Abstract
As the largest human energy reservoir, adipocytes drive an intense dialog with other cells/organs throughout the body to regulate the size of adipose tissue and to communicate with other metabolic tissues and the brain to regulate energy supply. Adipokines have long been described as mediators of this crosstalk, participating in obesity‐associated complications. Recently, adipocyte‐derived extracellular vesicles (Ad‐EVs) have emerged as new key actors in this communication due to their powerful capacity to convey complex messages between cells. Ad‐EVs convey specific subpopulations of RNA, proteins, and lipids from their parental cells, and can transfer these cargoes into various recipient cells, modulating their metabolism and cell cycle. In healthy individuals, Ad‐EVs actively participate in adipose tissue remodeling to compensate energy supply variations by exchanging information between adipocytes or stroma‐vascular cells, including immune cells. Besides this, recent evidence points out that Ad‐EV secretion and composition from dysfunctional adipocytes are strongly impacted within adipose tissue where they modulate local intercellular communication, contributing to inflammation, fibrosis, abnormal angiogenesis, and at distance with other cells/tissues intrinsically linked to fat (muscle, hepatocytes and even cancer cells). Additionally, some data even suggests that Ad‐EVs might have a systemic action. In this review, we will describe the particular properties of Ad‐EVs and their involvement in health and diseases, with a particular focus on metabolic and cardiovascular diseases as well as cancer.
Keywords: adipose tissue, cancer, cardiovascular diseases, diabetes, Exosomes, immune response, obesity
1. INTRODUCTION
Fat cells, or adipocytes, are very large cells (mean diameter of 100 µm), whose major function is the control of lipid storage and release. Apart from this, they have developed the ability to sense, manage, and send signals to provide a continuous energy supply to meet the demands of the organism. Adipocytes are major endocrine cells, releasing hundreds of bioactive molecules known as adipokines. More recently, extracellular vesicles (EVs) have emerged as mediators of adipocyte intercellular communication with organs including brain, liver, muscle, the immune system as well as within the adipose tissue (AT) itself. EV cargo exchange between cells, including lipids, proteins and RNAs, is now regarded as an alternative pathway for cell‐to‐cell communication. 1
As obesity incidence is growing alarmingly, understanding how adipocytes communicate with other cell types is a major health issue. AT expansion in response to chronic excess of nutriments is coupled to AT dysfunction and associated with adipokine and adipocyte‐derived EV (Ad‐EVs) release, contributing to the development of various obesity‐associated diseases. Whereas the role of soluble factors has been extensively studied and reviewed during the past decade, understanding how adipocytes use Ad‐EVs to communicate is an ongoing challenge in the field, and constitutes the basis of this review.
2. THE ADIPOSE TISSUE ORGAN AND ITS CELLULAR HETEROGENEITY
AT has evolved to be the storage compartment for excess energy in vertebrates, representing 10–15% of total body weight in a healthy human. AT is a multi‐depot organ and constitutes the main energy supply in the body to meet an organism's crucial survival needs including fuel for metabolism, lactation, thermogenesis, and immune responses. Initially considered as an inert fat depot, AT is now recognized as a dynamic endocrine organ that secretes various adipokines (hormones, cytokines, lipids…), which control nutrient homeostasis, energy balance, and inflammation. Although the functional cells of AT are indeed the adipocytes, cells composing the stroma‐vascular fraction, namely stem cells, pre‐adipocytes, macrophages, neutrophils, lymphocytes, and endothelial cells, also participate in AT plasticity.
Two classical fat depots are generally distinguished: white AT (WAT) and brown AT (BAT). In humans, WAT distributes at a subcutaneous level (buttocks, thighs, and abdomen) and viscerally, surrounding the inner organs (omental, mesenteric, and retroperitoneal depots), thereby providing insulation and protection. White adipocytes are characterized by an unilocular lipid droplet filling the cytoplasm where energy surplus is stored as triacylglycerols. These highly expandable cells, whose size varies according to triacylglycerol storage from 10 µm up to 150 µm in obese patients, store or mobilize lipids in case of energy demand or starvation. During obesity, all WAT depots are significantly enhanced, through a process that concomitantly induces an increase in adipocyte size (hypertrophy) and number (hyperplasia). BAT, on the other hand, is composed of brown adipocytes characterized by multilocular lipid droplets and possessing numerous mitochondria. Marked expression of the uncoupling protein‐1 (UCP1) enables brown adipocytes to uncouple via oxidative phosphorylation from ATP synthesis to generate heat. Therefore, BAT displays the unique capability to use stored lipids for heat production through the process of non‐shivering thermogenesis. 2 Moreover, recent data illustrated the transdifferentiating potential of adipocytes to meet physiological functions. Thus, chronic cold exposure induces the apparition of immersed UCP1+‐brown like adipocytes within WAT, referred to as beige or brite adipocytes. 2 During pregnancy and lactation, subcutaneous white adipocytes are converted to milk‐producing glands, also called pink adipocytes. 3 Alternatively, the high‐amount of fat retrieved in bone marrow take on a morphology that resembles white adipocytes but displays specific features that led them to be termed yellow adipocytes. 4
Chronic nutrient overload occurring during obesity is moreover signaled by a profound remodeling of fat with AT hypertrophy leading to local hypoxia, inflammation, oxidative or endoplasmic reticulum stresses as well as increased AT immune cell infiltration. Additionally, this pathological AT hypertrophy has been shown to impair angiogenesis and drive fibrosis. Collectively, this ominous triad can therefore contribute to AT dysfunction. 5 The resulting AT remodeling is associated with a chronic low‐grade inflammation, which promotes the development of insulin resistance, a major metabolic dysfunction related to obesity.
AT therefore appears as an organ with an extraordinary plasticity, whose composition evolved according to physiological, environmental, and nutritional conditions. Although composed of different fat depots, all studies published so far have focused on the role of Ad‐EVs derived from white adipocytes only, which are summarized in this review.
3. ADIPOCYTE‐DERIVED EXTRACELLULAR VESICLE CHARACTERISTICS
Extracellular vesicles are nanovesicles released from all cells. Based on their mode of biogenesis, composition, and size, EVs can be divided into subgroups consisting of exosomes (EXOs), microvesicles (MVs), and apoptotic bodies (ABs) (Figure 1).
FIGURE 1.
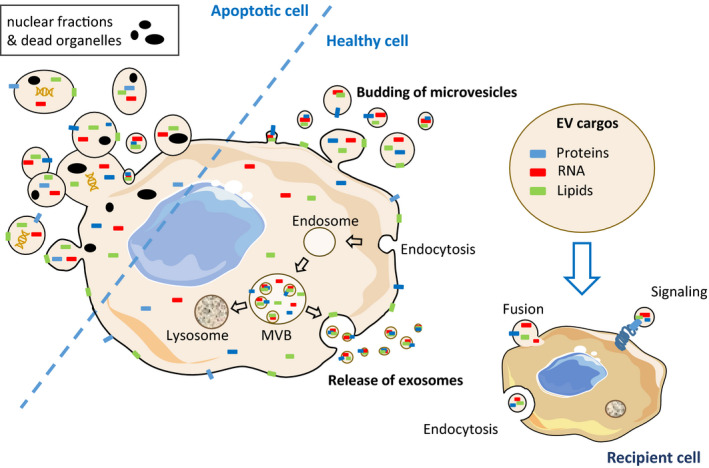
Major populations of extracellular vesicles (EVs) released from cells. Apoptotic bodies are produce during apoptosis and can contain fragmented DNA, RNA, nuclear, or organelle materials. Exosomes and microvesicles are released by live cells. EVs can target recipient cells either through receptor binding/activation (signaling), direct membrane fusion, endocytosis/pinocytosis, or internalization
EXOs are nanosized (50–100 nm) vesicles formed by the inward budding of the limiting membrane of the late endosomes, leading to the generation of multivesicular bodies (MVB) containing intraluminal vesicles (ILVs). ILVs are either released as exosomes in the extracellular milieu by fusion of the MVB with the plasma membrane, or degrade if MVBs fuse with the lysosomes. The molecular mechanisms that regulate the fate of MVBs are not completely understood. 6 EXO lipid composition reflects that of endosomes that is 2–3 times enrichment in EXOs compared with cells in terms of cholesterol, sphingomyelin, glycosphingolipids, and phosphatidylserine, whereas expressing less phosphatidylcholine than their cells of origin. 7 The proteins sequestered in EXOs are mainly involved in their biogenesis, and these include proteins involved in ILV formation from the ESCRT (Endosomal Sorting Complex Required for Transport), tetraspanins, proteins targeted for their degradation or release, or signaling proteins owing to the central role of endosomes in various cell events. 8 In addition to lipids and proteins, EXOs also contain nucleic acids. Some specific mechanisms have been identified for loading into EXOs, but until now, no generic pathway can be utilized to explain the sorting of RNAs into EXOs. 9
The two other types of EVs (MVs and ABs) have larger sizes than EXOs. ABs (500 nm–5 µm) are generated from the disassembly of apoptotic cells. They contain nanoliters of material from fragmented cells including RNAs, lipids from the plasma membranes, and sometimes DNA. 10 MVs (100–500 nm) are formed by cell membrane budding possibly in response to the increased intracellular calcium levels (Figure 1). The loss of cell membrane asymmetry in phospholipid distribution regulates membrane flippase, floppase and scramblase activities, leading to phosphatidylserine and phosphatidylethanolamine exposure on the outer membrane leaflet, and the activation of contractile proteins involved in MV release. 11 Phosphatidylserine is a negatively charged phospholipid, and thus results in the higher clotting capacity of MVs compared with EXOs and ABs. 12 MVs, like ABs, contain plasma membrane‐associated proteins, lipids, and RNAs from the cytosol.
