryland; 2020. COVID-19 World Symptom Survey Data API. https://covidmap.umd.edu/api.html Accessed December 2020. [Google Scholar]
- 100.YouGov. London, UK: YouGov; 2020. Personal measures taken to avoid COVID-19. https://today.yougov.com/topics/international/articles-reports/2020/03/17/personal-measures-taken-avoid-covid-19 Accessed December 2020. [Google Scholar]
- 101.Driessche KV, et al. Face masks in the post-COVID-19 era: a silver lining for the damaged tuberculosis public health response? Lancet Respir Med. 2021;9(4):340–342. doi: 10.1016/S2213-2600(21)00020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.World Health Organization. Geneva, Switzerland: WHO; 2019. WHO guidelines on tuberculosis infection prevention and control, 2019 update. [PubMed] [Google Scholar]
- 103.Dharmadhikari AS, et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2012;185(10):1104–1109. doi: 10.1164/rccm.201107-1190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martinez L, et al. Paediatric tuberculosis transmission outside the household: challenging historical paradigms to inform future public health strategies. Lancet Respir Med. 2019;7(6):544–552. doi: 10.1016/S2213-2600(19)30137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ragonnet R, et al. Profiling Mycobacterium tuberculosis transmission and the resulting disease burden in the five highest tuberculosis burden countries. BMC Med. 2019;17(1):208. doi: 10.1186/s12916-019-1452-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCreesh N, White RG. An explanation for the low proportion of tuberculosis that results from transmission between household and known social contacts. Sci Rep. 2018;8(1):5382. doi: 10.1038/s41598-018-23797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martinez L, et al. Transmission of Mycobacterium Tuberculosis in households and the community: a systematic review and meta-analysis. Am J Epidemiol. 2017;185(12):1327–1339. doi: 10.1093/aje/kwx025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McIntosh AI, et al. Partitioning the risk of tuberculosis transmission in household contact studies. PLoS One. 2019;14(10):e0223966. doi: 10.1371/journal.pone.0223966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Glynn JR, et al. Whole genome sequencing shows a low proportion of tuberculosis disease is attributable to known close contacts in rural Malawi. PLoS One. 2015;10(7):e0132840. doi: 10.1371/journal.pone.0132840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brooks-Pollock E, et al. Epidemiologic inference from the distribution of tuberculosis cases in households in Lima, Peru. J Infect Dis. 2011;203(11):1582–1589. doi: 10.1093/infdis/jir162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zelner JL, et al. Age-specific risks of tuberculosis infection from household and community exposures and opportunities for interventions in a high-burden setting. Am J Epidemiol. 2014;180(8):853–861. doi: 10.1093/aje/kwu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Middelkoop K, et al. Transmission of tuberculosis in a South African community with a high prevalence of HIV infection. J Infect Dis. 2015;211(1):53–61. doi: 10.1093/infdis/jiu403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Verver S, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet. 2004;363(9404):212–214. doi: 10.1016/S0140-6736(03)15332-9. [DOI] [PubMed] [Google Scholar]
- 114.Andrews JR, et al. Integrating social contact and environmental data in evaluating tuberculosis transmission in a South African township. J Infect Dis. 2014;210(4):597–603. doi: 10.1093/infdis/jiu138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wilkinson D, et al. Molecular epidemiology and transmission dynamics of Mycobacterium tuberculosis in rural Africa. Trop Med Int Health. 1997;2(8):747–753. doi: 10.1046/j.1365-3156.1997.d01-386.x. [DOI] [PubMed] [Google Scholar]
- 116.Marquez C, et al. The age-specific burden and household and school-based predictors of child and adolescent tuberculosis infection in rural Uganda. PLoS One. 2020;15(1):e0228102. doi: 10.1371/journal.pone.0228102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buu TN, et al. Tuberculosis acquired outside of households, rural Vietnam. Emerg Infect Dis. 2010;16(9):1466–1468. doi: 10.3201/eid1609.100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.The Global Fund. Geneva, Switzerland: Global Fund; 2020. COVID-19 response mechanism. https://www.theglobalfund.org/en/covid-19/response-mechanism/ Accessed January 2021. [Google Scholar]
- 119.Siedner MJ, et al. Protocol: Leveraging a demographic and health surveillance system for Covid-19 Surveillance in rural KwaZulu-Natal. Wellcome Open Res. 2020;5(109):1–15. doi: 10.12688/wellcomeopenres.15949.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kadota JL, et al. Impact of shelter-in-place on TB case notifications and mortality during the COVID-19 pandemic. Int J Tuberc Lung Dis. 2020;24(11):1212–1214. doi: 10.5588/ijtld.20.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]


