Abstract
Life evolved in the presence of alternating periods of light and dark that accompany the daily rotation of the Earth on its axis. This offered an advantage for organisms able to regulate their physiology to anticipate these daily cycles. In each light-sensitive organism studied, spanning single-celled bacteria to complex mammals, there exist timekeeping mechanisms able to control physiology over the course of 24 hours. Endowed with internal timekeeping, organisms can put their previously stored energy to the most efficient use, selectively ramping up biological processes at specific times of day or night according to when they’ll be needed. Humans have evolved to be more active during the day (diurnal), likely due to the increased opportunities for foraging or hunting in our evolutionary past, and this daily activity is accompanied by an upregulation of genes involved in metabolism to increase the energy available for such behaviours. Remarkably, this happens without conscious thought, due to a complex organism-wide signalling apparatus known as the circadian clock network, that conveys time information between cells and tissues.
A clock in every cell
Circadian clocks are self-sustaining timekeepers, able to continue on repeat, cycling through a biological program of events lasting approximately 24h (hence circadian-from Latin circa, ‘about’ and diem, ‘day’). Clocks tick due to a biological feedback loop present in almost every cell of the body. Interlocked cycles of gene expression and protein production drive daily rhythms in nearly all aspects of cellular function. At the centre of this feedback loop in mammals are two nuclear proteins - the aptly named CLOCK (Circadian Locomotor Output Cycles Protein Kaput) and its counterpart BMAL1 (Brain and muscle Arnt-like protein-1), which bind specific regions of DNA called E-boxes and promote nearby gene expression (Figure 1). During the night-time in humans (i.e. rest phase), CLOCK/BMAL1 bind E-boxes within regulatory regions of the genes Per (Period, 1-3) and Cry (Cryptochrome, 1-2). Per and Cry messenger RNAs are produced then translated into PER and CRY proteins in the cytoplasm. As the day begins, PER and CRY interact and pass back into the nucleus. PER/CRY then repress CLOCK/BMAL1, switching off circadian gene expression including their own. Towards the end of the day, enzymes phosphorylate the PER/CRY complex, which targets PER/CRY for degradation and frees up CLOCK/BMAL1 to start the cycle again. This core feedback loop cooperates with a host of additional regulatory mechanisms to form a cellular clock that drives daily rhythmic expression of a large fraction of mammalian genes. Not only does the clock control gene expression, essentially every other subcellular process has been shown to exhibit some level of daily regulation through a variety of cooperative signalling mechanisms (See Further Reading).