Taken together, when considering the diversity of the material encompassed in EVs, they will likely interact via different processes with the recipient cell and send a far more complex signal in comparison to the one mediated by individual soluble molecules. In addition to their lipid and protein content, the three EV subtypes also differ in their RNA content (for a recent review, see 13 ).
Given the overlapping size and composition between EXOs/MVs/ABs, it is very difficult to obtain pure populations of each subtype after EV isolation. Using more stringent isolation methods, recent works have shown the existence of subpopulations of vesicles within the EXO population, further complicating their functional characterization. 13 , 14 , 15 , 16 , 17 Therefore, to consider isolation bias, we will use the generic term of EVs in this review (respectively large and small EV for those obtained at 10,000 g and 100,000 g), but we recommend the reader carefully check the Material and Methods of each mentioned article to identify the origin of the vesicles mentioned.
3.1. Adipocyte‐derived extracellular vesicle specificities
3.1.1. Models & strategies for isolation and characterization
When studying EVs produced by a specific cell type, identifying the nature of the EV releasing cell is imperative. This is a major concern for adipocytes since as are no in vitro cellular models that fully recapitulate in vivo adipocyte metabolism and morphology. Therefore, establishing comparative studies between the different adipocyte models can contribute to improving the significance of findings. Adipocyte models used for Ad‐EV isolation include primary adipocytes 18 as well as adipocytes differentiated in vitro from primary pre‐adipocytes 19 , 20 or from pre‐adipose cell lines, most notably 3 T3‐L1 21 , 22 and 3 T3‐F442A cells. 23 Adipogenic cell lines are often used as a model despite (i) their inability to fully recapitulate the hormonal and metabolic characteristics or the diversity of primary adipocytes, (ii) their inability to be used to study the impact of obesity, and (iii) their in vitro growth independent from their natural microenvironment. 24 Primary adipocytes obtained by collagenase digestion of AT followed by flotation isolation can circumvent these limitations, although such enzymatic treatment may induce important cell stress. Furthermore, isolated fat cells lack the ability to proliferate and can only be maintained in culture for few days. 25 To improve their viability, 3D culture systems using collagen or fibronectin can be used. 26 Alternatively, AT explants can be used as a starting material. In this case, EVs retrieved in the conditioned medium do not only originate from adipocytes, but are also derived from all stromal cells, with some vesicles staying trapped in the extracellular matrix. Here, potential contamination by lipoproteins can also be avoided. 19 , 27 Finally, EVs can also be isolated from biological fluids, especially when studying the impact of obesity on circulating EVs. 28 Despite evidence highlighting the presence of adipokine‐associated EVs in blood, 28 , 29 , 30 there are currently no markers robust enough to primarily allow the identification and quantification of circulating Ad‐EVs, rendering their detection so far controversial and elusive. 30 , 31
Regarding Ad‐EV separation and enrichment, the vast majority of studies employs “classical” isolation methods, including centrifugation‐based protocols or commercial kits that are mainly adapted for small EV subtype isolation. Such techniques do not exclude contamination by non‐EV structures, including lipoprotein particles that would co‐precipitate especially when bio‐fluids like blood plasma 32 , 33 or AT explants‐conditioned media 34 are used as EV sources. To evaluate such potential contaminations, the presence of lipoprotein markers such as APOB and APOE may be determined by western blot analysis. Additional separation of the 100,000 g pellet could also be performed by utilizing size exclusion chromatography, for example, to further purify EVs. 18 , 35 More recently, AF4 (Asymmetric‐Flow Field‐Flow Fractionation) technology has been proposed to better isolate EVs from other particles such as lipoproteins. 36 Interestingly, a recent paper reported that Ad‐EVs isolated by an alternative protocol involving filtration and size fractionation are superior to the use of “standard purification strategies” that may be inefficient due to the high lipid content of these EVs. 31 By using such methods, the authors manage to obtain an Ad‐EV concentration 10 to 100 times higher as compared with the numerous published articles on Ad‐EVs that make use of the more “classical” protocols. Such discrepancy is indeed difficult to explain, inasmuch as the “standard purification strategies for exosome purification” used in this study is not described. Nonetheless, the high Ad‐EV purification yield might be overestimated due to the co‐isolation of adipocyte lipid droplets as testified by the high lipid droplet marker perilipin‐1 signal detected in the Ad‐EV isolated fraction.
3.1.2. Ad‐EV content
As previously indicated, EV cargo includes proteins, nucleic acids, and lipids. Several studies have analyzed Ad‐EV content using cell lines or primary adipocytes as cell sources based on targeted or untargeted methodologies. Most of these studies have focused on small EVs, whereas we analyzed both 3 T3‐L1 adipocyte‐derived large and small EV contents. 22 Among proteins conveyed by small Ad‐EVs, adipocyte‐specific cargo, including adiponectin FABP4/aP2 or perilipin‐1, have been identified apart from classical EV markers. Such results highlight a potential new EV secretion pathway for adipokines, whose contribution and regulation would need to be further explored. 37 This is indeed highly interesting because here, we identified large EVs as a specific sorting pathway for the cytokine Macrophage Migration Inhibitory Factor (MIF) and that EV‐associated MIF effects relies on a non‐canonical signaling pathway that influences MIF tautomerase activity. 28 Importantly, using comprehensive isotope‐based proteomics, we also revealed that less than one third of small Ad‐EV proteins are effectively transferred to melanoma target cells. Some abundant proteins within Ad‐EVs were not transferred, suggesting a highly selective transfer and/or uptake of EV cargo. 18 Besides, microRNA (miRNA) content identification has revealed the presence of many miRNAs, like miR‐27a 38 and miR‐34a, 39 which may affect target cell behavior. However, none of them seems to be specific to Ad‐EVs. Finally, although lipid content has been less studied, Ad‐EV phospholipid lipid composition is unsurprisingly close to that of adipocyte plasma membrane. 21 , 22 Fatty acid (FA) profiling has moreover revealed the particular enrichment of palmitic acid, an FA known to participate in modifying insulin sensitivity, within small Ad‐EVs in comparison with EVs from other cell sources. 21 In obesity, AT is subjected to chronic stress such as hypoxia or inflammation that can modify EV content in terms of both quality and quantity. 18 , 23 , 31 As Ad‐EV content in normal and pathologic conditions has been previously listed in other review articles, 40 , 41 , 42 , 43 it will not be detailed here.
3.1.3. Modulation of Ad‐EV‐specific content & functions in healthy context
AT homeostasis varies throughout the day and depends on food intake, which in itself depends on circadian rhythms and energy requirements. Recent data indicate that within the AT, not only the biogenesis of Ad‐EVs but also the selective sorting of proteins, lipids, and miRNAs into the EVs and their consequent release plays a fundamental role in regulating the global storage of fat in AT, as well as the ratio of large and small adipocytes present in the tissue. In this context, it has been demonstrated that Ad‐EVs released from large adipocytes are able to induce lipid synthesis and differentiation of small adipocytes. 44 The rate of internalization of Ad‐EVs derived from large adipocytes was higher than internalization of Ad‐EVs derived from small adipocytes, suggesting that Ad‐EVs from hypertrophied fat cells may participate in recruiting small adipocytes for AT expansion. 44 Others have demonstrated that Ad‐EVs can also be internalized by AT‐derived stem cells, promoting adipogenesis. 45
Different studies have highlighted a change in Ad‐EV composition along the adipogenic process, likely recapitulating parental cell phenotype. These small adipocytes released Ad‐EVs enriched in proteins for cell growth and homeostasis, namely PREF‐1 and PPARγ, and arachidonic acid, 21 whereas large adipocyte‐derived small Ad‐EVs were enriched in proteins involved in fibrotic processes/inflammation, signaling pathways, membrane‐mediated processes, metabolic pathways and cardiolipin. 46 They have been reported to be also enriched in miRNAs that are known to be involved in adipogenesis (let‐7b, miR‐103, miR‐146b, miR148a). 47 The data 48 demonstrated that some of these proteins (i.e., TRPML1) are both involved in the membrane fusion between the MVB and the plasma membrane and in adipocyte differentiation. Beside proteins and RNAs, the quantity of phosphatidylethanolamine, phosphatidylinositol, sphingomyelin, and cardiolipin appeared to increase with differentiation whereas phosphatidylserine decreased. 21
The identification of the precise EV cargo‐driven biological effects observed in healthy individuals remains so far elusive. Microarray analysis has revealed that Ad‐EVs isolated from differentiated adipocytes contain 7000 mRNAs, mainly involved in metabolic processes, and 140 microRNAs. 47 Both mRNAs (involved in FA esterification, lipid droplet biogenesis or coding for adipokines) and miRNAs were found to be transferred into small adipocytes, and associate with increased lipogenesis and adipocyte cell size. 49 Moreover, Sano et al suggested that enzymes contained in small Ad‐EVs released by large adipocytes (i.e., acetyl‐CoA carboxylase, glucose‐6‐phosphate dehydrogenase, and fatty acid synthase) could also be transferred into small adipocytes. 50 However, none of these two studies firmly demonstrated the functionality of transferred proteins and RNAs in the recipient cells.
The function of AT is to maintain systemic metabolic homeostasis despite fluctuations in nutrient availability during the day, and this homeostasis depends on food intake (fed vs fasted state), the quality and quantity of the diet (high‐fat diet vs normal diet) and hormonal regulation (e.g., insulin, glucagon, etc). Different studies evidenced that these parameters regulating energy fluctuations influence Ad‐EV composition and function. Recent data indicated that treatment of adipocytes with supra‐physiological concentrations of insulin for 48 h induced the release of a new population of small Ad‐EVs enriched in 116 proteins (including MMP2, FABP4/aP2, TGFBI), many of them known to interfere with insulin signaling pathways. 46 However, insulin affects neither small EV release 46 , 51 nor EV protein/lipid ratio. 46 These data rather suggest that alternation of fed/fasted conditions modulate Ad‐EV composition. In line with this hypothesis, proteomic analyses of the whole population of small EVs released from AT under fasted state showed a significant enrichment in proteins involved in lipid and amino acid metabolisms. 51 Interestingly, FABP4/aP2 is recruited to MVB sorting pathways under lipolytic conditions, which identify small Ad‐EVs as an alternative secretory route for this lipid transporter. 37
The quantity and the quality of lipids in the diet are also likely to affect EV release and composition. For instance, we have found that a palmitate‐enriched diet triggered the secretion of small EVs highly enriched in this lipotoxic FA from skeletal muscle, which changed their biological properties and perturbed skeletal muscle homeostasis. 52 Lipidomic analyses of small Ad‐EVs released from AT explants from obese mice fed a high‐fat diet also revealed a strong enrichment in palmitate, in addition to stearate, in comparison with Ad‐EVs from mice fed a standard chow diet. 53 in vitro, the treatment of adipocytes with palmitate or oleate 22 , 46 led to adipocyte hypertrophy, which may suggest that these FA‐enriched Ad‐EVs could also play a part in lipid storage enhancement. Small Ad‐EVs released from hypertrophic adipocytes have twice the protein content compared with control Ad‐EVs. 46 These proteins are mainly involved in energy and protein metabolism, cell growth, and signal transduction. Moreover, long chain omega‐3 fatty acid docosahexaenoic acid (DHA) treatment enhances the secretion of small Ad‐EVs enriched in adiponectin 54 that may contribute to the increased transfer of this insulin‐sensitive adipokine into Ad‐EV recipient cells. In addition, palmitate, but not oleate, also increases both small and large Ad‐EV release. 22 Interestingly, small Ad‐EV protein contents from adipocytes treated either with oleate or palmitate exhibited different profiles, suggesting that the nature of the lipid diet might specifically modulate Ad‐EV composition. 46 Finally, high level of intracellular cholesterol also affects the formation of MVB and the release of small EVs. 55 The mechanism by which lipid composition of the diet affects Ad‐EV release and composition, and participates in AT homeostasis, definitely deserves further investigation.
It was also suggested that small Ad‐EVs could modulate body energy intake. When injected intravenously, Ad‐EVs from lean mice decreased food intake and weight in obese mice. 56 Although data from this study did not provide conclusions on the direct or indirect effects of Ad‐EVs on the hypothalamus, they demonstrate that Ad‐EVs could regulate hypothalamic mTOR signaling following their uptake by hypothalamic anorexigenic POMC neurons. In addition, it was hypothesized that Ad‐EVs derived from AT mesenchymal stem cells may delay progression of nonalcoholic fatty liver disease through the release of anti‐fibrotic miR‐122 into circulation 57 and that small Ad‐EVs miR‐99b released by AT could regulate hepatic Fgf21 expression, thereby improving glucose tolerance. 30
Since the discovery that small Ad‐EVs could be taken up by peripheral blood monocytes, 53 many studies have demonstrated the role of Ad‐EVs in the inflammatory response associated with the development of AT. 41 However, inflammation is not solely a pathophysiological phenomenon associated with obesity, but is also an essential adaptive response for healthy AT development. 58 Moreover, small EVs from adipose‐derived stem cells (ADSC) of healthy lean animals attenuate inflammation within AT by inducing M2 macrophage polarization, thereby promoting AT beiging and breakdown of fat to facilitate metabolic homeostasis. 59 Such crosstalk between immune cells and adipocytes could imply the transfer of different cargos including the mRNA encoding the adipose‐related proteins resistin, PPARγ and adiponectin, 60 miRNA (e.g., miR‐155), 61 and lipids. 31
Altogether, Ad‐EVs derived from healthy adipocytes favor AT homeostasis and function by promoting adipose lipid storage and insulin sensitivity (Figure 2).
FIGURE 2.