Figure 1. The molecular clockwork.
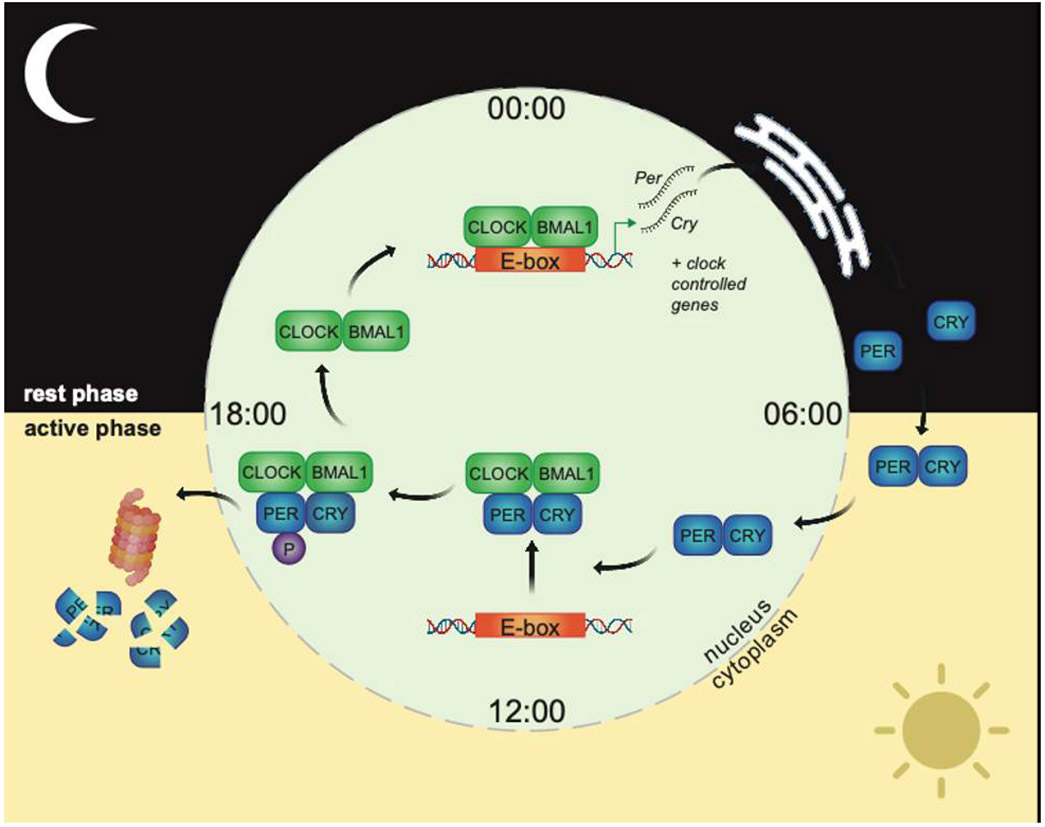
The transcription factors CLOCK and BMAL1 bind DNA E-boxes as a pair, inducing expression of Per and Cry mRNAs. After export to the endoplasmic reticulum, Per/Cry mRNAs are translated into PER/CRY proteins, which build up in the cytoplasm before passing back into the nucleus and repressing CLOCK/BMAL1 transcriptional activity by several mechanisms, including direct binding and dissociation of CLOCK/BMAL from DNA. Phosphorylation (P) of PER2 by specific kinases triggers degradation of the PER/CRY complex by the ubiquitin-proteasome pathway. CLOCK/BMAL1 are subsequently derepressed, and the cycle starts anew. Additional mechanisms, including auxiliary feedback loops, chromatin topology, protein modifications and mRNA processing, work in concert with the core loop to drive 24h hour rhythms at the genome, proteome and signalling level. Figure created using Biorender.com
One of the most important features of circadian clocks is their ability to adapt to changes in the environment. Within the body, specific signals derived from the external environment can act as zeitgebers (‘time-givers’ in German) and reset the timing of cellular clocks. This phenomenon is critical, allowing clock timing to be realigned in response to changing conditions. In addition, cellular clocks throughout the body must also remain synchronised to one another to promote healthy physiological function. Achieving alignment of clocks therefore represents a colossal signalling challenge for organisms, the mechanisms of which are still being revealed.
The circadian network
The first level of functional organisation of cellular clocks occurs at the tissue level; clocks within a particular tissue must behave synchronously in order to cohesively regulate organ function. In the pancreas for example, insulin-producing β cells influence clock timing in glucagon-emitting α cells, so that insulin and glucagon release is timed appropriately in response to food. Despite the importance of cell-to-cell clock coordination within tissues, how this is achieved is not yet well understood. To date, research suggests that such coordination may depend on either the exchange of soluble signalling factors between cells or the physical contacts between cells themselves.