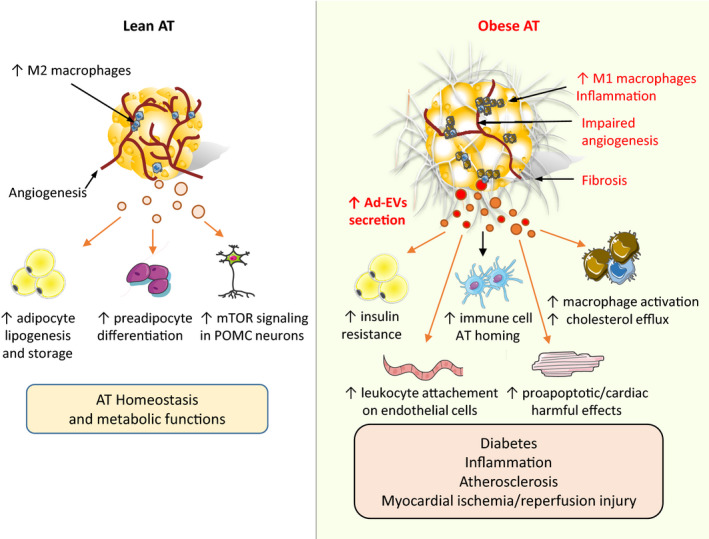
Comparative effects of adipocyte‐derived EVs derived from a healthy lean or dysfunctional obese adipose tissue. Obesity triggers important adipose tissue (AT) remodeling including M1 macrophage infiltration, insufficient angiogenic potential, and fibrosis, all contributing to the pathogenesis of dysfunctional adipose tissue (AT). Secretion of adipocyte‐derived EVs (Ad‐EVs) is enhanced in obesity, and Ad‐EV contents likely reflect the pathophysiological status of their parental cells. Ad‐EVs can be transferred to multiple recipient cells and thereby contribute to AT homeostasis and metabolic function in healthy conditions, whereas they participate to obesity–metabolic dysfunction development when derived from obese AT. Evidences supporting these mechanisms are detailed in the text
4. ADIPOCYTE‐DERIVED EXTRACELLULAR VESICLES ARE INVOLVED IN DISEASES
4.1. Ad‐EVs and obesity‐associated metabolic diseases
Metabolic diseases refer to all the diseases caused by dysfunctional metabolic activity and are often linked to dysfunctions affecting several organs, signifying lipid, inflammatory, and endocrine modifications. The coexistence of different metabolic disorders, including hyperglycemia, dyslipidemia, hypertension, obesity, and insulin resistance, therefore defines the metabolic syndrome. Combination of these risk factors significantly increases the risk for cardiovascular diseases and cancer. Of note, visceral obesity is considered as one of the most deleterious factors in metabolic syndrome and is associated with chronic low‐grade inflammation state.
4.1.1. Ad‐EV production is enhanced in obesity
Ad‐EV secretion has been shown to be significantly enhanced in genetically modified or high‐fat diet‐fed obese rodent models 23 , 53 or following obesity‐related stress conditions in vitro such as hyperglycemia/hyperinsulinemia, hyperlipidemia, or hypoxia. 46 , 62 Increase in circulating EVs in obese mice and humans has also been observed in many studies in comparison with healthy controls, whereas energy restriction or bariatric surgery leads to a decrease in plasma EV concentrations, suggesting that Ad‐EVs could contribute to increases in the EV circulating pool. 20 , 28 , 63 Nonetheless, in the absence of specific Ad‐EV markers, it is difficult to ascertain that such a rise is only due to these vesicles.
4.1.2. Obese Ad‐EVs are mediators of insulin‐resistance and inflammation
Different studies recently pointed out the potential role of Ad‐EVs in metabolic complications associated with obesity (see Figure 2 and below for detailed mechanisms). Intravenous delivery of small Ad‐EVs or AT macrophage (ATM) EVs derived from obese mice both induce systemic insulin‐resistance in lean mice and lead to altered insulin signaling in metabolic organs, including visceral AT, liver and muscle, and enhances the inflammatory profile of the treated animals. 53 , 64 Simulating obesity dysfunctions in vitro by exposing adipocytes to high‐glucose, high‐insulin, high‐lipid or inflammatory stimuli as well as hypoxia, stimulate Ad‐EV production. 22 , 46 , 62 Noticeably, small Ad‐EVs from hypoxic or obese adipocytes impair insulin‐signaling pathways in adipocytes in vitro, as illustrated by AKT pathway alterations. 46 , 62 Alternatively, obese small Ad‐EVs induce BMDM‐macrophage activation with increased homing properties to AT or liver, illustrating concomitant inflammatory response. 53
4.1.3. Molecular Ad‐EV cargos and metabolic dysfunctions
Different cargos transported by Ad‐EVs from obese subjects have been suggested to mediate such metabolic dysfunction. This includes adipokines, enriched in pathological EVs, which actively participate in the development of insulin‐resistance. 46 , 65 Notably, heat treatment of hypoxic small Ad‐EVs abolishes the inhibitory effects of small EVs on glucose transport, pointing toward the thermolabile‐dependent effects of these Ad‐EVs, which might be related to EV‐associated enzymatic activity. 62 Besides, miRNA profile analysis revealed the specific enrichment of Ad‐EVs in mRNA and microRNA (miRNA). 30 , 47 One such study even suggested that small Ad‐EVs might play a role as the main conveyor of circulating miRNA, because transplantation of AT into mice with an AT‐specific knockout of the miRNA‐processing enzyme Dicer restores the level of numerous circulating miRNAs associated with glucose tolerance improvement. 30 Among the miRNAs specifically enriched in Ad‐EVs, miR‐155‐associated Ad‐EVs induce M1 macrophage polarization and regulate insulin sensitivity. 61 , 64 Interestingly, miR‐155 targets two essential genes of adipocyte differentiation, the adipogenic transcription factor CEBPβand PPARγ, and has also been shown to regulate brown lineage commitment, thereby controlling the development of brown and beige fat cells. 66 Finally, small Ad‐EVs were recently presented as an alternative pathway to lipolysis for local lipid release, being able to modulate tissue macrophage differentiation and function. 31 Accordingly, we demonstrated that small Ad‐EVs are laden with enzymatic machinery, especially from mitochondrial origin, as well as FAs, which can be transferred to melanoma cells. 18 Whether Ad‐EVs are able to enclose and transfer mitochondria as recently described for activated monocytes‐EVs for instance 67 would need to be further explored because a transfer of adipocyte mitochondria to macrophages has been very recently demonstrated as a key process regulating systemic metabolic homeostasis. 68
4.1.4. Complex interplay between Ad‐EVs, immune cells and metabolic disturbances
Altogether, these data suggest that Ad‐EVs produced in the pathophysiological context of obesity are able to signal to metabolic organs, thereby triggering local and systemic inflammation and insulin‐resistance. These two metabolic responses are intrinsically linked and questions if this is a direct effect of Ad‐EVs on metabolic tissues or an indirect effect via EV‐activated immune cell modulation. This last scheme is supported by the fact that Ad‐EVs act as “find‐me” signals for the recruitment of monocytes and macrophages, enhancing macrophage infiltration within AT. 69 Moreover, human obese AT‐derived EVs fail to alter insulin signaling pathway in hepatocytes and muscle cells, in contrast to healthy adipocytes, which may reflect the necessity of primary Ad‐EV‐induced macrophage activation. 65 The diabetic environment favors a vicious cycle when it comes to EV production, which translates into significantly higher EV circulating levels in diabetic patients with altered cargo content that are prone to be internalized by leukocytes and to alter leukocyte function. 70 Accordingly, correlations between EV circulating levels and homeostatic model assessment of insulin resistance HOMA‐IR have been reported. 20 , 28 Further studies definitely would be needed to elucidate the interplay between Ad‐EVs and the neighboring adipose environment, including immune cells, and the precise mechanisms underlying metabolic dysfunctions.
4.2. Ad‐EVs and cardiovascular diseases
Despite the improvement in the management of risks factors, cardiovascular diseases (CVD) remain the principal cause of deaths worldwide. So far, efficient treatment to treat CVD is lacking. EVs have emerged as a new potential promising treatment in several contexts such as cancer and CVD. EVs released from hypertrophic adipocytes can impair endothelial cell function, suggesting a possible role for Ad‐EVs in obesity‐related atherosclerosis. 71 Evidence has been abundant in the validation of this concept. Inflammatory small Ad‐EVs promote leukocyte attachment to vascular endothelial cells, suggesting a role of such EVs in the setting of atherosclerosis. 72 Ad‐EVs interfere in the regulation of macrophage cholesterol homeostasis by increasing macrophage cholesterol efflux, thereby promoting the development of atherosclerosis. 73 Moreover, in vivo data have revealed that small Ad‐EVs can promote atherosclerosis plaque vulnerability and atherosclerosis by inducing angiogenesis of the vasa vasorum in diabetic atheroprone mice. 74
Recently, in vivo studies focusing on the role of Ad‐EVs in the homeostasis of cardiomyocytes revealed that Ad‐EVs derived from diabetic adipocytes were transferred into cardiomyocytes in the context of ischemia reperfusion. This transfer involves the Ad‐EV cargo miR‐130b‐3p. By acting on downstream targets such as AMPKα1/α2, Birc6, and Ucp3, the transfer of this miRNA leads to deleterious effect on cardiac function, suggesting that small Ad‐EVs act as pro‐deleterious messengers. 75 By contrast, different studies have suggested the beneficial effects of ADSC‐EVs in cardiac recovery, rendering them a great potential for use as therapeutic tools in regenerative therapies. 76 Attesting to their beneficial effects, small ADSC‐EVs have also been shown to reduce apoptosis in cardiomyocytes that were subjected to oxidative stress. 77 Furthermore, miR‐93‐5p‐enriched small ADSC‐EVs can prevent cardiac injury by interfering with autophagy and inflammation, leading to beneficial outcomes in the context of acute myocardial infarction and resulting injury. 78 Alternatively, ADSC‐EVs injections in a deoxycorticosterone acetate (DOCA)‐salt hypertensive rat model prevented cardio–renal dysfunction by limiting hypertension and subsequent damage. 79 Altogether, the precise role of Ad‐EVs, apart from ADSC‐EVs, is still poorly understood in the context of CVD and will require further investigations.
4.3. Ad‐EVs as major actors in cancer
The tumor microenvironment is composed of stromal cells, including fibroblasts, immune cells and endothelial cells, and the extracellular matrix. 80 Stromal cells are reprogramed by malignant cells to promote tumor growth and resistance to treatment. 80 , 81 This crosstalk must be highly coordinated, depending on an active communication between tumor and stromal cells that rely, at least in part, on EVs. Indeed, EVs play a key role in local and systemic cell‐to‐cell communication in cancer, being involved in communication between tumor cells as well as between tumor and stromal cells for tumor growth, metastasis, immune tolerance, and treatment resistance. 82
Apart from stromal cells, adipocytes were long forgotten though many cancers develop in the vicinity of AT. Today, the role of adipocytes in tumor initiation, progression, and drug resistance is clearly recognized. This deleterious association between adipocytes and tumor cells depend on systemic and local effects. 83 , 84 First, adipokines such as leptin, adiponectin, and interleukin 6 have been shown to modulate tumor progression. 85 More recently, a vicious cycle has been described at the invasive front of tumors, where adipocytes are in close proximity to cancer cells. This suggests that tumor secretions hence induce adipocyte activation. These activated adipocytes, named cancer‐associated adipocytes (CAAs), are delipidated and present a decreased expression of adipose markers as well an increased secretion of inflammatory cytokines and matrix remodeling proteases. 86 , 87 , 88 CAAs are also able to modify tumor cell properties, leading to heightened aggressiveness. Interestingly, the FAs released by adipocyte lipolysis are taken up by cancer cells to fuel their metabolism, further contributing to tumor progression. 89 , 90 , 91 EVs have recently emerged as new key actors in the dialog that takes place between tumor cells and adipocytes (see Figure 3 and below for detailed mechanisms).
FIGURE 3.
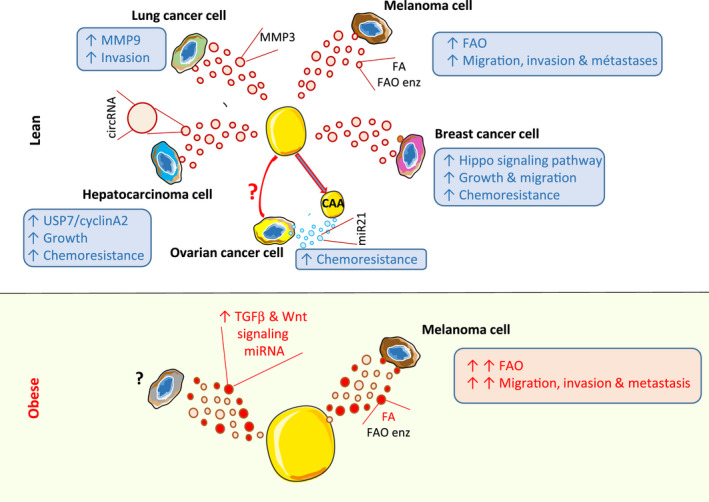
Adipocyte‐derived EVs contribute to cancer aggressiveness, a process amplified in obesity. Adipocytes secrete extracellular vesicles (Ad‐EVs) that are taken up by tumor cells, leading to increased aggressiveness. The associated mechanism and EV cargos involved are indicated. In obese individuals, the secretion of Ad‐EVs is modified quantitatively and qualitatively, exacerbating their deleterious effects. Enz, enzymes; FA, Fatty acids; FAO, fatty acid oxidation
4.3.1. EVs from “naïve” adipocytes
The first evidence linking Ad‐EVs and cancer was published in 2016. In this study, using different adipocyte models (3 T3‐F442A adipocytes and subcutaneous/visceral primary adipocytes), we have shown that, in prostate cancer and melanoma models, small Ad‐EVs enhance tumor cell migration, invasion, and lung metastases. 23 The pro‐invasive potential of small Ad‐EVs has further been confirmed in lung cancer, using 3 T3‐L1 Ad‐EVs. 92 Other studies have shown that small Ad‐EVs from 3 T3‐L1 adipocytes also promote hepatocellular carcinoma growth and increase chemo‐resistance. 93 , 94 Finally, small Ad‐EVs from in vitro differentiated mesenchymal stem cells increase breast cancer cell growth, migration, and chemo‐resistance. 95 Nevertheless, mechanisms that orchestrate Ad‐EV effects are diverse. In melanoma models, small Ad‐EVs transfer both the enzymes and the substrate (FAs) to tumor cells to perform fatty acid oxidation (FAO). 18 , 23 In line with this, melanoma cells treated with Ad‐EVs exhibit increased FAO that is required for heightened migration. Mechanistically, the FAs, which are transported by EVs from adipocytes to melanoma cells, are stored in lipid droplets, and then mobilized to fuel FAO by lipophagy. Finally, heightened FAO fuels tumor aggressiveness by increasing the migration of melanoma cells through a mechanism dependent on mitochondrial dynamics. In hepatocellular carcinoma, Zhang et al have revealed that small Ad‐EVs modify tumor cell behavior through the transport of circular RNAs (circRNAs) related to deubiquitylation. 93 In tumor cells, the horizontal transfer of these circRNAs led to miR‐34a suppression by a sponge effect and, consequently, activated the USP7/Cyclin A2 signaling pathway in tumor cells, leading to increased aggressiveness. In lung cancer, the transfer of MMP3 protein by small Ad‐EVs activated MMP9 activity in tumor cells, inducing increased aggressiveness. 92 Finally, in breast carcinoma, using a transcriptomic analysis of small Ad‐EV‐treated MCF7, the authors identified several activated pathways, including the Hippo signaling pathway. They have then confirmed its implication in Ad‐EV pro‐tumoral effects. 95
The diversity of Ad‐EV‐related molecular mechanisms favoring tumor progression therefore questions whether these processes co‐exist in the cell or if they are specific to a cell‐type/tumor type, which yet remains to be elucidated.
4.3.2. EVs from cancer‐associated adipocytes
Whereas many studies have analyzed the impact of EVs released by “naïve” adipocytes on tumor cells, strikingly, only one publication deals with EVs released by CAAs. In this study, using next‐generation sequencing, Au Yeung et al have shown that, in advanced ovarian cancer, miR21 is more abundant in EVs shed by CAAs compared with normal Ad‐EVs. Furthermore, this miRNA was transferred to epithelial ovarian carcinoma cells where it suppresses apoptosis through targeting APAF1, resulting in paclitaxel resistance. 96 Further research into this issue is needed to gain a deeper understanding of how CAA‐derived EVs influence cancer cell behavior.