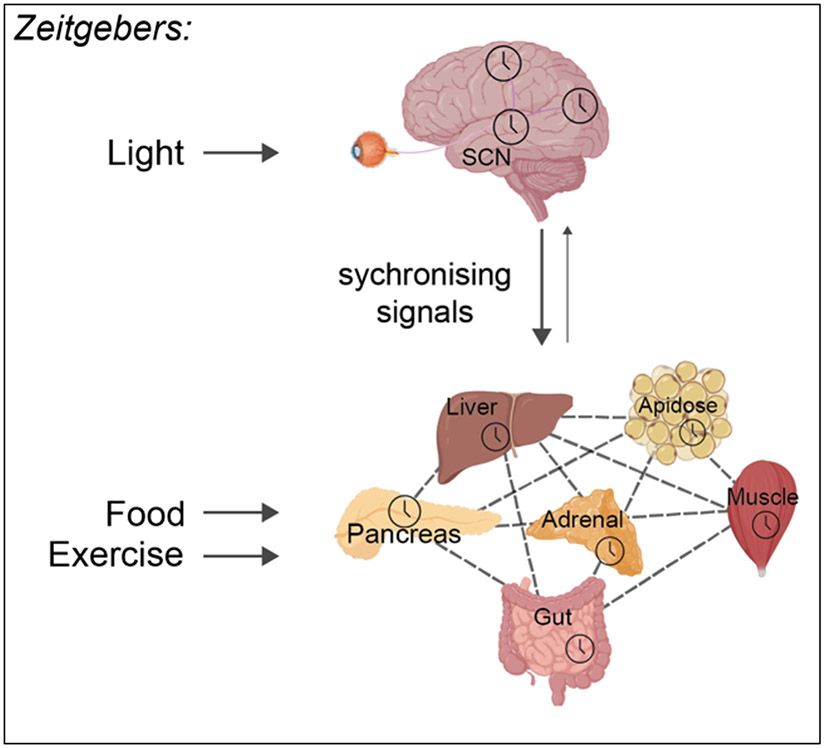
At the next level of organisation, tissue clocks in different organs form a network. In mammals, the clock network is arranged as a hierarchy, with the central clock in the hypothalamic suprachiasmatic nucleus (SCN) at the top (Figure 2). The SCN clock communicates with other brain clocks, and clocks in the rest of the body (i.e. periphery) and can synchronise their rhythms.
Figure 2. Mammalian circadian network organisation.
Light is the major zeitgeber for the central clock in the SCN, readjusting timing daily to be aligned with the light/dark cycle. The SCN communicates with clocks in the rest of the body to synchronise their timing. Clocks in peripheral tissues can also influence each other’s timing, through release of soluble factors in the bloodstream. For peripheral tissues, external factors such as food intake or exercise are important zeitgebers. Signalling from the SCN to peripheral tissues is considered dominant, though peripheral clocks can influence central clock function in certain contexts. Figure created using Biorender.com
Top-down synchronisation
The SCN is made up of a mere 45,000 neurons (less than 0.0001% of the 86 billion neurons in the human brain) which work together with their supporting glial cell counterparts to form the body’s central clock. The dominant zeitgeber for the central clock is light; neuronal input from the retina allows daily readjustment of SCN clock timing to align with the light-dark cycle. At a cellular level, this timing shift is thought be caused by light changing the electrical firing activity of SCN neurons which increases the expression of PER proteins, the negative regulators of BMAL1 shown in Figure 1. The SCN clock then passes on time-of-day information to other brain regions via neuronal connections and release of neuropeptides. To communicate with the periphery, the SCN uses diverse routes including neuronal connections, hormonal release from nearby endocrine glands and control of daily behaviours such as the sleep-wake cycle (Box 1).
Box 1: Synchronising signals from brain to peripheral clocks.

A key synchronising signal for peripheral tissues such as liver, muscle and adipose (fat), is the daily release of hormones called glucocorticoids from adrenal glands, secretory organs that sit on top of the kidneys. The SCN signals to the adrenals directly through neuronal connections and indirectly via control of hormonal release from the nearby pituitary gland. Using these routes, SCN timing cues are translated into daily oscillations in the amount of glucocorticoids released into the bloodstream. Binding of glucocorticoids to their receptors in peripheral tissues then synchronises cellular clocks through triggering a cascade of intracellular signalling events that culminate in changes in the timing of circadian gene expression.
The SCN also influences peripheral clocks indirectly though control of the sleep-wake cycle and its associated behaviours. During waking hours, mammals are more active and consume the majority of their calories, whereas during sleep, there is low movement and food intake is mostly absent. This cycle of feeding and fasting is a major zeitgeber for clocks in peripheral tissues (Box 2). One way feeding signals to clocks in peripheral tissues is by stimulating release of hormones such as insulin into the bloodstream. Similar to the synchronising effect of light in the SCN, insulin alters the timing of peripheral clocks through modulating the levels of the negative regulators of BMAL1. Decades of research has now clearly established that the SCN can impact timing in the rest of the body in a variety of ways. Yet recent evidence shows that the SCN clock does not act alone in this endeavour; peripheral clocks themselves play decisive roles in influencing each other’s timing.