4.3.3. Ad‐EVs in obesity‐related cancers
Epidemiological studies have shown that excess body weight is an established risk factor for many cancer incidence 97 and progression. 98 Indeed, preclinical studies have confirmed these deleterious associations (for a recent review, see 84 ). Several mechanisms drive the pro‐tumoral effect of obesity. First, endocrine processes involving alterations in insulin/IGF1, hormone signaling or adipokines have been shown to link obesity and cancer. Furthermore, the chronic inflammation observed in obese patients adversely influences tumor progression. In addition to systemic effects, several studies have revealed that bidirectional dialog existing between adipocytes and tumor cells is heightened by obesity. 84 , 99 , 100 As previously discussed, obesity quantitatively and qualitatively modifies Ad‐EV secretion, which results in the amplification of their effects on tumor aggressiveness. Accordingly, adipocytes isolated from obese individuals possess a higher ability to stimulate tumor aggressiveness that is dependent on metabolic remodeling of tumor cells toward FAO as previously described. Indeed, we have shown that, in obesity, small Ad‐EVs are enriched in FAs, leading to increased lipid accumulation in melanoma cells that provide more fuel for FAO and, consequently further increase in melanoma migration. 18 Additionally, in the studies mentioned above, Wang et al hypothesized that small Ad‐EV‐dependent MMP3 transfer in lung cancer cells is increased in obese patients. 92 Similarly, in hepatocellular carcinoma, 93 Zhang et al observed that the circRNAs involved in the process of deubiquitylation are upregulated in hepatocellular cancer patients with higher body fat ratios, and proposed that it could be associated with an increased transfer of these RNAs. Finally, two studies have shown that obese visceral adipocytes secrete small EVs containing miRNAs involved in the regulation of the TGF‐β and Wnt/β‐catenin pathways. 27 , 101 Although not directly demonstrated in cancer, these miRNAs conveyed by Ad‐EVs derived from obese patients may also contribute to the deleterious effects of Ad‐EV on cancer aggressiveness.
To conclude, the effect of obese patient‐derived Ad‐EVs on cancer development is due to both an increase in their local concentrations and to specific modifications of their lipid, RNA, and protein content that may act synergistically to amplify the adverse effects of Ad‐EVs on cancer cells.
5. CONCLUSIONS AND PERSPECTIVES
All the works cited in this review clearly indicate that Ad‐EVs participate both in the adaptation of AT during energy flow variations and in the development of pathologies associated with metabolic and cardiovascular diseases as well as cancers. These data suggest that targeting this EV‐mediated dialog could contribute to reduce the deleterious effects of Ad‐EVs.
Strategies limiting EV generation have already been implemented by inhibiting molecular actors of their biogenesis such as ESCRT proteins, tetraspanins, or ceramides. However, targeting tetraspanins has been shown to result in adverse pulmonary and pathologies associated with aging. 102 , 103 Reducing ceramide production can be a promising strategy because lowering its lipid synthesis pathway using an inhibitor of its derivative sphingosine 1‐phosphate has proven to ameliorate nonalcoholic steatohepatitis in a mouse model. 104 Furthermore, inhibiting ceramide production with nSMase inhibitor GW4869 have been shown to hamper muscle degeneration in mdx mice. 105 Although the composition and the biological action of EVs from these treated animals were not determined, it presents a proof‐of‐concept that modulation of EV release might be a therapeutic approach to regulate deleterious organ cross‐talk associated with the transfer of EV cargos (e.g., the transfer of harmful lipids during the development of metabolic diseases associated with high‐fat diets). Considering the ability of EVs to convey FAs, it could also be interesting to study the beneficial effects of diet‐derived lipids in terms of EV composition, to determine whether it could counteract obesity‐associated palmitate EV enrichment.
Although Ad‐EVs have mostly been reported to elicit detrimental effects, experimental data using AT‐stromal cell‐derived EVs suggest that they can deliver beneficial effects. For instance, small ADSC‐EVs exert a beneficial effect on insulin sensitivity through their ability to reduce AT inflammation and to induce AT beiging, 59 and their regenerative properties in connection with cardiovascular disease. 76 Alternatively, ATM‐derived small EVs isolated from lean mice improve glucose tolerance and insulin sensitivity when administered to obese individuals. 64 Finally, injection of blood small EVs derived from the blood of 3‐month‐old mice has been shown to reverse the expression of aging‐associated biomarkers of 18‐month‐old mice. 106
Indeed, understanding how Ad‐EV structure and function can be modulated in a beneficial way at an organismal level and in all fat depots poses the current challenge.
CONFLICT OF INTEREST
There are no conflicts of interest to declare.
AUTHOR CONTRIBUTIONS
All the authors wrote and edited the paper.
ACKNOWLEDGMENTS
This work was supported by Société Française de Dermatologie et de Pathologie Sexuellement Transmissible, Fondation ARC pour la Recherche sur le Cancer and Ligue Contre le Cancer (LN). SLL’s financial supports are Société Francophone du Diabète, INSERM, Université d’Angers. XL’s financial supports are ANR‐DFG grant Cardinal «Non‐coding RNAs and post‐ischemic cardiac remodeling » 2016‐2020 (ANR‐16‐CE92‐0032‐02) and ANR JCJC Extrema «EXtracellular vesicle TRansfEr of MicroRNA and Atherosclerosis » 2016‐2020 (ANR‐16‐CE14‐0007‐01) and ANR ZENITH PRCE 2020. SR is supported by a grant from ANR (ZENiTH‐ANR‐PRCE 2020). Authors acknowledge S. Mazlan S and S. Chatterjee S for English corrections.
This article is part of the Extracellular Vesicles and Homeostasis Special Collection.
REFERENCES
- 1. Valadi H, Ekström K, Bossios A, et al. Exosome‐mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654‐659. [DOI] [PubMed] [Google Scholar]
- 2. Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24‐36. [DOI] [PubMed] [Google Scholar]
- 3. Cinti S. Pink adipocytes. Trends Endocrinol Metab. 2018;29(9):651‐666. [DOI] [PubMed] [Google Scholar]
- 4. Attané C, Estève D, Chaoui K, et al. Human bone marrow is comprised of adipocytes with specific lipid metabolism. Cell Rep. 2020;30(4):949‐958 e6. [DOI] [PubMed] [Google Scholar]
- 5. Crewe C, An YA, Scherer PE. The ominous triad of adipose tissue dysfunction: inflammation, fibrosis, and impaired angiogenesis. J Clin Invest. 2017;127(1):74‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Desdin‐Mico G, Mittelbrunn M. Role of exosomes in the protection of cellular homeostasis. Cell Adh Migr. 2017;11(2):127‐134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res. 2017;66:30‐41. [DOI] [PubMed] [Google Scholar]
- 8. Villasenor R, Kalaidzidis Y, Zerial M. Signal processing by the endosomal system. Curr Opin Cell Biol. 2016;39:53‐60. [DOI] [PubMed] [Google Scholar]
- 9. O’Brien K, Breyne K, Ughetto S, et al. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21(10):585‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu X, Lai Y, Hua ZC. Apoptosis and apoptotic body: disease message and therapeutic target potentials. Biosci Rep. 2019;39(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muralidharan‐Chari V, Clancy JW, Sedgwick A, et al. Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci. 2010;123(Pt 10):1603‐1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lentz BR. Exposure of platelet membrane phosphatidylserine regulates blood coagulation. Prog Lipid Res. 2003;42(5):423‐438. [DOI] [PubMed] [Google Scholar]
- 13. Crescitelli R, Lässer C, Szabó TG, et al. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J Extracell Vesicles. 2013;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tauro BJ, Greening DW, Mathias RA, et al. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell‐derived organoids. Mol Cell Proteomics. 2013;12(3):587‐598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113(8):E968‐E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Laulagnier K, Vincent‐Schneider H, Hamdi S, et al. Characterization of exosome subpopulations from RBL‐2H3 cells using fluorescent lipids. Blood Cells Mol Dis. 2005;35(2):116‐121. [DOI] [PubMed] [Google Scholar]
- 17. Lai RC, Tan SS, Yeo RWY, et al. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles. 2016;5:29828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clement E, Lazar I, Attané C, et al. Adipocyte extracellular vesicles carry enzymes and fatty acids that stimulate mitochondrial metabolism and remodeling in tumor cells. EMBO J. 2020;39(3):e102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kranendonk MEG, Visseren FLJ, van Balkom BWM, et al. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 2014;22(5):1296‐1308. [DOI] [PubMed] [Google Scholar]
- 20. Eguchi A, Lazic M, Armando AM, et al. Circulating adipocyte‐derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl). 2016;94(11):1241‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Connolly KD, Guschina I, Yeung V, et al. Characterisation of adipocyte‐derived extracellular vesicles released pre‐ and post‐adipogenesis. J Extracell Vesicles. 2015;4:29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Durcin M, Fleury A, Taillebois E, et al. Characterisation of adipocyte‐derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 2017;6(1):1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lazar I, Clement E, Dauvillier S, et al. Adipocyte exosomes promote melanoma aggressiveness through fatty acid oxidation: a novel mechanism linking obesity and cancer. Cancer Res. 2016;76(14):4051‐4057. [DOI] [PubMed] [Google Scholar]
- 24. Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78(3):783‐809. [DOI] [PubMed] [Google Scholar]
- 25. Lafontan M. Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways. Am J Physiol Cell Physiol. 2012;302(2):C327‐C359. [DOI] [PubMed] [Google Scholar]
- 26. Elsdale T, Bard J. Collagen substrata for studies on cell behavior. J Cell Biol. 1972;54(3):626‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koeck ES, Iordanskaia T, Sevilla S, et al. Adipocyte exosomes induce transforming growth factor beta pathway dysregulation in hepatocytes: a novel paradigm for obesity‐related liver disease. J Surg Res. 2014;192(2):268‐275. [DOI] [PubMed] [Google Scholar]
- 28. Amosse J, Durcin M, Malloci M, et al. Phenotyping of circulating extracellular vesicles (EVs) in obesity identifies large EVs as functional conveyors of Macrophage Migration Inhibitory Factor. Mol Metab. 2018;18:134‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Connolly KD, Wadey RM, Mathew D, et al. Evidence for adipocyte‐derived extracellular vesicles in the human circulation. Endocrinology. 2018;159(9):3259‐3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Thomou T, Mori MA, Dreyfuss JM, et al. Adipose‐derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542(7642):450‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flaherty SE, Grijalva A, Xu X, et al. A lipase‐independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363(6430):989‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuana Y, Levels J, Grootemaat A, et al. Co‐isolation of extracellular vesicles and high‐density lipoproteins using density gradient ultracentrifugation. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brennan K, Martin K, FitzGerald SP, et al. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci Rep. 2020;10(1):1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee J‐E, Moon P‐G, Lee I‐K, et al. Proteomic analysis of extracellular vesicles released by adipocytes of Otsuka long‐Evans Tokushima fatty (OLETF) rats. Protein J. 2015;34(3):220‐235. [DOI] [PubMed] [Google Scholar]
- 35. Böing AN, van der Pol E, Grootemaat AE, et al. Single‐step isolation of extracellular vesicles by size‐exclusion chromatography. J Extracell Vesicles. 2014;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field‐flow fractionation. Nat Cell Biol. 2018;20(3):332‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ertunc ME, Sikkeland J, Fenaroli F, et al. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res. 2015;56(2):423‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yu Y, Hongwei D, Wei S, et al. Adipocyte‐derived exosomal MiR‐27a induces insulin resistance in skeletal muscle through repression of PPARgamma. Theranostics. 2018;8(8):2171‐2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pan Y, Hui X, Hoo RLC, et al. Adipocyte‐secreted exosomal microRNA‐34a inhibits M2 macrophage polarization to promote obesity‐induced adipose inflammation. J Clin Invest. 2019;129(2):834‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Yu M, Tian W. Physiological and pathological impact of exosomes of adipose tissue. Cell Prolif. 2016;49(1):3‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gao X, Salomon C, Freeman DJ. Extracellular vesicles from adipose tissue‐a potential role in obesity and type 2 diabetes? Front Endocrinol (Lausanne). 2017;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou Y, Tan C. miRNAs in adipocyte‐derived extracellular vesicles: multiple roles in development of obesity‐associated disease. Front Mol Biosci. 2020;7:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim Y, Kim OK. Potential roles of adipocyte extracellular vesicle‐derived miRNAs in obesity‐mediated insulin resistance. Adv Nutr. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Müller G, Schneider M, Biemer‐Daub G, et al. Upregulation of lipid synthesis in small rat adipocytes by microvesicle‐associated CD73 from large adipocytes. Obesity (Silver Spring). 2011;19(8):1531‐1544. [DOI] [PubMed] [Google Scholar]
- 45. Zhang Y, Yu M, Dai M, et al. miR‐450a‐5p within rat adipose tissue exosome‐like vesicles promotes adipogenic differentiation by targeting WISP2. J Cell Sci. 2017;130(6):1158‐1168. [DOI] [PubMed] [Google Scholar]
- 46. Camino T, Nerea L‐B, Belén B, et al. Vesicles shed by pathological murine adipocytes spread pathology: characterization and functional role of insulin resistant/hypertrophied adiposomes. Int J Mol Sci. 2020;21(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ogawa R, Tanaka C, Sato M, et al. Adipocyte‐derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem Biophys Res Commun. 2010;398(4):723‐729. [DOI] [PubMed] [Google Scholar]
- 48. Kim MS, Muallem S, Kim SH, et al. Exosomal release through TRPML1‐mediated lysosomal exocytosis is required for adipogenesis. Biochem Biophys Res Commun. 2019;510(3):409‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Müller G, Schneider M, Biemer‐Daub G, et al. Microvesicles released from rat adipocytes and harboring glycosylphosphatidylinositol‐anchored proteins transfer RNA stimulating lipid synthesis. Cell Signal. 2011;23(7):1207‐1223. [DOI] [PubMed] [Google Scholar]
- 50. Sano S, Izumi Y, Yamaguchi T, et al. Lipid synthesis is promoted by hypoxic adipocyte‐derived exosomes in 3T3‐L1 cells. Biochem Biophys Res Commun. 2014;445(2):327‐333. [DOI] [PubMed] [Google Scholar]
- 51. Crewe C, Joffin N, Rutkowski JM, et al. An endothelial‐to‐adipocyte extracellular vesicle axis governed by metabolic state. Cell. 2018;175(3):695‐708.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aswad H, Forterre A, Wiklander OPB, et al. Exosomes participate in the alteration of muscle homeostasis during lipid‐induced insulin resistance in mice. Diabetologia. 2014;57(10):2155‐2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Deng ZB, Poliakov A, Hardy RW, et al. Adipose tissue exosome‐like vesicles mediate activation of macrophage‐induced insulin resistance. Diabetes. 2009;58(11):2498‐2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DeClercq V, d'Eon B, McLeod RS. Fatty acids increase adiponectin secretion through both classical and exosome pathways. Biochim Biophys Acta. 2015;1851(9):1123‐1133. [DOI] [PubMed] [Google Scholar]
- 55. Sobo K, Le Blanc I, Luyet P‐P, et al. Late endosomal cholesterol accumulation leads to impaired intra‐endosomal trafficking. PLoS One. 2007;2(9):e851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gao J, Li X, Wang Y, et al. Adipocyte‐derived extracellular vesicles modulate appetite and weight through mTOR signalling in the hypothalamus. Acta Physiol (Oxf). 2020;228(2):e13339. [DOI] [PubMed] [Google Scholar]
- 57. Baranova A, Maltseva D, Tonevitsky A. Adipose may actively delay progression of NAFLD by releasing tumor‐suppressing, anti‐fibrotic miR‐122 into circulation. Obes Rev. 2019;20(1):108‐118. [DOI] [PubMed] [Google Scholar]
- 58. Wernstedt Asterholm I, Tao C, Morley TS, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20(1):103‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhao H, Shang Q, Pan Z, et al. Exosomes from adipose‐derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67(2):235‐247. [DOI] [PubMed] [Google Scholar]
- 60. Yang J, Hagen J, Guntur KV, et al. A next generation sequencing based approach to identify extracellular vesicle mediated mRNA transfers between cells. BMC Genom. 2017;18(1):987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang Y, Mei H, Chang X, et al. Adipocyte‐derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR‐155. J Mol Cell Biol. 2016;8(6):505‐517. [DOI] [PubMed] [Google Scholar]