Box 2: The feeding-fasting cycle and peripheral clocks.
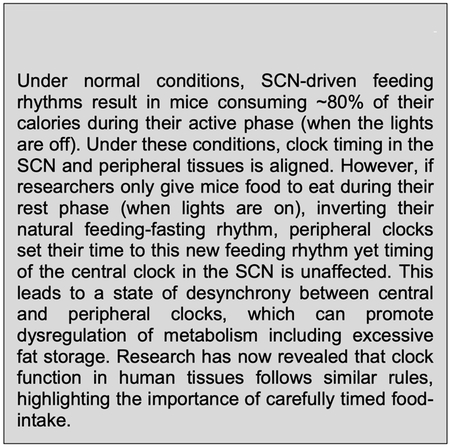
The power of peripheral clocks
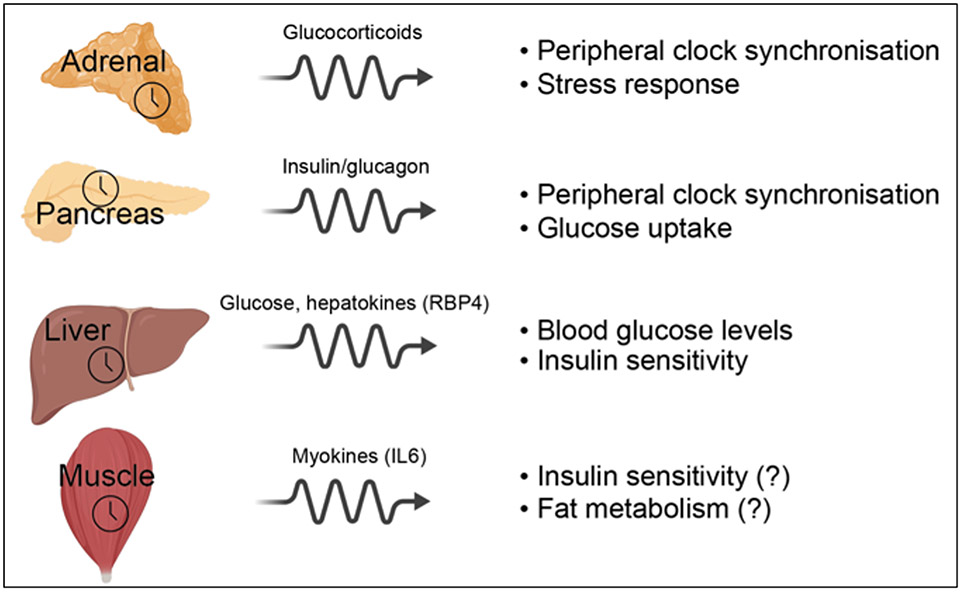
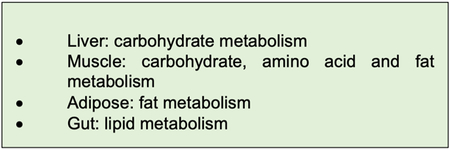
Clocks in the periphery regulate important aspects of metabolic function in each tissue (Box 3). Research is now revealing that signalling between these peripheral clocks is critical in regulating clock timing as well as metabolic output. For example, the local pancreatic clock controls the responsiveness of the pancreas to food-intake, so that the amount of insulin released differs according to the time of day that food is ingested. As insulin can act as a synchroniser in the periphery, the pancreatic clock thereby influences peripheral clock timing and metabolic output. A similar phenomenon occurs with the adrenal glands, in which the local clock determines whether glucocorticoids are released, according to the time of day that SCN input is received.
Box 3: Major metabolic roles of peripheral clocks.
Uncovering additional signals that relay timing information between peripheral clocks is an area of ongoing research. Two potential key players in peripheral clock-to-clock signalling are the liver and muscle (Figure 3). The liver clock controls blood glucose levels, thereby influencing systemic energy use through changing the amount of glucose available for other tissues. In addition, the liver clock controls secretion of small proteins called hepatokines, which so far have been shown to influence the ability of other tissues to take up glucose after a meal. How such liver-clock driven signals affect clocks in other peripheral tissues is not yet well defined. Like the liver, the muscle has its own arsenal of signalling proteins, in this case called myokines. Boosted by exercise, myokines have been shown to regulate energy use in other tissues. Like hepatokines, our understanding of the cellular mechanisms employed by myokines is hazy at best. Intriguingly though, from experiments on muscle cells grown in culture, the muscle clock has been shown to affect the daily rhythmic release of myokines. Excitingly, the influence of the muscle clock on myokine release in live animals remains unexplored.