- 62. Mleczko J, Ortega FJ, Falcon‐Perez JM, et al. Extracellular vesicles from hypoxic adipocytes and obese subjects reduce insulin‐stimulated glucose uptake. Mol Nutr Food Res. 2018;62(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hubal MJ, Nadler EP, Ferrante SC, et al. Circulating adipocyte‐derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25(1):102‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ying W, Riopel M, Bandyopadhyay G, et al. Adipose tissue macrophage‐derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell. 2017;171(2):372‐384; e12. [DOI] [PubMed] [Google Scholar]
- 65. Kranendonk ME, Visseren FLJ, van Herwaarden JA, et al. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring). 2014;22(10):2216‐2223. [DOI] [PubMed] [Google Scholar]
- 66. Chen Y, Siegel F, Kipschull S, et al. miR‐155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun. 2013;4:1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Puhm F, Afonyushkin T, Resch U, et al. Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circ Res. 2019;125(1):43‐52. [DOI] [PubMed] [Google Scholar]
- 68. Brestoff JR, Wilen CB, Moley JR, et al. Intercellular mitochondria transfer to macrophages regulates white adipose tissue homeostasis and is impaired in obesity. Cell Metab. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Eguchi A, Mulya A, Lazic M, et al. Microparticles release by adipocytes act as "find‐me" signals to promote macrophage migration. PLoS One. 2015;10(4):e0123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Freeman DW, Hooten NN, Eitan E, et al. Altered extracellular vesicle concentration, cargo, and function in diabetes. Diabetes. 2018;67(11):2377‐2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Muller G. Microvesicles/exosomes as potential novel biomarkers of metabolic diseases. Diabetes Metab Syndr Obes. 2012;5:247‐282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wadey RM, Connolly KD, Mathew D, et al. Inflammatory adipocyte‐derived extracellular vesicles promote leukocyte attachment to vascular endothelial cells. Atherosclerosis. 2019;283:19‐27. [DOI] [PubMed] [Google Scholar]
- 73. Barberio MD, Kasselman LJ, Playford MP, et al. Cholesterol efflux alterations in adolescent obesity: role of adipose‐derived extracellular vesical microRNAs. J Transl Med. 2019;17(1):232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang F, Chen F‐F, Shang Y‐Y, et al. Insulin resistance adipocyte‐derived exosomes aggravate atherosclerosis by increasing vasa vasorum angiogenesis in diabetic ApoE(‐/‐) mice. Int J Cardiol. 2018;265:181‐187. [DOI] [PubMed] [Google Scholar]
- 75. Gan LU, Xie D, Liu J, et al. Small extracellular microvesicles mediated pathological communications between dysfunctional adipocytes and cardiomyocytes as a novel mechanism exacerbating ischemia/reperfusion injury in diabetic mice. Circulation. 2020;141(12):968‐983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Fleury A, Martinez MC, Le Lay S. Extracellular vesicles as therapeutic tools in cardiovascular diseases. Front Immunol. 2014;5:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu Z, Yueqiao X, Wan Y, et al. Exosomes from adipose‐derived mesenchymal stem cells prevent cardiomyocyte apoptosis induced by oxidative stress. Cell Death Discov. 2019;5:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu J, Jiang M, Deng S, et al. miR‐93‐5p‐containing exosomes treatment attenuates acute myocardial infarction‐induced myocardial damage. Mol Ther Nucleic Acids. 2018;11:103‐115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lindoso RS, Lopes JA, Binato R, et al. Adipose mesenchymal cells‐derived EVs alleviate DOCA‐salt‐induced hypertension by promoting cardio‐renal protection. Mol Ther Methods Clin Dev. 2020;16:63‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309‐322. [DOI] [PubMed] [Google Scholar]
- 81. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423‐1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ciardiello C, Cavallini L, Spinelli C, et al. Focus on extracellular vesicles: new frontiers of cell‐to‐cell communication in cancer. Int J Mol Sci. 2016;17(2):175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Park J, Euhus DM, Scherer PE. Paracrine and endocrine effects of adipose tissue on cancer development and progression. Endocr Rev. 2011;32(4):550‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Duong MN, Geneste A, Fallone F, et al. The fat and the bad: Mature adipocytes, key actors in tumor progression and resistance. Oncotarget. 2017;8(34):57622‐57641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484‐498. [DOI] [PubMed] [Google Scholar]
- 86. Dirat B, Bochet L, Dabek M, et al. Cancer‐associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455‐2465. [DOI] [PubMed] [Google Scholar]
- 87. Andarawewa KL, Motrescu ER, Chenard M‐P, et al. Stromelysin‐3 is a potent negative regulator of adipogenesis participating to cancer cell‐adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65(23):10862‐10871. [DOI] [PubMed] [Google Scholar]
- 88. Iyengar P, Espina V, Williams TW, et al. Adipocyte‐derived collagen VI affects early mammary tumor progression in vivo, demonstrating a critical interaction in the tumor/stroma microenvironment. J Clin Invest. 2005;115(5):1163‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zhang M, Di Martino JS, Bowman RL, et al. Adipocyte‐derived lipids mediate melanoma progression via FATP proteins. Cancer Discov. 2018;8(8):1006‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang YY, Attané C, Milhas D, et al. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2(4):e87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nieman KM, Kenny HA, Penicka CV, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Wang J, Wu Y, Guo J, et al. Adipocyte‐derived exosomes promote lung cancer metastasis by increasing MMP9 activity via transferring MMP3 to lung cancer cells. Oncotarget. 2017;8(47):81880‐81891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang H, Deng T, Ge S, et al. Exosome circRNA secreted from adipocytes promotes the growth of hepatocellular carcinoma by targeting deubiquitination‐related USP7. Oncogene. 2019;38(15):2844‐2859. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94. Liu Y, Tan J, Ou S, et al. Adipose‐derived exosomes deliver miR‐23a/b to regulate tumor growth in hepatocellular cancer by targeting the VHL/HIF axis. J Physiol Biochem. 2019;75(3):391‐401. [DOI] [PubMed] [Google Scholar]
- 95. Wang S, Su X, Xu M, et al. Exosomes secreted by mesenchymal stromal/stem cell‐derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res Ther. 2019;10(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Au Yeung CL, Co N‐N, Tsuruga T, et al. Exosomal transfer of stroma‐derived miR21 confers paclitaxel resistance in ovarian cancer cells through targeting APAF1. Nat Commun. 2016;7:11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Renehan AG, Tyson M, Egger M, et al. Body‐mass index and incidence of cancer: a systematic review and meta‐analysis of prospective observational studies. Lancet. 2008;371(9612):569‐578. [DOI] [PubMed] [Google Scholar]
- 98. Calle EE, Rodriguez C, Walker‐Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625‐1638. [DOI] [PubMed] [Google Scholar]
- 99. Park J, Morley TS, Kim M, et al. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455‐465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Lengyel E, Makowski L, DiGiovanni J, et al. Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer. 2018;4(5):374‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ferrante SC, Nadler EP, Pillai DK, et al. Adipocyte‐derived exosomal miRNAs: a novel mechanism for obesity‐related disease. Pediatr Res. 2015;77(3):447‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Jin Y, Takeda Y, Kondo Y, et al. Double deletion of tetraspanins CD9 and CD81 in mice leads to a syndrome resembling accelerated aging. Sci Rep. 2018;8(1):5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Takeda Y, He P, Tachibana I, et al. Double deficiency of tetraspanins CD9 and CD81 alters cell motility and protease production of macrophages and causes chronic obstructive pulmonary disease‐like phenotype in mice. J Biol Chem. 2008;283(38):26089‐26097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Mauer AS, Hirsova P, Maiers JL, et al. Inhibition of sphingosine 1‐phosphate signaling ameliorates murine nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G300‐G313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Matsuzaka Y, Tanihata J, Komaki H, et al. Characterization and functional analysis of extracellular vesicles and muscle‐abundant miRNAs (miR‐1, miR‐133a, and miR‐206) in C2C12 myocytes and mdx mice. PLoS One. 2016;11(12):e0167811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lee BR, Kim J‐H, Choi E‐S, et al. Effect of young exosomes injected in aged mice. Int J Nanomedicine. 2018;13:5335‐5345. [DOI] [PMC free article] [PubMed] [Google Scholar]