Figure 3. Peripheral clock signalling output.
Local clocks in peripheral tissues control the rhythmic release of soluble signalling factors into the bloodstream. For many peripheral tissues, the effect of clock-controlled output on timing in other tissues is not well defined. Figure created using Biorender.com
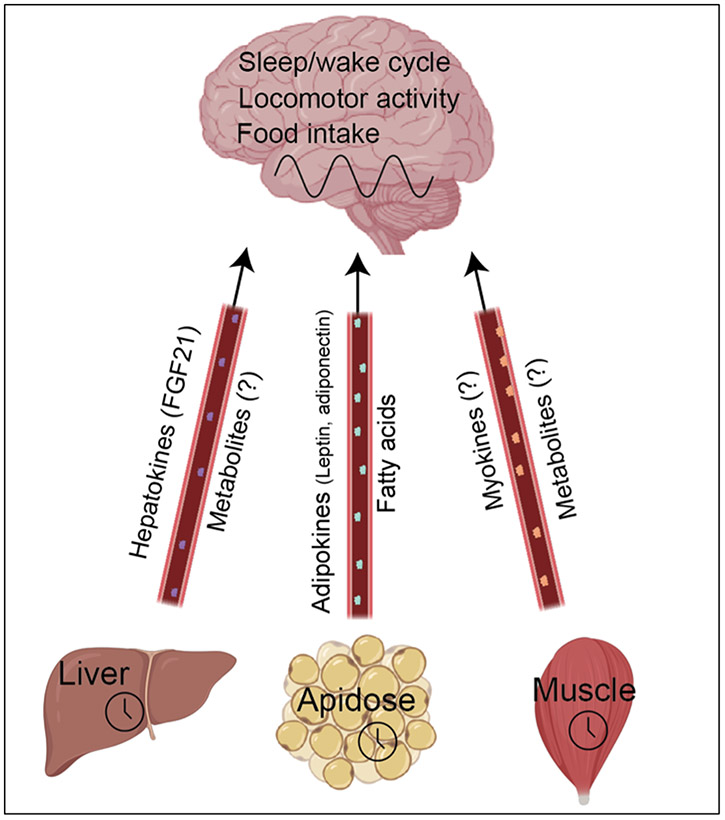
Peripheral clock feedback to the brain
Apart from their direct roles in synchronising each other, peripheral clocks can also signal back to the brain and regulate daily control of behavioural cycles (and thereby indirectly affect their own timing). Using mice in which peripheral clocks have been turned off in specific tissues, researchers have revealed that the liver, adipose, and muscle clocks can all affect behavioural cycles in this way (Figure 4). In adipose tissue for example, the local clock influences secretion of the hormone leptin, which travels in the bloodstream to the hypothalamus and supresses food intake. In addition, peripheral clocks control the secretion of metabolites, chemical intermediates in energy use, many of which can cross the blood-brain barrier and therefore potentially affect brain function. In cases such as the muscle, the identity of molecules that signal back to the brain to regulate circadian timekeeping remains a mystery.
Figure 4. Peripheral clock signalling to brain.
Recent research has revealed that local peripheral clocks can feedback to the brain and alter timing of circadian behavioural cycles, through as-of-yet poorly defined mechanisms. Figure created using Biorender.com
Future directions
Very few examples of peripheral clock communication have been described to date. However, the recent development of genetic mouse models in which clocks are active only in specific tissues, in otherwise ‘clock-less’ mice, offers a new approach to studying links between peripheral clocks. Using this methodology, work from our laboratory has revealed that peripheral clocks certainly cannot go it alone; mice with only a liver clock have just 20% of their normal rhythmic liver function. The next step is to restore clocks in multiple tissues to identify the key routes of communication that support the majority of circadian function in our organs.
Human relevance
Modern lifestyles present our circadian networks with a variety of zeitgebers on a daily basis, acting on central clocks, peripheral clocks, or both. If we mistime our exposure to zeitgebers, e.g. by eating late at night or disrupting our sleep-wake cycle through use of light-emitting mobile devices before bed, this can lead to desynchronisation between brain and peripheral clocks. Desynchronization also happens every time we experience jet lag, as peripheral clocks align to the new environment almost immediately whereas the SCN clock takes much longer to adjust (approximately 1 day per hour of time difference). While jet lag may cause some discomfort short term, long term or repeated desynchronisation may be highly detrimental for our health; night-shift workers, for example, are at a higher risk for obesity, metabolic diseases and cancer. However, there are reasons to be optimistic. The importance of circadian rhythms in overall health is increasingly being recognised, as is the notion that our circadian rhythms can be harnessed to treat disease more effectively. The emerging field of circadian medicine, for example, has revealed that the effectiveness of drugs can be increased by administration at certain times of the day. It is our hope that by understanding how timekeeping between tissues is achieved, it will reveal new ways to promote synchronous circadian function and optimum health.
Biography

Jacob Smith is a postdoctoral researcher is the laboratory of Professor Paolo Sassone-Corsi at University of California, Irvine. Jacob’s research is aimed at uncovering new communication routes for clock-dependent signalling between organs. jacob.smith@uci.edu

Paolo Sassone-Corsi is a professor of circadian biology at University of California, Irvine. Research from his laboratory has revealed the role of peripheral clocks and nutrition in plasticity of circadian function. psc@uci.edu
Further reading
- Koronowski KB, Kinouchi K, Welz PS, Smith JG, Zinna VM, Shi J, Samad M, Chen S, Magnan CN, Kinchen JM, Li W3, Baldi P, Benitah SA, Sassone-Corsi P. Defining the Independence of the Liver Circadian Clock. Cell. 2019. May 30;177(6):1448–1462.e14. doi: 10.1016/j.cell.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosby P, Hamnett R, Putker M, Hoyle NP, Reed M, Karam CJ, Maywood ES, Stangherlin A, Chesham JE, Hayter EA, Rosenbrier-Ribeiro L, Newham P, Clevers H, Bechtold DA, O'Neill JS. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell. 2019. May 2;177(4):896–909.e20. doi: 10.1016/j.cell.2019.02.017. Epub 2019 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke H, Asher G. Crosstalk between metabolism and circadian clocks (Review) Nat Rev Mol Cell Biol. 2019. April;20(4):227–241. doi: 10.1038/s41580-018-0096-9. [DOI] [PubMed] [Google Scholar]
- Dyar KA, Lutter D, Artati A, Ceglia NJ, Liu Y, Armenta D, Jastroch M, Schneider S, de Mateo S, Cervantes M, Abbondante S, Tognini P, Orozco-Solis R, Kinouchi K, Wang C, Swerdloff R, Nadeef S, Masri S, Magistretti P, Orlando V, Borrelli E, Uhlenhaut NH, Baldi P, Adamski J, Tschöp MH, Eckel-Mahan K, Sassone-Corsi P. Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell. 2018. September 6;174(6):1571–1585.e11. doi: 10.1016/j.cell.2018.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin L, Loizides-Mangold U, Skarupelova S, Pulimeno P, Chanon S, Robert M, Bouzakri K, Modoux C, Roux-Lombard P, Vidal H, Lefai E, Dibner C. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol Metab. 2015. August 6;4(11):834–45. doi: 10.1016/j.molmet.2015.07.009. eCollection 2015 November. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock (Review) Cell. 2015. March 26;161(1):84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Paschos GK, Ibrahim S, Song WL, Kunieda T, Grant G, Reyes TM, Bradfield CA, Vaughan CH, Eiden M, Masoodi M, Griffin JL, Wang F, Lawson JA, Fitzgerald GA. Obesity in mice with adipocyte-specific deletion of clock component Arntl. Nat Med. 2012. December;18(12):1768–77. doi: 10.1038/nm.2979. Epub 2012 November 11. [DOI] [PMC free article] [PubMed] [Google Scholar]